Abstract
Background and aims
Monitoring the immune response against SARS-CoV-2 is pivotal in the evaluation of long-term vaccine efficacy. Immunoglobulin G (IgG) antibodies represent an advisable tool to reach this goal, especially for the still poorly defined antibody trend induced by the new class of mRNA vaccines against SARS-CoV-2.
Materials and methods
Anti-Spike RBD IgG antibodies were monitored in a cohort of healthcare workers at CRO Aviano, National Cancer Institute, through MAGLUMI® chemiluminescence assay, at 1 and 4 months after full-schedule of BNT162b2 or mRNA-1273 vaccination.
Results
At 1 month after vaccination, 99.9% of 767 healthcare workers showed a reactive antibody response, which was inversely correlated with age, and positively associated with a previous history of COVID-19, and mRNA-1273 vaccination. Serological response was maintained in 99.6% of the 516 subjects monitored also at follow-up. An antibody decay from 559.8 AU/mL (IQR 359.7–845.7) to 92.7 AU/mL (IQR 65.1–148.6; p < 0.001) was observed, independently from age and sex.
Conclusion
Our data supported the ability of SARS-CoV-2 mRNA vaccines to induce at least a 4 months-lasting IgG response, even outside the rules of clinical trials. The antibody decay observed at follow-up suggested to deepen the immune response characterization to identify subjects with low anti-SARS-CoV-2 immunity possibly requiring a vaccination boost.
Keywords: SARS-CoV-2, COVID-19, IgG antibodies, mRNA vaccination, Antibody decay
Abbreviations: COVID-19, COronaVIrus Disease 19; RBD, Receptor Binding Domain; Ig, Immunoglobulin; HCW, HealthCare Workers; AU, Arbitrary Units; IQR, InterQuartile Range
1. Introduction
Clinical trials evaluating the efficacy of mRNA-based SARS-CoV-2 vaccines looked at the first occurrence of symptomatic COronaVIrus Disease 19 (COVID-19) with onset at least 7–14 days after the second vaccine injection, among participants who were seronegative at baseline [1], [2]. However, at present, there is an urgent need to evaluate the immune response kinetics with the aim of monitoring the vaccination coverage. A valuable biomarker of vaccine efficacy is represented by the induction of an effective immune response, which could be monitored through different parameters, as the virus-specific antibody production. In the natural infection by SARS-CoV-2 the major antigenic target of human antibodies is the Spike protein and in particular the Receptor Binding Domain (RBD) [3], [4], [5]. The RBD is responsible for the binding to the angiotensin-converting enzyme 2, the main receptor recognized by the virus to enter into the target cells [3], and is the primary target of SARS-CoV-2-neutralizing antibodies [6]. The Spike protein is encoded by both the mRNA-based vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), currently in use to prevent SARS-CoV-2 infection [1], [2]. Thus, antibody response against this protein and, in particular, the RBD represents a valuable tool to monitor the vaccine efficacy [7].
Several clinical laboratory assays are now available to track the immune response, by evaluating the presence and the titer of Spike-RBD-specific Immunoglobulin (Ig) M, IgG, and IgA antibodies [5], [8]. Recent evidence suggests that measuring SARS-CoV-2 IgM is needless and probably misleading, due to the low concordance of results obtained with different technologies [9], while IgA seem to predominate only at the early phase of disease, then decaying faster than IgG [10]. Conversely, IgG showed a slower decline [5] and a strong correlation with sera neutralizing activity [11].
Antibody decay represents a physiological response after natural infection and vaccination and should be monitored to evaluate the percentage of population losing the protective immunity [12]. Currently, still little is known about the decay rate of antibody titer after mRNA-based SARS-CoV-2 vaccination, and available data are limited to a low number of individuals [13], [14], [15] or a short follow-up [16], even if literature is growing quickly. Studies performed on SARS-CoV-2 infected individuals reported IgG antibody appearance at the fourth day after symptom onset, a peak of the levels in the second and third week of the illness [9], and then an antibody persistence from 9 to 11 months after the natural infection [5], [17]. In particular, a longitudinal analysis monitoring COVID-19 convalescent plasma donors showed a decrease in IgG levels overtime, but reported that 91.4% of donors had detectable IgG levels up to 11 months after recovery, thus asserting that seroreversion to negative was uncommon [5]. Preliminary studies on vaccinated individuals showed that antibodies elicited by the mRNA-1273 vaccine persisted through 6 months after the second dose, estimating a half-life of 52 days calculated with a model assuming a steady decay rate over time, or even 109 days using a model assuming that decay rate decrease over time [13]. Another study reported an exponential growth of SARS-CoV-2 Spike specific IgG immediately after vaccination, reaching a plateau 18–21 days after the first dose, and then 7 days after the second vaccination dose, and maintaining the 58% of peak values till more than 100 days of followup [7]. Conversely, Spike-specific IgA decreased quickly, falling to the 18% of peak levels within 100 days of follow-up [7].
