Abstract
The rhythmic and spontaneously generated electrical excitation that triggers the heartbeat originates in the sinoatrial node (SAN). SAN automaticity has been thoroughly investigated, which has uncovered fundamental mechanisms involved in cardiac pacemaking that are generally categorised into two interacting and overlapping systems: the ‘membrane’ and ‘Ca2+ clock’. The principal focus of research has been on these two systems of oscillators, which have been studied primarily in single cells and isolated tissue, experimental preparations that do not consider mechanical factors present in the whole heart. SAN mechano-sensitivity has long been known to be a contributor to SAN pacemaking—both as a driver and regulator of automaticity—but its essential nature has been underappreciated. In this review, following a description of the traditional ‘clocks’ of SAN automaticity, we describe mechanisms of SAN mechano-sensitivity and its vital role for SAN function, making the argument that the ‘mechanics oscillator’ is, in fact, the ‘grandfather clock’ of cardiac rhythm.
Keywords: Mechano-electric coupling, Stretch, Pacemaking, Calcium clock, Membrane clock, Heart rate
Introduction
Perhaps not surprising for such a critical element to life, the heart can independently maintain its function. Even when removed from the body, it continues to beat and intrinsically regulate its activity. This is possible as the electrical excitation that initiates the heartbeat is produced within the organ itself (which, incidentally, is one of the principal reasons that heart transplantation is also possible). The heart’s intrinsic pacemaker, the sinoatrial node (SAN), rhythmically generates action potentials (AP) that propagate through the myocardium, causing the heart to contract. The myogenic origin of cardiac excitation was first identified nearly 140 years ago by Walter Gaskell (Gaskell 1882), and its anatomical location within the heart a few decades later by Keith and Flack (Keith and Flack 1907). In more than a century since, an entire field of research investigating SAN automaticity has emerged, which has taught us much about what drives pacemaker function, regulation, and dysfunction. Yet, our understanding is far from complete, and fundamental questions remain unanswered.
Perhaps the most heavily studied—but still most highly contested—questions regarding the SAN relate to the mechanism(s) responsible for its automaticity: what keeps it ticking? There is still no consensus as to the relative importance of the various subcellular mechanisms involved in spontaneously generating the SAN AP (Lakatta and DiFrancesco 2009; Noble et al. 2010; Rosen et al. 2012). Yet, despite contradictory perspectives regarding the importance of individual cellular components for SAN automaticity, there is now general agreement that SAN pacemaking consists of a robust, dynamic, and flexible system characterised by multiple integrated subsystems and contributors.
The SAN AP differs from the AP of working cardiomyocytes in multiple ways, the most important for automaticity being the period of spontaneous diastolic depolarisation (SDD, rather than diastolic rest), during which membrane potential gradually depolarises from its most negative value (maximum diastolic potential, MDP) towards the threshold for AP generation (Bartos et al. 2015). The slope of SDD is the key to determining the frequency of SAN firing, and thus heart rate (Mangoni and Nargeot 2008). Two predominant systems contributing to SDD have been identified and extensively studied: the so-called ‘membrane clock’ (consisting of sarcolemmal ionic currents) and the ‘calcium (Ca2+) clock’ (comprising intracellular Ca2+ cycling) (Lakatta et al. 2008; Difrancesco 2010), which are mutually entrained to form a system of coupled oscillators capable of generating SAN automaticity (Lakatta et al. 2010).
This understanding of pacemaker function, however, has been developed based largely on investigations of mechanisms in isolated cells (and to a lesser degree, isolated tissue), which neglects factors acting in the whole heart. There are various important extracardiac neurohumoral factors that influence heart rate by acting directly on mechanisms of SAN automaticity, including those released locally by the autonomic nervous system and those released into the bloodstream by the endocrine system (MacDonald et al. 2020b). There are also intracardiac factors that acutely affect SAN function, perhaps the most well established being stretch, which is a major determinant of in vivo heart rate (Quinn and Kohl 2012).
In this review, after outlining the principal components of the two classical ‘clocks’ of SAN automaticity and their mutual entrainment, we briefly summarise the primary mechanisms of SAN mechano-sensitivity and the critical contribution of SAN stretch to pacemaking, making an argument for its role as the ‘grandfather clock’ of cardiac rhythm (Fig. 1).
Fig. 1.
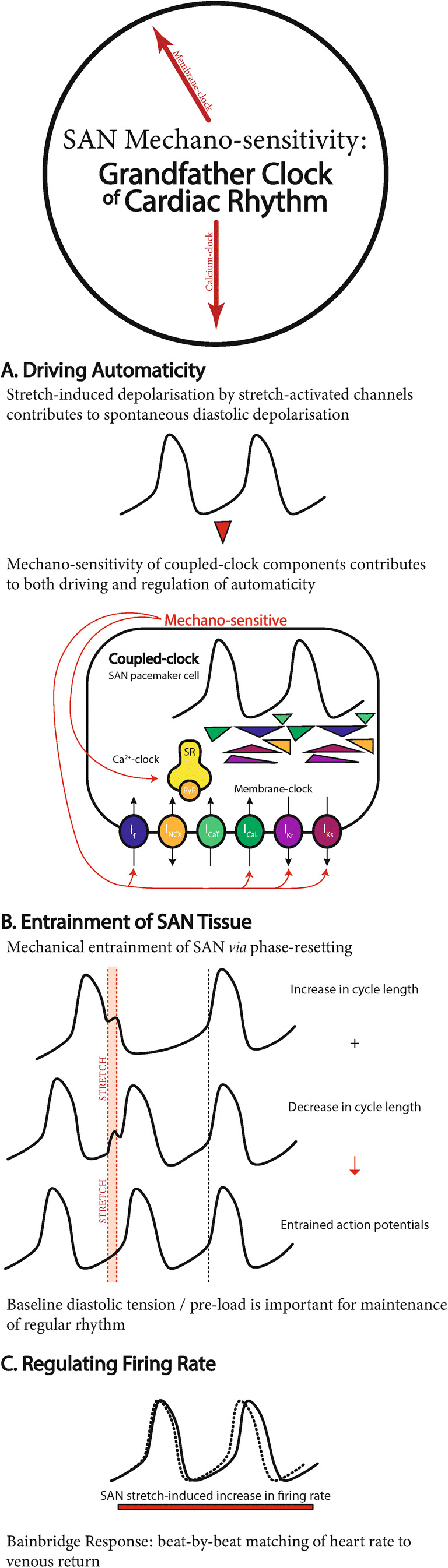
The grandfather clock of cardiac rhythm. Summary of the role of mechano-sensitivity in sinoatrial node (SAN) A automaticity, B entrainment, and C regulation. For expanded figures of the coupled-clock system, please refer to Lakatta et al. (2010), Quinn and Kohl (2012), and Bartos et al. (2015)
The classical understanding of cardiac pacemaking
Membrane clock
During early SDD, the membrane clock is driven by the “funny” current (If), an inward cation current that becomes increasingly activated as membrane potential becomes more negative (Bartos et al. 2015). It is passed through hyperpolarisation-activated cyclic nucleotide-gated (HCN) channels (Difrancesco 2010), of which there are four isoforms. HCN isoforms 1, 2, and 4 are expressed throughout the human heart and more prominently in the SAN than the atria, particularly HCN1, which in humans is almost exclusively expressed in the SAN (Li et al. 2015). The vital importance of HCN channels for pacemaking has been corroborated in HCN knockout mice, which display the hallmarks of SAN dysfunction, including prolonged recovery, prolonged conduction time, bradycardia, sinus dysrhythmia, and recurrent sinus pauses (Fenske et al. 2013; DiFrancesco et al. 2021).
