Abstract
Biosynthesis/metabolism, perception/signaling, and transport are three essential aspects of the actions of phytohormones. Jasmonates (JAs), including jasmonic acid (JA) and related oxylipins, are implicated in the regulation of a range of ecological interactions, as well as developmental programs to integrate these interactions. Jasmonoyl-isoleucine (JA-Ile) is the most bioactive JAs, and perception of JA-Ile by its coreceptor, the Skp1-Cullin1-F-box-type (SCF) protein ubiquitin ligase complex SCFCOI1-JAZ, in the nucleus derepresses the transcriptional repression of target genes. The biosynthesis and metabolism of JAs occur in the plastid, peroxisome, cytosol, endoplasmic reticulum, and vacuole, whereas sensing of JA-Ile levels occurs in the nucleus. It is increasingly apparent that a number of transporters, particularly members of the jasmonates transporter (JAT) family, located at endomembranes as well as the plasma membrane, constitute a network for modulating and coordinating the metabolic flux and signaling of JAs. In this review, we discuss recent advances in the metabolism, signaling, and especially the transport of JAs, focusing on intracellular compartmentation of these processes. The roles of transporter-mediated cell-cell transport in driving long-distance transport and signaling of JAs are also discussed.
Key words: jasmonates, metabolism, signaling, transport, cellular compartmentation
The complex intracellular compartmentation of JA synthesis, metabolism, and signaling provides a new regulatory layer for coordinating JA metabolic flux and signaling dynamics. A number of transporters located at endomembranes and the plasma membrane constitute a network that facilitates the transport of JAs. Here, the metabolism, signaling, and transport of JAs are reviewed through the lens of their intra- and intercellular distributions.
Introduction
Oxylipins are substances derived from oxygenated fatty acids that are produced ubiquitously in almost all living organisms, either by the activity of at least one mono- or di-oxygenase enzyme or by autonomously occurring chemical reactions (Wasternack and Feussner, 2018). In green lineages, only simple oxylipin molecules have been detected in prokaryotic organisms (e.g., cyanobacteria), but a multitude of more than 500 different molecules have been found in eukaryotic plants, ranging from algae and mosses to angiosperms. Jasmonates (JAs) are a class of oxylipin phytohormones derived from polyunsaturated fatty acids (PUFAs), preferentially α-linolenic acid (α-LeA), with jasmonic acid (JA) as the prototypical member (Browse, 2009; Wasternack et al., 2013). JAs have been previously defined as JA and its diverse metabolites derived from methylation, esterification, conjugation, sulfation, decarboxylation, glycosylation, hydroxylation, and carboxylation (Wasternack and Hause, 2013; Wasternack and Strnad, 2018). More recently, an alternative, and probably ancient, pathway has been shown to produce JA from dinor-OPDA (2,3-dinor-12-oxo-10,15(Z)-phytodienoic acid [dn-OPDA]) (Chini et al., 2018), which also acts as the bioactive ligand in Marchantia polymorpha (Monte et al., 2018, 2019). Thus, OPDA (12-oxo-10,15(Z)-phytodienoic acid), dn-OPDA, and their derivatives are also viewed as JAs (Park et al., 2013; Howe, 2018).
