Abstract
Background
Attempting to minimize radiation exposure during catheter ablation of atrial fibrillation (AF) for patients, operators and medical staffs should be performed. This study aimed to investigate the feasibility and safety of a metal interference alert guided septal approach using 3 intracardiac echocardiography viewing positions for near-zero fluoroscopy AF ablation procedures.
Methods/results
A total of 668 procedures among 608 consecutive patients with AF (67.2 ± 7.3 years, 408 males) who underwent catheter ablation were retrospectively evaluated and divided into 2 groups, near-zero group (n = 42) and conventional group (n = 595). In the near-zero group, a metal interference alert guided septal approach with 3 different catheter intracardiac echocardiography positions to minimize the fluoroscopy time was applied, and a left atrial access with 2 long sheaths from a single septal puncture without fluoroscopy was successfully achieved in 41 out of 42 cases. The total fluoroscopy time was significantly shorter in the near-zero group than that in the conventional group (0.5 ± 2.0 vs. 21.4 ± 12.9 min p < 0.0001). The total procedure time and time to the septal puncture were both significantly longer in the near-zero group than those in the conventional group (131.4 ± 40.2 vs. 116.6 ± 46.4p = 0.0453, 31.6 ± 9.2 vs. 19.9 ± 10.2 min, p < 0.0001), The ablation time did not differ between the 2 groups (Near-zero: 99.8 ± 41.0 vs. Conventional: 96.8 ± 44.3 min, p = 0.6663). There were no significant differences in the complication rate between the 2 groups (Near-zero: 0 vs. Conventional 14 case, p = 0.6151).
Conclusion
A metal interference alert guided septal approach using 3 intracardiac echocardiography viewing positions was feasible and safe for a near-zero fluoroscopy catheter ablation of AF.
Keywords: Atrial fibrillation, Near-zero fluoroscopy, Catheter ablation, Iintracardiac echocardiography
1. Introduction
Catheter ablation of atrial fibrillation (AF) has been widely performed especially in patients with drug refractory symptomatic AF [1]. Catheter ablation procedures have usually relied on fluoroscopic imaging for the atrial septal puncture and catheter manipulation. Recent advancements in 3-dimensional (3D) mapping systems with integration of fluoroscopy imaging systems can help to reduce the fluoroscopy time as well as radiation exposure time [2]. However, along with the increasing volume of AF ablation procedures, the total radiation dosage has not become neglectable even with a shorter exposure time per session. An increasing radiation exposure time might be associated with the potential risk of cataracts and malignancies, not only for operators but also the medical staffs in electrophysiology labs [3], [4]. Therefore, effort to reduce the radiation exposure should gain attention even in the 3D mapping system era [2], [5], [6].
Several studies on catheter ablation of AF without using fluoroscopy have been reported [7], [8], [9], [10]. Recently, a meta-analysis revealed that a low and zero fluoroscopy catheter ablation of AF had a similar clinical efficacy and safety and shorter procedure time than the conventional contact force approach [11]. Intracardiac echocardiography (ICE), which is commonly used for the atrial septal puncture and construction of the LA geometry with anatomical landmarks, helps reduce the radiation exposure during the ablation procedure. However, for several procedures including the atrial septal puncture, coronary sinus (CS) catheter insertion, and advancement of the ablation catheter from the right atrium (RA) to the left atrium (LA) fluoroscopy is usually used during a conventional ablation. A metal interference alert is displayed in the contact force window on the 3D-mapping system when the catheter touches the guidewire, which might be useful for minimizing the fluoroscopy during the atrial septal puncture. The purpose of this study was to investigate the feasibility and safety of a near-zero AF ablation procedure using 3 positions of the ICE catheter with a metal interference alert guided septal approach.
