Abstract
This experiment was undertaken to investigate the effects of dietary trans-anethole (TA) at 5 levels (0, 200, 400, 600, and 800 mg/kg of diet) on the growth performance, apparent nutrient digestibility and intestinal barrier function in broilers. Three hundred twenty 1-day-old Arbor Acres broilers were randomly divided into the 5 dietary treatments with 8 replicates each for 42 d. Dietary TA supplementation increased (P < 0.05) average daily feed intake (ADFI), but had no effects (P > 0.05) on average daily gain (ADG), feed/gain (F/G), and body weight (BW) of broilers throughout the entire experimental period. The apparent metabolizable energy (AME) and nitrogen-corrected apparent metabolizable energy (AMEn), the apparent total tract digestibility of dry matter (DM), crude protein (CP), organic matter (OM), and gross energy (GE) showed a quadratic increase (P < 0.05) with the increasing TA concentration in the diet. The apparent ileal digestibility of Lys, Met, Leu, Thr, Ala, Tyr, and Pro were higher (P < 0.05) in birds fed TA diets compared with control group. Dietary supplementation of 400 mg/kg of TA increased (P < 0.05) mRNA levels of jejunal and ileal Na+/glucose co-transporter (SGLT1) on d 21 and d 42, oligopeptide transporter 1 (PepT1) on d 42, and ileal mRNA expressions of occludin (OCLN), claudin-1 (CLDN-1), and mucin 2 (MUC2), villus height (VH), crypt depth (CD), and VH:CD on d 21, as well as jejunal zonula-occludens-1 (ZO-1) and ileal mucin 2 on d 42. Linear or quadratic responses of the jejunal CD and villus VH:CD ratio occurred (P < 0.01) with increasing dietary TA concentration on d 42. The inclusion of 400 mg/kg TA decreased (P < 0.05) cecal Escherichia coli population on d 21 and d 42, but increased (P < 0.05) Bifidobacterium population on d 21 and ileal Bifidobacterium on d 42. In conclusion, 400 mg/kg of TA is the optimum concentration for increasing nutrient utilization and intestinal barrier function of broilers.
Key words: broiler, trans-anethole, growth performance, nutrient digestibility, intestinal barrier function
INTRODUCTION
A favorable intestinal environment plays a key role in nutrient absorption and development of intestinal immune status (Clemente et al., 2012). It is widely confirmed that poultry performance is directly related to gastrointestinal function and health (Paraskeuas and Mountzouris, 2019). Intestinal health depends on the continuous interaction between diet and intestinal integrity, morphology, microbiota, and immunity (Du et al., 2016). Antibiotics were used in poultry diets for improving intestinal health and curing pathogen infection for many years. However, the prohibition on antibiotics has accelerated the research on seeking suitable natural alternatives with similar beneficial effects. Phytogenic feed additives (PFA) are reported to modulate gut health by positively affecting 4 interacting points as mentioned above, and then enhance livestock performance (Paraskeuas and Mountzouris, 2019; Pu et al., 2020; Xu et al., 2020).
Trans-anethole (TA), a main constituent of many essential oils of medicinal aromatic plants of more than 20 species (e.g., fennel, anise, and star anise), is a volatile terpenoid with anise flavor and easy to be deteriorated when exposed to light and high temperature. TA has been generally recognized as safe by the United States Food and Drug Administration (FDA) and widely used as an odorant in foods, cosmetics, alcoholic beverage, and perfumes (Sheikh et al., 2015; Aprotosoaie et al., 2016). Some data recorded in animal and cell line suggested that TA possess beneficial effects as a sensory additive in animal feed, including antimicrobial (Hançer Aydemir et al., 2018; Wieczyńska and Cavoski, 2018), antioxidant (Sá et al., 2018; Sá et al., 2020), anti-inflammatory (Kim et al., 2017; Zhang et al., 2018), ameliorating obesity (Kang et al., 2018; Rhee et al., 2018), and ameliorating hyperglycemia (Sheikh et al., 2015). In addition, some experimental data reported that TA had beneficial effects on cardiovascular and chronic diseases. The data recorded by Seo et al. (2018) firstly showed that TA could prevent hypertension induced by chronic exposure to both restraint stress and nicotine in rats. In vitro study showed that TA treatment suppressed the adipogenic differentiation of human mesenchymal stem cells (hMSCs), induced white adipocytes browning, and promoted lipid catabolism (Kang et al., 2018; Rhee et al., 2018), indicating its beneficial effects on obesity diseases. Based on its many biological activities, we hypothesized that TA may be used for growth promoters by improving the nutrient digestibility and intestinal barrier function in broilers. Therefore, the present study is first to investigate the effects of TA on the growth performance, nutrient digestibility, and intestinal barrier integrity in broilers.
MATERIALS AND METHODS
Preparation of Trans-anethole
The TA was kindly provided by Nanjing Dilger Medical Technology Co., Ltd (D105737, Nanjing, China). The analyzed purity of TA was 98.35%. The TA was stored in glass bottles in the dark and stored at 4°C until use.
Experimental Design, Birds and Management
This trial was carried out using a total of 320 one-day-old Arbor Acres broiler chicks (mixed sex) with similar initial body weight (39.75 ± 0.47 g) following the protocols of Animal Care approved by the Nanjing Agricultural University Animal Nutrition Research Institute (No. SYXK-2017-0027). The birds provided by a commercial hatchery (Yantai Land Animal Husbandry Co., Ltd, Yantai, China) were randomly allocated to 5 dietary treatments with 8 replicates of 8 birds each. Birds were fed mash corn-soybean meal based diets supplemented with 0, 200, 400, 600, and 800 mg/kg of TA respectively for 42 d in two phases (1–21 d and 22–42 d). The experimental diets were formulated to meet nutrient requirements recommended by Feeding Standard of chicken of the People's Republic of China (NY/T 33-2004). The ingredients and chemical composition of the basal diet are shown in Table 1. Four g/kg of Titanium dioxide (Zhejiang Jinghai New Material Co., Ltd, Quzhou, China) was externally added to the grower diet as an indicator for the ileal apparent amino acid digestibility measurement. TA was firstly mixed with soybean oil and then mixed with other ingredients. The experimental diet was prepared every 14 d and was kept in airtight containers prior to feeding.
Table 1.
Ingredients and nutrient composition of the basal diet1 (%).
| Item | Starter (1–21 d) | Grower (22–42 d) |
|---|---|---|
| Ingredients | ||
| Corn | 55.60 | 54.40 |
| Expanded soybean meal (46% CP) | 29.00 | 24.15 |
| Cottonseed meal | 2.50 | 3.00 |
| Wheat flour | 4.00 | 4.00 |
| Hydrolyzed feather meal | 1.50 | 1.50 |
| Soybean oil | 2.00 | 7.25 |
| Dicalcium phosphate | 0.90 | 0.80 |
| Limestone | 1.50 | 1.50 |
| Bentonite | 1.00 | 1.00 |
| Premix2 | 2.00 | 2.00 |
| Titanium dioxide | 0.00 | 0.40 |
| Chemical composition, analyzed | ||
| ME, calculated (Kcal/kg) | 2,894 | 3,234 |
| CP | 21.50 | 19.51 |
| Calcium | 0.96 | 0.84 |
| Total phosphorus | 0.66 | 0.55 |
| Lys | 1.45 | 1.40 |
| Met | 0.54 | 0.50 |
| Thr | 0.91 | 0.80 |
The experimental diet was the same basal diet supplemented with 0, 200, 400, 600, 800 mg of trans-anethole/kg of the basal diet.