Jasmonoyl-isoleucine (JA-Ile) acts as the major bioactive JAs to activate core JA signaling by binding with its coreceptor, the Skp1-Cullin1-F-box-type (SCF) protein ubiquitin ligase complex SCFCOI1-JAZ (Xie et al., 1998; Chini et al., 2007; Thines et al., 2007; Sheard et al., 2010). This JA-Ile-mediated core JA signaling (referred to as COI1-dependent or JA-Ile signaling hereafter) plays important roles primarily in regulating a plethora of ecological interactions with the abiotic and biotic environment, particularly defense against herbivores and necrotrophic pathogens (Zhang et al., 2017; Wang et al., 2019a). JA-Ile signaling is also implicated in the modulation of symbiotic interactions with arbuscular mycorrhiza and in the root nodule symbiosis (Shigeyama et al., 2012; Liu et al., 2018; Song et al., 2019; Basso and Veneault-Fourrey, 2020). To coordinate plant growth and development with ecological interactions, JA-Ile signaling is also involved in the regulation of various developmental processes, including seed germination, root growth and architecture, tuber and trichome formation, and particularly the development of reproductive organs (Wasternack and Hause, 2013; Huang et al., 2017). In addition, a putative role of the JA precursor cis-12-OPDA (Taki et al., 2005), the volatile cis-jasmone (CJ), and the hydroxylated JA (12-OH-JA) has been proposed, independent of JA-Ile-signaling pathways, although further research is required to define such a role (Wasternack et al., 2013; Wasternack and Feussner, 2018; Monte et al., 2020). The biosynthesis, metabolism/catabolism, and signaling of JA-Ile occur in various intracellular compartments, including the plastid/chloroplast, peroxisome, cytosol, vacuole, endoplasmic reticulum (ER), and nucleus. Thus, the distribution of JA precursors (e.g., OPDA), JA, and JA derivatives (e.g., JA-Ile) in these diverse subcellular compartments plays an essential role in regulating the metabolic flux and signaling of JAs. This view has been demonstrated by the characterization of carriers that participate in the export of OPDA from the plastids (JASSY, Guan et al., 2019) and the import of OPDA into the peroxisomes (COMATOSE 1 [CTS1], Theodoulou et al., 2005). The jasmonates transporter (JAT) family of eight members with diverse subcellular localizations at the plasma membrane (PM) and endomembranes has been identified in a clade of half-molecule ATP-binding cassette G (ABCG) transporters in Arabidopsis thaliana (Arabidopsis hereafter) (Li et al., 2017, 2020; Wang et al., 2019a). Among these JATs, AtJAT1/AtABCG16 has a dual localization at the PM and nuclear envelope (NE) and mediates the efflux of JA across the PM and the influx of JA-Ile into the nucleus. AtJAT1 is involved in regulating the distribution of JA-Ile between the nucleus and the cytosol and of JA between the cytosol and the apoplasm, thereby affecting JA metabolism and JA-Ile signaling (Li et al., 2017). Furthermore, the PM-localized and preferentially phloem-expressed AtJAT3 and AtJAT4 mediate the cellular import of JA and play an important role in wound-induced systemic resistance by driving long-distance transmission of wound-induced JA from leaf to leaf (Li et al., 2020). These findings highlight the important roles of transporters in the regulation of intra- and intercellular movement of JAs.
The biosynthesis, metabolism, and signaling of JAs have been extensively reviewed in several excellent reviews (Wasternack, 2007; Browse, 2009; Shan et al., 2012; Wasternack and Hause, 2013; Wasternack and Song, 2017; Howe et al., 2018; Koo, 2018; Wasternack and Feussner, 2018; Zhai et al., 2020). Here, we provide updated information on these processes, focusing on their intracellular compartmentation. We discuss recent advances in transporter modulation of the intra- and intercellular distributions of JAs and its effects on the regulation of their metabolic flux, signaling, and long-distance transmission.
Biosynthesis of OPDA/DINOR-OPDA in the chloroplast/plastid
The biosynthetic pathway and catalyzing enzymes of JA have been elucidated primarily in the model plant Arabidopsis and have been reviewed extensively (Heitz et al., 2016; Koo, 2018; Wasternack and Feussner, 2018; Wasternack and Hause, 2013; Wasternack and Song, 2017). We therefore address the biosynthesis of JA briefly, emphasizing the impact of intracellular compartmentation of JAs on their metabolic flux. As summarized in Figure 1, JA biosynthesis begins in the plastid/chloroplast, primarily from esterified α-LeA (18:3) in galactolipids of the chloroplast membrane or free fatty acids, probably released by functionally redundant members of the phospholipase (PLA) family. Although the first substrate is yet to be identified, the formation of JA takes place by one of the seven different branches of the so-called lipoxygenase (LOX) pathway (Wasternack, 2006). LOXs are nonheme-iron-containing dioxygenases that form fatty acid hydroperoxides from polyunsaturated substrates and can be subdivided into 9-LOXs and 13-LOXs according to the carbon atom at which molecular oxygen is introduced (Bannenberg et al., 2009a). In Arabidopsis, the 13-LOX members AtLOX 2, 3, 4, and 6 are localized in the chloroplast, whereas the 9-LOX members (e.g., AtLOX1 and 5) are probably localized in the cytosol (Glauser et al., 2009; Caldelari et al., 2011; Chauvin et al., 2013). The distribution of α-LeA between the chloroplast and cytosol can thus modulate the metabolic flux between 13-LOX- and 9-LOX-derived oxylipins (Figure 1).