2. Methods
2.1. Study population
This study included 602 consecutive patients (67.2 ± 7.3 years old, 408 males) with AF who underwent catheter ablation from May 2019 to December 2020 at Nippon Medical School Hospital. There were 668 procedures in total among 602 enrolled patients. Twenty-seven cases underwent coronary angiography during the AF ablation session, and the 4 remaining cases underwent a cava-tricuspid isthmus linear ablation for common atrial flutter prior to the atrial septal puncture. Those 31 procedures were excluded from this study because they affected the procedure time and fluoroscopy time. We divided the remaining cases into 2 groups (Conventional group, 595 cases and Near-zero fluoroscopy group, 42 cases, Supplemental Fig. 1). In the near-zero group, the AF ablation procedure was performed by a metal interference alert guided septal approach with 3 different catheter positions of the ICE. In the conventional group, the insertion of a long sheath and catheter manipulation, CS catheter insertion, and transseptal puncture were performed by fluoroscopic guidance.
The total procedure time was defined as the duration from initiation of the local anesthesia for the vascular access sites to the removal of all sheaths at the end of the session. The total procedure time, time to the atrial septal puncture, total fluoroscopy time, radiation dosage, and procedure related complications were retrospectively analyzed. This study was approved by the institutional ethics committee of the Nippon Medical School Hospital (B-2020–275).
2.2. Vascular access
A 9Fr 25 cm sheath, 8.5Fr SL-10 sheath, and long deflectable sheath (Agilis®, Abbott, Saint Paul, MN, USA) were inserted from the right femoral vein and a 7Fr 11 cm sheath from the right internal jugular vein. An ICE catheter (Soundstar®, Biosense Webster, Diamond Bar, CA) was introduced into the right atrium through a 9Fr 25 cm sheath. The atrial transseptal puncture was performed with a radiofrequency needle (NRG™ RF Transseptal Needle, Baylis Medical, Toronto Canada) and SL-1 sheath, which was replaced by a multielectrode mapping catheter (Pantaray®, Biosense Webster). An ablation catheter (THERMOCOOL SMARTTOUCH®, Biosense Webster) was introduced through the Agilis long sheath. A 6Fr Duo-decapolar catheter (BeeAT®, Japan Lifeline, Tokyo) was advanced into the distal coronary sinus through the right internal jugular vein sheath with the proximal electrodes placed on the lateral wall of the RA.
2.3. SVC view and respiratory adjustment
An ICE catheter was introduced via a 25 cm 9Fr sheath and advanced and positioned in the right atrium where the fossa ovalis was visualized (conventional view). To visualize the SVC (SVC View), the ICE catheter was manipulated with a posterior tilt and rightward deflection from the conventional view (Movie 1, Supplemental Fig. 2). If the SVC was obscure, the ICE catheter was advanced 5 ∼ 10 mm to where the tip of the ICE catheter became in contact with the right atrial (RA) free wall. A respiratory adjustment (ACURESP®) could be obtained in this SVC view. After completing the respiratory adjustment, the contour of the SVC was drawn as a landmark for the catheter manipulation. In addition, the guidewires placed through the other 2 long sheaths from the femoral vein and jugular vein sheath could be identified in the SVC view (Supplemental Fig. 2). The long sheaths placed from the femoral vein could be gently advanced while referring to the SVC view.
2.4. LA geometry and landmark tag
The left atrium (LA) and pulmonary vein (PV) geometry and several landmark tags including of the aorta, coronary sinus (OS) ostium, and tricuspid valve were obtained from the conventional view. The contour of the aorta and tags of the tricuspid valve were displayed in the CARTO image. The ostium of the coronary sinus (CS) was usually first visualized as a quarter moon, which was a useful landmark for the CS catheter insertion. The two tricuspid valve tags were useful for the manipulation of the ICE catheter in order to pass the tricuspid valve (Supplemental Figure 3). The contours of the LA endocardial surface including the LIPV ostium, LA posterior wall, and carina between the RSPV and RIPV were obtained. If the patients underwent a 3D-CT or MRI, a merge was performed.
2.5. SVC, RA, and CS geometry
The ablation catheter was advanced into the SVC and distal CS for a geometry construction. Those procedures allowed the visualization of the CS catheter (BeeAT®) from the SVC to the CS on the CARTO system. The CS catheter was introduced from the right jugular vein and advanced to a distal CS site. The contact force value was useful for the manipulation of the ablation catheter within the CS to prevent a cardiac perforation.
