Abstract
Amplified-fragment length polymorphism (AFLP) is a whole-genome fingerprinting method based on selective amplification of restriction fragments. The potential of the method for the characterization of mycoplasmas was investigated in a total of 50 strains of human and animal origin, including Mycoplasma genitalium (n = 11), Mycoplasma pneumoniae (n = 5), Mycoplasma hominis (n = 5), Mycoplasma hyopneumoniae (n = 9), Myco plasma flocculare (n = 5), Mycoplasma hyosynoviae (n = 10), and Mycoplasma dispar (n = 5). AFLP templates were prepared by the digestion of mycoplasmal DNA with BglII and MfeI restriction endonucleases and subsequent ligation of corresponding site-specific adapters. The amplification of AFLP templates with a single set of nonselective primers resulted in reproducible fingerprints of approximately 60 to 80 fragments in the size range of 50 to 500 bp. The method was able to discriminate the analyzed strains at species and intraspecies levels as well. Each of the tested Mycoplasma species developed a banding pattern entirely different from those obtained from other species under analysis. Subtle intraspecies genomic differences were detected among strains of all of the Mycoplasma species analyzed. The extent of polymorphism varied markedly between the analyzed mycoplasmas, comprising pattern similarity levels from 61.7% detected among M. dispar strains to 95.9% detected among M. genitalium strains. The results of the present study provide evidence of the high discriminatory power of AFLP analysis, suggesting the possible applicability of this method to the molecular characterization of mycoplasmas.
The members of the genus Mycoplasma (class Mollicutes) are the smallest organisms capable of self-replication. They lack the rigid cell wall present in other eubacteria and have an exceptionally small chromosome with low G+C content (28). All known mycoplasmas are parasites which usually exhibit a rather strict host and tissue specificity, and many of them are of clinical importance in human and veterinary medicine (23, 35).
In spite of a wide array of analytical methods being available, the characterization of mycoplasma isolates below the species level remains a rather demanding task. Generally the low-level discriminatory power of serological methods greatly limits their applicability to the typing of mycoplasmas. Protein profiling methods like isoenzyme analysis (32), one- and two-dimensional gel electrophoresis (24, 30), and immunoblotting (4) possess the required discriminatory power but are time-consuming, require large samples for analysis, and may be difficult to interpret. Various DNA-based methods have been used for intraspecies differentiation of mycoplasma isolates. They include restriction endonuclease analysis (27), field inversion gel electrophoresis (12), restriction fragment length polymorphism (8), PCR-mediated restriction fragment length polymorphism (34), arbitrarily primed PCR (15), and insertion sequence typing (6). However, most of the genetic typing assays also have drawbacks in that they may require a relatively large amount of high-quality DNA or may be difficult to reproduce and standardize between laboratories. Thus, the need for high-resolution analytical tools which will facilitate the typing of mycoplasmas on a routine basis still exists.
A recently developed technique called amplified-fragment length polymorphism (AFLP) (41) is a whole-genome fingerprinting method based on selective amplification of restriction fragments (40). The AFLP reaction is a multistep procedure which in an elegant manner combines the power of PCR with the informativeness of restriction enzyme analysis. The procedure includes the preparation of an AFLP template where genomic DNA is digested with two restriction endonucleases which produce cohesive fragment ends and cut DNA with different frequencies (rare cutter [RC] and frequent cutter [FC]). Following digestion, genomic restriction fragments are modified by ligation of synthetic, double-stranded oligonucleotide adapters (RC adapter and FC adapter) with ends complementary to those of the restriction fragments. Thus, after the ligation step, genomic restriction fragments have termini of known sequences. Such an AFLP template is submitted to a highly stringent PCR amplification with primers fully complementary to their targets (RC primer and FC primer). Labelling one of the AFLP primers (preferably the RC primer) allows the detection of only a subset of the hundreds of amplified restriction fragments.
AFLP usually yields more complex banding patterns than most of the available DNA fingerprinting methods, which may likely increase discrimination between strains under analysis. The strategy of using two restriction enzymes and selective amplification provides an extraordinary flexibility in designing the typing protocols optimal for the given microbial species and the chosen detection system as well. These features, combined with the possibility for automation and a high-throughput analysis, make AFLP an interesting alternative to the currently used whole-genome fingerprinting techniques.
In the present study, we report the development of an AFLP-based procedure suitable for the inter- and intraspecies differentiation of Mycoplasma species of human and animal origin.
MATERIALS AND METHODS
Bacterial strains, cultural conditions, and DNA extraction.
Mycoplasma strains used in this study are listed in Table 1. Mycoplasma genitalium strains were grown in modified Friis’s medium (20). Mycoplasma pneumoniae and Mycoplasma hominis strains were grown on modified Hayflick’s medium (25). Strains of Mycoplasma hyopneumoniae, Mycoplasma flocculare, and Mycoplasma dispar were cultivated as described earlier (22). Mycoplasma hyosynoviae strains were cultivated in modified Hayflick’s medium enriched with arginine and bacteriological mucine (14). All field isolates of the examined mycoplasmas were identified by the disc growth inhibition test performed with corresponding polyclonal rabbit hyperimmune antisera. DNA samples of the M. pneumoniae strains were prepared by a standard procedure (33). Genomic DNA from M. genitalium and M. hominis strains was extracted from cell pellets by using the Easy-DNA kit (Invitrogen, Carlsbad, Calif.) according to the manufacturer’s instruction. DNA from all animal mycoplasmas was extracted by a standard procedure (33) from 50 ml of broth cultures harvested in late log phase. Following extraction, DNA was resuspended in 500 μl of TE buffer (10 mM Tris-HCl [pH 7.6], 1 mM EDTA) and stored at 4°C until analysis. The quality and quantity of the extracted DNA were assessed by electrophoresis in 1% agarose gels and by ethidium bromide staining. The DNA concentration of individual samples was not adjusted prior to further analysis.