Although some have asserted that If is the predominant driver of SDD and the primary pacemaking mechanism (Difrancesco and Noble 2012), inward transmembrane Ca2+ currents also contribute to SDD. They are passed through both transient (T-type) and long-lasting (L-type) Ca2+ channels (Mangoni and Nargeot 2008). Cav3.1 T-type and Cav1.3 L-type Ca2+ channels contribute to the early portion of SDD, as they become activated at a relatively low membrane potential (Mangoni et al. 2003). SDD ends at the threshold for Cav1.2 L-type Ca2+ channels (approximately −40 mV), at which point their activation generates the upstroke of the SAN AP (Mesirca et al. 2015). Although fast sodium (Na+) channels do not trigger the upstroke in SAN cells (as they do in working myocytes), they are heterogeneously expressed at low levels throughout the SAN and appear to make some contribution to automaticity (Lei et al. 2005).
Repolarising currents are also fundamental to the membrane clock’s contribution to pacemaking; in fact, prior to the identification of If, their decay at the end of the SAN AP was thought by many to be the main driver of SAN automaticity. The rapid and slow delayed rectifier potassium (K+) currents (IKr and IKs) repolarise SAN myocytes to their MDP, but at the same time, their total current is continuously reduced. This repolarisation allows for a simultaneous increase in the activation of If, driving depolarisation (Mangoni and Nargeot 2008). Importantly, this depolarisation is not prevented by inwardly rectifying K+ channels (which stabilise and maintain the negative resting membrane potential of working cardiomyocytes), as those channels are minimally expressed or absent in SAN myocytes (Bartos et al. 2015).
So, overall, the balance of depolarising inward and repolarising outward membrane clock currents is one of the main determinants of SDD slope and largely responsible for the oscillations that drive SAN AP firing, which ultimately establishes heart rate.
Ca2+ clock
Intracellular Ca2+ cycling has also been shown to be a major contributor to SDD and SAN automaticity (Bartos et al. 2015). Local Ca2+ releases (LCR) from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyR) result in an increase in cytosolic Ca2+ concentration. Some of this Ca2+ is returned to the SR by the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), while the remainder is extruded from the cell by the Na+-Ca2+ exchanger (NCX)—with 1 Ca2+ ion exiting for 3 Na+ ions entering—which generates an electrogenic, depolarising current (Lakatta et al. 2008, 2010). Unlike Ca2+ sparks released from the SR at rest in working cardiomyocytes, LCR during SDD in the SAN are rhythmic, larger in amplitude, and longer in duration (Sirenko et al. 2013). This may be partly explained by the fact that while LCR were originally thought to be spontaneous, they are actually, at least in part, triggered by Ca2+ influx via Cav1.3 L-type Ca2+ channels (Torrente et al. 2016). The importance of diastolic intracellular Ca2+ cycling in SAN myocytes is further enhanced by the fact that they have higher basal cyclic adenosine monophosphate (cAMP) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) levels than working cardiomyocytes, which results in greater phosphorylation of Ca2+ handling proteins (L-type Ca2+ channels, RyR, and phospholamban) and an increase in their activity (Vinogradova et al. 2000, 2006).
Overall, the rate and amplitude of intracellular LCR, and the balance between Ca2+ reuptake into the SR by SERCA and extrusion via NCX, are important determinants of SDD slope, and thus of heart rate (Vinogradova and Lakatta 2009).
Coupled-oscillator system
The combined actions of the membrane and Ca2+ clocks form a robust and redundant system for SAN automaticity. These individually defined ‘clocks’ are tightly coupled, as the action of one influences the other (Bogdanov et al. 2006; Mattick et al. 2007; Lakatta et al. 2008). Changes in membrane potential driven by the membrane clock influence intracellular Ca2+ balance, while LCR, as part of the Ca2+ clock, activate NCX, which is located in the cell membrane and directly alters its potential. The activity of the two clocks is further coordinated through entrainment by mutual intracellular regulatory mechanisms (MacDonald et al. 2020b). In fact, the clocks are so tightly coupled and interdependent one must question whether it is even productive or beneficial to distinguish between them; the oscillatory nature of the SAN is the result of the combined activity of the various components of the membrane and Ca2+ clocks, even though none alone are independently oscillatory. None of If, trans-sarcolemmal Ca2+ or Na+ flux, activation of NCX by LCR, or the decay of IK can independently produce the rhythmic membrane potential oscillations that result in SAN automaticity. Also, the SAN continues to fire even with the loss of individual clock components, indicating a protective redundancy. Therefore, the system driving SAN automaticity is best thought of as a system of coupled oscillators (rather than individual ‘clocks’).
It is important to recognise, however, that the activity of an individual pacemaker cell in well coupled SAN tissue will not be able to excite the entire node on its own. Thus, not only are the cellular contributors to automaticity within SAN cells mutually entrained but so must be the activity of individual cells within SAN tissue, resulting in their synchronous excitation (Jalife 1984). One mechanism by which this tissue-level entrainment of cellular activity may occur is through a cyclic stretch of the SAN, as the right atrium fills with blood during each heartbeat, which directly influences cell-level automaticity due to the inherent mechano-sensitivity of SAN myocytes (Quinn and Kohl 2012, 2021). In fact, as described below, SAN mechano-sensitivity might itself be considered a ‘mechanics clock’ (or better a ‘mechanics oscillator’), as stretch effects are a key driver—and perhaps a master controller—of cardiac pacemaking.