Supplied per kilogram of diet: vitamin A, 11,500 IU; cholecalciferol, 3,500 IU; vitamin E, 30 mg; vitamin K3, 5 mg; thiamin, 3.38 mg; riboflavin, 9.0 mg; pyridoxine, 8.96 mg; vitamin B12, 0.025 mg; choline chloride, 800 mg; calcium pantothenate, 13 mg; niacin, 45 mg; biotin, 0.15 mg; folic acid, 1.20 mg; Mn, 60 mg; Fe, 66.5 mg; Zn, 88 mg; Cu, 8.8 mg; I, 0.70 mg; Se, 0.288 mg.
All of the diets were fed as mash and the birds had free access to feed and water. All of the birds were kept in wire cages in a temperature-controlled environment and the temperature was gradually reduced from 35°C on the first day to 22°C by 0.5°C per day until the end of the experiment.
Growth Performance Parameters
Data on the body weight (BW) and feed intake of birds of each cage were recorded weekly and used for calculating the average daily feed intake (ADFI), average daily gain (ADG), and feed/gain (F/G). Mortalities and health status were visually recorded daily to correct feed consumption.
Sample Collection
Three birds per pen were collected for excreta on d 35 for the measurement of nutrients apparent total tract digestibility (ATTD). Total excreta from each individual were collected for 3 d after 7 d of adaption period. Afterward, the daily excreta of each replicate (3 birds) were mixed, weighed and placed in excreta collection trays in the same time during collection period and stored at 4°C until nutrients analysis. Feathers and shredded dry skin were removed carefully from the excreta. At 39 d of age, the above birds were slaughtered by cervical dislocation for ileum digesta sampling 12 h after fasting in order to determine the apparent ileal digestibility (AID) of amino acids. The digesta from the Meckel's diverticulum to the ileocecal junction were immediately collected. The collected digesta was immediately freeze-dried, ground through 0.25-mm mesh, and mixed thoroughly until analysis of titanium and amino acid concentration.
At 21 and 42 d of age, 8 birds per group with average BW of its replicate were selected, stunned and subsequently sacrificed by cervical dislocation. Approximately 1.5 cm of middle jejunal and ileal segments were fixed in 4% paraformaldehyde for histomorphological analysis. Digesta in the ileum and cecum were collected into sterile 1.5 mL freezing tube for microbiota analysis. The another 3-cm jejunum and ileum segments were dissected and flushed with ice-cold sterile saline, then kept into 2 mL freezing tube until later analysis. The samples of intestinal segments and digesta were rapidly frozen in liquid nitrogen, later stored at −80°C for further analysis.
Chemical Analysis for Nutrient Digestibility
The samples of feed and excreta were analyzed according to the procedures of Association of Official Analytical Chemists (AOAC, 2000). The content of crude protein (CP) in the fresh samples was determined as 6.25 × Kjeldahl nitrogen. The contents of dry matter (DM), ether extract (EE), organic matter (OM) and gross energy (GE) were assayed by freeze-dried samples which were smashed to 40 meshes by grinder. The GE in the feed and excreta was determined using adiabatic bomb calorimeter (WHR-15, Changxing High Grade Educational Equipment Development Co. Ltd, Changxing, China). The apparent metabolizable energy (AME) and nitrogen-corrected apparent metabolizable energy (AMEn) value of the experimental diets was calculated according to Sibbald (1976). The ileal digesta samples were analyzed for amino acids using automatic amino acid analyzer (LA8080, Hitachi high-technologies Co. Ltd, Tokyo, Japan). Titanium concentration in the diet and ileal digesta samples was determined according to Short et al. (1996).
Morphological Measurements of the Jejunum and Ileum
The paraformaldehyde-fixed samples were dehydrated in graded ethanol, transparentized with xylol, and embedded in paraffin. The cross sections (5 μm) of intestinal segments were prepared with a microtome and stained with hematoxylin-eosin (H&E). A total of 10 well-oriented villus and crypts for each H&E-stained sections were randomly selected for measuring the villus height (VH), crypt depth (CD) with light microscope (Olympus CX31, Tokyo, Japan) and Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD). The mean of each cross section was used for statistical analysis.
RNA Extraction and Reverse Transcription-PCR Analysis
Extraction of total RNA was performed using the Trizol reagent (9108, TaKaRa Biotechnology, Dalian, Liaoning, China) according to the manufacturer's instructions. The purity and concentration of RNA was determined by microspectrophotometer (NanoDrop-1000, Thermo Fisher Scientific, Waltham, UK). Subsequently, 500 ng of total RNA from each sample was reversely transcribed to cDNA using PrimeScript RT reagent Kit with gDNA Eraser (RR036A, TaKaRa Biotechnology). The synthetic cDNA was stored at −20°C until Real-time fluorescent quantitative PCR analysis and the amplified products were distinguished on 1% agarose gels.
Real-Time Fluorescent Quantitative PCR
Real-time PCR was performed in 96 well microplates using ChamQ SYBR qPCR Master Mix Kit (Q311-02, Vazyme-innovation in enzyme technology, Nanjing, China) based on Applied Biosystems 7500 Real-time PCR System. The primers which are listed on Table 2 were commercially synthesized by Sangon Biotechnology Co., Ltd (Shanghai, China). The relative mRNA abundance of target genes was calculated using the 2-ΔΔCt method with endogenous reference gene (β-actin).
Table 2.
Gene-specific primers and GenBank numbers of chickens.
| Gene1 | GeneBank ID | Primer sequences (5’→3’) | Product size (bp) |
|---|---|---|---|
| SGLT1 | NM_001293240.1 | F:TGTGGGCATAGCAGGAACAG | 141 |
| R:TACTCCGGCATTGTCACCAC | |||
| PepT1 | NM_204365.1 | F:TTCCCATGGAGTCAACAGGC | 146 |
| R:GGCTGCTGCATTCTTGATGG | |||
| OCLN | NM_205128.1 | F:ATGCACCCACTGAGTGTTGG | 93 |
| R:GAGGTGTGGGCCTTACACAG | |||
| ZO-1 | XM_015278981.2 | F:AGCCCCTTGGTAATGTGTGG | 87 |
| R:TTGGGCGTGACGTATAGCTG | |||
| CLDN1 | NM_001013611.2 | F:GGTATGGCAACAGAGTGGCT | 91 |
| R:CAGCCAATGAAGAGGGCTGA | |||
| MUC2 | JX284122.1 | F:TGTGGTCTGTGTGGCAACTT | 128 |
| R:GTGACATCAGGGCACACAGA | |||
| β-Actin | NM_205518.1 | F:ACCGGACTGTTACCAACACC | 116 |
| R:CCTGAGTCAAGCGCCAAAAG |
Abbreviations: F, forward; R, reverse.
