Abstract
Background
The role of SARS-Cov-2-infected persons who develop symptoms after testing (presymptomatics) or not at all (asymptomatics) in the pandemic spread is unknown.
Objectives
To determine infectiousness and probable contribution of asymptomatic persons (at the time of testing) to pandemic SARS-CoV-2 spread.
Data sources
LitCovid, medRxiv, Google Scholar, and WHO Covid-19 databases (to 31 March 2021) and references in included studies.
Study eligibility criteria
Studies with a proven or hypothesized transmission chain based either on serial PCR cycle threshold readings and/or viral culture and/or gene sequencing, with adequate follow-up.
Participants
People exposed to SARS-CoV-2 within 2–14 days to index asymptomatic (at time of observation) infected individuals.
Interventions
Reliability of symptom and signs was assessed within contemporary knowledge; transmission likelihood was assessed using adapted causality criteria.
Methods
Systematic review. We contacted all included studies' corresponding authors requesting further details.
Results
We included 18 studies from a diverse setting with substantial methodological variation (this field lacks standardized methodology). At initial testing, prevalence of asymptomatic cases was 12.5–100%. Of these, 6–100% were later determined to be presymptomatic, this proportion varying according to setting, methods of case ascertainment and population. Nursing/care home facilities reported high rates of presymptomatic: 50–100% (n = 3 studies). Fourteen studies were classified as high risk of, and four studies as at moderate risk of symptom ascertainment bias. High-risk studies may be less likely to distinguish between presymptomatic and asymptomatic cases. Six asymptomatic studies and four presymptomatic studies reported culturing infectious virus; data were too sparse to determine infectiousness duration. Three studies provided evidence of possible and three of probable/likely asymptomatic transmission; five studies provided possible and two probable/likely presymptomatic SARS-CoV-2 transmission.
Conclusion
High-quality studies provide probable evidence of SARS-CoV-2 transmission from presymptomatic and asymptomatic individuals, with highly variable estimated transmission rates.
Keywords: Asymptomatic cases, Levels of evidence, Presymptomatic cases, SARS-CoV-2, Transmission
Introduction
Prevention of transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the subsequent impact on associated illness and deaths are global priorities according to WHO [1]. However, the transmission of the SARS-CoV-2 is not completely understood, nor are the roles played by cases who remain without symptoms throughout their infected period (asymptomatics) or those who go on to develop symptoms at a later date (presymptomatics). The distinction is important as public health and social measures (PHSMs) for restricting transmission are usually aimed at those exhibiting symptoms, whereas the size of the transmission threat posed by asymptomatic persons is not well understood, because data are limited. To date, several reviews have identified significant limitations in methods to ascertain transmission potential, such as reliance on a single binary polymerase chain reaction (PCR) testing [[2], [3], [4], [5], [6]] and lack of standardized methods. Assessment of transmission potential requires clinical, epidemiologic, molecular and laboratory evidence into a framework that identifies higher-quality evidence to reduce the uncertainty over transmission dynamics. A robust framework requires comprehensive and serial screening for symptoms [2,6,7] with high level confirmatory evidence of infection including viral culture or possibly longitudinal serial PCRs, to indicate the presence of replicating and/or infectious virus and/or gene sequencing (GS) with confirmation of identical or similar sequences and a credible epidemiologic link [7].
Objectives
To assess the transmission potential of SARS-CoV-2 from pre and asymptomatic individuals, we set out to address the following questions:
-
1
Are asymptomatic or presymptomatic PCR-positive individuals infectious?
-
2.
If so, what proportion are infectious, and what is the duration of infectiousness?
-
3.
What is the relationship between infectiousness and PCR cycle threshold (Ct)?
-
4.
Is there evidence of a chain of transmission that establishes asymptomatic and/or presymptomatic transmission of SARSCoV-2?
Materials and methods
This review is part of a series of living reviews [[8], [9], [10], [11], [12]] and will be updated as new and important research is published. The review protocol is available at medRxiv [13].
Search strategy
All searches were conducted to 31 March 2021 – for details of the searches please see the supplementary material (Appendix A).
Inclusion criteria
Studies were eligible for inclusion if they reported the following information:
Population
People exposed to SARS-CoV-2 within 2–14 days (incubation time) of close contact or suspected community or institutional exposure to index asymptomatic (at the time of observation) infected individuals, as defined in the study.
Reference
Secondary case infected with a confirmed or probable case definition with transmission outcome confirmed by the results of viral culture or serial qRT-PCR with or without GS [7].
Design
Prospective or retrospective observational studies, including case series and ecological designs, or interventional studies including randomised trials and clinical reports, outbreak reports, case–control studies and experimental studies, studies incorporating models based on observed data. We excluded studies reporting solely predictive modelling as well as single case reports giving no information on secondary cases. Studies not reporting data by symptom status were excluded.
We included all identified studies satisfying our overall inclusion criteria. To assess the chain of transmission (question 4), we included only studies with (a) documentation of the likelihood of transmission; (b) presence of infectious virus from viral culture (defined as encompassing any of several methods whereby one can detect exponential virus growth in cell culture in combination with a method that can uniquely identify the replicating agent as being SARS-CoV-2) and/or documentation of phylogenetics (i.e., genetic sequence lineage); and/or (c) adequate follow-up and reporting of symptoms and signs [14,15] (please see supplementary material, Appendix B for explanations). One review author wrote to the corresponding author of included studies to request further details. We did not assess the possible mode(s) of transmission.
Quality assessment
We are unaware of quality assessment and reporting criteria for transmission studies. Previous studies have used adapted observational checklists [6], but we required robust indicators of the presence of replicating and/or infectious virus to estimate the proportion of virus shedding in quantifiable terms and verification of the circumstances and relationship between exposure and outcome (chain of transmission). We therefore developed methods. As we were looking at the possible transition between lack of symptoms and their onset, assessment of the precise definition of symptoms and signs (set in the knowledge at the time each study was carried out), and whether the follow-up was adequate were considered essential for determining the SARS-CoV-2 transmission from pre and asymptomatically infected individuals. Incorporating the latter two criteria in the methods was important to minimize bias by applying objective defined symptom criteria, a defined time period of symptom assessment both before and after the testing period as well as the method of ascertainment.
To assess the quality of the methods for symptom ascertainment, one reviewer (E.S.) extracted the information on the criteria to define those classified as symptomatic, asymptomatic and presymptomatic. We also included the authors' responses to requests for additional information in our assessment of bias. One reviewer (C.J.H.) categorized the potential for bias as high, moderate, or low which was independently checked by a second reviewer (T.J.). Reasons for the bias assessment for each study were also recorded. Disagreements were resolved through discussion with the help of a third reviewer (J.C.).
Data extraction
Search yields were screened in duplicate and data from included studies were extracted into templates including study characteristics, methodological aspects of studies and a summary of the main findings. Two reviewers also extracted data on the inclusion criteria for the review and data for the transmission analysis. We followed PRISMA reporting guidelines as indicated for systematic or scoping reviews where applicable [16]. Data extraction was performed by one reviewer (E.S.) and independently checked by a second reviewer (T.J.). In cases of disagreement, a third reviewer (C.J.H.) arbitrated.