TABLE 1.
Mycoplasma strains analyzed by AFLP fingerprinting
| Organism | Strain | Origin of specimen | Country of origin | Reference |
|---|---|---|---|---|
| M. genitalium | G-37T | Human urethra | United Kingdom | 38 |
| M2288 | Human urethra | Denmark | 20 | |
| M2300 | Human urethra | Denmark | 20 | |
| M2321 | Human urethra | Denmark | 20 | |
| M2341 | Human urethra | Denmark | 20 | |
| M-30 | Human urethra | United States | 38 | |
| UTMB-10G | Human joint | United States | 37 | |
| R32G | Human throat | United States | 3 | |
| TW 10-5G | Human throat | United States | 3 | |
| TW 10-6G | Human throat | United States | 3 | |
| TW 48-5G | Human throat | United States | 3 | |
| M. pneumoniae | MP 5 | Human throat | Denmark | Clinical isolate |
| MP 9 | Human throat | Denmark | Clinical isolate | |
| M1581 | Human throat | Denmark | Clinical isolate | |
| M1999 | Human throat | Denmark | Clinical isolate | |
| M2018 | Human throat | Denmark | Clinical isolate | |
| M. hominis | M612 | Human cervix | Denmark | Clinical isolate |
| M635 | Human cervix | Denmark | Clinical isolate | |
| M651 | Human cervix | Denmark | Clinical isolate | |
| MH2335 | Human cervix | Denmark | Clinical isolate | |
| MH2354 | Human urethra | Denmark | Clinical isolate | |
| M. hyopneumoniae | JT | Porcine lung | United Kingdom | 16 |
| Mp 98 | Porcine lung | Denmark | Field strain | |
| Mp 228 | Porcine lung | Denmark | Field strain | |
| Mp 241 | Porcine lung | Denmark | Field strain | |
| Mp 448 | Porcine pericardium | Denmark | 5 | |
| Mp 474 | Porcine pericardium | Denmark | 5 | |
| Mp 529 | Porcine pericardium | Denmark | 5 | |
| Mp 724 | Porcine lung | Denmark | Field strain | |
| Mp 806 | Porcine lung | Denmark | Field strain | |
| M. flocculare | Ms42T | Porcine lung | Denmark | 26 |
| Mp 17 | Porcine lung | Denmark | Field strain | |
| Mp 212 | Porcine lung | Denmark | Field strain | |
| Mp 530 | Porcine lung | Denmark | Field strain | |
| Mp 548 | Porcine lung | Denmark | Field strain | |
| M. hyosynoviae | S16T | Porcine joint | United States | 31 |
| M60 | Porcine lung | Denmark | 13 | |
| Mp 6 | Porcine joint | Denmark | Field strain | |
| Mp 96 | Porcine joint | Denmark | Field strain | |
| Mp 110 | Porcine joint | Denmark | Field strain | |
| Mp 393 | Porcine joint | Denmark | Field strain | |
| Mp 404 | Porcine joint | Denmark | Field strain | |
| Mp 908 | Porcine tonsil | Denmark | Field strain | |
| Mp 909 | Porcine tonsil | Denmark | Field strain | |
| Mp 912 | Porcine tonsil | Denmark | Field strain | |
| M. dispar | 462/2T | Bovine lung | United Kingdom | 17 |
| Mb 331 | Bovine lung | Denmark | Field strain | |
| Mb 341 | Bovine lung | Denmark | Field strain | |
| Mb 358 | Bovine lung | Denmark | Field strain | |
| Mb 372 | Bovine lung | Denmark | Field strain |
AFLP template preparation. (i) DNA digestion.
Five microliters of the DNA samples containing approximately 200 to 600 ng of genomic DNA was simultaneously digested with 10 U of BglII and 10 U of MfeI (New England Biolabs, Beverly, Mass.) at 37°C for 2 h in a restriction buffer containing 10 mM Tris-acetate (pH 7.5), 10 mM Mg acetate, 50 mM K acetate, 5 mM dithiothreitol, and 50 ng of bovine serum albumin per μl (40). The total reaction volume was 20 μl.
(ii) Adapter preparation and ligation conditions.
Oligonucleotides used for the preparation of AFLP adapters are listed in Table 2. Double-stranded adapters were assembled in separate vessels by mixing equimolar amounts of corresponding oligonucleotides. The mixtures were incubated for 10 min at 65°C and cooled for 15 min at room temperature. Following digestion, a 5-μl sample of genomic DNA was transferred to a new tube containing 2 pmol of the BGL adapter, 20 pmol of the MFE adapter, 1 U of T4 DNA ligase, 2 μl of a 10× ligase buffer (United States Biochemical, Cleveland, Ohio), and 8 μl of restriction buffer. The total reaction volume was 20 μl. Ligation was carried out overnight at room temperature.
TABLE 2.
Adapter and primer oligonucleotides used for AFLP reaction
| Oligonucleotide | Sequence |
|---|---|
| BGL adapter | 5′ CGGACTAGAGTACACTGTC 3′ |
| 5′ GATCGACAGTGTACTCTAGTC 3′ | |
| MFE adapter | 5′ AATTCCAAGAGCTCTCCAGTAC 3′ |
| 5′ TAGTACTGGAGAGCTCTTGG 3′ | |
| AFLP primers | |
| BGL2F-0 | 5′ GAGTACACTGTCGATCT 3′ (nonselective BGL primer) |
| MFE1-0 | 5′ GAGAGCTCTTGGAATTG 3′ (nonselective MFE primer) |
Amplification of modified DNA.
