Abstract
Introduction
There is paucity of data on the outcomes of hospitalization for bicuspid aortic valve (BAV)-related aortopathies.
Methods
We queried the NIS database (2012–2016) for hospitalizations for elective thoracic aortic repair or acute aortic syndrome (AAS) among those with BAV versus trileaflet aortic valve (TAV).
Results
Our analysis yielded 38,010 hospitalizations for elective aortic repair, of whom 34.4% had BAV, as well as 81,875 hospitalizations for thoracic AAS, of whom 1.1% had BAV. Hospitalizations for BAV were younger and had fewer comorbidities compared with their TAV counterparts. The number of hospitalizations for BAV during the observational period was unchanged. After propensity matching, elective aortic repair for BAV was associated with lower mortality (0.5% versus 1.7%, odds ratio = 0.28; 95% CI 1.5–0.50, p < 0.001), use of mechanical circulatory support, acute stroke, and shorter length of hospital stay compared with TAV. After propensity matching, AAS among those with BAV had a greater incidence of bleeding events, blood transfusion, cardiac tamponade, ventricular arrhythmias, and a longer length of hospital stay compared with TAV. Among those with BAV, predictors of lower mortality if undergoing elective aortic repair included larger hospitals and teaching hospitals. Predictors of higher mortality in patients with AAS included heart failure, chronic kidney disease, and coronary artery disease.
Conclusion
Data from a national database showed no change in the number of hospitalizations for BAV-related aortopathy, with relatively lower incidence of AAS. Compared with TAV, elective aortic repair for BAV is associated with lower mortality, while BAV-related AAS is associated with higher in-hospital complications.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40119-021-00237-3.
Keywords: Aortic aneurysm, Aortic repair, Acute aortic syndrome
Key Summary Points
| Hospitalizations for bicuspid aortic valve (BAV) were more commonly to be for elective aortic repair rather than acute aortic syndrome (AAS), and they were more likely to be younger with fewer comorbidities compared with their trileaflet aortic valve (TAV) counterparts. |
| During the study year there was no significant change in the number of hospitalizations for BAV, contrasting with a rising trend in hospitalizations for TAV in both the elective thoracic aortic aneurysm (TAA) repair and AAS cohorts. |
| Admissions with BAV undergoing elective TAA repair were associated with lower mortality, acute stroke, use of mechanical circulatory support device (MCS), and shorter length of hospital stay. |
| Admissions with BAV hospitalized for AAS had higher rates of bleeding events, cardiac tamponade, ventricular arrhythmias, and longer length of hospital stay. |
| Among admissions with BAV undergoing elective aortic repair, the predictors of lower mortality included large-sized hospitals and teaching hospitals, while predictors of higher mortality among admissions with AAS included history of heart failure, fluid/electrolytes abnormalities, chronic kidney disease (CKD), and coronary artery disease (CAD). |
Introduction
Bicuspid aortic valve (BAV) is the most common congenital valvular cardiac anomaly, occurring in 1–2% of the population [1]. Patients with BAV have significant comorbidities which may increase patient mortality compared with other congenital cardiac abnormalities [1]. BAV is commonly associated with aortopathies including thoracic aortic aneurysm (TAA) and aortic coarctation, with an estimated prevalence of 79% in certain reports [2–4]. While the underlying pathophysiology is not fully explained, genetic and acquired mechanism have been postulated to contribute to aortopathies in patients with BAV [5]. TAA and subsequent aortic dissection is a feared complication in patients with BAV and associated with significant mortality [1, 5]. Despite limited reports on the exact lifetime risk of AAS in patients with BAV, several reports suggest an eight-fold higher risk than their counterparts with trileaflet aortic valve (TAV) [6]. The management of patients with aortopathies associated with BAV include routine surveillance and identification of high risk subjects who will warrant prophylactic surgical aortic intervention [7]. The decision to intervene is usually based on balancing the risk of disease progression to AAS versus the operative risk of aortic repair. Moreover, other to consider include associated valvular dysfunction, prior thoracic surgical procedures, as well as the surgical experience of the treating center [8]. Such complex decision-making mandates a thorough evaluation of the outcomes of elective aortic for BAV as well outcomes of BAV-related AAS. The primary goal of the present analysis was to evaluate trends and outcomes of patients with BAV hospitalized for elective aortic root and acute aortic syndromes in comparison with patients with TAV.
Methods
We analyzed data from the National Inpatient Sample (NIS) database (2012–2016) that is distributed by the Healthcare Cost and Utilization Project (HCUP) [9]. The NIS is the largest all-payer publicly available inpatient care database in the USA; it includes patients covered by Medicare, Medicaid, private insurance, and those who are uninsured. In 2012, the NIS database was updated to include 20% of hospital admissions in the USA systemically sampled from all hospitals [10]. Prior studies were published using data from the NIS to describe national trends and outcomes of cardiovascular diseases [11, 12]. The NIS presents data using the International Classification of Diseases, Ninth Edition (ICD-9) up to September 2015, while data from October 2015 up to December 2016 are reported using ICD-10 codes. Using appropriate discharge weights, data from the NIS can be used to obtain national estimates. This study was exempt from institutional review board evaluation, since it contains de-identified data that are publicly available. Hence, no informed patient consent was required.
We interrogated the NIS database (2012–2016) to identify admissions for TAA repair or acute thoracic aortic syndrome (AAS) using the corresponding ICD-9 and ICD-10 codes. To identify admissions for elective TAA repair, admissions with codes for TAA were included, then patients aged at least 18 years old with procedural codes for surgical aortic repair and an elective indicator for the index hospitalization were selected. Admissions for AAS were identified by including those with codes for thoracic aortic dissection or thoracic aortic rupture plus an urgent indicator in the index admission. We excluded admissions with missing vital status or any of the propensity matching variables and those receiving endovascular aortic repair. To identify admissions for patients with BAV, we used ICD-9 diagnostic codes (746.3, 746.4) and ICD-10 diagnostic codes (Q23.0 and Q23.1) for BAV. Those codes have been previously utilized to identify patients with BAV [13, 14] and have been validated to have good specificity and positive predictive value to identify BAV [15].
