Abstract
All cells sense force and build their cytoskeleton to optimize function. How is this achieved? Two major systems are involved. The first is that load deforms specific protein structures in a proportional and orientation-dependent manner. The second is post-translational modification of proteins as a consequence of signaling pathway activation. These two processes work together in a complex way so that local subcellular assembly as well as overall cell function are controlled. This review discusses many cell types but focuses on striated muscle. Detailed information is provided on how load deforms the structure of proteins in the focal adhesions and filaments, using α-actinin, vinculin, talin, focal adhesion kinase, LIM domain-containing proteins, filamin, myosin, titin, and telethonin as examples. Second messenger signals arising from external triggers are distributed throughout the cell causing post-translational or chemical modifications of protein structures, with the actin capping protein CapZ and troponin as examples. There are numerous unanswered questions of how mechanical and chemical signals are integrated by muscle proteins to regulate sarcomere structure and function yet to be studied. Therefore, more research is needed to see how external triggers are integrated with local tension generated within the cell. Nonetheless, maintenance of tension in the sarcomere is the essential and dominant mechanism, leading to the well-known phrase in exercise physiology: “use it or lose it.”
Keywords: Integrin, Mechanotransduction, Mechanobiology, Costamere, Adhesome, Sarcomere
Introduction
All cells sense force and build their cytoskeleton to optimize function. How is this achieved? Two major systems are involved. The first is that load deforms specific protein structures in a proportional and orientation-dependent manner. The second is post-translational modification of proteins as a consequence of signaling pathway activation. These two processes work together in a complex way so that local subcellular assembly as well as overall cell function are controlled. This review discusses many cell types but focuses on striated muscle.
Myocytes are specialized contractile cells that possess the particular feature of producing significant anisotropic force. This differentiates them from other cell types that are limited to sensing mechanical forces and developing isotropic forces. Newton’s Third law of motion states that when one object exerts a force on a second object, that second object exerts a force that is equal in magnitude and opposite in direction on the first object. Therefore, there is a pair of forces acting on the interacting objects regardless of the source of the force. Thus, in all cells, the forces can be divided into exogenous, generated exterior to the cell, and endogenous, generated internally (Gautel and Djinović-Carugo 2016; Ward and Iskratsch 2020; Yang et al. 2015).
Cardiac and skeletal myocytes possess highly evolved and organized cytoskeletal arrays called sarcomeres, composed primarily of actin and myosin along with a vast number of associated proteins that provide directed contraction. The forces of a muscle fiber are generated by the myosin motors in the thick filaments acting upon the thin filaments. These forces deform many proteins in the connected sarcomeric structure, such as titin that supports the spring-like properties of the sarcomere. Importantly, force is transmitted laterally via the Z-discs to the extracellular environment at the integrin focal adhesion complex, known as the costamere in muscle. Conversely, there are forces generated externally that propagate via the costamere to the interior cytoskeleton (Samarel et al. 2013). At the cell membrane, the integrins, stretch-activated ion channels (Jiang et al. 2021), cadherins, the nuclei, and many other elements are deformed by force, which all contribute to the mechanotransduction response, collectively named “tensegrity” (Ingber 2006). Thus, no matter from which direction the force comes, it is detected, and signals are then transmitted downstream throughout the cells through YAP1 (Dupont et al. 2011). Thus, both inside-out and outside-in mechanical forces lead to deformation of a milieu of structural proteins embedded in the cytoskeleton of myocytes because of Newton’s Third Law and the structural connectivity of all these cytoskeletal proteins. However, biology might well introduce some asymmetric responses depending on orientation of the sensor, direction of the force, and which chemical signaling pathways become activated.
Cells also respond to external signals when ligands or hormones bind to transmembrane proteins to trigger conformational changes in cell membrane receptors that activate signals downstream. This sets up a chain reaction that amplifies the signal by second messengers pathways specific to each source of stimulus. For example, second messengers, such as Ca2+, cyclic adenosine monophosphate (cAMP), and inositol 1,4,5-trisphosphate (IP3), activate phosphatases and acetyltransferases that phosphorylate and acetylate cytoskeletal proteins (Russell and Solís 2021). In turn, these modifications promote rapid assembly of the cytoskeleton or initiate contraction and motility in cells. Other destinations of these signals converge in transcription factors that promote their migrations in and out of the nucleus to alter gene expression. Many of these signals are discussed in this review with a focus on those most relevant to striated muscle cells. Many other proteins critically involved in mechanotransduction and mechanosensation could have been selected, such as fibronectin from the extracellular matrix, the cortical actin and spectrin cytoskeleton, or the lamins in the nucleus.
The focus of this review is to aid in understanding how the cells adapt by integrating the physical forces of altered work with the chemical signaling pathways. Some key structural mechanosensors and relevant signaling pathways that modify protein conformation are described in detail. Finally, several unanswered questions in the complex field of muscle growth and plasticity are identified, showing why there is still a need to tackle research questions with a multidisciplinary approach.
Load deforms structure of proteins in the focal adhesions, named costamere in muscle
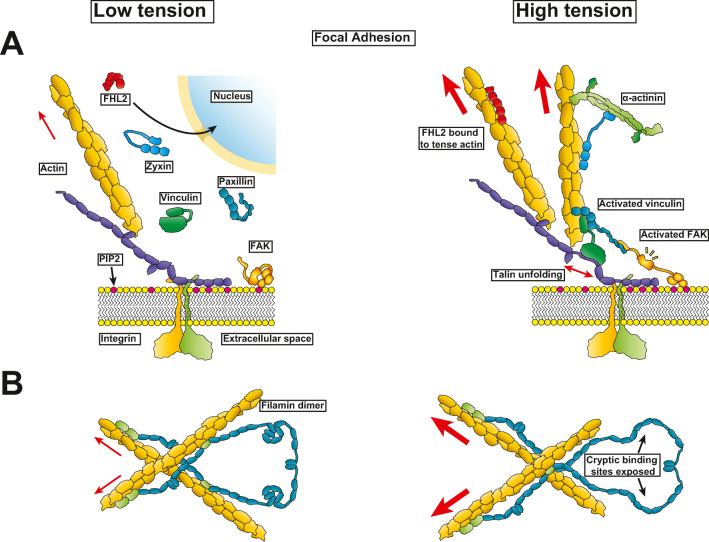
All mammalian adherent cells, including striated muscle cells, possess a vast set of cytoskeletal proteins that provide structural stability for cell anchoring and also response to mechanical forces. The response to mechanical forces leads to cytoskeletal protein deformations to unleash new functionalities that vary with each protein. These tension-driven conformational changes in the protein may elicit specific biochemical reactions (Wang et al. 2019). The downstream effects from mechanical deformation of protein domains can lead to chemical post-translational modification or to the non-covalent binding/unbinding or binding partners such as proteins, or small molecules that act as ligands. Proteins with well-studied load transduction mechanisms in muscle will be the focus of this review. Many of these proteins are found in the focal adhesion on the cytoplasmic side of the transmembrane integrin. The focal adhesion is a major location for force detection and subsequent protein deformation, see Fig. 1A. Shown here before and after loading are focal adhesion kinase (FAK), paxillin, vinculin, talin, zyxin, and four-and-a-half LIM domains 2 (FHL2). Other members of the LIM domain-containing proteins are discussed in detail below. Filamin is the example selected from the cytoskeleton. Note dystrophin is a very important component lying under the sarcolemma and provides mechanical strength, and mechanoprotection, to it. However, its role in mechanosignaling could be indirect as loss of dystrophin in skeletal muscles is reported to cause aberrant signaling from increased cytoskeletal stress (Iyer et al. 2019). Readers are encouraged to consult the dedicated references (Gao and McNally 2015).
Fig. 1.
Structural deformation at rest compared to loaded states at the focal adhesion and costamere. (A) Talin binds to activated integrin at the focal adhesion (costamere in muscle) to stabilize actin filaments. With increased load, talin rod repeats unfold permitting vinculin association and strengthening actin anchoring. Other proteins like paxillin and focal adhesion kinase (FAK) are also recruited. High actin tension leads to recruitment of LIM-containing proteins like zyxin and FHL2. (B) Filamin dimers crosslink actin filaments. Interactions between the Ig-like domains become disrupted with increasing mechanical loads on actin. This untangling leads to exposure of cryptic binding sites of proteins like integrins
Vinculin
Vinculin is a member of the cadherin and integrin cell adhesion multiprotein complex where it bridges to actin and talin in a load-dependent manner. Structurally, vinculin is composed of a head domain that binds to talin and a tail domain that binds to F-actin and PIP2 (Izard and Brown 2016). Vinculin exists in an autoinhibited state, comprised of interactions between the vinculin head and tail domains, that become activated with the unfolding of the talin rod domains. Vinculin activation, the state in which vinculin binds to actin and talin with high affinity, may occur via several mechanisms including direct interaction with talin, force-mediated activation, PIP2 binding and phosphorylation; however, the specific mechanisms involving vinculin activation and recruitment to the focal adhesions remain unsolved (Bays and DeMali 2017; Khan and Goult 2019). Nevertheless, the general mechanism of vinculin attachment to actin and load bearing is unidirectional with increasing binding stability of the F-actin-vinculin interaction with increasing loads applied to F-actin towards the pointed end direction (Huang et al. 2017). In cardiac myocytes, vinculin localizes to costameres and adherens junctions. While costameric adhesion implies a classic focal adhesion multiprotein complex involving integrin attachment, vinculin binding to adherens junctions is anchored via N-cadherins along with the α/β-catenins, where α-catenin has been shown to be required for vinculin recruitment (Lyon et al. 2015; Merkel et al. 2019).
Metavinculin is a splice variant of vinculin that contains an additional peptide extension at the C-terminal (Byrne et al. 1992). While vinculin is ubiquitously expressed, metavinculin expression is limited to smooth, cardiac, and skeletal muscles (Witt et al. 2004). In general, vinculin dimerization is a step implicated in the activation of vinculin. Metavinculin dimers can bind two PIP2 molecules while the vinculin dimers only bind one. Interestingly, a metavinculin mutant containing a R975W substitution involved in dilated and hypertrophic cardiomyopathy fails to dimerize, which could decrease the ability of metavinculin to stabilize cell adhesions (Chinthalapudi et al. 2016). In vitro studies on the metavinculin point mutations A934V and ΔL954 associated with DCM and the R975W mutation associated with both dilated and hypertrophic cardiomyopathy have shown that all mutants exhibit an increased F-actin bundling capacity (Sarker et al. 2019). Metavinculin expression in the heart is much lower than vinculin (Witt et al. 2004) making it intriguing to study how this mutation may affect cardiomyocyte remodeling leading to hypertrophy.