However, several clinical factors could influence the generation of an effective immune response as well as the maintenance of an immunological memory during time. Even if no significant differences were reported for age and sex in the main clinical trials evaluating the efficacy of mRNA-based vaccines [1], [2], several studies described a reduced efficacy of vaccination in vulnerable subjects as cancer patients, kidney transplant recipients, and hemodialysis patients [18], [19].
On these backgrounds, the main aim of the present study was to monitor the induction of an IgG antibody response after mRNA-based vaccination against SARS-CoV-2 in a real-life setting involving healthcare workers (HCW), measuring the antibody titer 1 and 4 months after a complete vaccination schedule, to evaluate the antibody decay rate with an automated and highly reproducible system. This analysis could contribute to better define the duration of protection against COVID-19 after vaccination, also considering the possible need for a vaccination boost.
2. Material and methods
2.1. Subjects and blood sample collection
Seven hundred and sixty-seven HCW were tested, as part of occupational health surveillance, for anti-SARS-CoV-2 antibodies 1 month after two vaccination doses (BNT162b2 [n = 722; 94.1%] or mRNA-1273 [n = 43; 5.6%] vaccines [unknown: n = 2; 0.3%]). Among these, 516 (BNT162b2, n = 515; 99.8%; mRNA-1273, n = 1; 0.2%) were tested also 4 months after the final dose (median 105, range 90–175 days after the first evaluation). For all the included subjects, surveillance for SARS-CoV-2 infection was carried out through a molecular assay on a nasopharyngeal swab every 15–30 days. All subjects expressed their informed consent for the conservation and use of biological samples, which are stored in the Institute Biobank.
2.2. Serological tests
Serum was obtained from a 4.9 ml blood sample after centrifugation at 3600 rpm for 10 min at room temperature, then frozen and maintained at −80 °C till usage. Serum samples were thawed at 37 °C for 10 min and after vortexing they were analysed for the detection of IgG against SARS-CoV-2 Spike RBD through the MAGLUMI® SARS-CoV-2 S-RBD IgG kit on a MAGLUMI 800 analyzer (Snibe Diagnostic, Shenzen New Industries Biomedical Engineering Co. Ltd., Shenzen, China) under manufacturer’s instructions. Briefly, the immunoassay evaluates the IgG concentration by means of a calibration curve (instrument-specifically generated by 2-point calibration, based on a 10-point master curve provided by the MAGLUMI system) in chemiluminescence. Results are expressed as arbitrary units (AU)/mL within a linear range of 0.180–100 AU/mL (defined by the Limit of Detection and the maximum of the master curve). Presence of reactivity was established for values ≥1.1 AU/mL, absence of reactivity for values <0.9 AU/mL; values between 0.9 and 1.1 AU/mL were considered undetermined [9]. In case of IgG concentration >100 AU/mL, serum samples were automatically diluted by the analyzer and the antibody titer calculated again by the analyzer software. The Coefficient of Variation (CV) varied from 11.82% for values of 0.550 AU/ML, to 8.51% for 2.421 AU/mL, till 4.89% for 5.109 AU/mL.
2.3. Statistical analysis
Categorical data are reported as absolute frequencies and percentages, continuous variables are reported as median and interquartile range (IQR). Due to the skewed distribution of values, difference in IgG values between groups was accessed with Kruskal-Wallis or Mann-Whitney test as appropriate. Paired differences between the two assessments were analyzed with Wilcoxon signed rank test. Association between continuous variables was measured using Spearman’s correlation coefficient (rho). Chi-squared test was used to evaluate association between categorical variables. In box plot graphics, the horizontal line represents the median value, the box the interquartile range and the whiskers the lower and the higher value included in the following interval: 1st quartile − 1.5x(3rd-1st quartile) and 3rd quartile + 1.5x(3rd −1st quartile); values outside this interval are considered outliers (dots). A p-value <0.05 was considered statistically significant.
3. Results
3.1. Detection of SARS-CoV-2 S-RBD IgG 1 month after vaccination
Globally, 767 HCW were tested for the presence of IgG specific for SARS-CoV-2 Spike-RBD 1 month after receiving the second dose of the preventive vaccination. 557 (72.6%) were female, 210 (27.4%) male, with a median age of 46 (IQR 35 – 55). Overall, a reactive antibody response was registered in the 99.9% (766/767) of the subjects, showing an IgG value ≥1.1 AU/mL. The only negative subject was affected by a lymphoproliferative disease under therapeutic treatment.