Data synthesis and reporting
We summarized data narratively and reported the outcomes as stated in the paper. To answer our first and second questions, we tabulated the results of viral culture from those studies attempting it and expressed the positive results as a percentage of the studies' population by setting (Table 1 ). To answer the third question, we assessed the relationship between Cts and viral culture results.
Table 1.
Numbers of asymptomatic and presymptomatic cases at the time of testing and infectious statusa
| Study | Asymptomatic at the time of testing, n/N (%) | Asymptomatic infectious, n/N (%) | Presymptomatic infectious, n/N (%) | Population/setting |
|---|---|---|---|---|
| Arons | 27/48 (56.3) | 1/3 (33.3) | 17/24 (70.8) | Skilled nursing home facility |
| Borges | 39/48 (81.3) | N/A | N/A | Non-COVID-19 hospital ward |
| Cordery | 3/13 (23.1) | No viral growth | Children and young people in school settings | |
| De Laval | 3/24 (12.5) | N/A | N/A | Military support facility cases and contacts |
| Ferreria | N/A | N/A | N/A | Healthcare workers |
| Gettings | 30/139 (21.6) | N/A | N/A | Students and staff in school |
| Hershow | 18/56 (32.1) | N/A | N/A | Students and staff in elementary schools |
| Jeffery Smith | 16/16 (100) | N/A | N/A | Care home |
| Lewis | 2/12 (16.7) | N/A | No culturable and potentially infectious virus could be isolated | Households |
| Murata | 90/90 (100) | 7/39 (17.9) | Passengers disembarking from the Diamond Princess cruise ship | |
| Pray | 7/39 (17.9) | 6/32 (18.8) | University campus | |
| Soto | 8/30 (26.7) | N/A | N/A | Emergency childcare centre contacts |
| Speake | 22/29 (75.9) | 4/11 (36.4) | N/A | Passengers on a flight |
| Surie | 11/17 (65) | One participant became symptomatic on day 3 after the first possible (RT-PCR positive) test and was culture-positive on the day of the initial test. One severely immunocompromised participant shed replication-competent virus for 19 days from the positive test (17 days from symptom onset)b | Nursing home residents | |
| Taylor | 17/24 (70.8) | 6/7 (85.7) | Army barracks | |
| Van Hensbergen | 3/19 (15.8)c | N/A | N/A | Long-term care facility |
| Wallace | 49/111 (44.1) | 12/52 (23.1) | 2/3 (66.7) | Detainees in a detention centre |
N/A = culture not attempted.
The studies report replicating SARS-CoV-2 from viral culture.
Data in Fig. 1 in the paper identifies the immunocompromised patient as participant Q and the individual who became symptomatic on day 3 as participant K.
Asymptomatic (potentially presymptomatic).
To answer our fourth question, we tabulated our assessment of the probability of transmission for asymptomatic and presymptomatic cases (Table 2 ).
Table 2.
Characteristics of included studies
| Study (the calendar time period of collection) | Study population | Brief description of study | Screening/testing methods for COVID-19 infection | Case definition(s) including cut-off values for PCR or other laboratory tests | Symptom ascertainment, including follow-up ascertainment methods | Evidence of the chain of transmission (probability) | Notes |
|---|---|---|---|---|---|---|---|
| Arons (Feb-March 2020) | Skilled nursing home facility; residents with mean age 78 years, Washington, USA. | Repeated point prevalence survey in a care home. First case was identified then a survey done 10 days later, followed by a second survey 7 days subsequently. | NP and OP swabs taken and subjected to testing for SARS-CoV-2, using rRT-PCR, viral culture and gene sequencing. | Positive testing residents were categorized as symptomatic with typical symptoms (fever, cough, or shortness of breath), symptomatic with only atypical symptoms, presymptomatic or asymptomatic. | Standardized symptom: assessment form completed by nurses for each resident tested, on the survey day. Interview and medical records were used to ascertain symptoms for the previous 14 days. Asymptomatic positive-testing residents were reassessed for symptoms 7 days later. |
Probable/Likely Viral growth was observed for specimens obtained from 17/24 presymptomatic residents, 24 presymptomatic residents had a median rRT-PCR Ct value of 23.1. More than half of residents with positive test results were asymptomatic at the time of testing and most likely contributed to the transmission. Staff and residents were being actively screened for signs and symptoms and either promptly isolated (residents) or excluded from work (staff) if any were present. |
Direct evidence about transmission not presented, indirect evidence reported about spread within the care home. Cognitive impairment was present in 28/48 (58%) patients that were positive for SARS-CoV-2, reducing the reliability of their self-reporting. |
| Borges (Summer 2020) | Non-COVID-19 hospital ward patients and staff, Lisbon and Tagus valley, Portugal. | Investigation of a nosocomial outbreak, with in-depth contact tracing and testing. After cases were identified within the hospital, 348 HCWs and 92 patients were screened. Laboratory tests were performed in 245 individuals | NP and OP swabs were collected from patients and HCWs. Positive SARS-CoV-2 RNA samples were subjected to virus genome sequencing. | Not reported. | Symptoms of fever, cough or shortness of breath, were recorded at the time of testing. | N/A | The transmission was not tracked according to symptomatology. Study participants were not subjected to serial PCR testing and culture was not attempted. Asymptomatic is not clearly defined, and only referred to as at the time of testing, so may not be persistently asymptomatic. |
| Cordery (Oct-Dec 2020) | School staff, pupils and their households, London, UK | Sequential longitudinal sampling of infected children, their contacts, and surfaces at school and home. Nose and throat swabs were taken, faecal samples were collected where possible. Samples with Ct value < 30 were inoculated into Vero cells for culture. | Case follow-up: separate nose, throat, and hand swabs, saliva samples and gingival crevicular fluid swabs were obtained from each case up to 5 times within 14 days, then weekly over a second period of up to 14 days; also, faecal samples collected where available. Close contact follow-up: combined nose–throat swabs and gingival crevicular fluid swabs were obtained from each participating contact on the same day or as soon as possible (<48 hr) after case sampling, then weekly for up to 28 days. Samples with high viral load (Ct value < 30) were inoculated into Vero cells for culture. | Combined nose–throat swab testing positive by RT-PCR. | Symptom description and contact history of cases were collected by questionnaire, completed by parent or guardian. |
Probable/Likely Three asymptomatic cases were detected in week 2 of screening. No evidence of wider transmission among children remaining in school, except the 1 unexpected cluster of 3 asymptomatic cases in 1 school in the same class. In 1 of the asymptomatic cases, the viral load rose on repeat testing to >4 million copies per swab and another asymptomatic household member was identified as infected (case A Cts: 26.3, 22.3,28.2). The case remained asymptomatic however despite viral shedding continuing for at least a week after an initial positive test. |
Environmental sampling was also done in homes and schools. |
| De Laval (Feb–March 2020) | Military support facility staff, France | Outbreak investigation using a testing strategy according to pre-test probability, after identification of a severely ill index case. Case finding and contact tracing with testing of at-risk contact persons who had any relevant symptoms. Only symptomatics were tested. | NP and OP swabs were taken. Samples positive by PCR were gene sequenced. One month subsequently, serology was done on all staff, which was potentially able to indicate past infections that ppts did not report symptoms for (i.e., asymptomatic). | Confirmed cases were participants with positive RT-PCR test results and/or positive serology | The interviewer-administered a standardized questionnaire using an in-depth interview to ascertain symptoms and date of onset, also information about contacts in the 14 days prior to symptom onset. |