The oligonucleotide primers used for the amplification of the modified restriction fragments (AFLP primers) are listed in Table 2. The BGL2F-0 primer was labelled at the 5′ end with 6-carboxyfluorescein (FAM) (Oswell DNA Services, Ltd., Southampton, United Kingdom). The amplification was performed in a final volume of 50 μl. The reaction mixture contained 2 μl of 10-fold-diluted ligation product, a 200 μM concentration of each of the four deoxyribonucleoside triphosphates, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 65 ng of BGL2F-0 primer, 65 ng of MFE1-0 primer, and 1.5 U of Taq DNA polymerase (Gibco BRL, Gaithersburg, Md.). The amplification was performed in a programmable thermocycler by using an initial denaturation step at 94°C for 3 min followed by 30 cycles consisting of denaturation at 94°C for 60 s, annealing at 54°C for 60 s, and extension at 72°C for 90 s. The final cycle included a 10-min extension step at 72°C.
AFLP fragment detection and analysis.
Amplification products were detected on an ABI 373A automated sequencer (Perkin-Elmer, Norwalk, Conn.). Detection mixtures consisted of 2 μl of PCR products diluted 1:20 in sterile water, 2 μl of deionized formamide, 0.3 μl of internal-lane size standard labelled with TAMRA dye (GeneScan 500 TAMRA; Applied Biosystems, Foster City, Calif.), and 0.7 μl of loading buffer (supplied with the size standard). The mixtures were heated at 94°C for 2 min, cooled on ice, and electrophoresed on 6% denaturing polyacrylamide gels for 9 h by using plates with 24 cm of well-to-read distance. Data collection, preprocessing, fragment sizing, and pattern analysis were done by using 672 GENESCAN 1.2.2-1 fragment analysis software (Applied Biosystems).
For the purpose of numerical analysis, the background level was subtracted from the raw AFLP data with Genotyper 1.1.1 software (Applied Biosystems). The normalized data were converted with MWtoGel software and imported in GelCompar 4.0 (both from Applied Maths, Kortrijk, Belgium). Levels of similarity between fingerprints were calculated by using the band-based Dice similarity coefficient (SD). Clustering of fingerprints was performed with the unweighted pair group method by using average linkages.
Sequencing.
Particular genomic segments of three M. genitalium strains (M. genitalium G-37T, M. genitalium UTMB-10G, and M. genitalium M2288) were PCR amplified and analyzed by sequencing. Two microliters of DNA sample was added to 48 μl of a prepared reaction mixture containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, the four deoxynucleosides (100 μM each), 65 ng of each of the oligonucleotide primers (Table 3), and 0.5 U of Taq polymerase (Perkin-Elmer) and covered with paraffin oil. Samples were subjected to an initial denaturation step at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min in a thermocycler. To ensure complete strand extension, the reaction mixture was kept at 72°C for 10 min after the final cycle. Amplicons were purified with the QIAquick spin PCR purification kit (Qiagen, Hilden, Germany) and sequenced on an ABI 373A automatic sequencer by using the AmpliTaq FS dye terminator kit (Perkin-Elmer) according to the manufacturer’s instructions.
TABLE 3.
Oligonucleotide primers used for amplification of genomic segments of M. genitalium strains and sequencing
| Primer | Sequence | Explanation |
|---|---|---|
| MG-D1-F | 5′ CTTACCCTTAAAGTTTGTTG 3′ | Primer pair amplifies 288-bp fragment of MG 166 of M. genitalium G-37T |
| MG-D1-R | 5′ CTAATGGTGATTTCAGTG 3′ | |
| MG-D2-F | 5′ GGTTTAAACAATGTACAGG 3′ | Primer pair amplifies 410-bp fragment of MG 075 of M. genitalium G-37T |
| MG-D2-R | 5′ TAGGTGTATGACTCGTTAGC 3′ | |
| MG-D4-F | 5′ GCAAGTTTGAAAAAGTAAGTG 3′ | Primer pair amplifies 650-bp fragment of MG 095 and downstream noncoding region of M. genitalium G-37T |
| MG-D4-R | 5′ GAAAAAAGTGAAAGTAGTAAGG 3′ |
RESULTS
Complexity of the AFLP fingerprints.
In this study AFLP was used for genotyping a total of 50 Mycoplasma strains of human and animal origin (Table 1). The detection system was chosen to provide an optimal separation and a uniform sizing of fragments of 50 to 500 bp. Under the conditions used, all of the analyzed strains developed complex banding patterns within the aforementioned size range. Fingerprints of the lowest complexity were those obtained from M. hyosynoviae and M. flocculare strains, consisting of approximately 60 AFLP fragments. M. dispar, M. hominis, and M. pneumoniae strains showed patterns containing about 70 fragments, while M. genitalium and M. hyopneumoniae strains developed fingerprints with 80 and more fragments.
Reproducibility of the AFLP reaction.