2.6. CS catheter insertion
A duo-decapolar CS catheter was used in this study. The distal 8 electrodes (i.e., the 4 CS pairs of bipolar electrodes) were shown on the CARTO3 image. The CS catheter was introduced into the right ventricle (RV) through the tricuspid valve while referring to the intracardiac electrograms and TA tags. The CS catheter were pulled back with counter-clockwise rotation until the tip of the catheter reached the contour of the CS ostium. After that, the CS catheter was gently advanced to a distal site in the CS (Supplemental Movie 2, Supplemental Figure 3). The CS ostium tag visualized by ICE certainly indicated the correct location and made the insertion of the CS catheter easy and accurate.
2.7. Atrial septal puncture
The SVC view could clearly display the guidewire for the SL-1 long-sheath, and assisted in advancing the long sheath over the guidewire into the SVC. The tip of the long sheath was positioned just above the SVC-RA junction. A saline injection could identify the location of the tip of the SL-1 sheath. The RF needle within the dilator of the long sheath was advanced and inserted into the SL-1 sheath. The SVC view could visualize the tip of the SL-1 sheath within the SVC. The RF needle system with the SL-1 sheath was gently pulled back with a clockwise rotation to the fossa ovalis. After the tip of the RF needle reached the fossa ovalis, the ICE view was changed from the SVC view to the conventional view and the position of the RF needle site within the fossa ovalis was adjusted.
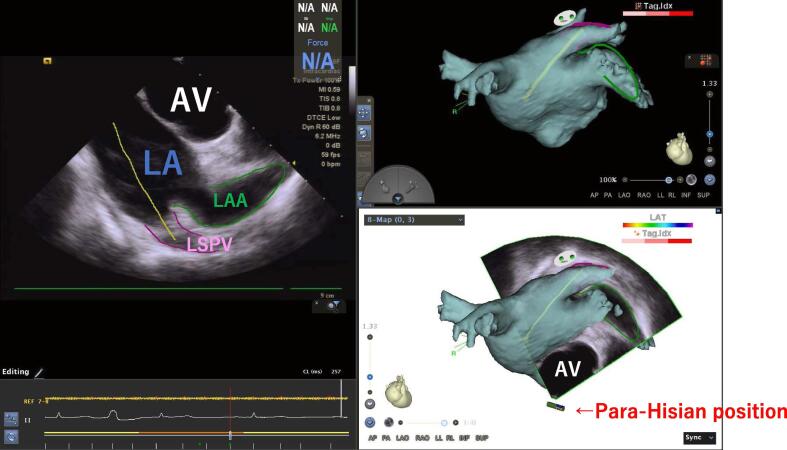
RF current was delivered at a suitable transseptal puncture site on the atrial septum in the conventional view. A saline injection from the RF needle was able to confirm that the tip of the RF needle was in the LA. After the SL-1 sheath was advanced into the LA while referring to the conventional ICE view, a guidewire was advanced through the SL-1 dilator toward the left superior PV (Supplemental Movie 3). However, sometimes the guidewire became lodged and coiled in the left atrial appendage (LAA). It was important to confirm that the guidewire was surely positioned within the LSPV before advancing the long sheath and dilator over the guidewire and dilating the septal puncture site. The para-Hisian view was useful for differentiating whether the position of the guidewire was in the LSPV or LAA (Supplemental Movie 4). The catheter was advanced to the Para-Hisian position by referring to the TA tags. After the tip of the Soundstar ICE catheter passed over the tricuspid valve, the deflection of ICE catheter was released at the Para-Hisian position. The ICE catheter was rotated clockwise until the LA and LAA were visualized. In that view, a transverse section of the aortic valve, visualizing the 3 aortic cusps, was also confirmed (Fig. 1). The Para-Hisian view could differentiate the left PVs from the LAA, which might be difficult to identify by the conventional view. In the Para-Hisian view the ridge between the LAA and left PV was also identified, and the contour of the posterior LA, LSPV, and LAA was created. The tag on the ridge was a useful landmark for the catheter manipulation and determination of the ablation site on the anterior side of the left PVs. The long sheath was advanced and pulled back to dilate the septal puncture site.
Fig. 1.