Talin
Talin is large focal adhesion protein composed of 4.1 (F), ezrin (E), radixin (R), moesin (M) or the FERM domain that interacts with integrin, and a rod domain that interacts with both actin and vinculin. The formation of the focal adhesion is in part mediated by the activation of integrins via simultaneous interactions with extracellular matrix ligands and interactions with the talin FERM domain, specifically via the integrin binding site 1 in the FERM F3 domain (Anthis et al. 2009). A recent crystal structure shows the inhibited state of talin is in a globular conformation (Dedden et al. 2019). Upon activation, talin extends exposing two actin-binding sites in the rod domain (Hemmings et al. 1996) and a third actin-binding domain in the FERM domain (Lee et al. 2004). Talin also recruits the type 1 phosphatidylinositol phosphate kinase by the FERM domain of talin that catalyzes the local synthesis of PIP2 from phosphatidylinositol 4-phosphate (Di Paolo et al. 2002; Ling et al. 2002). A secondary type of actin anchoring to talin is by the unfolding of the rod domains in talin that become vinculin-binding sites. R3, the fist talin domain to unfold, is held by internal non-covalent interactions and unfolded with mechanical forces over 5 pN (Yao et al. 2014). This mechanical unfolding leads to the recruitment of activated vinculin that then acts as a bridge to link actin and talin (del Rio et al. 2009; Izard et al. 2004). Unfolding of talin leads to the exposure of up to 11 vinculin binding sites (Gingras et al. 2005). When a talin 1 Förster Resonance Energy Transfer (FRET) sensor, which exhibits FRET response by forces under 5 pN, is expressed in neonatal rat ventricular myocytes a cyclic FRET response is observed in spontaneously contracting myocytes at physiologic levels of substrate stiffness of 6 kPa. However, when the stiffness is similar to levels seen in fibrotic tissue near 130 kPa or at the very soft 1 kPa, the cyclic FRET response is lost, indicating an active sensing of tension at focal adhesions (Pandey et al. 2018). It is unknown how these results translate to adult cardiomyocytes that mainly express the talin 2 isoform which has different properties than talin 1 such as increased binding of vinculin at low strain forces (Manso et al. 2013).
Focal adhesion kinase
Focal adhesion kinase (FAK) contains an N-terminal FERM domain, which is a module localizing various proteins at the interface between the plasma membrane and the cytoskeleton. Next is the tyrosine kinase domain, then a 220-residue proline-rich region, and finally a C-terminal focal adhesion targeting (FAT) domain. The globular domains of FAK have been characterized (Arold et al. 2002; Ceccarelli et al. 2006; Hayashi et al. 2002). FAK is autoinhibited in the cytosol but becomes activated (i.e., the kinase domain is able to phosphorylate substrates) when bound to integrin and other proteins in the focal adhesion complex, such as talin, vinculin, and paxillin (Fig. 1A). FAK is the first protein recruited to the focal adhesion when the cell experiences an external mechanical stimulus, unfolding it to reveal the Tyr-397 residue, and initiating the autophosphorylation and focal adhesion-mediated signaling (Bauer et al. 2019). The crystal structure of the FERM and kinase region shows how FAK autoinhibition is achieved by interactions between the FERM and the catalytic domain (Lietha et al. 2007). At the N-terminus, FAK undergoes S910 phosphorylation via PKCδ and Src-dependent pathways that are important for cell spreading and sarcomere reorganization (Chu et al. 2011). An excellent review of how force affects FAK structure and mechanotransduction provides more details (Tapial Martínez et al. 2020).
FAK plays a major role in normal response to local forces, including matrix stiffness. Various cells types grown on stiffer substrates generate higher forces in the focal adhesions, and FAK activity also increases (Seong et al. 2013). Furthermore, FAK localization to focal adhesions and phosphorylation occur in response to increased muscle fiber loading, which regulates skeletal muscle differentiation and muscle size (Graham et al. 2015). FAK phosphorylation increased when cultured heart cells were mechanically strained (Torsoni et al. 2003). Senyo et al. demonstrated that while ERK phosphorylation increases with transverse and longitudinal stretching of neonatal rat ventricular myocytes, FAK phosphorylation increases only when myocytes are stretched in the transverse direction (Senyo et al. 2007). This differential response of FAK to the force direction may play a role in diseases that cause altered tissue stiffness such as cancer and heart disease (Urciuoli and Peruzzi 2020). For example, aberrant mechanotransduction in the heart is tightly linked to the local forces at the focal adhesions and FAK activation (Samarel 2014). Stiffness arising from fibrosis is a major signal to trigger hypertrophy in the overloaded myocardium (Franchini et al. 2000; Knöll et al. 2003; Samarel 2014). As such, FAK could be involved in eliciting counterproductive activation of pro-hypertrophic signaling pathways as those involved in dilated cardiomyopathy due to the disproportional gain in the local tissue stiffness from collagen deposition.
LIM domain-containing proteins
The Lin11, Isl- 1, and Mec-3 (LIM) domain family is a large group of proteins that have in common the zinc-finger motif (Li et al. 2012). Functionally, these proteins are better characterized as regulatory proteins in the cytosol and cytoskeleton that also may play a dual role as transcription factors. Many of these proteins are found associated with the sarcomere, particularly to the Z-disc (Frank et al. 2006; Hoshijima 2006; Solís and Solaro 2021).
A subset of this LIM family of proteins exhibits mechanosensing via mechanisms recently discovered. One of these is the sensing of actin tension via tandem LIM domains. Winkelman et al. reported that those proteins containing at least three tandem LIM domains such as zyxin, paxillin, tes, and enigma tend to accumulate in stress fibers undergoing high force (Winkelman et al. 2020). Similarly, the proteins zyxin, paxillin, and FHL tend to accumulate in stress fibers of cells. These properties were recapitulated in vitro as demonstrated by the increased binding of FHL3 to F-actin under tension by surface-immobilized myosin V and myosin VI that engage in pulling F-actin in opposite directions (Sun et al. 2020).
The function of the tandem LIM-domains as mechanosensors and transcription factors is best exemplified by Four-and a half LIM domains 2 (FHL2) also known as DRAL (down-regulated in rhabdomyosarcoma) (Genini et al. 1997). FHL2 has a dual function as a mechanosensor by binding to focal adhesions and as a transcription factor by regulating p21 expression. The role of FHL2 as a mechanosensor is demonstrated by its decreased association to focal adhesions in the preence of a Rho-associated kinase (ROCK) inhibitor with a concomitant migration of FHL2 from the cytoplasm into the nucleus (Nakazawa et al. 2016). This accumulation in the nucleus is also observed under low substrate stiffness of under 10 kPa FHL2 in which nuclear accumulation is facilitated by FAK phosphorylation at Y93 (Nakazawa et al. 2016). The role of FHL2 as a transcription factor stems from its ability to interact with RNA polymerase II to upregulate p21 gene expression involved in inhibition of growth. In striated muscles, FHL2 has been reported to interact with ERK-2 (Purcell et al. 2004), the muscle α7β1 integrin receptor (Samson et al. 2004), FAK (Nakazawa et al. 2016), the Z-discs (Purcell et al. 2004), and the titin M-band and N2B regions (Lange et al. 2002; Radke et al. 2007). Upregulation of FHL2 appears to suppress hypertrophic stimuli in vitro by blocking ERK-2 transcriptional activity in the nucleus (Purcell et al. 2004). FHL2 overexpression blunts the hypertrophic effects of phenylephrine and aortic banding (Friedrich et al. 2014). Interestingly, FHL2 appears to be downregulated in patients with hypertrophic cardiomyopathy (Friedrich et al. 2014). Despite this evidence, FHL2 mice knockdowns have shown mixed results with reports of no effects in hearts even when subjected to aortic banding (Chu et al. 2000), to significant enhancement of hypertrophy from chronic infusion of isoproterenol (Kong et al. 2001). Such prior findings should be further studied in light of the better understanding of tandem LIM domains as mechanosensors that bind to F-actin under high tension.
Filamin
Filamins constitute a family of immunoglobulin (Ig)-like domain proteins composed of filamins A, B, and C with the main function being to provide structural support by crosslinking actin (Razinia et al. 2012). Filamin A and B are ubiquitously expressed in mammalian cells while filamin C is restricted to striated muscles (Mao and Nakamura 2020). Filamins can dimerize via the Ig repeat 24 at their C-terminus (Pudas et al. 2005) while their actin-binding capacity is provided by two calponin homology domains located at the N-terminus (Iwamoto et al. 2018). Filamins have been shown to interact with other proteins like integrins while competing with talin for the cytoplasmic tail of the integrin β subunit (Kiema et al. 2006). Filamin A and B have an additional actin binding site at the Ig repeat 10 that is absent in filamin C (Suphamungmee et al. 2012). The role of filamin as mechanical sensor is illustrated by its differential binding of partners in absence and in presence of strain (see Fig. 1B). For example, under low levels of load filamin A interacts with FilGAP. However, increasing loads via its interaction with actin leads to unbinding of FilGAP as a result of the untangling of the filamin Ig-repeats (Mao and Nakamura 2020). Taken together, filamins are important strain sensors that orchestrate the activation of signaling pathways from their untangling response to increasing actin tension.
Load deforms structure of proteins in the sarcomere
Sarcomeres constitute the main frame of the structure. Sarcomeres consist to thin filaments made from actin, troponin, and tropomyosin while thick filaments have myosin heavy chains and myosin binding protein C (MyBP-C). These two filaments are organized in a regular pattern by proteins at the Z-disc like α-actinin, CapZ, and telethonin that support thin filaments and by the M-bad proteins like obscurin and myomesin that support thick filaments. Titin extends along both filaments starting at the Z-disc and ending and the M-line. Like costameres discussed above, the sarcomeres have very dynamic load-bearing proteins that deform in response to mechanical loads. Some of the most relevant proteins including myosin, α-actinin, titin, and telethonin are discussed in this section.
α-actinin
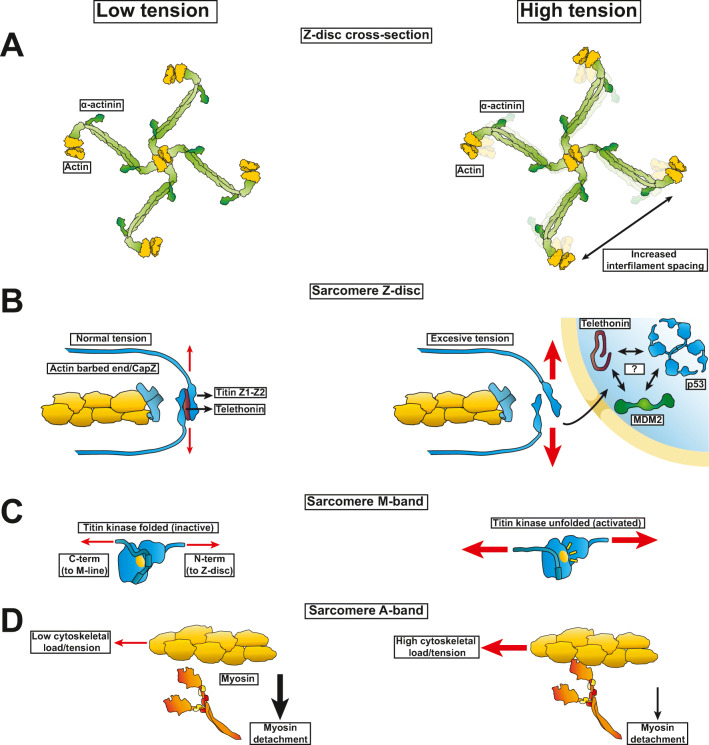
Located at the Z-disc, α-actinin is ubiquitously found in mammalian cells as a dimer that bundles parallel actin filaments. There are four α-actinin isoforms of which isoforms 2 and 3 are found in striated muscles (Sjöblom et al. 2008). The crystal structure of the α-actinin dimer shows an antiparallel assembly in which the calmodulin-like domains at the C-terminus bind to the neck region next to the actin-binding domain (Ribeiro Euripedes de Jr et al. 2014). This binding interaction renders the calmodulin-like domains available to interact with the titin Z-repeats. During muscle contraction the Z-disc mesh constituted by α-actinin and actin filaments experiences reversible deformations (Gautel and Djinović-Carugo 2016). Early electron microscope images in cardiac (Goldstein et al. 1989) and skeletal soleus muscles (Goldstein et al. 1988) have attributed a small square-lattice to the relaxed state, with a smaller actin separation, and a basketweave-lattice to the contractile or unstimulated muscles, with a larger actin separation. However, recent electron microscopy reconstructions have attributed a basketweave-lattice to the relaxed state, showing a smaller actin separation, and a diamond-shaped lattice to the contractile state, showing a larger actin separation (Fig. 2A) (Oda and Yanagisawa 2020). These structures may be specific to each muscle type, as the basketweave-lattice conformation has also been found in relaxed muscles of plainfin midshipman fish (Porichthys notatus) (Burgoyne et al. 2019). Another recent electron microscope reconstruction of single psoas myofibrils in the rigor state found a rhomboid pattern in the Z-discs (Wang et al. 2021). While the skeletal and cardiac muscles greatly differ in protein isoform content and ultrastructure, the diamond-shaped pattern seen in the contracting porcine cardiac muscle preparations are similar to those of rigor soleus muscles (Oda and Yanagisawa 2020; Wang et al. 2021). More studies are needed to clearly define the relaxed and contractile conformations of the Z-disc lattice for each muscle type.
