Abstract
Wooden breast (WB) is a muscle disorder affecting modern commercial broiler chickens that leads to a palpable firm pectoralis major muscle and causes severe reduction in meat quality, resulting in substantial economic losses for the poultry industry. Most studies have focused on the regulatory mechanisms underlying this defect with respect to the gene and protein expression levels as well as the levels of metabolites. MicroRNAs (miRNAs) play critical roles in human muscular disorders, such as the Duchenne muscular dystrophy, by regulating the muscle regeneration or fibrosis processes. In this study, we investigated the miRNAs and related pathways that play important roles in the development of WB. We generated the miRNA expression profiles of the pectoralis major muscle samples from 3 WB-affected and 3 nonaffected chickens selected from a commercial broiler population via small RNA sequencing. A total of 578 miRNAs were identified in the chicken breast muscles from the initial analysis of the sequencing data. Of these, 23 miRNAs were significantly differentially expressed (false discovery rate [FDR] <0.05, log2|Foldchange| >1), including 20 upregulated and 3 downregulated miRNAs in the WB group compared to the normal group. Moreover, functional enrichment of the predicted target genes of differential miRNAs indicated that these miRNAs were involved in biological processes and pathways related to energy metabolism, apoptosis, focal adhesion, and development of blood vessels. Four differentially expressed miRNAs were validated by quantitative real-time polymerase chain reaction (qRT-PCR). We also highlighted several differentially expressed miRNAs, such as gga-miR-155, gga-miR-29c, and gga-miR-133, for their potential roles in the regulation of the development of WB. To the best of our knowledge, this is the first study investigating the miRNA expression profile of the breast muscle associated with WB. The findings of this study can be used to explore the potential molecular mechanisms of other muscle disorders in broilers and provide valuable information for chicken breeding.
Key words: myopathy, wooden breast, deep sequencing, miRNA
INTRODUCTION
To meet the growing global demand for chicken meat, genetic selection of commercial broilers was done, which has led to a remarkable progress in enhancing the growth rate and muscle mass of these broilers in the past decades (Havenstein et al., 2003; Velleman, 2018). However, the highly intensive selection resulted in an increased incidence of pectoral myopathies and muscle abnormalities, such as deep pectoral myopathy, white striping, and wooden breast (WB) as well as the development of pale, soft, and exudative meat (Soglia and Mazzoni, 2018; Velleman, 2018). WB is an emerging muscle disorder in fast-growing broilers, which is characterized by the hardness of the pectoralis major muscle, often accompanied by pale color and white striping (Sihvo et al., 2014; Sihvo et al., 2017). Histologically, breast muscle with WB showed severe polyphasic myodegeneration with regeneration, accumulation of connective and fat tissue, or fibrosis. WB myopathies not only decrease the visual appearance of meat but also negatively affect the meat quality traits, leading to substantial economic losses in the poultry industry. In general, breast muscles affected by WB usually exhibit high pH, low water-holding/water-binding capacity, high fat and collagen content, and low amounts of proteins and ash (Mudalal et al., 2015; Soglia et al., 2015; Chatterjee et al., 2016; Zhang et al., 2020; Oliveira et al., 2021).
To understand the etiology of WB, many studies have been carried out to compare the differences in the gene and protein expression levels as well as the metabolite content between the WB and normal muscles (Mutryn et al., 2015; Kuttappan et al., 2017; Abasht et al., 2019; Pampouille et al., 2019; Papah and Abasht, 2019; Bordini et al., 2021). For example, Mutryn et al. (2015) found over 1500 differentially expressed genes between the birds affected and unaffected by WB, suggesting hypoxia, oxidative stress, and cellular repair mechanisms in the WB-affected tissues. A recent study on WB proteome profiles demonstrated increased apoptosis and protein synthesis, intense contraction, and high oxidative stress in WB broilers (Zhang et al., 2021). Papah and Abasht (2019) confirmed the existence of a slow myofiber phenotype and provided mechanistic insights into the increased lipid uptake and metabolism in WB. However, the exact mechanism underlying this defect remains unknown.
MicroRNAs (miRNAs) are 18 to 24 nucleotide (nt) long noncoding regulatory RNAs that target mRNAs for degradation or inhibition of translation at the post-transcriptional level (Bartel, 2004). Some miRNAs are associated with various chicken muscle functions, such as miR-454 (postnatal development), mir-27b (myoblast differentiation), and miR-140 (intramuscular fat deposition) (Chen et al., 2020; Zhang and He, 2021; Zhang et al., 2018). miRNAs also play important regulatory roles in human muscle disorders (Kirby et al., 2015). For example, miR-206 promotes skeletal muscle regeneration and delays the progression of Duchenne muscular dystrophy (Liu et al., 2012). However, little is known about the relationship between miRNAs and WB myopathy in broilers. In a previous study by Zambonelli et al. (2016), only 5 differentially expressed miRNAs (DEmiRNAs) between the WB-affected and normal samples were identified by microarray expression profiling.
In this study, we performed high-throughput sequencing to compare the miRNA expression levels between the WB and normal breast muscles to systematically reveal the candidate miRNAs associated with WB. Our findings provide a better understanding of the underlying mechanisms of various muscle disorders in broilers.
MATERIALS AND METHODS
Ethics Statement
All animal experiments were approved by the Animal Care and Use Committee at the Poultry Institute, Chinese Academy of Agricultural Science (Approval ID: S20191010, Yangzhou, China). All the experiments in this study were performed as per the relevant guidelines and regulations set by the Ministry of Agriculture and Rural Affairs of the People's Republic of China.
Sample Selection and Preparation
Ross 308 broilers were used in the present study. All birds were given free access to both feed and water, and housed according to the optimal industry-growing standards. At 42 d of age, 80 hens were euthanized in a slaughterhouse via electrical stunning and exsanguination. After euthanasia, the pectoralis major muscle samples were collected from them to identify 12 WB and 12 normal (NOR) breast filets via palpation according to the criteria proposed by Sihvo et al. (2014) and Papah et al.(2017). In brief, WB filets were remarkably hardened diffusely or on focally extensive areas, often covered with a layer of turbid or scattered petechiae. At the same time, the NOR breast muscles exhibited no detectable hardness.