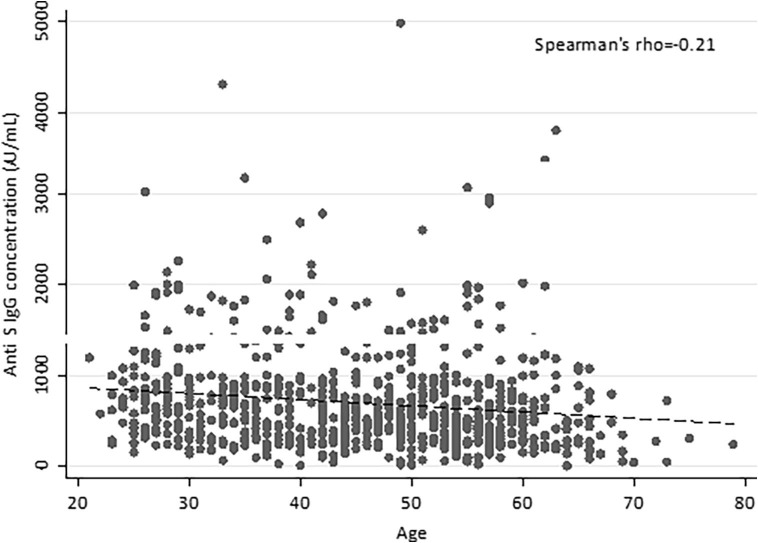
No difference in the antibody titer was highlighted between men and women in the global case study (p = 0.166) (see Supplementary Data, Supplementary Table 1), while the IgG concentration negatively correlated with age (Spearman’s rho = −0.21, p < 0.001) (Fig. 1 ), with median values of 716.1 AU/mL for subjects < 30 years (IQR 470.9–1034.8) and 450.3 AU/mL for individuals ≥ 60 years (IQR 231.3–741.8).
Fig. 1.
Correlation of anti-SARS-CoV-2 IgG levels and age at 1 month after vaccination. The antibody titers of 767 HCW are plotted against age; the dashed line represents the linear trend. Anti-S, anti-Spike RBD; AU, arbitrary units.
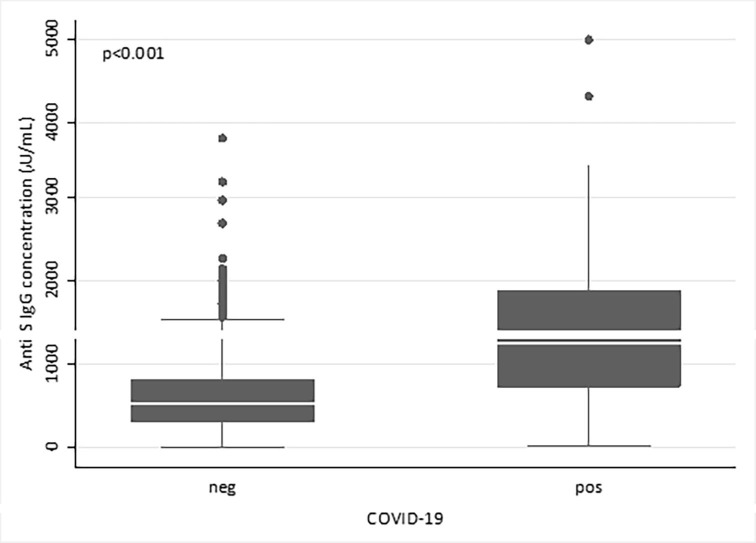
SARS-CoV-2 infection before first dose of vaccination was detected in 64/767 (8.3%) subjects through molecular swab analysis; in these subjects the antibody titer appeared significantly higher (median value 1249.3 AU/mL, IQR 726.3–1881.5) than that measured in infection-free individuals (median value 538.2 AU/mL, IQR 312.1–809; p < 0.001). No differences were noticed in subjects reporting COVID-19 symptoms (46/64, 71.9%; median value 1230.45 AU/mL, IQR 767–1900.9) compared to asymptomatic patients (median value 1308.45 AU/mL, IQR 710.8–1842).
Then, we compared antibody titers between patients receiving the BNT162b2 vaccine (n = 722), and those vaccinated with mRNA-1273 (n = 43). The two populations were similar in terms of age and sex (Table 1 ), but those receiving the mRNA-1273 showed a higher prevalence of patients with previous SARS-CoV-2 infection (n = 42, 5.8% for BNT162b2; n = 21, 48.8% for mRNA-1273). Thus, we stratified patients based on previous infection and interestingly observed that higher levels of antibodies were detected after mRNA-1273 vaccine compared to BNT162b2 type both in infection-naïve cases (BNT162b2, median value 532.55 AU/mL, IQR 310.85–804.55; mRNA-1273, median value 736.95 AU/mL, IQR 484.8–988; p = 0.016), and in those with a history of COVID-19 (BNT162b2, median value 1072.65 AU/mL, IQR 503.1–1606.7; mRNA-1273, median value 1813.4 AU/mL, IQR 865.4–2500; p = 0.019) (Table 1). In the mRNA-1273 cohort a significantly higher antibody titer was observed in case of SARS-CoV-2 infection (median value 1813.4 AU/mL, IQR 865.4–2500) in comparison to infection-free individuals (median value 736.95 AU/mL, IQR 484.8–988; p = 0.002) (Fig. 2 ).