SGLT1, Na+/glucose co-transporter; PepT1, Oligopeptide transporter 1; OCLN, Occludin; ZO-1, Zonula occludens-1; CLDN-1, Claudin-1; MUC2, Mucin-2.
Bacterial DNA Extraction and Quantification
The extraction of total bacterial genomic DNA from digesta samples of ileum and cecum was performed by TIANamp Stool DNA Kit (DP328, Tiangen Biotechnology Co., Ltd, Beijing, China) following manufacturer's instruction. Subsequently, RT-qPCR procedure was conducted to estimate the amount of Escherichia coli, Lactobacillu and Bifidobacterium using ChamQ SYBR qPCR Master Mix Kit (Q311-02, Vazyme Biotechnology Co., Ltd, Nanjing, China), and Applied Biosystems 7500 Real-time PCR System. As shown in Table 3, the primers targeting the 16S rRNA gene were found from the relevant literature (Walter et al., 2001; Bartosch et al., 2004; Rinttilä et al., 2004) and commercially synthesized via Sangon Biotechnology Co., Ltd. Specific standard curves were established for the quantification of above bacteria referred to Jiao et al. (2013). The bacteria copies were expressed as log10 cells/g of digesta for statistical analysis (Chen et al., 2013).
Table 3.
Primer and probe sequences used for quantitative real-time PCR.
| Item | Primer sequence (5’→3’) | References |
|---|---|---|
| Escherichia coli | F:CATTGACGTTACCCGCAGAAGAAGC | Bartosch et al. (2004) |
| R: CTCTACGAGACTCAAGCTTGC | ||
| Lactobacillus | F:AGCAGTAGGGAATCTTCCA | Walter et al. (2001) |
| R: CACCGCTACACATGGAG | ||
| Bifidobacterium | F:TCGCGTC(C/T)GGTGTGAAAG | Rinttilä et al. (2004) |
| R: CCACATCCAGC(A/G)TCCAC |
Abbreviations: F, forward; R, reverse.
Statistical Analysis
All data were analyzed by one-way ANOVA using the GLM procedure of SAS (SAS Institute, 2001) and checked normality and homogeneity of variances before statistical analysis. The data on performance parameters and nutrient digestibility was analyzed on a pen basis, whereas data on intestinal barrier integrity was analyzed on individual bird. Values are presented as means with a standard error (SEM). Significant effects were analyzed using Tukey's HSD test and declared at P < 0.05. In addition, orthogonal polynomial contrasts were performed to investigate the linear and quadratic effects of dietary TA supplementation level, and the significance was declared at P < 0.05.
RESULTS
Growth Performance
All of the birds were healthy and no mortality appeared. As shown in Table 4, overall growth of birds was not affected (P > 0.05) by the inclusion of TA. However, the ADFI of birds supplemented with TA was higher (P < 0.05) than that of control birds during the grower phase (d 22 to d 42) and entire trial period (d 1–d 42). All birds had similar ADG, F/G, and BW in either phase or the entire period of the experiment. Furthermore, TA inclusion level had no linear (P > 0.05) or quadratic (P > 0.05) effects on ADFI, ADG, and F/G, whereas linearly increased (P = 0.022) or tended (P = 0.072) to quadratically increase ADG of d 22 to d 42.
Table 4.
The growth performance parameters of broilers fed diets with different concentration of TA supplementation1.
| Item2 | Dietary TA concentration, mg/kg |
SEM | Effects (P-value) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| 1–21 d | |||||||||
| ADFI, g | 37.51 | 38.67 | 38.08 | 37.68 | 37.92 | 0.219 | 0.511 | 0.919 | 0.667 |
| ADG, g | 28.49 | 29.48 | 29.45 | 28.61 | 29.54 | 0.183 | 0.194 | 0.358 | 0.554 |
| F/G, g/g | 1.32 | 1.31 | 1.29 | 1.32 | 1.29 | 0.008 | 0.601 | 0.292 | 0.575 |
| 22–42 d | |||||||||
| ADFI, g | 115.37b | 115.67b | 123.75ab | 124.36a | 122.13ab | 0.992 | 0.013 | 0.235 | 0.459 |
| ADG, g | 73.56 | 73.97 | 73.87 | 74.48 | 74.44 | 0.367 | 0.263 | 0.022 | 0.072 |
| F/G, g/g | 1.58 | 1.56 | 1.66 | 1.66 | 1.64 | 0.007 | 0.602 | 0.775 | 0.597 |
| 1–42 d | |||||||||
| ADFI, g | 78.30b | 81.10ab | 83.60a | 80.25ab | 81.67ab | 0.673 | 0.029 | 0.637 | 0.623 |
| ADG, g | 51.27 | 51.91 | 52.32 | 51.56 | 53.25 | 0.302 | 0.289 | 0.098 | 0.243 |
| F/G, g/g | 1.53 | 1.56 | 1.59 | 1.56 | 1.53 | 0.007 | 0.579 | 0.508 | 0.458 |
| BW, g | |||||||||
| 0 d | 39.86 | 39.67 | 39.44 | 40.33 | 39.47 | 0.108 | 0.108 | 0.888 | 0.970 |
| 21 d | 646.88 | 654.38 | 653.75 | 651.88 | 662.38 | 2.356 | 0.370 | 0.091 | 0.239 |
| 42 d | 2191.69 | 2207.78 | 2205.06 | 2215.88 | 2225.61 | 9.222 | 0.267 | 0.229 | 0.491 |
Means within a row with different letters differ significantly (P < 0.05).
Data are means for 8 replicates of 8 birds per replicate.
Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; F/G, feed/gain.
Nutrient Digestibility
The addition of TA had positive effects (P < 0.05) on AME and AMEn, ATTD of DM, CP, and GE (Table 5). Furthermore, the AME and AMEn, digestibility of DM, CP, OM, and GE were quadratically increased (P < 0.05) with increasing TA concentration. No significant effects of TA inclusion on the ATTD of EE and OM were seen (P > 0.05).
Table 5.
The total tract apparent digestibility of DM, CP, EE, OM, GE, AME, and AMEn of broilers fed diets with different concentration of TA supplementation1.
| Item | Dietary TA concentration, mg/kg |
SEM | Effects (P-value) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| DM, % | 72.65ab | 73.31ab | 75.43a | 74.21ab | 71.28b | 0.491 | 0.045 | 0.578 | 0.017 |
| CP, % | 54.69b | 62.25a | 61.13a | 61.31a | 59.19ab | 0.766 | 0.007 | 0.137 | 0.004 |
| EE, % | 78.75 | 78.97 | 81.31 | 77.02 | 74.74 | 1.345 | 0.485 | 0.228 | 0.237 |
| OM, % | 77.22 | 79.69 | 78.86 | 77.85 | 76.57 | 0.431 | 0.184 | 0.268 | 0.032 |
| GE, % | 73.55b | 76.83ab | 79.12a | 78.76a | 75.85ab | 0.635 | 0.018 | 0.134 | 0.003 |
| AME, Kcal/kg | 3,295b | 3,442ab | 3,544a | 3,530a | 3,398ab | 0.119 | 0.018 | 0.134 | 0.003 |
| AMEn, Kcal/kg | 3,143b | 3,253ab | 3,341a | 3,344a | 3,229ab | 0.112 | 0.048 | 0.142 | 0.009 |
Abbreviations: AMEn, nitrogen-corrected apparent metabolizable energy; CP, crude protein; DM, dry matter; GE, gross energy; OM, organic matter.