The contribution of SAN stretch to cardiac pacemaking
The physiological importance of stretch effects on heart rate
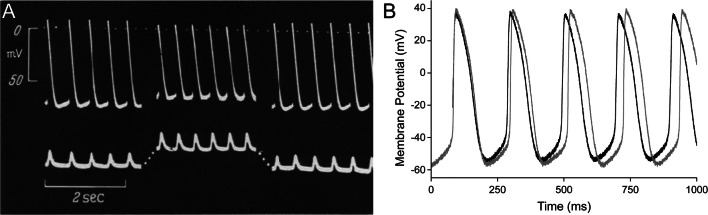
The direct effects of stretch on SAN activity were first established by Starzinsky and von Bezold, who showed in rabbits with severed connections between the heart and the autonomic nervous system that an increase in venous return caused sinus tachycardia (Starzinsky and von Bezold 1867). More generally known, however, is the work of Francis Bainbridge, who demonstrated that right atrial distention by intravenous fluid injection caused an acute increase in heart rate in anaesthetised dogs (Bainbridge 1915). This response was later corroborated in humans by passively lifting the legs of healthy subjects to increase venous return to the heart—without a simultaneous change in arterial pressure—which similarly increased heart rate (Roddie et al. 1957; Donald and Shepherd 1978). This effect is now commonly known as the ‘Bainbridge response’. An acute increase in heart rate or SAN beating rate in response to sustained atrial or SAN stretch has been shown to also occur in a multitude of experimental animals across the invertebrate (Sénatore et al. 2010) and vertebrate (Pathak 1973) phyla, and most recently in zebrafish (MacDonald et al. 2017). Interestingly, this is not the case in the mouse SAN, however, where beating rate tends to decrease with sustained stretch (Cooper and Kohl 2005). This species difference in the heart rate response to stretch can be explained by the relation of the reversal potential of the stretch-activated channels involved (discussed further below) to the species-specific action potential morphology (Cooper and Kohl 2005; MacDonald et al. 2020a); however, that explanation is yet to be experimentally verified. Regardless, the evolutionary conservation of the heart rate response to stretch demonstrates the fundamental nature of stretch effects on SAN automaticity. While originally considered to be an extracardiac, neurohumorally mediated effect, an increase in beating rate with stretch is observed not only in intact animals but also in isolated hearts and right atrial tissue (Blinks 1956), the SAN (Deck 1964), and single SAN cells (Cooper et al. 2000) (Fig. 2), indicating that intracardiac mechanisms are key contributors. For a comprehensive summary of the clinically and experimentally observed effects of stretch on SAN function, please see (Quinn and Kohl 2012, 2021).
Fig. 2.
Stretch-induced increase in the beating rate of isolated sinoatrial node preparations. A Intracellular sharp electrode recording of transmembrane potential (top) and applied and generated force (bottom; passive stretch and active contraction pointing upwards) in spontaneously beating cat isolated sinoatrial node (SAN) tissue (from Deck 1964) and B patch-clamp recording of transmembrane potential in a spontaneously beating rabbit isolated SAN cell (light curve, before stretch; dark curve, during stretch) (from Cooper et al. 2000). Both show an increase in beating rate during stretch, accompanied by a reduction in the absolute value of maximum diastolic and maximum systolic potential
The acute increase in SAN automaticity that occurs with distension of the right atrium (the Bainbridge response) is a critical regulator of heart rate, which along with stretch-induced changes in ventricular stroke volume via the Frank–Starling mechanism allows the heart to match cardiac output (= heart rate × stroke volume) to changes in venous return on a beat-by-beat basis (Quinn and Kohl 2016). The Bainbridge response also opposes the baroreceptor response (the Bezold–Jarisch or depressor reflex, which reduces heart rate when arterial blood pressure is increased, von Bezold and Hirt 1867; Jarisch and Richter 1939), thus preventing excessive bradycardia or overdistension of the right atrium, while helping to maintain cardiac output and adequate circulation during hemodynamic changes that increase both venous return and arterial pressure. Thus, the increase in heart rate with SAN stretch is vital for maintaining balanced cardiovascular system performance, while also matching the throughput of the left and right sides of the heart over any period of time (Quinn and Kohl 2012; Quinn 2015). Being a fundamental autoregulatory mechanism of cardiac function, it is perhaps not surprising that the mechanisms of the SAN stretch response are not only intrinsic to the heart but in fact to SAN myocytes themselves (Cooper et al. 2000), reflecting their inherent mechano-sensitivity.
Mechanisms of SAN mechano-sensitivity
Although the cellular mechanisms of the SAN stretch response remain incompletely understood (Quinn and Kohl 2012, 2021), it is clear that they relate to acute feedback of the heart’s mechanical status to its electrical activity, a process known as “mechano-electric feedback” or “mechano-electric coupling” (Quinn et al. 2014; Quinn and Kohl 2021). Clinical and experimental observations of the acute effects of SAN stretch can generally be explained by evoking a mechano-sensitive trans-sarcolemmal current with a reversal potential between the MDP and maximum systolic potential (MSP) of SAN myocytes. Cation non-selective stretch-activated channels (SACNS), with a reversal potential between −20 and 0 mV (Guharay and Sachs 1984) would pass such a current. In fact, a stretch of single SAN myocytes results in the activation of a current with a reversal potential of approximately −11 mV (Cooper et al. 2000) and its pharmacological block with Grammostola spatulata mechanotoxin-4 (GsMTx-4) reduces the increase in SAN beating rate seen with stretch (Cooper and Kohl 2005).
Nevertheless, the molecular identity of SACNS in the SAN has not yet been determined (Peyronnet et al. 2016) and one must be cautious not to fall into a ‘plausibility trap’ by assuming its critical importance (Quinn and Kohl 2011), as there are several other mechano-sensitive components within SAN cells that may also contribute to the stretch response. In particular, mechano-sensitivity of membrane and Ca2+ clock components (Quinn and Kohl 2012) may partly mediate the effects of SAN stretch on automaticity.
To start, If has been shown to be mechano-sensitive. In cell expression systems, the activation, deactivation, and current amplitude of HCN channels are altered by mechanical stimuli (Calloe et al. 2005; Lin et al. 2007), which results in a frequency-dependent alteration in the rate of cell excitation (Lin et al. 2007). Other components of the membrane clock have also been shown to be mechano-sensitive, including L-type Ca2+ channels (Calabrese et al. 2002; Lyford et al. 2002), fast Na+ channels, and delayed rectifier K+ channels (Morris 2011). Components of the Ca2+ clock have likewise been shown to be mechano-sensitive in other cardiac cell types, as axial stretch of ventricular myocytes results in an increase of Ca2+ sparks (Iribe et al. 2009). Lowered extracellular Ca2+ and pharmacological inhibition of SERCA (which prevents the reuptake of Ca2+ into the SR) or of RyR (which prevents Ca2+ release from the SR) results in a reduction in the SAN stretch response (Arai et al. 1996). These findings, along with the immediate change in SAN cell beating rate that occurs with acute changes in intracellular Ca2+ concentration (Yaniv et al. 2011), support the potential importance of the mechano-sensitivity of Ca2+ handling in the response of the SAN to stretch. Ultimately, if any of the above mechanically induced changes seen in other cell types occur in SAN myocytes, they could make significant contributions to SAN mechano-sensitivity, and while the specific mechanism(s) leading to the acute response of the SAN to stretch remain unclear, it seems reasonable to assume that like the coupled-oscillator system driving automaticity, multiple mechanisms may be involved. What is clear is that stretch generally leads to an increase in SAN beating rate, which may be important for in vivo SAN function.
Mechanical entrainment of SAN activity
In vivo, during atrial diastole, the ventricles are contracting, pulling the atrioventricular valve plane apically and causing a stretch of the atrial tissue containing the SAN (Hales et al. 2012). The SAN is then stretched further as blood returns to the heart and fills the right atrium. Peak SAN stretch levels coincide with the period of SDD, as membrane potential is moving towards the threshold for AP generation, so any stretch-induced depolarising currents will act to mechanically ‘prime’ pacemaker cells for excitation. This allows for a beat-by-beat adaptation of heart rate to changes in venous return, such as occur with exercise, alterations in posture, or modulation of thorax-abdomen pressure gradients caused by respiratory activity (Quinn and Kohl 2012).