Fig. 2.
Striated muscle proteins showing structural deformation at rest compared to loaded in the sarcomere. (A) The α-actinin and actin lattice is deformed when muscles go from a relaxed to contracting state leading to an increased actin-actin separation. (B) Titin N-terminal domain anchoring at the Z-disc exhibits a unilateral resistance to increased loads in the direction of contractile force by thin and thick filament sliding. (C) The titin kinase domain of striated muscle unfolds with high tension from actomyosin contraction leading to autophosphorylation and activation of hypertrophic signaling pathways. With excessive loads telethonin leads to p53 downregulation possibly via direct interactions with p53 and its cognate E3 ligase MDM2. (D) Myosin detachment rate from actin post-power stroke decreases with increased loads against the direction of the myosin level arm swinging
Telethonin
Telethonin (or T-cap) is a titin N-terminal cross-linker found in striated muscles in the Z-disc that exhibits unidirectional resistance to force when complexed to titin (Fig. 2B). Each telethonin links two titin Z1-Z2 immunoglobulin-like domains by adopting a beta-sheet conformation that displays the two titin N-termini in a palindromic geometry (Zou et al. 2006). When the two titin N-termini are pulled from their C-terminus, as normally occurs during active muscle contraction, the resistance to disrupt the non-covalent interactions between telethonin and the two titin Z1-Z2 ends can be as high as half the force needed to break a covalent bond. These high forces are attributed to the selective clustering of hydrogen bonds to the C-terminus side of the Z1-Z2 domain that prevent shear motion similar to a hook (Bertz et al. 2009). Even though the titin-telethonin complex is stable in the absence of any mechanical forces, the complex is more susceptible to breaking when the forces are applied in the opposite direction. The implications for this directionality are clearly imposed by the stretching forces during sarcomere shortening. Telethonin appears not to be essential for normal muscle function since mouse knockouts are viable and heart failure only develops when these mice are subject to ventricular overload that causes sarcomeric disarray (Knöll et al. 2011). However, telethonin global knockouts in mice revealed increased skeletal muscle stiffness accompanied by upregulation of myostatin (Markert et al. 2010). The role of telethonin may be to act as a regulator of the stress response since upregulation of telethonin decreases the levels of the proapoptotic tumor suppressor p53 (Knöll et al. 2011).
Live cell imaging of telethonin-GFP constructs show that telethonin is incorporated late in the process of de novo formation of new sarcomeres, or myofibrillogenesis, as pre-myofibrils lack incorporation of telethonin until striated sarcomeres form (Wang et al. 2005). This is despite that near 15% of titin is found in a soluble fraction (Rudolph et al. 2019). Given the large size of titin, the odds of arranging two titin N-termini in close proximity may be too low except for when two titin N-termini are incorporated at close proximity in sarcomeres. Telethonin is a substrate for protein kinase D phosphorylation in Ser-157 and Ser-161 and normal phosphorylation is necessary for maintenance of T-tubule stability and normal Ca2+ signaling during contraction (Candasamy et al. 2014). Taken together this evidence shows that telethonin is accomplishing a secondary role in sarcomere assembly rather than working as a sensor for pathologic mechanical stress in assembled sarcomeres.
Titin
Titin is the largest protein in mammals, with a near 3 MDa mass that spans a half-sarcomere (~1 μm in cardiac muscle and ~1.2 μm in skeletal) from Z-disc to M-band. Near the C-terminus located at the M-band, titin harbors a serine-threonine kinase domain termed titin kinase, which is susceptible to activation via mechanical unfolding (Gräter et al. 2005; Puchner et al. 2008). Autoinhibition is controlled by the C-terminal autoinhibitory tail that becomes unfolded with mechanical strains of near ~30 pN at 37°C (Puchner et al. 2008). Interestingly, Ca2+/calmodulin can also release the inhibition of the C-terminal autoinhibitory tail in vitro, possibly due to direct binding, leading to activation of the kinase (Mayans et al. 1998). Upon unfolding of the autoinhibitory tail (Fig. 2C), titin kinase binds ATP to initiate autophosphorylation of the autoinhibitory tyrosine (Puchner et al. 2008). This titin kinase domain appears to form a signalosome composed by sequential interactions between titin kinase, NBR1, p62, MuRF2, and SRF (Lange et al. 2005). NBR functions as a cargo receptor in autophagy and interacts with p62 (also known as SQSTM1), which functions as an autophagy receptor for the degradation of ubiquitinated proteins (Sánchez-Martín and Komatsu 2018). In turn, p62 interacts with the E3 ubiquitin ligase MURF2 (also known as TRIM55) necessary for normal metabolism of myofibrillar proteins (Lodka et al. 2016; Witt et al. 2008; Witt et al. 2005). Lastly, MuRF2 interacts with SRF in the nucleus. SRF is an ubiquitous transcription factor involved in development that interacts with the myocardin-related transcription factor family responsible for the gene regulation of several contractile proteins (Miano et al. 2007; Olson and Nordheim 2010). In conjunction, arrest of beating in cardiomyocytes promotes nuclear translocation of sarcomeric MuRF2 and concomitant release of SRF from the nucleus into the cytoskeleton (Lange et al. 2005). As such, on one hand it appears that the mechanical feedback on the titin kinase signalosome provides a direct link to the regulation of gene expression via selective exclusion of the transcription factor SRF between the cytoplasm and the nucleus. On the other hand, it is p62 that may be directly regulating protein turnover and quality control via selective autophagy (Danieli and Martens 2018). The conjunction of these signals could be responsible for the differential effects of different regimes of mechanical stimuli such as increased hypertrophy from eccentric contractions relative to contralateral and isometric muscle contractions in skeletal muscles (Barash et al. 2004).
Myosin
The myosin superfamily is composed of 15 classes of myosins (Sellers 2000).These ATPase motor proteins convert high-energy phosphate bonds from ATP into mechanical force leading to a swinging motion of the myosin lever arm (Forkey et al. 2003). The swinging motion of this lever arm leads to useful work by moving cellular cargoes along actin fibers as in the case of the progressive motor myosin V (Mehta et al. 1999) or by ensembles of myosin II motors that exhibit short-lived attachment to actin and assemble into thick filaments to promote muscle contraction (Hanson and Huxley 1953; Huxley and Niedergerke 1954; Huxley 1953). Unlike a normal ATPase enzyme, the chemo-mechanical reaction cycle of myosin is sensitive to mechanical forces that act in opposite direction of the lever arm swinging motion (Capitanio et al. 2012; Sung et al. 2015). This strain-dependence describes an exponential relationship between the externally applied force and the myosin detachment rate and it is in part due to inhibition of ADP release from myosin (Greenberg et al. 2014; Mentes et al. 2018). This load-dependent change in myosin behavior is shown diagrammatically, Fig. 2D. Interestingly, progressive myosin motors like myosin VI appear to be less sensitive to strains compared to non-progressive myosins (Altman et al. 2004). Drugs like omecamtiv mecarbil that increase the attachment lifetime of myosin in the pre-power-stroke state (Planelles-Herrero et al. 2017; Woody et al. 2018) render myosin less sensitive to changes in the external load (Liu et al. 2018). In summary, myosins have a very distinctive response to strain depending on its class and drugs have been shown to modulate the force to detachment rate relationship.
Second messenger signals distributed throughout the cell cause post-translational or chemical modifications of protein structures
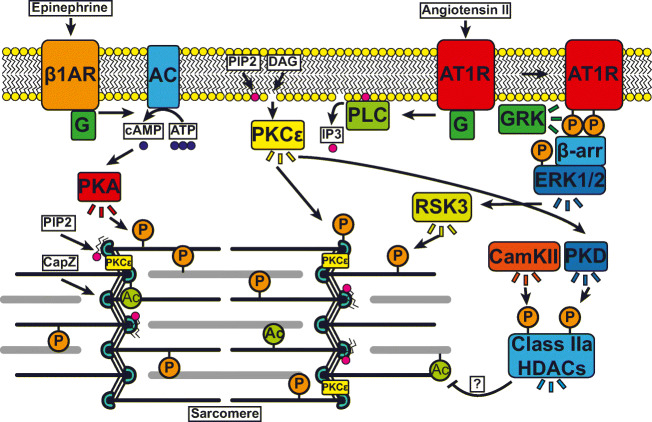
In addition to the localized physical forces discussed above, all cells respond to chemical stimuli initiated by a trigger or first messenger, usually the binding of a ligand to a cell membrane receptor such as the integrins (Humphries 2000) or the G protein–coupled receptors (Pfleger et al. 2019; Weis and Kobilka 2018). This results in amplification into a signaling cascade of second messengers, which diffuse throughout the cell as a series of molecular events. In cardiac muscles, ligands such as angiotensin II and epinephrine activate angiotensin II type 1 receptors (AT1R) and β1 adrenergic receptors (β1AR), respectively, while voltage change activates the calcium release. Downstream signals and their destinations are shown in Fig. 3. These extracellular signals lead to the activation of second messengers such as cyclic AMP (cAMP), inositol 1,4,5-trisphosphate (IP3), diacylglycerol (DAG), and cytosolic Ca2+ release. Note that the initial signal is amplified in many cascading events such as β-arrestin for angiotensin II (Ryba et al. 2017). In all striated muscles, the excitation-contraction process begins with the depolarization of the membrane that activates the voltage-sensitive L-type calcium channels that promote Ca2+ release across the sarcolemma and the T-tubules. These Ca2+ transients activate the ryanodine receptors that enable sarcoplasmic Ca2+ release. The Ca2+ pulse activates thin filaments by binding to troponin C to promote actomyosin contraction. The secondary messengers activate enzymes such as protein kinase A (PKA), protein kinase C (PKC), protein kinase D (PKD), RSK3, and Ca2+/calmodulin-dependent protein kinase II (CaMKII), that (de)-phosphorylate, (de)-acetylate cytoskeletal proteins to match muscle function to physiologic demand.
Fig. 3.