Table 1.
Comparison of clinical parameters and antibody titers between HCW vaccinated with BNT162b2 or mRNA-1273.
| BNT162b2 | mRNA-1273 | p-value* | |
|---|---|---|---|
| No of subjects | 722 | 43 | |
| Age, median (IQR) | 46 (35–55) | 48 (37–54) | 0.671 |
| Sex | |||
| Female, n (%) | 526 (72.9) | 30 (69.8) | 0.659 |
| Male, n (%) | 196 (27.1) | 13 (30.2) | |
| Anti-S IgG titers measured at 1 month after vaccination in absence of COVID-19, median (IQR) | 532.55 (310.85–804.55) AU/mL | 736.95 (484.8–988) AU/mL | 0.016 |
| Anti-S IgG titers measured at 1 month after vaccination in case of prior COVID-19, median (IQR) | 1072.65 (503.1–1606.7) AU/mL | 1813.4 (865.4–2500) AU/mL | 0.019 |
IQR: interquartile range; Anti-S, anti-Spike RBD.
Mann-Whitney test for age and anti-S IgG titers, Chi-squared test for sex.
Fig. 2.
Antibody response to vaccination in subjects with or without previous SARS-CoV-2 infection at 1 month after vaccination. Comparison of the antibody titers specific for SARS-CoV-2 between infection-free HCW (neg; n = 703) and HCW with previous SARS-CoV-2 infection (pos, n = 64) after receiving vaccination. Anti-S, anti-Spike RBD; AU, arbitrary units.
3.2. Detection of SARS-CoV-2 S-RBD IgG 4 months after vaccination
Antibody titer was evaluated a second time, 4 months after vaccination, in a subgroup of 516 HCW belonging to the global case study. Those presenting both evaluations (n = 516) and subjects with a single antibody test (n = 251) were similar for sex distribution, age and IgG concentration measured 1 month after vaccination (see Supplementary Data, Supplementary Table 2). The median time between the first and the second evaluation was 105 days (range 90–175 days), no correlation between the antibody titer and different blood sample timing was observed (Spearman’s rho = 0.04, p = 0.332).
At 4 months after vaccination, 99.6% of the subjects (514/516) showed a reactive antibody response against Spike-RBD. The two non-reactive (one undetermined and one negative) subjects were affected by an autoimmune disease. As for the first evaluation, also the IgG titer measured 4 months after vaccination showed a negative correlation with age (Spearman’s rho = −0.18, p < 0.001).
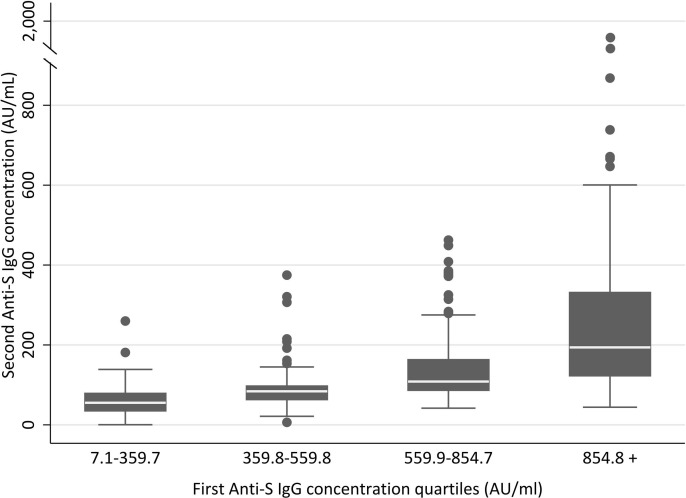
A reduction in the antibody titer was observed for almost all subjects, except for 1 case that showed a positive molecular swab for SARS-CoV-2 between the first and the second IgG evaluation, and did not display antibody decrease (not shown). Considering only subjects with both evaluations, median values were 559.8 AU/mL (IQR 359.7–845.7) for the first assessment vs 92.7 AU/mL (IQR 65.1–148.6) for the second (p < 0.001). Values of the second IgG in each quartile of the first IgG are represented in Fig. 3 .
Fig. 3.
Dynamics of antibody titer reduction at 4 months after vaccination. Distribution of the second antibody titer evaluation based on each quartile of the first antibody level. Anti-S, anti-Spike RBD; AU, arbitrary units.
The antibody decay was evaluated as percentage of decrease from the IgG value measured 1 month after vaccination and the corresponding level detected 3 months later. No association was observed between antibody decay and age (Spearman’s rho = −0.02), sex (women, median value 82.6%, IQR 74.8–86.5; men, median value 81.5%, IQR 73.5–87.6; p = 0.996), while a slightly lower decrease was observed for subjects in the first quartile of levels of IgG measured 1 month after vaccination (Kruskal-Wallis p = 0.004), compared with other quartiles, with median levels ranging from 77.5% for the first quartile (IQR 70.4–85.6) to 83.0% (IQR 75.0–88.3) of the last quartile.
