Bobillo et al performed a retrospective analysis of early-stage diffuse large B-cell lymphoma (DLBCL) in the rituximab era. Although overall outcomes are excellent, extranodal DLBCL has inferior survival, and patients benefit from radiotherapy in the setting of positive positron emission tomography findings after chemoimmunotherapy.
Key Points
Extranodal stage I DLBCLs have worse outcomes than nodal stage I DLBCLs.
Positron emission tomography at the end of immunochemotherapy may help select extranodal patients for RT consolidation.
Visual Abstract
Abstract
This retrospective study aimed to better define the characteristics and outcomes of extranodal stage I diffuse large B-cell lymphoma (DLBCL) in the rituximab era. Patients diagnosed with stage I DLBCL from 2001 to 2015 treated with rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) or R-CHOP–like regimens with or without radiation (RT) were included. We identified 1955 patients with newly diagnosed DLBCL, of whom 341 had stage I and were eligible for this analysis. Extranodal presentation was observed in 224 (66%) patients, whereas 117 (34%) had nodal involvement. The most common extranodal sites were as follows: bone, 21%; stomach, 19%; testis, 9%; intestine, 8%; breast, 8%. Overall, 69% extranodal patients and 68% nodal patients received RT. Median follow-up was 5.5 years (interquartile range, 4.3-8.2). Ten-year overall survival (OS) and disease-free survival were 77% (95% confidence interval [CI], 67%-83%) and 77% (95% CI, 68%-85%). In the multivariable analyses, extranodal involvement was associated with worse OS (hazard ratio [HR], 3.44; 95% CI, 1.05-11.30) and progression-free survival (PFS; HR, 3.25; 95% CI, 1.08-9.72) compared with nodal involvement. Consolidation RT was associated with better OS (HR, 0.26; 95% CI, 0.12-0.49) and PFS (HR, 0.35; 95% CI, 0.18-0.69) in the extranodal population; however, the benefit was no longer observed in patients that were positron emission tomography (PET) negative at the end of immunochemotherapy. Relapses occurred usually late (median, 37 months), and the most common sites were the lymph nodes (31%) and the central nervous system (27%). Extranodal stage I DLBCL had a worse outcome than nodal stage 1 DLBCL. End of immunochemotherapy PET results may help select extranodal patients for consolidation RT.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of lymphoma, accounting for 30% to 40% of all new diagnoses of non-Hodgkin lymphoma. 1 DLBCL presents as stage I in 15% to 20% of patients and arises in extranodal sites in approximately 50%. 2,3 The characteristics and outcomes of stage I DLBCL have been described in retrospective studies over the past years. 2-6 However, these studies have several limitations: the majority were conducted in the pre-rituximab era, some merged different histologies, and most of them did not use positron emission tomography (PET) to confirm localized disease. 2-6 Furthermore, stage I and II DLBCLs were often analyzed together, and neither the outcome of stage I DLBCL nor the extranodal population was reported separately. 2-7
National Comprehensive Cancer Network (NCCN) guidelines for limited stage DLBCL allows options such as a short or extended course of immunochemotherapy followed or not by radiation therapy (RT). 8-12 In the modern era, 2 randomized trials have explored the best treatment in limited stage DLBCL. 13,14 The 02-03 trial from the Lymphoma Study Association (LYSA) group demonstrated that 4 to 6 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) was similar to 4 to 6 cycles of R-CHOP plus RT in patients who achieved a complete response (CR) assessed by PET after 4 cycles of immunochemotherapy. 13 More recently, the FLYER trial conducted by the German High-Grade Non-Hodgkin Lymphoma Study Group/German Lymphoma Alliance, showed that 4 cycles of R-CHOP plus 2 doses of rituximab was not inferior to 6 cycles of R-CHOP in patients with stage I to II DLBCL. 14 Patients were younger than 60 years and had an international prognostic index (IPI) of 0, and only 40% had extranodal involvement. Whether patients with stage I DLBCL and extranodal disease may benefit from this approach is currently unknown.
Against this background, we performed a retrospective study including patients with stage I DLBCL treated at Memorial Sloan Kettering Cancer Center (MSKCC) in the rituximab era. This study aimed to describe the clinical characteristics and outcomes of patients with stage I DLBCL and to compare the characteristics and outcomes between the nodal and extranodal patients. We also analyzed the outcomes among different treatments and examined the role of radiation therapy.