Signaling pathways that affect protein modifications at their destination in muscle. In cardiac muscles, the ligands epinephrine and angiotensin II bind to the β1 adrenergic receptors (β1AR) and angiotensin II type 1 receptor (AT1R), respectively, activating second messengers such as cyclic AMP (cAMP) produced by adenylyl cyclase (AC), inositol 1,4,5-trisphosphate (IP3), and diacylglycerol (DAG) produced by phospholipase C (PLC). The signaling cascades proceed to activate other kinase enzymes, such as protein kinase A (PKA), protein kinase C epsilon (PKCε), protein kinase D (PKD) Ca2+/calmodulin-dependent protein kinase II (CaMKII), and class IIa histone deacetylases (HDAC) that (de)-phosphorylate, (de)-acetylate cytoskeletal proteins. After ligand binding, G-protein-couple receptor inactivation begins by G protein-coupled receptor kinase(GRK) phosphorylation to promote β-arrestin. This leads to activation of other kinases such as extracellular signal-regulated kinases 1 and 2 (ERK1/2) and p90 ribosomal S6 kinase 3 (RSK3) that modify sarcomeric proteins. Modifications by the signals for phosphorylation (P), acetylation (Ac), binding to phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), and PKCε binding at sarcomeric destinations are shown at the Z-disk where the actin capping protein CapZ is modified, and in the thin filament. Collectively, signals are integrated by the sarcomeric proteins to match muscle function to physiologic demand
Excellent reviews on signaling are plentiful (Nakamura and Sadoshima 2018). Many discuss these pathways in detail but do not usually describe their mechanism of action on arrival at a destination. On arrival the signals modify charge of an amino acid or make other chemical changes, which alter interactions with neighboring proteins resulting in a regulation of cell function. Some of the major signaling pathways are mitogen-activated protein kinase (MAPK/ERK), cyclic nucleotide (cAMP)-dependent, and phosphoinositide (IP3) /diacyl glycerol (DAG). Through these pathways, the most common effects of the second messengers are to alter charge on an amino acid by adding phosphate via phosphorylation catalyzed by protein kinases (MAPK, PKA, PKC), or by removing phosphate with a phosphatase (Lorenzen-Schmidt et al. 2016). Also generated from IP3 is the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) (Li et al. 2014), which does not make a covalent bond with the amino acid but binds to alter function. In addition, many second messengers are ions, with calcium being the most important one in striated muscle.
There are numerous examples for how each of these cascading signals alters the protein structure when the chemical modification occurs at the destination of one or more of these signals. In this section, the focus is on the well-studied examples of CapZ in the Z-disc and troponin (Tn) as case studies. Many other cytoskeletal proteins are also destinations of the signals described that in turn modify protein function by covalent and non-covalent means. Readers are referred to specialized literature in structural proteins like muscle LIM protein (MLP) (Buyandelger et al. 2011), α-actinin (Sjöblom et al. 2008), myosin light chain (Kamm and Stull 2011), myosin binding protein C (MyBP-C) (Heling et al. 2020), and titin (LeWinter and Granzier 2010) .
Actin capping protein, CapZ
Assembly or disassembly of thin filaments is largely controlled by blocking or adding actin subunits to the fast-growing barbed end with regulation by a mushroom-like capping protein comprised of α- and β-subunits (Edwards et al. 2014). In striated muscle, the capping protein is named CapZ for its location at the Z-disc (Caldwell et al. 1989). The COOH termini of the α- and β-subunits of CapZ act like two “tentacles” critical for actin capping and thin filament assembly of the sarcomere (Wear et al. 2003). Cryo-electron microscopy and crystallography have defined the structure of CapZ (Narita et al. 2006; Yamashita et al. 2003). If only the β-tentacle but not the α-tentacle is bound to the actin, CapZ is able to ‘wobble’, which may expose additional binding sites for other regulatory molecules (Kim et al. 2007; Kim et al. 2010). The α-subunit plays an additional role in capping of the CapZ dimer to the thin filament (Takeda et al. 2010) but more attention has been paid to the β-tentacle.
What mechanism underlies the regulation of CapZ binding to actin? Post-translational modifications by phosphorylation and acetylation, and also the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) generated from IP3. It has become clear that the control of the dynamic exchange of the cap on and off the barbed end is regulated by modification of CapZ received in response mechanical and neurohormonal cues (Pyle et al. 2004). A series of papers, using FRAP and other techniques, showed modification is achieved when PKCε and PIP2 signals from activated pathways reach the CapZ β-subunit to alter its binding dynamics to actin at the tentacle, possibly by the phosphorylation at S-263 (Hartman et al. 2009; Kim et al. 2007; Wear and Cooper 2004). Growing cells on a stiff surface also activates FAK and PIP2 signaling pathways to affect CapZ (Li et al. 2016; Li et al. 2014). A bout of exercise delivered via cyclic strain to cardiac myocytes growing on a flexible membrane rapidly initiates an increase of CapZ and actin capping dynamics which abates in few hours after the stimulation ends, and is dependent on PKCε at the β-tentacle (Lin et al. 2015; Lin et al. 2013). Mass spectrometry identified CapZβ1 phosphorylation on S204 and acetylation on K199, two residues which are near the actin-binding surface (Lin et al. 2016). The acetylation is dependent on HDAC3, and HDAC inhibition might provide a therapeutic target (McKinsey 2012).
Troponin
The troponin (Tn) heterotrimer composed by troponin I (TnI), troponin (TnC), and troponin (TnT) has a dual function as an inhibitor of muscle contraction and as destination of regulatory signaling pathways in striated muscles. As an inhibitor of muscle contraction, TnC is the Ca2+ binding element of Tn that interacts with the TnI switch peptide to promote the dissociation of the TnI inhibitory and mobile domain regions from actin (Solis et al. 2018; Tripet et al. 1997; Van Eyk et al. 1993; Yamada et al. 2020). TnC binds Ca2+ via its EF-hand motifs, which are ubiquitous Ca2+ and Mg2+ binding elements in nature. While both cardiac and skeletal TnC have four EF-hand motifs, only three of these motifs are functional Ca2+/Mg2+ binding sites in cardiac TnC (Grabarek 2011). During muscle contraction intracellular Ca2+ levels rise from ~0.1 to ~1 mM sufficient for the exchange of Mg2+ for Ca2+ at the EF-hand motifs to increase the probability of opening of an hydrophobic patch in TnC that binds to the TnI switch peptide (Solís and Solaro 2021). The Ca2+ binding affinity and dissociation rate of TnC can be tuned by post-translational modifications from intracellular signals. The best-studied modification is the S23/24 phosphorylation by PKA in the cardiac TnI isoform that increases the dissociation rates of Ca2+ from TnC by allosteric mechanisms. Tn possesses multiple phosphorylation sites that arise from other kinases such as PKCα, PKCβII, PKCε, PKC δ, and PKG, and PKD1 (Solaro et al. 2013). In addition to phosphorylation, cardiac TnC has been reported to be S-nitrosylated at Cys84 leading to decreased myocardial sensitivity to Ca2+ (Irie et al. 2015). The specific contribution of these signals was illustrated by the expression of a PKA inhibitor peptide in cardiomyocytes in mice. PKA inhibition in these mice showed blunted β-adrenergic receptor regulation of heart chronotropy and contractility in addition to altered Ca2+ handling (Zhang et al. 2019). The susceptibility of myofibrillar proteins to acetylation led to study the effects of an acetyl-mimetic modification in cardiac TnI K132 that showed decreased Ca2+ sensitivity in thin filaments and faster relaxation of myofibrils (Lin et al. 2020). Other modifications may have deleterious effects in Tn such as irreversible glycation of the TN complex due to diabetic cardiomyopathies (Janssens et al. 2018). All these modifications have been compiled to some extent for cardiac TnC (Reinoso et al. 2020). In summary, this shows the wide variety of muscle regulatory mechanism at play in a well-studied protein like Tn. The functional characterization of these signals in other myofibrillar proteins is still in its infancy.
How are mechanical and chemical signals integrated by muscle proteins? Unanswered questions in muscle adaptation.
Use it or lose it: How does load alter sarcomere assembly or degradation?
Exercise matters. It is known that remodeling of muscle is regulated by the kind of exercise a muscle performs (Russell et al. 2000). The whole heart or skeletal muscle respond to being loaded or unloaded, but this also happens at the level of a subcellular region of a cell in culture. Loading or neurohumoral stimulation (Koshman et al. 2010) stabilizes the sarcomeric structure, decreases the dynamic protein exchange (Hartman et al. 2009; Mkrtschjan et al. 2018; Solís and Russell 2019), and extends the half-life before the degradation of the muscle protein. Sarcomeric actin’s half-life is 20 days, myosin heavy chain is 7–10 days, but the troponin I, T, and C are 3.2, 3.5, and 5.3 days, respectively (Martin 1981). Myosin heavy chain and actin synthesis decrease and degradation increases as sarcomeres disappear (Byron et al. 1996), but these changes reverse when cells are loaded again (Simpson et al. 1996). Importantly, once the sarcomeres disassemble, the monomers can be tagged by ubiquitin and half-life reduced to minutes in both cardiac and skeletal muscles (Samarel et al. 1992). Sarcomere degradation ensues through the ubiquitin proteosome pathway (Wang et al. 2020). Studies on skeletal muscle atrophy using proteomic analysis have reported that myofibrillar proteins, including α-actinin and myosin, exhibit decreased acetylation and increased ubiquitination on cast immobilized rat hind limbs (Ryder et al. 2015). Unanswered questions remain in understanding how tension locks the proteins in place, while reduced work allows disassembly (Chopra et al. 2018). Overall, the mechanical and chemical stimuli govern cardiomyocyte size and contractility, Fig. 4. But exactly how do higher levels of chemical stimulation interact with direct mechanical conformational changes?
Fig. 4.
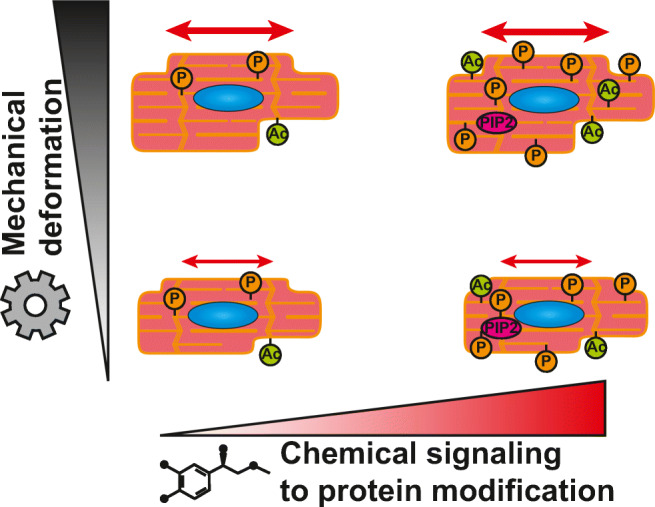
Integration of mechanical deformation and chemical signals in striated muscle. The effects of mechanical load and post-translational modification are shown diagrammatically with the resulting protein modifications by the signals for phosphorylation (P), acetylation (Ac), and binding of phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) to sarcomeric destinations. Mechanical loading alone triggers muscle hypertrophy, as does activation of the chemical signaling pathways alone. The mechanical loading is effective at the subcellular level near to the deformed proteins while the chemical signals is distributed throughout the cells. Thus, both local and whole cell response can be regulated
How is longitudinal vs. transverse growth regulated?
It is generally thought that to make a muscle fiber longer when strained, new sarcomeres are added most easily at the ends of the cells where the semi-crystalline arrays need not be disrupted (Russell et al. 2010), or at any position in the cells if sarcomeres are broken leaving an opportunity for repair (Dix and Eisenberg 1991a; Dix and Eisenberg 1991b; Yu and Russell 2005). Earlier studies addressed the question of how pre-myofibrils are formed and how translation of muscle proteins is distributed for the sarcomrere assembly process (Eisenberg et al. 1991; Rhee et al. 1994; Russell et al. 1992). Live cell imaging of tagged sarcomeric proteins show how these processes occur in developing muscle (Sanger et al. 2010; Yang et al. 2016), which may differ from adult remodeling after damage.
