Abstract
The proto-oncogene Fli-1 encodes a transcription factor of the ets family whose overexpression is associated with multiple virally induced leukemias in mouse, inhibits murine and avian erythroid cell differentiation, and induces drastic perturbations of early development in Xenopus. This study demonstrates the surprisingly sophisticated regulation of Fli-1 mRNA translation. We establish that two FLI-1 protein isoforms (of 51 and 48 kDa) detected by Western blotting in vivo are synthesized by alternative translation initiation through the use of two highly conserved in-frame initiation codons, AUG +1 and AUG +100. Furthermore, we show that the synthesis of these two FLI-1 isoforms is regulated by two short overlapping 5′ upstream open reading frames (uORF) beginning at two highly conserved upstream initiation codons, AUG −41 and GUG −37, and terminating at two highly conserved stop codons, UGA +35 and UAA +15. The mutational analysis of these two 5′ uORF revealed that each of them negatively regulates FLI-1 protein synthesis by precluding cap-dependent scanning to the 48- and 51-kDa AUG codons. Simultaneously, the translation termination of the two 5′ uORF appears to enhance 48-kDa protein synthesis, by allowing downstream reinitiation at the 48-kDa AUG codon, and 51-kDa protein synthesis, by allowing scanning ribosomes to pile up and consequently allowing upstream initiation at the 51-kDa AUG codon. To our knowledge, this is the first example of a cellular mRNA displaying overlapping 5′ uORF whose translation termination appears to be involved in the positive control of translation initiation at both downstream and upstream initiation codons.
The proto-oncogene Fli-1 was first identified as a common proviral insertion site (Friend leukemia insertion site 1), observed in 75% of erythroleukemia cell clones induced by Friend murine leukemia virus (MuLV) in mice (3, 4). It encodes a transcription factor belonging to the ETS family, the founding member of which is the v-ets oncogene, an oncogenic version of c-ets1, transduced by the E26 leukemogenic virus (26, 36). More than 30 different members of the ETS family have been identified to date (25). All members of this family share a highly conserved ETS DNA binding domain responsible for the fixation to a core consensus purine-rich sequence, GGA(A/T), found in a wide variety of viral and cellular transcriptional regulatory regions (28, 47). ETS proteins are involved in the regulation of many different biological processes ranging from morphogenesis and eye development in Drosophila melanogaster to hematopoietic differentiation in mammals. In addition, many genes of the ets family participate in various oncogenic processes when activated as a result of chromosomal translocations or proviral insertions (see reference 13 for a review).
In addition to Friend erythroleukemia, activation of the Fli-1 gene by proviral insertion has been reported in several non-B and non-T leukemias induced by the Cas-Br-E virus (5) and in granulocytic leukemia induced by the Graffy MuLV (9). In all these cases, the proviral insertions occur in the 5′ region of the Fli-1 gene and are responsible for the overexpression of a normal FLI-1 protein. The Fli-1 gene also is rearranged in a majority of cases of the Ewing family of tumors that share t(11;22) chromosome translocation (8). In these cases, the translocation is responsible for the production of an abnormal fusion protein, EWS–FLI-1, which harbors the N-terminal part of the EWS protein and the C-terminal part of FLI-1, including the ETS DNA binding domain, and which displays altered transactivation properties compared to normal FLI-1 (1, 32, 37). Recently, we showed that the transcription of the Fli-1 gene is positively regulated by the SPI-1/PU.1 transcription factor (43), another ETS protein involved in erythroleukemia induced by the spleen focus-forming virus component of the Friend viral complex (35, 41, 49). We also showed that overexpression of FLI-1 inhibits the chemically induced erythroid terminal differentiation of spleen focus-forming virus-infected cells (43). Similarly, it has been shown that overexpression of FLI-1 also inhibits the erythropoietin-dependent erythroid differentiation of one other mouse erythroleukemia cell line (44) as well as avian primary erythroblasts (39). In this latter case, inhibition of avian erythroid differentiation by FLI-1 is associated with increased proliferation, reduced apoptosis upon erythropoietin withdrawal, and deregulation of cyclin D2 and D3 gene expression (39). The FLI-1 protein also displays antiapoptotic activity in NIH 3T3 cells (50) and functionally interferes with nuclear hormone receptors (7). The molecular targets of FLI-1 involved in all these processes remain unknown.
The Fli-1 gene is normally expressed in cells of various types during the early development of Xenopus (34), mice (33), and chickens (29). In all three species, the Fli-1 gene is expressed in endothelial and neural crest cells. Further detailed studies with chickens (29) have shown that Fli-1 gene expression in neural crest cells is restricted to mesenchymal lineages derived from neural crest cells at the end of their migration. The Fli-1 gene is also expressed in cartilage cells derived from mesoderm and could be expressed in the putative precursor of hematopoietic cells and angioblasts (29). Fli-1−/− transgenic mice have been established which, surprisingly, display a very discrete phenotype characterized by thymic hypocellularity (33). However, these Fli-1−/− mice are still able to produce an abnormal FLI-1TP protein, modified in its N-terminal part, which could substitute for the normal FLI-1 protein functions. Overexpression of FLI-1, obtained by injection of synthetic Fli-1 mRNA into Xenopus embryos, leads to dramatic development anomalies of the anteroposterior and dorsoventral polarities, of tissue differentiation, particularly in eye and cartilage development, and of erythroid differentiation including ectopic erythroid differentiation and hemangioma (42). In contrast, very subtle anomalies limited to the abnormal proliferation of B-lymphoid cells have been observed in transgenic mice displaying a twofold overexpression of FLI-1 (52). The unexpectedly discrete phenotype of these transgenic mice is most probably due to a FLI-1 overexpression lower than that obtained in Xenopus embryos as well as to counterselection of transgenic embryos expressing higher levels. Taken together, these data strongly suggest that the Fli-1 gene most probably plays important roles in multiple differentiation and cell migration processes during development, which remain to be precisely identified.
Despite multiple unresolved questions concerning the normal functions of the Fli-1 gene and its role in leukemia, it is already clear that its expression needs to be tightly controlled in vivo. To date, three different promoters of the Fli-1 gene have been identified. One of these promoter was first identified in erythroleukemia cell lines induced by Friend MuLV. It is responsible for transcription initiating at position −398 upstream from the ATG triplet specifying the translation initiation of Fli-1 mRNA (2, 43). A second promoter has been identified 1.4 kb upstream. This promoter directs the synthesis of an alternatively spliced Fli-1 mRNA, named Fli-1b, which lacks exon 1 due to direct splicing between new exon 1b and exon 2 (10). This alternative Fli-1b mRNA, which to date has been detected only in two human leukemic pre-B-cell lines, is responsible for the synthesis of a short FLI-1 protein by translation initiating in exon 2 instead of exon 1. More recently, we have identified a third promoter which is responsible for transcription initiating at position −204 and whose activity is positively regulated by transcription factor SPI-1/PU.1 (43). Given the specific expression of Spi-1 in the hematopoietic tissue, this latter promoter might be responsible for the specific expression and function of the Fli-1 gene in hematopoiesis, but this possibility remains to be demonstrated.
All known Fli-1 mRNA isoforms harbor more than 200 nucleotides (nt) of 5′ untranslated region (5′ UTR). This suggests strongly that the 5′ UTR is involved in additional controls of Fli-1 gene expression at the posttranscriptional or translational level (19, 22, 46, 48). The present study was undertaken to investigate this latter possibility.
MATERIALS AND METHODS
Plasmid construction.
All DNA templates used to direct the synthesis of the different Fli-1 mRNAs were derived from a common wild-type construct harboring the mouse Fli-1 cDNA (from positions −165 to +1484 with respect to ATG +1) cloned into the single NotI site of plasmid pBSK+ (Stratagene, Ozyme, Montigny le Bretonneux, France). Mutants harboring deletions in the 5′ UTR were constructed by PCR using a common reverse primer located in the coding sequence (CCTGGGCAATGCCATGGAAG) and the forward primers ATAAGAATGCGGCCGCCCGGGTCAATGTGTGGAATA (mut −49), ATAAGAATGCGGCCGCAATATTGGGGGGCTCGGCTG (mut −33), ATAAGAATGCGGCCGCACTTGGCCAAATGGACGGGA (mut −10), and ATAAGAATGCGGCCGCGGCAGATATGAACTGCTTCGG (mut +93). In all cases, the amplified cDNA fragments were cut by NotI and BamHI and used to replace the corresponding 5′ NotI-BamHI fragment from the wild-type construct. The other point mutations were introduced as recommended by the manufacturer, using the QuickChange site-directed mutagenesis kit (Stratagene) and oligonucleotides CTCCCCAAGGCAGATCTTACTGCTTCGGGGAGT (mutATG+100), GCTGTAACCGGGTCACTTTGTGGAATATTGGGG (mut −41), AACCGGGTCAATGTCTTGAATATTGGGGGGCTC (mut −37), AACCGGGTCACTTTCTTGAATATTGGGGGGCTC (mut −41/−37), GGACGGGACTATCAAGGAGGCTCTG (mut S1), GCTCTGTCTGTGGTCAGTGACGATC (mut S2), GGACGGGACTATCAAGGAGGCTCTGTCTGTGGTCAGTGACGATC (mut S12), GTCTGTGGTCAGCGACGATCAGTCCCTTTTCGATTCAGCATACGG (mut S14), and CCCAAGGCAGATATGCCTGCTTCGGGGAGTCCCGACTACGGGCAGCCC (mut S16). All mutated Fli-1 cDNAs were verified by sequencing between the SacI and BamHI restriction sites and then subcloned back to the parental pBSK+ Fli-1 cDNA plasmid. Expression vectors pCI Fli−165, pCI Fli−41/−37, pCI FliS12, pCI Fli-10, pCI Fli+93, and pCI Fli-10 mutATG+100 were obtained by subcloning the corresponding Fli-1 cDNA under the control of the cytomegalovirus (CMV) promoter into the single NotI site of pCI Neo vector (Promega, Charbonnières, France).