Patients and methods
Patients
We retrospectively reviewed the records of all newly diagnosed patients with DLBCL at MSKCC from 2001 to 2015 treated with frontline R-CHOP or R-CHOP-like regimens. In all cases, the pathology diagnosis was confirmed by expert hematopathologists at MSKCC according to the World Health Organization classification of hematopoietic and lymphoid tumors. 1 To be eligible for this study, patients were required to have staging with PET/computed tomography (CT) and bone marrow biopsy. Involvement of lymph nodes, spleen, thymus, or Waldeyer ring was considered nodal, and involvement of other organs was considered extranodal. Patients with primary central nervous system lymphoma, primary mediastinal B-cell lymphoma, or transformation of a previous indolent lymphoma were excluded. Bulky disease was defined as any mass exceeding 7 cm. The Hans algorithm 15 was used to classify patients as germinal center B-cell–like phenotype (GCB) or non-germinal center B-cell–like (non-GCB). The stage-modified IPI (SM-IPI) includes 4 risk factors: age greater than 60 years, stage II disease, elevated lactate dehydrogenase, and poor performance status (Eastern Cooperative Oncology Group ≥2). 8 Central nervous system (CNS) prophylaxis was administered by physician preference. This study was approved by the institutional reviewed board at MSKCC and was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
Characteristics were compared using χ2 and Fisher’s exact tests for categorical variables and Student t tests and Wilcoxon rank sum tests for continuous variables. Progression-free survival (PFS) was defined as the time from diagnosis until progression, relapse, or death of any cause; disease-free survival (DFS) was defined as the time from complete response until progression or lymphoma-related death; and overall survival (OS) was defined as the time from diagnosis until death of any cause. Time to second malignancy was calculated from time to initial diagnosis until diagnosis of second cancer. Patients that did not have an event were censored at the end of follow-up.
To compare OS, PFS, and DFS across patient characteristics, we applied inverse probability of treatment weights, derived to balance patient characteristics across treatment groups using a multinomial propensity score analysis via the twang package in R. 16 The basis for the propensity score is a generalized boosted model with an outcome of treatment group, adjusted for age, sex, SM-IPI, bulky, nodal disease, and cell of origin. 17 Weighted log-rank tests were implemented to test for differences in survival across treatment groups and to examine the univariable association between variables and survival. Variables that were significant in the univariable analyses (P < .05) were included in weighted multivariable Cox proportional hazard models. Hazard ratios (HRs) and 95% confidence intervals (CIs) were used to summarize the association between variables and survival. Subanalyses were performed among extranodal patients to examine clinical characteristics associated with survival in this population. Sample sizes and event rates were too small to perform subanalyses among nodal patients. Sensitivity analysis excluding patients with testicular lymphoma was performed to explore survival outcomes.
To evaluate PET status (positive vs negative) at the end of chemotherapy and the role of RT based on PET response on PFS and OS, we performed landmark survival analyses restricted to patients who had a PET/CT performed after chemotherapy and before RT by 6 months after diagnosis. We applied a nonparametric analysis of competing risks and used Gray’s test to compare the cumulative incidence of second neoplasm by nodal status and receipt of RT.
All statistical analyses were performed in SASv9.4 (SAS Institute, Cary, NC) and R version 3.5.3. 18
Results
Patient characteristics
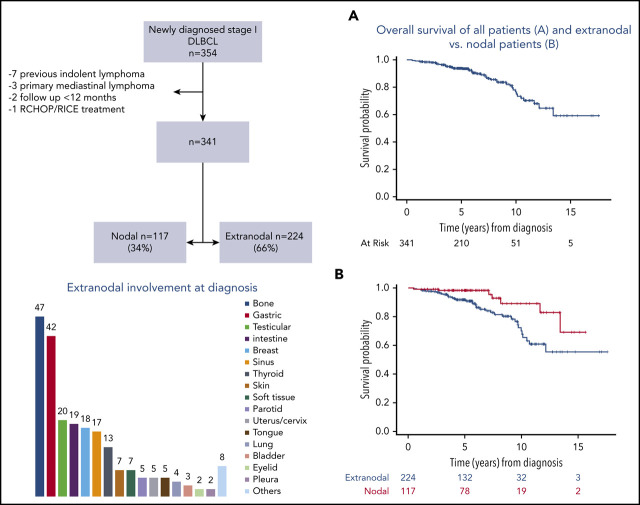
We identified 1955 patients newly diagnosed with DLBCL and treated with frontline R-CHOP–like regimens from 2001 to 2015 at MSKCC. Among them, 354 had stage I DLBCL (Figure 1). We excluded 13 patients for the following reasons: previous indolent lymphoma (n = 7), primary mediastinal B-cell lymphoma (n = 3), follow-up less than 12 months (n = 2), and treatment with R-CHOP-RICE (rituximab, ifosfamide, carboplatin, and etoposide) (n = 1). Three hundred forty-one patients were included in the analyses: 224 (66%) with extranodal stage I DLBCL and 117 (34%) with nodal stage I DLBCL.
Figure 1.
Consort diagram.
Clinical characteristics of extranodal and nodal patients were similar, and they are listed in Table 1. The median age at presentation was 61 years (range, 21-88 years) in extranodal patients and 58 years (range, 18-88 years) in nodal patients. The extranodal sites are detailed in Table 2, and the most common were as follows: bone (n = 47, 21%), stomach (n = 42, 19%), testes (n = 20, 9%), intestine (n = 19, 8%), and breast (n = 18, 8%). Fifty-two (23%) extranodal and 32 (27%) nodal patients had complete surgical removal before treatment. Cell of origin (COO) was determined by immunohistochemistry using the Hans algorithm 15 in 249 patients. GCB was the most common phenotype in extranodal (n = 98, 60%) and nodal (n = 62, 72%) patients. Ki67 staining was determined in 237 patients with a median value of 80% (range, 10%-100%). Six patients were classified as high-grade B-cell lymphoma: 4 extranodal and 2 nodal. Fluorescence in situ hybridization studies for MYC and BCL2 and/or BCL6 rearrangements were performed in 34 patients. Three patients had a double-hit lymphoma: 2 extranodal and 1 nodal. Fourteen patients (6%) in the extranodal group and 15 (13%) in the nodal group presented with a concurrent indolent histology at diagnosis.