Muscle filaments are highly anisotropic and mechanosensors in the focal adhesions and other locations must also be oriented in order to explain detection of directionality and response in length and width regulation. What and where are these mechanosensors, and what signaling pathways do they activate? Lengthening sarcomeres by strain leads to the addition of new sarcomeres to return to the optimal sarcomere length at the single neonatal myocyte level within hours (Mansour 2004) where a PKCε-dependent signaling pathway appears critical in linking external mechanical stimulation to the internal environment. The focal adhesion complex, through FAK, is also necessary for the cardiac remodeling process. Transverse strain of aligned myocytes increases phosphorylation of FAK (Y397pFAK), and ERK1/2 (Thr183)/Tyr185) more than longitudinal strain (Senyo et al. 2007). Also, paxillin (pY31) phosphorylation increases with longitudinal strain. These findings implicate a primary conformational change of proteins in the focal adhesion, perhaps leading to access of cryptic sites, as well as activation of signaling pathways.
The ERK pathway was further implicated in a transgenic mouse model (Kehat et al. 2011). Recently a target for ERK in the asymmetrical cardiac myocyte hypertrophy has been described as being modulated by serum response factor phosphorylation (Li et al. 2020), regulated by RSK3 (p90 ribosomal S6 kinase type 3) and protein phosphatase 2A (PP2A) at signalosomes organized by the scaffold protein muscle A-kinase anchoring protein β (mAKAPβ). These scaffold sensors are situated near the cell membrane, but sarcomere assembly is regulated at more distant sites (Taneja et al. 2020). This suggests that a signaling pathway is amplifying some local protein deformation event. It is known local protein dynamics are regulated in a localized load-dependent manner as detected by FRAP as cited above. How does local mechanical deformation of specific proteins interact with whole cell signaling events? Note that other oriented sensors may remain to be identified.
How does a lengthening versus a shortening contraction regulate hypertrophy?
First it is necessary to point out that the terms concentric and eccentric are used in the opposite manner for skeletal and cardiac muscle. Skeletal refers to changes in the muscle itself while the cardiac refers to the position of the heart in the chest. Therefore, the terms lengthening and shortening contractions are preferable (Russell et al. 2000). Other frequently used terms in exercise physiology for lengthening contractions are resistance or strength training, which increase muscle fiber cross sectional area, mass and total force a muscle can produce (Hughes et al. 2018). To put these forces into perspective for the heart, one can describe that the internal forces generated by contraction are dominant during systole, while those external forces coming from the stiffness of the heart outside prevail during diastole (Davis et al. 2016). To date, there is no mechanistic explanation for these findings. It seems possible that different signaling pathways are activated by external forces other than from internal ones so that inside-out and outside-in directions may produce different outcomes. Furthermore, muscle strained in different directions have anisotropically configured proteins loosened or deformed permitting altered exchange dynamics, and the opportunity for local sarcomere assembly. How do local controls become amplified or diminished depending on the signaling milieu of the cell cytoplasm?
How does straining of heart cells enhance the next contraction, the Frank Starling effect?
A specialized mechanical response to force exerted by the heart is summarized by the Frank-Starling principle, which is not seen in skeletal muscle. The Frank-Starling law of the heart states that increasing diastolic filling volume leads to an increasing systolic pressure development. In other words, straining of the heart ventricular walls before contraction magnifies the contractile force of the ventricles during the next heartbeat. This is a hormone-independent response of the heart, and it is a distinctive feature of cardiac muscles (Konhilas et al. 2002a). The Frank-Starling responses are found at the level of cardiac muscle slices, single cardiomyocytes and engineered cardiac tissues (Hirt et al. 2014; Narolska et al. 2006; Pitoulis et al. 2021).
To date, the precise mechanism that drives the Frank-Starling Law of the heart is not known. However, what is known are the biochemical and physiologic interventions that modify this length-force response relationship down to the scale of myofibrils. Readers are encouraged to consult dedicated reviews (de Tombe et al. 2010; Kawai and Jin 2021; Sequeira and van der Velden 2017). Briefly, some of the key evidence suggests (i) increased Ca2+ sensitivity of thin filaments when stretching myofibrils (Hibberd and Jewell 1982); (ii) the increased thin filament sensitivity is not driven by changes in thin and think filament interspace distance (Konhilas et al. 2002b); (iii) increased myofibrillar lengthening increases number of myosin heads in the disordered relaxed state available to bind to actin (Farman et al. 2011; Reconditi et al. 2017); (iv) phosphorylation of proteins like troponin I, myosin light chain, and myosin-binding protein C alter the length-force relationship (Kampourakis et al. 2016; Kumar et al. 2015); and (v) the influence of titin strain at varying sarcomere lengths (Ait-Mou et al. 2016; Cazorla et al. 2001). This just shows how much progress has been made but also points to the need to solve this very challenging and health-relevant mechanism involving the concerted response of cytoskeletal proteins to mechanical feedback.
What restoring forces return sarcomere to resting length on relaxation?
One usually finds a statement that contraction occurs when the intracellular level of calcium rises and relaxation occurs when it declines. The mechanism for calcium activation of troponin, discussed above, is well understood, and leads to the development of force for contraction. However, the nature of the restoring force that returns the sarcomere to its resting length is still unclear. Mere removal of calcium does not generate a force in the opposite direction by reversing the myosin motor with further energy consumption. In whole organs, the restoring force comes partially from the recoil of surrounding connective tissue, but isolated cells return to rest length meaning that sarcomeric elements are also involved. There are several good candidates discussed throughout this review where energy could be stored in the deformed sarcomeric protein structures to provide the recoil force for lengthening on relaxation. The myofilament could be subject to a reduction in the lattice spacing with loading but no evidence to suggest this provides a restoring force (Konhilas et al. 2002a). Titin does have spring-like regions situated to assist in sarcomere lengthening (LeWinter and Granzier 2010). The Z-disk undergoes major structural change of α-actinin during contraction, mainly in the transverse direction but some force vector is also longitudinal making this worth considering. Indeed, the number of α-actinin molecules is the major determinant of the width of the Z-disk in fast, slow and cardiac muscles, each performing at a different strength and speed, but all able to relax (Eisenberg 1983). Understanding the drivers of increased restoring forces acting during myofibrillar relaxation could be a therapeutic strategy to treat diastolic heart diseases such as heart failure with preserved ejection fraction. Many unanswered questions remain unsolved and much of the merit to study these problems is to understand human diseases to improve health.
Summary
This review focuses on how load deforms specific structures in a proportional and orientation-dependent manner in striated muscle, thereby affecting local, subcellular interactions between proteins. An equally important mechanism by which protein behavior is affected occurs throughout the whole cell is a consequence of signaling pathway activation and the subsequent post-translational modifications. The two processes of mechanical protein deformation and signals reaching the protein work together in a complex way so that local subcellular assembly is controlled as well as overall cell function. However, more research is needed to understand how externally triggered signaling pathways are integrated with tension generated locally within the cell. Nonetheless, maintenance of tension in the sarcomere is the essential and dominant mechanism, leading to the well-known phrase in exercise physiology: “use it or lose it.”
Acknowledgements
This work was supported by the National Institutes of Health grants HL62426 (to B. Russell, Project 2) and HL151825 (to C. Solís). The authors declare no competing financial interests.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ait-Mou Y, Hsu K, Farman GP, Kumar M, Greaser ML, Irving TC, de Tombe PP. Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc Natl Acad Sci U S A. 2016;113:2306–2311. doi: 10.1073/pnas.1516732113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D, Sweeney HL, Spudich JA. The mechanism of myosin VI translocation and its load-induced anchoring. Cell. 2004;116:737–749. doi: 10.1016/s0092-8674(04)00211-9. [DOI] [PubMed] [Google Scholar]
- Anthis NJ, et al. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 2009;28:3623–3632. doi: 10.1038/emboj.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arold ST, Hoellerer MK, Noble ME. The structural basis of localization and signaling by the focal adhesion targeting domain. Structure (London, England : 1993) 2002;10:319–327. doi: 10.1016/s0969-2126(02)00717-7. [DOI] [PubMed] [Google Scholar]
- Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol. 2004;286:C355–C364. doi: 10.1152/ajpcell.00211.2003. [DOI] [PubMed] [Google Scholar]
- Bauer MS, et al. Structural and mechanistic insights into mechanoactivation of focal adhesion kinase. Proc Natl Acad Sci U S A. 2019;116:6766–6774. doi: 10.1073/pnas.1820567116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays JL, DeMali KA. Vinculin in cell-cell and cell-matrix adhesions. Cell Mol Life Sci CMLS. 2017;74:2999–3009. doi: 10.1007/s00018-017-2511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertz M, Wilmanns M, Rief M. The titin-telethonin complex is a directed, superstable molecular bond in the muscle Z-disk. Proc Natl Acad Sci U S A. 2009;106:13307–133310. doi: 10.1073/pnas.0902312106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne T, et al. Three-dimensional structure of the basketweave Z-band in midshipman fish sonic muscle. Proc Natl Acad Sci. 2019;116:15534–15539. doi: 10.1073/pnas.1902235116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyandelger B, Ng KE, Miocic S, Piotrowska I, Gunkel S, Ku CH, Knöll R. MLP (muscle LIM protein) as a stress sensor in the heart. Pflugers Arch - Eur J Physiol. 2011;462:135–142. doi: 10.1007/s00424-011-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne BJ, Kaczorowski YJ, Coutu MD, Craig SW (1992) Chicken vinculin and meta-vinculin are derived from a single gene by alternative splicing of a 207-base pair exon unique to meta-vinculin. J Biol Chem 267:12845–12850. 10.1016/S0021-9258(18)42353-8 [PubMed]
- Byron KL, Puglisi JL, Holda JR, Eble D, Samarel AM. Myosin heavy chain turnover in cultured neonatal rat heart cells: effects of [Ca2+]i and contractile activity. Am J Phys. 1996;271:C01447–C01456. doi: 10.1152/ajpcell.1996.271.5.C01447. [DOI] [PubMed] [Google Scholar]
- Caldwell JE, Heiss SG, Mermall V, Cooper JA. Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry. 1989;28:8506–8514. doi: 10.1021/bi00447a036. [DOI] [PubMed] [Google Scholar]