In vitro transcription and translation.
All DNA templates were linearized by EcoRI digestion and used to synthesize the corresponding Fli-1 mRNA by using T3 RNA polymerase. Uncapped mRNAs were synthesized using the AmpliScribe T3 transcription kit (Epicentre Technologies, TEBU, Le Perray-en-Yvelines, France), whereas capped mRNAs were synthesized using the mMessage mMachine T3 kit (Ambion, Clinisciences, Montrouge, France) as recommended by the manufacturers. Capped and uncapped mRNAs were further purified on MicroSpin S-300 HR columns (Pharmacia Biotech, Orsay, France) to eliminate excess unincorporated free nucleotides and cap analog. Both capped and uncapped mRNAs were recovered by ethanol precipitation, resuspended in water, and quantified by UV spectrophotometry. Aliquots of each synthetic mRNA were carefully analyzed by formaldehyde denaturing agarose gel electrophoresis to ensure quality and quantitation. A 1-μg sample of each mRNA was then incubated for 1 h at 30°C in 25 μl of commercial rabbit reticulocyte lysate (Flexi-Rabbit Reticulocyte Lysate System; Promega) in the presence of 1 μCi of [35S]methionine (Amersham, Les Ulis, France) per μl. Reactions were stopped by adding an equal volume of 2× Laemmli denaturing buffer (24), and the mixtures were boiled for 10 min and stored at −80°C until analysis. Equal amounts of the reaction products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography of the dried gels. Quantitative analyses of the results were carried out using a GS-525 molecular imager (Bio-Rad, Yvry sur Seine, France) and Molecular Analyst software (Bio-Rad).
Cell culture and transfection.
Mouse Friend erythroleukemia cell lines were kindly provided by F. Moreau-Gachelin or F. Wendling. These cell lines, as well as NIH 3T3 cells were grown in Iscove's modified Dulbecco's medium (IMDM; Gibco-BRL Life Technologies, Gergy Pontoise, France) supplemented with 10% fetal calf serum (FCS; Roche Diagnostic, Meylan, France). The mouse pre-B cell line 70Z/3 (kindly provided by E. Schneider) was grown in RPMI 1640 medium (Gibco-BRL) supplemented with 10% FCS and 20 μM α-thioglycerol. Transfections of NIH 3T3 cells were performed in six-well culture plates, with each well containing 106 cells which were incubated with 3 μg of plasmid DNA and 15 μg of DAC-30 (Eurogentec, Seraing, Belgium) for 5 h in 2 ml of IMDM containing only 5% FCS. At the end of this period, this medium was replaced with 2 ml of medium containing 10% FCS and the cells were grown for a further 48 h before analysis of the expression of Fli-1 mRNA by Northern blotting and that of FLI-1 proteins by Western blotting.
Western blot analyses.
Western blot analyses of FLI-1 proteins were performed on total-cell lysates using commercial Fli-1 antibody (1/200 dilution of rabbit polyclonal antibody) (no. sc-356; Santa Cruz Biotechnology, Santa Cruz, Calif.) revealed by classical enhanced chemiluminescence (ECL+; Amersham) and autoradiography. Semiquantitative analyses were performed by densitometry of the autoradiograms. More precise quantitative analyses of FLI-1 proteins expressed in NIH 3T3 transfected cells were performed using a Fluorimager 595 (Molecular Dynamics) after the Western blot was revealed by enhanced chemical fluorescence (Amersham) instead of ECL.
RNA analyses.
Total RNA from cell lines was prepared using RNA-plus (Quantum Biotechnologies, Montreuil, France) as specified by the manufacturer. A 1-μg portion of total RNA was denatured for 10 min at 65°C and reverse transcribed for 1 h at 37°C in a final volume of 20 μl containing 200 U of Moloney MuLV reverse transcriptase (Gibco-BRL), 0.5 μM random hexamers, 0.5 mM each deoxynucleoside triphosphate, 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 50 mM Tris-HCl (pH 8.3), and 20 U of RNasin (Promega). Reverse transcriptions were terminated by heating for 10 min at 90°C and subsequent chilling on ice. PCR amplifications were performed using 5 μl of the reverse transcription product in a final volume of 50 μl of 1× PCR buffer (50 mM KCl, 20 mM Tris HCl [pH 8.4], 1.5 mM MgCl2, 0.05% W1 [Gibco-BRL]) containing 0.2 mM each deoxynucleoside triphosphate, 0.2 μM (each) sense and antisense primers, and 2.5 U of Taq DNA polymerase (Gibco-BRL). The PCRs were performed using an initial 5-min denaturing step at 95°C followed by 30 cycles of 30 s at 94°C, 30 s at 67°C, and 1 min at 72°C and terminated by a 10-min elongation step at 72°C. Primers 3′ex2 (CCCGTAGTCAGGACTCCCCG) and 5′ex1b (CACCGCCACTCCAGGTCTGG) were used to amplify Fli-1b transcripts, whereas primers 3′ex2 and 5′ex1 (AGGGGGCACTCAGAGAGG) were used to amplify other Fli-1 transcripts initiated at position −398 or −204. Amplified DNA products were analyzed by agarose gel electrophoresis and visualized by ethidium bromide fluorescence. Northern blot analyses of Fli-1 mRNA expression in NIH 3T3 transfected cells were performed using 40 μg of total RNA, the Express hybridization solution (Clonetech, Palo Alto, Calif.), and a radiolabeled Fli-1 cDNA probe. Specific signals of the expected size were quantified using a GS-525 molecular imager.
RESULTS
Two different FLI-1 protein isoforms are synthesized through alternative translation initiations of the same Fli-1 transcripts.
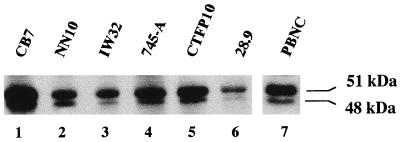
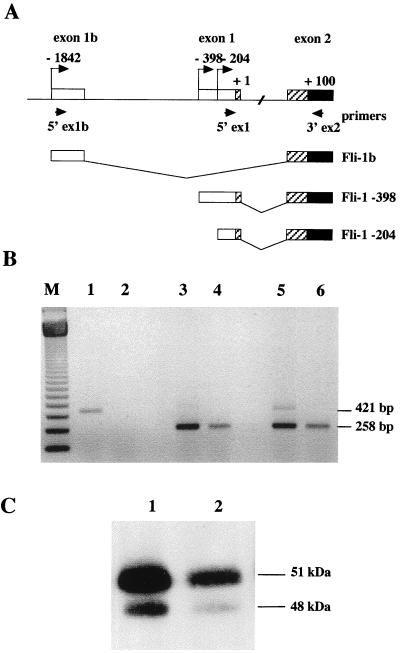
According to the longest open reading frame (ORF) identified in cloned Fli-1 cDNAs, the product of the Fli-1 gene is usually referred to as a single protein of 452 amino acids with a predicted molecular mass of 51 kDa. This predicted size roughly corresponds to that of the major protein obtained after in vitro translation of synthetic Fli-1 mRNA and to that of the major protein revealed by Fli-1-specific antibodies in Western blot analyses of cell lysates. However, in virtually every cell line analyzed so far, as illustrated for Friend erythroleukemic cells (Fig. 1, lanes 1 to 6) and normal mouse peripheral blood nucleated cells (lane 7), Western blot analyses also indicate the presence of another minor protein of about 48 kDa, whose origin remains unclear. Recently, a new variant of human Fli-1 mRNA has been described, whose synthesis is initiated 1.4 kb upstream of the other known transcription initiation sites at positions −398 and −204 (2, 10, 43). This mRNA lacks exon 1 because of its splicing between new exon 1b and normal exon 2 (10). To date, this variant mRNA has been detected only in two human pre-B leukemic cell lines and is responsible for the synthesis of a FLI-1 protein isoform, named FLI-1b, initiated at AUG +100 in exon 2 instead of AUG +1 in exon 1. The size of this FLI-1b isoform roughly corresponds to the 48-kDa short isoform observed in Friend erythroleukemia cells. We therefore decided to investigate whether this Fli-1b mRNA was expressed in Friend erythroleukemia cells. For that purpose, we designed two mouse primers, one forward primer located in exon 1b (5′ex1b) and one reverse primer located in exon 2 (3′ex2), for directing the amplification of a 421-bp cDNA fragment from the mouse Fli-1b mRNA by reverse transcription-PCR (RT-PCR) (Fig. 2A). A single fragment of the expected size was observed in the mouse pre-B-cell line 70Z/3 (30) (Fig. 2B, lane 1), indicating that, like human Fli-1b mRNA, mouse Fli-1b mRNA is expressed in pre-B leukemic cells and can account for the synthesis of the FLI-1b protein isoform in these latter cells (Fig. 2C, lane 1). In contrast, no amplification of the specific Fli-1b cDNA could be obtained from Friend erythroleukemia cells (Fig. 2B, lane 2), although control experiments using the same reverse primer in exon 2 and a forward primer in exon 1 (5′ex1) led to the expected specific 258-bp fragment corresponding to other Fli-1 transcripts (lanes 4 and 6).