Table 1.
Patients characteristics at diagnosis
| Variables | All, N (%) | Extranodal, n (%) | Nodal, n (%) | P * |
|---|---|---|---|---|
| Number, n | 341 | 224 (66) | 117 (34) | |
| Median age (range), y | 60 (18-88) | 61 (21-88) | 58 (18-88) | .27 |
| Male | 169 (50) | 111 (50) | 58 (50) | .95 |
| ECOG | ||||
| 0-1 | 332 (97) | 216 (96) | 116 (99) | .17 |
| ≥2 | 9 (3) | 8 (4) | 1 (1) | |
| Bulky (>7 cm) | 32 (10) | 21 (11) | 11 (9) | |
| Missing | 36 (11) | 35 (16) | 1 (1) | .70 |
| B symptoms | 10 (3) | 5 (2) | 5 (4) | .32 |
| Serum LDH | ||||
| Above normal | 69 (22) | 48 (23) | 21 (20) | .67 |
| Missing | 25 (7) | 13 (6) | 12 (10) | |
| SM-IPI score | ||||
| Missing | 25 (7) | 12 (5) | 13 (11) | † |
| 0 | 127 (37) | 80 (36) | 47 (40) | |
| 1 | 146 (43) | 100 (45) | 46 (39) | |
| 2 | 39 (11) | 29 (13) | 10 (9) | |
| 3 | 4 (1) | 3 (1) | 1(1) | |
| Treatment | ||||
| R-CHOP × 3-4 | 47 (14) | 28 (13) | 19 (16) | <.001 |
| R-CHOP × 3-4 + RT | 172 (50) | 98 (44) | 74 (63) | |
| R-CHOP × 6 | 60 (18) | 41 (18) | 19 (16) | |
| R-CHOP × 6 + RT | 62 (28) | 57 (25) | 5 (4) | |
| Combined modality | ||||
| Yes | 234 (69) | 155 (69) | 79 (68) | .75 |
| No | 107 (31) | 69 (31) | 38 (32) | |
| RT dose, cGy | ||||
| Median | 3060 | 3060 | 3060 | .28 |
| Range | 3000-4500‡ | 3000-4500 | 3000-4500 | |
| Complete excision before treatment | 84 (25) | 52 (23) | 32 (27) | .4 |
| CNS prophylaxis | 48 (14) | 47 (21) | 1 (1) | <.001 |
| Cell of origin | ||||
| Germinal center | 160 (47) | 98 (44) | 62 (53) | .06 |
| Non–germinal center | 89 (26) | 65 (29) | 24 (21) | |
| Missing | 92 (27) | 61 (27) | 31 (26) |
cGy, centigray; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase.
P values calculated excluding missing values.
P value cannot be calculated because of sparse cells.
One patient cannot complete the planned RT and received a total dose of 2700 cGy. One patient with concomitant breast lymphoma and breast cancer received a dose of 6000 cGy.
Table 2.
Distribution of extranodal sites at diagnosis
| Extranodal sites | n (%) |
|---|---|
| Bone | 47 (21) |
| Stomach | 42 (19) |
| Testis | 20 (9) |
| Intestine | 19 (8) |
| Breast | 18 (8) |
| Sinus/nose | 17 (8) |
| Thyroid | 13 (6) |
| Skin | 7 (3) |
| Soft tissue | 7 (3) |
| Parotid | 5 (2) |
| Uterus/cervix/vagina | 5 (2) |
| Tongue | 5 (2) |
| Lung | 4 (2) |
| Bladder | 3 (1) |
| Eyelid | 2 (1) |
| Pleura | 2 (1) |
| Larynx | 1 (0.5) |
| Gallbladder | 1 (0.5) |
| Prostate | 1 (0.5) |
| Pericardium | 1 (0.5) |
| Bucal submucosa | 1 (0.5) |
| Liver | 1 (0.5) |
| Gingiva | 1 (0.5) |
| Lacrimal | 1 (0.5) |
Treatment and response
Patients were treated with 4 different approaches as summarized in Table 1. The most common treatment in both groups was R-CHOP × 3-4 + RT: 98 (44%) extranodal and 74 (63%) nodal. Patients with extranodal involvement were more likely to receive 6 cycles of chemotherapy with or without RT than nodal patients. Overall, 155 (69%) extranodal and 79 (68%) nodal patients received consolidative RT (P = .75). Fifty-three (63%) patients with complete surgical removal and 181 (74%) patients without complete tumor excision received RT consolidation. All patients with testicular lymphoma received R-CHOP plus RT and intrathecal methotrexate. Extranodal patients were more likely to receive CNS prophylaxis than nodal patients. Specific sites and type of CNS prophylaxis are detailed in supplemental Table 1, available on the Blood Web site.