Means within a row with different letters differ significantly (P < 0.05).
Data are means for 8 replicates of 3 birds per replicate.
As shown in Table 6, means of the AID of Lys, Met, Leu, and Thr were higher (P < 0.05) in the TA supplemented groups compared with control group. Additionally, there was a quadratic effect (P < 0.05) of the supplemented TA on the AID of Lys, and Leu. The TA inclusion level had a linear (P < 0.05) or quadratic (P < 0.05) effect on AID of Met, Ile, Thr, and Val.
Table 6.
The apparent ileal digestibility of essential amino acids of broilers fed diets with different concentration of TA supplementation1.
| Item,% | Dietary TA concentration, mg/kg |
SEM | Effects (P-value) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| Lys | 85.73b | 86.10b | 87.65a | 85.73b | 85.53b | 0.118 | <0.001 | 0.519 | 0.002 |
| Met | 78.10b | 81.56a | 82.95a | 82.33a | 81.36a | 0.345 | 0.001 | 0.013 | <0.001 |
| Arg | 93.30 | 93.25 | 93.12 | 93.64 | 92.78 | 0.147 | 0.477 | 0.537 | 0.611 |
| His | 82.37 | 83.89 | 83.70 | 83.89 | 83.53 | 0.232 | 0.221 | 0.168 | 0.088 |
| Leu | 82.43b | 82.69b | 84.07a | 83.65ab | 83.05ab | 0.190 | 0.036 | 0.130 | 0.033 |
| Ile | 77.29b | 78.46ab | 78.27ab | 79.02ab | 80.14a | 0.304 | 0.071 | 0.005 | 0.020 |
| Phe | 83.81 | 84.05 | 84.28 | 84.20 | 83.80 | 0.242 | 0.956 | 0.940 | 0.725 |
| Thr | 75.27b | 75.84ab | 76.06ab | 76.95a | 76.63ab | 0.217 | 0.041 | 0.015 | 0.044 |
| Val | 75.78b | 76.32ab | 76.63ab | 77.39ab | 77.97a | 0.266 | 0.078 | 0.005 | 0.018 |
Means within a row with different letters differ significantly (P < 0.05).
Data are means for 8 replicates of 3 birds per replicate.
Gene Expression of Jejunal and Ileal Glucose and Amino Acid Transporters
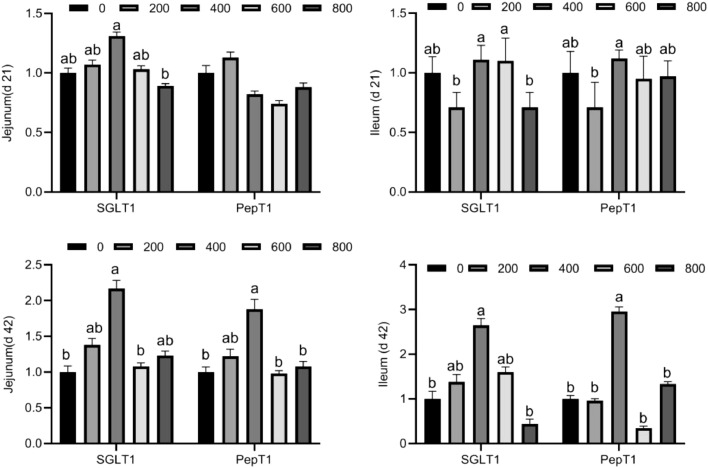
Compared with the control group, the expression of Na+/glucose co-transporter (SGLT1) and Oligopeptide transporter 1 (PepT1) in the jejunum and ileum of broilers was higher (P < 0.05) with 400 mg/kg of TA administration (Figure 1). TA inclusion had no effect (P > 0.05) on PepT1 expression in the jejunum of broilers on d 21. Additionally, TA inclusion level significantly affected jejunal and ileal expression levels of SGLT1 and PepT1. The inclusion of 800 mg/kg TA showed lowest SGLT1 and PepT1 expression compared with all other groups.
Figure 1.
The gene expression of glucose and amino acid transporters in the jejunum and ileum of broilers. Values are means (n = 8), with their standard errors represented by vertical bars. a-bMeans within a row with different letters differ significantly (P < 0.05). SGLT1, Na+/glucose co-transporter; PepT1, Oligopeptide transporter 1.
Barrier Integrity Related Gene Expression in Jejunum and Ileum
As shown in Table 7, compared with control group, TA supplemented at 400 mg/kg increased (P < 0.05) the mRNA abundance of occludin (OCLN), claudin-1 (CLDN-1), and mucin 2 (MUC2) in ileum of broilers on d 21, and jejunal zonula occludens-1 (ZO-1) and ileal MUC2 on d 42. Strangely, TA supplementation decreased (P < 0.05) ileal mRNA level of CLDN-1 on d 42. In addition, linear or quadratic responses of jejunal expression of CLDN-1 and MUC2, and ileal expression of OCLN on d 21, and ileal TJ protein expression except ZO-1 on d 42 occurred (P < 0.05) with increasing dietary TA concentration. There was also a quadratic (P < 0.05) effect of TA on ileal expression of CLDN-1 on d 21, and jejunal expression of MUC2 on d 42.
Table 7.
The mRNA expression of TJ protein in the jejunum and ileum of broilers fed diets with different concentration of TA supplementation1.
| Item2 | Dietary TA concentration, mg/kg |
SEM | Effects (P-value) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| 21 d Jejunum |
|||||||||
| OCLN | 1.00 | 0.67 | 1.33 | 0.47 | 0.74 | 0.096 | 0.072 | 0.186 | 0.223 |
| ZO-1 | 1.00 | 0.97 | 0.95 | 0.99 | 1.21 | 0.034 | 0.141 | 0.440 | 0.096 |
| CLDN-1 | 1.00ab | 0.91ab | 1.18a | 0.64b | 0.69b | 0.046 | 0.007 | 0.014 | 0.023 |
| MUC2 | 1.00a | 1.09a | 0.68ab | 0.57b | 0.53b | 0.071 | 0.043 | 0.006 | 0.022 |
| Ileum | |||||||||
| OCLN | 1.00b | 1.19ab | 1.46a | 1.17b | 1.16b | 0.136 | 0.019 | 0.008 | 0.013 |
| ZO-1 | 1.00 | 0.97 | 1.02 | 0.87 | 0.99 | 0.026 | 0.396 | 0.485 | 0.714 |
| CLDN-1 | 1.00b | 0.92b | 1.47a | 1.43a | 0.14c | 0.042 | <0.001 | 0.105 | <0.001 |
| MUC2 | 1.00b | 0.78b | 1.52a | 1.07ab | 0.82b | 0.060 | 0.007 | 0.743 | 0.717 |
| 42 d | |||||||||
| Jejunum | |||||||||
| OCLN | 1.00 | 0.87 | 1.15 | 0.85 | 1.33 | 0.185 | 0.916 | 0.615 | 0.807 |
| ZO-1 | 1.00b | 1.15ab | 1.36a | 1.13ab | 1.06b | 0.062 | 0.028 | 0.844 | 0.229 |
| CLDN-1 | 1.00 | 0.30 | 0.31 | 0.48 | 0.68 | 0.104 | 0.207 | 0.542 | 0.076 |
| MUC2 | 1.00 | 0.85 | 1.02 | 0.79 | 0.58 | 0.058 | 0.132 | 0.958 | 0.046 |
| Ileum | |||||||||
| OCLN | 1.00a | 1.14a | 1.01a | 0.51ab | 0.18b | 0.106 | 0.033 | 0.015 | 0.042 |
| ZO-1 | 1.00ab | 0.73ab | 1.31a | 0.86ab | 0.41b | 0.078 | 0.014 | 0.352 | 0.652 |
| CLDN-1 | 1.00a | 0.39b | 0.19b | 0.15b | 0.16b | 0.065 | 0.007 | <0.001 | <0.001 |
| MUC2 | 1.00ab | 0.95ab | 1.90a | 1.38ab | 0.52b | 0.124 | 0.017 | 0.747 | 0.866 |
Means within a row with different letters differ significantly (P < 0.05).