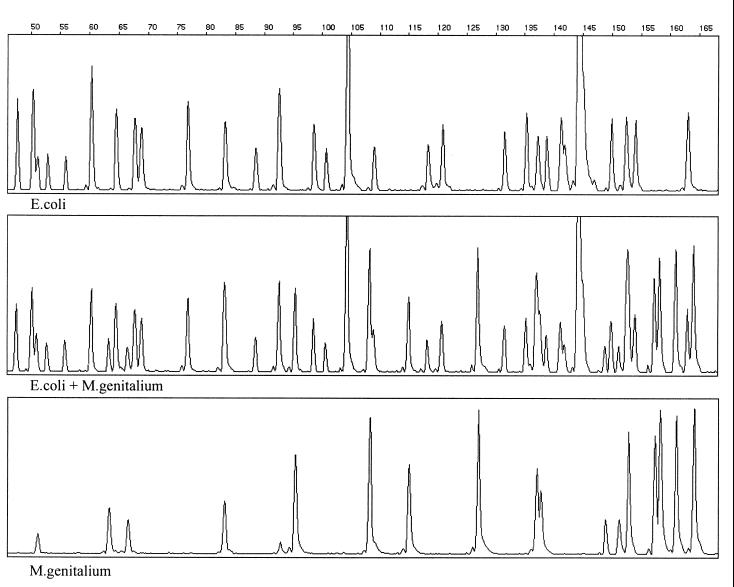
Reproducibility tests were based on repeated analysis of identical samples. DNA samples from the 11 M. genitalium and the 10 M. hyosynoviae strains were submitted to the AFLP procedure repeated in triplicate, while DNA samples from other mycoplasmas were analyzed in duplicate. Further, from each of the type and reference strains of M. hyosynoviae (S16T and M60, respectively) three subcultures were made. DNAs prepared from these six subcultures were also included in the reproducibility test. The repeated analysis revealed indistinguishable banding patterns for all of the identical samples being analyzed (data are shown only for M. genitalium G-37T [Fig. 1]). The reproducibility of the AFLP fingerprints was also tested with regard to changes of annealing temperatures in the amplification step. No difference in banding patterns of M. genitalium G-37T could be detected after four separate amplifications with annealing temperatures of 54, 56, 58, and 60°C (data not shown). The stability of the AFLP patterns of individual samples was retained, even when artificially created mixtures of AFLP templates of different complexities were analyzed. A simultaneous amplification of AFLP templates of M. genitalium G-37T and a field isolate of Escherichia coli, with adjusted DNA concentrations, yielded a hybrid fingerprint containing all fragments present in the AFLP fingerprints of the individual samples (Fig. 2).
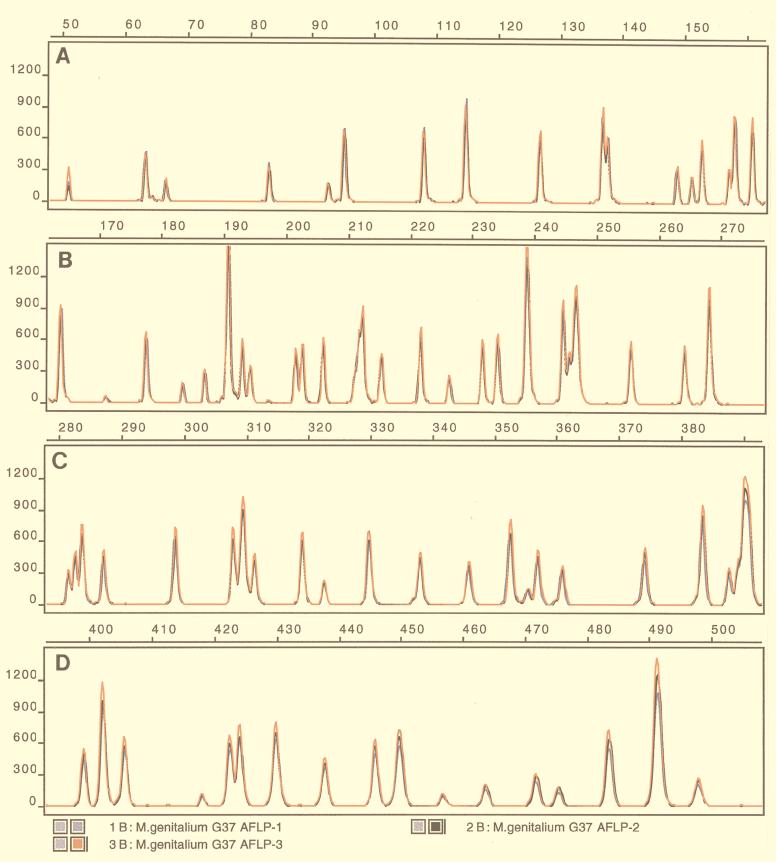
FIG. 1.
AFLP fingerprint of M. genitalium G-37T. BglII plus MfeI AFLP templates were prepared on three occasions with the same batch of genomic DNA of M. genitalium G-37T. Amplification products obtained from each experiment (blue, black, and red patterns) were detected on an ABI 373A sequencer by GeneScan 1.2.2.-1 software. The complete AFLP patterns are superimposed and divided into four parts (A to D). The fragment size scale (base pairs) is indicated above each panel. y axes indicate relative amounts of amplicons (in fluorescence units).
FIG. 2.
Genescan-derived electropherogram traces of AFLP templates of different complexity. AFLP templates of a field isolate of Escherichia coli and M. genitalium G-37T were prepared by the digestion of genomic DNAs with BglII and MfeI and subsequent ligation of corresponding adapters. PCR products of individual samples and a mixture of the AFLP templates, containing adjusted DNA concentrations, were detected on an ABI 373A sequencer. The hybrid pattern (middle panel) contains all of the bands detected in individual AFLP templates. The fragment size scale (base pairs) is indicated above the top panel.
AFLP of Mycoplasma species.