The para-Hisian view differentiated between an LAA and LSPV anatomical location. The green and purple lines show the LAA and LSPV, respectively. The contour of the guidewire (yellow) indicates the LSPV direction. LA; left atrium, LAA; left atrial appendage, AV; aortic valve, LSPV; left superior pulmonary vein. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.8. Metal interference alert guided approach
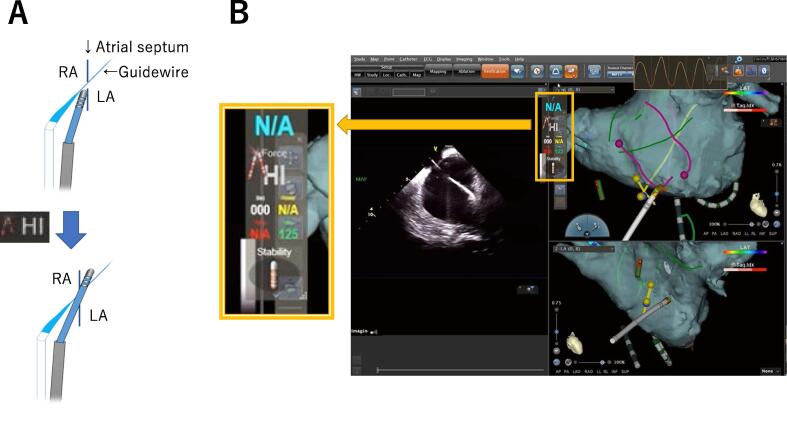
After the atrial septal puncture procedure, the guidewire was left in the LSPV and the SL-1 sheath was pulled back into the RA, and the point of intersection between the guidewire and atrial septum was tagged (Fig. 2). The contour of the atrial septum and guidewire from the RA to the LA was also drawn. A second long sheath (Agilis®) was introduced into the LA with the metal interference alert guided approach using the contact force technology. When the distal tip of the ablation catheter touched the guidewire at the septal puncture site, a metal interference alert (Fig. 2A) with a high contact force value was displayed (Fig. 2B). Advancing the ablation catheter at this time allowed it to pass into the LA through the septal puncture site. When the tip of the catheter pushed on the fossa ovalis at a site other than the septal puncture site, the contact force gradually increased and reached a high contact value alert without displaying a metal interference alert (Supplemental Movie 5). This method was useful for identifying the correct catheter position at the septal puncture site. In addition, the green marker displayed at the tip of the catheter helped manipulate the catheter and easily identify the location of the ablation catheter in the LA.
Fig. 2.
Metal interfere alert with a “Hi” contact force displayed while the guidewire and ablation catheter touch at the septal puncture site. The ICE image confirmed that the ablation catheter had passed the septal puncture site. LA; left atrium, RA; right atrium, ICE; Intracardiac echocardiography.
2.9. Statistical analysis
The data are expressed as the mean ± standard deviation for continuous variables and absolute frequencies and percentages for categorical variables. For the continuous variables and categorical variables, differences between the groups were compared with a Student’s t-test and Fisher’s exact test, respectively. All analyses were performed with GraphPad Prism version 9.0 software (GraphPad Software, Inc., California).
3. Results
3.1. Patient characteristics
The patient characteristics are shown in Table 1. The present study consisted of 602 patients, 408 (67.8%) of whom were male. In total, 41.1% had paroxysmal AF, 31.6% had non-paroxysmal AF and 27.3% had a prior history of an AF ablation.
Table 1.
Patient characteristics.
| Patient Characteristics | ||||
|---|---|---|---|---|
| Age | (y/o) | 67.2 | ± | 13.5 |
| Male | (%) | 67.8 | ||
| Hight | (cm) | 163.7 | ± | 28.0 |
| Weight | (kg) | 66.6 | ± | 13.6 |
| Body mass index | (kg/m2) | 24.6 | ± | 5.0 |
| Left atrial diameter | (mm) | 39.7 | ± | 7.4 |
| LV ejection fraction | (%) | 64.3 | ± | 7.9 |
| Hypertension | (%) | 60.1 | ||
| Diabetes mellitus | (%) | 17.4 | ||
| Heart failure | (%) | 21.4 | ||
| History of a stroke or TIA | (%) | 14.0 | ||
| CHADS2 score | 1.5 | ± | 1.4 | |
| CHA2DS2-VASc score | 2.4 | ± | 1.6 | |
LV; left ventricular, TIA; transient ischemic attack.