The whole left breast muscles of the selected birds were packaged and stored at 4°C until further meat quality analysis. A 0.5 × 0.5 × 3 cm3 section in the cranial part of the right pectoral muscle, parallel to the muscle fiber, was removed and fixed in 10% formalin buffer for histological analysis. From the same location, a piece of muscle tissue was collected, immediately frozen in liquid nitrogen, and stored at –80°C for RNA extraction.
Histological Evaluation
Histological examination was performed to ensure that the selected samples exhibited the NOR and WB characteristics. The tissue samples were fixed in formalin by immersion, embedded in paraffin, and cut into at 4 µm thick sections. The muscle samples were oriented longitudinally and transversally and the slides were stained with hematoxylin and eosin (H&E).
Measurement of Meat Quality
All 24 filets were used to evaluate the quality of the meat. At 24 h postmortem, the ultimate pH of the breast muscle was evaluated using a portable pH meter (FE20; Mettler Toledo, Zurich, Switzerland) via direct insertion of the glass electrode into the muscles. The color parameters based on the lightness (L*), redness (a*), and yellowness (b*) of the muscles were measured at the same time on the internal face of the muscle using an NH310 portable colorimeter (3nh technology, Shenzhen, China). Drop loss of the hanging muscle was determined after 24 h of storage at 2°C and it was zip-locked in a plastic bag. The shear force of the raw meat was measured using a digital meat tenderness meter (C1LM3; Northeast Agricultural University, Harbin, China).
Small RNA Sequencing
Six WB and 6 NOR muscles randomly selected from 24 filets were used for small RNA sequencing. Total RNA from the 12 filets (6 NOR and 6 WB) was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA). The RNA concentration and integrity were estimated using the NanoDrop 2000 (Thermo Fisher Scientific, Waltham MA) and Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA), respectively. RNA molecules in a size range of 18 to 30 nt were enriched by polyacrylamide gel electrophoresis (PAGE). After the addition of the 3′- and 5′- adapters, the ligation products were reverse transcribed by PCR amplification, and 140 to 160 bp PCR products were enriched to generate a cDNA library using the TruSeq Small RNA Library Prep Kit (Illumina, San Diego, CA). The library insert size was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Finally, the qualified cDNA library was sequenced using Illumina HiSeq 2500 by Gene Denovo Biotechnology Co., Ltd. (Guangzhou, China).
Bioinformatics Analysis
Clean reads were obtained from the raw data after removing the reads with poly N, 5′-adapter contaminants, those without 3′-adapter or insert tags, and other low-quality reads. After the filtering and trimming steps, high-quality (18–35 nt) clean reads were mapped to the chicken genome Galgal v.6.0, using Bowtie2 (Langmead et al., 2009). The mapped small RNA reads were then searched against the miRbase 22.1 database (http://www.mirbase.org/). Sequences matching the Gallus gallus miRBase were considered to be known miRNAs. Based on their genome positions and hairpin structures, novel miRNA candidates were also predicted using Mireap (v.0.2) (Hafner et al., 2008). To improve the reliability for miRNA identification, the cut-off value of the sequence read count was set at >10 in all samples.
The identified known and novel miRNA expression levels were calculated and normalized to transcripts per million (TPM). DEmiRNAs between the WB and NOR groups were analyzed using edgeR (Robinson et al., 2010), and log2 |foldchange| >1 with a false discovery rate (FDR) <0.05 were considered significant. Target genes of DEmiRNAs were predicted by integrating 2 different tools: TargetScan v.7.2 (http://www.targetscan.org/vert_72/) and miRDB (http://mirdb.org/). Only the genes expressed in the chicken muscle tissues were regarded as potential target genes based on our previous gene expression data (Liu et al., 2020).
Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed on the target genes for the identified DEmiRNAs using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/). Biological processes and KEGG pathways were considered significant at P < 0.05.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analyses
Total RNA for sequencing was reverse transcribed into cDNA using the miRNA 1st-Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). qRT-PCR was then performed using the Agilent Mx3000P system (Agilent Technologies, Palo Alto, CA) with the miRNA Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). The U6 small nuclear RNA (snRNA) was used as the internal control. Primers were synthesized by Sangon Inc. (Shanghai, China) and are listed in Table 1. The relative expression levels of miRNAs were quantified using the 2−ΔΔCt method.
Table 1.
Primer sequences for quantitative real-time polymerase chain reaction (qRT-PCR) validation.
| miRNA | Type | Sequences (5’-3’) |
|---|---|---|
| gga-miR-146b-5p | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCGCCTA |
| F | CGCGTGAGAACTGAATTCCA | |
| R | AGTGCAGGGTCCGAGGTATT | |
| gga-miR-21-5p | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA |
| F | GCGCGTAGCTTATCAGACTGA | |
| R | AGTGCAGGGTCCGAGGTATT | |
| gga-miR-142-3p | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACAAC |
| F | GCGTGGCAGTGTCTTAGCTG | |
| R | AGTGCAGGGTCCGAGGTATT | |
| gga-miR-133a-3p | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAGCT |
| F | CGTTGGTCCCCTTCAACC | |
| R | AGTGCAGGGTCCGAGGTATT | |
| gga-miR-3530-5p | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCCACAA |
| F | CGGCTCTGCTCGCACCA | |
| R | AGTGCAGGGTCCGAGGTATT | |
| novel-m0077-3p | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTTCGG |
| F | CGGCGCACACACGCGG | |
| R | AGTGCAGGGTCCGAGGTATT | |
| U6 | RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCGGCT |
| F | GAGCCCGAAGACCAACAAAG | |
| R | AGTGCAGGGTCCGAGGTATT |
Statistical Analysis
All statistical analyses were conducted using the IBM SPSS Statistics v.20.0 software (IBM Corporation, Armonk, NY). Statistical significance was set at P < 0.05.