We described the temporal trends in hospitalizations with elective TAA repair and AAS for those with BAV versus TAV. We also reported comparative outcomes for hospitalizations with elective aortic repair in BAV versus not, and for hospitalizations with AAS in BAV versus not. The primary outcome in this study is the in-hospital mortality. Secondary outcomes included cardiac arrest, cardiogenic shock, use of mechanical circulatory support device (MCS), acute kidney injury (AKI), hemodialysis for AKI, acute myocardial infarction (MI), acute stroke, ventricular arrhythmias, complete heart block, permanent pacemaker implantation, respiratory complications, discharge to skilled nursing facilities, and length of hospital stay. Baseline characteristics and clinical outcomes were identified using the appropriate ICD-9 and ICD-10 codes, Clinical Classifications Software (CCS) codes, and Elixhauser comorbidities as reported by the Healthcare Cost and Utilization Project (HCUP) (Supplemental Table 1).
A logistic regression analysis was used to identify predictors of mortality among patients with BAV hospitalized for elective TAA repair and those hospitalized with AAS. The model included all baseline characteristics and hospital characteristics. A univariate regression analysis was conducted first, and significant predictors were then included in a multivariable regression model. We employed propensity score methodology to match hospitalizations for elective TAA repair with BAV to those with TAV using a 1:1 ratio. A similar propensity matching model was conducted to match hospitalizations for AAS with BAV to those with TAV. The matching was performed using MatchIt R package (R software) [16]. Nearest neighbor technique was adopted to match each case to a control which is closest in terms of calculated propensity score, with a caliper width of 0.2. The propensity score was calculated from the following 26 matching variables: age, gender, race, diabetes mellitus, hypertension, obesity, history of heart failure, chronic lung disease, peripheral arterial disease, pulmonary circulation disorders, chronic liver disease, chronic kidney disease (CKD), chronic anemia, fluids/electrolytes disturbance, coagulopathy, hypothyroidism, history of smoking, history of implantable cardiac defibrillator, history of cardiac pacemaker, carotid artery disease, prior stroke, prior percutaneous coronary intervention (PCI), prior CABG, hospital bed-size, hospital region, and hospital teaching status.
Categorical variables were presented as numbers and percentages and analyzed using chi-square test. Continuous variables were presented as mean ± standard deviation or median and interquartile range (IQR) depending on the skewness of distribution. Continuous variables were analyzed using Student t test or Mann–Whitney U test. To analyze the temporal changes during the study period, we used time series plots and interrupted time series regression models. All outcomes were analyzed using the complex samples facility of SPSS to account for hospital strata, clustering, and weights. All analyses were conducted using the appropriate weighting samples in accordance with HCUP regulations [17]. Associations were considered significant if the two-tailed p value was less than 0.05. We used the SPSS software (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp Released 2016) for all statistical analyses.
Results
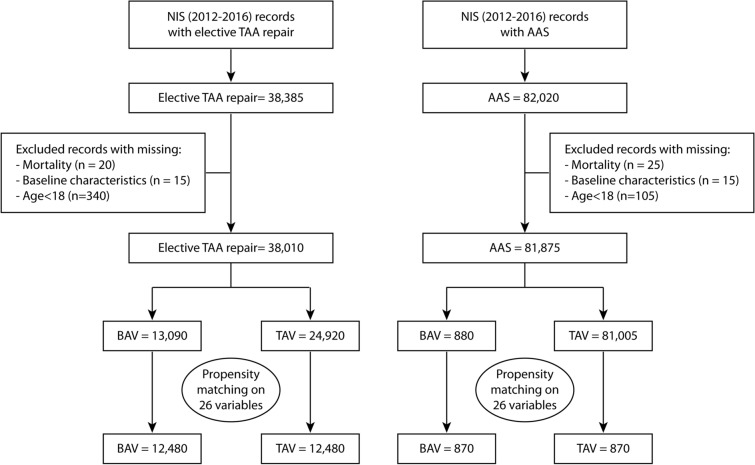
From 2012 to 2016, our analysis included 38,385 hospitalizations for elective TAA repair and 82,020 hospitalizations for thoracic AAS. After exclusion of cases with missing mortality, missing baseline characteristics, and those aged less than 18 years old, our final analysis included 38,010 hospitalizations for elective aortic repair and 81,875 hospitalizations for AAS. Study flow sheet as outlined in Fig. 1.
Fig. 1.
Study flowsheet
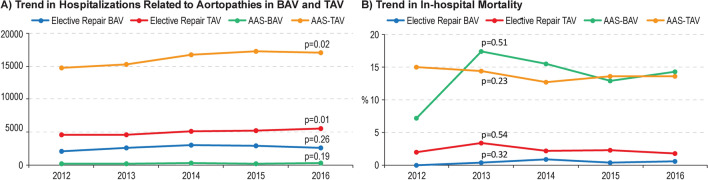
Among the elective TAA repair group, 13,090 (34.4%) had BAV and 24,920 (65.6%) had TAV. The number of hospitalizations for those with BAV was 2030 in 2012 compared with 2595 in 2016 with no change in the temporal trend (Ptrend = 0.26), while there was a significant rise in the number of hospitalizations with TAV from 4520 in 2012 to 5540 in 2016 (Ptrend = 0.01). Overall in-hospital mortality for elective aortic repair in BAV was 0.46%, with no change in the temporal trend during study years (Ptrend = 0.32). The overall in-hospital mortality for elective TAA repair in TAV was 2.31% with no change in the trend (Ptrend = 0.54) (Fig. 2). Baseline characteristics for the elective TAA repair cohort is outlined in Table 1. Before matching, those with BAV were younger (55.5 ± 13.3 versus 63.5 ± 15.3, p < 0.001), less likely to be female (23.9% versus 31.3%, p < 0.001), and with fewer comorbidities including hypertension, diabetes, CKD, chronic lung disease, coronary artery disease (CAD), prior PCI, or prior CABG. After matching, standardized mean differences between both groups were less than 10% (Supplemental Fig. 1).
Fig. 2.
Temporal trend in the number and in-hospital mortality of hospitalizations for elective TAA repair and AAS
Table 1.