Figure 1.
Intracellular compartmentation of the biosynthesis, metabolism, and signaling of JAs.
The biosynthesis of JA occurs sequentially in the plastid/chloroplast and peroxisome/cytosol from α-LeA (the main route indicated by blue arrows) or from hexadecatrienoic acid (16:3) (summarized from knowledge obtained primarily in Arabidopsis). Some compounds in the pathway are substrates of enzymes located in distinct intracellular compartments. These constitute nodes (metabolic branching points indicated by red dots) through which metabolic flux can be diverted toward distinct end products. Peroxisomal JA is then transported to the cytosol, where diverse JA derivatives are produced, including the bioactive JA-Ile and possibly 12-OH-JA-Ile, which then enter the nucleus to activate core JA signaling by reprogramming gene expression. JA-Ile may move into the ER and is deactivated by hydrolysis or deconjugation. The endomembranes of the plastid, peroxisome, vacuole, and ER are shown by green, orange, blue, and purple lines, respectively (double-layer membranes are indicated by double lines); the PM is shown by double black lines representing the phospholipid bilayer, and enzymes localized at these intracellular compartments are indicated by letters in the same color. The NE with a double membrane and nuclear pore is shown. Biologically active compounds are indicated by red letters. Also shown are JASSY and OPDAT1, localized at the outer and inner membrane of the chloroplast, respectively, and probably involved in the efflux of OPDA, and CTS localized at the peroxisomal membrane and involved in the import of OPDA.
In the chloroplast, the oxygenated product of 13-LOX acting on α-LeA is (13S)-hydroperoxy octadecatrienoic acid (13-HPOT), which acts as the substrate for cytochrome P450 enzymes of the CYP74 family, allene oxide synthase (AOS), hydroperoxide lyase (HPL), epoxyalcohol synthase, and divinyl ether synthase (DES). AOS is the next enzyme that acts in the octadecanoid pathway for JA biosynthesis by forming unstable allylic epoxides that spontaneously hydrolyze in water to 13-hydroxy-12-oxo-octadecadienoic acid (α-ketols) and 9-hydroxy-12-oxo-octadecadienoic acid (γ-ketols) and cyclize to a racemic mixture of cis-(+) and cis-(−) enantiomers (Song and Brash, 1991; Song et al., 1993; Harms et al., 1998; Hause et al., 2000; Farmer and Goossens, 2019). The cis-(+) isomer of OPDA is then rapidly formed, which requires the proximity of allene oxide cyclase (AOC) to the AOS product. The stereochemistry of the pentenyl side chain and the carboxylic acid side chain in positions 3 and 7, respectively, of the pentanone ring in the nascent JA is established in the AOC-catalyzed step and retained in later steps (Ziegler et al., 2000; Schaller et al., 2008; Stenzel et al., 2012). Although α-LeA is the predominant PUFA in the chloroplast membrane, hexadecatrienoic acid (16:3) also undergoes similar reactions for conversion to dn-OPDA. By contrast, α-LeA in the cytosol can be oxygenated by 9-LOX and subsequently by 9-AOS to generate α-ketols and γ-ketols or alternatively oxygenated by α-dioxygenase (α-DOX) (Blee, 2002; Hamberg et al., 2005; Bannenberg et al., 2009b; Christensen et al., 2015). Therefore, the distribution of α-LeA between the chloroplast/plastid and the cytosol can also significantly affect metabolic flux between JAs and other oxylipins by competing for the same substrates. However, the mechanism by which plastid export of α-LeA is modulated has yet to be elucidated.