- Candasamy AJ, et al. Phosphoregulation of the titin-cap protein telethonin in cardiac myocytes. J Biol Chem. 2014;289:1282–1293. doi: 10.1074/jbc.M113.479030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio M, et al. Ultrafast force-clamp spectroscopy of single molecules reveals load dependence of myosin working stroke. Nat Methods. 2012;9:1013–1019. doi: 10.1038/nmeth.2152. [DOI] [PubMed] [Google Scholar]
- Cazorla O, Wu Y, Irving TC, Granzier H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res. 2001;88:1028–1035. doi: 10.1161/hh1001.090876. [DOI] [PubMed] [Google Scholar]
- Ceccarelli DF, Song HK, Poy F, Schaller MD, Eck MJ. Crystal structure of the FERM domain of focal adhesion kinase. J Biol Chem. 2006;281:252–259. doi: 10.1074/jbc.M509188200. [DOI] [PubMed] [Google Scholar]
- Chinthalapudi K, Rangarajan ES, Brown DT, Izard T. Differential lipid binding of vinculin isoforms promotes quasi-equivalent dimerization. Proc Natl Acad Sci U S A. 2016;113:9539–9544. doi: 10.1073/pnas.1600702113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A, et al. Force generation via β-cardiac Myosin, Titin, and α-Actinin drives cardiac sarcomere assembly from cell-matrix adhesions. Dev Cell. 2018;44:87–96.e85. doi: 10.1016/j.devcel.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PH, Bardwell WM, Gu Y, Ross J, Jr, Chen J. FHL2 (SLIM3) is not essential for cardiac development and function. Mol Cell Biol. 2000;20:7460–7462. doi: 10.1128/mcb.20.20.7460-7462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M, et al. Serine-910 phosphorylation of focal adhesion kinase is critical for sarcomere reorganization in cardiomyocyte hypertrophy. Cardiovasc Res. 2011;92:409–419. doi: 10.1093/cvr/cvr247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli A, Martens S (2018) p62-mediated phase separation at the intersection of the ubiquitin-proteasome system and autophagy. J Cell Sci 131. 10.1242/jcs.214304 [DOI] [PMC free article] [PubMed]
- Davis J, et al. A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell. 2016;165:1147–1159. doi: 10.1016/j.cell.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48:851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedden D, Schumacher S, Kelley CF, Zacharias M, Biertümpfel C, Fässler R, Mizuno N. The architecture of Talin1 reveals an autoinhibition mechanism. Cell. 2019;179:120–131.e113. doi: 10.1016/j.cell.2019.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, et al. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Eisenberg BR. Distribution of myosin mRNA during development and regeneration of skeletal muscle fibers. Dev Biol. 1991;143:422–426. doi: 10.1016/0012-1606(91)90093-i. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Eisenberg BR. Redistribution of myosin heavy chain mRNA in the midregion of stretched muscle fibers. Cell Tissue Res. 1991;263:61–69. doi: 10.1007/bf00318400. [DOI] [PubMed] [Google Scholar]
- Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Edwards M, Zwolak A, Schafer DA, Sept D, Dominguez R, Cooper JA. Capping protein regulators fine-tune actin assembly dynamics. Nat Rev Mol Cell Biol. 2014;15:677–689. doi: 10.1038/nrm3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B (1983) Section 10. Quantitative ultrastructure of mammalian skeletal muscle. Handbook of Physiology Peachey LD and Adrian RH, editors Bethesda, MD: American Physiological Society 73112. 10.1002/cphy.cp100103
- Eisenberg BR, Goldspink PH, Wenderoth MP. Distribution of myosin heavy chain mRNA in normal and hyperthyroid heart. J Mol Cell Cardiol. 1991;23:287–296. doi: 10.1016/0022-2828(91)90065-t. [DOI] [PubMed] [Google Scholar]
- Farman GP, Gore D, Allen E, Schoenfelt K, Irving TC, de Tombe PP. Myosin head orientation: a structural determinant for the Frank-Starling relationship. Am J Phys Heart Circ Phys. 2011;300:H2155–H2160. doi: 10.1152/ajpheart.01221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkey JN, Quinlan ME, Shaw MA, Corrie JE, Goldman YE. Three-dimensional structural dynamics of myosin V by single-molecule fluorescence polarization. Nature. 2003;422:399–404. doi: 10.1038/nature01529. [DOI] [PubMed] [Google Scholar]
- Franchini KG, Torsoni AS, Soares PH, Saad MJ. Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ Res. 2000;87:558–565. doi: 10.1161/01.res.87.7.558. [DOI] [PubMed] [Google Scholar]
- Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. Journal of Molecular Medicine (Berlin, Germany) 2006;84:446–468. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Friedrich FW, et al. FHL2 expression and variants in hypertrophic cardiomyopathy. Basic Res Cardiol. 2014;109:451. doi: 10.1007/s00395-014-0451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao QQ, McNally EM. The dystrophin complex: structure, function, and implications for therapy. Compr Physiol. 2015;5:1223–1239. doi: 10.1002/cphy.c140048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautel M, Djinović-Carugo K. The sarcomeric cytoskeleton: from molecules to motion. J Exp Biol. 2016;219:135–145. doi: 10.1242/jeb.124941. [DOI] [PubMed] [Google Scholar]
- Genini M, Schwalbe P, Scholl FA, Remppis A, Mattei MG, Schäfer BW. Subtractive cloning and characterization of DRAL, a novel LIM-domain protein down-regulated in rhabdomyosarcoma. DNA Cell Biol. 1997;16:433–442. doi: 10.1089/dna.1997.16.433. [DOI] [PubMed] [Google Scholar]
- Gingras AR, Ziegler WH, Frank R, Barsukov IL, Roberts GC, Critchley DR, Emsley J. Mapping and consensus sequence identification for multiple vinculin binding sites within the talin rod. J Biol Chem. 2005;280:37217–37224. doi: 10.1074/jbc.M508060200. [DOI] [PubMed] [Google Scholar]
- Goldstein MA, Michael LH, Schroeter JP, Sass RL. Structural states in the Z band of skeletal muscle correlate with states of active and passive tension. J Gen Physiol. 1988;92:113–119. doi: 10.1085/jgp.92.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MA, Michael LH, Schroeter JP, Sass RL. Two structural states of Z-bands in cardiac muscle. Am J Phys. 1989;256:H552–H559. doi: 10.1152/ajpheart.1989.256.2.H552. [DOI] [PubMed] [Google Scholar]
- Grabarek Z. Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Biochim Biophys Acta. 2011;1813:913–921. doi: 10.1016/j.bbamcr.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ZA, Gallagher PM, Cardozo CP. Focal adhesion kinase and its role in skeletal muscle. J Muscle Res Cell Motil. 2015;36:305–315. doi: 10.1007/s10974-015-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräter F, Shen J, Jiang H, Gautel M, Grubmüller H. Mechanically induced titin kinase activation studied by force-probe molecular dynamics simulations. Biophys J. 2005;88:790–804. doi: 10.1529/biophysj.104.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg MJ, Shuman H, Ostap EM. Inherent force-dependent properties of β-cardiac myosin contribute to the force-velocity relationship of cardiac muscle. Biophys J. 2014;107:L41–l44. doi: 10.1016/j.bpj.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Huxley HE. Structural basis of the cross-striations in muscle. Nature. 1953;172:530–532. doi: 10.1038/172530b0. [DOI] [PubMed] [Google Scholar]
- Hartman TJ, Martin JL, Solaro RJ, Samarel AM, Russell B. CapZ dynamics are altered by endothelin-1 and phenylephrine via PIP2- and PKC-dependent mechanisms. Am J Physiol Cell Physiol. 2009;296:C1034–C1039. doi: 10.1152/ajpcell.00544.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Vuori K, Liddington RC. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat Struct Biol. 2002;9:101–106. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- Heling L, Geeves MA, Kad NM. MyBP-C: one protein to govern them all. J Muscle Res Cell Motil. 2020;41:91–101. doi: 10.1007/s10974-019-09567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings L, et al. Talin contains three actin-binding sites each of which is adjacent to a vinculin-binding site. J Cell Sci. 1996;109(Pt 11):2715–2726. doi: 10.1242/jcs.109.11.2715. [DOI] [PubMed] [Google Scholar]
- Hibberd MG, Jewell BR. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol. 1982;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: state of the art. Circ Res. 2014;114:354–367. doi: 10.1161/circresaha.114.300522. [DOI] [PubMed] [Google Scholar]
- Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Phys Heart Circ Phys. 2006;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.nature.com/articles/srep20674 (n.d.) - supplementary-information
- Huang DL, Bax NA, Buckley CD, Weis WI, Dunn AR. Vinculin forms a directionally asymmetric catch bond with F-actin. Science. 2017;357:703–706. doi: 10.1126/science.aan2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DC, Ellefsen S, Baar K (2018) Adaptations to Endurance and Strength Training. Cold Spring Harbor Perspectives in Medicine 8. 10.1101/cshperspect.a029769 [DOI] [PMC free article] [PubMed]
- Humphries MJ (2000) Integrin structure. Biochem Soc Trans 28:311–339. 10.1042/bst0280311 [PubMed]
- Huxley HE. Electron microscope studies of the organisation of the filaments in striated muscle. Biochim Biophys Acta. 1953;12:387–394. doi: 10.1016/0006-3002(53)90156-5. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Niedergerke R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954;173:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Irie T, et al. S-Nitrosylation of Calcium-Handling Proteins in Cardiac Adrenergic Signaling and Hypertrophy. Circ Res. 2015;117:793–803. doi: 10.1161/circresaha.115.307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto DV, Huehn A, Simon B, Huet-Calderwood C, Baldassarre M, Sindelar CV, Calderwood DA. Structural basis of the filamin A actin-binding domain interaction with F-actin. Nat Struct Mol Biol. 2018;25:918–927. doi: 10.1038/s41594-018-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SR, Shah SB, Ward CW, Stains JP, Spangenburg EE, Folker ES, Lovering RM. Differential YAP nuclear signaling in healthy and dystrophic skeletal muscle. Am J Physiol Cell Physiol. 2019;317:C48–c57. doi: 10.1152/ajpcell.00432.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard T, Brown DT. Mechanisms and Functions of Vinculin Interactions with Phospholipids at Cell Adhesion Sites. J Biol Chem. 2016;291:2548–2555. doi: 10.1074/jbc.R115.686493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard T, Evans G, Borgon RA, Rush CL, Bricogne G, Bois PR. Vinculin activation by talin through helical bundle conversion. Nature. 2004;427:171–175. doi: 10.1038/nature02281. [DOI] [PubMed] [Google Scholar]
- Janssens JV, Ma B, Brimble MA, Van Eyk JE, Delbridge LMD, Mellor KM. Cardiac troponins may be irreversibly modified by glycation: novel potential mechanisms of cardiac performance modulation. Sci Rep. 2018;8:16084. doi: 10.1038/s41598-018-33886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, et al. The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction. Nat Commun. 2021;12:869. doi: 10.1038/s41467-021-21178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Signaling to myosin regulatory light chain in sarcomeres. J Biol Chem. 2011;286:9941–9947. doi: 10.1074/jbc.R110.198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampourakis T, Sun YB, Irving M. Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proc Natl Acad Sci U S A. 2016;113:E3039–E3047. doi: 10.1073/pnas.1602776113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Jin JP (2021) Mechanisms of Frank-Starling law of the heart and stretch activation in striated muscles may have a common molecular origin. J Muscle Res Cell Motil. 10.1007/s10974-020-09595-2 [DOI] [PMC free article] [PubMed]
- Kehat I, et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. doi: 10.1161/circresaha.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RB, Goult BT. Adhesions assemble!-autoinhibition as a major regulatory mechanism of integrin-mediated adhesion. Front Mol Biosci. 2019;6:144. doi: 10.3389/fmolb.2019.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiema T, et al. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell. 2006;21:337–347. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Kim K, McCully ME, Bhattacharya N, Butler B, Sept D, Cooper JA. Structure/function analysis of the interaction of phosphatidylinositol 4,5-bisphosphate with actin-capping protein: implications for how capping protein binds the actin filament. J Biol Chem. 2007;282:5871–5879. doi: 10.1074/jbc.M609850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Cooper JA, Sept D. The interaction of capping protein with the barbed end of the actin filament. J Mol Biol. 2010;404:794–802. doi: 10.1016/j.jmb.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöll R, Hoshijima M, Chien K. Cardiac mechanotransduction and implications for heart disease. Journal of Molecular Medicine (Berlin, Germany) 2003;81:750–756. doi: 10.1007/s00109-003-0488-x. [DOI] [PubMed] [Google Scholar]