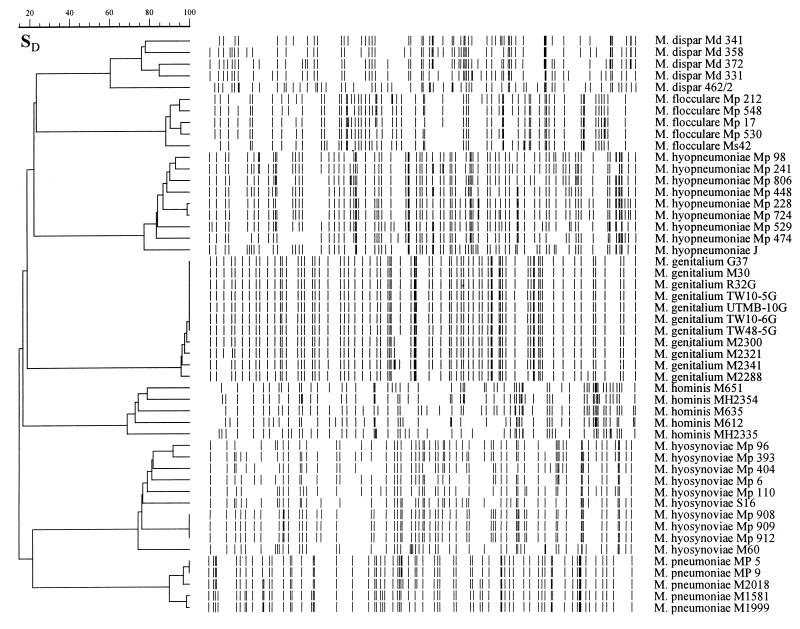
The discriminatory power of the AFLP technique was investigated by analysis of groups of isolates belonging to seven distinct Mycoplasma species (Table 1). Under the conditions used, the method was able to discriminate the analyzed strains at both species and intraspecies levels. Cluster analysis of AFLP data revealed seven groups, each group consisting of strains belonging to a single species (Fig. 3). M. dispar strains showed five AFLP patterns, which clustered at a linkage level of 61.7%. M. flocculare strains showed five different AFLP patterns, forming a cluster with a linkage level of 88.2%. The nine analyzed M. hyopneumoniae strains clustered at a linkage level of 77.4%. The 10 analyzed M. hyosynoviae strains revealed eight AFLP profiles, which grouped at a linkage level of 74.4%. Indistinguishable banding patterns were obtained from the three field isolates (Mp 908, Mp 909, and Mp 912) recovered from different animals in a single herd. The M. hominis and M. pneumoniae strains showed five and four different AFLP patterns, which clustered at linkage levels of 69 and 90%, respectively. M. pneumoniae MP 5 and MP 9 showed indistinguishable banding patterns. The 11 analyzed M. genitalium strains developed five AFLP profiles, which clustered at a linkage level of 95.9%. The strains which could not be differentiated with the method used were G-37T, M-30, R32G, UTMB-10G, TW 10-5G, TW 10-6G, and TW 48-5G.
FIG. 3.
Digitized chromosomal AFLP fingerprints of the Mycoplasma strains of human and animal origin. SD, band-based Dice similarity coefficient (%). The dendrogram was constructed by using the unweighted pair group method with average linkages.
Identification of AFLP in M. genitalium.
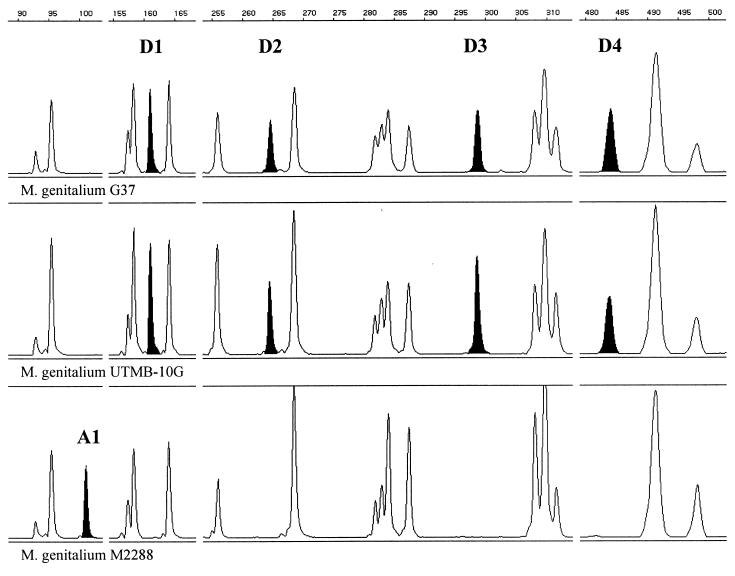
The Danish urethral isolate of M. genitalium M2288 showed a unique AFLP profile which differed in five positions from the fingerprint obtained from reference strain G-37T. The difference consisted of one additional fragment (at the position of 101 bp) (Fig. 4, A1) and four missing fragments (positions of 161, 264, 299, and 484 bp) (Fig. 4; D1, D2, D3, and D4, respectively). The chromosomal positions of the four AFLP fragments present in the reference pattern of M. genitalium G-37T (Fig. 4; D1, D2, D3, and D4) were predicted after computer-assisted analysis of the distribution of BglII and MfeI restriction sites in the published genomic sequence of this strain (GenBank accession no. L43967) (11), and the fragments were identified as parts of MG 166 (rpL6; Fig. 4; D1), MG 075 (hypothetical protein; Fig. 4; D2), and MgPar 4 (repetitive sequence; Fig. 4; D3), while the fourth fragment (Fig. 4; D4) begins in MG 095 (hypothetical protein) and ends 456 bp downstream in the noncoding region.
FIG. 4.
Segments of AFLP patterns obtained from M. genitalium G-37T, UTMB-10G, and M2288. The fragment size scale (base pairs) is indicated above each segment. AFLP fragments are highlighted in black and designated as A1, D1, D2, D3, and D4.