Baseline characteristics in the unmatched and matched cohorts for elective TAA repair
| Characteristic | Unmatched cohort | P value | Matched cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BAV (n = 13,090) | TAV (24,920) | BAV (n = 12,480) | TAV (n = 12,480) | ||||||
| Age | 55.5 ± 13.3 | 63.5 ± 15.3 | < 0.001 | 56.16 ± 13.2 | 57.84 ± 13.4 | ||||
| Female sex | 3125.001 | 23.9% | 7809 | 31.3% | < 0.001 | 3015.001 | 24.2 | 2864 | 23.0% |
| Race | |||||||||
| White | 10,599 | 81.0% | 19,290 | 77.4% | < 0.001 | 10,104 | 81.0% | 10,119 | 81.1% |
| Black | 150 | 1.1% | 1365 | 5.5% | 145 | 1.2% | 160 | 1.3% | |
| Hispanic | 505 | 3.9% | 894 | 3.6% | 485 | 3.9% | 480 | 3.8% | |
| Asian/Pacific Islander | 235 | 1.8% | 730 | 2.9% | 235 | 1.9% | 225 | 1.8% | |
| Native American | 60 | 0.5% | 65 | 0.3% | 45 | 0.4% | 50 | 0.4% | |
| Other races | 405 | 3.1% | 815 | 3.3% | 385 | 3.1% | 435 | 3.5% | |
| Coagulopathy | 3945 | 30.1% | 8010 | 32.1% | 0.080 | 3800 | 30.4% | 3889 | 31.2% |
| Valvular disease | 85 | 0.6% | 270 | 1.1% | 0.063 | 85 | 0.7% | 80 | 0.6% |
| Obesity | 2280 | 17.4% | 4245 | 17.0% | 0.673 | 2154 | 17.3% | 2235 | 17.9% |
| Fluid/electrolyte disorders | 4780 | 36.5% | 9239 | 37.1% | 0.659 | 4580 | 36.7% | 4639 | 37.2% |
| Hypertension | 8055 | 61.5% | 18,629 | 74.8% | < 0.001 | 7865 | 63.0% | 8229 | 65.9% |
| Hypothyroidism | 1179 | 9.0% | 2820 | 11.3% | 0.001 | 1149 | 9.2% | 1135 | 9.1% |
| Chronic kidney disease | 595 | 4.5% | 2269 | 9.1% | < 0.001 | 595 | 4.8% | 550 | 4.4% |
| Chronic liver disease | 230 | 1.8% | 370 | 1.5% | 0.371 | 215 | 1.7% | 245 | 2.0% |
| Chronic lung disease | 1850 | 14.1% | 5120 | 20.5% | < 0.001 | 1800 | 14.4% | 2194 | 17.6% |
| Coronary artery disease | 3615 | 27.6% | 10,125 | 40.6% | < 0.001 | 3595 | 28.8% | 3855 | 30.9% |
| Diabetes mellitus | 1530 | 11.7% | 3520 | 14.1% | 0.003 | 1495 | 12.0% | 1639 | 13.1% |
| Anemia | 1345 | 10.3% | 3209 | 12.9% | 0.001 | 1305 | 10.5% | 1404 | 11.3% |
| Hx of ICD | 50 | 0.4% | 245 | 1.0% | 0.005 | 20 | 0.4% | 26 | 0.5% |
| Hx of cardiac pacemaker | 175 | 1.3% | 650 | 2.6% | < 0.001 | 175 | 1.4% | 205 | 1.6% |
| Prior PCI | 360 | 2.8% | 1175 | 4.7% | < 0.001 | 360 | 2.9% | 435 | 3.5% |
| Prior CABG | 110 | 0.8% | 730 | 2.9% | < 0.001 | 110 | 0.9% | 80 | 0.6% |
| Prior stroke | 430 | 3.3% | 1375 | 5.5% | < 0.001 | 430 | 3.4% | 425 | 3.4% |
| Hx of smoking | 1779 | 13.6% | 3950 | 15.9% | 0.011 | 1734 | 13.9% | 1744 | 14.0% |
| Hx of heat failure | 45 | 0.3% | 245 | 1.0% | 0.003 | 45 | 0.4% | 45 | 0.4% |
| Pulmonary circ. disease | 20 | 0.2% | 90 | 0.4% | 0.107 | 20 | 0.2 | 15 | 0.1% |
| Peripheral vascular disease | 8829 | 67.5% | 13,100 | 52.6% | < 0.001 | 8344 | 66.5% | 8344 | 66.5% |
| Hospital bed-size | |||||||||
| Small-sized | 764 | 5.8% | 1449 | 5.8% | 0.991 | 714 | 5.7% | 740 | 5.9% |
| Medium-sized | 2374 | 18.1% | 4494 | 18.0% | 2264 | 18.1% | 2279 | 18.3% | |
| Large-sized | 9950 | 76.0% | 18,975 | 76.1% | 9500 | 76.1% | 9460 | 75.8% | |
| Hospital region | |||||||||
| Northeast | 2555 | 19.5% | 5175 | 20.8% | < 0.001 | 2435 | 19.5% | 2480 | 19.9% |
| Midwest or North Central | 4189 | 32.0% | 6845 | 27.5% | 3994 | 32.0% | 3880 | 31.1% | |
| South | 3614 | 27.6% | 7849 | 31.5% | 3454 | 27.7% | 3534 | 28.3% | |
| West | 2729 | 20.9% | 5049 | 20.3% | 2594 | 20.8% | 2584 | 20.7% | |
| Hospital teaching status | |||||||||
| Rural | 145 | 1.1% | 350 | 1.4% | 0.143 | 140 | 1.1% | 130 | 1.0% |
| Urban non-teaching | 1739 | 13.3% | 3615 | 14.5% | 1684 | 13.5% | 1765 | 14.1% | |
| Urban teaching | 11,205 | 85.6% | 20,954 | 84.1% | 10,655 | 85.4% | 10,584 | 84.8% | |
PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, Hx history
Among the AAS group, 880 (1.1%) had BAV and 81,005 (98.9%) had no BAV. The number of hospitalizations with AAS in BAV did not change during the study period (Ptrend = 0.19), while there was a rise in the number of hospitalizations with AAS in TAV from 14,760 in 2012 to 17,065 in 2016 (Ptrend = 0.02). The overall in-hospital mortality for AAS in BAV was 13.6%, and for AAS in TAV was 13.8% with no change in the temporal trend during the study years (Ptrend = 0.51 and Ptrend = 0.23, respectively) (Fig. 2). Baseline characteristics of AAS cohort are outlined in Table 2. Before matching, those with BAV were younger (53.1 ± 14.2 versus 65.2 ± 15.3, p < 0.001) less likely to be women (19.9% versus 40.9%, p < 0.001), and had fewer comorbidities. After matching, standardized mean differences between both groups were less than 10%.