FIG. 1.
Western blot analysis of FLI-1 proteins in total-cell lysates prepared from several Friend erythroleukemia cell lines (lanes 1 to 6) and from peripheral blood nucleated cells of a normal mouse (lane 7).
FIG. 2.
The mouse Fli-1b transcript is expressed in the leukemic pre-B-cell line 70Z/3 but not in the Friend erythroleukemia cell line 745-A. (A) Schematic representation of the 5′ genomic structure of the mouse Fli-1 gene and of the 5′ ends of known transcript isoforms. Coordinates of transcription initiation sites are given with respect to the last AUG located in exon 1 (AUG +1), usually described to initiate the large FLI-1 protein isoform. Open boxes represent 5′UTR sequences, and the solid box represents the common FLI-1 coding sequence of all Fli-1 transcript isoforms. The sequences which are alternatively coding (−398 and −204 transcripts) or noncoding (transcript Fli-1b) are indicated by hatched boxes. Small horizontal arrows indicate the position of the primers used in RT-PCR. (B) RT-PCR analysis of Fli-1 transcripts expressed in the pre-B-cell line 70Z/3 (lanes 1, 3, and 5) and in the erythroleukemia cell line 745-A (lanes 2, 4, and 6). All the RT-PCR amplifications were performed with equal amounts of total RNA and the common primer 3′ex2 with either primer 5′ex1b (lanes 1 and 2), primer 5′ex1 (lanes 3 and 4), or both (lanes 5 and 6). The lengths of specific Fli-1b cDNA (421 bp) or Fli-1 −398 and −204 cDNAs (258 bp) are given to the right. M, 123-bp ladder. (C) Control Western blot analysis of FLI-1 protein isoforms expressed in the pre-B-cell line 70Z/3 (lane 1) and the erythroleukemia cell line 745-A (lane 2).
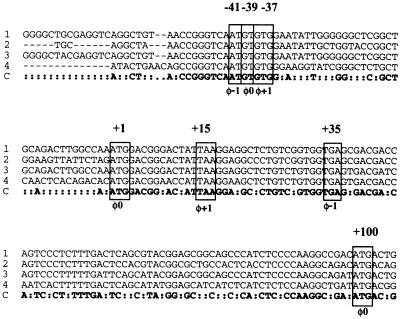
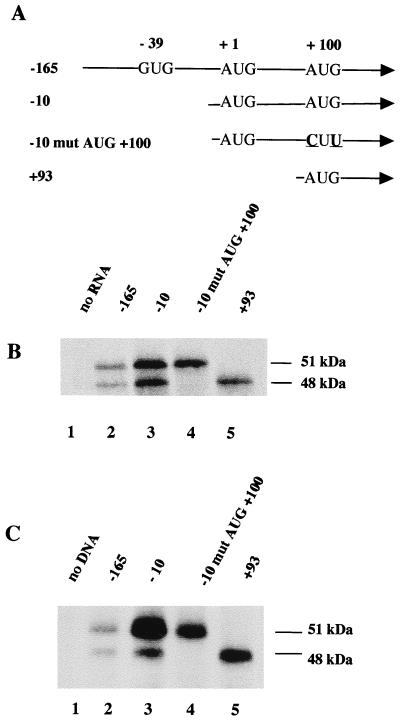
The above data raised the possibility that the two FLI-1 isoforms expressed in Friend erythroleukemia cells might be synthesized by alternative translation initiation of the same Fli-1 transcripts harboring classical exon 1. Interestingly, we noticed the presence of a highly conserved 16-nt motif located between positions −49 and −33 preceding the AUG +1 initiation codon in exon 1 of mouse, human, Xenopus, or quail Fli-1 mRNA (Fig. 3). This motif includes three putative overlapping initiation codons: one AUG at position −41 and two GUG at positions −39 and −37 (Fig. 3). Of these three putative initiation codons, GUG −39 is located in the same frame as that of the FLI-1 coding frame starting at AUG +1 and could initiate the synthesis of a FLI-1 protein with a predicted molecular mass of 52.4 kDa. Given the uncertainty of the molecular mass of FLI-1 proteins determined by Western blot, this suggested that the two FLI-1 isoforms observed in Friend erythroleukemia cells could result from alternative translation at either GUG −39, AUG +1, or AUG +100. In vitro translation with synthetic Fli-1 mRNA harboring progressive deletions in the exon 1 5′ UTR or point mutations disrupting AUG +100 (Fig. 4A) was used to distinguish between these possibilities. We found that Fli-1 mRNA starting at position −10 produces at least 10-fold more FLI-1 proteins than does Fli-1 mRNA starting at position −165 but still produces both FLI-1 isoforms (Fig. 4B, compare lanes 2 and 3). These data indicate that GUG −39 is not responsible for the synthesis of any FLI-1 isoform. In contrast, Fli-1 mRNA starting at position +93 did not produce the 51-kDa isoform but still produced the 48-kDa isoform (lane 5). Furthermore, Fli-1 mRNA starting at position −10 but carrying two point mutations disrupting AUG +100 still produced the 51-kDa protein but no longer produced the 48-kDa protein (lane 4). We therefore concluded that alternative translation initiating at codons AUG +1 and AUG +100 is responsible for the synthesis of the 51- and 48-kDa isoforms, respectively.
FIG. 3.
Partial alignment of Fli-1 cDNA sequences from human (row 1), quail (row 2), mouse (row 3), and frog (row 4). Row C contains the consensus sequence. The coordinates of conserved translation initiation or termination codons (boxed) are given on top of the alignment. The phase of each conserved codon is indicated below the alignment.
FIG. 4.
Identification of the initiation codons involved in the synthesis of the 51- and 48-kDa FLI-1 proteins. (A) Schematic drawing of the 5′ ends of synthetic Fli-1 cDNA used to synthesize Fli-1 mRNA by in vitro transcription. Point mutations are underlined. (B) Equal amounts of each of these synthetic Fli-1 mRNA were used to program rabbit reticulocyte lysates in the presence of [35S]methionine. Translation products were then separated by SDS-PAGE, transferred to a nitrocellulose membrane, and revealed by autoradiography. (C) Western blot analysis of FLI-1 proteins synthesized in NIH 3T3 cells under transient expression of the transfected pCI Neo vector (Promega) carrying the indicated Fli-1 cDNA.
To test whether alternative translation initiation of Fli-1 mRNA could also occur in vivo, the four Fli-1 cDNAs starting at position −165, −10 (with or without the AUG +100 mutation), or +93 were subcloned into the expression vector pCI Neo under the control of the CMV promoter. NIH 3T3 cells were then transfected by the resulting vectors, and the transient expression of FLI-1 proteins was analyzed by Western blotting (Fig. 4C). As expected from the analysis of in vitro translation of the corresponding mRNA, we found low levels of both isoforms in cells transfected by pCI Fli−165 vector (Fig. 4C, lane 2), higher levels of both isoforms in cells transfected with pCI Fli−10 vector (lane 3), and only the 51- or 48-kDa isoform in cells transfected, respectively, with pCI Fli−10 mutAUG+100 (lane 4) or pCI Fli+93 vector (lane 5). Taken together, these data establish that the same Fli-1 transcripts can produce the two different FLI-1 isoforms through alternative translation initiation at codons AUG +1 and AUG +100 both in vitro and in vivo. This finding led us to investigate the underlying mechanism responsible for this alternative translation initiation of Fli-1 transcripts.
Differential regulation of the synthesis of the 51- and 48-kDa FLI-1 isoforms by conserved region −49/−33.