The aforementioned genomic regions were suspected to contain a sequence variability which accounted for the absence of the four fragments in the AFLP pattern of M. genitalium M2288.
Chromosomal segments of M. genitalium G-37T and M. genitalium M2288, which corresponded to AFLP fragments D1, D2, and D4, were amplified with primers flanking the associated BglII and MfeI restriction sites (Table 3) and analyzed by sequencing. The AFLP fragment D3 (Fig. 4), putatively identified as part of the repetitive element MgPar 4, was not analyzed. M. genitalium UTMB-10G, which showed an AFLP pattern identical to that of the type strain, was also analyzed.
The DNA sequence of the amplicons D1, D2, and D4 all concurred with the sequence predicted in the computer analysis of the published G-37T sequence. The PCR products representing D1, D2, and D4 AFLP fragments of M. genitalium G-37T and M. genitalium UTMB-10G showed identical sequences, while the corresponding products obtained from M. genitalium M2288 contained changes in the associated MfeI sites: the CAATTG sequence of the MfeI site was changed to CAGTTG, AAATTG, and CAATTA in D1, D2, and D4, respectively. The changes in the MfeI sites in the D1 and D4 genomic regions of strain M2288 did not affect amino acid coding and were the only variance from the analogous regions of G-37T and UTMB-10G (data not shown). The amplified D2 region of M2288 revealed a larger sequence variability than the corresponding regions obtained from M. genitalium G-37T and UTMB-10G. The difference comprised mutations at 10 positions, seven of which accounted for changes in amino acid coding (data not shown).
DISCUSSION
AFLP fingerprinting has been shown to be a sensitive method for the molecular characterization of various bacterial species (1, 10, 18, 19, 21, 39). However, in order to be used to its full potential, the technique may require a certain tailoring to account for genomic differences of various organisms (genome size, G+C content, and DNA modification). The choice of the restriction enzymes obviously has great impact on the results of the AFLP reaction. In this study two six-base cutters were selected, of which MfeI cuts mycoplasmal DNA more frequently than BglII does. BglII and MfeI together break up the M. genitalium G-37T chromosome into fewer than 600 fragments with a cutting frequency ratio of approximately 1:2. The amplification of AFLP fragments was performed by using a single set of so-called nonselective primers (no selective nucleotides at the 3′ ends of the primers). Yet, selectivity of amplification (the key feature of the AFLP reaction) is achieved through a predominant amplification of BglII-MfeI fragments in the presence of BglII-BglII and MfeI-MfeI fragment fractions. An inefficient amplification of BglII-BglII and MfeI-MfeI fragment fractions may be explained by the formation of stem-loop structures, due to inverted repeats present at the ends of these fragments, which compete with primer annealing (40).
As revealed by cluster analysis, AFLP fingerprints of the analyzed mycoplasmas retain attributes of species specificity, leading to the conclusion that the method may be used as an additional tool for species identification (e.g., mycoplasmas isolated from atypical hosts). However, this approach is currently of limited applicability, due to the lack of an appropriate database of reference patterns. The high-level discriminatory power of the method makes it useful for studies of intraspecies diversity of mycoplasmas. The different extent of intraspecies polymorphism detected in this study suggests various degrees of genetic diversity among the analyzed species. A small number of AFLP fragments detected among M. genitalium strains support previous findings reported by Razin et al. (29) on the substantial genetic homogeneity of this species. It has been shown previously that clinical isolates of M. pneumoniae can be classified in two distinct groups, based upon divergence in the major cytadhesin gene (36). Numerical analysis of the M. pneumoniae genomic fingerprints obtained in the present study also showed segregation into what could seem like two groups. However, analysis of more strains is needed in order to draw conclusions regarding the chromosomal variation within this species. The results of AFLP analysis of the five randomly chosen clinical isolates of M. hominis provide further evidence of high-level intraspecies variability of this organism and are generally in agreement with the data obtained by using other molecular typing methods (7, 9). The genetic diversity of M. hyopneumoniae has been examined by different techniques and reported previously (2, 12), but studies of intraspecies variation at the DNA level of M. flocculare, M. hyosynoviae, and M. dispar have not been reported. The results of AFLP analysis indicate a moderate (M. flocculare) to high (M. hyosynoviae and M. dispar) level of intraspecies heterogeneity of those mycoplasma species. However, presently it is unknown whether the observed variations have any biologically relevant role or just reflect a rapid evolutionary process.
Different molecular mechanisms may account for the observed polymorphism including point mutations which introduce or remove restriction sites and thus AFLP fragments, genomic rearrangements, and insertion or deletion events between restriction sites, which alter the size of the individual fragments. The availability of the entire chromosomal sequence of M. genitalium G-37T allowed us to examine the nature of certain polymorphisms detected among M. genitalium strains by comparing fingerprinting and sequencing data at the whole-genome level. This approach was based on the amplification and sequencing of analogous chromosomal regions of two strains having particular AFLP fragments (G-37T and UTMB-10G) and one strain (M2288) showing absence of the corresponding fragments. The sequence of the AFLP fragments analyzed concurred completely with the sequence predicted by computer analysis of the G-37T genomic DNA sequence, suggesting that variant AFLP patterns truly reflect variations in the genomes of the analyzed strains. Further, in two of the three sequenced fragments (D1 and D4), the only variation observed was a single point mutation in the recognition site of one of the enzymes, a mutation that did not alter the putative amino acid sequence. To the contrary, in the third fragment (D2) multiple variations were seen. These differences may reflect the different selective pressure of different parts of the chromosome. The distribution of variation is not obvious, as D4 consists partly of a noncoding region while D1 and D2 are derived from putative coding regions.