3.2. Procedural outcomes
In all 42 cases in the near-zero group, the CS catheter was successfully inserted and placed without using fluoroscopy. Among those 42 cases in near-zero group, 41 (97.6%) a left atrial access was successfully achieved without fluoroscopy by the metal interference alert guided septal approach. In total 28 out of 42 cases (66.7%) achieved a complete zero fluoroscopy ablation. Thirteen cases required a short duration of fluoroscopy. Those cases required fluoroscopy for the advancement of the long sheath after the septal puncture because of an unclear atrial septum using the conventional view.
The remaining one case in the near-zero fluoroscopy group had a thick atrial septum (≈9mm), which made it difficult to cross the atrial septum with the metal interference alert guided method. Even with the catheter probing technique using fluoroscopy it failed to pass the atrial septum. We gave up obtaining LA access for a 2nd sheath, and the ablation procedure was performed with a single long sheath. The total fluoroscopy time was 12.3 min in that case. However, the total fluoroscopy time was significantly shorter in the near-zero group than that in the conventional group (0.5 ± 2.0 vs. 21.4 ± 12.9 min p < 0.0001). The radiation dosage was significantly smaller in the near zero group (2.0 ± 11.2 vs. 32.3 ± 64.0 mGy, p = 0.0023). The total procedure time (131.4 ± 40.2 vs. 116.6 ± 46.4 min, p = 0.0453) and septal puncture time to the left atrial access (31.6 ± 9.2 vs. 19.9 ± 10.2 min vs. p < 0.0001) were significantly longer in the near-zero group. On the other hand, the ablation time did not differ between the 2 groups (near-zero: 99.8 ± 41.0 vs. conventional: 96.8 ± 44.3 min, p = 0.6663). Table 2.
Table 2.
Procedure outcomes.
| Conventional group | Near-zero group | p value | ||||||
|---|---|---|---|---|---|---|---|---|
| Total procedure time | (min) | 116.6 | ± | 46.4 | 131.4 | ± | 40.2 | 0.0453 |
| Septal puncture time | (min) | 19.9 | ± | 10.2 | 31.6 | ± | 9.2 | <0.0001 |
| Ablation time | (min) | 96.8 | ± | 44.3 | 99.8 | ± | 41.0 | 0.6663 |
| Total fluoroscopy time | (min) | 21.4 | ± | 12.9 | 0.5 | ± | 2.0 | <0.0001 |
| Radiation dosage | (mGy) | 32.3 | ± | 64.0 | 2.0 | ± | 11.3 | 0.0023 |
Among 42 patients with near-zero fluoroscopy group, 34 and 3 patients underwent cardiac CT and MRI (Image group), respectively. Remaining 5 patients performed neither CT nor MRI and rule out of the LA thrombus was performed by transesophageal echocardiography (No image group). There were no significant differences in the total procedure time (Image: 123 ± 27 vs. No image: 194 ± 57 min, p = 0.0696), septal puncture time (Image: 16 ± 9 vs. No image: 19 ± 6 min, p = 0. 0810), total fluoroscopy time (Image: 0.5 ± 2.1 vs. No image: 0.2 ± 0.4 min, p = 0.3733) and radiation dosage (Image: 2.3 ± 11.8 vs. No image: 0.1 ± 0.2 mGy, p = 0.2886) regardless of the with or without pre-procedural cardiac imaging.
There were 14 procedural related complications in the conventional group (2.4%), cardiac tamponade in 3, hematomas at the puncture site in 2, atrio-ventricular fistula in 1, transient diaphragmatic paralysis in 3, nasal bleeding in 3, aspiration pneumonia in 1, and a minor cerebral infarction in 1, however, there were no procedural related complications in near-zero group. There were no statistically significant differences in the complication rate between the 2 groups (p = 0.6151).