RESULTS
Histological Observation
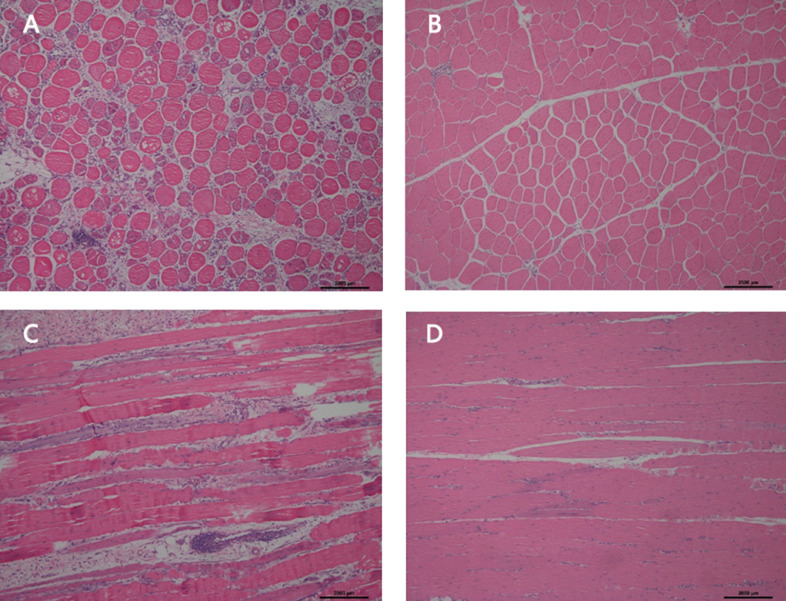
The breast muscles of the selected Ross 308 broilers were stained with H&E. As shown in Figure 1, multifocal myodegeneration and necrosis were observed in the pectoralis major muscles of the WB-affected birds. In comparison to the NOR group, myofibers of WB samples exhibited variable diameters and loss of characteristic polygonality. The degenerated muscle fibers were separated or replaced by interstitial connective tissues with inflammatory cells. The cellular infiltrates surrounding veins were observed in the WB samples.
Figure 1.
Histological observations of the wooden breast (WB) and normal (NOR) pectoralis major muscles. (A) Transverse section of the WB muscle; (B) Transverse section of the NOR muscle; (C) Longitudinal section of the WB muscle; (D) Longitudinal section of the NOR muscle.
Meat Quality Test
Detailed information on the meat quality traits is provided in Table 2. Filets affected by WB exhibited higher pH values (P < 0.01) than the normal filets. Regarding color, higher values for the lightness (L*) parameter were observed in the WB samples (P < 0.01). However, no differences (P > 0.05) were observed in the redness (a*) and yellowness (b*) parameters among the groups. Drip losses were higher (P < 0.05) in the muscles affected by WB than the normal muscles. The shear force was not affected by myopathy (P > 0.05).
Table 2.
Meat quality of the normal (NOR) and wooden breast (WB) pectoralis major muscles of broilers.
| Category | ||||
|---|---|---|---|---|
| Trait | NOR | WB | SEM | P-value |
| pH | 5.71 | 5.85 | 0.04 | 0.008 |
| Lightness(L*) | 50.13 | 58.60 | 1.90 | <0.0001 |
| Redness(a*) | 9.81 | 8.65 | 1.03 | 0.274 |
| Yellowness(b*) | 10.25 | 11.38 | 0.82 | 0.188 |
| Drip loss (%) | 1.43 | 1.62 | 0.07 | 0.016 |
| Shear force(N) | 15.42 | 14.18 | 1.71 | 0.479 |
Abbreviations: NOR, normal breast; WB, wooden breast.
Overview of miRNA Sequencing Data
We used the Illumina deep sequencing method to analyze the small RNA populations in 12 libraries. Deep sequencing of 12 samples produced 93.39 million and 65.33 million raw sequence reads from NOR and WB samples, respectively (Table S1). After filtering the low-quality reads and adaptor sequences, 77.28 million and 52.98 million clean reads remained in the NOR and WB samples, respectively. Length distribution analyses showed that the majority of small RNAs ranged from 21 to 24 nt in length (>84.9%), and the small RNAs with 22 nt were the most abundant (> 35.5%). Over 63.7% of the sequences in the NOR group and 64.2% of the sequences in the WB group were successfully mapped to the chicken reference genome.
Identification of Chicken miRNAs in Breast Muscles
After the alignment of mapped reads to the chicken reference mature miRNA collections, a total of 387 known miRNAs were identified from the 12 libraries (Table S2). The read number of the top 20 miRNAs accounted for 84.4% of the total reads. Expression profile analyses revealed that most miRNAs were expressed in a small proportion of miRNA genes. In addition, 191 novel miRNAs were predicted in this study (Table S3). The sequencing frequencies of the novel miRNAs were much lower than those of the known miRNAs.