Treatment response was assessed by PET/CT in 316 (93%) patients and by CT or magnetic resonance imaging in the remaining cases. Three hundred thirty-seven (99%) patients achieved a CR, 99% extranodal and 98% nodal, and 3 patients progressed. One patient died of infection during therapy.
Survival outcomes
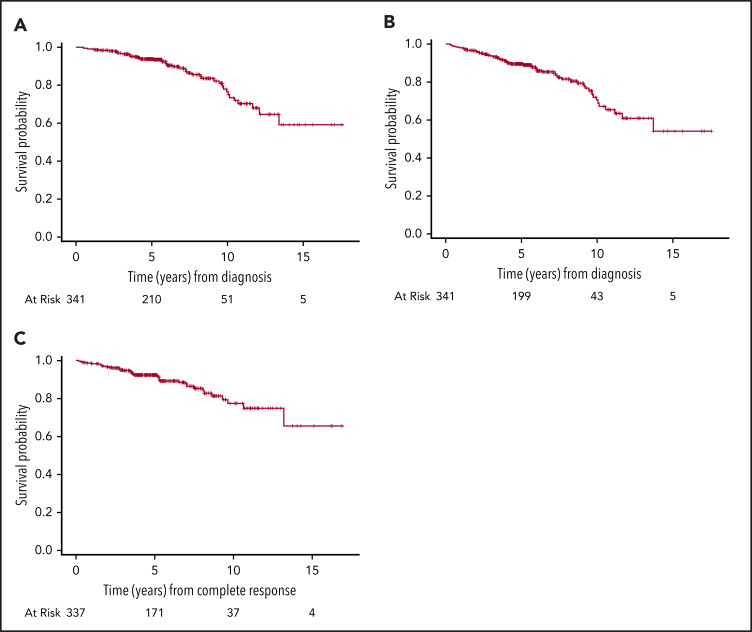
The median follow-up for surviving patients was 5.5 years (interquartile range, 4.3-8.2). Forty-five patients died: 38 extranodal and 7 nodal. The most common cause of death was lymphoma related: extranodal, n = 15; nodal, n = 2. The 5- and 10-year OS of the entire cohort was 94% (95% CI, 91%-96%) and 77% (95% CI, 67%-83%), and the 5- and 10-year DFS were 92% (95% CI, 89%-95%) and 77% (95% CI, 68%-85%), respectively (Figure 2).
Figure 2.
Outcomes of 341 patients with stage I DLBCL. (A) OS. (B) PFS. (C) DFS.
Propensity score analyses.
We compared survival using a multinomial propensity score weighted analyses across treatment groups adjusted for age, sex, SM-IPI, bulky, nodal disease, and COO. All the variables that were significant in the univariable weighted analyses (supplemental Table 2) were included in the weighted multivariable analysis (supplemental Table 3). In the multivariable analyses, we found patients with extranodal disease had an inferior OS (HR, 3.44; 95% CI, 1.05-11.30; P = .04) and PFS (HR, 3.25; 95% CI, 1.08-9.72; P = .04) than patients with nodal involvement, with a 10-year OS and PFS rates of 70% (95% CI, 58-79) and 63% (95% CI, 50-73), respectively, for extranodal patients compared with 89% (95% CI, 74-96) and 85% (95% CI, 70-92) for nodal patients. There were no significant differences in DFS between nodal and extranodal patients (Figure 3). High SM-IPI (2-3 risk factors) was associated with worse OS (HR, 5.68; 95% CI, 2.79-11.59; P < .001), PFS (HR, 3.14; 95% CI, 1.31-7.49; P = .01) and DFS (P = .01 in univariable analyses) compared with low SM-IPI (0-1 risk factors; supplemental Figure 1). There were no significant differences in OS, PFS, or DFS among the 4 treatment regimens. However, patients who received RT had a superior OS (HR, 0.24; 95% CI, 0.12-0.49; P < .001) compared with patients who did not receive RT.
Figure 3.
Outcomes of the whole series according to the site of involvement, nodal or extranodal. (A) OS. (B) PFS. (C) DFS.
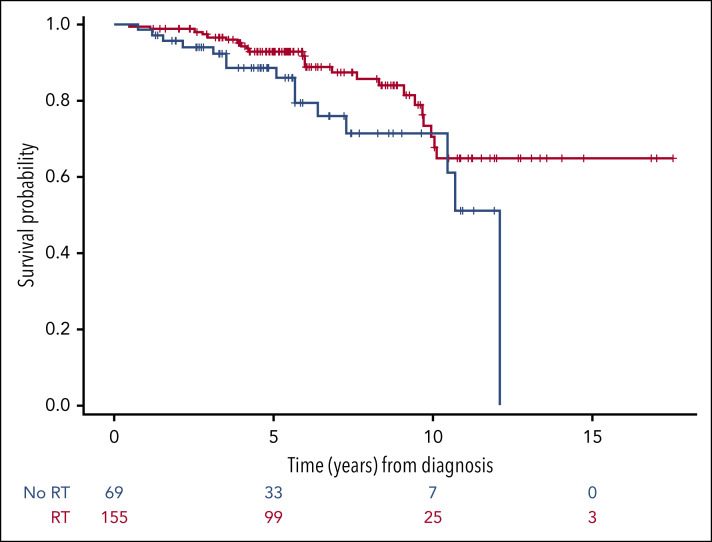
In multivariable analyses performed in the extranodal population (supplemental Table 4), RT was associated with better OS (HR, 0.26; 95% CI, 0.12-0.53; P < .001) and PFS (HR, 0.35; 95% CI, 0.18-0.69; P = .003) compared with no RT (Figure 4). SM-IPI was also an independent prognostic factor for OS and PFS. Among the nodal group, too few events occurred to perform multivariable analyses. In the univariable analyses, bulky disease and high SM-IPI were associated with inferior PFS. No variables were significantly associated with OS.