Production of JA in the peroxisome or cytosol
OPDA produced in the chloroplast may be conjugated with GSH by chloroplast-localized glutathione S-transferase 6 (GST6) to form OPDA-GSH, which is proposed to be transported into the vacuole as the storage form of OPDA (Ohkama-Ohtsu et al., 2011). However, for JA synthesis, OPDA or dn-OPDA generated by the 13-LOX/AOS pathway must leave the chloroplast and enter the peroxisome. Due to its lipophilic nature, protonated OPDAH can diffuse across the chloroplast envelope along its gradient. However, as a weak acid (pKa ∼4.77), deprotonated OPDA− is the predominant molecular form at pH ∼7.2–7.3 in the chloroplast stroma and cytosol, thereby hampering the movement of OPDA across the chloroplast envelope into the cytosol. Recently, JASSY, a Bet v1-like protein localized at the outer membrane of the chloroplast envelope, was shown to bind with OPDA and function as a membrane channel (Guan et al., 2019). Loss-of-function jassy mutants show greatly compromised JA accumulation when wounded, which in turn results in increased susceptibility to cold treatment and pathogen attack. Unlike other mutants in JA synthesis and signaling, the jassy mutant is fertile and accumulates a small amount of JA, indicating that basal OPDAH diffusion under normal conditions may be sufficient for JA-dependent floral development (Guan et al., 2019). Furthermore, a plastid inner envelope-localized protein, OPDAT1, has been identified in Populus trichocarpa and may participate in the export of OPDA (Zhao et al., 2021). Given that the C16 structure of dn-OPDA is less lipophilic than the C18 structure of OPDA, whether these carriers are also involved in the export of dn-OPDA remains to be determined.
OPDA released from the chloroplast into the cytosol must move into the peroxisome where JA is synthesized. Because the OPDA− anion can be trapped in the relatively alkaline peroxisome (pH ∼8.4), OPDA can enter the peroxisome by diffusion of OPDAH along its gradient. However, active transport may facilitate and accelerate the proximal entry of OPDA, even against its gradient, for JA synthesis. Consistently, the full-molecule ATP-binding cassette D group (ABCD) transporter CTS has been suggested to participate in OPDA import into the peroxisome in Arabidopsis (Theodoulou et al., 2005). The loss-of-function cts mutants have reduced, but not abolished, JA levels that do not result in the male sterility that is characteristic of mutants in JA biosynthesis and signaling (Theodoulou et al., 2005), indicating the existence of basal diffusion in peroxisomal uptake of OPDA. Consistent with a proposed role of CTS in accelerating the peroxisomal entry of OPDA for rapid and efficient JA production, cts mutants accumulate a modest amount of JA with a significant time delay in response to wounding. Intriguingly, the impaired peroxisomal import of OPDA in cts leads to elevated OPDA levels in the cytosol, which have been proposed to activate a signaling pathway that is probably independent of JA-Ile signaling (Dave and Graham, 2012; Maynard et al., 2018). Therefore, CTS-mediated distribution of OPDA between the cytosol and the peroxisome plays an important role in regulating the homeostasis and signaling between OPDA and JA. Alternatively, cytosolic OPDA may be conjugated with isoleucine to form cis-(+)-OPDA-Ile, which also appears to be a JA-Ile-independent signal (Flokova et al., 2016; Wasternack and Hause, 2016). However, the transport activity and substrates of CTS remain to be confirmed in planta.