Table 2.
Baseline characteristics in the unmatched and matched cohorts for AAS in BAV versus TAV
| Characteristic | Unmatched cohort | P value | Matched cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BAV (n = 880) | TAV (n = 81,005) | BAV (n = 870) | TAV (n = 870) | ||||||
| Age | 53.07 ± 14.2 | 65.19 ± 15.3 | < 0.001 | 53.29 ± 14.1 | 54.57 ± 15.7 | ||||
| Female sex | 175 | 19.9% | 33,144 | 40.9% | < 0.001 | 175 | 20.1% | 125 | 14.4% |
| Race | |||||||||
| White | 655.001 | 74.4% | 46,869 | 57.9% | < 0.001 | 645 | 74.1% | 655 | 75.3% |
| Black | 55 | 6.2% | 17,730 | 21.9% | 55 | 6.3% | 45 | 5.2% | |
| Hispanic | 50 | 5.7% | 5060 | 6.2% | 50 | 5.7% | 60 | 6.9% | |
| Asian/Pacific Islander | NR | 1.1% | 2920 | 3.6% | NR | 1.1% | NR | 1.1% | |
| Native American | NR | 1.1% | 220 | 0.3% | NR | 1.1% | – | – | |
| Other races | 25 | 2.8% | 2849 | 3.5% | 25 | 2.9% | 15 | 1.7% | |
| Coagulopathy | 280 | 31.8% | 14,715 | 18.2% | < 0.001 | 275 | 31.6% | 285 | 32.8% |
| Obesity | 115 | 13.1% | 11,739 | 14.5% | < 0.001 | 115 | 13.2% | 90 | 10.3% |
| Fluid and electrolyte disorders | 400 | 45.5% | 32,329 | 39.9% | 0.124 | 395 | 45.4% | 365 | 42.0% |
| Hypertension | 540 | 61.4% | 64,395 | 79.5% | < 0.001 | 535 | 61.5% | 545 | 62.6% |
| Hypothyroidism | 40 | 4.5% | 8754 | 10.8% | 0.007 | 40 | 4.6% | 50 | 5.7% |
| Chronic kidney disease | 95 | 10.8% | 16,670 | 20.6% | 0.002 | 90 | 10.3% | 105 | 12.1% |
| Chronic liver disease | 10 | 1.1% | 2045 | 2.5% | 0.238 | NR | 1.1% | NR | 1.1% |
| Chronic lung disease | 90 | 10.2% | 18,630 | 23.0% | < 0.001 | 90 | 10.3% | 130 | 14.9% |
| Coronary artery disease | 175 | 19.9% | 23,549 | 29.1% | 0.006 | 170 | 19.5% | 175 | 20.1% |
| Diabetes mellitus | 75 | 8.5% | 13,264 | 16.4% | 0.005 | 75 | 8.6% | 75 | 8.6% |
| Anemia | 125 | 14.2% | 16,860 | 20.8% | 0.030 | 125 | 14.4% | 195 | 22.4% |
| Hx of ICD | – | – | 1135 | 1.4% | 0.113 | – | – | NR | 1.1% |
| Hx of cardiac pacemaker | 15 | 1.7% | 2010 | 2.5% | 0.508 | 15 | 1.7% | 15 | 1.7% |
| Prior PCI | 20 | 2.3% | 3920 | 4.8% | 0.113 | 20 | 2.3% | 25 | 2.9% |
| Prior CABG | NR | 0.6% | 5119 | 6.3% | 0.002 | NR | 0.6% | NR | 1.1% |
| Prior stroke | NR | 0.6% | 6199 | 7.7% | < 0.001 | NR | 0.6% | NR | 1.1% |
| Hx of smoking | 210 | 23.9% | 18,644 | 23.0% | 0.792 | 210 | 24.1% | 210 | 24.1% |
| Hx of heat failure | 20 | 2.3% | 8790 | 10.9% | < 0.001 | 20 | 2.3% | 45 | 5.2% |
| Pulmonary circulation disease | 20 | 2.3% | 2170 | 2.7% | 0.740 | 15 | 1.7% | 35 | 4.0% |
| Peripheral vascular disease | 320 | 36.4% | 37,029 | 45.7% | 0.013 | 315 | 36.2% | 335 | 38.5% |
| Hospital bed-size | |||||||||
| Small-sized | 50 | 5.7% | 6314 | 7.8% | 0.298 | 50 | 5.7% | 65 | 7.5% |
| Medium-sized | 155 | 17.6% | 16,684 | 20.6% | 155 | 17.8% | 165 | 19.0% | |
| Large-sized | 675 | 76.7% | 58,005 | 71.6% | 665 | 76.4% | 640 | 73.6% | |
| Hospital region | |||||||||
| Northeast | 210 | 23.9% | 15,300 | 18.9% | 0.137 | 210 | 24.1% | 225 | 25.9% |
| Midwest or North Central | 210 | 23.9% | 18,909 | 23.3% | 205 | 23.6% | 225 | 25.9% | |
| South | 250 | 28.4% | 29,539 | 36.5% | 250 | 28.7% | 230 | 26.4% | |
| West | 210 | 23.9% | 17,255 | 21.3% | 205 | 23.6% | 190 | 21.8% | |
| Hospital teaching status | |||||||||
| Rural | – | – | 2435 | 3.0% | < 0.001 | – | – | NR | 0.6% |
| Urban non-teaching | 110 | 12.5% | 14,594 | 18.0% | 110 | 12.6% | 140 | 16.1% | |
| Urban teaching | 770 | 87.5% | 63,974 | 79.0% | 760.001 | 87.4% | 725 | 83.3% | |
| Valvular disease | 175 | 9.4% | 7605 | 19.9% | < 0.001 | 165 | 19.0% | 205 | 23.6% |
PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, ICD implantable cardiac defibrillator, Hx history; NR Not reportable per HCUP regulations
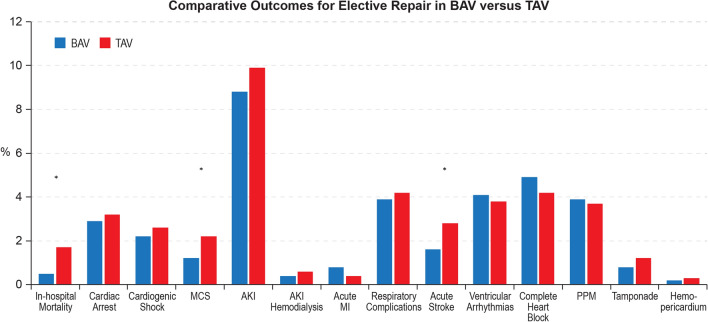
After propensity matching, in-hospital mortality for elective TAA repair in BAV was significantly lower compared with TAV (0.5% versus 1.7%, OR = 0.28; 95% CI 1.5–0.50, p < 0.001). No difference was observed between the BAV and TAV groups in the rate of cardiac arrest (OR = 0.89; 95% CI 0.65–1.20, p = 0.44), cardiogenic shock (OR = 0.83; 95% CI 0.59–1.16, p = 0.28), AKI (OR = 0.88; 95% CI 0.73–1.06, p = 0.16), hemodialysis for AKI (0.4% versus 0.6%, OR = 0.67; 95% CI 0.32–1.39, p = 0.28), acute MI (0.8% versus 0.4%, OR = 1.91; 95% CI 0.91–3.99, p = 0.08), respiratory complications (3.9% versus 4.2%, OR = 0.93; 95% CI 0.72–1.21, p = 0.59), bleeding events (45.0% versus 46.0%, OR = 0.96; 95% CI 0.86–1.07, p = 0.48), blood transfusions (32.9% versus 33.1%, OR = 0.99; 95% CI 0.88–1.11, p = 0.85), ventricular arrhythmias (4.1% versus 3.8%, OR = 1.08; 95% CI 0.82–1.41, p = 0.60), complete heart block (4.9% versus 4.2%, OR = 1.19; 95% CI 0.92–1.55, p = 0.19), permanent pacemaker insertions (3.9% versus 3.7%, OR = 1.05; 95% CI 0.79–1.38, p = 0.76), hemopericardium (0.2% versus 0.3%, OR = 0.57; 95% CI 0.17–1.96, p = 0.37) and cardiac tamponade (0.8% versus 1.2%, OR = 0.68; 95% CI 0.39–1.16, p = 0.15). Hospitalizations for AAS in BAV were associated with lower utilization of MCS (1.2% versus 2.2% OR = 0.51; 95% CI 0.33–0.78, p = 0.01), acute stroke (1.6% versus 2.8%, OR = 0.57; 95% CI 0.39–0.84, p = 0.01), discharges to nursing facilities (7.5% versus 11.1%, OR = 0.65; 95% CI 0.53–79, p < 0.001), and shorter median length of stay (6 (IQR 2) versus 6 (IQR 3) days, p < 0.001) (Supplemental Table 2, Fig. 3).
Fig. 3.
Comparative outcomes of hospitalizations for elective aortic repair in BAV versus TAV
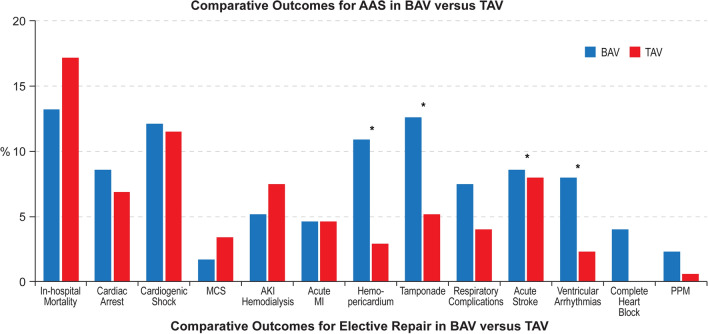
After propensity matching, no difference was observed in in-hospital mortality among hospitalizations for AAS in BAV versus TAV (13.2% versus 17.2%, OR = 0.73; 95% CI 0.44–1.22, p = 0.22). No difference was also observed in the incidence of cardiac arrest (8.6% versus 6.9%, OR = 1.27; 95% CI 0.65–2.51, p = 0.48), cardiogenic shock (12.1% versus 11.5%, OR = 1.06; 95% CI 0.64–1.75, p = 0.83), use of MCS (1.7% versus 3.4%, OR = 0.49; 95% CI 0.16–1.56, p = 0.22), AKI (35.6% versus 38.5%, OR = 0.88; 95% CI 0.60–1.31, p = 0.53), hemodialysis for AKI (5.2% versus 7.5%, OR = 0.68; 95% CI 0.30–1.52, p = 0.34), acute MI (4.6% versus 4.6%, OR = 1.00; 95% CI 0.46–2.16, p > 0.999), respiratory complications (7.5% versus 4.0%, OR = 1.93; 95% CI 0.89–4.16, p = 0.09), acute stroke (8.6% versus 8.0%, OR = 1.08; 95% CI 0.58–2.02, p = 0.81), permanent pacemaker implantation (2.3% versus 0.6%, OR = 4.07; 95% CI 0.44–37.79, p = 0.18), and discharges to nursing facilities (17.2% versus 15.5%, OR = 1.13; 95% CI 0.69–1.87, p = 0.62). Hospitalizations for AAS in BAV were associated with a higher incidence of hemopericardium (10.9% versus 2.9%, OR = 4.14; 95% CI 1.96–8.76, p = 0.01) and cardiac tamponade (12.6% versus 5.2%, OR = 2.65; 95% CI 1.47–4.81, p < 0.01), bleeding events (47.1% versus 30.5%, OR = 2.04; 95% CI 1.38–3.00, p < 0.001), blood transfusion (37.9% versus 24.1%, OR = 1.92; 95% CI 1.30–2.84, p = 0.01), ventricular arrhythmias (8.0% versus 2.3%, OR = 3.72; 95% CI 1.40–9.90, p = 0.01), complete heart block (4.0% versus 0%, OR = 1.04; 95% CI 1.03–1.06, p = 0.01), and longer median length of stay (8 (IQR 8) versus 7 (IQR 9) days, p = 0.02) (Supplemental Table 3, Fig. 4).