4. Discussion
The present study showed the feasibility and safety of a near-zero fluoroscopy catheter ablation using a novel metal interference alert guided septal approach with 3 positions for viewing the ICE view. Various techniques and methods for a zero or near-zero catheter ablation of AF have been reported. Near-zero or zero fluoroscopic ablation techniques performed with only 1 long sheath and a single transseptal puncture [12], [13] or 2 long sheaths with a double transseptal puncture have been previously reported [7], [14]. Catheter ablation with only one long sheath requires a catheter exchange of the mapping and ablation catheters each time. The method with 2 long sheaths and a double transseptal puncture requires repeating the placement of the transseptal puncture needle and transseptal puncture procedure. The present technique using the metal interference alert method allowed placing 2 long sheaths with a single transseptal puncture, which enabled an immediate access of the ablation catheter into the LA after a single transseptal puncture procedure, leading to a shorter procedure time without using fluoroscopy.
The time to the septal puncture was longer (≈11 min) in the near-zero group than that in the conventional group. In the near-zero group, it took more time to create the geometry of the RA and CS with the ablation catheter and to manipulate the CS catheter without fluoroscopy. The decision of where the septal puncture site should be also took slightly more time in the near-zero group. That additional time until the transseptal puncture might be improved after an operator learning curve. There were no differences in the procedure time after the atrial septal puncture (i.e., ablation time).
4.1. Benefit of a 25 cm sheath (9Fr) for the ICE catheter
There were several reasons for the benefit of using a 25 cm sheath for the ICE catheter. First, there are no major branches from the inferior vena cava at the distal end of the 25 cm sheath. Second, the 25 cm sheath allowed at least a 20 cm free length of the catheter from the distal end of the sheath, resulting in a lesser contact force on the cardiac wall or structures within the RA and RV during the catheter manipulation. The 25 cm sheath with the ICE catheter allowed us a flexible, prompt, and accurate catheter manipulation and a movement to the 3 catheter positions for the ICE (i.e. conventional, SVC, and Para-Hisian view) during the AF ablation procedure.
4.2. CS catheter insertion
We used CS catheter with a defibrillation function which usually inserted from right jugular vein. Therefore, right jugular vein was chosen for the blood access. Although, the jugular vein approach has a potential risk of pneumothorax and neck hematoma, we carefully performed puncture and there were no procedural complications associated with blood access from right jugular vein. In addition, it has been reported that operator radiation exposure for insertion of CS catheter was significantly increased in jugular vein approach as compared with femoral vein approach [15]. The CS catheter insertion is sometimes difficult that might increase the radiation exposure. One reason is that the ostium of the CS is invisible on fluoroscopy. In the present study, a conventional CS catheter could be visualized on the 3D mapping system and the CS ostium and tricuspid valve landmarks could also be identified. Therefore, an accurate catheter manipulation and catheter insertion into the CS could be performed. Indeed, in all of the cases in the near-zero fluoroscopy group, the CS catheter could successfully be inserted into the CS without fluoroscopy.
4.3. Advantages of the Para-Hisian view
After the septal puncture procedure, the guidewire was advanced into the left superior PV to dilate the septal puncture site by advancing the long sheath. Fluoroscopy easily identified the location of the guidewire and whether it was in the left superior PV or LA appendage. However, with the conventional ICE view, it is sometimes difficult to identify whichever the guidewire is positioned in the anterior (LAA) or posterior (left PV) direction. The advantage of the Para-Hisian view was that it could visualize the ridge and differentiate the left PVs from LAA. The tag on the ridge between LAA and left PVs was useful for the catheter manipulation during the catheter ablation of the left PVs.
In addition, after the 2 long sheaths were placed in the LA, the real time LV wall motion was visualized in the Para-Hisian view, which could help identify even a small amount of a pericardial effusion in the case of a cardiac perforation. Cardiac tamponades occur at a rate of 0.45% even in high-volume centers with experienced physicians [16]. The real time Para-Hisian view enabled us to continuously monitor the ventricular wall motion and make an early detection of pericardial effusion prior to the development of cardiac tamponade during the procedure.
4.4. Metal interference alert methods
The contact force technology has increased the efficacy and safety of catheter ablation [17]. When 2 long sheaths are inserted into the LA with a single transseptal puncture, a second long sheath is usually advanced into the LA by a fluoroscopic guided catheter probing technique or guidewire probing technique. However, it takes more time to navigate the ablation catheter and manipulate the long sheath with fluoroscopy. In addition, it has been reported that the catheter probing technique has a risk of an atrial septal dissection/perforation leading to cardiac tamponade [18].
