Abstract
A 69-year-old man visited our hospital due to an abnormal shadow on a chest X-ray. Chest CT showed a mass shadow in his left lower lobe accompanied by an infiltrative shadow in the right upper lobe. Thorough examination led to a diagnosis of pulmonary squamous cell lung carcinoma, stage IIIB (T3N2M0). Combination treatment with chemotherapy and programmed cell death receptor 1 (PD-1) inhibitor was started, leading to a partial response. However, his pre-existing pulmonary infiltrative shadow progressed during the maintenance treatment with PD-1 inhibitor, and sputum culture revealed Mycobacterium abscessus infection. Thus, exacerbation of pre-existing nontuberculous mycobacterial pulmonary disease (NTM-PD) resulting from treatment with PD-1 inhibitor was suspected. Then, treatment with PD-1 inhibitor was discontinued, and he underwent pulmonary resection after antibiotic therapy against Mycobacterium abscessus infection. Recently, special attention has been paid to the association of Mycobacterium tuberculosis (TB) infection and treatment with immune checkpoint inhibitors (ICIs) in TB-endemic areas. This case also emphasizes the importance of realizing the risk of NTM infection when treating patients with ICIs, especially in NTM-endemic areas.
Keywords: Non-small-cell lung cancer, Immune checkpoint inhibitor, PD-1 inhibitor, Nontuberculous mycobacterial pulmonary disease, Exacerbation
1. Introduction
The most recent progress in the treatment of patients with non-small-cell lung cancer (NSCLC) is the development of immune checkpoint inhibitors (ICIs), especially programmed cell death receptor 1 (PD-1) or programmed cell death receptor ligand 1 (PD-L1) inhibitor [1,2]. PD-(L)1 inhibitor with or without chemotherapy is the current standard of care for patients with treatment-naïve advanced NSCLC, raising concerns about increases of infectious diseases [3], and a growing number of reports have suggested the association of Mycobacterium tuberculosis (TB) infection with PD-(L)1 inhibitors [[4], [5], [6]]. Here, we report a patient with NSCLC whose pre-existing nontuberculous mycobacteria pulmonary disease (NTM-PD) exacerbated during treatment with PD-1 inhibitor and chemotherapy.
2. Case report
A 69-year-old man was referred to our hospital due to an abnormal shadow on a chest X-ray (Fig. 1A). Computed tomography (CT) showed a lung mass, 3.3 × 3.0 cm, in the left lower lobe (Fig. 2A) accompanied by an infiltrative shadow in the right upper lobe (Fig. 2B). Transbronchial tumor biopsy demonstrated squamous cell carcinoma. Positron emission tomography (PET) revealed mediastinal lymph node metastases, whereas no distant metastases were detected. There were no apparent brain metastases on magnetic resonance imaging (MRI). Hence, he was diagnosed with squamous cell lung carcinoma, stage IIIB (T3N2M0). Immunohistochemistry for PD-L1 revealed that his tumor proportion score (TPS) was 100%. Since curative radiation therapy was inappropriate because of the large radiation field, combination treatment with chemotherapy (nab-paclitaxel + carboplatin) and PD-1 inhibitor (pembrolizumab) was started. After four cycles of the treatment, he achieved a partial response (Fig. 2C); however, after two cycles of maintenance treatment with pembrolizumab, he developed a cough, and chest X-ray revealed progression of his pre-existing infiltrative shadow in the right upper lung field (Fig. 1B). His sputum culture was positive for Mycobacterium abscessus. Thus, exacerbation of pre-existing NTM-PD due to treatment with PD-1 inhibitor was suspected. Therefore, pembrolizumab was discontinued and antibiotic therapy with imipenem, amikacin, and clarithromycin was started. He then underwent right upper lobectomy and after that pembrolizumab was resumed concurrently with antibiotic therapy. However, disease progression was confirmed after three additional cycles of pembrolizumab. Thereafter, pembrolizumab was terminated and he is now receiving cytotoxic chemotherapy without recurrence of Mycobacterium abscessus.