Identification of DEmiRNAs
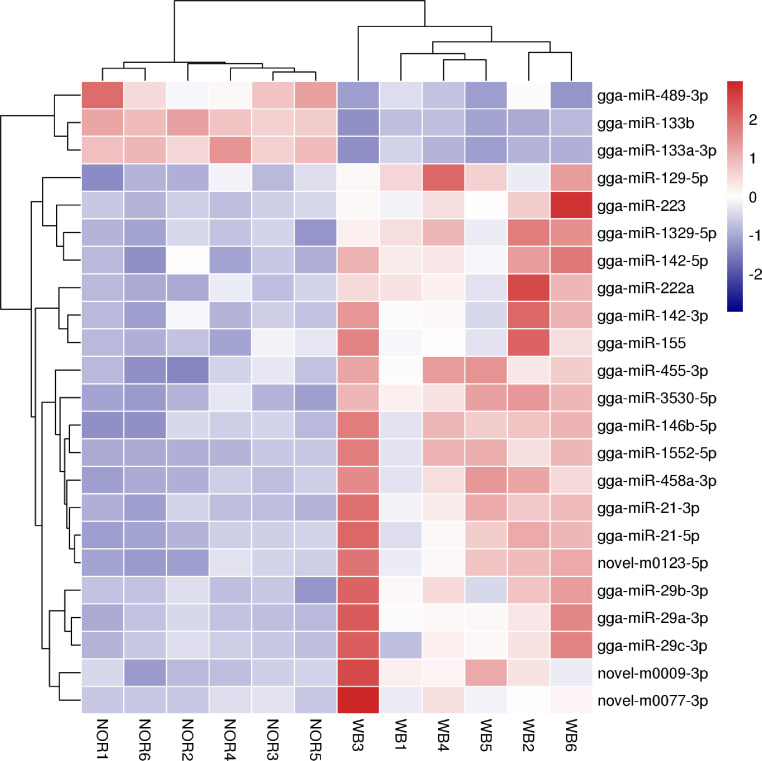
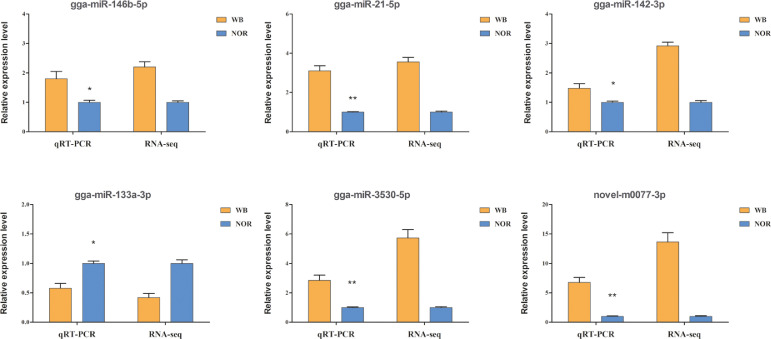
After applying a stringent filter approach to compare the miRNA expression levels between the WB and NOR samples (FDR < 0.05, log2|Foldchange| > 1), we identified 23 DEmiRNAs, 20 of which were known and the remaining were novel (Table 3). The majority (20 out of 23) of all DEmiRNAs were upregulated, while 3 miRNAs, including gga-miR-489-3p, gga-miR-133b, and gga-133a-3p, were downregulated in the WB muscles compared to the NOR muscles. Several highly expressed miRNAs (TPM > 10,000) were identified as DEmiRNAs, including gga-miR-146b-5p, gga-miR-21-5p, and gga-miR-133a-3p. However, none of them matched the DEmiRNAs identified in previous microarray studies related to WB (Zambonelli et al., 2016), which may be due to the differences in the breeds and examination methods used. A heatmap was created to visualize the expression pattern of the DEmiRNAs in the NOR and WB muscles (Figure 2). Additionally, hierarchical clustering resulted in a clear discrimination of the 12 birds into the correct group of origin. qRT-PCR analysis was performed to validate the 6 DEmiRNAs from RNA-seq, including gga-miR-146b-5p, gga-miR-21-5p, gga-miR-142-3p, gga-miR-133a-3p, gga-miR-3530-5p, and novel-m0077-3p. As shown in Figure 3, the expression patterns of all 6 miRNAs were in good agreement with the sequencing data in terms of their direction and relative fold changes, suggesting the reliability of the RNA-seq approach.
Table 3.
Differentially expressed miRNAs identified in the breast muscles of NOR and WB chickens.
| miRNA | WB (TPM) | NOR (TPM) | log2FC (WB/NOR) | FDR | Type |
|---|---|---|---|---|---|
| gga-miR-223 | 245.84 | 64.76 | 1.92 | 0.0000 | Up |
| gga-miR-21-3p | 2,862.65 | 738.48 | 1.95 | 0.0000 | Up |
| gga-miR-146b-5p | 39,883.76 | 18056.15 | 1.14 | 0.0001 | Up |
| gga-miR-21-5p | 45,122.03 | 12724.5 | 1.83 | 0.0001 | Up |
| gga-miR-3530-5p | 12.95 | 2.25 | 2.52 | 0.0001 | Up |
| gga-miR-1552-5p | 26.87 | 9.7 | 1.47 | 0.0002 | Up |
| gga-miR-222a | 357.81 | 145.34 | 1.3 | 0.0003 | Up |
| gga-miR-458a-3p | 73.68 | 25.58 | 1.53 | 0.0004 | Up |
| gga-miR-142-5p | 2,654.68 | 1177.66 | 1.17 | 0.0026 | Up |
| gga-miR-129-5p | 82.01 | 37.2 | 1.14 | 0.0028 | Up |
| gga-miR-155 | 88.34 | 34.57 | 1.35 | 0.0028 | Up |
| gga-miR-142-3p | 109.72 | 37.55 | 1.55 | 0.0029 | Up |
| gga-miR-455-3p | 72.91 | 36.29 | 1.01 | 0.0036 | Up |
| gga-miR-29c-3p | 50.24 | 20.53 | 1.29 | 0.0038 | Up |
| gga-miR-29a-3p | 52.29 | 21.71 | 1.27 | 0.0047 | Up |
| novel-m0077-3p | 3.07 | 0.23 | 3.77 | 0.0058 | Up |
| novel-m0009-3p | 10.98 | 3.82 | 1.53 | 0.0072 | Up |
| gga-miR-1329-5p | 9.55 | 4.29 | 1.16 | 0.0304 | Up |
| gga-miR-29b-3p | 10.98 | 4.7 | 1.23 | 0.0442 | Up |
| novel-m0123-5p | 2.21 | 0.66 | 1.75 | 0.0442 | Up |
| gga-miR-489-3p | 5.41 | 14.54 | -1.43 | 0.0092 | Down |
| gga-miR-133a-3p | 18,101.01 | 42964.55 | -1.25 | 0.0346 | Down |
| gga-miR-133b | 1,498.12 | 3274.01 | -1.13 | 0.0442 | Down |
The miRNA expression level was calculated and normalized to transcripts per million (TPM).
Figure 2.
Hierarchically clustered heat map of the differentially expressed microRNAs (DEmiRNAs). Red and blue represent the upregulated and downregulated expression of DEmiRNAs in WB muscles, respectively. Color density indicates the level of fold change. Abbreviations: NOR, normal; WB, wooden breast.
Figure 3.
Validation of 6 DEmiRNAs in WB and NOR muscles via quantitative real-time polymerase chain reaction (qRT-PCR). For qRT-PCR, the expression levels of DEmiRNAs were shown as 2−ΔΔCt. For RNA-seq, the transcripts per million (TPM) were used to calculate the gene expression levels. Data are expressed as the mean±standard deviation (SD). Abbreviations: DEmiRNAs, differentially expressed microRNAs; NOR, normal; WB, wooden breast.