A metal interference alert is displayed in the case of a catheter-catheter interaction or catheter-guide wire interaction. Therefore, in the present method, displaying the metal interference alert ensured that the ablation catheter touched the guidewire implying there was no cardiac perforation risk. Therefore, a “Hi” contact force with a metal interference alert (Fig. 2) indicated that the ablation catheter was touching the atrial septum and guidewire, indicating a correct position at the transseptal puncture site. The ablation catheter can be advanced toward the LA at that time. A “Hi” contact force without a metal interference alert indicated that the position of the ablation catheter was located in an incorrect position on the atrial septum. In that case, the ablation catheter should never be pushed to obtain LA access.
4.5. Pre-procedural cardiac imaging
Cardiac imaging prior to the ablation procedure was performed not only for the rule out a left atrial thrombus but also obtaining anatomy of the atrium and PVs which was important information for the safety and accurate procedure. Total procedure time and septal puncture time tended to shorter in the patients with obtaining pre-procedure cardiac imaging than those without, although there was not statistically significant. The anatomical information by pre-procedure cardiac imaging might be beneficial for the procedure of the near-zero fluoroscopy.
5. Limitations
This study was a retrospective analysis, and there was a variety of the patient background characteristics including 1st session vs. re-session, paroxysmal AF vs. non-paroxysmal AF, and several ablation strategies depending on the operator, which might have affected the total procedure time. The main scope of the present study was to investigate the feasibility and safety of a near zero fluoroscopy catheter ablation with 2 long sheaths in the LA through one transseptal puncture by a metal interference alert method and 3 ICE views. In the present study, we showed only procedural feasibility and safety of the near-zero fluoroscopy method. Moreover, near-zero fluoroscopy group consisted of only 42 cases which was relatively small as compared with conventional group. Our institute plays a role in teaching and training hospital for medical students and residents. It is important to show the anatomical information and catheter position and manipulation by fluoroscopy for education. Therefore, we performed catheter ablation of AF with conventional method by using fluoroscopy in majority of the cases. Further prospective randomized study will be needed to clarify the efficacy of the short and long-term prognoses of the present methods.
It is important to keep in mind the reduction of the fluoroscopy time for patients and the medical staffs in the EP lab during ablation procedures [18]. In addition to the several previously reported methods and techniques for a zero or near-zero fluoroscopy catheter ablation of AF, the metal interference guided approach with 3 catheter positions for the ICE was useful to reduce the fluoroscopy time resulting in a zero or near-zero fluoroscopy for the catheter ablation of AF. Complete zero fluoroscopy was not the final goal of the catheter ablation. It is important to keep in mind that fluoroscopy should be used if there is no confidence in the guidewire and catheter manipulation for a safer ablation procedure.
6. Conclusion
The metal interference alert guided septal approach with 3 ICE views was useful and safe for achieving a near-zero fluoroscopy AF ablation procedure.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Mr. John Martin for his linguistic assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100896.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomstrom-Lundqvist C. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) European Heart J. 2020 [Google Scholar]
- 2.Heidbuchel H., Wittkampf F.H., Vano E., Ernst S., Schilling R., Picano E. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014;16:946–964. doi: 10.1093/europace/eut409. [DOI] [PubMed] [Google Scholar]
- 3.Picano E., Piccaluga E., Padovani R., Antonio Traino C., Grazia Andreassi M., Guagliumi G. Risks Related To Fluoroscopy Radiation Associated With Electrophysiology Procedures. J. Atrial Fibrillation. 2014;7:1044. doi: 10.4022/jafib.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovoor P., Ricciardello M., Collins L., Uther J.B., Ross D.L. Risk to patients from radiation associated with radiofrequency ablation for supraventricular tachycardia. Circulation. 1998;98:1534–1540. doi: 10.1161/01.cir.98.15.1534. [DOI] [PubMed] [Google Scholar]