Possible Three cases were asymptomatic. Contact tracing results did not identify any transmission from asymptomatic to symptomatic cases in this cluster. |
Only symptomatic individuals had swabs collected. Serology done 1 month later was able to identify participants who remained asymptomatic. |
| Ferreria (April–May 2020) | HCWs, a large hospital, Toronto, Canada | Over a 6-week period, HCWs were prospectively enrolled and underwent 1 to 6 serial NP swabs for SARS-CoV-2 PCR testing; study participants were required to be asymptomatic and not have a previous diagnosis of COVID-19. | Serial NP swabs were taken and subjected to PCR. Serological testing for IgG was performed on a subset of asymptomatic HCWs with no prior known exposure to SARS-CoV-2. Genome sequencing was performed on positive swab specimens. |
PCR Ct count cut off not reported. For serology, ratio of ppt sample: calibration interpreted as: <0.8 negative; 0.8 to <1.0 borderline; and 1.1 IgG positive. |
Symptoms compatible with COVID-19 included fever, headache, new or worsening cough, shortness of breath, sore throat, rhinorrhoea, diarrhoea, anosmia, myalgias and conjunctivitis. | N/A | The main aim of the study was to assess the prevalence of asymptomatic, positive-testing HCWs. |
| Francis (until November 2020) | Patients attending hospital, and associated staff contacts, Nottingham, UK | Hospital patients were screened on admission irrespective of symptomatology; hospital staff were tested if symptomatic or a local outbreak occurred. | NP swabs were subjected to RT-PCR; positive tests from samples with some epidemiological evidence of linkage were subject to genome sequencing; also surveillance of sequences was done using samples with PCR Ct < 30. | Not reported. | No methods for symptom assessment were reported. | N/A | The study was set up to examine how whole genome sequencing can help identify and control outbreaks. Clusters of infections are reported, with evidence on epidemiology, PCR, and genome sequencing. Transmission from specific individuals not reported; transmission from individuals established to be asymptomatic therefore not reported. |
| Gettings (Dec 2020–Jan 2021) | Students and staff in schools, Georgia, USA | Index cases and their close contacts in schools were identified by the school and public health staff. Epidemiology and WGS were used to identify transmission patterns. | In-school contacts: symptoms assessed, RT-PCR test on anterior nasal swab offered. WGS was done on PCR- positive samples with a Ct of <32 cycles. | Case is defined as a student or staff member who attended school in person within ≤2 days before testing PCR or antigen test positive. | Symptoms at the time of testing were recorded, and for 14 days, daily through daily text-message based symptom monitoring. | N/A | The study aimed to assess the extent and settings of transmission in and related to schools. |
| Hershow (Dec 2020–Jan 2021) | Students and staff in elementary schools reopening after pandemic related closure, Utah, USA. | The screening was offered to close contacts of identified index cases. Samples were collected 5–10 days after exposure. | Saliva samples (or nasal samples, if saliva is not available) are subjected to RT-PCR. WGS was performed on positive samples. | Index case defined as a student or staff member with laboratory-confirmed SARS-CoV-2 infection who had attended in-person school while infectious for at least 1 day. |
Symptoms and exposures information were collected by questionnaire. |
Possible Low transmission in schools despite substantial community transmission. Among the five persons with school-associated cases, 3 persons were asymptomatic and 3 were exposed to asymptomatic index patients; 4 cases were attributed to student-to-student transmission, and 1 was to student-to-teacher transmission. |
Community transmission was relatively high at the time. In-school mask use and 3ft/2 m distancing were in place. Reports low transmission in schools despite substantial community transmission. |
| Jeffery Smith (April 2020) | Care homes, London, UK | Study designed to look at asymptomatic transmission, using serology and comparing findings from 7 care homes without outbreaks (single case or no cases) to 6 care homes with recognised outbreaks (2 cases or more). | 7 non-outbreak homes investigated with nasal swabbing for SARS-CoV-2 RT-PCR and serology for SARS-CoV-2 antibodies five weeks later. WGS was performed on RT-PCR positive samples. | Case definition not reported. | Staff self-reported symptom status during preceding 14 days and at the time of swabbing; residents' symptoms were recorded by staff. Daily monitoring of each care home by study staff to identify any newly symptomatic individuals. Typical COVID-19 symptoms classed as fever 37.8°C, shortness of breath/cough; atypical symptoms included (but not restricted to) new confusion, reduced alertness, fatigue, lethargy, reduced mobility and diarrhoea. |
Possible The finding of asymptomatic SARS-CoV-2 infection in care homes that did not report a single case of COVID-19 and genomic evidence of a small cluster of staff and residents infected with the same SARS-CoV-2 lineage in care home F. It was not possible to extract direct information on transmission from identified asymptomatic or pre-symptomatic index cases identified to contacts. |
This study aimed to investigate asymptomatic transmission; it is phase 3 of a series of investigations in care homes. |
| Lewis (April 2020) | Households, Utah, USA | Within-household transmission study following identification of an index case within each household. | NP swabs were taken daily, blood samples at day 0 and day 14. Swabs tested by RT-PCR. All positive or inconclusive according to PCR were subjected to viral culture. | Symptoms classified as classic (cough, shortness of breath, or discomfort while breathing), non-classic (>2 of measured or subjective fever, chills, headache, myalgia, sore throat, loss of taste, or loss of smell), and asyndromic (symptoms other than classic or non-classic). PCR Ct values were categorized as low (<20), medium (20–30), and high (>30). | Index patients and household members completed a daily symptom diary; |
Unlikely Five households enrolled. Eligibility entailed an identified positive index case resulting from testing due to symptom onset within each household; secondary transmission was observed in 2 households. WGS for the second household (HH 05-00 symptomatic) indicated the likely chain of transmission was from 05-00 and/or 05-03 (symptomatic) who had genetically identical infections and were exposed to the same community contact. WGS indicates that the infections across all 4 household members in HH-2 were essentially genetically identical, suggesting that the index case, 02–00 (symptomatic), transmitted to all remaining household members. |
|
| Murata (Feb 2020) | Passengers disembarking from the Diamond Princess cruise ship, Japan | Observational study of a cohort of asymptomatic passengers and crew members who tested positive for SARS-CoV-2 and their cabin-mates who tested negative and were transferred from the cruise ship to on-shore hospitals in Japan for isolation. | Screening RT-PCR of nasopharyngeal or throat swabs. Samples with 2 or more positive PCR test results were subjected to viral culture. | Ct value of 40 is used as a cut-off for positivity. | Asymptomatic status was determined at the time of testing based on the absence of fever (temperature of ≥37.5°C) and clinical symptoms (cough, dyspnoea, chest pain, sore throat, and nasal discharge) by physicians and nurses | N/A | This study was designed to examine the shedding of viable viruses from asymptomatic carriers. |