The cyclopentenone ring of OPDA imported into the peroxisome is then reduced by the flavin-dependent oxidoreductase OPDA reductase 3 (OPR3) to yield 3-oxo-2-(20-[Z]-pentenyl)-cyclopentane-1-octanoic acid (OPC-8) (Sanders et al., 2000; Schaller and Weiler, 1997; Stintzi and Browse, 2000). There are six OPR members in the Arabidopsis genome, but only OPR3 is targeted to the peroxisome (Strassner et al., 2002). Prior to undergoing the β-oxidation cycle, OPC-8 is activated by esterification to CoA catalyzed by OPC-8 coenzyme A ligase1 (OPCL1), a member of the acyl activating enzymes (AAEs) (Koo et al., 2006). OPC-8 is then converted to JA via three rounds of peroxisomal β-oxidation catalyzed sequentially by acyl-CoA oxidase (ACX) (Cruz Castillo et al., 2004; Pinfield-Wells et al., 2005; Schilmiller et al., 2007), multifunctional protein (MFP) (Delker et al., 2007), and L-3-ketoacyl-CoA thiolase (KAT) (Afitlhile et al., 2005; Cruz Castillo et al., 2004), ultimately removing six carbons from the carboxy-terminal carbon chain. In addition, peroxisomal OPDA may be converted to dn-OPDA, which is also produced in the chloroplast from hexadecatrienoic acid (16:3) or converted to iso-OPDA (Figure 1). Recently, an alternative pathway for JA synthesis has been revealed in the opr3-3 mutant in which OPR3 activity is completely depleted. OPDA and hexadecatrienoic acid-derived dn-OPDA are metabolized to tetranor-OPDA (tn-OPDA) and 4,5-didehydro-JA (4,5-ddh-JA), which is thought to be reduced to JA by OPR2 after release into the cytosol. It has been proposed that this OPR2-dependent and OPR3-independent JA biosynthesis pathway may be ancient and may have been retained in vascular plants by distinct processes or under certain environmental conditions, although the OPR3-dependent route is preferred (Chini et al., 2018; Wasternack and Hause, 2018). In addition, the JA produced has been proposed to undergo dehydration and decarbonation to generate CJ in the peroxisome (Koch et al., 1997), although an origin of CJ from iso-OPDA has also been proposed (Dabrowska and Boland, 2007).
Metabolism of JAS in the cytosol
The cytosol is enclosed by the PM and acts not only as the matrix for a large proportion of the chemical reactions of metabolism but also as the medium for metabolite transport between the cytosol and apoplasm, and particularly among various subcellular compartments enclosed by endomembranes, where distinct chemical reactions occur (Staehelin, 2015). Peroxisomal JA is then translocated to the cytosol and can be converted into active, partially active, and inactive compounds. The metabolism of JA has been reviewed extensively (Wasternack and Hause, 2013; Koo, 2018; Wasternack and Feussner, 2018; Wasternack and Strnad, 2018). At least 12 metabolic pathways converting JA or a derivative formed from JA are known, and the catalytic enzymes that have been identified to date are presented in Figure 1. Among their reactions are conjugation with amino acids, hydroxylation, carboxylation, decarboxylation, methylation, esterification, sulfation, and O-glycosylation.
Although some JAs are active in distinct stress response and developmental processes, JA-Ile is the most biologically active of the JAs, and its perception activates core JA signaling. The conjugation of JA with isoleucine has been shown to be catalyzed in the cytosol by jasmonoyl-isoleucine synthetase (JAR1), a member of the GH3 family (Hsieh et al., 2000; Staswick and Tiryaki, 2004), a group of acyl acid-amido synthetases with 19 members in Arabidopsis. Crystallization showed that JAR1 produced only the (3R,7R) configuration of JA-Ile ((−)-JA-Ile), even though (3R,7S)-JA-Ile ((+)-7-iso-JA-Ile) is the more active JA-Ile configuration (Westfall et al., 2012). However, the Arabidopsis jar1 mutant exhibits a residual amount (10%–20%) of JA-Ile that is sufficient to induce JA-responsive gene expression in damaged leaves and to sustain male fertility, indicating that other GH3 members may also be involved in the formation of JA-Ile (Staswick, 2009; Suza and Staswick, 2008). In addition, JA-Ala, JA-Val, JA-Leu, and JA-Met have also been identified as endogenous bioactive JA conjugates via CORONATINE INSENSITIVE1 (COI1)-dependent signaling (Yan et al., 2016). In addition to conjugation with Ile to form JA-Ile, JA can also undergo glycosylation, hydroxylation, methyl esterification, and sulfonation (Figure 1) (Wasternack and Hause, 2013; Wasternack and Song, 2017; Wasternack and Feussner, 2018). Recently, members of the 2-oxoglutarate Fe(II)-dependent oxygenase gene family, named JASMONATE-INDUCED OXYGENASEs (JOXs) (Smirnova et al., 2017) or JASMONIC ACID OXIDASES (JAOs) (Caarls et al., 2017), have been found in Arabidopsis and are involved in the direct hydroxylation of JA to 12-OH-JA, thereby contributing to the inactivation of JA.