Fig. 4.
Comparative outcomes of hospitalizations for AAS in BAV versus TAV
Multivariable regression analysis identified clinical and hospital predictors of higher in-hospital mortality among those with BAV. Among hospitalizations for elective TAA repair in BAV, predictors of lower mortality included large-sized hospitals (OR = 0.43; 95% CI 0.21–0.88, p = 0.02) and teaching hospitals (OR = 0.55; 95% CI 0.34–0.89, p = 0.02). While among hospitalizations for AAS in BAV, predictors of higher mortality included history of heart failure (OR = 13.38; 95% CI 6.59–27.16, p < 0.001), fluids/electrolytes disturbances (OR = 2.33; 95% CI 1.53–3.53, p < 0.001), CKD (OR = 2.41; 95% CI 1.51–3.82, p < 0.001), and CAD (OR = 1.46; 95% CI 1.01–2.13, p = 0.05) (Supplemental Tables 4 and 5).
Discussion
In this large national analysis including approximately 120,000 hospitalizations, we evaluated the temporal trends and outcomes of elective TAA repair and AAS for BAV versus TAV. The salient study findings are (1) hospitalizations for BAV were more commonly to be for elective aortic repair rather than AAS, and they were more likely to be younger with fewer comorbidities compared with their TAV counterparts; (2) during the study year there was no significant change in the number of hospitalizations for BAV, contrasting with a rising trend in hospitalizations for TAV in both the elective TAA repair and AAS cohorts; (3) after propensity matching, admissions with BAV undergoing elective TAA repair were associated with lower mortality, acute stroke, use of MCS, and shorter length of hospital stay; (4) after propensity matching, admissions with BAV hospitalized for AAS had higher rates of bleeding events, blood transfusion, cardiac tamponade, hemopericardium, ventricular arrhythmias, and longer length of hospital stay compared with TAV; (5) among admissions with BAV undergoing elective aortic repair, the predictors of lower mortality included large-sized hospitals and teaching hospitals, while predictors of higher mortality among admissions with AAS included history of heart failure, fluid/electrolytes abnormalities, CKD, and CAD.
Among the most feared complications in patients with BAV are aortopathies and associated AAS. Aortopathies in patients with BAV have a distinctly different clinical course compared with aortopathies in patients with TAV. Aortic root dilatation often begins in childhood among patients with BAV [18, 19]. Across different age groups, patients with BAV have aortic annulus, sinus, and ascending aorta that are larger than their counterparts with TAV [20, 21]. Studies have suggested that aortopathies in TAV are mostly related to atherosclerosis whereas aortopathies in patients with BAV include medial cystic degeneration and tissue necrosis [22].
In this analysis, patients with BAV were more likely to be hospitalized for elective aortic repair, constituting 34.4% of all hospitalizations for elective aortic repair, while hospitalizations for AAS were far less common among the BAV group (1% of all hospitalizations with AAS). Others have also suggested low incidence of acute dissection in patients with BAV [23]. In the Olmsted County study, 416 patients with BAV were followed for 16 years and they reported incidence of acute dissection of 3.1% [1]. In a Canadian study including 642 patients with BAV, the frequency of aortic dissection was 0.1% per patient-year of follow-up [23].
Our analysis demonstrated that patients with BAV admitted for AAS were younger (53.1 versus 65.2 years) and with fewer comorbidities than their TAV counterparts. Etz et al. conducted a retrospective analysis including 460 patients who underwent surgical repair for acute aortic dissection. Etz et al. showed that among patients with acute dissection, those with BAV were almost 15 years younger (46.7 versus 61.6 years) and had fewer comorbidities compared with those with TAV [6]. Similar findings were also reported in a smaller observational analysis inclusive of 100 patients with acute type A dissection [22]. We also demonstrated that among those referred for elective TAA repair, patients with BAV were almost 8 years younger, and with significantly lower comorbidities compared to those with TAV. In the Olmsted County study, patients with BAV underwent aortic surgeries at younger age than the general population [24]. Also, reports have suggested earlier and probably faster aortic dilatation among patients with BAV compared with TAV [6, 25]. The lower incidence of traditional risk factors for AAS, including hypertension among those with BAV, is probably related to different disease pathology for AAS among patients with BAV.
Our analysis demonstrated that elective TAA surgical repair among patients with BAV had excellent in-hospital safety, with significantly lower in-hospital mortality compared with their TAV counterparts before (0.5% versus 2.3%) and after matching (0.5% versus 1.7%). Limited reports are available on the safety outcomes for elective TAA surgical repair among patients with BAV. In an older study by Nazer et al., their single-center experience showed a 2.1% hospital mortality for elective aortic surgery in patients with BAV [26]. The reported in-hospital mortality in our analysis for elective aortic surgery in those with TAV groups seems in accordance with other contemporary reports [27], and lower than other older reports [28, 29]. We also demonstrated lower in-hospital acute stroke among the BAV group, and shorter length of hospital stay compared with TAV. The lower stroke rates might be also related to the lower atherosclerotic disease burden associated with aortopathies in patients with BAV versus patients with TAV [22].