Target Gene Prediction and Functional Enrichment of DEmiRNAs
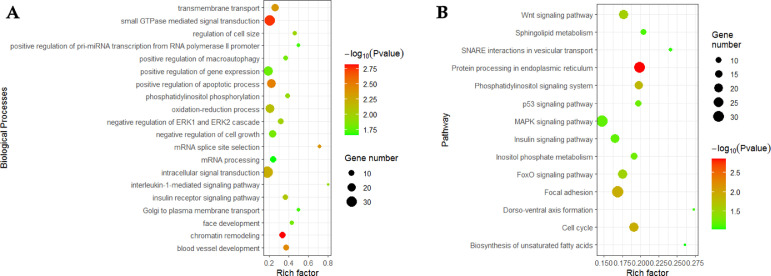
The target genes of known miRNAs were predicted to understand the biological functions of DEmiRNAs. A total of 1,472 target genes were predicted for the DEmiRNAs (Table S4). In comparison with the published transcriptome data (Zambonelli et al., 2016; Papah et al., 2018; Pampouille et al., 2019; Praud et al., 2020; Bordini et al., 2021), a total of 26 target genes of DEmiRNAs matched with crucial genes related to the development of WB (Table 4). These genes are involved in many functions, such as lipid metabolism, collagen metabolism, energy metabolism, hypoxia, and oxidative stress responses. Furthermore, all predicted target genes of the DEmiRNAs were mapped to the GO and KEGG databases. In GO enrichment, 37 enriched biological processes were mainly involved in the regulation of the apoptotic process, development of blood vessels, and energy metabolism. In KEGG analysis, a total of 14 pathways were identified, including focal adhesion, cell cycle, Wnt, and FoxO signaling pathways (Figure 4 and Table S5).
Table 4.
The interactions between the candidate miRNA-target genes related to WB by comparison between the results of the present and previous studies.
| Target gene | miRNA |
|---|---|
| Lipid metabolism | |
| ADIPOQ | gga-miR-133a-3p, gga-miR-133b, gga-miR-155 |
| PDK4 | gga-miR-21-3p |
| Collagen metabolism | |
| COL27A1 | gga-miR-29a-3p |
| COL3A1 | gga-miR-29a-3p, gga-miR-29c-3p |
| COL4A1 | gga-miR-21-5p, gga-miR-29a-3p |
| COL4A2 | gga-miR-29a-3p, gga-miR-29b-3p, gga-miR-29c-3p |
| COL5A1 | gga-miR-29a-3p |
| COL6A2 | gga-miR-29a-3p, gga-miR-29b-3p, gga-miR-29c-3p |
| COL6A3 | gga-miR-133a-3p, gga-miR-133b, gga-miR-29a-3p, gga-miR-29b-3p, gga-miR-29c-3p |
| COL9A1 | gga-miR-29a-3p, gga-miR-29b-3p, gga-miR-29c-3p |
| Energy metabolism | |
| PRKAG2 | gga-miR-146b-5p |
| TIGAR | gga-miR-146b-5p |
| STAT3 | gga-miR-29a-3p, gga-miR-29b-3p |
| HDAC4 | gga-miR-29a-3p, gga-miR-29b-3p, gga-miR-29c-3p |
| LDHA | gga-miR-1329-5p |
| LDHB | gga-miR-1329-5p |
| Hypoxia and oxidative stress | |
| HIF1A | gga-miR-1329-5p |
| POSTN | gga-miR-222a |
| Vascular development | |
| SERPINE2 | gga-miR-458a-3p |
| THBS1 | gga-miR-133a-3p, gga-miR-133b, gga-miR-3530-5p |
| SDK1 | gga-miR-21-3p |
| Myofiber degeneration and regeneration | |
| TNFRSF1A | gga-miR-142-3p, gga-miR-155, gga-miR-29a-3p, gga-miR-29b-3p, gga-miR-29c-3p, gga-miR-455-3p |
| DCN | gga-miR-1329-5p |
| HSPD1 | gga-miR-1329-5p |
| PLCD1 | gga-miR-21-3p, gga-miR-133a-3p, gga-miR-133b |
| MMP16 | gga-miR-146b-5p, gga-miR-21-3p |
Figure 4.
Gene ontology (GO) and pathway analysis of DEmiRNAs. (A) The top 20 significantly enriched GO terms of the target genes for DEmiRNAs in biological processes. (B) The significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of target genes for DEmiRNAs. The size and color of each bubble represent the number of genes enriched in the term/pathway and the enrichment significance, respectively. Abbreviation: DEmiRNAs, differentially expressed microRNAs.
DISCUSSION
WB myopathy has been identified as an emerging myopathy in fast-growing broilers in recent years (Brot et al., 2016; Xing et al., 2020). Considering its adverse impact on the poultry industry, an improvement in the knowledge regarding the molecular mechanisms involved in the onset of this defect is extremely important. Recent transcriptomic and proteomic studies have described the changes in the muscle gene expression levels and identified candidate genes as well as key pathways related to the pathogenesis of WB (Mutryn et al., 2015; Zambonelli et al., 2016; Papah et al., 2018; Pampouille et al., 2019). Previous studies in human medicine have indicated that miRNAs may be crucial regulators of myopathies (Chen et al., 2009; Parkes et al., 2015). Therefore, this study was conducted to investigate the miRNA expression pattern under WB conditions and depict the miRNA-mediated mechanism involved in the development of WB to understand the molecular basis of myopathy and promote the healthy production of broilers.
In this study, we examined the miRNA expression profiles associated with the occurrence of WB using RNA-seq. We screened 6 WB and 6 unaffected ROSS 308 broilers using direct and histological evaluations. In addition, the meat quality of the selected samples in our study was in concordance with previous studies, suggesting the reliability of our sampling method (Mudalal et al., 2015; Soglia et al., 2015; Zhang et al., 2020).