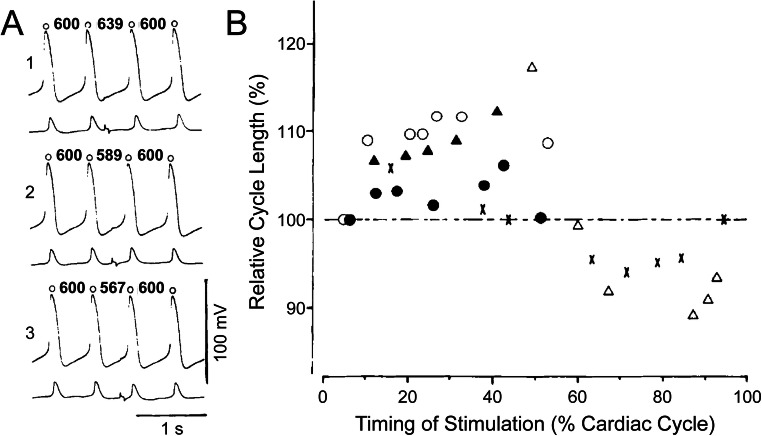
At the tissue level, a stretch of the SAN may play another important role. While the majority of SAN cells will experience stretch-induced depolarisation during a similar period of SDD, cells that are not firing synchronously will experience it at some other point in the cardiac cycle. The response to this ‘out-of-phase’ stretch may act to entrain (or reset) the electrical activity of those cells via a phenomenon known as ‘phase-resetting’, so that excitation is more uniformly timed across the entire SAN. It has been shown that injection of a subthreshold (i.e. non-excitatory) depolarising current pulse into spontaneously beating SAN cells (as would occur with SAN stretch) can result in an increase or a decrease in their beating rate, depending on the timing of the stimulation within the cardiac cycle (Anumonwo et al. 1991), which can entrain SAN cell activity (Verheijck et al. 1998). Phase-resetting behaviour, in response to an externally applied, subthreshold electrical stimulus, has been shown to also occur in SAN tissue (Fig. 3) (Sano et al. 1978; Jalife and Antzelevitch 1979) and has been corroborated by computational modelling (Ypey et al. 1982; Reiner and Antzelevitch 1985; Guevara and Jongsma 1990; Anumonwo et al. 1991; Coster and Celler 2003; Krogh-Madsen et al. 2004; Tsalikakis et al. 2007; Huang et al. 2011). Through this phenomenon, subthreshold depolarisation of SAN cells generated intrinsically by stretch may act to normalise heterogeneous electrical activity across non-synchronous cells and help prevent abnormally fast or arrhythmic groups of cells from overtaking pacemaking by their entrainment (Abramovich-Sivan and Akselrod 1999). Since the SAN is constantly subjected to oscillating cyclic stretch in vivo, the stretch may thus act to specifically enhance SDD and to ‘smooth out’ differences in automaticity between cells across the node, thus stabilising rhythm (Ushiyama and Brooks 1977).
Fig. 3.
Phase resetting in the sinoatrial node (SAN). A Application of subthreshold square-wave pulse in the early (1), middle (2), and late (3) phase of the cardiac cycle in the rabbit isolated SAN (lower tracings in each section are action potentials from the SAN region close to the atrium to show time of stimulus artefacts) and B the relationship between cycle length and time of stimulation in the cardiac cycle, showing that subthreshold depolarising current pulses result in an increase or a decrease in cycle length, depending on the timing of the stimulation within the cardiac cycle. From Sano et al. (1978)
SAN mechano-sensitivity: the grandfather clock of cardiac rhythm
Maintenance of baseline heart rate and rhythm via SAN mechano-sensitivity
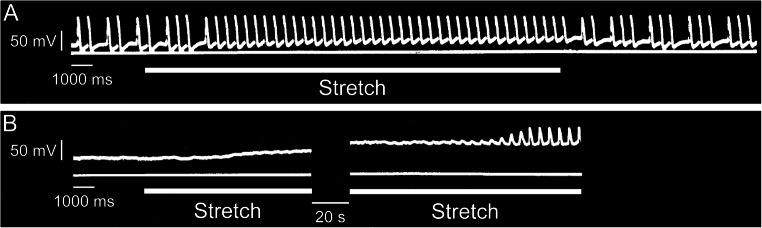
Even though it was discovered over 110 years ago (Keith and Flack 1907), our understanding of the SAN continues to develop. For instance, it has just recently been revealed that there are two distinct and competing pacemaker regions within the SAN, localised near the superior and inferior vena cava, which preferentially drive fast and slow heart rates, helping explain previous observations of pacemaker shifts in response to various physiological inputs (Brennan et al. 2020). With the importance of re-evaluating previous experimental evidence in mind, we propose that the strong emphasis put on the contributions of the membrane and Ca2+ oscillators to SAN automaticity have meant that a critical contributor—the mechanics oscillator—has been largely overlooked. While the importance of the Bainbridge response as a beat-by-beat regulator of heart rate in response to changes in venous return is well established, the fundamental importance of diastolic stretch and mechanical oscillations to SDD and SAN automaticity are underappreciated (yet critical), as SAN mechano-sensitivity may be involved in maintaining the regularity of baseline rhythm and entraining myocytes across the node. The contribution of mechanical load to SDD was first established in 1964 by Klaus Deck, using microelectrode recordings of membrane potential during an equi-biaxial stretch of cat and rabbit isolated SAN tissue (Deck 1964). The critical nature of a ‘minimal’ diastolic tension for the generation and stabilisation of rhythmic SAN excitation was confirmed soon after, as slack isolated SAN tissue often shows irregular or no rhythm, and the application of physiological levels of baseline stretch restores regular activity (Fig. 4) (Brooks et al. 1966; Lange et al. 1966; Ushiyama and Brooks 1977). In such cases, it is apparent that missed beats or SAN quiescence are due to the failure of other pacemaker mechanisms to sustain SDD in the absence of a sufficient mechanical preload, which when applied restores function through a positive shift in MDP towards AP threshold, resulting in regular, spontaneous excitation of the SAN. The importance of an adequate preload for SAN pacemaking may in fact be present from the very first heartbeat during embryonic development, as fluid pressure buildup in the quiescent cardiac tube appears to be a prerequirement for the initiation of spontaneous cardiac excitation during ontogenesis (Rajala et al. 1976, 1977; Chiou et al. 2016).
Fig. 4.