4. Discussion
In the present study, we monitored the amount of Spike-RBD specific IgG antibodies in a cohort of HCW during a quite long follow-up after vaccination with mRNA-based vaccines BNT162b2 or mRNA-1273. As other investigations on Italian cohorts in real-life settings and outside the rules of clinical trials [20], [21], [16], we reported a high efficacy of the vaccination, detecting a positive antibody production in more than 99.5% of cases both 1 month after the second vaccination dose, and also at a further follow-up of 4 months. The only 3 cases with no detectable antibodies or undetermined values referred to vulnerable subjects affected by hematological or autoimmune diseases. Studies evaluating the BNT162b2 vaccine efficacy in vulnerable people reported the development of anti-Spike SARS-CoV-2 antibodies in the 88.9% of hemodialysis patients, the 17.8% of kidney transplant recipients [19], and the 55% of cancer patients [18]. In particular, the lack of seroconversion was associated with age over 65 and treatment by chemotherapy [18], thus supporting the need to monitor the antibody trend especially in these patients after vaccination.
Our results revealed an inverse correlation between age and antibody titer, measured both at 1 and 4 months after the second vaccination dose. The same observation was reported in other Italian cohorts, showing reduced levels of antibodies in subjects aged >65 years compared to younger patients [20], [21], even if this difference was not always maintained overtime [16]. The main difference between these studies and our analysis is the time of observation: we monitored the antibody titer at a longer follow-up, still reporting an inverse correlation between age and the amount of antibodies 4 months after vaccination. A reduced induction of a protective immunity against SARS-CoV-2 after mRNA-based vaccines was noticed in particular in individuals above 70–75 years [22], which are under-represented in our cohort.
No differences were instead revealed for sex, for any class of age, with male and female showing a comparable antibody titer 1 month after vaccination, and similar levels of antibody decay. Literature in this regard is still conflicting, reporting in some cases a higher initial antibody level in women compared to men [23], while others showed no differences due to sex [16].
The clinical parameter that seemed to mainly influence the antibody level in our cohort was the documented previous infection by SARS-CoV-2. People affected by the virus before vaccination showed higher levels of Spike-RBD antibodies compared to infection-naïve individuals 1 month after vaccination. This data was reported also in other cohorts of subjects [21], [16]. We could not evaluate whether this difference was maintained also at a longer follow-up, because of the reduced number of subjects with a previous infection monitored 4 months after vaccination. Other studies reported discordant data in this regard, showing both a decline of the antibody titer more accentuated in subjects with a previous infection, compared to individuals not infected [21], or conversely a lack of drop in antibody at 3 months after the first injection if people were seropositive before vaccination [24]. Interestingly, in our cohort the antibody decay observed between the first and the second analyses was only slightly dependent on the first IgG level, showing comparable percentages of decrease in all the classes of subjects divided by first IgG level. We thus hypothesized that the higher level of antibodies observed 1 month after vaccination in pre-infected individuals should not influence the subsequent decay. However, to definitively confirm this idea we should implement the number of antibody evaluations at 4 months after vaccination and further extend the time of follow-up. We documented only 1 case of SARS-CoV-2 infection between the first and the second antibody evaluation, that was the only subject not showing an antibody decay. Even if preliminary, this observation is consistent with other studies showing an increase in antibody titer months after vaccination in subjects exposed to the virus, thus suggesting a natural boost of the immune response [17].
Intriguingly, subjects previously experiencing asymptomatic versus symptomatic infections did not show a different antibody titer 1 month after vaccination. Consistently, lower IgG levels were observed in absence of symptoms compared to symptomatic cases only during acute and early convalescent phase of natural infection, then smoothing out over a longer time [17].
Interestingly, both by means of previous or absence of COVID-19, mRNA-1273 elicited higher antibody titers compared to BNT162b2 1 month after vaccination in our cohort of vaccinees. As recently reported [25], these data suggested a stronger immunogenicity for the mRNA-1273 formulation that could be probably related to the increased amount of mRNA administered with this vaccine [1], [2]. Unfortunately, we could not perform the same analysis also at 4 months after vaccination due to the paucity of subjects undergoing antibody titration in the group of mRNA-1273 vaccinees.