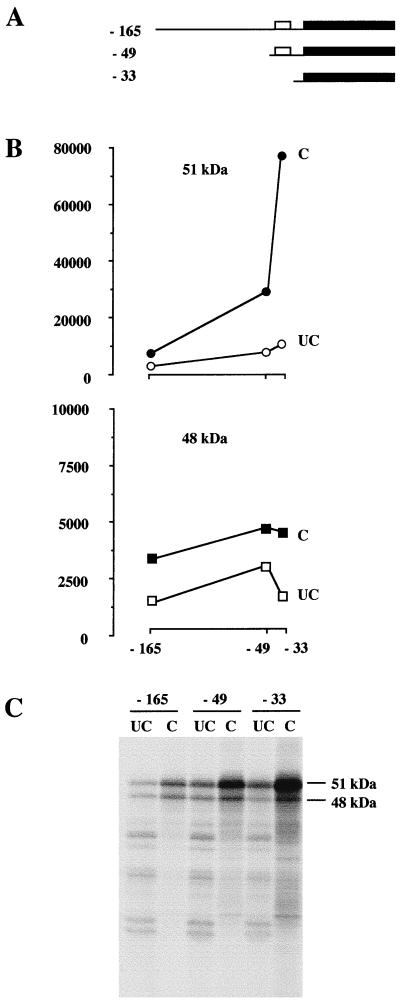
We recently described a new promoter of the mouse Fli-1 gene responsible for transcription initiating at position −204 (43). This promoter was identified by transfection assays using a luciferase reporter gene placed under the control of the mouse Fli-1 gene −270/−41 region. During the course of this study, we noticed that no luciferase activity could be obtained using the same luciferase reporter gene placed under the control of the −270/−31 region instead of the −270/−41 region (data not shown), thus indicating a strong inhibitory effect of the −41/−31 region. This −41/−31 region overlaps the highly conserved −49/−33 region, which includes three putative upstream initiation codons (Fig. 3). These data suggested that the highly conserved −49/−33 region might inhibit translation. To address this question, we used three different synthetic Fli-1 mRNA starting at position −165, −49, or −33 (Fig. 5A). Equal amounts of capped or uncapped versions of each of these Fli-1 mRNA were incubated in rabbit reticulocyte lysate, and translation products were analyzed by gel electrophoresis and autoradiography (Fig. 5C). The 51- and 48-kDa isoforms synthesized were then quantified by scanning densitometry with a molecular imager (Fig. 5B). Deletion of the −165/−49 region led to a similar increase in synthesis of both FLI-1 isoforms from capped and uncapped mRNA. Deletion of the −49/−33 region led to a further marked increase in synthesis of the 51-kDa isoform. In contrast, the same deletion of the −49/−33 region led to a reproducible decrease in synthesis of the 48-kDa protein. Taken together, these results indicate that the conserved −49/−33 region is involved in the negative control of synthesis of the major 51-kDa isoform and the positive control of synthesis of the minor 48-kDa isoform.
FIG. 5.
Differential regulation of the synthesis of the 51- and 48-kDa FLI-1 isoforms by the conserved region −49/−33. Capped and uncapped Fli-1 mRNAs harboring progressive deletions of the 5′UTR were synthesized by in vitro transcription. Equal amounts of each of these synthetic Fli-1 mRNA were used to program rabbit reticulocyte lysates in the presence of [35S]methionine. Translation products were separated by SDS-PAGE. Labeled translation products were then visualized by autoradiography, and radioactive signals corresponding to the 51- and 48-kDa proteins were quantified using a Molecular Imager. (A) Schematic representation of synthetic Fli-1 mRNA. The open boxes represent the −49/−33 conserved region in 5′UTR, and the solid boxes represent coding sequences. (B) Graphical representation of the results of the quantitation of the 51-kDa (top) or 48-kDa (bottom) isoforms synthesized with the capped (C) or uncapped (UC) versions of each Fli-1 mRNA. The y axis shows arbitrary units of radioactivity, and the x axis shows the type of mRNA used for translation. (C) Autoradiography of the gel.
Differential regulation of 51- and 48-kDa isoform synthesis by the conserved region −49/−33 is mediated by upstream AUG −41 and GUG −37 codons.
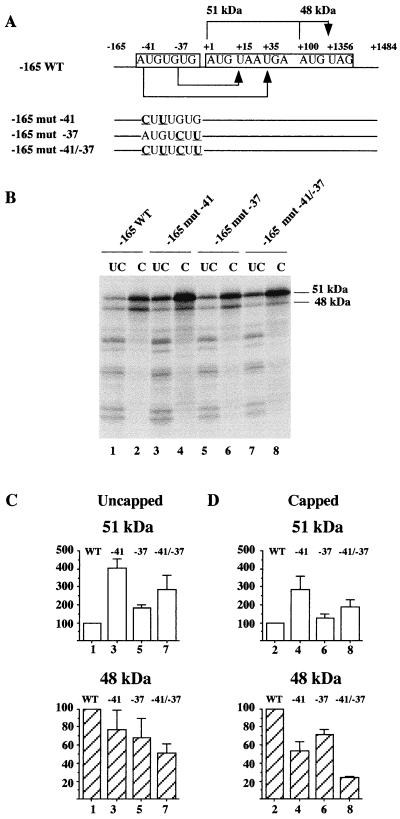
The two upstream codons, AUG −41 and GUG −37, which are included in the conserved regulatory region −49/−33 of the 5′ UTR of mouse Fli-1 mRNA define two short ORFs terminating, respectively, at the stop codons UGA +35 and UAA +15 (Fig. 3 and 6A). The locations of these two stop codons, between AUG +1 and AUG +100, are conserved in human, Xenopus, and quail Fli-1 mRNAs. We therefore hypothesized that the regulatory function of the −49/−33 conserved region might be mediated by these two short ORFs. To test this hypothesis, we used three synthetic Fli-1 mRNAs harboring point mutations disrupting either the AUG −41 codon, the GUG −37 codon, or both (Fig. 6A). Equal amounts of capped or uncapped Fli-1 mRNAs were incubated in rabbit reticulocyte lysate, and the translation products were analyzed by SDS-PAGE and autoradiography (Fig. 6B). Mutation of the AUG −41 codon led to a fourfold increase in synthesis of the 51-kDa isoform and concomitantly to a 20% decrease in synthesis of the 48-kDa isoform from uncapped mRNA (Fig. 6C, lane 3). Mutation of the GUG −37 codon also led to an increase (twofold) in synthesis of the 51-kDa isoform and to a decrease (35%) in synthesis of the 48-kDa isoform (lane 5). Similar effects of these mutations were observed on synthesis of both isoforms from capped mRNA (Fig. 6D, lanes 4 and 6). Therefore, these results indicated that both upstream initiation codons, AUG −41 and, to a lesser extent, GUG −37, contribute simultaneously to the negative regulation of synthesis of the 51-kDa isoform and to the positive regulation of synthesis of the 48-kDa isoform.
FIG. 6.
Differential regulation of the synthesis of the 51- and 48-kDa FLI-1 isoforms by the conserved region −49/−33 is mediated by upstream codons, AUG −41 and GUG −37. Capped or uncapped versions of Fli-1 mRNA with or without mutations of AUG −41 or GUG −37 codons or with both mutations were synthesized by in vitro transcription. Equal amounts of each of these synthetic Fli-1 mRNAs were used to program rabbit reticulocyte lysates in the presence of [35S]methionine. Translation products were separated by SDS-PAGE. Labeled translation products were then visualized by autoradiography, and radioactive signals corresponding to the 51- and 48-kDa proteins were quantified using a Molecular Imager. (A) Schematic representation of synthetic Fli-1 mRNA. Point mutations are underlined. The two short 5′ uORFs beginning at AUG −41 or GUG −37 and ending, respectively, at UGA +35 and UAA +15 are indicated by arrows under the drawing of the wild-type mRNA. Similarly, the two in-frame FLI-1 ORF starting at AUG +1 or AUG +100 and ending at UAG +1356 are indicated by arrows above the drawing of the wild-type mRNA. (B) Autoradiogram of the gel. (C and D) Graphical representation of the results obtained with uncapped and capped versions of the same mRNA. Results are expressed as the percentage of 51-kDa (white bars) or 48-kDa (hatched bars) isoforms produced by the indicated mutated mRNA compared to the wild type (means and standard deviations of three different experiments).
As expected, the combined mutations of both upstream codons on the same capped or uncapped mRNA led to a greater reduction in synthesis of the 48-kDa isoform (Fig. 6D, lane 8; Fig. 6C, lane 7) than did mutations at either AUG −41 or GUG −37 alone (Fig. 6D, lanes 4 and 6; Fig. 6C, lanes 3 and 5). This indicated that both upstream initiation codons contribute to the positive regulation of synthesis of the 48-kDa isoform in a roughly additive manner. In contrast, the positive effect of combined mutations at both upstream codons on synthesis of the 51-kDa isoform was unexpectedly lower (30 to 40% less) than the positive effect of the mutation at AUG −41 alone (Fig. 6C and D, compare lanes 3 and 7 and lanes 4 and 8). The surprising observation was indeed the positive contribution of GUG −37, evidenced by comparison of mutant −41/−37 (carrying no upstream initiation codon) and mutant −41 (carrying only GUG −37). The observation that GUG −37 positively contributes to synthesis of the 51-kDa protein was in complete contrast to the rule that upstream codons are usually preferentially used to initiate translation at the expense of downstream codons. This led us to investigate whether this positive contribution could be mediated through the translation termination process of the 5′ upstream ORF (uORF) initiated at GUG −37.