| Pray (Sept–Oct 2020) | University campuses, Wisconsin, USA | Evaluated performance of an antigen (immunoassay) test compared with RT-PCR for SARS-CoV-2 detection among asymptomatic and symptomatic persons. | Nasal swabs were collected from all consenting participants and tested using rapid antigen and RT-PCR. All specimens testing positive were subjected to viral culture. | Case definition cited; laboratory test cut-off values not reported. | Cross-sectional study, no follow-up. | N/A | Study was designed to investigate performance of a rapid antigen test, using RT-PCR as the standard. Onward transmission was not investigated. |
| Soto (April–May 2020) | Emergency childcare centre contacts, Quebec, Canada | Outbreak study in an emergency childcare centre, including 120 children, employees and household contacts of confirmed COVID-19 cases. | NP swabs were subjected to RT-PCR. Nucleic acids were extracted from NP samples and subjected to reverse transcription for phylogenetic analyses. | Algorithm for deciding cases is reported; PCR Ct cut-offs not reported. | Definition of symptoms collected not reported and asymptomatic not defined | N/A | Epidemiology (social network analysis) and phylogeny were used. Unclear but assume screening of all children and staff at the childcare centre; report states that within household contacts, only symptomatics were tested. |
| Speake (March–April 2020) | Passengers on a 5-hr domestic flight, Australia; some passengers had arrived from abroad, including from cruise ships. | To investigate the possible transmission of SARS-CoV-2 on a commercial airline flight, using whole genome sequencing to support evidence on chains of transmission. | PCR testing was applied to throat swabs and bilateral NP or deep nasal swabs from symptomatic individuals. Genome sequencing performed where possible (if multiple samples were available from a participant, samples with lowest Ct values used). Virus culture was attempted for all samples sent to 1 of the 2 laboratories used. | Case definition according to symptomatology and/or a closely matching virus genomic sequence. | Symptoms compatible with COVID-19 led to testing. | N/A | People were only tested for SARS-CoV-2 if they had significant symptoms (testing capacity not sufficient to include people without symptoms). |
| Surie (June–Aug 2020) | Convenience sample of elderly care home residents with underlying health conditions, Arkansas, USA | To estimate the infectious period of SARS-CoV-2 in elderly care home residents with underlying conditions, using symptom recording. 17/39 nursing home residents (all PCR positive, all eventually symptomatic) were followed prospectively to examine viral shedding duration, and viral culture was done to assess infectivity. | OP and anterior nares swabs and saliva samples collected and tested using RT-PCR. All positive samples were subjected to viral culture. Where CPE in viral culture was observed RT-PCR was used to confirm the presence of SARS-CoV-2. Collection of blood (for serology) attempted at enrolment and at visit days 6, 12, 21, and 42. | Not reported. | Symptoms of shortness of breath, cough, malaise, muscle pain, dizziness, diarrhoea, vomiting, sore throat and headache assessed by HCW (before enrolment). At enrolment and each subsequent visit, participants were interviewed using the CDC standard list of symptoms, to which chest and abdominal pain were added. Participants followed for 42 days after enrolment in study. Symptom assessment, medical record review, sample collection done at each study visit. |
Possible Whole-genome sequencing on eligible specimens (Ct < 30) showed there were only 2–3 single nucleotide variant differences among the entire set of sequenced genomes, which implied they were likely from the same source and a single introduction to the nursing home. There were 6 symptomatic participants, only 1 was among the 9 (53%) participants who had replicable, infectious virus isolated. The authors consider that the findings underscore the potential role of pre-symptomatic carriers in transmission. |
Infectivity was defined as isolation of replication-competent virus from a specimen in cell culture |
| Taylor (March–May 2020) | Outbreak investigation at army barracks, London, UK | Study to monitor SARS-CoV-2 infection and antibodies in soldiers, their family and civilians; also to correlate SARS-CoV-2 infection and antibody positivity with clinical symptoms and signs. | Screening of army personnel, their families and civilian contacts was done twice 36 days apart. Nasal swabs, throat swabs and blood samples taken. Respiratory samples subjected to rtRT-PCR. Positive samples with Ct < 35 subjected to WGS and to virus isolation on Vero E6 cells; virus detection confirmed by CPE up to 14 days after inoculation. Serum samples analysed for SARS-CoV-2 antibodies. | Not reported | Participant recalled symptom onset and timing, assessed by questionnaire (including fever, cough, sore throat, runny nose, sneezing, breathless, drowsy, lethargic, seizures, coma, muscle aches, rash, vomiting, diarrhoea, loss of appetite, conjunctivitis, headache, loss of smell, loss of taste, blurred vision, other (state). |
Probable/Likely There were 4 cases that all remained asymptomatic throughout with 0 base difference (genetically indistinguishable), these 4 cases had a link through 1 common workplace location. There were another 4 (different) cases although 2 later developed symptoms also with 0 base difference, other than visiting the same shop and using a common entrance to the barracks no common links in the workplace/barracks setting could be found. |
No onward transmission from asymptomatics documented; samples from asymptomatic individuals were assessed for infectivity via viral culture, but timeline of symptoms to exclude previous or subsequent symptoms is not reported. |
| Van Hensbergen (March 2020) | 99 residents of a long-term care facility, aged 64 to 97 years, The Netherlands. | Cross-sectional outbreak investigation in a care home. | Throat and NP swabs taken for PCR testing. Cycle count numbers determined. WGS performed on samples. Residents with relevant symptoms or recent contact or epidemiological history were tested; additionally, 12 random samples from residents without symptoms were taken. | Clinical case definition reported. | On the day of survey, HCWs performed semi-structured oral interviews of all residents, to collect information on age, sex, new or unusual signs and symptoms of disease, complemented with comorbidity information from their patient records and taking their temperature (rectally) in the morning and the evening (subfebrile: 37.5–38°C; fever: 38.0°C and above). Symptoms: fever, subfebrile temperature, cough, fatigue, malaise, vomiting, loss of appetite, nausea and dizziness | N/A | Some residents had some impaired cognition, which may have prevented full recording of signs and symptoms. |
| Wallace (May–June 2020) | Detainees in a detention centre, Louisiana, USA. | Prospective cohort study using serial testing of detainees initiated 2 to 4 weeks after identification of SARS-CoV-2 infection in staff and detainees. Dormitories had shared toilets and bathroom facilities. | NP swabs were tested by rRT-PCR. Ct < 40 considered positive and those specimens were subjected to culture; also nucleic acid extracted and sequenced. | Ct < 40 considered positive | Symptom questionnaire, self-administered on each survey day. |
Probable/Likely 12/52 asymptomatic had positive viral culture results. A large number of asymptomatic infections, and shedding of replication-competent virus in asymptomatic participants. The phylogeny indicates within-dormitory transmission. Individuals are described as asymptomatic, but they could have been presymptomatic. |
Authors state: “… detained persons might have limited recall of mild symptoms and symptom timing, particularly symptoms occurring >2 weeks before testing, potentially resulting in an overestimation of the prevalence of asymptomatic infection.” |
HCW, healthcare worker; N/A, not applicable/not assessed; NP, nasopharyngeal, OP, oropharyngeal; WGS, whole-genome sequencing.