Our data clearly showed that 4 months after the vaccination completion, the antibody titer experienced a significant decrease compared to the levels detected 3 months earlier. As already mentioned, the antibody decay is usually observed months after any vaccination, however little is still known regarding the antibody level required to ensure immunological protection to SARS-CoV-2 or the time of seroreversion to negative. It seems that the rate of antibody loss after SARS-CoV-2 infection is similar to that of endemic human coronavirus, showing a biphasic decay of neutralizing antibodies with a first rapid decay, mainly due to the decrease in IgA and IgM responses, and then a slower decrease attributed to IgG antibodies [26]. We only evaluated the IgG antibody titer, due to the longer half-life of this class of antibodies, the higher reliability of the detection methods in comparison to the other isotypes, and the strong correlation with the sera neutralizing activity, as demonstrated by Padoan et al [11]. Few studies reported a half-life of IgG neutralizing antibody titers ranging from 100 to 120 days after SARS-CoV-2 infection or vaccination, with a stabilization thereafter, assuming that the residual level of antibodies can function immediately to neutralize incoming virus [27], [26]. Others hypothesized that a level of 20% of the initial antibody titer after infection is associated with 50% protection against asymptomatic or mild infection, and 3% of the initial titer is required for 50% protection against severe infection [28]. Thus, monitoring the antibody titer throughout time with consecutive evaluations could represent a valuable tool to watch over the vaccine efficacy and the maintenance of an immune protection. In particular, the measurement performed 1 month after vaccination could early identify non-responders to the vaccine, while the evaluation of the antibody titer 4–6 months after vaccination could identify those requiring an eventual vaccination boost [16], [29], finally optimizing the vaccination schedule and thus the world-wide diffusion of vaccines.
Our analysis presented some limitations as the lower number of subjects analyzed at 4 months after vaccination compared to the global case study, in particular the absence of subjects vaccinated through the mRNA-1273 vaccine, and the paucity of vulnerable subjects, which may respond with a lesser extent to vaccines. Furthermore, our results were generated through a single antibody detection assay, while previous studies reported different antibody trends in patients with non severe COVID-19 when using multiple detection assays [30]. Moreover, the antibody response provides only a partial view of the immune protection induced by vaccination, since a major role is played by the cellular immune response. Indeed, CD4+ and CD8+ T responses specific for SARS-CoV-2 have been documented after natural SARS-CoV-2 infection and seem to persist longer than neutralizing antibodies [31], [23], [32], [26]. T-cell immunity could contribute particularly in individuals with weak or absent antibody response, as suggested by people affected by agammaglobulinemia or pharmaceutical B cell depletion, that experience uncomplicated COVID-19 after SARS-CoV-2 infection [33], [34], [35], [23]. Since T-cells and antibodies have distinct memory kinetics, it is not possible to predict T cell responses by measuring antibodies [32], thus the quantification of Spike-RBD-specific T cells should be performed together with serological analysis through dedicated assays. We thus plan to perform a serological and cellular combined analysis and extend it to a higher number of individuals including vulnerable subjects, in order to provide a more complete view of the protective coordinated immunity against the virus and thus propose an accurate tool to better define vaccine efficacy and coverage.
CRediT authorship contribution statement
Giulia Brisotto: Conceptualization, Investigation, Writing – original draft. Elena Muraro: Conceptualization, Investigation, Writing – original draft. Marcella Montico: Formal analysis, Visualization. Chiara Corso: Methodology, Validation. Chiara Evangelista: Methodology. Mariateresa Casarotto: Methodology. Cristina Caffau: Resources. Roberto Vettori: Resources. Maria Rita Cozzi: Resources. Stefania Zanussi: Resources, Writing – review & editing. Matteo Turetta: Investigation, Resources, Data curation, Writing – review & editing. Federico Ronchese: Supervision, Project administration, Funding acquisition. Agostino Steffan: Conceptualization, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors thank all the employees of the Centro di Riferimento Oncologico (CRO), Aviano, IRCCS, for their outstanding commitment and devotion during this complicated pandemic phase. The authors are grateful to the staff of the CRO-Biobank for their support in sample collection and management.