- 5.Osorio J., Bubien R.S., Ruff J.D., Brenyo A.J., Rajendra A., Gidney B.A. Single Day Observational Experience at High Volume Ablation Programs: What is the Impact to Practicing Electrophysiologists? Journal of atrial fibrillation. 2018;11:2059. doi: 10.4022/jafib.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canpolat U., Faggioni M., Della Rocca D.G., Chen Q., Ayhan H., Vu A.A. State of Fluoroless Procedures in Cardiac Electrophysiology Practice. J Innov Card Rhythm Manag. 2020;11:4018–4029. doi: 10.19102/icrm.2020.110305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baykaner T., Quadros K.K., Thosani A., Yasmeh B., Mitra R., Liu E. Safety and efficacy of zero fluoroscopy transseptal puncture with different approaches. Pacing and clinical electrophysiology : PACE. 2020;43:12–18. doi: 10.1111/pace.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy V.Y., Morales G., Ahmed H., Neuzil P., Dukkipati S., Kim S. Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart rhythm : the official journal of the Heart Rhythm Society. 2010;7:1644–1653. doi: 10.1016/j.hrthm.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Zei P.C., Quadros K.K., Clopton P., Thosani A., Ferguson J., Brodt C. Safety and Efficacy of Minimal- versus Zero-fluoroscopy Radiofrequency Catheter Ablation for Atrial Fibrillation: A Multicenter, Prospective Study. J. Innov. Card Rhythm Manag. 2020;11:4281–4291. doi: 10.19102/icrm.2020.111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naruse Y., Keçe F., de Riva M., Watanabe M., Wijnmaalen A.P., Dehnavi R.A. Effect of Non-fluoroscopic Catheter Tracking on Radiation Exposure during Pulmonary Vein Isolation: Comparison of Four ablation systems. J. Atrial Fibrillation. 2018;11:2068. doi: 10.4022/jafib.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H.D., Abid Q.U., Ravi V., Sharma P., Larsen T., Krishnan K. Meta-analysis of pulmonary vein isolation ablation for atrial fibrillation conventional vs low- and zero-fluoroscopy approaches. J. Cardiovasc. Electrophysiol. 2020;31:1403–1412. doi: 10.1111/jce.14450. [DOI] [PubMed] [Google Scholar]
- 12.Cha M.J., Lee E., Oh S. Zero-fluoroscopy catheter ablation for atrial fibrillation: a transitional period experience. J Arrhythm. 2020;36:1061–1067. doi: 10.1002/joa3.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang G., Cheng L., Liang Z., Zhang J., Dong R., Hang F. Zero-fluoroscopy transseptal puncture guided by right atrial electroanatomical mapping combined with intracardiac echocardiography: A single-center experience. Clin. Cardiol. 2020;43:1009–1016. doi: 10.1002/clc.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lurie A., Amit G., Divakaramenon S., Acosta J.G., Healey J.S., Wong J.A. Outcomes and Safety of Fluoroless Catheter Ablation for Atrial Fibrillation. CJC Open. 2021;3:303–310. doi: 10.1016/j.cjco.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W., Yao Y., Zhang S., He D.S. Comparison of operator radiation exposure during coronary sinus catheter placement via the femoral or jugular vein approach. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2011;13:539–542. doi: 10.1093/europace/euq515. [DOI] [PubMed] [Google Scholar]
- 16.Michowitz Y., Rahkovich M., Oral H., Zado E.S., Tilz R., John S. Effects of sex on the incidence of cardiac tamponade after catheter ablation of atrial fibrillation: results from a worldwide survey in 34 943 atrial fibrillation ablation procedures. Circulation Arrhythmia Electrophysiology. 2014;7:274–280. doi: 10.1161/CIRCEP.113.000760. [DOI] [PubMed] [Google Scholar]
- 17.Macle L., Frame D., Gache L.M., Monir G., Pollak S.J., Boo L.M. Atrial fibrillation ablation with a spring sensor-irrigated contact force-sensing catheter compared with other ablation catheters: systematic literature review and meta-analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-023775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehrmann H., Schneider J., Jadidi A.S., Park C.I., Schiebeling-Romer J., Allgeier J. Transseptal access for left atrial ablation: the catheter-probing techniques are not without risk. J. Cardiovasc. Electrophysiol. 2014;25:479–484. doi: 10.1111/jce.12356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.