Figure 4.
OS of the 224 patients with extranodal disease treated with or without RT consolidation. Patients who were treated with combined modality had a better OS compared with patients treated with immunochemotherapy alone (HR, 0.26; 95% CI, 0.12-0.53).
Finally, we performed a survival subanalysis excluding patients with testicular lymphoma (n = 20), who usually present worse outcomes. We observed extranodal patients continued to have an inferior OS and PFS than nodal patients. Similar to the original analysis, extranodal patients who received RT presented better OS and PFS than those who did not (supplemental Table 5).
Landmark analyses based on PET response after chemotherapy.
We performed a landmark analysis of OS restricted to 304 patients who were alive at 6 months after diagnosis (n = 1 excluded) and had a PET/CT at the end of chemotherapy before RT (n = 36 excluded). Thirty-two patients (10.5%) had a PET-positive scan, of which 24 (75%) received RT. Six patients (25%) had a biopsy before RT. The presence of a PET-positive scan was not associated with inferior OS (P = .27) either in the whole cohort or in the extranodal group (P = .94). In the landmark analysis of PFS including 301 patients alive and without disease 6 months after diagnosis, the presence of a PET-positive scan after chemotherapy was not associated with worse PFS in the whole cohort (P = .85) or in the extranodal population (P = .63) (supplemental Figure 2). These findings could be explained by the high number of patients with a PET-positive scan who received RT consolidation (75%). Furthermore, some sites such as bone might show persistent PET activity because of inflammatory changes rather than residual disease, although biopsies were not performed in all cases to confirm this.
We also explored the role of RT in patients with PET-negative scan at the end of chemotherapy (n = 272), and we did not observe a statistically significant difference in OS (P = .11) or PFS (P = .21) by use of RT. Finally, we performed the same analyses in the extranodal population (n = 171), and results were consistent (Figure 5). We sought to evaluate the role of RT and the optimal number of R-CHOP cycles in patients with IPI = 0; however, because of the low number of events, these subanalyses could not be performed.
Figure 5.
Landmark analyses in patients with PET that was negative at the end of immunochemotherapy according to the administration of RT consolidation or not. OS (n = 272) (A) and PFS (n = 272) (B) of the whole cohort; OS (n = 171) (C) and PFS (n = 171) (D) of patients with extranodal involvement.
Patterns of relapse
Seventeen (8%) patients in the extranodal group and 6 (5%) patients in the nodal group relapsed at a median time of 36 (range, 4 months to 13 years) and 37 months (range, 9 months to 10 years), respectively. Relapse according to initial site is presented in supplemental Figure 4. Sixteen (61%) patients relapsed or progressed as localized disease, 5 involving the initial site and 11 involving a distant site. Six patients (26%) relapsed 5 years after treatment completion. Relapses occurred outside of the radiation field in all patients who received RT. PET scan at the end of treatment was positive in 2 of 23 patients. The most common sites of relapse were the lymph nodes (n = 8, 31%) and the CNS (n = 7, 27%). Patients who relapsed in the CNS had initial involvement of testes (n = 2), breast (n = 2), and lymph nodes (n = 3), and 3 of 7 had received CNS prophylaxis. The COO of patients with CNS relapse was GCB (n = 3), non-GCB (n = 2), and missing (n = 2).
Second neoplasms
After a median follow-up of 5.5 years, 28 (8%) patients developed a second solid malignancy: 16 (7%) extranodal and 12 (10%) nodal. Eight (2%) patients developed a second myeloid neoplasm at a median time of 3.9 years. Six of 7 patients with available cytogenetics presented chromosomal aberrations commonly associated with therapy-related myeloid neoplasms including partial loss of 5q, loss of chromosome 7, and inversion 16. There were no statistically significant differences in the cumulative incidence of second malignancies between extranodal and nodal or between patients treated with and without RT (supplemental Figure 3).
Discussion
To the best of our knowledge, this is the largest retrospective study limited to stage I DLBCL in the rituximab era. We confirm stage I DLBCL frequently arises in extranodal organs, and, although outcomes are overall very favorable, the involvement of extranodal sites is associated with worse prognosis. In accordance with previous population-based reports, extranodal DLBCL accounted for 66% of all stage I in our series. 2,3,19 These numbers are higher compared with recent series of limited-stage DLBCL, probably because they also included patients with stage II. The most common sites as expected were bone, stomach, and testes. 2,3,19
The outcome of the whole series was excellent with an OS at 5 years of 94% similar to other rituximab era studies. 13 The findings that extranodal involvement was associated with worse survival are in agreement with previous reports of stage I DLBCL in the pre-rituximab era. 2,3 However, in the modern era, 2 retrospective studies described similar outcomes between nodal and extranodal patients. 6,19 Notably, these studies were limited by a small sample size, the lack of baseline PET in most patients, and the absence of treatment adjustment in a multivariable analyses. One of the limitations of our study is that patients received four different treatment regimens. Moreover, PET response criteria have changed over the last 15 years. To reduce some of this bias, we included only patients staged with PET, and a propensity score analysis was performed to control for potential confounders associated with different treatments, making our results more informative.