Effects of physiological levels of baseline stretch on isolated sinoatrial node (SAN) beating rate. Floating microelectrode recordings of transmembrane potential in cat isolated SAN, showing a stretch-induced shift of the maximum diastolic potential towards less negative values, resulting in A restoration of regular rhythm in a SAN with irregular activity at slack length or B initiation of spontaneous excitation in a previously quiescent SAN. In both examples, tissue length was increased by ~40% from slack, with periods of stretch indicated by the lower horizontal lines. From Lange et al. (1966)
Implications of SAN mechano-sensitivity in cardiac disease and in ageing
A consequence of the apparent vital role of stretch for SAN automaticity is that it may be an important consideration in some forms of SAN dysfunction. The SAN stretch response has been shown to be influenced by tissue structure and stiffness (MacDonald et al. 2020a), so there may be a stretch-dependent link between SAN dysfunction and structural or mechanical changes that occur with advanced age and in many cardiac pathologies. Stretch-induced SAN dysfunction with ageing and in disease may be further exasperated by changes in SAN mechano-sensitivity secondary to electrical remodelling (Kistler et al. 2004; Morris and Kalman 2014; Csepe et al. 2015) or by an increase in electrically-coupled, mechano-sensitive fibroblasts (Kohl et al. 1994; Kohl and Noble 1996; Quinn et al. 2016) with increased fibrosis, which will also affect the normal patterns of stretch during the cardiac cycle, leading to an altered stretch response. Furthermore, the stabilisation of heart rate by stretch appears to be functional only within a certain range, as excessive stretch results in irregular rhythms (Lange et al. 1966) and multifocal activity (Hoffman and Cranefield 1960), which may in part account for SAN dysfunction in pathologies associated with atrial volume overload (Sparks et al. 1999; Morton et al. 2003; Sanders et al. 2003).
Conclusions and future research
The SAN is a vital piece of tissue for sustaining life, and thus its electrical activity is driven by a system of integrated and redundant mechanisms to ensure it continues to operate under a wide variety of (patho-)physiological conditions. SAN stretch is one fundamental contributor to pacemaking, as it: (A) drives SAN automaticity by contributing to SDD through SACNS and/or mechano-sensitivity of coupled-clock components; (B) entrains pacemaker cells across the SAN via phase-resetting caused by their mechanically induced subthreshold depolarisation, with the level of baseline stretch being important for the stability of rhythm generation; and (C) regulates SAN firing rate through the Bainbridge response, by which stretch results in the beat-by-beat matching of heart rate to venous return (Fig. 1).
One important consequence of a critical role for SAN mechano-sensitivity in pacemaking is the need for its consideration in future experimental investigations of SAN function. For instance, in isolated, Langendorff perfused hearts that are not in working mode (so have no atrial filling), the Bainbridge response is not engaged, meaning the effect of stretch on diastolic depolarisation is not present (which could partly account for the generally slower heart rate seen in isolated compared to in vivo hearts). Often, mechanical uncouplers (e.g. blebbistatin) are used in these preparations, which affect mechanics by preventing contraction (while preserving electrical activity). The loss of contraction should have no effect on heart rate, as it will not change the amount of stretch experienced by the SAN and in fact, might cause an increase in SAN stretch if there is a buildup of fluid in the atria that is no longer ejected. But in all cases, it is important to recognise that cells in tissue are always under some level of baseline mechanical load, which in isolated hearts (with or without mechanical uncouplers) may contribute to the regularity of SAN firing. Targeted manipulation of mechanical activity and baseline load in healthy and diseased whole hearts (e.g. working vs. non-working Langendorff, with or without blebbistatin) may be a means to further explore the importance of the various contributions of stretch to SAN (dys-)function in the whole heart.
Another potential means of exploring the influences of stretch on SAN function is computational modelling (Quinn and Kohl 2013). Highly complex, three-dimensional, electro-mechanically coupled whole heart models now exist (Travanova 2011; Niederer et al. 2019), which could be modified to include the hypothesised subcellular mechanisms of SAN mechano-sensitivity (i.e. SACNS or mechano-sensitivity of coupled-clock components) to gain further experimentally inaccessible insight into the relative importance of the different effects of stretch on SAN function, as well as which mechano-sensitive mechanisms can account for experimentally observed effects, to help identify the most likely mechano-sensitive candidates for experimental follow-up.
SAN mechano-sensitivity may also represent an unappreciated therapeutic target for the treatment of SAN dysfunction. If subcellular mechanisms responsible for stretch effects can be identified, then they may be pharmacologically manipulated as an anti-arrhythmic therapy for the restoration of normal cardiac rhythm. There is also the potential to directly target SAN mechanics in diseases where it has been disrupted, using novel devices or biomaterials to normalise stretch and restore normal function.
In summary, SAN automaticity is driven by the combined actions of multiple oscillators that drive SDD and ultimately cause membrane potential to cross the threshold for AP generation. The importance of diastolic load and cyclic stretch for SAN function has been previously underappreciated. They may in fact be crucial for the stabilisation of pacemaking through the mechanical priming and entrainment of SAN cells and through their effect on the activity of other mechanisms contributing to SAN automaticity, suggesting SAN mechano-sensitivity is the ‘grandfather clock’ of cardiac rhythm (Fig. 1).
Abbreviations
- AP
Action potential
- Ca2+
Calcium
- CaMKII
Calcium/calmodulin-dependent protein kinase II
- cAMP
Cyclic adenosine monophosphate
- HCN
Hyperpolarisation-activated cyclic nucleotide-gated channels