Synthesis of both FLI-1 isoforms is positively regulated by the conserved stop codons, UAA +15 and UGA +35.
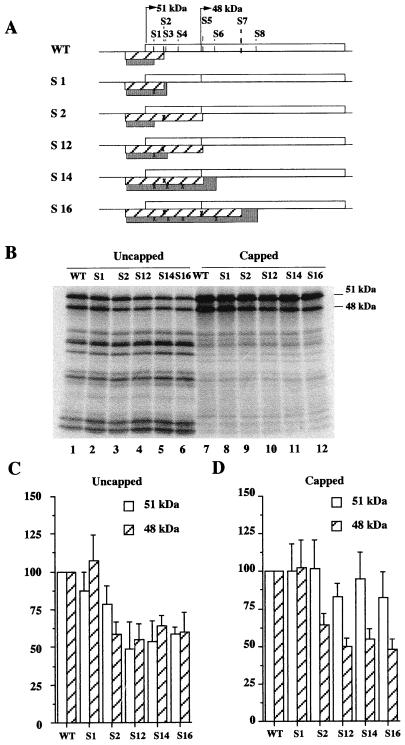
The contribution of the translation termination process of the two 5′ uORF beginning at AUG −41 and GUG −37 was addressed by the use of three synthetic Fli-1 mRNAs carrying point mutations disrupting either stop codon UAA +15 (mut S1), stop codon UGA +35 (mut S2), or both (mut S12) (Fig. 7A). The disruption of codon UGA +35 led to a decrease in synthesis of the 48-kDa protein from capped or uncapped mRNA (Fig. 7C and D, lanes S2). Although the disruption of codon UAA +15 alone had no significative effect (lanes S1), the decrease in synthesis of the 48-kDa protein reached 50% after disruption of both UAA +15 and UGA +35 codons (lanes S12). These data thus indicate that at least 50% of the synthesis of the 48-kDa protein from capped or uncapped mRNA is dependent on the translation termination of the two short 5′ uORF, mainly that initiated by AUG −41.
FIG. 7.
Synthesis of both FLI-1 isoforms is positively regulated by the conserved UAA +15 and UGA +35 stop codons. The same experiment as in Fig. 6 was performed using the different mRNA shown in panel A, in which the overlap of the two 5′ uORFs initiated at either AUG −41 (hatched boxes) or GUG −37 (grey boxes) and the FLI-1 ORF (open boxes) was progressively increased by the mutations of the corresponding in-frame stop codons (crosses). These stop codons are numbered from 1 to 8 depending on their increasing distance from AUG +1. Stop codons S1 (UAA +15, changed to CAA), S3 (UGA +39, changed to CGA), S4 (UGA +57, changed to CGA) S6 (UGA +120, changed to CGA) and S8 (UGA +210) are located in the same frame as the GUG −37 codon, whereas stop codons S2 (UGA +35, changed to UCA), S5 (UGA +101, changed to UGC), and S7 (UGA +185) are located in the same frame as AUG −41 codon. (B) Autoradiogram of the gel showing the different proteins synthesized; the positions of the 51- and 48-kDa FLi-1 isoforms are indicated to the right. (C and D) Graphical representations of the quantitative analysis of the production of the 51-kDa and the 48-kDa isoforms from uncapped or capped versions of the same mRNA. Results are expressed as the percentage of the amounts of either 51- or 48-kDa isoforms produced by the indicated mRNA compared to the wild type (means and standard deviations of three different experiments).
The same two mutations of the UAA +15 and UGA +35 stop codons also led to a significant reduction of synthesis of the 51-kDa protein, reaching a 50% reduction when combined on the same uncapped mRNA (Fig. 7C, lane S12). Similarly, although they individually had no detectable effect, they also led to a 20% reduction of synthesis of the 51-kDa protein when combined on the same capped mRNA (Fig. 7D, lane S12). These data thus indicate that synthesis of the 51-kDa isoform is also dependent on the translation termination of the two short 5′ uORF initiated by AUG −41 and GUG −37.
In the next experiments, we tried to determine whether the proportion of synthesis of the 51-kDa protein which appeared to be dependent on the translation termination at UAA +15 and UGA +35 stop codons could be underestimated due to an eventual rescue of the mutations at these stop codons by further downstream in-frame stop codons. For that purpose, we investigated the effect of simultaneous mutations of the next two (Fig. 7A, mutant S14) or the next four (Fig. 7A, mutant S16) stop codons which are present in the same reading frames as AUG −41 or GUG −37. No further significant reduction in the synthesis of the 51-kDa isoform could be observed even after mutations of all of the next four in-frame stop codons from either capped or uncapped mRNA (Fig. 7D and C, lanes S14 and S16). These data thus indicate that none of the four other stop codons located up to the position +120 seems to be able to mediate a positive effect on the synthesis of the 51-kDa isoform. The possibility that some other stop codon located downstream of position +120 may exert a positive effect was not further investigated.
Regulation of FLI-1 protein synthesis by the two short 5′ uORF in vivo.
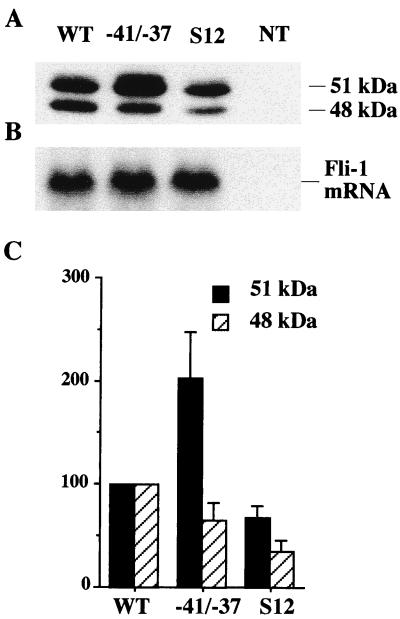
Taken together, the above results indicated that the two short 5′ uORF are involved in both positive and negative regulation of synthesis of the FLI-1 proteins in vitro. However, to appreciate the biological significance of these results, it was important to verify that these regulations do occur in vivo. For that purpose, the wild-type Fli-1 cDNA as well as the two mutants carrying either the two mutations disrupting upstream codons AUG −41 and GUG −37 or the two mutations disrupting stop codons UAA +15 and UGA +35 were subcloned into expression vector pCI Neo under the control of the CMV promoter. NIH 3T3 cells were transfected by each of the resulting expression vectors, and transiently expressed FLI-1 proteins as well as Fli-1 mRNA were quantified respectively by Western blotting (Fig. 8A) and Northern blotting (Fig. 8B). The same amount of Fli-1 mRNA was obtained in cells transfected by either one of the three expression vectors, indicating that the stability of Fli-1 mRNA is not affected by mutations disrupting either the upstream codons or the stop codons (Fig. 8B). In contrast, reproducible and significant effects were observed on the synthesis of the two isoforms of FLI-1 proteins. Mutations at codons AUG −41 and GUG −37 led to an up to twofold increase in synthesis of the 51-kDa protein and to a 20% decrease in synthesis of the 48-kDa protein (Fig. 8A and C, lanes −41/−37). Mutation at stop codons UAA +15 and UGA +35 led to a more than twofold decrease in synthesis of the 48-kDa protein and to a reproducible 20% decrease in synthesis of the 51-kDa protein (Fig. 8A and C, lanes S12). Overall, these results established that the mutations disrupting either initiation or stop codons of the two short 5′ uORF led to the same effects on the synthesis of FLI-1 proteins in vivo as that observed using capped versions of the same synthetic Fli-1 mRNAs in vitro.
FIG. 8.
Regulation of Fli-1 mRNA translation by conserved 5′ uORFs in vivo. NIH 3T3 cells were transfected by Fli-1 expression vectors carrying either the wild-type cDNA sequence (lane WT), mutations at both upstream AUG −41 and GUG −37 codons (lane −41/−37), or mutations at both stop codons UAA +15 and UGA +35 (lane S12). NT, nontransfected cells. (A) Western blot analysis of FLI-1 proteins produced in transfected cells. (B) Northern blot analysis of Fli-1 mRNA. (C) Quantitative analysis of the production of the 51- and 48-kDa proteins. Relative values are expressed as percentages of the amount of proteins produced by the wild-type sequence (means and standard deviations of four different transfections).
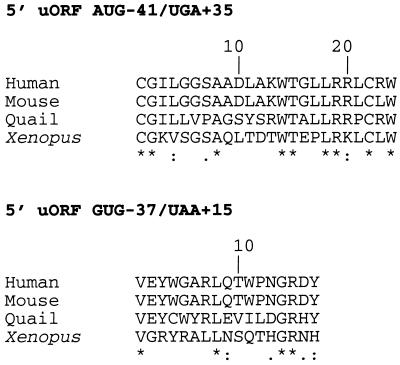
The coding sequences of the two 5′ uORF are very poorly conserved during evolution.