Metabolism of JAS in the vacuole and ER
Many chemicals, in particular plant secondary metabolites, use the vacuole as the site for cellular storage, and this compartmentation plays an important role in regulating their homeostasis (de Brito Francisco and Martinoia, 2018). A temporal link between the rapid wound-induced upsurge in JA/JA-Ile and the decline in free OPDA in distal undamaged leaves suggests that OPDA availability is important for rapid JA production (Koo et al., 2009). This OPDA may have been stored in the vacuole where OPDA-GSH has been suggested to be imported (Ohkama-Ohtsu et al., 2011). The vacuolar auxin transporter facilitator WAT1 has been identified in Arabidopsis and is required for auxin homeostasis and signaling (Ranocha et al., 2013). Whether the vacuolar import and export of OPDA are mediated by transporters and the roles of OPDA compartmentation in the vacuole remain to be verified experimentally.
ER is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs, known as cisternae, and tubular structures, which sometimes branch and are reticular in appearance (Brandizzi, 2021). This ER network allows for an increased surface area to be devoted to transport functions. As illustrated in the wound response, JA-Ile levels increase and decline quickly in leaves, indicating the presence of metabolic pathways to turn overJA-Ile. Two major pathways for JA-Ile catabolism have been described and reviewed (Koo, 2018; Wasternack and Strnad, 2018) (Figure 1). The ω-oxidation pathway involves the oxidation of the terminal carbon at the methyl end of the pentenyl side chain of JA-Ile to generate 12-hydroxy-JA-Ile (12-OH-JA-Ile), followed by its subsequent oxidation to 12-COOH-JA-Ile. The hydrolytic pathway hydrolyzes the amide bond of JA-Ile to form JA and Ile. Members of the CYP94 gene family (CYP94B1, CYP94B3, CYP94C1) hydroxylate JA-Ile, leading to the formation of 12-OH-JA-Ile, and CYP94C1 can further carboxylate 12-COOH-JA-Ile (Heitz et al., 2012; Koo et al., 2011, 2014). IAR3 and ILL6 of the ILR1-like amidohydrolase (IAH) family catalyze the hydrolysis of JA-Ile or 12-OH-JA-Ile to JA or 12-OH-JA (Bhosale et al., 2013; Widemann et al., 2013; Zhang et al., 2016). JA-Ile binds with its coreceptor complex in the nucleus (compare the following and Figure 2), and therefore JA-Ile must first enter the nucleus to activate JA-Ile signaling. Because the membranes of the ER are continuous with the outer nuclear membrane, it is not surprising that the catabolism of JA-Ile occurs in the ER (Koo et al., 2014). In addition, recent studies provide evidence that 12-OH-JA-Ile is also bioactive, activating COI1-dependent signaling (Hazman et al., 2019; Jimenez-Aleman et al., 2019; Poudel et al., 2019). The partitioning of JA-Ile biosynthesis and catabolism in the cytosol and ER, respectively, provide a further layer of regulation for JA-Ile homeostasis and thereby its signaling.
Figure 2.
Sensing of JA-Ile levels and signaling dynamics in the nucleus.
Sensing of nuclear JA-Ile levels by the transcriptional states (repression, depression, and activation) of JA-responsive genes. At low JA-Ile levels (top left), the transcription of JA-responsive genes is repressed owing to the accumulated JAZ proteins that bind and repress MYCs, involving indirect recruitment of TPL via JAZ-bound NINJA. This repressed state is then derepressed by elevated JA-Ile levels in response to environmental and developmental cues (top right), which promotes the binding of JA-Ile (red dot) with JAZ in the SCFCOI1 coreceptor, polyubiquitylation of JAZ, and subsequent degradation of JAZ by the 26S proteasome. The degradation of JAZ unmasks the MED25 binding site on MYC to engage the Mediator complex and recruit additional coactivators (e.g., HAC1 and LUH), resulting in the formation of the transcription preinitiation complex with RNA polymerase II (Pol II) and the activation of JA-responsive genes (bottom). Because JA-responsive genes encode JA-Ile catabolic enzymes and/or stable JAZ proteins, negative feedback loops are established to terminate JA responses with a characteristic time delay, eventually restoring the repressed state. Nuclear JA-Ile levels are modulated by JAT1, in turn regulating the derepression and activation states. AtJAT1-mediated nuclear entry of JA-Ile concentrates and partitions JA-Ile in the nucleus, driving the equilibrium between JA and JA-Ile in the direction of JA-Ile in the cytosol, thus rapidly and efficiently activating JA-Ile signaling. By contrast, JAT1-mediated cellular export removes JA from the cytosol, driving the equilibrium between JA and JA-Ile in the direction of JA, attenuating JA-Ile signaling and facilitating the cell-cell transport of JA from source to sink cells. MED25, Mediator subunit 25; HAC1, histone acetyltransferase 1; LUH, Leunig homolog; Pol Ⅱ, RNA polymerase II; TPL, Topless.