Data are means for 8 replicates of 1 bird per treatment.
Abbreviations: OCLN, occludin; ZO-1, zonula occludens-1; CLDN-1, claudin-1; MUC2, mucin 2.
Morphological Measurements of Jejunum and Ileum
Broilers consuming TA-containing diets had considerably higher (P < 0.05) ileal VH and VH:CD ratio than control group, whereas had no differences (P > 0.05) on jejunal VH, CD, and VH:CD ratio on d 21 (Table 8). The lower CD and higher VH:CD ratio in the jejunum of broilers on d 42 was observed (P < 0.05) in TA group compared with the control group. In addition, the TA showed a linear (P < 0.05) or quadratic (P < 0.05) effect on the ileal VH and VH:CD ratio on d 21, and jejunal CD and VH:CD ratio (P < 0.01) on d 42.
Table 8.
Intestinal morphology of broilers fed diets with different concentration of TA supplementation1.
| Item2 | Dietary TA concentration, mg/kg |
SEM | Effects (P-value) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| 21 d Jejunum |
|||||||||
| VH, μm | 360.37 | 363.81 | 360.48 | 337.05 | 367.96 | 9.382 | 0.855 | 0.859 | 0.890 |
| CD, μm | 126.77 | 135.96 | 135.43 | 133.4 | 126.34 | 2.239 | 0.488 | 0.831 | 0.187 |
| VH:CD | 2.86 | 2.68 | 2.68 | 2.53 | 2.93 | 0.072 | 0.421 | 0.984 | 0.246 |
| Ileum | |||||||||
| VH, μm | 236.82b | 274.24ab | 300.18a | 281.93ab | 299.06a | 5.421 | 0.005 | 0.002 | 0.002 |
| CD, μm | 94.49b | 109.25a | 100.92ab | 92.22b | 94.13b | 1.601 | 0.011 | 0.169 | 0.086 |
| VH:CD | 2.50b | 2.52b | 2.99ab | 3.08a | 3.19a | 0.061 | 0.001 | <0.001 | 0.003 |
| 42 d Jejunum |
|||||||||
| VH, μm | 384.41 | 362.9 | 420.45 | 445.23 | 364.95 | 14.849 | 0.370 | 0.099 | 0.136 |
| CD, μm | 115.33a | 113.90ab | 100.79b | 101.07b | 100.97b | 1.833 | 0.028 | 0.004 | 0.009 |
| VH:CD | 3.33b | 3.19b | 4.17a | 4.41a | 3.61ab | 0.133 | 0.035 | 0.002 | 0.007 |
| Ileum | |||||||||
| VH, μm | 279.26 | 322.91 | 307.69 | 290.93 | 284.81 | 7.590 | 0.424 | 0.678 | 0.316 |
| CD, μm | 87.66 | 89.51 | 83.90 | 86.40 | 80.69 | 1.255 | 0.254 | 0.067 | 0.152 |
| VH:CD | 3.19 | 3.61 | 3.67 | 3.37 | 3.53 | 0.098 | 0.541 | 0.538 | 0.489 |
Means within a row with different letters differ significantly (P < 0.05).
Data are means for 8 replicates of 1 bird per treatment.
CD, crypt depth; VH, villus height.
Microflora Populations in the Ileal and Cecal Digesta
As shown in Table 9, dietary supplementation of TA resulted in lower (P < 0.05) amount of cecal E. coli and higher Bifidobacterium, whereas had no effect (P > 0.05) on ileal microflora population compared with the control group on d 21. Additionally, birds fed TA diets tended (P = 0.058) to have higher amount of Lactobacillu in the cecal digesta on d 21. On d 42, the inclusion of TA increased (P < 0.05) the amount of ileal and cecal Lactobacillu, and ileal Bifidobacterium, but decreased (P < 0.05) the E. coli compared with the control group. Furthermore, linear (P < 0.01) or quadratic (P < 0.01) responses of E. coli quantity, and quadratic (P < 0.01) response of Bifidobacterium quantity in the cecal digesta occurred with increasing dietary TA concentration on d 21. At 42 d of age, the amount of E. coli in the ileum was quadratically (P = 0.006) decreased, and the Lactobacillu in the cecum was linearly (P = 0.030) increased.
Table 9.
Microflora population in the ileum and cecum content of broilers fed diets with different concentration of TA supplementation1.
| Item, log10 cells/g digesta | Dietary TA concentration, mg/kg |
SEM | Effects (P-value) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | ANOVA | Linear | Quadratic | ||
| 21 d Ileum |
|||||||||
| Escherichia coli | 9.72 | 9.94 | 9.69 | 9.83 | 9.82 | 0.175 | 0.992 | 0.940 | 0.996 |
| Lactobacillu | 11.18 | 11.54 | 11.97 | 11.6 | 11.8 | 0.127 | 0.370 | 0.156 | 0.208 |
| Bifidobacterium | 7.07 | 6.76 | 7.01 | 7.2 | 7.28 | 0.119 | 0.693 | 0.303 | 0.453 |
| Cecum | |||||||||
| Escherichia coli | 12.18a | 10.66b | 9.42c | 9.36c | 11.16ab | 0.175 | <0.001 | 0.001 | 0.003 |
| Lactobacillu | 9.02b | 9.42ab | 9.56ab | 10.61a | 8.83b | 0.195 | 0.058 | 0.585 | 0.123 |
| Bifidobacterium | 8.75b | 8.53b | 10.41a | 10.88a | 9.00b | 0.165 | <0.001 | 0.162 | 0.004 |
| 42 d | |||||||||
| Ileum | |||||||||
| Escherichia coli | 9.09a | 7.72b | 7.80b | 8.06b | 8.40ab | 0.129 | 0.014 | 0.329 | 0.006 |
| Lactobacillu | 9.03ab | 8.37bc | 9.67a | 8.55bc | 7.77c | 0.179 | 0.027 | 0.103 | 0.070 |
| Bifidobacterium | 6.81ab | 6.89ab | 7.28a | 6.87ab | 6.67b | 0.074 | 0.032 | 0.363 | 0.649 |
| Cecum | |||||||||
| Escherichia coli | 10.08a | 10.12a | 9.27b | 9.72ab | 9.89ab | 0.135 | 0.028 | 0.425 | 0.295 |
| Lactobacillu | 12.02b | 11.92b | 12.86ab | 13.47a | 12.11b | 0.190 | 0.042 | 0.030 | 0.073 |
| Bifidobacterium | 8.12 | 8.54 | 8.64 | 8.27 | 7.90 | 0.092 | 0.094 | 0.622 | 0.456 |
Means within a row with different letters differ significantly (P < 0.05).