Funding
This work was supported by the Medical Direction of the Centro di Riferimento Oncologico (CRO), IRCCS, Aviano Institute.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2021.10.035.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., C4591001 Clinical Trial Group Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. COVE Study Group, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W., Wang Q., Tan L.i., Wu W., Tang S., Xiong Z., Zheng S., McAdam A.J. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58(6) doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Giorgi V., West K.A., Henning A.N., Chen L.N., Holbrook M.R., Gross R., Liang J., Postnikova E., Trenbeath J., Pogue S., Scinto T., Alter H.J., Cantilena C.C. Naturally acquired SARS-CoV-2 immunity persists for up to 11 months following infection. J. Infect. Dis. 2021:jiab295. doi: 10.1093/infdis/jiab295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ju B., Zhang Q.i., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584(7819):115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 7.Wisnewski A.V., Campillo Luna J., Redlich C.A., Ansari A.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PloS One. 2021;16(6):e0249499. doi: 10.1371/journal.pone.0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byazrova M., Yusubalieva G., Spiridonova A., Efimov G., Mazurov D., Baranov K., Baklaushev V., Filatov A. Pattern of circulating SARS-CoV-2-specific antibody-secreting and memory B-cell generation in patients with acute COVID-19. Clin. Transl. Immunol. 2021;10(2) doi: 10.1002/cti2.v10.210.1002/cti2.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porru S., Monaco M.G.L., Carta A., Spiteri G., Parpaiola M., Battaggia A., Galligioni G., Ferrazzi B., Lo Cascio G., Gibellini D., Peretti A., Brutti M., Tardivo S., Ghirlanda G., Verlato G., Gaino S., Peserico D., Bassi A., Lippi G. SARS-CoV-2 Infection in Health Workers: Analysis from Verona SIEROEPID Study during the Pre-Vaccination Era. Int. J. Environ. Res. Public Health. 2021;18:6446. doi: 10.3390/ijerph18126446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claër L., Quentric P., Fadlallah J., Devilliers H., Ghillani P., Gunn C., Hockett R., Mudumba S., Guihot A., Luyt C.-E., Mayaux J., Beurton A., Fourati S., Bruel T., Schwartz O., Lacorte J.-M., Yssel H., Parizot C., Dorgham K., Charneau P., Amoura Z., Gorochov G. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13:eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padoan A., Bonfante F., Cosma C., Di Chiara C., Sciacovelli L., Pagliari M., Bortolami A., Costenaro P., Musso G., Basso D., Giaquinto C., Plebani M. Analytical and clinical performances of a SARS-CoV-2 S-RBD IgG assay: comparison with neutralization titers. Clin. Chem. Lab. Med. 2021;59:1444–1452. doi: 10.1515/cclm-2021-0313. [DOI] [PubMed] [Google Scholar]
- 12.Antia A., Ahmed H., Handel A., Carlson N.E., Amanna I.J., Antia R., Slifka M. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018;16:e2006601. doi: 10.1371/journal.pbio.2006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doria-Rose N., Suthar M.S., Makowski M., O’Connell S., McDermott A.B., Flach B., Ledgerwood J.E., Mascola J.R., Graham B.S., Lin B.C., O’Dell S., Schmidt S.D., Widge A.T., Edara V.-V., Anderson E.J., Lai L., Floyd K., Rouphael N.G., Zarnitsyna V., Roberts P.C., Makhene M., Buchanan W., Luke C.J., Beigel J.H., Jackson L.A., Neuzil K.M., Bennett H., Leav B., Albert J., Kunwar P. mRNA-1273 Study Group, Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N. Engl. J. Med. 2021;384(23):2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terpos E., Trougakos I.P., Karalis V., Ntanasis-Stathopoulos I., Gumeni S., Apostolakou F., Sklirou A.D., Gavriatopoulou M., Skourti S., Kastritis E., Korompoki E., Papassotiriou I., Dimopoulos M.A. Kinetics of Anti-SARS-CoV-2 Antibody Responses 3 Months Post Complete Vaccination with BNT162b2; A Prospective Study in 283 Health Workers. Cells. 2021;10:1942. doi: 10.3390/cells10081942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trougakos I.P., Terpos E., Zirou C., Sklirou A.D., Apostolakou F., Gumeni S., Charitaki I., Papanagnou E.-D., Bagratuni T., Liacos C.-I., Scorilas A., Korompoki E., Papassotiriou I., Kastritis E., Dimopoulos M.A. Comparative kinetics of SARS-CoV-2 anti-spike protein RBD IgGs and neutralizing antibodies in convalescent and naïve recipients of the BNT162b2 mRNA vaccine versus COVID-19 patients. BMC Med. 2021;19:208. doi: 10.1186/s12916-021-02090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padoan A., Dall'Olmo L., Rocca F.D., Barbaro F., Cosma C., Basso D., Cattelan A., Cianci V., Plebani M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta. 2021;519:60–63. doi: 10.1016/j.cca.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorigatti I., Lavezzo E., Manuto L., Ciavarella C., Pacenti M., Boldrin C., Cattai M., Saluzzo F., Franchin E., Del Vecchio C., Caldart F., Castelli G., Nicoletti M., Nieddu E., Salvadoretti E., Labella B., Fava L., Guglielmo S., Fascina M., Grazioli M., Alvisi G., Vanuzzo M.C., Zupo T., Calandrin R., Lisi V., Rossi L., Castagliuolo I., Merigliano S., Unwin H.J.T., Plebani M., Padoan A., Brazzale A.R., Toppo S., Ferguson N.M., Donnelly C.A., Crisanti A. SARS-CoV-2 antibody dynamics and transmission from community-wide serological testing in the Italian municipality of Vo’. Nat. Commun. 