Sensing of JA-ILE levels and signaling dynamics in the nucleus
The nucleus is a key organelle in eukaryotic cells whose main functions are the control of gene transcription and the mediation of DNA replication. The nucleus is enclosed by the NE, which is composed of a double membrane, providing a site for transcription in the nucleoplasm that is segregated from the site of translation in the cytoplasm. The NE is impermeable to large molecules such as proteins and RNA, whose movement between the cytoplasm and the nucleoplasm occurs by active transport through the nuclear pore complex (NPC) (Staehelin, 2015). In animals, nuclear receptors bind with their cognate hormonal ligands in either the cytoplasm (type I nuclear receptors; e.g., steroid receptors) or nucleus (type II nuclear receptors; e.g., triiodothyronine receptors) (Tata, 2002; Puzianowska-Kuznicka et al., 2013). The receptors for auxin, JA, gibberellic acid (GA), and abscisic acid (ABA) are also localized in the nucleus and have been proposed to bind with their ligands in the nucleus (Santner and Estelle, 2009; Lumba et al., 2010; Takeuchi et al., 2021).
The perception and signaling of JA-Ile have been reviewed extensively (Browse, 2009; Chini et al., 2016; Howe et al., 2018; Ueda et al., 2020; Zhai et al., 2020) and are briefly summarized in Figure 2 to highlight the requirement for nuclear entry of JA-Ile. Sensing levels of the dynamic bioactive JAs, predominantly the most active naturally occurring isomer, (+)-7-iso-JA-Ile, leads to the prompt transition of transcriptional states between repression and activation, mediated by the interplay between the transcriptional activator MYC and repressor JAZ (JASMONATE-ZIM DOMAIN) proteins. MYC2 and its close relatives, such as Arabidopsis MYC3 and MYC4, are subclade IIIe members of the basic helix-loop-helix (bHLH) protein family. These transcription factors (MYCs) bind to G-box motifs to regulate the expression of a large portion of JA-responsive genes (Fernández-Calvo et al., 2011; Qi et al., 2011, 2015). By contrast, JAZ proteins, members of the TIFY (ZIM domain) protein family, play a critical role in repressing the activity of MYC transcription factors. At low JA-Ile levels, JAZ proteins bind directly with MYCs and, via JAZ-bound NOVEL INTERACTOR OF JAZ (NINJA) adaptor protein and potentially other corepressors, indirectly recruit the TOPLESS (TPL) scaffolding protein, which silences gene expression through interactions with histones, histone deacetylase (HDA), and the Mediator complex. This repressor complex inhibits the transcriptional activity of MYC transcription factors (Figure 2). Elevated JA-Ile accumulation, however, promotes the binding of its bioactive form (+)-7-iso-JA-Ile and JAZ to the COI1 component of the SCF E3 ubiquitin ligase complex (SCFCOI1), the polyubiquitylation of JAZs, and the subsequent degradation of JAZs by the 26S proteasome (Browse, 2009; Chini et al., 2007; Fonseca et al., 2009; Sheard et al., 2010; Thines et al., 2007). JAZ degradation unmasks the MED25 binding site on MYC, allowing the formation of the transcription preinitiation complex with RNA polymerase II and thereby activating core JA signaling (An et al., 2017; Chen et al., 2012; Wang et al., 2019b). According to this model, the preexisting SCFCOI1-JAZ coreceptor of JA-Ile must be assembled in the nucleus (Figure 2). Consistently, both COI1 and JAZ proteins are localized in the nucleus, and their nuclear localization was not altered in the mutants of the other interacting partner (Chini et al., 2007; Withers et al., 2012). Semi-quantitative electron microscopic autoradiography using atjar1 mutant cells in which formation of the biologically active JA-Ile is largely impaired (Suza and Staswick, 2008), shows that JA-Ile, but not JA, enters the nucleus (Li et al., 2017). Hence, nuclear entry of JA-Ile is essential for efficient and rapid activation of core JA signaling.