The current study identified 20 upregulated and 3 downregulated miRNAs in WB muscles, which may play important roles in the occurrence of this disease. Among the 23 DEmiRNAs, several have been shown to be involved in the muscular disorders in humans. For instance, 3 upregulated miRNAs identified in our study, miR-155, miR-146b, and miR-222 are consistently upregulated across 10 primary muscle disorders (Eisenberg et al., 2007). In addition, dysregulation of miR-21 (Zanotti et al., 2015), miR-29c (Cappella et al., 2018), and miR-223 (Inoue et al., 2013), has been associated with human myopathies, including Duchenne muscular dystrophy, myotonic dystrophy, and dermatomyositis. Interestingly, these DEmiRNAs are known to be linked to avian innate immunity (Chen and Ali Abdalla, 2020). As inflammation is observed in both human muscular disorders and WB, the miRNAs identified here might play similar roles in the immune response of different myopathies.
Moreover, the 2 downregulated miRNAs, gga-miR-133a-3p and gga-miR-133b, in the WB samples are both from the miR-133 family. As one of the miRNAs specifically expressed in muscles (myomiRs), miR-133 plays key roles in the proliferation and differentiation of myoblasts (Yu et al., 2014). Decreased expression levels of miR-133 are detected in the skeletal muscles of the patients with muscular dystrophy (Greco et al. 2009). Taetzsch et al. (2021) reported that miR-133 could slow the pathogenesis of Duchenne muscular dystrophy. In the present study, miR-133 was predicted to suppress the expression of phospholipase C delta 1 (PLCD1), which is a key member of the muscle regeneration process (Hindi et al., 2013). Taken together, our findings indicate that miR-133 might regulate the development of WB by inhibiting the muscle regeneration; however, further studies are required to elucidate this mechanism. miRNAs can regulate the gene expression by binding to the 3′-untranslated region of mRNAs (Bartel, 2009). In the current study, 1,472 target genes were identified among all the DEmiRNAs. GO and KEGG analyses revealed that DEmiRNAs participated in the regulation of the apoptotic process, development of blood vessels, energy metabolism, focal adhesion, cell cycle, Wnt, and FoxO signaling pathways. In addition, several target genes of DEmiRNAs have also been highlighted for regulating WB development in previous transcriptome studies (Zambonelli et al., 2016; Papah et al., 2018; Pampouille et al., 2019; Praud et al., 2020; Bordini et al., 2021). Based on the functional enrichment analysis and the information in the existing literature, some miRNAs and their target genes may be strong candidates for regulating onset of WB. For example, dysregulation of energy metabolism is considered to be an essential feature of WB myopathy (Papah et al., 2018; Baldi et al., 2020). In the present study, energy metabolism-related processes, including glycolytic and oxidative reduction processes, were found to be significantly enriched. We found that a group of glycolytic genes, including the protein kinase AMP-activated noncatalytic subunit gamma 2 (PRKAG2), TP53-induced glycolysis regulatory phosphatase (TIGAR), histone deacetylase 4 (HDAC4), and the signal transducer and activator of transcription 3 (STAT3), were predicted to be targeted by the gga-miR-146-5p and gga-miR-29 families. In our previous study on the type of muscle fibers, gga-miR-146b-5p was found to be downregulated in the glycolytic muscle and involved in energy metabolism (Liu et al., 2020). Inhibition and overexpression analyses demonstrate that miR-29a and miR-29c regulate the uptake and metabolism of glucose (Massart et al., 2017). In addition, gga-miR-1329-5p was found to be upregulated in the WB muscles, directly targeting the lactate dehydrogenase A (LDHA) and lactate dehydrogenase B (LDHB) genes. Zhao et al., 2020 reported that the altered expression levels of LDHA and LDHB may contribute to the dysregulation of lactate metabolism in WB muscles. Based on the findings of the present and previous studies, it can be speculated that gga-miR-29, gga-miR-146b-5p, and gga-miR-1329-5p may play significant roles in the development of WB by regulating the energy metabolism pathway.
Hypoxia within the breast muscle is a likely cause of WB development in broilers (Soglia et al., 2021). In response to hypoxic stress, the hypoxia-responsive genes, including the hypoxia inducible factor 1 subunit alpha (HIF1A), become differentially expressed in WB muscles (Mutryn et al., 2015; Malila et al., 2019). The current study showed that gga-miR-1329-5p could inhibit the expression of HIF1A, suggesting that this miRNA is associated with myopathy via the hypoxic response mechanism. The replacement of muscle fiber by collagen was regarded as a symbolic pathological change in WB muscles and this change was also observed in the samples used in this study (Sihvo et al., 2017; Velleman et al., 2017). Among the target genes, a group of collagen coding genes (COL3A1, COL4A1, COL4A2, COL5A1, COL6A2, COL6A3, COL9A1, and COL27A1) was predicted to be the target genes of the identified DEmiRNAs, including gga-miR-29a-3p, gga-miR-29c-3p, gga-miR-29b-3p, gga-miR-21-5p, gga-miR-133a-3p, and gga-miR-133b. Moreover, miR-21 and miR-29 may regulate myopathy by inhibiting the expression levels of COL1A1, COL6A1, and COL3A1 in Duchenne muscular dystrophy (Zanotti et al., 2015). Although only some of the 8 collagen genes were differentially expressed in the previous transcriptome data (Papah et al., 2018), these miRNAs may act as important regulators of the WB myopathy.
CONCLUSION
To the best of our knowledge, this is the first study to perform miRNA expression profiling of the broilers with WB. Using differential expression analysis and functional enrichment, we identified several candidate miRNAs associated with the development of WB. These results will provide insights into the potential underlying mechanisms of several muscle abnormalities observed in fast-growing chicken breeds.
Acknowledgments
ACKNOWLEDGMENTS
Financial support for this study was provided by the Chinese Agricultural Research System (No. CARS-41), earmarked fund for Jiangsu Agricultural Industry Technology System (JATS[2020]356), Special Fund for Major Breeding Programs in Jiangsu Province (PZCZ201728), and Special Fund for Independent Innovation of Agricultural Science and Technology in Jiangsu Province of China (CX(20)3003).
DISCLOSURES
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101496.