The results of this study clearly indicate the high potential of the AFLP method to differentiate mycoplasma isolates at both species and subspecies levels. Highly reproducible and easy to perform, AFLP provides a rich source of molecular markers, useful for studies of epidemiology, pathogenicity, and genetic variation in natural populations of Mycoplasma species. The combination of AFLP with powerful detection systems, such as automated DNA sequencers, enables a uniform data collection and analysis, as well as the storage of biological data in electronic form, which make them comparable in long time frames and feasible for interlaboratory exchange.
ACKNOWLEDGMENTS
We are grateful to Joseph G. Tully and David Taylor-Robinson for providing the M. genitalium strains. Katja Kristensen and Ulla Amtoft are acknowledged for excellent technical assistance.
This work was supported by a grant from the Danish Ministry of Food, Agriculture and Fisheries.
REFERENCES
- 1.Aarts H J M, van Lith L A J T, Keijer J. High-resolution genotyping of Salmonella strains by AFLP-fingerprinting. Lett Appl Microbiol. 1998;26:131–135. doi: 10.1046/j.1472-765x.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 2.Artiushin S, Minion F C. Arbitrarily primed PCR analysis of Mycoplasma hyopneumoniae field isolates demonstrates genetic heterogeneity. Int J Syst Bacteriol. 1996;46:324–328. doi: 10.1099/00207713-46-1-324. [DOI] [PubMed] [Google Scholar]
- 3.Baseman J B, Dallo S F, Tully J G, Rose D L. Isolation and characterization of Mycoplasma genitalium strains from the human respiratory tract. J Clin Microbiol. 1988;26:2266–2269. doi: 10.1128/jcm.26.11.2266-2269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benkirane A, Amghar S, Kirchhoff H. Analysis of membrane proteins of Mycoplasma capricolum strains by SDS-PAGE and immunoblotting. Zentbl Vetmed Reihe B. 1993;40:119–124. doi: 10.1111/j.1439-0450.1993.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 5.Buttenschøn J, Friis N F, Aalbæk B, Jensen T K, Iburg T, Mousing J. Microbiology and pathology of fibrinous pericarditis in Danish slaughter pigs. J Vet Med Ser A. 1997;44:271–280. doi: 10.1111/j.1439-0442.1997.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheng X, Nicolet J, Poumarat F, Regalla J, Thiaucourt F, Frey J. Insertion element IS1296 in Mycoplasma mycoides subsp. mycoides small colony identifies a European clonal line distinct from African and Australian strains. Microbiology. 1995;141:3221–3228. doi: 10.1099/13500872-141-12-3221. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen C, Christiansen G, Rasmussen O F. Heterogeneity of Mycoplasma hominis as detected by a probe for atp genes. Isr J Med Sci. 1987;23:591–594. [PubMed] [Google Scholar]
- 8.Christiansen G, Andersen H. Heterogeneity among Mycoplasma hominis strains as detected by probes containing parts of ribosomal ribonucleic acid genes. Int J Syst Bacteriol. 1988;38:108–115. [Google Scholar]
- 9.Christiansen G, Andersen H, Birkelund S, Freundt E A. Genomic and gene variation in Mycoplasma hominis strains. Isr J Med Sci. 1987;23:595–602. [PubMed] [Google Scholar]
- 10.Desai M, Tanna A, Wall R, Efstratiou A, George R, Stanley J. Fluorescent amplified-fragment length polymorphism analysis of an outbreak of group A streptococcal invasive disease. J Clin Microbiol. 1998;36:3133–3137. doi: 10.1128/jcm.36.11.3133-3137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser C M, Gocayne J D, White O, Adams M D, Clayton R A, Fleischmann R D, Bult C J, Kerlavage A R, Sutton G, Kelly J M, Fritchman J L, Weidman J F, Small K W, Sandusky M, Fuhrmann J, Nguyen D, Utterback T R, Saudek D M, Phillips C A, Merrick J M, Tomb J-F, Dougherty B A, Bott K F, Hu P-C, Lucier T S, Peterson S N, Smith H O, Hutchison III C A, Venter J C. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 12.Frey J, Haldimann A, Nicolet J. Chromosomal heterogeneity of various Mycoplasma hyopneumoniae field strains. Int J Syst Bacteriol. 1992;42:275–280. doi: 10.1099/00207713-42-2-275. [DOI] [PubMed] [Google Scholar]
- 13.Friis N F. A new porcine mycoplasma species: Mycoplasma suidaniae. Acta Vet Scand. 1970;11:487–490. doi: 10.1186/BF03547974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friis N F, Ahrens P, Larsen H. Mycoplasma hyosynoviae isolation from the upper respiratory tract and tonsils of pigs. Acta Vet Scand. 1991;32:425–429. doi: 10.1186/BF03546943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geary S J, Forsyth M H, Aboul Saoud S, Wang G, Berg D E, Berg C M. Mycoplasma gallisepticum strain differentiation by arbitrary primer PCR (RAPD) fingerprinting. Mol Cell Probes. 1994;8:311–316. doi: 10.1006/mcpr.1994.1042. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin R F W, Pomeroy A P, Whittlestone P. Characterisation of Mycoplasma suipneumoniae: a mycoplasma causing enzootic pneumonia of pigs. J Hyg. 1967;65:85–96. doi: 10.1017/s0022172400045563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gourlay R N, Leach R H. A new mycoplasma species isolated from pneumonic lungs of calves (Mycoplasma dispar sp. nov.) J Med Microbiol. 1970;3:111–123. doi: 10.1099/00222615-3-1-111. [DOI] [PubMed] [Google Scholar]