Our analysis showed that patients with BAV admitted with AAS suffered more complications compared to their TAV counterparts, and these included bleeding events, cardiac tamponade, and hemopericardium. Hemopericardium and cardiac tamponade complicating acute dissection usually occur in the setting of blood leaking into the pericardial space from the false lumen with dissection extending back to the aortic root [30]. In their analysis, Etz et al. found that the primary entry point for aortic dissection was more likely to involve the aortic root or the tubular ascending aorta in patients with BAV, while in those with TAV it was more likely to involve the aortic arch [6]; this difference might explain the higher incidence of tamponade and hemopericardium in the BAV group in our analysis. Also, Etz et al. found a numerically higher bleeding-related mortality among patients with BAV and AAS compared with TAV (11.1% versus 4.4%), although this was statistically non-significant [6]. This might be related to the different pathophysiology in patients with BAV, with more diffuse aortopathy with predominant medial necrosis/ degeneration [6, 22]. Also, aortic stenosis in cases of BAV is associated with coagulation disorders and increased bleeding diathesis [31].
Our analysis identified clinical and hospital predictors for mortality among patients with BAV in both cohorts. Among patients with BAV undergoing elective aortic repair, large-sized hospitals and teaching hospitals were both predictors of lower in-hospital mortality. This finding is in accordance with prior studies showing better outcomes of aortic surgeries among those with BAV in centers of excellence. This was also adopted by the current American College of Cardiology (ACC) guidelines which gave a class II-A recommendation for aortic repair in asymptomatic patients with BAV with low surgical risk if the diameter of the aortic root is ≥ 5.0 cm, provided the surgery is performed by an experienced surgical team in a center of expertise [7]. Among those with BAV presenting with AAS, predictors of higher mortality included history of heart failure, CKD, CAD, and fluids/electrolytes abnormalities. Beside traditional clinical predictors, the use of machine learning models has been proposed in risk-stratifying patients with aortic aneurysms and predicting risk of AAS. Future studies are warranted to develop machine learning models for predicting adverse outcomes among patients with BAV-related aortopathy [32, 33].
This current analysis is the largest to date evaluating the trends and outcomes of elective TAA repair and AAS among patients with BAV. The strength of the analysis comes from the national representativeness and large sample size. Our study results suggest that compared with patients with TAV, patients with BAV have favorable outcomes with elective TAA surgical repair, especially when performed in large-sized and teaching hospitals. Among both BAV and TAV, presentations with AAS suffered significantly higher mortality, with even higher morbidities among those with BAV. These findings highlight the importance of patient screening and identification of high-risk subjects with BAV and thoracic aortic aneurysm who should be referred for elective TAA repair.
This analysis has certain limitations. The NIS is an administrative database that is liable to documentation and coding errors. However, the NIS has been internally and externally validated [34, 35]. Also, the NIS is time discrete, and reported outcomes are only pertinent to certain hospitalization, with no long-term data. Our study cohort were identified using ICD codes, but our analysis was limited by the lack of imaging data to verify these diagnoses. The use of ICD codes for identifying patients with BAV has been previously demonstrated to have specificity of 90.3% and positive predictive value of 85.6%, but only moderate negative predictive value (68%) [15]. The use of both ICD-9 and ICD-10 coding systems represents a limitation of the current analysis because of the possible variability in frequencies and observations across the two coding systems. Also, the available ICD codes do not allow one to identify the location of TAA (i.e., ascending, arch, or descending TAA). Among those with acute dissection, we were unable to differentiate the type of acute dissection (i.e. type A versus type B dissection). Other useful information was irretrievable through this database, including data on medications, laboratory data, and procedural details. Being an observational analysis, there is always the possibility of selection bias and unmeasured confounders. However, we have conducted robust propensity matching and regression analysis to reduce allocations bias. Despite the aforementioned limitations, our study addresses an important knowledge gap in the literature regarding the outcomes of hospitalizations.
Conclusions
Real-world data from a national database showed that hospitalizations for BAV-related aortopathy were more commonly to be for elective aortic repair rather than AAS, and they were more likely to be younger with fewer comorbidities compared with their TAV counterparts. Elective aortic repair for BAV is safe and associated with lower in-hospital mortality compared with TAV. Hospitalizations for AAS in those with BAV were associated with higher in-hospital complications compared with TAVR.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
AE contributed to study conception, writing the article, statistical analysis, critical revision of the article and final approval of the article. AAM contributed to study conception, writing the article, critical revision of the article and final approval of the article. IYE contributed to study conception, data collection, writing the article, critical revision of the article and final approval of the article. MAO contributed to data collection, writing the article, critical revision of the article and final approval of the article. AE contributed to data collection, writing the article, critical revision of the article and final approval of the article. GOO contributed to data collection, writing the article, critical revision of the article and final approval of the article. RG contributed to data collection, writing the article, critical revision of the article and final approval of the article. SJC contributed to data collection, writing the article, critical revision of the article and final approval of the article. DP contributed to data collection, analysis and interpretation, writing the article, critical revision of the article and final approval of the article. EJ contributed to study conception, data collection, writing the article, critical revision of the article and final approval of the article. HJ contributed to study conception, data collection, writing the article, critical revision of the article and final approval of the article, and logistic support.
Funding
No funding or sponsorship was received for this study or publication of this article.
Disclosures
Ayman Elbadawi, Karim Mahmoud, Islam Y. Elgendy, Mohmed A. Omer, Ahmed Elsherbeny, Gbolahan O. Ogunbayo, Scott J. Cameron, Ravi Ghanta, David Paniagua, Ernesto Jimenez, Hani Jneid have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors. This study was exempt from institutional review board evaluation, since it contains de-identified data that are publicly available. Hence, no informed patient consent was required. The data was obtained from a publicly available database.
Prior Presentation
This manuscript is based on work that has been previously presented at the American College of Cardiology meeting as a poster presentation.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- 1.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 2.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119:880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 3.Russo CF, Mazzetti S, Garatti A, et al. Aortic complications after bicuspid aortic valve replacement: long-term results. Ann Thorac Surg. 2002;74:S1773–S1776. doi: 10.1016/S0003-4975(02)04261-3. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DP, Xu C, Plappert T, et al. Increased ascending aortic wall stress in patients with bicuspid aortic valves. Ann Thorac Surg. 2011;92:1384–1389. doi: 10.1016/j.athoracsur.2011.04.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies RR, Kaple RK, Mandapati D, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. 2007;83:1338–1344. doi: 10.1016/j.athoracsur.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 6.Etz CD, von Aspern K, Hoyer A, et al. Acute type A aortic dissection: characteristics and outcomes comparing patients with bicuspid versus tricuspid aortic valve. Eur J Cardiothorac Surg. 2014;48:142–150. doi: 10.1093/ejcts/ezu388. [DOI] [PubMed] [Google Scholar]
- 7.Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;67:724–731. doi: 10.1016/j.jacc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Losenno KL, Goodman RL, Chu MW. Bicuspid aortic valve disease and ascending aortic aneurysms: gaps in knowledge. Cardiol Res Pract. 2012;2012:145202. 10.1155/2012/145202. [DOI] [PMC free article] [PubMed]
- 9.Elixhauser A. Clinical classifications for health policy research: version 2: Software and User's Guide. US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1996.