SM-IPI was initially introduced by the Southwest Oncology Group to better stratify patients with limited-stage DLBCL in the pre-rituximab era. 8 In the rituximab era, SM-IPI was validated by our group in a retrospective study including stages I and II and has been recently associated with event-free survival (EFS) in the LYSA 0203 trial. 7 We confirmed that SM-IPI is a powerful predictor for survival in stage I DLBCL, although stage II was eliminated as a risk factor, establishing 2 groups, one with 0 to 1 risk factors with an excellent OS at 5 years of 96% (95% CI, 92-98) and other with 2 to 3 risk factors with worse outcomes and 5-year OS of 76% (95% CI, 58-87).
NCCN guidelines for limited-stage DLBCL recommend a short or extended course of immunochemotherapy followed or not by RT based on randomized trials conducted in the pre-rituximab era. 8-12,20 In our series, 4 regimens were administered based on physician’s decision, and survival was similar across the treatment groups. Interestingly, we observed that extranodal patients may benefit from RT consolidation, especially those who did not achieve a PET CR after immunochemotherapy. Recently, the UNFOLDER randomized trial by the German High-Grade Non-Hodgkin Lymphoma Study Group/German Lymphoma Alliance reported longer EFS in extranodal patients who received 6 cycles of R-CHOP-14 or -21 plus RT compared with non-RT (3-year 84% vs 68%, P = .001). However, this difference was because of a higher rate of PR in the non-RT arm that required additional treatment, mostly RT. 21 In the same way, some retrospective and population-based studies yielded similar results showing better OS and PFS in patients with early-stage DLBCL receiving RT consolidation. 22
In the PET/CT era, the role of RT has been questioned in patients with early-stage DLBCL who achieved a PET CR. 13,23 Recently, the randomized 02-03 trial conducted by the LYSA group compared R-CHOP 4 to 6 cycles followed by RT or not in patients with stage I to II nonbulky DLBCL, achieving a PET CR after 4 cycles of R-CHOP. They observed similar outcomes in the 2 groups with an EFS at 5 years of 92% in the RT arm vs 89% in the non-RT arm. 13 However, whether RT could be spared in patients with extranodal involvement remained uncertain because only 39% of patients in this trial had extranodal disease. Moreover, patients with breast, skin, or ovarian involvement were excluded. In our study, radiation consolidation did not result in a better outcome for patients who achieved a PET CR with chemotherapy. Interestingly, the same results were observed in the extranodal group, suggesting that RT could potentially be omitted if PET CR is achieved, with the exception of testicular lymphoma, where contralateral testicular radiation appears to reduce relapse. 24-26
The optimal number of R-CHOP cycles for limited-stage DLBCL has been addressed in the FLYER trial. 14 This study included 592 patients with nonbulky, limited stage that were randomized to receive R-CHOP × 6 or R-CHOP × 4 + 2 doses of rituximab. They showed very good outcomes in the 2 arms with a similar 3-year EFS of 89%. Notably, all patients in this study were younger than 60 years and had very favorable prognostic factors, and again only 32% had extranodal involvement. 14 In a same way, the preliminary results of 2 PET-directed studies 27,28 showed an excellent outcome in patients with limited-stage DLBCL who received 4 cycles of R-CHOP and achieved a PET CR after cycle 3, with no need for RT consolidation. Interestingly, these studies included patients with risk factors, and around 45% had extranodal involvement, although sites were not specified. 27,28 In our series, patients with nodal disease presented very good outcomes, with a 10-year DFS of 89% (95% CI, 75-95), suggesting that a short course of chemotherapy could be a good treatment as proposed in previous studies, especially if PET CR is achieved. The extranodal population, as discussed above, presented worse prognosis; unfortunately, because of the low number of events, we could not performed further subanalyses to compare short vs extended R-CHOP in the extranodal group. Thus, whether a short course of chemotherapy is the most appropriate treatment of patients with extranodal disease should be addressed in prospective trials.
Finally, late relapses in advanced stage DLBCL are uncommon; however, they seem to be more frequent in limited stage. The recent long-term analysis of the SWOG S8736 study reported a pattern of continuous relapse after 5 years. 29 Late relapses continue to occur in the modern era, as observed in our study, with 26% of patients relapsing after 5 years, and other studies. 19,29,30 In contrast, the LYSA 02-03 trial described only 4 patients relapsing beyond 5 years, probably reflecting a more favorable-risk population. 13 We observed 27% of relapses occurred in the CNS, which is similar to the 32% recently reported in stage I DLBCL. 19 Our data show the testes and breast are high-risk sites for CNS recurrence, 24-26 suggesting the need for CNS prophylaxis in patients with breast involvement, which is not routinely performed.