Data are means for 8 replicates of 1 bird per treatment.
DISCUSSION
The positive effects of TA in gastrointestinal health of broilers, such as enhancing nutrient utilization (Jamroz and Kamel, 2002), delaying gastric emptying (Asano et al., 2016), gastroprotector activity (Freire et al., 2005), antimicrobial (Senatore et al., 2013; Wieczyńska and Cavoski, 2018; Kwiatkowski et al., 2019), and anti-inflammatory (Paraskeuas et al., 2017; Paraskeuas and Mountzouris, 2019) properties, had been widely reported. In this study, the results revealed that the inclusion of TA had no significant effects on ADG, BW and F/G, whereas the ADFI was significantly increased. Similar with our results, the growth performance of broilers was not affected by inclusion of plant essential oils containing TA (Amad et al., 2011; Hafeez et al., 2016). However, Ding et al. (2020) observed that the ADFI in White Leghorn broilers was quadratically increased with increasing dietary star anise oil (SAO) levels. Prior to that, the results found by us indicated that inclusion of SAO tended to quadratically increased ADFI of laying hens (Yu et al., 2018). This may be due to the aromatic anise flavor of SAO which could stimulate appetite, thereby increasing the ADFI (Ertas et al., 2005; Wang et al., 2011). Moreover, the experimental conditions, hygiene, animal age, diet type, and altered microbiota may also affect the performance response of broilers to TA (Goel et al., 2008).
Previously, we found that SAO may have nutrient releasing effects of broiler diets (Yu et al., 2019). Similarily, it was detected that the essential oil compound consisting of thymol and anethole significantly increased the ileal nutrient digestibility in broilers (Amad et al., 2011). The results of this study demonstrated that the inclusion of TA significantly increased the apparent digestibility of protein, energy and amino acids especially the AID of lysine and methionine as well as the transcript abundance of SGLT1 and PepT1 compared with control group. In accordance with that, Reyer et al. (2017) indicated that compound essential oils containing star anise induced a dose-dependent membrane recruitment of SGLT1 and PepT1 in Caco-2 cells. Kreydiyyeh et al. (2003) demonstrated that 0.05% SAO significantly increased jejunal glucose absorption, and TA is responsible for its biological effects. Moreover, a positive impact of TA on transcriptional level of lipid and carbohydrate metabolism was well demonstrated (Sheikh et al., 2015; Kang et al., 2018; Song et al., 2020), which provide evidences for the possible molecular mechanism of TA increasing nutrient digestibility of broilers. Taken together, the increase in nutrient digestibility in the present study can be attributed to the interaction of TA with the nutrient transport absorption function of intestinal epithelium. Additionally, the digestive stimulant functions of TA may contribute to a reduction of the protein, energy and amino acids concentration in the diets of broilers. The underlying mechanisms of promoting digestion of TA require more in-depth characterization.
Our results also found that dietary TA administration enhanced gene expression of TJ protein, improved intestinal morphology, and modulated ileal and cecal microbiota populations in broilers. The intestinal barrier integrity is performed by a layer of epithelial cells and TJ protein. TJ protein is mainly composed of transmembrane protein compounds such as OCLN, ZO cytosolic proteins, and the CLDN family (Paraskeuas and Mountzouris, 2019). Furthermore, MUC2 secreted by goblet cells has beneficial effects on intestinal barrier protection, thus playing a key role in intervening intestinal inflammation (Moughan et al., 2013). In the current study, the inclusion of TA led to higher levels of OCLN, CLDN-1, ZO-1, and MUC2 in the jejunum and ileum compared with control group. The results indicated that TA may enhance intestinal barrier function of broilers via increasing the mRNA levels of TJ protein and mucins. Similarly, previous studies reported the gastroprotector and mucous protective properties of TA (Schmeda-Hirschmann et al., 2002; Freire et al., 2005). This indicated that TA may protect the gastrointestinal mucous against aggressive factors. We also noted that TA supplementation decreased ileal mRNA level of CLDN-1. As we know, there is discrepancy between mRNA and protein levels of TJ protein due to post-translational modification (Zhang et al., 2020). Hence, this unreasonable result is inexplicable and requires to be confirmed by determination of protein level of CLDN-1. Some data showed that SAO containing 90% of TA increased the secretions of intestinal mucous and absorption surface area in the intestine (Jang et al., 2007; Amad et al., 2011). This is consistent with our results that VH and VH: CD was significantly increased by TA inclusion, which revealed a higher absorption surface area in the intestine. On the other hand, the antimicrobial activities of TA were widely reported, such as Staphylococcus aureus strains (Kwiatkowski et al., 2019), Pseudomonas aeruginosa (Hançer Aydemir et al., 2018), E. coli (Yi et al., 2021), Klebsiella pneumonia (Senatore et al., 2013) and so on. The antimicrobial properties of TA may be beneficial for gut microflora composition and favorable in intestinal health. Similar with that, the results of this study demonstrated that the cecal E. coli amount was significantly decreased whereas the Lactobacillu and Bifidobacterium were clearly increased. Some literature reported that the influence of PFA on intestinal microbiota population is directly linked with phytogenic composition and supplementation concentration (Cross et al., 2007; Mountzouris et al., 2011). We have concluded that the antimicrobial mechanisms of TA can be attributed to the degradation of cell wall, destruction of cytoplasmic membrane, or denaturing of membrane proteins, which result in ions, DNA, protein, and glucose leakage, lipid damage and finally cell death (Yu et al., 2020). Obviously, the increased nutrient digestibility in this study may also be attributed to the enhanced intestinal barrier function by TA in broilers.
Apparently, our findings indicated that the inclusion of 400 mg/kg TA appeared better nutrient digestibility and intestinal barrier function of broilers. However, 800 mg/kg TA had adverse effects on that, which is consistent with our previous finding that high concentration of SAO decreased the nutrient digestibility of laying hens (Yu et al., 2019). Previous studies reported that dietary phytogenic compounds concentration could affect broiler response (Ciftci et al., 2005; Soltan et al., 2008). The results conducted by Ding et al. (2020) also observed a dose-dependent efficacy of SAO on enhancing growth performance and antioxidant status of broilers. It was also reported that the addition concentration of PFA could affect the efficacy (Mountzouris et al., 2011; Reyer et al., 2017). As mentioned earlier, TA is the main active constituent of SAO which is faint yellow with a highly anise flavor. The strong pungent anise smell may influence the diet's taste and broilers’ appetite, thereby lead to stress response of broilers when added to the diet at a high concentration. On the basis of that, an inferior effect of high concentration of TA on nutrient utilization and intestinal barrier integrity was detected. This phenomenon suggested that the nutrient digestibility and intestinal barrier integrity might response to TA inclusion in a dose dependent manner.