Due to the diversity of kits, methods, and reagents between different laboratories, we did not aggregate quantitative data (such as viral culture results or Ct values) [17].
We reported subgroups of results by setting and detailed the included papers (e.g., care homes, detention centres, educational settings, hospitals, households and passengers). To assign likelihood of transmission, two reviewers (C.J.H., T.J.) independently used the existing WHO Uppsala Monitoring Centre (UMC) framework standardized case causality assessment [18] and adapted it for SARS-CoV-2 transmission. The causality categories included certain, probable/likely, possible, unlikely or unclear (please see supplementary material, Appendix C for the adapted criteria).
Proof of viral replication does not automatically translate into infectiousness. Probability of infectiousness can only be determined once all transmission evidence is assessed.
Clarification was sought from study authors and where there was disagreement consensus was reached by discussion.
Results
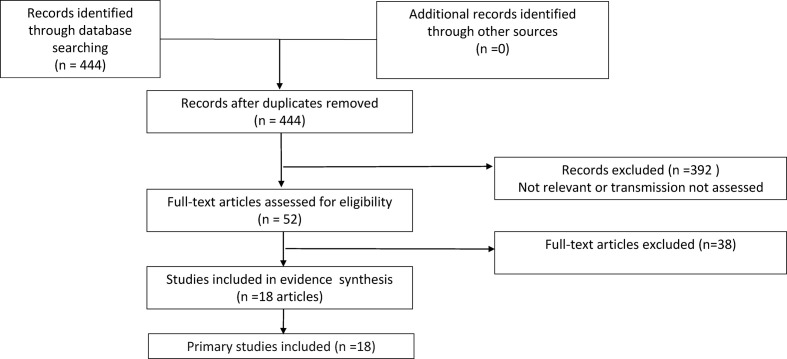
The literature searches identified 444 records for screening for inclusion in this review (Fig. 1 ): 388 studies were excluded after title and abstract screening. A further 39 studies were excluded on full-text analysis (see Fig. 1 for the reasons for exclusion), leaving 18 studies in the review (please see supplementary material, Appendix D). We then wrote to 18 corresponding authors (of 18 papers) and received 17 responses. After receiving the responses, we included 18 studies in the review (Table 2).
Fig. 1.
Flow chart for asymptomatic transmission.
Of the 18 included studies, seven were done in the USA [[19], [20], [21], [22], [23], [24], [25]], seven in Europe [[26], [27], [28]], of which four were in the UK [[29], [30], [31], [32]], two in Canada [33,34], one in Japan (cruise ship) [35] and one in Australia (flight) [36]. Most studies were completed in 2020, with 11 in the first half of the year [19,22,25,27,28,30,31,[33], [34], [35], [36]], and four in the second half [23,24,26,29]; two studies were done between December and January 2021 [20,21].
Four studies were done in long-term care facilities [19,24,28,30], and one among patients in a hospital [26]. Three studies were based in schools with or without associated households [20,21,29]. Other settings included an emergency childcare centre (children and staff) [34], a detention centre [25], passengers on a flight [36], passengers disembarking a cruise ship [35], staff in an army barracks [31], a military facility [27], healthcare workers in a hospital [33], households [22] and a university campus [23]. Studies included varying designs, including cross-sectional, repeat surveys, or symptom-responsive screening designs; and studies addressed varying research questions.
Nasopharyngeal/oropharyngeal/throat samples were collected and tested by RT-PCR in all studies except one [21], a school-based study that used saliva samples tested by RT-PCR [21]. Samples were subjected to viral culture in nine studies [19,[22], [23], [24], [25],29,31,35,36], and GS was applied in ten [20,21,25,26,28,30,31,33,34,36]. Two studies performed serology to assess immunological response [27,33].
Sixteen studies reported carrying out GS for phylogenetic assessment to assess possible alternative sources of infection [[19], [20], [21], [22],[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]].
Quality assessment
Thirteen studies were classified as high risk, and four studies at moderate risk of symptom ascertainment bias. Studies at high and moderate risk of bias lessen the confidence level in distinguishing between presymptomatic and asymptomatic cases.
Studies with a documented index case by serial viral culture, or evidence from prospective Ct/quantification cycle (Cq)/increasing viral load, or a comprehensive epidemiological investigation on transmission coupled with GS, were infrequent [22,25,29,34].
The supplementary material (Appendix E) shows the quality of symptom assessment. While each individual study has strong methodological aspects, the current lack of standardized methodology and clear reporting criteria creates methodological challenges. Examples include differences in measurement thresholds (e.g., temperature above, 37.5, 37.8 or 38°C), symptoms collected, and the mode and timing of data collection (self-report, checklist or structured questionnaire, interview or chart review). Fourteen studies report the criteria for symptomatic status, four [19,25,33,35] for asymptomatic assessment, and four [19,22,25,33] for presymptomatic status.
Timings of the data collection, both before and after a positive test, and the length of follow-up are crucial for determining symptomatic status and to avoid recall bias. In the study by Surie et al. [24] (moderate bias risk), each participant was followed for 42 days from enrolment. At the first PCR-positive test, 11 (65%) participants did not report any symptoms, but all subsequently became symptomatic (one on day 25).
A checklist or symptom assessment form was used by eight studies [19,[21], [22], [23],25,27,29,31], interviews by six [19,24,25,27,28,30], and one study used text message-based symptom monitoring [20].
Infectiousness of asymptomatic and presymptomatic cases (at the time of testing), their proportion and setting
Nine studies [19,[22], [23], [24], [25],29,31,35,36] assessed the infectious status of asymptomatics (n = 7) and presymptomatics (n = 3) [19,24,25]. Six of the asymptomatic studies and all three presymptomatic studies reported on the presence of replicating, infectious virus (Table 1) with varying methodologies and none reported any quantification of the amount of virus.
The overall proportion asymptomatics at the time of testing varied significantly across studies (range 12.5–100%) depending on the setting, the methods of case ascertainment and the population. The proportion of asymptomatics considered infectious varied from 17.9% (cruise ship passengers) to 86% (in army barracks) but the lack of reported quantification makes interpretation difficult. Six studies reported presymptomatic cases (n = 50 participants) [19,22,24,25,30,33]. Three of these were done in nursing/care home facilities and reported high rates of asymptomatic individuals: 50% (8/16) [30], 89% (24/27) [19] and 100% (11/11) [24].
In three studies in the elderly, the authors reported atypical or subtle symptoms, including new confusion, reduced alertness, fatigue, lethargy, reduced mobility and diarrhoea [19,24,30], or difficulty distinguishing acute from chronic symptoms.