Appendix. Supplementary materials
REFERENCES
- Abasht B., Zhou N., Lee W.R., Zhuo Z., Peripolli E. The metabolic characteristics of susceptibility to wooden breast disease in chickens with high feed efficiency. Poult. Sci. 2019;98:3246–3256. doi: 10.3382/ps/pez183. [DOI] [PubMed] [Google Scholar]
- Baldi G., Yen C.N., Daughtry M.R., Bodmer J., Bowker B.C., Zhuang H., Petracci M., Gerrard D.E. Exploring the factors contributing to the high ultimate pH of broiler pectoralis major muscles affected by wooden breast condition. Front. Physiol. 2020;11:343. doi: 10.3389/fphys.2020.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordini M., Zappaterra M., Soglia F., Petracci M., Davoli R. Weighted gene co-expression network analysis identifies molecular pathways and hub genes involved in broiler White Striping and Wooden Breast myopathies. Sci. Rep. 2021;11:1776. doi: 10.1038/s41598-021-81303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot S.D., Perez S., Shivaprasad H.L., Baiker K., Polledo L., Clark M., Grauroma L. Wooden breast lesions in broiler chickens in the UK. Vet. Rec. 2016;178 doi: 10.1136/vr.103561. vetrec-2015-103561. [DOI] [PubMed] [Google Scholar]
- Cappella M., Perfetti A., Cardinali B., Garcia-Manteiga J.M., Carrara M., Provenzano C., Fuschi P., Cardani R., Renna L.V., Meola G., Falcone G., Martelli F. High-throughput analysis of the RNA-induced silencing complex in myotonic dystrophy type 1 patients identifies the dysregulation of miR-29c and its target ASB2. Cell Death Dis. 2018;9:729. doi: 10.1038/s41419-018-0769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Zhuang H., Bowker B.C., Rincon A.M., Sanchez-Brambila G. Instrumental texture characteristics of broiler pectoralis major with the wooden breast condition. Poult. Sci. 2016;95:2449–2454. doi: 10.3382/ps/pew204. [DOI] [PubMed] [Google Scholar]
- Chen J.F., Callis T.E., Wang D.Z. microRNAs and muscle disorders. J. Cell Sci. 2009;122:13–20. doi: 10.1242/jcs.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Zhang S., Xu Z., Gao J., Mishra S.K., Zhu Q., Zhao X., Wang Y., Yin H., Fan X., Zeng B., Yang M., Yang D., Ni Q., Li Y., Zhang M., Li D. MiRNA profiling in pectoral muscle throughout pre- to post-natal stages of chicken development. Front. Genet. 2020;11:570. doi: 10.3389/fgene.2020.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Ali Abdalla B. Epigenetic regulation by non-coding RNAs in the avian immune system. Life (Basel) 2020;10:148. doi: 10.3390/life10080148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg I., Eran A., Nishino I., Moggio M., Lamperti C., Amato A.A., Lidov H.G., Kang P.B., North K.N., Mitrani-Rosenbaum S., Flanigan K.M., Neely L.A., Whitney D., Beggs A.H., Kohane I.S., Kunkel L.M. Distinctive patterns of microRNA expression in primary muscular disorders. Proc. Natl. Acad. Sci. 2007;104:17016–17021. doi: 10.1073/pnas.0708115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco S., De Simone M., Colussi C., Zaccagnini G., Fasanaro P., Pescatori M., Cardani R., Perbellini R., Isaia E., Sale P., Meola G., Capogrossi M.C., Gaetano C., Martelli F. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009;23:3335–3346. doi: 10.1096/fj.08-128579. [DOI] [PubMed] [Google Scholar]
- Hafner M., Landgraf P., Ludwig J., Rice A., Ojo T., Lin C., Holoch D., Lim C., Tuschl T. Identification of microRNAs and other small regulatory RNAs using cDNA library sequencing. Methods. 2008;44:3–12. doi: 10.1016/j.ymeth.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- Hindi S.M., Tajrishi M.M., Kumar A. Signaling mechanisms in mammalian myoblast fusion. Sci. Signal. 2013;6:re2. doi: 10.1126/scisignal.2003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Jinnin M., Yamane K., Makino T., Kajihara I., Makino K., Honda N., Nakayama W., Fukushima S., Ihn H. Down-regulation of miR-223 contributes to the formation of Gottron's papules in dermatomyositis via the induction of PKCɛ. Eur. J. Dermatol. 2013;23:160–167. doi: 10.1684/ejd.2013.1959. [DOI] [PubMed] [Google Scholar]
- Kirby T.J., Chaillou T., McCarthy J.J. The role of microRNAs in skeletal muscle health and disease. Front. Biosci. 2015;20:37–77. doi: 10.2741/4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttappan V.A., Bottje W., Ramnathan R., Hartson S.D., Coon C.N., Kong B.W., Owens C.M., Vazquez-Añon M., Hargis B.M. Proteomic analysis reveals changes in carbohydrate and protein metabolism associated with broiler breast myopathy. Poult. Sci. 2017;96:2992–2999. doi: 10.3382/ps/pex069. [DOI] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Williams A.H., Maxeiner J.M., Bezprozvannaya S., Shelton J.M., Richardson J.A., Bassel-Duby R., Olson E.N. microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J. Clin. Invest. 2012;122:2054–2065. doi: 10.1172/JCI62656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang M., Shan Y., Ji G., Ju X., Tu Y., Sheng Z., Xie J., Zou J., Shu J. miRNA-mRNA network regulation in the skeletal muscle fiber phenotype of chickens revealed by integrated analysis of miRNAome and transcriptome. Sci. Rep. 2020;10:10619. doi: 10.1038/s41598-020-67482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malila Y., Thanatsang K., Arayamethakorn S., Uengwetwanit T., Srimarut Y., Petracci M., Strasburg G.M., Rungrassamee W., Visessanguan W. Absolute expressions of hypoxia-inducible factor-1 alpha (HIF1A) transcript and the associated genes in chicken skeletal muscle with white striping and wooden breast myopathies. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massart J., Sjögren R.J.O., Lundell L.S., Mudry J.M., Franck N., O'Gorman D.J., Egan B., Zierath J.R. Altered miR-29 expression in type 2 diabetes influences glucose and lipid metabolism in skeletal muscle. Diabetes. 2017;66:1807–1818. doi: 10.2337/db17-0141. [DOI] [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- Mutryn M.F., Brannick E.M., Fu W., Lee W.R., Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genom. 