CONCLUSIONS
Inclusion of 400 mg/kg TA increased intestinal transcription abundance of SGLT1 and PepT1 transporters and intestinal barrier integrity, which may be the underlying mechanisms of TA exerting its promoting digestion functions.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (No.2018YFD0501101); and the National Natural Science Foundation of China (No. 31802094).
DISCLOSURES
The authors declare that there is no conflict of interest.
REFERENCES
- Amad A.A., Manner K., Wendler K.R., Neumann K., Zentek J. Effects of a phytogenic feed additive on growth performance and ileal nutrient digestibility in broiler chickens. Poult. Sci. 2011;90:2811–2816. doi: 10.3382/ps.2011-01515. [DOI] [PubMed] [Google Scholar]
- Aprotosoaie A.C., Costache I.I., Miron A. Anethole and its role in chronic diseases. Adv. Exp. Med. Biol. 2016;929:247–267. doi: 10.1007/978-3-319-41342-6_11. [DOI] [PubMed] [Google Scholar]
- Asano T., Aida S., Suemasu S., Mizushima T. Anethole restores delayed gastric emptying and impaired gastric accommodation in rodents. Biochem. Biophys. Res. Commun. 2016;472:125–130. doi: 10.1016/j.bbrc.2016.02.078. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists . 17th ed. Assoc. Offic. Anal. Chem.; Washington, DC: 2000. Official Methods of Analysis. [Google Scholar]
- Bartosch S., Fite A., Macfarlane T., McMurdo M.E. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004;70:3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chen D.W., Michiels J., Smet S.D. Dietary fiber affects intestinal mucosal barrier function by regulating intestinal bacteria in weaning piglets. Br. J. Nutr. 2013;110:1837–1848. doi: 10.1017/S0007114513001293. [DOI] [PubMed] [Google Scholar]
- Ciftci M., Guler T., Dalkilic B., Ertas O.N. The effect of anise oil (pimpinella anisum L.) on broiler performance. Int. J. Poult. Sci. 2005;4:851–855. [Google Scholar]
- Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D.E., McDeVitt R.M., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Ding X., Yang C.W., Yang Z.B., Ren X.J., Wang P.P. Effects of star anise (Illicium verum Hook.f) oil on the nuclear factor E2–related factor 2 signaling pathway of chickens during subclinical Escherichia coli challenge. Poult. Sci. 2020;99:3092–3101. doi: 10.1016/j.psj.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:2–10. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertas O.N., Guler T., Ciftci M., Dalkilic B., Simsek G. The effect of an essential oil Mix derived from oregano, clove and anise on broiler performance. Int. J. Poult. Sci. 2005;4:879–884. [Google Scholar]
- Freire R.S., Morais S.M., Catunda-Junior F.E.A., Pinheiro D.C.S.N. Synthesis and antioxidant, anti-inflammatory and gastroprotector activities of anethole and related compounds. Bioorgan. Med. Chem. 2005;13:4353–4358. doi: 10.1016/j.bmc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- Goel G., Makkar H.P., Becker K. Changes in microbial community structure, methanogenesis and rumen fermentation in response to saponin-rich fractions from different plant materials. J. Appl. Microbiol. 2008;105:770–777. doi: 10.1111/j.1365-2672.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- Hafeez A., Männer K., Schieder C., Zentek J. Effect of supplementation of phytogenic feed additives (powdered vs. encapsulated) on performance and nutrient digestibility in broiler chickens. Poult. Sci. 2016;95:622–629. doi: 10.3382/ps/pev368. [DOI] [PubMed] [Google Scholar]
- Hançer Aydemir D., Çifci G., Aviyente V., Boşgelmez-Tinaz G. Quorum-sensing inhibitor potential of trans-anethole against Pseudomonas aeruginosa. J. Appl. Microbiol. 2018;125:731–739. doi: 10.1111/jam.13892. [DOI] [PubMed] [Google Scholar]
- Jamroz D., Kamel C. Plant extracts enhance broiler performance. In non- ruminant nutrition: antimicrobial agents and plant extracts on immunity, health and performance. J. Anim. Sci. 2002;80:41–46. [Google Scholar]
- Jang I., Ko Y., Kang S., Lee C. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed. Sci. Technol. 2007;134:304–315. [Google Scholar]
- Jiao J.Z., Wang P.P., Tang S.X., Zhou C.S., Tan Z.L. Quantity and distribution characteristics of functional microorganisms in gastrointestinal tract of Liuyang black goats. Acta Vet. Zootech. Sinica. 2013;44:1590–1599. [Google Scholar]
- Kang N.H., Mukherjee S., Min T., Kang S.C., Yun J.W. Trans-anethole ameliorates obesity via induction of browning in white adipocytes and activation of brown adipocytes. Biochim. 2018;151:1–13. doi: 10.1016/j.biochi.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Kim K.Y., Lee H.S., Seol G.H. Anti-inflammatory effects of trans -anethole in a mouse model of chronic obstructive pulmonary disease. Biomed. Pharmacother. 2017;91:925–930. doi: 10.1016/j.biopha.2017.05.032. [DOI] [PubMed] [Google Scholar]
- Kreydiyyeh S.I., Usta J., Knio K., Markossian S., Dagher S. Aniseed oil increases glucose absorption and reduces urine output in the rat. Life. Sci. 2003;74:663–673. doi: 10.1016/j.lfs.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski P., Grygorcewicz B., Pruss A., Wojciuk B., Dołęgowska B., Giedrys-Kalemba S., Sienkiewicz M., Wojciechowska-Koszko I. The effect of subinhibitory concentrations of trans-anethole on antibacterial and antibiofilm activity of mupirocin against mupirocin-resistant staphylococcus aureus strains. Microb. Drug. Resist. 2019;25:1424–1429. doi: 10.1089/mdr.2019.0101. [DOI] [PubMed] [Google Scholar]
- Moughan P.J., Rutherfurd S.M., Balan P. Kiwifruit, mucins, and the gut barrier. Adv. Food. Nutr. Res. 2013;68:169–185. doi: 10.1016/B978-0-12-394294-4.00009-2. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Paraskevas V., Tsirtsikos P., Palamidi I., Steiner T., Schatzmayr G., Fegeros K. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Anim. Feed. Sci. Technol. 2011;168:223–231. [Google Scholar]
- Paraskeuas V., Fegeros K., Hunger C., Theodorou G., Mountzouris K.C. Dietary inclusion level effects of a phytogenic characterised by menthol and anethole on broiler growth performance, biochemical parameters including total antioxidant capacity and gene expression of immune-related biomarkers. Anim. Prod. Sci. 2017;57:33–41. [Google Scholar]
- Paraskeuas V.V., Mountzouris K.C. Modulation of broiler gut microbiota and gene expression of toll-like receptors and tight junction proteins by diet type and inclusion of phytogenics. Poult. Sci. 2019;98:2220–2230. doi: 10.3382/ps/pey588. [DOI] [PubMed] [Google Scholar]