Duration of infectiousness in asymptomatics and presymptomatics
Asymptomatic
Murata et al. [35] examined SARS-CoV-2 cell infectivity in samples longitudinally obtained from asymptomatic carriers and infectious virus evidence from seven was reported within 7 days after the initial positive PCR test, except for one person who demonstrated cell infectivity at day 6 and day 15 with negative intervening cultures (Table 2). The specimen at day 15 (Ct 30.3) was from a 70-year-old Japanese female with diabetes mellitus and hypertension who had intermittent RT-PCR positivity >21 days, which raises concerns of the reliability of the culture [35].
Presymptomatic
In the study by Arons et al. [19], 27 residents were classified as asymptomatic (15 reported no symptoms, and 12 stable chronic symptoms). In the 7 days after their initial positive test, 24 asymptomatic residents (89%) had onset of symptoms. The median time to symptom onset was four days (interquartile range 3–5) [19].
Lewis et al. [22] did not observe infectiousness in the two presymptomatic individuals identified. The 33-year-old woman (case 02-01) reported symptoms the day after a positive test, and a 7-year-old girl reported symptoms after 2 days (case 02-03). In the study by Surie et al. [24], 9/17 participants (53% of a convenience sample of 90 infected) had replicating and/or infectious virus isolated. One severely immunocompromised participant shed replicating and/or infectious virus for 19 days from the positive test (17 days from symptom onset) [24]. One became symptomatic on day 3 after a positive RT-PCR test having been culture positive on the day of the initial test. The patient was hospitalized on day 5 and died [24].
Relationship between infectiousness and PCR cycle threshold in asymptomatics and in presymptomatics
Asymptomatics
The median Ct of culture-positive individuals in the study by Murata et al. [35] were significantly associated with the presence of replicating and/or infectious virus Ct 24.6 (IQR 20.4–25.2) vs. culture-negative Ct 35.9 (IQR 33.5–37.1), p < 0.001. In the study by Wallace et al. [25], Cts for symptomatic (median 32.7, range 19.7–36.3) were comparable with asymptomatic, (median 32.9, range 19.8–36.9). The median Ct of culture-positive was 24.4 (IQR 21.5–28.0; range 19.8–33.7) and in the two culture-positive presymptomatics Cts were 20 and 31.1). In the study by Cordery et al. [29], viral loads were reported as low in two-thirds of the cases (given E gene Ct 34.5 and 35.6). In one case, the initial Ct was 26.3 that fell to 22.3 on day 4 (suggesting infectiousness) before increasing to 28.2 by day 8 [29].
Presymptomatic
In the study by Lewis et al. [22], symptom onset in one patient (case 02-01) was associated with progression from a high Ct (>30) to a medium value (Ct 20–30); symptom onset led to progression to a low value (<20) suggesting active viral replication. In the other case (case 02-02, girl aged 7) the Ct remained in the range 20–30. Both cases reported high Cts (>30) on day 14. In the study by Surie et al. [24], infectious virus could not be cultured above a Ct of 29. In the study by Arons et al. [19], the Ct values by symptom status were similar (asymptomatic, 25.5; presymptomatic, 23.1; atypical symptoms, 24.2; typical symptoms, 24.8).
Evidence of transmission from asymptomatic and presymptomatic cases of SARS-CoV-2
Asymptomatics
Nine studies provided insufficient information [20,23,26,28,[32], [33], [34], [35], [36]] and were classified as unclear regarding evidence of transmission.
In three studies the probability of transmission was classified as possible [21,27,30], in three probable/likely [25,29,31]. In Wallace et al. [25], 46 individuals were reported as asymptomatic, but the methodological limitations including self-reporting, recall bias and an incarcerated population raise concerns as to the veracity of the asymptomatic status.
Presymptomatic
The probability of transmission from five studies was classified as possible in two studies [24,30], unlikely in one [22] and probable/likely in two [19,25]. In one study it was unclear [33].
Discussion
Limited reliable epidemiological, clinical and laboratory evidence shows that individuals asymptomatic at the time of testing and presymptomatic cases can shed replicating SARS CoV-2 which will then infect some of the contacts (infectious virus). It was not possible to pool data to estimate the proportion of positive-testing individuals that remained asymptomatic, nor the overall infectious proportion, due to substantial methodological differences and widely discrepant estimates for the proportion of individuals asymptomatic at testing who subsequently developed symptoms (i.e., were truly presymptomatics). Caution should be applied to previously published summary estimates, due to heterogeneity in settings, the methods of case ascertainment (including follow-up), testing and source populations.
Differences in proportion of asymptomatic and presymptomatic cases will occur as a consequence of single point-in-time testing (with no or selective follow-up) and use of different signs/symptoms definitions.
Single or point binary PCR testing (especially with no Ct reported) cannot give information on infectivity, as the work of Murata et al. shows [35]. A follow-up of up to 21 days after the first PCR test of 90 apparently asymptomatic cases from the Diamond Princess with repeated PCR tests, taken in conjunction with the clinical picture and reporting of serial (i.e., on the same subject) Cts, identified 39 as asymptomatic subjects with more than two consecutive or non-consecutive positive PCR test results at the hospital, seven considered to demonstrate cell infectivity.
The serial trend of Ct values, which is linked to the probability of culturing viruses [5,37], is thus predictive of likely individual infectiousness, allowing adequate measures to be taken to interrupt transmission. We do not have sufficient data to explore the likelihood of infectiousness by age and risk group, but the evidence presented in this review shows that a variable but appreciable percentage of asymptomatic subjects develop symptoms, which a single assessment will not identify. The labelling of a subject as ‘asymptomatic’ based on a single observation can be misleading. We cannot be certain of the duration of infectiousness but note that there do not seem to be large differences in median Cts between potentially infectious asymptomatic and presymptomatic subjects, consistent with observations from a Manitoba series [37].
Our decision to rely only on studies reporting genome sequencing and/or viral culture of samples to indicate infectious potential, and epidemiological tracing to identify onward transmission meant that we eschewed quantity for quality and precision, unlike two previous reviews [38,39]. Four variables, namely clinical history, laboratory confirmation, sequencing and epidemiological investigation, narrow transmission uncertainty. Sequencing ascertained phylogenetics and lack of contamination or co-infection, while culture indicated whether infectious transmission potential was present. PCR identified those infectious with SARS-CoV-2 RNA. The included studies represent strong efforts from their authors to address the issue of asymptomatic and presymptomatic transmission. A 94% response rate to reviewers' queries is very unusual in systematic reviews [40]. The willingness shown by the corresponding authors in responding to all our queries and providing extra information should be harnessed to establish an international effort to standardize methods and reporting of viral transmission studies, drawing together the epidemiological, clinical and virological disciplines.
Human challenge studies have major safety and ethical concerns [41,42] and definitive proof is therefore difficult to obtain. Studies included here have provided probable and possible evidence. Two studies [19,25] were assessed as likely to show transmission of infectious virus in different settings (nursing home and detention centre) from both asymptomatic and presymptomatic cases with the limitation of symptom ascertainment bias in these difficult populations.
As with other respiratory viruses, a better understanding of transmission dynamics is essential for pandemic planning. If a substantial proportion of transmission occurs from infectious truly asymptomatic individuals, control measures such as quarantine and contact tracing might have lesser value, especially if the duration of infectiousness is brief, but infectivity high, which can be seen with children and influenza [43].