2015;16:1–19. doi: 10.1186/s12864-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira R.F., Mello J.L.M., Ferrari F.B., Cavalcanti E.N.F., Souza R.A., Pereira M.R., Giampietro-Ganeco A., Villegas-Cayllahua E.A., Fidelis H.A., Favero M.S., Amoroso L., Souza P.A., Borba H. Physical, chemical and histological characterization of pectoralis major muscle of broilers affected by wooden breast myopathy. Animals (Basel) 2021;11:596. doi: 10.3390/ani11030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampouille E., Hennequet-Antier C., Praud C., Juanchich A., Brionne A., Godet E., Bordeau T., Fagnoul F., Le Bihan-Duval E., Berri C. Differential expression and co-expression gene network analyses reveal molecular mechanisms and candidate biomarkers involved in breast muscle myopathies in chicken. Sci. Rep. 2019;9:14905. doi: 10.1038/s41598-019-51521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papah M.B., Abasht B. Dysregulation of lipid metabolism and appearance of slow myofiber-specific isoforms accompany the development of Wooden Breast myopathy in modern broiler chickens. Sci. Rep. 2019;9:17170. doi: 10.1038/s41598-019-53728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of wooden breast disease in modern broiler chickens. Avian Pathol. 2017;46:623–643. doi: 10.1080/03079457.2017.1339346. [DOI] [PubMed] [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Gene expression profiling of the early pathogenesis of wooden breast disease in commercial broiler chickens using RNA-sequencing. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes J.E., Day P.J., Chinoy H., Lamb J.A. The role of microRNAs in the idiopathic inflammatory myopathies. Curr. Opin. Rheumatol. 2015;27:608–615. doi: 10.1097/BOR.0000000000000225. [DOI] [PubMed] [Google Scholar]
- Praud C., Jimenez J., Pampouille E., Courousse N., Godet E., Le Bihan-Duval E., Berri C. Molecular phenotyping of white striping and wooden breast myopathies in chicken. Front. Physiol. 2020;11:633. doi: 10.3389/fphys.2020.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Lindén J., Airas N., Immonen K., Valaja J., Puolanne E. Wooden breast myodegeneration of pectoralis major muscle over the growth period in broilers. Vet. Pathol. 2017;54:119–128. doi: 10.1177/0300985816658099. [DOI] [PubMed] [Google Scholar]
- Soglia F., Petracci M., Davoli R., Zappaterra M. A critical review of the mechanisms involved in the occurrence of growth-related abnormalities affecting broiler chicken breast muscles. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soglia F., Mazzoni M. Spotlight on avian pathology: current growth-related breast meat abnormalities in broilers. Avian Pathol. 2018;48:1–3. doi: 10.1080/03079457.2018.1508821. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mudalal S., Babini E., Di Nunzio M., Mazzoni M., Sirri F., Cavani C., Petracci M. Histology, composition, and quality traits of chicken pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2015;95:651. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Taetzsch T., Shapiro D., Eldosougi R., Myers T. The microRNA miR-133b functions to slow Duchenne muscular dystrophy pathogenesis. J. Physiol. 2021;599:171–192. doi: 10.1113/JP280405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velleman S.G. Recent developments in breast muscle myopathies associated with growth in poultry. Annu. Rev. Anim. Biosci. 2018;15:289–308. doi: 10.1146/annurev-animal-020518-115311. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L., Tonniges J.R. Fibrillar collagen organization associated with broiler wooden breast fibrotic myopathy. Avian Dis. 2017;61:481. doi: 10.1637/11738-080217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Xing T., Zhao X., Zhang L., Li J.L., Zhou G.H., Xu X.L., Gao F. Characteristics and incidence of broiler chicken wooden breast meat under commercial conditions in China. Poult. Sci. 2020;99:620–628. doi: 10.3382/ps/pez560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Lu Y., Li Z., Wang Q. microRNA-133: expression, function and therapeutic potential in muscle diseases and cancer. Curr. Drug Targets. 2014;15:817–828. doi: 10.2174/1389450115666140627104151. [DOI] [PubMed] [Google Scholar]
- Zambonelli P., Zappaterra M., Soglia F., Petracci M., Sirri F., Cavani C., Davoli R. Detection of differentially expressed genes in broiler pectoralis major muscle affected by white striping – wooden breast myopathies. Poult. Sci. 2016;95:2771. doi: 10.3382/ps/pew268. [DOI] [PubMed] [Google Scholar]
- Zanotti S., Gibertini S., Curcio M., Savadori P., Pasanisi B., Morandi L., Cornelio F., Mantegazza R., Mora M. Opposing roles of miR-21 and miR-29 in the progression of fibrosis in Duchenne muscular dystrophy. Biochim. Biophys. Acta. 2015;1852:1451–1464. doi: 10.1016/j.bbadis.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Zhang G., He M. MicroRNA-27b-3p targets the myostatin gene to regulate myoblast proliferation and is involved in myoblast differentiation. Cells. 2021;17:423. doi: 10.3390/cells10020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Li D.-H., Li F., Sun J.-W., Jiang R.-R., Li Z.-J., Han R.-L., Li G.-X., Liu X.-J., Kang X.-T., Sun G.-R. Integrated analysis of MiRNA and genes associated with meat quality reveals that Gga-MiR-140-5p affects intramuscular fat deposition in chickens. Cell Physiol. Biochem. 2018;46:2421–2433. doi: 10.1159/000489649. [DOI] [PubMed] [Google Scholar]
- Zhang X., To K.V., Jarvis T.R., Campbell Y.L., Hendrix J.D., Suman S.P., Li S., Antonelo D.S., Zhai W., Chen J., Zhu H., Schilling M.W. Broiler genetics influences proteome profiles of normal and woody breast muscle. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang P., Xu X., Xia T., Li Z., Zhao T. Effect of wooden breast myopathy on water-holding capacity and rheological and gelling properties of chicken broiler breast batters. Poult. Sci. 2020;99:3742–3751. doi: 10.1016/j.psj.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Kogut M.H., Genovese K.J., Hsu C.Y., Lee J.T., Farnell Y.Z. Altered expression of lactate dehydrogenase and monocarboxylate transporter involved in lactate metabolism in broiler wooden breast. Poult. Sci. 2020;99:11–20. doi: 10.3382/ps/pez572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.