- 10.Cost H, Project U. HCUP 3: a federal state industry partnership in health data. Sponsored by the Agency for Health Care Policy and Research The HCUP. 2003. Accessed 10 July 2020.
- 11.Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2019;12:1825–1836. doi: 10.1016/j.jcin.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Elbadawi A, Elgendy IY, Mahmoud K, et al. National trends and outcomes of percutaneous coronary intervention in patients≥ 70 years of age with acute coronary syndrome (from the National Inpatient Sample Database) Am J Cardiol. 2018;123:25–32. doi: 10.1016/j.amjcard.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Andell P, Li X, Martinsson A, et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. 2017;103:1696–1703. doi: 10.1136/heartjnl-2016-310894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elbadawi A, Saad M, Elgendy IY, et al. Temporal trends and outcomes of transcatheter versus surgical aortic valve replacement for bicuspid aortic valve stenosis. JACC Cardiovasc Interv. 2019;12:1811–1822. doi: 10.1016/j.jcin.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 15.Khan A, Ramsey K, Ballard C, et al. Limited accuracy of administrative data for the identification and classification of adult congenital heart disease. J Am Heart Assoc. 2018;7:e007378. doi: 10.1161/JAHA.117.007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho DE, Imai K, King G, Stuart E. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42. 10.18637/jss.v042.i08.
- 17.Houchens R, Ross D, Elixhauser A. Using the HCUP National Inpatient Sample to estimate trends. 2015. 2017. Accessed 10 July 2020.
- 18.Beroukhim RS, Kruzick TL, Taylor AL, Gao D, Yetman AT. Progression of aortic dilation in children with a functionally normal bicuspid aortic valve. Am J Cardiol. 2006;98:828–830. doi: 10.1016/j.amjcard.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Gurvitz M, Chang R-K, Drant S, Allada V. Frequency of aortic root dilation in children with a bicuspid aortic valve. Am J Cardiol. 2004;94:1337–1340. doi: 10.1016/j.amjcard.2004.07.130. [DOI] [PubMed] [Google Scholar]
- 20.Morgan-Hughes GJ, Roobottom CA, Owens PE, Marshall AJ. Dilatation of the aorta in pure, severe, bicuspid aortic valve stenosis. Am Heart J. 2004;147:736–740. doi: 10.1016/j.ahj.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 21.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 22.Eleid MF, Forde I, Edwards WD, et al. Type A aortic dissection in patients with bicuspid aortic valves: clinical and pathological comparison with tricuspid aortic valves. Heart. 2013;99:1668–1674. doi: 10.1136/heartjnl-2013-304606. [DOI] [PubMed] [Google Scholar]
- 23.Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–1325. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 24.Michelena H, Desjardins V, Avierinos J. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776–2784. doi: 10.1161/CIRCULATIONAHA.107.740878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Januzzi JL, Isselbacher EM, Fattori R, et al. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD) J Am Coll Cardiol. 2004;43:665–669. doi: 10.1016/j.jacc.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 26.Nazer RI, Elhenawy AM, Fazel SS, Garrido-Olivares LE, Armstrong S, David TE. The influence of operative techniques on the outcomes of bicuspid aortic valve disease and aortic dilatation. Ann Thorac Surg. 2010;89(6):1918–1924. doi: 10.1016/j.athoracsur.2010.02.070. [DOI] [PubMed] [Google Scholar]
- 27.Zafar MA, Li Y, Rizzo JA, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155:1938–1950. doi: 10.1016/j.jtcvs.2017.10.140. [DOI] [PubMed] [Google Scholar]
- 28.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–28. doi: 10.1016/S0003-4975(01)03236-2. [DOI] [PubMed] [Google Scholar]
- 29.Clouse WD, Hallett JW, Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ., III Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA. 1998;280:1926–1929. doi: 10.1001/jama.280.22.1926. [DOI] [PubMed] [Google Scholar]
- 30.Isselbacher EM, Cigarroa JE, Eagle KA. Cardiac tamponade complicating proximal aortic dissection. Is pericardiocentesis harmful? Circulation. 1994;90:2375–2378. doi: 10.1161/01.CIR.90.5.2375. [DOI] [PubMed] [Google Scholar]
- 31.Thompson JL, III, Schaff HV, Dearani JA, et al. Risk of recurrent gastrointestinal bleeding after aortic valve replacement in patients with Heyde syndrome. J Thorac Cardiovasc Surg. 2012;144:112–116. doi: 10.1016/j.jtcvs.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 32.Hata A, Yanagawa M, Yamagata K, et al. Deep learning algorithm for detection of aortic dissection on non-contrast-enhanced CT. Eur Radiol. 2021;31:1151–1159. doi: 10.1007/s00330-020-07213-w. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Zhang C, Zhang G, et al. A study of aortic dissection screening method based on multiple machine learning models. J Thorac Dis. 2020;12:605. doi: 10.21037/jtd.2019.12.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whalen D, Houchens R, Elixhauser A. 2004 HCUP Nationwide Inpatient Sample (NIS) comparison report. HCUP Method Series Report 2007-03. Accessed 10 July 2020.
- 35.Elbadawi A, Olorunfemi O, Ogunbayo GO, et al. Cardiovascular outcomes with surgical left atrial appendage exclusion in patients with atrial fibrillation who underwent valvular heart surgery (from the National Inpatient Sample Database) Am J Cardiol. 2017;119:2056–2060. doi: 10.1016/j.amjcard.2017.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.