Policy recommendations
This review includes a limited body of evidence on which to base public health recommendations. High-quality research should be embedded into all public health interventions where substantial uncertainty exists, including the question of whether asymptomatic and presymptomatic individuals are important drivers of onward transmission of SARS-CoV-2, particularly in high-risk settings.
Research recommendations
The studies' design and the lack of a universal methods and reporting standard hinders interpretation. We recommend follow-up studies of at least 28 days' duration during epidemics with consolidated and comprehensive symptoms/signs assessment and repeated serial PCR testing. At least one study per sub-population and setting should be carried out. Graphic presentation of the results could be standardized as per the study by Lewis et al. [22]. All transmission studies should carry out gene sequencing to clarify viral lineage and clarify possible alternative sources of infection [44].
Conclusion
In summary, the results of published studies provide evidence of transmission of SARS-CoV-2 from presymptomatic and asymptomatic individuals. This study does not establish how frequently this is likely to occur, and estimated transmission rates were highly variable. Single point-in-time estimates and binary PCR testing alone cannot provide reliable information on symptom status and information on infectivity. The number of studies and asymptomatic and presymptomatic cases eligible for inclusion was low. More data and standardization of methods is needed to further reduce uncertainty.
Access to data
The data included in the review are from the relevant publications or from correspondence with authors via email. Although supplementary data were included in the review, no permission was sought to make public correspondence with the authors of the 18 included studies. The protocol for this review is accessible: T. Jefferson, A. Plüddemann, E.A. Spencer, J. Brassey, E.C. Rosca, I. Onakpoya, C. Heneghan, D.H. Evans, J. Conly. The evidence on transmission dynamics of COVID-19 from pre- and asymptomatic cases: protocol for a systematic review (Version 2). MedRxiv 2021.05.06.21256615; doi:https://doi.org/10.1101/2021.05.06.21256615.
Author contributions
T.J. and C.H. designed the protocol and wrote the draft manuscript. E.S., T.J., E.C.R. and C.H. extracted and checked screen and data. J.B. carried out searches. All authors contributed to the protocol and manuscript, and approved the final version.
Transparency declaration
Authors' disclosures are in Appendix F except for I.J.O. and E.A.S., who have no interests to disclose. Funding: C.H. has been PI on WHO funded transmission work and received funding from the University of Calgary and funding support from the NIHR SPCR.
Funding
This study is funded by the National Institute for Health Research School for Primary Care Research (NIHR SPCR) Project 569. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Acknowledgements
This review would not have been possible without the input of Drs Vitor Borges, Victoria Chu, Victor Ferreira, Frank De Laval, John Jernigan, Jenna R. Gettings, Mitch Van Hensenberg, Nathaniel Lewis, Shamez Ladhani, Matthew Loose, Suzanne McEvoy, Ian Pray, Hannah Taylor, Aki Sakurai, Shiranee Shriskandan, Julio Soto, Diya Surie and Megan Wallace. We are grateful for their help.
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.10.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
Multimedia component 2
Multimedia component 3
Multimedia component 4
Multimedia component 5
Multimedia component 6
References
- 1.WHO. Operational planning guidance to support country preparedness and response Geneva. World Health Organization; 2020. https://www.who.int/publications/i/item/draft-operational-planning-guidance-for-un-country-teams [cited 2021 8/16]. Available from: [Google Scholar]
- 2.Meyerowitz E.A., Richterman A., Bogoch I.I., Low N., Cevik M. Towards an accurate and systematic characterisation of persistently asymptomatic infection with SARS-CoV-2. Lancet Infect Dis. 2021;21:e163–e169. doi: 10.1016/S1473-3099(20)30837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byambasuren O., Cardona M., Bell K., Clark J., McLaws M.-L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. medRxiv. 2020:2020. doi: 10.3138/jammi-2020-0030. 05.10.20097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SARS-CoV-2, SARS-CoV-1 and MERS-CoV viral load dynamics, duration of viral shedding and infectiousness – a living systematic review and meta-analysis. medRxiv. 2020:2020. doi: 10.1016/S2666-5247(20)30172-5. 07.25.20162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jefferson T., Spencer E.A., Brassey J., Heneghan C. Viral cultures for Coronavirus Disease 2019 infectivity assessment: A systematic review. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buitrago-Garcia D., Egli-Gany D., Counotte M.J., Hossmann S., Imeri H., Ipekci A.M., et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heneghan C., Spencer E., Brassey J., Pluddeman A., Onakpoya I., et al. 2021. A hierarchical framework for assessing transmission causality of respiratory viruses. Preprints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heneghan C., Spencer E., Brassey J., Plüddemann A., Onakpoya I., Evans D., et al. SARS-CoV-2 and the role of airborne transmission: A systematic review [version 1; peer review: 1 approved with reservations, 2 not approved] F1000Research. 2021;10 [Google Scholar]
- 9.Heneghan C., Spencer E., Brassey J., Plüddemann A., Onakpoya I., Evans D., et al. SARS-CoV-2 and the role of orofecal transmission: A systematic review [version 1; peer review: 1 approved with reservations] F1000Research. 2021;10 doi: 10.12688/f1000research.51592.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onakpoya I., Heneghan C., Spencer E., Brassey J., Plüddemann A., Evans D., et al. SARS-CoV-2 and the role of close contact in transmission: A systematic review [version 1; peer review: awaiting peer review] F1000Research. 2021;10 doi: 10.12688/f1000research.52439.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onakpoya I., Heneghan C., Spencer E., Brassey J., Plüddemann A., Evans D., et al. SARS-CoV-2 and the role of fomite transmission: A systematic review [version 3; peer review: 2 approved] F1000Research. 2021;10 doi: 10.12688/f1000research.51590.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plüddemann A., Spencer E.A., Heneghan C.J., Brassey J., Onakpoya I.J., Rosca E.C., et al. SARS-CoV-2 and the role of vertical transmission from infected pregnant women to their fetuses: systematic review. medRxiv. 2021:2021. doi: 10.1101/2021.06.30.21259750. 06.30.21259750. [DOI] [Google Scholar]
- 13.Jefferson T., Plüddemann A., Spencer E., Brassey J., Rosca E., Onakpoya I., et al. The evidence on transmission dynamics of COVID-19 from pre- and asymptomatic cases: protocol for a systematic review (Version 2) medRxiv. 2021:2021. doi: 10.1101/2021.05.06.21256615. 05.06.21256615. [DOI] [Google Scholar]
- 14.Gwaltney J.M., Jr., Hendley J.O. Rhinovirus transmission: one if by air, two if by hand. Am J Epidemiol. 1978;107:357–361. doi: 10.1093/oxfordjournals.aje.a112555. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Genomic sequencing of SARS-CoV-2: a guide to implementation for maximum impact on public health. 2021. https://apps.who.int/iris/bitstream/handle/10665/338480/9789240018440-eng.pdf [cited 2021 8/15]. Licence: CC BY-NC-SA 3.0 IGO]. Available from: [Google Scholar]