In conclusion, our study confirms the good outcomes of patients with stage I DLBCL in the modern era. We observed that patients with extranodal involvement have an inferior OS and PFS than nodal patients. Patients with extranodal stage I DLBCL may benefit from RT consolidation. Conversely, in extranodal patients achieving a PET-negative CR after immunochemotherapy, RT could potentially be spared. The optimal number of chemotherapy cycles in patients with extranodal disease should be explored in prospective randomized trials, especially when RT is omitted. Finally, the late relapse pattern observed in this population suggests prolonged surveillance is needed.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work was supported in part by research funding from Fundación Alfonso Martín Escudero to S.B. and in part by National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30CA008748.
Footnotes
Presented at the 15th International Conference on Malignant Lymphoma, Lugano, Switzerland, 18-22 June 2019.
Data sharing requests should be sent to Sabela Bobillo (sbobillo@vhio.net).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.B. and A.Y. conceived the project, analyzed the data, and wrote the manuscript; J.A.L. and V.E.S. analyzed the data, wrote the manuscript, and provided the figures; and E.J., C.L.B., P.C.C., A.H., P.A.H., S.M.H., A.K., M.J.M., A.M., A.N., D.S., P.G., C.N.O., M.L.P., D.S., G.v.K., A.D.Z., J.Y., and A.D. treated patients and approved the manuscript.
Conflict-of-interest disclosure: J.A.L. reports salary support for Project Genomics Evidence Neoplasia Information Exchange (GENIE) through the American Association for Cancer Research. A.D. has received personal fees from Roche, Corvus Pharmaceuticals, Physicians' Education Resource, Seattle Genetics, Peerview Institute, Oncology Specialty Group, Pharmacyclics, Celgene, Novartis, and Takeda and research grants from the National Cancer Institute and Roche. C.L.B. received grant funding from Janssen, Novartis, Epizyme, Xynomics, Bayer, and BMS; served as a consultant for Life Sci, GLG, Celgene, Seattle Genetics, and Xynomics; and holds honorarium from Dava Oncology. P.H. receives research support from Portola, Novartis/GSK, Molecular Templates, and Janssen Pharmaceuticals and served as consultant for Karyopharm, Juno, Portola, Celgene, and AstraZeneca. S.M.H. received research funding from ADCT Therapeutics, Aileron, Forty-Seven, Verastem, Kyowa Hakko Kirin, Millennium Pharmaceuticals Inc., Celgene, Trillium, and Daiichii Sankyo and consults for Astex, Affimed, Merck Sharp and Dome, Kyowa Hakko Kirin Pharma, Corvus Pharmaceuticals Inc., Celgene, Portola Pharmaceuticals, Takeda Millennium, Innate Pharma, Verastem, Miragen Therapeutics Inc., Seattle Genetics, and ADCT. A.K. receives research funding from AbbVie Pharmaceuticals, Adaptive Biotechnologies, Pharmacyclics, and Seattle Genetics and serves on the advisory board for Celgene and Astra Zeneca. M.L.P. receives honoraria from Flagship Ventures, Novartis, Evelo, Seres Therapeutics, Jazz Pharmaceuticals, Therakos, Amgen, and Merck and consults for Merck and Pharmacyclics. A.N. receives honoraria from Janssen, Pharmacyclics, and Prime Oncology; consults for Medscape; serves on the advisory board for Janssen; serves on the speakers’ bureau for Prime Oncology; and receives research funding for Rafael Pharma and Pharmacyclics. A.M. receives research support from Seattle Genetics, Merck, Bristol-Myers Squibb, and Incyte and receives honorarium from Kyowa Hakko Kirin Pharma, Miragen Therapeutics, Takeda Pharmaceuticals, ADC Therapeutics, Seattle Genetics, Cell Medica, Bristol-Myers Squibb, and Erytech Pharma. D.S. consults for InPractice Elsevier and Seattle Genetics and is on the speaker’s bureau for Medical Crossfire. A.D.Z. consults for Genentech/Roche, Gilead, Celgene, Janssen, Amgen, Novartis, Adaptive Biotechnology, and Verastem; serves on the advisory board for MorphoSys, Gilead, Genentech, AbbVie, AstraZeneca, Pharmacyclics; and receives research funding from MEI Pharmaceuticals, Roche, Gilead, and Beigene. A.Y. receives research support from Janssen, Curis, Merck, BMS, Syndax, and Roche; receives honorarium from Janssen, AbbVie, Merck, Curis, Epizyme, Roche, and Takeda; and consults for Biopath, Xynomics, Epizyme, Roche, Celgene, and HCM. The remaining authors declare no competing financial interests.
Correspondence: Anas Younes, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 330, New York, NY 10065; e-mail: younesa@mskcc.org.