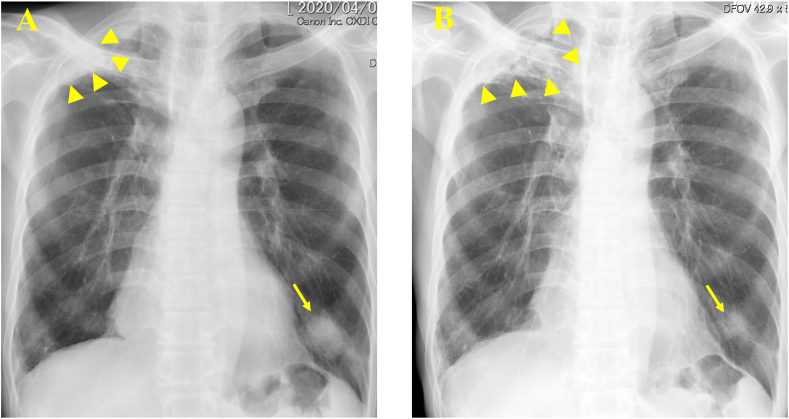
Fig. 1.
Chest radiographic examination. (A) A mass shadow in the left lower lung field (arrow) accompanied by an infiltrative shadow in the right upper lobe (arrowhead). (B) Shrinking of the mass shadow (arrow) with the progression of the pre-existing infiltrative shadow (arrowhead).
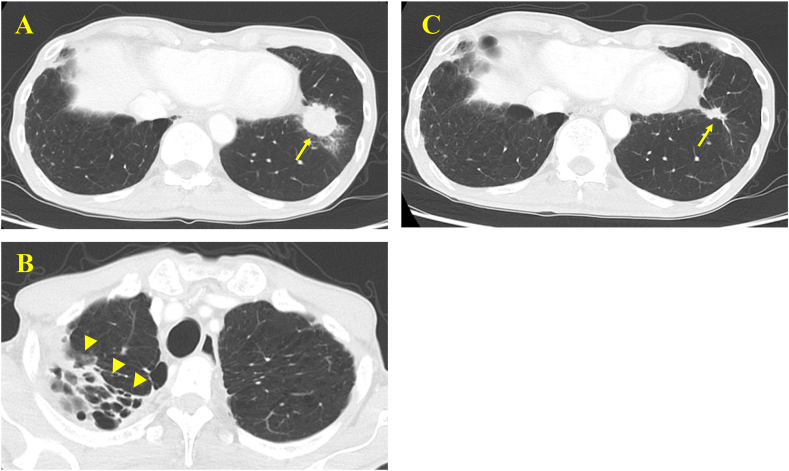
Fig. 2.
Chest computed tomography. (A) A lung mass in the left lower lobe (arrow). (B) An infiltrative shadow in the right upper lobe (arrowhead). (C) Shrinking of the mass shadow in the left lower lobe (arrow).
3. Discussion
Since the first publication of a case suggesting the association of TB infection and treatment with PD-(L)1 inhibitors in 2016, special attention has been paid to the association, especially in TB-endemic areas [7]. Although the precise mechanisms are not fully understood, it is postulated that PD-(L)1 inhibitors might unmask latent TB infection by overexpressing TB-specific T cells [8].
To our knowledge, only one case series (including 3 patients) has been reported regarding the association of NTM infection and treatment with PD-(L)1 inhibitors [9]; however, according to a retrospective review using the US Food and Drug Administration Adverse Events Reporting System (FAERS), there were 72 cases of TB infection and 13 cases of NTM infection resulting from treatment with PD-(L)1 inhibitors between January 2015 and March 2020. The reporting odds ratio (ROR) was measured to compare the risk of the infection between PD-(L)1 inhibitors and other drugs, and it was 1.79 (95% CI: 1.42–2.26, p < 0.0001) for TB infection and 5.49 (95% CI: 3.15–9.55, p < 0.0001) for NTM infection [10]. Therefore, it is clear that PD-(L)1 inhibitors increase the risk of NTM infection.