2021;12:4383. doi: 10.1038/s41467-021-24622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palich R., Veyri M., Marot S., Vozy A., Gligorov J., Maingon P., Marcelin A.-G., Spano J.-P. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021;32(8):1051–1053. doi: 10.1016/j.annonc.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertrand D., Hamzaoui M., Lemée V., Lamulle J., Hanoy M., Laurent C., Lebourg L., Etienne I., Lemoine M., Le Roy F., Nezam D., Plantier J.-C., Boyer O., Guerrot D., Candon S. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. JASN. 2021;32(9):2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariani M., Acquila M., Tripodi G., Spiazzi R., Castagnola E. Antibodies against Receptor Binding Domain of SARS-CoV-2 spike protein induced by BNT162b2 vaccine: results from a pragmatic, real-life study. J. Infect. Public Health. 2021 doi: 10.1016/j.jiph.2021.06.020. S1876-0341(21)00186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvagno G.L., Henry B.M., Pighi L., De Nitto S., Gianfilippi G.L., Lippi G. Three-month analysis of total humoral response to Pfizer BNT162b2 mRNA COVID-19 vaccination in healthcare workers. J. Infect. 2021;83(2):e4–e5. doi: 10.1016/j.jinf.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wall E.C., Wu M., Harvey R., Kelly G., Warchal S., Sawyer C., Daniels R., Hobson P., Hatipoglu E., Ngai Y., Hussain S., Nicod J., Goldstone R., Ambrose K., Hindmarsh S., Beale R., Riddell A., Gamblin S., Howell M., Kassiotis G., Libri V., Williams B., Swanton C., Gandhi S., Bauer D.LV. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet Lond. Engl. 2021;397(10292):2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., Beale S., Fong W.L.E., Patel P., Kovar J., Hayward A.C., Aldridge R.W. Virus Watch Collaborative, Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet Lond. Engl. 2021;398(10298):385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tré-Hardy M., Cupaiolo R., Wilmet A., Beukinga I., Blairon L. Waning antibodies in SARS-CoV-2 naïve vaccinees: Results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers. J. Infect. 2021;83(3):381–412. doi: 10.1016/j.jinf.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards N.E., Keshavarz B., Workman L.J., Nelson M.R., Platts-Mills T.A.E., Wilson J.M. Comparison of SARS-CoV-2 Antibody Response by Age Among Recipients of the BNT162b2 vs the mRNA-1273 Vaccine. JAMA Netw. Open. 2021;4(9):e2124331. doi: 10.1001/jamanetworkopen.2021.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cromer D., Juno J.A., Khoury D., Reynaldi A., Wheatley A.K., Kent S.J., Davenport M.P. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat. Rev. Immunol. 2021;21(6):395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muecksch F., Wise H., Batchelor B., Squires M., Semple E., Richardson C., McGuire J., Clearly S., Furrie E., Greig N., Hay G., Templeton K., Lorenzi J.C.C., Hatziioannou T., Jenks S., Bieniasz P.D. Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J. Infect. Dis. 2021;223:389–398. doi: 10.1093/infdis/jiaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 29.Lippi G., Henry B.M., Plebani M. Anti-SARS-CoV-2 Antibodies Testing in Recipients of COVID-19 Vaccination: Why, When, and How? Diagn. Basel Switz. 2021;11:941. doi: 10.3390/diagnostics11060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaffner A., Risch L., Weber M., Thiel S., Jüngert K., Pichler M., Wohlwend N., Lung T., Ritzler M., Hillmann D., Copeland S., Renz H., Paprotny M., Risch M. Sustained SARS-CoV-2 nucleocapsid antibody levels in nonsevere COVID-19: a population-based study. Clin. Chem. Lab. Med. 2020;59:e49–e51. doi: 10.1515/cclm-2020-1347. [DOI] [PubMed] [Google Scholar]
- 31.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., Marrama D., de Silva A.M., Frazier A., Carlin A.F., Greenbaum J.A., Peters B., Krammer F., Smith D.M., Crotty S., Sette A. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., Nakao C., Rayaprolu V., Rawlings S.A., Peters B., Krammer F., Simon V., Saphire E.O., Smith D.M., Weiskopf D., Sette A., Crotty S. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fallet B., Kyburz D., Walker U.A. Mild Course of COVID-19 and Spontaneous Virus Clearance in a Patient With Depleted Peripheral Blood B Cells Due to Rituximab Treatment. Arthritis Rheumatol. Hoboken NJ. 2020;72(9):1581–1582. doi: 10.1002/art.41380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wurm H., Attfield K., Iversen A.KN., Gold R., Fugger L., Haghikia A. Recovery from COVID-19 in a B-cell-depleted multiple sclerosis patient. Mult. Scler. Houndmills Basingstoke Engl. 2020;26(10):1261–1264. doi: 10.1177/1352458520943791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon D., Tascilar K., Schmidt K., Manger B., Weckwerth L., Sokolova M., Bucci L., Fagni F., Manger K., Schuch F., Ronneberger M., Hueber A., Steffen U., Mielenz D., Herrmann M., Harrer T., Kleyer A., Krönke G., Schett G. Brief Report: Humoral and cellular immune responses to SARS-CoV-2 infection and vaccination in B cell depleted autoimmune patients. Arthritis Rheumatol. Hoboken NJ. 2021 doi: 10.1002/art.41914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.