The alignments of the predicted sequences of peptides encoded by both 5′ uORF in human, mouse, quail, and Xenopus mRNAs are shown in Fig. 9. Only 9 out of the 24 amino acids (37%) encoded by the 5′ uORF beginning at AUG −41 and only 4 out of the 17 amino acids (23%) encoded by the 5′ uORF beginning at GUG −37 appear to be conserved among the four species. Further analysis of all these peptidic sequences failed to reveal any remarkable bias in amino acid composition and failed to reveal any significant homology to known motifs recorded in protein databases. This very poor conservation of peptidic sequences encoded by the two 5′ uORF is in marked contrast to the very high conservation of the location of their initiating and terminating codons in the Fli-1 mRNA sequence (Fig. 3), as well as to the very high conservation (>87%) of the first 33 amino acids of the 51-kDa isoform, which are missing in the 48-kDa isoform (29). This suggests in turn that there is a very low probability that the regulatory function of the two 5′ uORF may be mediated by the two short putative peptides they encode.
FIG. 9.
Alignment of the predicted amino acid sequences of the putative peptides encoded by the two conserved 5′ uORF in human, mouse, quail, and Xenopus mRNAs. Conserved amino acids are marked by asterisks.
DISCUSSION
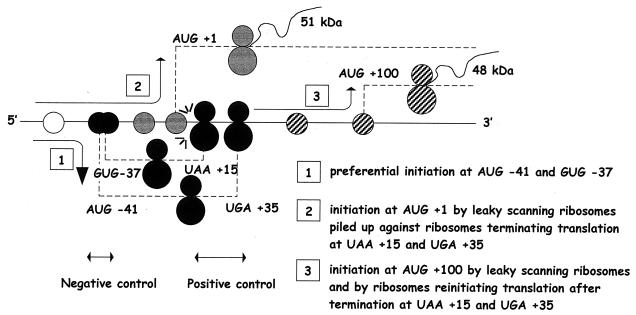
The present study establishes that the translation of Fli-1 mRNA leads to the synthesis of two FLI-1 protein isoforms through the use of two alternative and highly conserved in-frame initiation codons, AUG +1 and AUG +100. Furthermore, we show that the synthesis of these two FLI-1 isoforms is tightly controlled by two short overlapping 5′ uORF beginning at two highly conserved upstream initiation codons located in the 5′ UTR and terminating at two highly conserved stop codons located between the two alternative initiation codons AUG +1 and AUG +100. The most original finding of this study is that these two conserved 5′ uORF not only are involved in the negative control of the major large isoform synthesis but also are simultaneously involved in the positive control of synthesis of both isoforms. As discussed below, we suggest that this unique positive control is mediated by a classical termination-reinitiation process and by a new mechanism involving the interference between the termination process of the two 5′ uORF and leaky scanning (Fig. 10).
FIG. 10.
Diagram illustrating the mechanisms involved in the regulation of Fli-1 mRNA translation by the conserved 5′ uORF. Step 1: Given the minimal twofold stimulation of the 51-kDa protein synthesis induced by the disruption of AUG −41 and GUG −37, we can estimate that at least half of the 40S subunits loaded at the 5′ end of Fli-1 mRNA initiate translation at upstream AUG −41 and GUG −37 codons. The remaining 40S subunits go on their 3′ progression by leaky scanning. Step 2: Translation initiation at AUG +1 is facilitated by the piling up of leaky-scanning 40S subunits against ribosomes terminating the translation of the two 5′ uORF at stop codons UAA +15 and UGA +35. Step 3: At least half of the translation initiated at AUG +100 is due to ribosomes which reinitiate after translation termination at stop codons UAA +15 and UGA +35, while the remaining translation is due to scanning 40S subunits having bypassed all upstream initiation codons.
Translation of Fli-1 mRNA preferentially initiates at AUG −41.
The disruption of AUG −41 invariably leads to a marked increase in synthesis of the 51-kDa protein, thus indicating that the dominant effect of AUG −41 on translation initiated at downstream AUG +1 is negative. Importantly, we already know that AUG −41 is fully functional in vivo, since it is responsible for synthesis of the abnormal FLI-1TP protein produced by transgenic mice in which exon 2 of the Fli-1 gene has been replaced by a Neor gene (33). Taken together, these data are in agreement with the leaky-scanning model (23), which predicts that, given its first position, AUG −41 should be preferentially used to initiate translation but that, given its suboptimal nucleotide context, it can also be bypassed by 40S subunits, allowing translation initiation by downstream AUG +1. On the other hand, synthesis of the 51-kDa protein is only marginally affected by mutation at GUG −37. This observation is also in agreement with the second position of GUG −37 and with the current notion that GUG codons are usually less efficiently used to initiate translation than are AUG codons.
Half of the synthesis of the 48-kDa protein is made by a termination-reinitiation mechanism.
In apparent contrast to the leaky-scanning model, the disruptions of AUG −41 and GUG −37 invariably lead to a decrease in synthesis of the 48-kDa protein. Part of this discrepancy can be explained by taking into account that translation initiated by AUG −41 and GUG −37 terminates at stop codons UGA +35 and UAA +15, which are located upstream from AUG +100. Indeed, numerous data indicate that translation initiation can occur at AUG codons located downstream from short uORF through a termination-reinitiation mechanism (17, 18, 27). This termination-reinitiation mechanism has been extensively studied, particularly in the context of the yeast GCN4 mRNA (17). It has been demonstrated that it is mediated by forward scanning of mRNA by ribosomes after the termination of the uORF translation. Actually, we found that the disruptions of stop codons UAA +15 and UGA +35 led to a roughly twofold reduction of synthesis of the 48-kDa protein thus indicating that at least 50% of this synthesis is dependent on a termination-reinitiation mechanism. This termination-reinitiation mechanism therefore partially compensates for the loss of synthesis of the 48-kDa protein which is due to the preferential initiation at upstream AUG −41 and GUG −37 codons. Importantly, the leaky-scanning model predicts that recognition of AUG +1 must be increased when it is in the first position after the disruption of upstream AUG −41 and GUG −37. However, in contrast to AUG −41 and GUG −37, the loss of the 48-kDa protein due to the preferential initiation at upstream AUG +1 is not compensated by a termination-reinitiation mechanism. This, in turn, explains why the disruption of AUG −41 and GUG −37 leads to a decrease in synthesis of the 48-kDa protein.
At least 20% of the synthesis of the 51-kDa protein is dependent on the interference between the termination process of the two 5′ uORF and leaky scanning.
The disruptions of both stop codons UAA +15 and UGA +35 reproducibly lead to a decrease in synthesis of the 51-kDa protein, indicating that part of the translation initiated at AUG +1 is also dependent on the termination process of the two short 5′ uORF. Similar positive effects of stop codons on the translation at upstream codons have already been reported in a few cases, principally for artificial or viral mRNA (12, 14, 20, 38, 45). In most of the cases investigated, such a positive effect rapidly decreases with the distance between the stop codon and the upstream initiation codon and is completely abolished when this distance exceeds 100 nt (14, 20, 38). In agreement with these data, our data indicate that the decrease in synthesis of the 51-kDa protein is maximal only after disruption of both UAA +15 and UGA +35. One model, which has already been suggested to explain the positive effect of stop codons on upstream translation initiation, proposes that, as in the termination-reinitiation mechanism which can occur at downstream codons, terminating ribosomes are also able to scan the mRNA sequence in the 5′ direction and to reinitiate when they encounter upstream initiation codons (14, 20, 38, 45). However, to our knowledge, the reality of such a backscanning by terminating ribosomes has not yet been directly demonstrated.
At first glance, it seems difficult to reconcile the negative effect of disrupting codons UAA +15 and UGA +35 on synthesis of the 51-kDa protein with the opposite effect induced by the disruption of the corresponding upstream AUG −41 and GUG −37. This discrepancy can easily be explained by taking into account the fact that the ribosomes that initiate translation at AUG +1 are not the same ribosomes that initiate translation at upstream AUG −41 and GUG −37. Indeed, as suggested above, translation initiation at AUG +1 involves 40S subunits that have already bypassed AUG −41 and GUG −37 by leaky scanning. Furthermore, given its suboptimal nucleotide context (C at position −3), AUG +1 is itself also prone to be bypassed by scanning 40S subunits. Our observation that a significant amount of 48-kDa protein is still produced, even after the elimination of any possibility of termination-reinitiation, is a strong indication that leaky scanning over AUG +1 indeed occurs. Interestingly, it is already known that the recognition of a given AUG codon in a suboptimal context can be stimulated by blocking the scanning process immediately downstream from this AUG codon (23). By analogy, we thus suggest a new mechanism by which the recognition of AUG +1 by scanning 40S subunits may be facilitated through their piling up against terminating ribosomes transiently stalled at stop codons UAA +15 and UGA +35.