REFERENCES
- 1. World Health Organization . WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 2. Krol AD, Hermans J, Dawson L, et al. Treatment, patterns of failure, and survival of patients with Stage I nodal and extranodal non-Hodgkin’s lymphomas, according to data in the population-based registry of the Comprehensive Cancer Centre West. Cancer. 1998;83(8):1612-1619. [DOI] [PubMed] [Google Scholar]
- 3. Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation—a population-based study of 1575 cases. Br J Haematol. 2004;124(2):151-159. [DOI] [PubMed] [Google Scholar]
- 4. López-Guillermo A, Colomo L, Jiménez M, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005;23(12):2797-2804. [DOI] [PubMed] [Google Scholar]
- 5. Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, Noordijk EM. Primary extranodal non-Hodgkin’s lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol. 2003;14(1):131-139. [DOI] [PubMed] [Google Scholar]
- 6. Hui D, Proctor B, Donaldson J, et al. Prognostic implications of extranodal involvement in patients with diffuse large B-cell lymphoma treated with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Lymphoma. 2010;51(9):1658-1667. [DOI] [PubMed] [Google Scholar]
- 7. Kumar A, Lunning MA, Zhang Z, Migliacci JC, Moskowitz CH, Zelenetz AD. Excellent outcomes and lack of prognostic impact of cell of origin for localized diffuse large B-cell lymphoma in the rituximab era. Br J Haematol. 2015;171(5):776-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339(1):21-26. [DOI] [PubMed] [Google Scholar]
- 9. Reyes F, Lepage E, Ganem G, et al; Groupe d’Etude des Lymphomes de l’Adulte (GELA) . ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005;352(12):1197-1205. [DOI] [PubMed] [Google Scholar]
- 10. Bonnet C, Fillet G, Mounier N, et al; Groupe d’Etude des Lymphomes de l’Adulte . CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2007;25(7):787-792. [DOI] [PubMed] [Google Scholar]
- 11. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. [DOI] [PubMed] [Google Scholar]
- 12. Zelenetz AD, Gordon LI, Wierda WG, et al. Diffuse large B-cell lymphoma version 1.2016. J Natl Compr Canc Netw. 2016;14(2):196-231. [DOI] [PubMed] [Google Scholar]
- 13. Lamy T, Damaj G, Soubeyran P, et al; LYSA Group . R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood. 2018;131(2):174-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poeschel V, Held G, Ziepert M, et al; German Lymphoma Alliance . Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial. Lancet. 2019;394(10216):2271-2281. [DOI] [PubMed] [Google Scholar]
- 15. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275-282. [DOI] [PubMed] [Google Scholar]
- 16. Greg R, Dan M, Andrew M, Ann GB, Lane B twang: Toolkit for Weighting and Analysis of Nonequivalent Groups. R package version 1.5. 2017. Available at: https://CRAN.R-project.org/package=twang. Accessed April 2019.
- 17. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. R Computing Team . A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 19. Nijland M, Boslooper K, van Imhoff G, et al. Relapse in stage I(E) diffuse large B-cell lymphoma. Hematol Oncol. 2018;36(2):416-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfreundschuh M, Murauski M, Ziepert M, et al. Radiotherapy (RT) to bulky (B) and extralymphatic (E) disease in combination with 6xR-CHOP-14 or R-CHOP-21 in young good-prognosis DLBCL patients: Results of the 2x2 randomized UNFOLDER trial of the DSHNHL/GLA. J Clin Oncol. 2018;36(15 suppl):7574. [Google Scholar]
- 22. Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28(27):4170-4176. [DOI] [PubMed] [Google Scholar]
- 23. Sehn LH. Chemotherapy alone for localized diffuse large B-cell lymphoma. Cancer J. 2012;18(5):421-426. [DOI] [PubMed] [Google Scholar]
- 24. Validire P, Capovilla M, Asselain B, et al. Primary breast non-Hodgkin’s lymphoma: a large single center study of initial characteristics, natural history, and prognostic factors. Am J Hematol. 2009;84(3):133-139. [DOI] [PubMed] [Google Scholar]
- 25. Yhim HY, Kim JS, Kang HJ, et al. Matched-pair analysis comparing the outcomes of primary breast and nodal diffuse large B-cell lymphoma in patients treated with rituximab plus chemotherapy. Int J Cancer. 2012;131(1):235-243. [DOI] [PubMed] [Google Scholar]
- 26. Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol. 2011;29(20):2766-2772. [DOI] [PubMed] [Google Scholar]
- 27. Sehn LH, Scott DW, Villa D, et al. Long-term follow-up of a PET-guided approach to treatment of limited-stage diffuse large B-cell lymphoma (DLBCL) in British Columbia (BC) [abstract]. Blood. 2019;134(suppl 1):Abstract 401. [Google Scholar]
- 28. Persky DO, Li H, Stephens DM, et al. PET-directed therapy for patients with limited-stage diffuse large B-cell lymphoma—results of intergroup NCTN study S1001 [abstract]. Blood. 2019;134(suppl 1):Abstract 349. [Google Scholar]
- 29. Stephens DM, Li H, LeBlanc ML, et al. continued risk of relapse independent of treatment modality in limited-stage diffuse large B-cell lymphoma: final and long-term analysis of Southwest Oncology Group Study S8736. J Clin Oncol. 2016;34(25):2997-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Persky DO, Unger JM, Spier CM, et al; Southwest Oncology Group . Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26(14):2258-2263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.