A recent Japanese nationwide hospital-based survey demonstrated that the incidence rate for NTM-PD was 14.7 cases per 100,000 person-years in 2014, which was approximately three times higher than that in 2007 [11]. In another international survey, the annual prevalence of NTM-PD was estimated at 24.9 cases per 100,000 persons in Japan, but only 6.2 cases per 100,000 persons in Europe [12]. Physicians, especially in NTM-endemic areas such as Japan, should be aware of the risk of pre-existing NTM-PD exacerbation due to PD-(L)1 inhibitors. Furthermore, considering the expected high prevalence of latent NTM infection without NTM-PD in NTM-endemic areas, potential risk of NTM infection should be paid close attention in every patient receiving PD-(L)1 inhibitor in those areas. In fact, the aforementioned case series were from Japan, and none of the three patients had NTM-PD at the baseline but newly developed it during treatment with PD-(L)1 inhibitors [9].
Recently, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, another ICI, was introduced for the treatment of NSCLC [13,14], and the incidence of immune-related adverse events (irAEs) is expected to increase. Accordingly, more patients will receive steroids, which may also increase the risk of infectious disease including NTM.
4. Conclusion
We encountered an NSCLC patient whose pre-existing NTM-PD exacerbated during treatment with PD-1 inhibitor and chemotherapy. Physicians in endemic areas should be aware of the risk of NTM infection when treating patients with ICI.
Funding source
None declared.
Authors’ disclosures of potential conflicts of interest
None declared.
References
- 1.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujita K., Kim Y.H., Kanai K., Yoshida H., Mio T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir. Med. 2019;146:66–70. doi: 10.1016/j.rmed.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Fujita K., Terashima T., Mio T. Anti-PD1 antibody treatment and the development of acute pulmonary tuberculosis. J. Thorac. Oncol. 2016;11:2238–2240. doi: 10.1016/j.jtho.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Jensen K.H., Persson G., Bongaard A.-L., Pohl M. Development of pulmonary tuberculosis following treatment with anti-PD-1 for non-small cell lung cancer. Acta Oncol. 2018;57:1127–1128. doi: 10.1080/0284186X.2018.1433877. [DOI] [PubMed] [Google Scholar]
- 6.van Eeden R., Rapoport B.L., Smit T., Anderson R. Tuberculosis infection in a patient treated with nivolumab for non-small cell lung cancer: case report and literature review. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reungwetwattana T., Adjei A.A. Anti-PD-1 antibody treatment and the development of acute pulmonary tuberculosis. J. Thorac. Oncol. 2016;11:2048–2050. doi: 10.1016/j.jtho.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Picchi H., Mateus C., Chouaid C., Besse B., Marabelle A. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatment. Clin. Microbiol. Infect. 2018;24:216–218. doi: 10.1016/j.cmi.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Fujita K., Yamamoto Y., Kanai O., Okamura M., Nakatani K. Development of Mycobacterium avium complex lung disease in patients with lung cancer on immune checkpoint inhibitors, open. Forum. Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa067. ofaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand K., Sahu G., Burns E., Ensor A., Ensor J. Mycobacterial infections due to PD-1 and PD-L1 checkpoint inhibitors. ESMO. Open. 2020;5 doi: 10.1136/esmoopen-2020-000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Namkoong Ho, Kurashima A., Morimoto K., Hoshino Y., Hasegawa N. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan, emerg. Infect. Dis. 2016;22:1116–1117. doi: 10.3201/eid2206.151086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schildkraut J.A., Gallagher J., Morimoto K., Lange C., Haworth C. Epidemiology of nontuberculous mycobacterial pulmonary disease in Europe and Japan by Delphi estimation. Respir. Med. 2020;173:106164. doi: 10.1016/j.rmed.2020.106164. [DOI] [PubMed] [Google Scholar]
- 13.Hellmann M.D., Paz-Ares L., Bernabe Caro R., Zurawski B., Kim S.W. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 14.Paz-Ares L., Ciuleanu T.E., Cobo M., Schenker M., Zurawski B. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]