Two other indirect arguments are in favor of this piling-up model. (i) The first argument concerns the comparison of mutants −41/−37 and −41 (Fig. 6, compare lane 3 to lane 7 and lane 4 to lane 8), which indicates that synthesis of the 51-kDa protein can be slightly stimulated by the presence of GUG −37 as the only upstream initiation codon. This slight positive effect of GUG −37 cannot obviously be explained by backscanning even if all ribosomes terminating translation at UAA +15 could reinitiate at AUG +1. In contrast, according to the piling-up model proposed (Fig. 10), it is quite conceivable that each terminating ribosome transiently stalled at stop codon UAA +15 can induce more than one 40S subunit to recognize AUG +1, which otherwise would have bypassed this site. Two nonexclusive possibilities can explain why this positive contribution of GUG −37 is evidenced only after disruption of AUG −41. By modifying the nucleotide context of GUG −37 and placing it in first position, the disruption of AUG −41 may enhance the recognition of GUG −37, thus facilitating the detection of its slight positive effect on downstream initiation. Alternatively, the disruption of GUG −37, by modifying the nucleotide context of AUG −41, may enhance the preferential use of AUG −41, whose dominant negative effect may in turn mask the slight positive effect of GUG −37.
(ii) The second argument is based on our results showing that synthesis of the 51-kDa protein is less strongly affected by the disruption of stop codons UAA +15 and UGA +35 on capped than on uncapped mRNA. According to the piling-up model proposed, this difference could be explained if the bypassing of AUG +1 by leaky scanning occurs less frequently on capped than on uncapped mRNA. The observation that the capped version of the −33 Fli-1 mRNA leads to a greater excess of 51- over 48-kDa protein synthesis than does the uncapped version of the same mRNA (Fig. 5) is in agreement with that possibility.
A diagram summarizing the three steps involved in the translational regulation of Fli-1 mRNA by the conserved 5′ uORF is given in Fig. 10.
Biological significance of the translation regulation of Fli-1 mRNA by conserved 5′ uORF.
The high evolutionary conservation of the two 5′ uORF in Fli-1 mRNA strongly suggests that they have very important functions in vivo. Surprisingly, we found, like others, that both FLI-1 isoforms display similar transactivating properties (reference 10 and our unpublished data). We found also that, like the 51-kDa isoform (43), the 48-kDa isoform is able to inhibit the N,N′-hexamethylene- bis-acetamide (HMBA)-induced differentiation of MEL cells (data not shown). Obviously, these data do not exclude the possibility that these two different FLI-1 isoforms may have different functions in some particular cell contexts in vivo. However, one alternative explanation for the high conservation of 5′ uORF in Fli-1 mRNA might be more closely related to their translational regulatory function than to their property to allow the synthesis of two different FLI-1 isoforms per se. In that respect, it is noteworthy that most retroviral insertions leading to the activation of Fli-1 gene expression in leukemia occur precisely downstream of the conserved initiation codons of the two 5′ uORF, thus bypassing their negative control (5). One unique property of Fli-1 mRNA translation is to allow the constitutive repression of FLI-1 protein synthesis through the preferential translation initiation by upstream AUG −41 and GUG −37 while allowing their simultaneous positive regulation through the control of the translation termination process of the two short 5′ uORF. Indeed, it is already known that the efficiency of the termination-reinitiation mechanism is dependent on the phosphorylation state of eukaryotic initiation factor 2 (17), which is itself regulated by many external signals (17, 21). Furthermore, recent data obtained in experiments with yeast indicate that the translation termination process itself can also be regulated by variations in the environmental stress conditions (11). We therefore suggest that one of the main reasons for the conservation of the two 5′ uORF on Fli-1 mRNA might be to allow the rapid fine-tuning of the amounts of FLI-1 proteins by external signals while keeping these amounts below threshold levels which could be deleterious for cells in which the Fli-1 gene is transcribed. Alternatively or concomitantly, the two 5′ uORF might also be involved in the simultaneous control of Fli-1 mRNA stability through the activation of the nonsense mediated mRNA decay pathway (6, 15, 51). Fli-1 mRNAs are already known to display a high turnover in vivo (31, 43). Furthermore, according to current models (6, 15, 16, 51), it can be predicted that, given the location of UGA +35 less than 500 nt upstream of the next exon 2-exon 3 junction, Fli-1 mRNAs should be indeed prone to degradation through the nonsense mediated mRNA decay pathway depending on the control of the termination-reinitiation process at AUG +100. Experiments are in progress to investigate this latter possibility.
ACKNOWLEDGMENTS
We are very grateful to F. Moreau-Gachelin, F. Wendling, and E. Schneider for providing the different mouse Friend erythroleukemia cell lines and the 70Z/3 cell line, respectively. We are also very grateful to M. Lopez-Lastra and C. Gabus for technical advice on the synthesis of capped mRNAs and to J. J. Madjar and A. Greco for critical reading of the manuscript.
This study was supported by grants from the Université Lyon 1, the Centre National de la Recherche Scientifique, the Association pour la Recherche contre le Cancer (ARC grant 9764), the Ligue Nationale contre le Cancer (Comité national et Comités départementaux du Rhône, de la Drôme et de l'Yonne), and the Fondation de France.
REFERENCES
- 1.Baily R A, Bosselut R, Zucman J, Cormier F, Delattre O, Roussel M, Thomas G, Ghysdael J. DNA-binding and transcriptional activation properties of the EWS-FLI-1 fusion protein resulting from the t(11;22) translocation in Ewing sarcoma. Mol Cell Biol. 1994;14:3230–3241. doi: 10.1128/mcb.14.5.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbeau B, Bergeron D, Beaulieu M, Nadjem Z, Rassart E. Characterization of the human and mouse Fli-1 promoter regions. Biochim Biophys Acta. 1996;1307:220–232. doi: 10.1016/0167-4781(96)00060-7. [DOI] [PubMed] [Google Scholar]
- 3.Ben-David Y, Giddens E R, Bernstein A. Identification and mapping of a common proviral integration site Fli1 in erythroleukemia cells induced by Friend murine leukemia virus. Proc Natl Acad Sci USA. 1990;87:1332–1336. doi: 10.1073/pnas.87.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-David Y, Bernstein A. Friend virus induced erythroleukemia and the multistage of cancer. Cell. 1991;66:831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron D, Poliquin L, Houde J, Barbeau B, Rassart E. Analysis of proviruses integrated in Fli-1 and Evi-1 regions in Cas-Br-E MuLV-induced non-T-, non-B-cell leukemias. Virology. 1992;191:661–669. doi: 10.1016/0042-6822(92)90241-g. [DOI] [PubMed] [Google Scholar]
- 6.Culbertson M R. RNA surveillance: unforseen consequences for gene expression, inherited genetics disorders and cancer. Trends Genet. 1999;15:74–80. doi: 10.1016/s0168-9525(98)01658-8. [DOI] [PubMed] [Google Scholar]
- 7.Darby T G, Meibner J D, Rühlman A, Mueller V H, Scheibe R J. Functional interference between retinoic acid or steroid hormone receptors and the oncoprotein Fli-1. Oncogene. 1997;15:3067–3082. doi: 10.1038/sj.onc.1201503. [DOI] [PubMed] [Google Scholar]
- 8.Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, De Jong P, Rouleau G, Aurias A, Thomas G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 9.Denicourt C, Edouard E, Rassart E. Oncogene activation in myeloid leukemia by Graffy murine leukemia virus proviral integration. J Virol. 1999;73:4439–4442. doi: 10.1128/jvi.73.5.4439-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhulipala P D K, Lee L, Rao V N, Reddy E S P. Fli-b is generated by usage of differential splicing and alternative promoter. Oncogene. 1998;17:1149–1157. doi: 10.1038/sj.onc.1202030. [DOI] [PubMed] [Google Scholar]
- 11.Eaglestone S S, Cox B S, Tuite M F. Translation termination efficiency can be regulated in Saccharomyces cerevisiae by environmental stress through a prion-mediated mechanism. EMBO J. 1999;18:1974–1981. doi: 10.1093/emboj/18.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geballe A P, Morris D. Initiation codons within 5′-leaders of mRNAs as regulators of translation. Trends in Biochem Sci. 1994;19:159–165. doi: 10.1016/0968-0004(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 13.Ghysdael J, Boureux A. The ETS family of transcriptional regulators. In: Yaniv M, Ghysdael J, editors. Progress in gene expression. Vol. 1. Basel, Switzerland: Birkhauser Verlag; 1997. pp. 29–89. [Google Scholar]