- Knöll R, et al. Telethonin deficiency is associated with maladaptation to biomechanical stress in the mammalian heart. Circ Res. 2011;109:758–769. doi: 10.1161/circresaha.111.245787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Shelton JM, Rothermel B, Li X, Richardson JA, Bassel-Duby R, Williams RS. Cardiac-specific LIM protein FHL2 modifies the hypertrophic response to beta-adrenergic stimulation. Circulation. 2001;103:2731–2738. doi: 10.1161/01.cir.103.22.2731. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Irving TC, de Tombe PP. Length-dependent activation in three striated muscle types of the rat. J Physiol. 2002;544:225–236. doi: 10.1113/jphysiol.2002.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konhilas JP, Irving TC, de Tombe PP. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res. 2002;90:59–65. doi: 10.1161/hh0102.102269. [DOI] [PubMed] [Google Scholar]
- Koshman YE, Piano MR, Russell B, Schwertz DW. Signaling responses after exposure to 5 alpha-dihydrotestosterone or 17 beta-estradiol in norepinephrine-induced hypertrophy of neonatal rat ventricular myocytes. Journal of Applied Physiology (Bethesda, Md : 1985) 2010;108:686–696. doi: 10.1152/japplphysiol.00994.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Govindan S, Zhang M, Khairallah RJ, Martin JL, Sadayappan S, de Tombe PP. Cardiac myosin-binding Protein C and Troponin-I phosphorylation independently modulate myofilament length-dependent activation. J Biol Chem. 2015;290:29241–29249. doi: 10.1074/jbc.M115.686790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Auerbach D, McLoughlin P, Perriard E, Schäfer BW, Perriard JC, Ehler E. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci. 2002;115:4925–4936. doi: 10.1242/jcs.00181. [DOI] [PubMed] [Google Scholar]
- Lange S, et al. The Kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- Lee HS, et al. Characterization of an actin-binding site within the talin FERM domain. J Mol Biol. 2004;343:771–784. doi: 10.1016/j.jmb.2004.08.069. [DOI] [PubMed] [Google Scholar]
- LeWinter MM, Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010;121:2137–2145. doi: 10.1161/circulationaha.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Ponten F, dos Remedios CG. The interactome of LIM domain proteins: the contributions of LIM domain proteins to heart failure and heart development. Proteomics. 2012;12:203–225. doi: 10.1002/pmic.201100492. [DOI] [PubMed] [Google Scholar]
- Li J, Tanhehco EJ, Russell B. Actin dynamics is rapidly regulated by the PTEN and PIP2 signaling pathways leading to myocyte hypertrophy. Am J Phys Heart Circ Phys. 2014;307:H1618–H1625. doi: 10.1152/ajpheart.00393.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mkrtschjan MA, Lin YH, Russell B. Variation in stiffness regulates cardiac myocyte hypertrophy via signaling pathways. Can J Physiol Pharmacol. 2016;94:1–9. doi: 10.1139/cjpp-2015-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. Signalosome-regulated serum response factor phosphorylation determining myocyte growth in width versus length as a therapeutic target for heart failure. Circulation. 2020;142:2138–2154. doi: 10.1161/circulationaha.119.044805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129:1177–1187. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Li J, Swanson ER, Russell B. CapZ and actin capping dynamics increase in myocytes after a bout of exercise and abates in hours after stimulation ends. Journal of Applied Physiology (Bethesda, Md : 1985) 2013;114:1603–1609. doi: 10.1152/japplphysiol.01283.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-H, Swanson ER, Li J, Mkrtschjan MA, Russell B. Cyclic mechanical strain of myocytes modifies CapZβ1 post translationally via PKCε. J Muscle Res Cell Motil. 2015;36:329–337. doi: 10.1007/s10974-015-9420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Warren CM, Li J, McKinsey TA, Russell B. Myofibril growth during cardiac hypertrophy is regulated through dual phosphorylation and acetylation of the actin capping protein CapZ. Cell Signal. 2016;28:1015–1024. doi: 10.1016/j.cellsig.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, et al. Site-specific acetyl-mimetic modification of cardiac troponin I modulates myofilament relaxation and calcium sensitivity. J Mol Cell Cardiol. 2020;139:135–147. doi: 10.1016/j.yjmcc.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- Liu C, Kawana M, Song D, Ruppel KM, Spudich JA. Controlling load-dependent kinetics of β-cardiac myosin at the single-molecule level. Nat Struct Mol Biol. 2018;25:505–514. doi: 10.1038/s41594-018-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodka D, et al. Muscle RING-finger 2 and 3 maintain striated-muscle structure and function. J Cachexia Sarcopenia Muscle. 2016;7:165–180. doi: 10.1002/jcsm.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen-Schmidt I, Clarke SB, Pyle WG. The neglected messengers: control of cardiac myofilaments by protein phosphatases. J Mol Cell Cardiol. 2016;101:81–89. doi: 10.1016/j.yjmcc.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Lyon RC, Zanella F, Omens JH, Sheikh F. Mechanotransduction in cardiac hypertrophy and failure. Circ Res. 2015;116:1462–1476. doi: 10.1161/circresaha.116.304937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manso AM, et al. Talin1 has unique expression versus talin 2 in the heart and modifies the hypertrophic response to pressure overload. J Biol Chem. 2013;288:4252–4264. doi: 10.1074/jbc.M112.427484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour H, de Tombe PP, Samarel AM, Russell B (2004) Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Cepsilon and focal adhesion kinase. Circ Res 94(5):642–9. 10.1161/01.RES.0000121101.32286.C8. Epub 2004 Feb 12 [DOI] [PubMed]
- Mao Z, Nakamura F (2020) Structure and function of filamin C in the muscle Z-Disc. Int J Mol Sci 21. 10.3390/ijms21082696 [DOI] [PMC free article] [PubMed]
- Markert CD, et al. Functional muscle analysis of the Tcap knockout mouse. Hum Mol Genet. 2010;19:2268–2283. doi: 10.1093/hmg/ddq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AF (1981) Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem 256:964–968. 10.1016/S0021-9258(19)70073-8 [PubMed]
- Mayans O, et al. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998;395:863–869. doi: 10.1038/27603. [DOI] [PubMed] [Google Scholar]
- McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol. 2012;52:303–319. doi: 10.1146/annurev-pharmtox-010611-134712. [DOI] [PubMed] [Google Scholar]
- Mehta AD, Rock RS, Rief M, Spudich JA, Mooseker MS, Cheney RE. Myosin-V is a processive actin-based motor. Nature. 1999;400:590–593. doi: 10.1038/23072. [DOI] [PubMed] [Google Scholar]
- Mentes A, et al. High-resolution cryo-EM structures of actin-bound myosin states reveal the mechanism of myosin force sensing. Proc Natl Acad Sci U S A. 2018;115:1292–1297. doi: 10.1073/pnas.1718316115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel CD, Li Y, Raza Q, Stolz DB, Kwiatkowski AV. Vinculin anchors contractile actin to the cardiomyocyte adherens junction. Mol Biol Cell. 2019;30:2639–2650. doi: 10.1091/mbc.E19-04-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- Mkrtschjan MA, Solís C, Wondmagegn AY, Majithia J, Russell B. PKC epsilon signaling effect on actin assembly is diminished in cardiomyocytes when challenged to additional work in a stiff microenvironment. Cytoskeleton (Hoboken, NJ) 2018;75:363–371. doi: 10.1002/cm.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- Nakazawa N, Sathe AR, Shivashankar GV, Sheetz MP. Matrix mechanics controls FHL2 movement to the nucleus to activate p21 expression. Proc Natl Acad Sci U S A. 2016;113:E6813–e6822. doi: 10.1073/pnas.1608210113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita A, Takeda S, Yamashita A, Maéda Y. Structural basis of actin filament capping at the barbed-end: a cryo-electron microscopy study. EMBO J. 2006;25:5626–5633. doi: 10.1038/sj.emboj.7601395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narolska NA, et al. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res. 2006;99:1012–1020. doi: 10.1161/01.RES.0000248753.30340.af. [DOI] [PubMed] [Google Scholar]
- Oda T, Yanagisawa H. Cryo-electron tomography of cardiac myofibrils reveals a 3D lattice spring within the Z-discs. Commun Biol. 2020;3:585. doi: 10.1038/s42003-020-01321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, et al. Cardiomyocytes sense matrix rigidity through a combination of muscle and non-muscle myosin contractions. Dev Cell. 2018;44:326–336.e323. doi: 10.1016/j.devcel.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger J, Gresham K, Koch WJ. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat Rev Cardiol. 2019;16:612–622. doi: 10.1038/s41569-019-0220-3. [DOI] [PubMed] [Google Scholar]
- Pitoulis FG et al (2021) Remodelling of adult cardiac tissue subjected to physiological and pathological mechanical load in vitro. Cardiovasc Res. 10.1093/cvr/cvab084 [DOI] [PMC free article] [PubMed]
- Planelles-Herrero VJ, Hartman JJ, Robert-Paganin J, Malik FI, Houdusse A. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat Commun. 2017;8:190. doi: 10.1038/s41467-017-00176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchner EM, et al. Mechanoenzymatics of titin kinase. Proc Natl Acad Sci U S A. 2008;105:13385–13390. doi: 10.1073/pnas.0805034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudas R, Kiema TR, Butler PJ, Stewart M, Ylänne J. Structural basis for vertebrate filamin dimerization. Structure (London, England : 1993) 2005;13:111–119. doi: 10.1016/j.str.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Purcell NH, Darwis D, Bueno OF. Müller JM, Schüle R, Molkentin JD. Extracellular signal-regulated kinase 2 interacts with and is negatively regulated by the LIM-only protein FHL2 in cardiomyocytes. Mol Cell Biol. 2004;24:1081–1095. doi: 10.1128/mcb.24.3.1081-1095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle WG, La Rotta G, de Tombe PP, Sumandea MP, Solaro RJ (2006) Control of cardiac myofilament activation and PKC-betaII signaling through the actin capping protein, CapZ. J Mol Cell Cardiol 41(3):537–43. 10.1016/j.yjmcc.2006.06.006 [DOI] [PubMed]
- Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, Gotthardt M. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proc Natl Acad Sci U S A. 2007;104:3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razinia Z, Mäkelä T, Ylänne J, Calderwood DA. Filamins in mechanosensing and signaling. Annu Rev Biophys. 2012;41:227–246. doi: 10.1146/annurev-biophys-050511-102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reconditi M, et al. Myosin filament activation in the heart is tuned to the mechanical task. Proc Natl Acad Sci U S A. 2017;114:3240–3245. doi: 10.1073/pnas.1619484114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso TR et al (2020) A comprehensive guide to genetic variants and post-translational modifications of cardiac troponin C. J Muscle Res Cell Motil. 10.1007/s10974-020-09592-5 [DOI] [PMC free article] [PubMed]
- Rhee D, Sanger JM, Sanger JW. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton. 1994;28:1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- Ribeiro Ede A Jr, Pinotsis N, Ghisleni A, Salmazo A, Konarev PV, Kostan J, et al (2014) The structure and regulation of human muscle α-actinin. Cell 159(6):1447–60. 10.1016/j.cell.2014.10.056. Epub 2014 Nov 26. [DOI] [PMC free article] [PubMed]