- 16.Tricco A.C., Lillie E., Zarin W., O'Brien K.K., Colquhoun H., Levac D., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 17.IDSA, AMP IDSA and AMP joint statement on the use of SARS-CoV-2 PCR cycle threshold (Ct) values for clinical decision-making 2021. https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-amp-statement.pdf [cited 2021 8/16]. Available from:
- 18.WHO-UMC The use of the WHO-Uppsala Monitoring Centre (UMC) system for standardised case causality assessment. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf [cited 2021 8/16]. Available from:
- 19.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gettings J.R., Gold J.A.W., Kimball A., Forsberg K., Scott C., Uehara A., et al. SARS-CoV-2 transmission in a Georgia school district – United States, December 2020-January 2021. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershow R.B., Wu K., Lewis N.M., Milne A.T., Currie D., Smith A.R., et al. Low SARS-CoV-2 transmission in elementary schools – salt lake County, Utah, December 3, 2020-January 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:442–448. doi: 10.15585/mmwr.mm7012e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis N.M., Duca L.M., Marcenac P., Dietrich E.A., Gregory C.J., Fields V.L., et al. Characteristics and timing of initial virus shedding in severe acute respiratory syndrome coronavirus 2, Utah, USA. Emerg Infect Dis. 2021;27:352–359. doi: 10.3201/eid2702.203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pray I.W., Ford L., Cole D., Lee C., Bigouette J.P., Abedi G.R., et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses - Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1642–1647. doi: 10.15585/mmwr.mm695152a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surie D., Huang J.Y., Brown A.C., Gable P., Biedron C., Gilbert S.E., et al. Infectious period of severe acute respiratory syndrome coronavirus 2 in 17 nursing home residents-Arkansas, June-August 2020. Open Forum Infect Dis. 2021;8:ofab048. doi: 10.1093/ofid/ofab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace M., James A.E., Silver R., Koh M., Tobolowsky F.A., Simonson S., et al. Rapid transmission of severe acute respiratory syndrome coronavirus 2 in detention facility, Louisiana, USA, May-June, 2020. Emerg Infect Dis. 2021;27:421–429. doi: 10.3201/eid2702.204158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borges V., Isidro J., Macedo F., Neves J., Silva L., Paiva M., et al. Nosocomial outbreak of SARS-CoV-2 in a “non-COVID-19’ hospital ward: virus genome sequencing as a key tool to understand cryptic transmission. Viruses. 2021;13:604. doi: 10.3390/v13040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Laval F., Grosset-Janin A., Delon F., Allonneau A., Tong C., Letois F., et al. Lessons learned from the investigation of a COVID-19 cluster in Creil, France: effectiveness of targeting symptomatic cases and conducting contact tracing around them. BMC Infect Dis. 2021;21:457. doi: 10.1186/s12879-021-06166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Hensbergen M., den Heijer C.D.J., Wolffs P., Hackert V., Ter Waarbeek H.L.G., Oude Munnink B.B., et al. COVID-19: first long-term care facility outbreak in The Netherlands following cross-border introduction from Germany, March 2020. BMC Infect Dis. 2021;21:418. doi: 10.1186/s12879-021-06093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordery R., Reeves L., Zhou J., Rowan A., Watber P., Rosadas C., et al. Transmission of SARS-CoV-2 by children attending school. Interim report on an observational, longitudinal sampling study of infected children, contacts, and the environment. medRxiv. 2021:2021. 03.08.21252839. [Google Scholar]
- 30.Jeffery-Smith A., Dun-Campbell K., Janarthanan R., Fok J., Crawley-Boevey E., Vusirikala A., et al. Infection and transmission of SARS-CoV-2 in London care homes reporting no cases or outbreaks of COVID-19: prospective observational cohort study, England 2020. Lancet Reg Health Eur. 2021;3:100038. doi: 10.1016/j.lanepe.2021.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor H., Wall W., Ross D., Janarthanan R., Wang L., Aiano F., et al. Cross sectional investigation of a COVID-19 outbreak at a London Army barracks: neutralising antibodies and virus isolation. Lancet Reg Health Eur. 2021;2:100015. doi: 10.1016/j.lanepe.2020.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francis R.V., Billam H., Clarke M., Yates C., Tsoleridis T., Berry L., et al. The impact of real-time whole genome sequencing in controlling healthcare-associated SARS-CoV-2 outbreaks. medRxiv. 2021:2021. doi: 10.1093/infdis/jiab483. 04.15.21253894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira V.H., Chruscinski A., Kulasingam V., Pugh T.J., Dus T., Wouters B., et al. Prospective observational study and serosurvey of SARS-CoV-2 infection in asymptomatic healthcare workers at a Canadian tertiary care center. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soto J.C., Barakat M., Hutter J.A., Kiely M., Moreira S., Shapiro B.J., et al. Outbreak investigation of SARS-CoV-2 transmission in an emergency childcare centre. Can J Public Health. 2021;112:566–575. doi: 10.17269/s41997-021-00544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murata T., Sakurai A., Suzuki M., Komoto S., Ide T., Ishihara T., et al. Shedding of viable virus in asymptomatic SARS-CoV-2 carriers. mSphere. 2021;6(3) doi: 10.1128/mSphere.00019-21. e00019–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speake H., Phillips A., Chong T., Sikazwe C., Levy A., Lang J., et al. Flight-associated transmission of severe acute respiratory syndrome coronavirus 2 corroborated by whole-genome sequencing. Emerg Infect Dis. 2020;26:2872. doi: 10.3201/eid2612.203910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullard J., Funk D., Dust K., Garnett L., Tran K., Bello A., et al. Infectivity of severe acute respiratory syndrome coronavirus 2 in children compared with adults. CMAJ. 2021;193:E601–E606. doi: 10.1503/cmaj.210263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madewell Z.J., Yang Y., Longini I.M., Jr., Halloran M.E., Dean N.E. Household Transmission of SARS-CoV-2: A systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byambasuren O., Cardona M., Bell K., Clark J., McLaws M.L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. JAMMI. 2020;5:223–234. doi: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manca A., Cugusi L., Dvir Z., Deriu F. Non-corresponding authors in the era of meta-analyses. J Clin Epidemiol. 2018;98:159–161. doi: 10.1016/j.jclinepi.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Dawson L., Benbow N., Fletcher F.E., Kassaye S., Killelea A., Latham S.R., et al. Addressing ethical challenges in US-based HIV phylogenetic research. J Infect Dis. 2020;222:1997–2006. doi: 10.1093/infdis/jiaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamrozik E., Selgelid M.J. COVID-19 human challenge studies: ethical issues. Lancet Infect Dis. 2020;20:e198–e203. doi: 10.1016/S1473-3099(20)30438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrozou E., Mermel L.A. Does influenza transmission occur from asymptomatic infection or prior to symptom onset? Public Health Rep. 2009;124:193–196. doi: 10.1177/003335490912400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sintchenko V., Holmes E.C. The role of pathogen genomics in assessing disease transmission. BMJ. 2015;350:h1314. doi: 10.1136/bmj.h1314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Multimedia component 2
Multimedia component 3
Multimedia component 4
Multimedia component 5
Multimedia component 6