- 14.Gunnery S, Mäivali U, Mathews M B. Translation of uncapped mRNA involves scanning. J Biol Chem. 1997;272:21642–21646. doi: 10.1074/jbc.272.34.21642. [DOI] [PubMed] [Google Scholar]
- 15.Hentze M W. Translational regulation: versatile mechanisms for metabolic and developmental control. Curr Opin Cell Biol. 1995;7:393–398. doi: 10.1016/0955-0674(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 16.Hentze M W, Kulozik A. A perfect message: RNA surveillance and nonsense-mediated decay. Cell. 1999;96:307–310. doi: 10.1016/s0092-8674(00)80542-5. [DOI] [PubMed] [Google Scholar]
- 17.Hinnenbusch A. Translational regulation of yeast GCN4. J Biol Chem. 1997;272:21661–21664. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- 18.Hwang W H, Su T S. Translational regulation of hepatitis B virus polymerase gene by termination-reinitiation of an upstream minicistron in a length-dependent manner. J Gen Virol. 1998;79:2181–2189. doi: 10.1099/0022-1317-79-9-2181. [DOI] [PubMed] [Google Scholar]
- 19.Jackson R J, Wickens M. Translational controls impinging on the 5′-untranslated region and initiation factor proteins. Curr Opin Genet Dev. 1997;7:233–241. doi: 10.1016/s0959-437x(97)80133-5. [DOI] [PubMed] [Google Scholar]
- 20.Johansen H, Shumperli D, Rosenberg M. Affecting gene expression by altering the length and sequence of the 5′ leader. Proc Natl Acad Sci USA. 1984;81:7698–7702. doi: 10.1073/pnas.81.24.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleijn M, Scheper G C, Voorma H O, Thomas A A M. Regulation of translation initiation by signal transduction. Eur J Biochem. 1998;253:531–544. doi: 10.1046/j.1432-1327.1998.2530531.x. [DOI] [PubMed] [Google Scholar]
- 22.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Laudet V, Hänni C, Stéhelin D, Duterque-Coquillaud M. Molecular phylogeny of the ETS gene family. Oncogene. 1999;18:1351–1359. doi: 10.1038/sj.onc.1202444. [DOI] [PubMed] [Google Scholar]
- 26.Leprince D, Gegonne A, Coll J, de Taisne C, Schneeberger A, Lacrou C, Stéhelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983;306:395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- 27.Lukkonen M G M, Tan W, Shwartz S. Efficiency of reinitiation of translation on human immunodeficiency virus type 1 mRNAs is determined by the length of the upstream open reading frame and by the intercistronic distance. J Virol. 1995;69:4086–4094. doi: 10.1128/jvi.69.7.4086-4094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macleod K, Leprince D, Stéhelin D. The ets gene family. Trends Biochem Sci. 1992;17:251–256. doi: 10.1016/0968-0004(92)90404-w. [DOI] [PubMed] [Google Scholar]
- 29.Mager A M, Grapin-Botton A, Ladjali K, Meyer D, Wolff C M, Stiegler P, Bonnin M A, Remy P. The avian Fli gene is specifically expressed during embryogenesis in a subset of neural crest cells giving rise to mesenchyme. Int J Dev Biol. 1998;42:561–572. [PubMed] [Google Scholar]
- 30.Mains P E, Sibley C H. LPS-nonresponsive variants of mouse B cell lymphoma, 70Z/3: isolation and characterization. Somatic Cell Genet. 1983;9:699–720. doi: 10.1007/BF01539475. [DOI] [PubMed] [Google Scholar]
- 31.Mao X, Miesfeld S, Yang H, Leiden J M, Thompson C B. The FLI-1 and chimeric EWS-FLI-1 oncoproteins display similar DNA binding specificities. J Biol Chem. 1994;269:18216–18222. [PubMed] [Google Scholar]
- 32.May W A, Lessnick S L, Braun B S, Klemsz M, Lewis B C, Lunsford L B, Hromas R, Dennis C T. The Ewing's sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1. Mol Cell Biol. 1993;13:7393–7398. doi: 10.1128/mcb.13.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mélet F, Motro B, Rossl D J, Zhang L, Bernstein A. Generation of a novel Fli-1 protein by gene targeting leads to a defect in thymus development and a delay in Friend virus-induced erythroleukemia. Mol Cell Biol. 1996;16:2708–2718. doi: 10.1128/mcb.16.6.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer D, Stiegler P, Hindemlang C, Mager A M, Remy P. Whole-mount in situ hybridization reveals the expression of the Xl-Fli gene in several lineages of migrating cells in Xenopus embryos. Int J Dev Biol. 1995;39:909–919. [PubMed] [Google Scholar]
- 35.Moreau-Gachelin F, Wendling F, Molina T, Denis N, Titeux M, Grimber G, Briand P, Vainchenker W, Tavitian A. Spi-1/PU.1 transgenic mice develop multistep erythroleukemias. Mol Cell Biol. 1996;16:2453–2463. doi: 10.1128/mcb.16.5.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunn M F, Seeburg P H, Moscovici C, Duesberg P H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983;306:391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- 37.Ohno T, Rao V N, Reddy E S P. EWS/Fli-1 chimeric protein is a transcriptional activator. Cancer Res. 1993;53:5859–5863. [PubMed] [Google Scholar]
- 38.Peabody D S, Berg P. Effect of upstream reading frames on translation efficiency in simian virus 40 recombinants. Mol Cell Biol. 1986;6:2704–2711. doi: 10.1128/mcb.6.7.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira R, Tran Quang C, Lesault I, Dolznig H, Beug H, Ghysdael J. FLI-1 inhibits differentiation and induces proliferation of primary erythroblasts. Oncogene. 1999;18:1597–1608. doi: 10.1038/sj.onc.1202534. [DOI] [PubMed] [Google Scholar]
- 40.Pestova T V, Hellen C U T. Ribosome recruitement and scanning: what's new. Trends Biochem Sci. 1999;24:85–87. doi: 10.1016/s0968-0004(99)01356-0. [DOI] [PubMed] [Google Scholar]
- 41.Rao G, Rekhtman N, Cheng G, Krasilov T, Skoultchi A. Deregulated expression of the PU.1 transcription factor blocks murine erythroleukemia cell terminal differentiation. Oncogene. 1997;14:123–131. doi: 10.1038/sj.onc.1200807. [DOI] [PubMed] [Google Scholar]
- 42.Remy P, Sénan F, Meyer D, Mager A M, Hindelang C. Overexpression of the Xenopus Xl-fli gene during early embryogenesis leads to anomalies in head and heart development and erythroid differentiation. Int J Dev Biol. 1996;40:577–589. [PubMed] [Google Scholar]
- 43.Starck J, Doubeikovski A, Sarrazin S, Gonnet C, Rao G, Skoultchi A, Godet J, Dusanter-Fourt I, Morlé F. Spi-1/PU.1 is a positive regulator of the Fli-1 gene involved in inhibition of erythroid differentiation in Friend erythroleukemia cell lines. Mol Cell Biol. 1999;19:121–135. doi: 10.1128/mcb.19.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamir A, Howard J, Higgins R R, Li Y J, Berger L, Zacksenhaus E, Reis M, Ben-David Y. Fli-1, an Ets-related transcription factor, regulates erythropoietin-induced erythroid proliferation and differentiation: evidence for direct transcriptional repression of the Rb gene during differentiation. Mol Cell Biol. 1999;19:4452–4464. doi: 10.1128/mcb.19.6.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas K R, Capecchi M R. Introduction of homologous DNA sequences into mammalian cells induces mutations in the cognate gene. Nature. 1986;324:34–38. doi: 10.1038/324034a0. [DOI] [PubMed] [Google Scholar]
- 46.Van der Velden A W, Thomas A M. The role of 5′ untranslated region of an mRNA in translation regulation during development. Int J Biochem Cell Biol. 1999;31:87–106. doi: 10.1016/s1357-2725(98)00134-4. [DOI] [PubMed] [Google Scholar]
- 47.Wazylyk C, Hahn S L, Giovanne A. The ets family of transcription factors. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- 48.Willis A E. Translational control of growth factor and proto-oncogene expression. Int J Biochem Cell Biol. 1999;31:73–86. doi: 10.1016/s1357-2725(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 49.Yamada T, Kondoh N, Matsumoto M, Yoshida M, Maekawa A, Oikawa T. Overexpression of PU.1 induces growth and differentiation inhibition and apoptotic cell death in murine erythroleukemia cells. Blood. 1997;59:1383–1393. [PubMed] [Google Scholar]
- 50.Yi H K, Fujimara Y, Ouchida M, Prasad D D K, Rado V N, Reddy E S P. Inhibition of apoptosis by normal and aberrant Fli-1 and erg proteins involved in human solid tumors and leukemias. Oncogene. 1997;14:1259–1268. doi: 10.1038/sj.onc.1201099. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Sun X, Qian Y, Laduca J P, Maquat L E. At least one intron is required for the nonsense-mediated decay of triosephosphate isomerase mRNA: a possible link between nuclear splicing and cytoplasmic translation. Mol Cell Biol. 1998;18:5272–5283. doi: 10.1128/mcb.18.9.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Eddy A, Teng Y T, Fritzler M, Kluppel M, Mélet F, Bernstein A. An immunological disease in transgenic mice that overexpress Fli-1, a member of the ets family. Mol Cell Biol. 1995;15:6961–6970. doi: 10.1128/mcb.15.12.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]