- Rudolph F, et al. Resolving titin's lifecycle and the spatial organization of protein turnover in mouse cardiomyocytes. Proc Natl Acad Sci U S A. 2019;116:25126–25136. doi: 10.1073/pnas.1904385116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B, Solís C (2021) Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage. J Muscle Res Cell Motil. 10.1007/s10974-021-09596-9 [DOI] [PMC free article] [PubMed]
- Russell B, Wenderoth MP, Goldspink PH. Remodeling of myofibrils: subcellular distribution of myosin heavy chain mRNA and protein. Am J Phys. 1992;262:R339–R345. doi: 10.1152/ajpregu.1992.262.3.R339. [DOI] [PubMed] [Google Scholar]
- Russell B, Motlagh D, Ashley WW. Form follows function: how muscle shape is regulated by work. Journal of Applied Physiology (Bethesda, Md : 1985) 2000;88:1127–1132. doi: 10.1152/jappl.2000.88.3.1127. [DOI] [PubMed] [Google Scholar]
- Russell B, Curtis MW, Koshman YE, Samarel AM. Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. J Mol Cell Cardiol. 2010;48:817–823. doi: 10.1016/j.yjmcc.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryba DM, Li J, Cowan CL, Russell B, Wolska BM, Solaro RJ. Long-term biased beta-arrestin signaling improves cardiac structure and function in dilated cardiomyopathy. Circulation. 2017;135:1056–1070. doi: 10.1161/circulationaha.116.024482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder DJ, Judge SM, Beharry AW, Farnsworth CL, Silva JC, Judge AR. Identification of the Acetylation and Ubiquitin-Modified Proteome during the Progression of Skeletal Muscle Atrophy. PLoS ONE. 2015;10:e0136247. doi: 10.1371/journal.pone.0136247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarel AM. Focal adhesion signaling in heart failure. Pflugers Arch - Eur J Physiol. 2014;466:1101–1111. doi: 10.1007/s00424-014-1456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarel AM, Spragia ML, Maloney V, Kamal SA, Engelmann GL. Contractile arrest accelerates myosin heavy chain degradation in neonatal rat heart cells. Am J Phys. 1992;263:C642–C652. doi: 10.1152/ajpcell.1992.263.3.C642. [DOI] [PubMed] [Google Scholar]
- Samarel AM, Koshman Y, Swanson ER, Russell B. Biophysical forces modulate the costamere and Z-Disc for sarcomere remodeling in heart failure. In: Solaro RJ, Tardiff JC, editors. Biophysics of the failing heart: physics and biology of heart muscle. New York: Springer New York; 2013. pp. 141–174. [Google Scholar]
- Samson T, et al. The LIM-only proteins FHL2 and FHL3 interact with alpha- and beta-subunits of the muscle alpha7beta1 integrin receptor. J Biol Chem. 2004;279:28641–28652. doi: 10.1074/jbc.M312894200. [DOI] [PubMed] [Google Scholar]
- Sánchez-Martín P, Komatsu M (2018) p62/SQSTM1 - steering the cell through health and disease. J Cell Sci 131. 10.1242/jcs.222836 [DOI] [PubMed]
- Sanger JW, Wang J, Fan Y, White J, Sanger JM. Assembly and dynamics of myofibrils. J Biomed Biotechnol. 2010;2010:858606. doi: 10.1155/2010/858606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker M, et al. Cardiomyopathy mutations in metavinculin disrupt regulation of vinculin-induced F-Actin assemblies. J Mol Biol. 2019;431:1604–1618. doi: 10.1016/j.jmb.2019.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers JR. Myosins: a diverse superfamily. Biochim Biophys Acta. 2000;1496:3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- Senyo SE, Koshman YE, Russell B. Stimulus interval, rate and direction differentially regulate phosphorylation for mechanotransduction in neonatal cardiac myocytes. FEBS Lett. 2007;581:4241–4247. doi: 10.1016/j.febslet.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong J, et al. Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proc Natl Acad Sci U S A. 2013;110:19372–19377. doi: 10.1073/pnas.1307405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira V, van der Velden J. The Frank-Starling Law: a jigsaw of titin proportions. Biophys Rev. 2017;9:259–267. doi: 10.1007/s12551-017-0272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DG, Sharp WW, Borg TK, Price RL, Terracio L, Samarel AM. Mechanical regulation of cardiac myocyte protein turnover and myofibrillar structure. Am J Phys. 1996;270:C1075–C1087. doi: 10.1152/ajpcell.1996.270.4.C1075. [DOI] [PubMed] [Google Scholar]
- Sjöblom B, Salmazo A, Djinović-Carugo K. α-Actinin structure and regulation. Cell Mol Life Sci. 2008;65:2688. doi: 10.1007/s00018-008-8080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro RJ, Henze M, Kobayashi T. Integration of troponin I phosphorylation with cardiac regulatory networks. Circ Res. 2013;112:355–366. doi: 10.1161/circresaha.112.268672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís C, Russell B. CapZ integrates several signaling pathways in response to mechanical stiffness. J Gen Physiol. 2019;151:660–669. doi: 10.1085/jgp.201812199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís C, Solaro RJ (2021) Novel insights into sarcomere regulatory systems control of cardiac thin filament activation. J Gen Physiol 153. 10.1085/jgp.202012777 [DOI] [PMC free article] [PubMed]
- Solis C, Kim GH, Moutsoglou ME, Robinson JM. Ca(2+) and myosin cycle states work as allosteric effectors of troponin activation. Biophys J. 2018;115:1762–1769. doi: 10.1016/j.bpj.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, et al. Mechanosensing through direct binding of tensed F-Actin by LIM domains. Dev Cell. 2020;55:468–482.e467. doi: 10.1016/j.devcel.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J, et al. Harmonic force spectroscopy measures load-dependent kinetics of individual human β-cardiac myosin molecules. Nat Commun. 2015;6:7931. doi: 10.1038/ncomms8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suphamungmee W, Nakamura F, Hartwig JH, Lehman W. Electron microscopy and 3D reconstruction reveals filamin Ig domain binding to F-actin. J Mol Biol. 2012;424:248–256. doi: 10.1016/j.jmb.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, et al. Two distinct mechanisms for actin capping protein regulation--steric and allosteric inhibition. PLoS Biol. 2010;8:e1000416. doi: 10.1371/journal.pbio.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja N, Neininger AC, Burnette DT. Coupling to substrate adhesions drives the maturation of muscle stress fibers into myofibrils within cardiomyocytes. Mol Biol Cell. 2020;31:1273–1288. doi: 10.1091/mbc.E19-11-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapial Martínez P, López Navajas P, Lietha D (2020) FAK structure and regulation by membrane interactions and force in focal adhesions. Biomolecules 10. 10.3390/biom10020179 [DOI] [PMC free article] [PubMed]
- Torsoni AS, Constancio SS, Nadruz W, Jr, Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res. 2003;93:140–147. doi: 10.1161/01.res.0000081595.25297.1b. [DOI] [PubMed] [Google Scholar]
- Tripet B, Van Eyk JE, Hodges RS. Mapping of a second actin-tropomyosin and a second troponin C binding site within the C terminus of troponin I, and their importance in the Ca2+-dependent regulation of muscle contraction. J Mol Biol. 1997;271:728–750. doi: 10.1006/jmbi.1997.1200. [DOI] [PubMed] [Google Scholar]
- Urciuoli E, Peruzzi B (2020) Involvement of the FAK network in pathologies related to altered mechanotransduction. Int J M Sci 21. 10.3390/ijms21249426 [DOI] [PMC free article] [PubMed]
- Van Eyk JE, Strauss JD, Hodges RS, Rüegg JC (1993) A synthetic peptide mimics troponin I function in the calcium-dependent regulation of muscle contraction. FEBS Lett 323:223–228. 10.1016/0014-5793(93)81344-Y [DOI] [PubMed]
- Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton. 2005;61:34–48. doi: 10.1002/cm.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yan J, Goult BT. Force-dependent binding constants. Biochemistry. 2019;58:4696–4709. doi: 10.1021/acs.biochem.9b00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fan Y, Dube S, Agassy NW, Dube DK, Sanger JM, Sanger JW. Myofibril assembly and the roles of the ubiquitin proteasome system. Cytoskeleton (Hoboken, NJ) 2020;77:456–479. doi: 10.1002/cm.21641. [DOI] [PubMed] [Google Scholar]
- Wang Z, Grange M, Wagner T, Kho AL, Gautel M, Raunser S. The molecular basis for sarcomere organization in vertebrate skeletal muscle. Cell. 2021;184:2135–2150.e2113. doi: 10.1016/j.cell.2021.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M, Iskratsch T (2020) Mix and (mis-)match - The mechanosensing machinery in the changing environment of the developing, healthy adult and diseased heart. Biochim Biophys Acta, Mol Cell Res 2020:118436. 10.1016/j.bbamcr.2019.01.017 [DOI] [PMC free article] [PubMed]
- Wear MA, Cooper JA. Capping protein: new insights into mechanism and regulation. Trends Biochem Sci. 2004;29:418–428. doi: 10.1016/j.tibs.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Wear MA, Yamashita A, Kim K, Maéda Y, Cooper JA. How capping protein binds the barbed end of the actin filament. Curr Biol. 2003;13:1531–1537. doi: 10.1016/s0960-9822(03)00559-1. [DOI] [PubMed] [Google Scholar]
- Weis WI, Kobilka BK. The Molecular Basis of G Protein-Coupled Receptor Activation. Annu Rev Biochem. 2018;87:897–919. doi: 10.1146/annurev-biochem-060614-033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JD, Anderson CA, Suarez C, Kovar DR, Gardel ML. Evolutionarily diverse LIM domain-containing proteins bind stressed actin filaments through a conserved mechanism. Proc Natl Acad Sci U S A. 2020;117:25532–25542. doi: 10.1073/pnas.2004656117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S, Zieseniss A, Fock U, Jockusch BM, Illenberger S. Comparative biochemical analysis suggests that vinculin and metavinculin cooperate in muscular adhesion sites. J Biol Chem. 2004;279:31533–31543. doi: 10.1074/jbc.M314245200. [DOI] [PubMed] [Google Scholar]
- Witt SH, Granzier H, Witt CC, Labeit S. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol. 2005;350:713–722. doi: 10.1016/j.jmb.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Witt CC, Witt SH, Lerche S, Labeit D, Back W, Labeit S. Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2. EMBO J. 2008;27:350–360. doi: 10.1038/sj.emboj.7601952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody MS, Greenberg MJ, Barua B, Winkelmann DA, Goldman YE, Ostap EM. Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat Commun. 2018;9:3838. doi: 10.1038/s41467-018-06193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Namba K, Fujii T. Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat Commun. 2020;11:153. doi: 10.1038/s41467-019-14008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Maeda K, Maéda Y. Crystal structure of CapZ: structural basis for actin filament barbed end capping. EMBO J. 2003;22:1529–1538. doi: 10.1093/emboj/cdg167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zhang X, Guo Y, Meng F, Sachs F, Guo J. Mechanical dynamics in live cells and fluorescence-based force/tension sensors. Biochim Biophys Acta. 2015;1853:1889–1904. doi: 10.1016/j.bbamcr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, et al. Dynamic Myofibrillar Remodeling in Live Cardiomyocytes under Static Stretch. Sci Rep. 2016;6:20674. doi: 10.1038/srep20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Goult BT, Chen H, Cong P, Sheetz MP, Yan J. Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation. Sci Rep. 2014;4:4610. doi: 10.1038/srep04610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JG, Russell B. Cardiomyocyte remodeling and sarcomere addition after uniaxial static strain in vitro. The Journal of Histochemistry and Cytochemistry : Official Journal of the Histochemistry Society. 2005;53:839–844. doi: 10.1369/jhc.4A6608.2005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Cardiomyocyte PKA ablation enhances basal contractility while eliminates cardiac β-Adrenergic response without adverse effects on the heart. Circ Res. 2019;124:1760–1777. doi: 10.1161/circresaha.118.313417. [DOI] [PubMed] [Google Scholar]
- Zou P, et al. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature. 2006;439:229–233. doi: 10.1038/nature04343. [DOI] [PubMed] [Google Scholar]