- 18.Janssen P, Maquelin K, Coopman R, Tjernberg I, Bouvet P, Kersters K, Dijkshoorn L. Discrimination of Acinetobacter genomic species by AFLP fingerprinting. Int J Syst Bacteriol. 1997;47:1179–1187. doi: 10.1099/00207713-47-4-1179. [DOI] [PubMed] [Google Scholar]
- 19.Janssen P, Coopman R, Huys G, Swings J, Bleeker M, Vos P, Zabeau M, Kersters K. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology. 1996;142:1881–1893. doi: 10.1099/13500872-142-7-1881. [DOI] [PubMed] [Google Scholar]
- 20.Jensen J S, Hansen H T, Lind K. Isolation of Mycoplasma genitalium strains from the male urethra. J Clin Microbiol. 1996;34:286–291. doi: 10.1128/jcm.34.2.286-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keim P, Kalif A, Schupp J, Hill K, Travis S E, Richmond K, Adair D M, Hugh-Jones M, Kuske C R, Jackson P. Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. J Bacteriol. 1997;179:818–824. doi: 10.1128/jb.179.3.818-824.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobish M, Friis N F. Swine mycoplasmoses. Rev Sci Tech Off Int Epizoot. 1996;15:1569–1605. doi: 10.20506/rst.15.4.983. [DOI] [PubMed] [Google Scholar]
- 23.Krause D C, Taylor-Robinson D. Mycoplasmas which infect humans. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 417–444. [Google Scholar]
- 24.Leach R H, Costas M, Mitchelmore D L. Relationship between Mycoplasma mycoides subsp. mycoides (‘large-colony’ strains) and M. mycoides subsp. capri, as indicated by numerical analysis of one-dimensional SDS-PAGE protein patterns. J Gen Microbiol. 1989;135:2993–3000. doi: 10.1099/00221287-135-11-2993. [DOI] [PubMed] [Google Scholar]
- 25.Lind K, Lindhardt B Ø, Schütten H J, Blom J, Christiansen C. Serological cross-reaction between Mycoplasma genitalium and Mycoplasma pneumoniae. J Clin Microbiol. 1984;20:1036–1043. doi: 10.1128/jcm.20.6.1036-1043.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyling A, Friis N F. Serological identification of a new porcine mycoplasma species, M. flocculare. Acta Vet Scand. 1972;13:287–289. doi: 10.1186/BF03548589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poumarat F, Solsona M. Molecular epidemiology of Mycoplasma mycoides subsp. mycoides biotype small colony, the agent of contagious bovine pleuropneumonia. Vet Microbiol. 1995;47:305–315. doi: 10.1016/0378-1135(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 28.Razin S. Molecular biology and genetics of mycoplasmas (Mollicutes) Microbiol Rev. 1985;49:419–455. doi: 10.1128/mr.49.4.419-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razin S, Tully J G, Rose D L, Barile M F. DNA cleavage patterns as indicators of genotypic heterogeneity among strains of Acholeplasma and Mycoplasma species. J Gen Microbiol. 1983;129:1935–1944. doi: 10.1099/00221287-129-6-1935. [DOI] [PubMed] [Google Scholar]
- 30.Rodwell A W, Rodwell E S. Relationships between strains of Mycoplasma mycoides subspp. mycoides and capri studied by two-dimensional gel electrophoresis of cell proteins. J Gen Microbiol. 1978;109:259–263. doi: 10.1099/00221287-109-2-259. [DOI] [PubMed] [Google Scholar]
- 31.Ross R F, Karmon J A. Heterogeneity among strains of Mycoplasma granularum and identification of Mycoplasma hyosynoviae, sp. n. J Bacteriol. 1970;103:707–713. doi: 10.1128/jb.103.3.707-713.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salih M M, Ernø H, Simonsen V. Electrophoretic analysis of isoenzymes of mycoplasma species. Acta Vet Scand. 1983;24:14–33. doi: 10.1186/BF03546754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Sasaki T, Kenri T, Okazaki N, Iseki M, Yamashita R, Shintani M, Sasaki Y, Yayoshi M. Epidemiological study of Mycoplasma pneumoniae infections in Japan based on PCR-restriction fragment length polymorphism of the P1 cytadhesin gene. J Clin Microbiol. 1996;34:447–449. doi: 10.1128/jcm.34.2.447-449.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simecka J W, Davis J K, Davidson M K, Ross S E, Städtlander C T K-H, Cassell G H. Mycoplasma diseases of animals. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 391–415. [Google Scholar]
- 36.Su C J, Dallo S F, Baseman J B. Molecular distinctions among clinical isolates of Mycoplasma pneumoniae. J Clin Microbiol. 1990;28:1538–1540. doi: 10.1128/jcm.28.7.1538-1540.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tully J G, Rose D L, Baseman J B, Dallo S F, Lazzell A L, Davis C P. Mycoplasma pneumoniae and Mycoplasma genitalium mixture in synovial fluid isolate. J Clin Microbiol. 1995;33:1851–1855. doi: 10.1128/jcm.33.7.1851-1855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tully J G, Taylor-Robinson D, Cole R M, Rose D L. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;i:1288–1291. doi: 10.1016/s0140-6736(81)92461-2. [DOI] [PubMed] [Google Scholar]
- 39.Valsangiacomo C, Baggi F, Gaia V, Balmelli T, Peduzzi R, Piffaretti J. Use of amplified fragment length polymorphism in molecular typing of Legionella pneumophila and application to epidemiological studies. J Clin Microbiol. 1995;33:1716–1719. doi: 10.1128/jcm.33.7.1716-1719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kupier M, Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zabeau M, Vos P. Selective restriction fragment amplification: a general method for DNA fingerprinting. Publication 0534858A1. Munich, Germany: European Patent Office; 1993. [Google Scholar]