- Pu J., Chen D., Tian G., He J., Zheng P., Mao X., Yu J., Huang Z.Q., Luo J.Q., Luo Y.H., Yu B. Effects of benzoic acid, Bacillus coagulans and oregano oil combined supplementation on growth performance, immune status and intestinal barrier integrity of weaned piglets. Anim. Nutr. 2020;6:152–159. doi: 10.1016/j.aninu.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyer H., Zentek J., Männer K., Youssef I.M.I., Aumiller T., Weghuber J., Wimmers K., Mueller A.S. Possible molecular mechanisms by which an essential oil blend from star anise, rosemary, thyme, and oregano and saponins increase the performance and ileal protein digestibility of growing broilers. J. Agric. Food. Chem. 2017;65:6821–6830. doi: 10.1021/acs.jafc.7b01925. [DOI] [PubMed] [Google Scholar]
- Rhee Y., Moon J.H., Mo J., Pham T., Chung P. mTOR and ROS regulation by anethole on adipogenic differentiation in human mesenchymal stem cells. BMC Cell. Biol. 2018;19:12. doi: 10.1186/s12860-018-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinttilä T., Kassinen A., Malinen E., Krogius L., Palva A. Development of an extensive set of 16s rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004;97:1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute . SAS Inst. Inc.; Cary, NC: 2001. SAS User's Guide. Version 8.2. [Google Scholar]
- Sá N.A.R., Bruno J.B., Guerreiro D.D., Cadenas J., Alves B.G., Cibin F.W.S., Leal-Cardoso J.H., Gastal E.L., Figueiredo J.R. Anethole reduces oxidative stress and improves in vitro survival and activation of primordial follicles. Braz. J. Med. Biol. Res. 2018;51:e7129. doi: 10.1590/1414-431X20187129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá N.A.R., Vieira L.A., Ferreira A.C.A., Cadenas J., Bruno J.B., Maside C., Sousa F.G.C., Cibin F.W.S., Alves B.G., Rodrigues A.P.R., Leal-Cardoso J.H., Gastal E.L., Figueiredo J.R. Anethole supplementation during oocyte maturation improves in vitro production of bovine embryos. Reprod. Sci. 2020;27:1602–1608. doi: 10.1007/s43032-020-00190-x. [DOI] [PubMed] [Google Scholar]
- Schmeda-Hirschmann G., Rodriguez J., Astudillo L. Gastroprotective activity of the diterpene solidagenone and its derivatives on experimentally induced gastric lesions in mice. J. Ethnopharmacol. 2002;81:111–115. doi: 10.1016/s0378-8741(02)00054-5. [DOI] [PubMed] [Google Scholar]
- Senatore F., Oliviero F., Scandolera E., Taglialatela-Scafati O., Roscigno G., Zaccardelli M., De Falco E. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell] Fitoterapia. 2013;90:214–219. doi: 10.1016/j.fitote.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Seo E., Kang P., Seol G.H. Trans-anethole prevents hypertension induced by chronic exposure to both restraint stress and nicotine in rats. Biomed. Pharmacother. 2018;102:249–253. doi: 10.1016/j.biopha.2018.03.081. [DOI] [PubMed] [Google Scholar]
- Sheikh B.A., Pari L., Rathinam A., Chandramohan R. Trans-anethole, a terpenoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Biochim. 2015;112:57–65. doi: 10.1016/j.biochi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. [Google Scholar]
- Sibbald I.R. A bioassay for true metabolizable energy in feedingstuffs. Poult. Sci. 1976;55:303–308. doi: 10.3382/ps.0550303. [DOI] [PubMed] [Google Scholar]
- Soltan M.A., S.Shewita R., Elkatcha M.I. Effect of dietary anise seeds supplementation on growth performance, immune response, carcass traits and some blood parameters of broiler chickens. Int. J. Poult. Sci. 2008;7:1078–1088. [Google Scholar]
- Song A., Park Y.J., Kim B.Y., Lee S.G. Modulation of lipid metabolism by trans-anethole in hepatocytes. Molecules. 2020;25:4946. doi: 10.3390/molecules25214946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Hertel C., Tannock G.W., Lis C.M., Munro K., Hammes W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001;67:2578–2585. doi: 10.1128/AEM.67.6.2578-2585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.W., Hu W.T., Huang B.K., Qin L.P. Illicium verum: a review on its botany, traditional use, chemistry and pharmacology. J. Ethnopharmacol. 2011;136:10–20. doi: 10.1016/j.jep.2011.04.051. [DOI] [PubMed] [Google Scholar]
- Wieczyńska J., Cavoski I. Antimicrobial, antioxidant and sensory features of eugenol, carvacrol and trans-anethole in active packaging for organic ready-to-eat iceberg lettuce. Food. Chem. 2018;259:251–260. doi: 10.1016/j.foodchem.2018.03.137. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lahaye L., He Z., Zhang J., Yang C., Piao X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+) Anim. Nutr. 2020;6:269–277. doi: 10.1016/j.aninu.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Q.Y., Liu J.X., Zhang Y.F., Qiao H.Z., Chen F., Zhang S.H., Guan W.T. Anethole attenuates enterotoxigenic Escherichia coli-induced intestinal barrier disruption and intestinal inflammation via modification of TLR signaling and intestinal microbiota. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.647242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.Y., Wei J.D., Yang C.W., Yang Z.B., Yang W.R., Jiang S.J. Effects of star anise (Illicium verum Hook.f.) essential oil on laying performance and antioxidant status of laying hens. Poult. Sci. 2018;97:3957–3966. doi: 10.3382/ps/pey263. [DOI] [PubMed] [Google Scholar]
- Yu C.Y., Guo Y.X., Yang Z.B., Yang W.R., Jiang S.Z. Effects of star anise (Illicium verum Hook.f.) essential oil on nutrient and energy utilization of laying hens. Anim. Sci. J. 2019;90:880–886. doi: 10.1111/asj.13221. [DOI] [PubMed] [Google Scholar]
- Yu C.Y., Zhang J.F., Wang T. Star anise essential oil: chemical compounds, antifungal and antioxidant activities: a review. J. Essen. Oil. Res. 2020;33:1–22. [Google Scholar]
- Zhang S., Chen X., Devshilt I., Yun Q., Huang C., An L., Dorjbat S., He X. Fennel main constituent, trans-anethole treatment against LPS-induced acute lung injury by regulation of Th17/Treg function. Mol. Med. Rep. 2018;18:1369–1376. doi: 10.3892/mmr.2018.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Kou J., Wu Y.J., Wang M.M., Zhou X.M., Yang Y., Wu Z.L. Dietary genistein supplementation improves intestinal mucosal barrier function in Escherichia coli O78-challenged broilers. J. Nutri. Biochem. 2020;77 doi: 10.1016/j.jnutbio.2019.108267. [DOI] [PubMed] [Google Scholar]