- If
“Funny” current
- IKr
Rapid delayed rectifier potassium current
- IKs
Slow delayed rectifier potassium current
- K+
Potassium
- MDP
Maximum diastolic potential
- MSP
Maximum systolic potential
- NCX
Sodium-calcium exchanger
- LCR
Local calcium releases
- Na+
Sodium
- RyR
Ryanodine receptors
- SACNS
Cation non-selective stretch-activated channels
- SAN
Sinoatrial node
- SDD
Spontaneous diastolic depolarisation
- SERCA
Sarco/endoplasmic reticulum calcium-ATPase
- SR
Sarcoplasmic reticulum
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2016-04879 to T.A.Q.), the Heart and Stroke Foundation of Canada (G-18-0022185 to T.A.Q.), and the Canadian Institutes of Health Research (MOP 342562 to T.A.Q.). E.A.M. is supported by a British Heart Foundation Programme Grant (RG/20/6/35095).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abramovich-Sivan S, Akselrod S (1999) Phase response curve based model of the SA node: simulation by two-dimensional array of pacemaker cells with randomly distributed cycle lengths. Med Biol Eng Comput 37:482–491. 10.1007/BF02513334 [DOI] [PubMed]
- Anumonwo JMB, Delmar M, Vinet A, Michfaels DC, Jalife J (1991) Phase resetting and entrainment of pacemaker activity in single sinus nodal cells. Circ Res 68:1138–1153. 10.1161/01.res.68.4.1138 [DOI] [PubMed]
- Arai A, Kodama I, Toyama J (1996) Roles of Cl- channels and Ca2+ mobilization in stretch-induced increase of SA node pacemaker activity. Am J Physiol Heart Circ Physiol 270:H1726–H1735. 10.1152/ajpheart.1996.270.5.H1726 [DOI] [PubMed]
- Bainbridge FA (1915) The influence of venous filling upon the rate of the heart. J Physiol 50:65–84. 10.1113/jphysiol.1915.sp001736 [DOI] [PMC free article] [PubMed]
- Bartos DC, Grandi E, Ripplinger CM (2015) Ion channels in the heart. Compr Physiol 5:1423–1464. 10.1002/cphy.c140069 [DOI] [PMC free article] [PubMed]
- Blinks JR (1956) Positive chronotropic effect of increasing right atrial pressure in the isolated mammalian heart. Am J Phys 186:299–303. 10.1152/ajplegacy.1956.186.2.299 [DOI] [PubMed]
- Bogdanov KY, Maltsev VA, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD et al (2006) Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res 99:979–987. 10.1161/01.RES.0000247933.66532.0b [DOI] [PubMed]
- Brennan JA, Chen Q, Gams A, Dyavanapalli J, Mendelowitz D, Peng W et al (2020) Evidence of superior and inferior sinoatrial nodes in the mammalian heart. JACC Clin Electrophysiol 6:1827–1840. 10.1016/j.jacep.2020.09.012 [DOI] [PMC free article] [PubMed]
- Brooks CM, Lu HH, Lange G, Mangi R, Shaw RB, Geoly K (1966) Effects of localized stretch of the sinoatrial node region of the dog heart. Am J Phys 211:1197–1202. 10.1152/ajplegacy.1966.211.5.1197 [DOI] [PubMed]
- Calabrese B, Tabarean IV, Juranka P, Morris CE (2002) Mechanosensitivity of N-type calcium channel currents. Biophys J 83:2560–2574. 10.1016/S0006-3495(02)75267-3. 10.1016/S0006-3495(02)75267-3 [DOI] [PMC free article] [PubMed]
- Calloe K, Elmedyb P, Olesen SP, Jorgensen NK, Grunnet M (2005) Hypoosmotic cell swelling as a novel mechanism for modulation of cloned HCN2 channels. Biophys J 89:2159–2169. 10.1529/biophysj.105.063792 [DOI] [PMC free article] [PubMed]
- Chiou KK, Rocks JW, Chen CY, Cho S, Merkus KE, Rajaratnam A et al (2016) Mechanical signaling coordinates the embryonic heartbeat. Proc Natl Acad Sci U S A 113:8939–8944. 10.1073/pnas.1520428113 [DOI] [PMC free article] [PubMed]
- Cooper PJ, Kohl P (2005) Species- and preparation-dependence of stretch effects on sino-atrial node pacemaking. Ann N Y Acad Sci 1047:324–335. 10.1196/annals.1341.029 [DOI] [PubMed]
- Cooper PJ, Lei M, Cheng LX, Kohl P (2000) Selected contribution: axial stretch increases spontaneous pacemaker activity in rabbit isolated sinoatrial node cells. J Appl Physiol 89:2099–2104. 10.1152/jappl.2000.89.5.2099 [DOI] [PubMed]
- Coster ACF, Celler BG (2003) Phase response of model sinoatrial node cells. Ann Biomed Eng 31:271–283.10.1114/1.1553455 [DOI] [PubMed]
- Csepe TA, Kalyanasundaram A, Hansen BJ, Zhao J, Fedorov VV (2015) Fibrosis: a structural modulator of sinoatrial node physiology and dysfunction. Front Physiol 6:1–8. 10.3389/fphys.2015.00037 [DOI] [PMC free article] [PubMed]
- Deck KA (1964) Dehnungseffekte am spontansehlagenden, isolierten Sinusknoten. Pflugers Arch 280:120–130 [PubMed]
- Difrancesco D (2010) The role of the funny current in pacemaker activity. Circ Res 106:434–446. 10.1161/CIRCRESAHA.109.208041 [DOI] [PubMed]
- Difrancesco D, Noble D (2012) The funny current has a major pacemaking role in the sinus node. Heart Rhythm 9:299–301. 10.1016/j.hrthm.2011.09.021 [DOI] [PubMed]
- DiFrancesco ML, Mesirca P, Bidaud I, Isbrandt D, Mangoni ME (2021) The funny current in genetically modified mice. Prog Biophys Mol Biol. 10.1016/j.pbiomolbio.2021.06.003 [DOI] [PubMed]
- Donald DE, Shepherd JT (1978) Reflexes from the heart and lungs: physiological curiosities or important regulatory mechanisms. Cardiovasc Res 12:449–469. 10.1093/cvr/12.8.449 [PubMed]
- Fenske S, Krause SC, Hassan SIH, Becirovic E, Auer F, Bernard R et al (2013) Sick sinus syndrome in HCN1-deficient mice. Circulation 128:2585–2594. 10.1161/CIRCULATIONAHA.113.003712 [DOI] [PubMed]
- Gaskell WH (1882) Preliminary observations on the innervation of the heart of the tortoise. J Physiol 3:369–379. 10.1113/jphysiol.1882.sp000110 [DOI] [PMC free article] [PubMed]
- Guevara MR, Jongsma HJ (1990) Phase resetting in a model of sinoatrial nodal membrane: ionic and topological aspects. Am J Physiol Heart Circ Physiol 258:734–747. 10.1152/ajpheart.1990.258.3.H734 [DOI] [PubMed]
- Guharay F, Sachs F (1984) Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol 352:685–701. 10.1113/jphysiol.1984.sp015317 [DOI] [PMC free article] [PubMed]
- Hales PW, Schneider JE, Burton RAB, Wright BJ, Bollensdorff C, Kohl P (2012) Histo-anatomical structure of the living isolated rat heart in two contraction states assessed by diffusion tensor MRI. Prog Biophys Mol Biol 110:319–330. 10.1016/j.pbiomolbio.2012.07.014 [DOI] [PMC free article] [PubMed]
- Hoffman B, Cranefield P (1960) Electrophysiology of the heart. McGraw-Hill, New York
- Huang X, Mi Y, Qian Y, Hu G (2011) Phase-locking behaviors in an ionic model of sinoatrial node cell and tissue. Phys Rev E Stat Nonlin Soft Matter Phys 83:061917. 10.1103/PhysRevE.83.061917 [DOI] [PubMed]
- Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RAB et al (2009) Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res 104:787–795. 10.1161/CIRCRESAHA.108.193334 [DOI] [PMC free article] [PubMed]
- Jalife J (1984) Mutual entrainment and electrical coupling as mechanisms for synchronous firing of rabbit sino-atrial pace-maker cells. J Physiol 356:221–243. 10.1113/jphysiol.1984.sp015461 [DOI] [PMC free article] [PubMed]
- Jalife J, Antzelevitch C (1979) Phase resetting and annihilation of pacemaker activity in cardiac tissue. Science 206(80):695–697. 10.1126/science.493975 [DOI] [PubMed]
- Jarisch A, Richter H (1939) Die afferenten bahnen des veratrineffektes in den herznerven. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 193:355–371. 10.1007/BF01859921
- Keith A, Flack M (1907) The form and nature of the muscular connections between the primary divisions of the vertebrate heart. J Anat Physiol 41:172–189 [PMC free article] [PubMed]
- Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK et al (2004) Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol 44:109–116. 10.1016/j.jacc.2004.03.044 [DOI] [PubMed]
- Kohl P, Noble D (1996) Mechanosensitive connective tissue: potential influence on heart rhythm. Cardiovasc Res 32:62–68. 10.1016/S0008-6363(95)00224-3 [PubMed]
- Kohl P, Kamkin AG, Kiseleva IS, Noble D (1994) Mechanosensitive fibroblasts in the sinoatrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol 79:943–956. 10.1113/expphysiol.1994.sp003819 [DOI] [PubMed]
- Krogh-Madsen T, Glass L, Doedel EJ, Guevara MR (2004) Apparent discontinuities in the phase-resetting response of cardiac pacemakers. J Theor Biol 230:499–519. 10.1016/j.jtbi.2004.03.027 [DOI] [PubMed]
- Lakatta EG, DiFrancesco D (2009) What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol 47:157–170. 10.1016/j.yjmcc.2009.03.022 [DOI] [PMC free article] [PubMed]
- Lakatta EG, Vinogradova TM, Maltsev VA (2008) The missing link in the mystery of normal automaticity of cardiac pacemaker cells. Ann N Y Acad Sci 1123:41–57. 10.1196/annals.1420.006 [DOI] [PMC free article] [PubMed]
- Lakatta EG, Maltsev VA, Vinogradova TM (2010) A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res 106:659–673. 10.1161/CIRCRESAHA.109.206078 [DOI] [PMC free article] [PubMed]
- Lange G, Lu HH, Chang A, Brooks CM (1966) Effect of stretch on the isolated cat sinoatrial node. Am J Phys 211:1192–1196. 10.1152/ajplegacy.1966.211.5.1192 [DOI] [PubMed]
- Lei M, Goddard C, Liu J, Léoni AL, Royer A, Fung SSM et al (2005) Sinus node dysfunction following targeted disruption of the murine cardiac sodium channel gene Scn5a. J Physiol 567:387–400. 10.1113/jphysiol.2005.083188 [DOI] [PMC free article] [PubMed]
- Li N, Csepe TA, Hansen BJ, Dobrzynski H, Higgins RSD, Kilic A et al (2015) Molecular mapping of sinoatrial node HCN channel expression in the human heart. Circ Arrhythm Electrophysiol 8:1219–1227.10.1161/CIRCEP.115.003070 [DOI] [PMC free article] [PubMed]
- Lin W, Laitko U, Juranka PF, Morris CE (2007) Dual stretch responses of mHCN2 pacemaker channels: accelerated activation, accelerated deactivation. Biophys J 92:1559–1572.10.1529/biophysj.106.092478 [DOI] [PMC free article] [PubMed]
- Lyford GL, Strege PR, Shepard A, Ou Y, Ermilov L, Miller SM et al (2002) α1C (CaV1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol 283:C1001–C1008. 10.1152/ajpcell.00140.2002 [DOI] [PubMed]
- MacDonald EA, Stoyek MR, Rose RA, Quinn TA (2017) Intrinsic regulation of sinoatrial node function and the zebrafish as a model of stretch effects on pacemaking. Prog Biophys Mol Biol 130:198–211. 10.1016/j.pbiomolbio.2017.07.012 [DOI] [PubMed]
- MacDonald EA, Madl J, Greiner J, Ramadan AF, Wells SM, Torrente AG et al (2020a) Sinoatrial node structure, mechanics, electrophysiology and the chronotropic response to stretch in rabbit and mouse. Front Physiol 11:809. 10.3389/fphys.2020.00809 [DOI] [PMC free article] [PubMed]
- MacDonald EA, Rose RA, Quinn TA (2020b) Neurohumoral control of sinoatrial node activity and heart rate: insight from experimental models and findings from humans. Front Physiol 11:170. 10.3389/fphys.2020.00170 [DOI] [PMC free article] [PubMed]
- Mangoni ME, Nargeot J (2008) Genesis and regulation of the heart automaticity. Physiol Rev 88:919–982. 10.1152/physrev.00018.2007 [DOI] [PubMed]
- Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J et al (2003) Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A 100:5543–5548. 10.1073/pnas.0935295100 [DOI] [PMC free article] [PubMed]
- Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D (2007) Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the If pacemaker current. J Physiol 582:1195–1203. 10.1113/jphysiol.2007.133439 [DOI] [PMC free article] [PubMed]
- Mesirca P, Torrente AG, Mangoni ME (2015) Functional role of voltage gated Ca2+ channels in heart automaticity. Front Physiol 6:19. 10.3389/fphys.2015.00019 [DOI] [PMC free article] [PubMed]
- Morris C (2011) Pacemaker, potassium, calcium, sodium: stretch modulation of the voltage-gated channels. In: Kohl P, Sachs F, Franz M (eds) Cardiac Mechano-Electric Coupling and Arrhythmias. University Press, Oxford, Oxford, pp 42–49. 10.1093/med/9780199570164.003.0006
- Morris GM, Kalman JM (2014) Fibrosis, electrics and genetics - perspectives on sinoatrial node disease. Circ J 78:1272–1282. 10.1253/circj.cj-14-0419 [DOI] [PubMed]
- Morton JB, Sanders P, Vohra JK, Sparks PB, Morgan JG, Spence SJ et al (2003) Effect of chronic right atrial stretch on atrial electrical remodeling in patients with an atrial septal defect. Circulation 107:1775–1782. 10.1161/01.CIR.0000058164.68127.F2 [DOI] [PubMed]
- Niederer S, Lumens J, Trayanova NA (2019) Computational models in cardiology. Nat Rev Cardiol 2:100–111. 10.1038/s41569-018-0104-y [DOI] [PMC free article] [PubMed]
- Noble D, Noble PJ, Fink M (2010) Competing oscillators in cardiac pacemaking. Circ Res 106:1791–1797. doi: 10.1161/CIRCRESAHA.110.218875 [DOI] [PubMed]
- Pathak CL (1973) Autoregulation of chronotropic response of the heart through pacemaker stretch. Cardiology 58:45–64. 10.1159/000169618 [DOI] [PubMed]
- Peyronnet R, Nerbonne JM, Kohl P (2016) Cardiac mechano-gated ion channels and arrhythmias. Circ Res 118:311–329. doi: 10.1161/CIRCRESAHA.115.305043 [DOI] [PMC free article] [PubMed]
- Quinn TA (2015) Cardiac mechano-electric coupling: a role in regulating normal function of the heart? Cardiovasc Res 108:1–3. 10.1093/cvr/cvv203 [DOI] [PMC free article] [PubMed]
- Quinn TA, Kohl P (2011) Systems biology of the heart: hype or hope? Ann N Y Acad Sci 1245:40–43. 10.1111/j.1749-6632.2011.06327.x [DOI] [PubMed]
- Quinn TA, Kohl P (2012) Mechano-sensitivity of cardiac pacemaker function: pathophysiological relevance, experimental implications, and conceptual integration with other mechanisms of rhythmicity. Prog Biophys Mol Biol 110:257–268. 10.1016/j.pbiomolbio.2012.08.008 [DOI] [PMC free article] [PubMed]
- Quinn TA, Kohl P (2013) Combining wet and dry research: experience with model development for cardiac mechano-electric structure-function studies. Cardiovasc Res 97:601–611. 10.1093/cvr/cvt003 [DOI] [PMC free article] [PubMed]
- Quinn TA, Kohl P (2016) Rabbit models of cardiac mechano-electric and mechano-mechanical coupling. Prog Biophys Mol Biol 121:110–122. 10.1016/j.pbiomolbio.2016.05.003 [DOI] [PMC free article] [PubMed]
- Quinn TA, Kohl P (2021) Cardiac mechano-electric coupling: acute effects of mechanical stimulation on heart rate and rhythm. Physiol Rev 101:37–92. 10.1152/physrev.00036.2019 [DOI] [PubMed]
- Quinn TA, Kohl P, Ravens U (2014) Cardiac mechano-electric coupling research: fifty years of progress and scientific innovation. Prog Biophys Mol Biol 115:71–75. 10.1016/j.pbiomolbio.2014.06.007 [DOI] [PubMed]
- Quinn TA, Camelliti P, Rog-Zielinska EA, Siedlecka U, Poggioli T, O’Toole ET et al (2016) Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc Natl Acad Sci USA 113:14852–14857. 10.1073/pnas.1611184114 [DOI] [PMC free article] [PubMed]
- Rajala GM, Kalbfleisch JH, Kaplan S (1976) Evidence that blood pressure controls heart rate in the chick embryo prior to neural control. J Embryol Exp Morpholog 36:685–695. doi: 10.1242/dev.36.3.685 [PubMed]
- Rajala GM, Pinter MJ, Kaplan S (1977) Response of the quiescent heart tube to mechanical stretch in the intact chick embryo. Dev Biol 61:330–337. 10.1016/0012-1606(77)90302-5 [DOI] [PubMed]
- Reiner VS, Antzelevitch C (1985) Phase resetting and annihilation in a mathematical model of sinus node. Am J Physiol 249:H1143-H1153. doi: 10.1152/ajpheart.1985.249.6.H1143 [DOI] [PubMed]
- Roddie IC, Shepherd JT, Whelan RF (1957) Reflex changes in vasoconstrictor tone in human skeletal muscle in response to stimulation of receptors in a low-pressure area of the intrathoracic vascular bed. J Physiol 139:369–376. 10.1113/jphysiol.1957.sp005897 [DOI] [PMC free article] [PubMed]
- Rosen MR, Nargeot J, Salama G (2012) The case for the funny current and the calcium clock. Heart Rhythm 9:616–618. 10.1016/j.hrthm.2011.10.008 [DOI] [PubMed]
- Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB et al (2003) Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation 108:1461–1468. 10.1161/01.CIR.0000090688.49283.67 [DOI] [PubMed]
- Sano T, Sawanobori T, Adaniya H (1978) Mechanism of rhythm determination among pacemaker cells of the mammalian sinus node. Am J Physiol Heart Circ Physiol 235:H379–H384. 10.1152/ajpheart.1978.235.4.H379 [DOI] [PubMed]
- Sénatore S, Reddy VR, Sémériva M, Perrin L, Lalevée N (2010) Response to mechanical stress is mediated by the TRPA channel painless in the Drosophila heart. PLoS Genet 6:e1001088. 10.1371/journal.pgen.1001088 [DOI] [PMC free article] [PubMed]
- Sirenko S, Yang D, Li Y, Lyashkov AE, Lukyanenko YO, Lakatta EG et al (2013) Ca2+-dependent phosphorylation of Ca2+ cycling proteins generates robust rhythmic local Ca2+ releases in cardiac pacemaker cells. Sci Signal 6:ra6. 10.1126/scisignal.2003391 [DOI] [PMC free article] [PubMed]
- Sparks PB, Mond HG, Vohra JK, Jayaprakash S, Kalman JM (1999) Electrical remodeling of the atria following loss of atrioventricular synchrony. Circulation 100:1894–1900. 10.1161/01.cir.100.18.1894 [DOI] [PubMed]
- Starzinsky, von Bezold A (1867) Von dem einflusse des intracardialen blutdruckes auf die hauflgkeit der herzschlage. Untersuch Phys Lab 195–214
- Torrente AG, Mesirca P, Neco P, Rizzetto R, Dubel S, Barrere C et al (2016) L-type Cav1.3 channels regulate ryanodine receptor-dependent Ca2+ release during sino-atrial node pacemaker activity. Cardiovasc Res 109:451–461. 10.1093/cvr/cvw006 [DOI] [PubMed]
- Travanova NA (2011) Whole-heart modeling: applications to cardiac electrophysiology and electromechanics. Circ Res 108:113–128. 10.1161/CIRCRESAHA.110.223610 [DOI] [PMC free article] [PubMed]
- Tsalikakis DG, Zhang HG, Fotiadis DI, Kremmydas GP, Michalis K (2007) Phase response characteristics of sinoatrial node cells. Comput Biol Med 37:8–20. 10.1016/j.compbiomed.2005.09.011 [DOI] [PubMed]
- Ushiyama J, Brooks CMC (1977) Interaction of oscillators: effect of sinusoidal stretching of the sinoatrial node on nodal rhythm. J Electrocardiol 10:39–44. 10.1016/s0022-0736(77)80029-0 [DOI] [PubMed]
- Verheijck EE, Wilders R, Joyner RW, Golod DA, Kumar R, Jongsma HJ et al (1998) Pacemaker synchronization of electrically coupled rabbit sinoatrial node cells. J Gen Physiol. 111:95–112. 10.1085/jgp.111.1.95 [DOI] [PMC free article] [PubMed]
- Vinogradova TM, Lakatta EG (2009) Regulation of basal and reserve cardiac pacemaker function by interactions of cAMP-mediated PKA-dependent Ca2+ cycling with surface membrane channels. J Mol Cell Cardiol 47:456–474. 10.1016/j.yjmcc.2009.06.014 [DOI] [PMC free article] [PubMed]
- Vinogradova TM, Zhou Y, Bogdanov KY, Yang D, Kuschel M, Cheng H et al (2000) Sinoatrial node pacemaker activity requires Ca 2+ /calmodulin-dependent protein kinase II activation. Circ Res 87:760–767. 10.1161/01.res.87.9.760 [DOI] [PubMed]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D et al (2006) High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98:505–514. 10.1161/01.RES.0000204575.94040.d1 [DOI] [PubMed]
- von Bezold A, Hirt L (1867) Über die physiologischen wirkungen des essigsauren veratrins. Untersuchungen Aus Dem Physiol Lab Wurzbg 75–156
- Yaniv Y, Maltsev VA, Escobar AL, Spurgeon HA, Ziman BD, Stern MD et al (2011) Beat-to-beat Ca2+-dependent regulation of sinoatrial nodal pacemaker cell rate and rhythm. J Mol Cell Cardiol 51:902–905. 10.1016/j.yjmcc.2011.08.029 [DOI] [PMC free article] [PubMed]
- Ypey DL, Van Meerwijk WPM, de Bruin G (1982) Suppression of pacemaker activity by rapid repetitive phase delay. Biol Cybern 45:187–194. 10.1007/BF00336191 [DOI] [PubMed]