As a medium-strong acid with a predicted pKa of ∼3.61, JA-Ile exists almost entirely in the deprotonated JA-Ile− form at pH ∼7.3 in the cytosol; nuclear entry by JA-IleH diffusion across the NE would therefore be slow and along its gradient. By contrast, energized active transport would be rapid and against the JA-Ile gradient. To isolate JA-Ile transporters, a yeast-two-hybrid assay that detected JA-Ile-dependent binding of COI1 and JAZ3 was performed, and several NRT1/PTR FAMILY (NPF) proteins localized at the PM were identified (Chiba et al., 2015). Whereas NPF4.1/AIT3 exhibits JA-Ile transport activity in yeast cells, NPF2.10/GTR1 shows activity for JA-Ile and JA uptake in Xenopus oocytes (Saito et al., 2015). Similar to other NPFs, NPF4.1/AIT3 also showed transport activity for ABA and GAs in yeast cells (Chiba et al., 2015), and NPF2.10/GTR1 showed transport activity for GAs, NO3−, and glucosinolates (Saito et al., 2015). Furthermore, exogenous GA3, but not JA, restored male fertility to the gtr1 mutant (Saito et al., 2015). Thus, these NPFs transport a broad range of substrates, and their in planta roles in JA/JA-Ile transport and signaling remain to be evaluated.
The identification and characterization of Arabidopsis jasmonate transporter 1 (JAT1), which mediates the nuclear entry of JA-Ile, provides direct evidence for the nuclear entry of JA-Ile (Li et al., 2017). The finding that exogenous JA in the medium inhibits the growth of yeast cells has enabled the screening of putative JA transporters based on altered sensitivity of the transformants. Exploiting this screening system in yeast cells, a clade with eight members in the half-molecule G group of ABC transporters (ABCGs) has been identified as containing candidate jasmonate transporters (JATs) (Li et al., 2017, 2020; Wang et al., 2019a). Yeast cells expressing AtJAT1 exhibited enhanced resistance to exogenous JA (Li et al., 2017). In addition to the PM, unexpected localization at the NE was detected. The loss-of-function atjat1 mutants show substantial reductions in the expression of JA-responsive marker genes, JA-Ile-induced degradation of JAZ1, and resistance to the necrotrophic fungal pathogen Botrytis cinerea. Dual roles in mediating the nuclear import of JA-Ile and the cellular export of JA have been demonstrated for AtJAT1 in isolated nuclei and cultured suspension cells, respectively. The compromised JA-Ile signaling of atjat1 mutants is consistent with the reduced nuclear entry of JA-Ile, which outweighs the enhanced cytosolic accumulation of JA due to defective cellular JA export (Li et al., 2017). Furthermore, atjat1 plants, like jassy and cts mutants, do not exhibit the male sterility diagnostic of mutants in JA synthesis and signaling, indicative of basal diffusion of JA-IleH across the NE. However, JA-dependent male sterility is manifested when AtJAT1 expression is knocked out in the atjar1 background in which the formation of JA-Ile is largely impaired (Staswick et al., 2002; Suza and Staswick, 2008). This cooperation between AtJAT1 and AtJAR1 reveals that AtJAT1 also acts to maintain a critical nuclear JA-Ile level to activate JA signaling during normal floral development.
Characterization of AtJAT1 shows that, although basal diffusion does occur, JA-Ile enters the nucleus primarily by transporter-mediated active import. However, immunogold studies have revealed that AtJAT1 is likely to be localized at the inner membrane of the NE. The outer nuclear membrane is part of the ER membrane, and the perinuclear space between the inner and outer membranes is thus continuous with the ER lumen. The ER-localized PIN (PIN-FORMED) permease-like auxin transporters have been proposed to transport auxin between the ER lumen and the nucleoplasm (Bender et al., 2013; Mravec et al., 2009; Park et al., 2017; Skalicky et al., 2018; Viaene et al., 2013). Similarly, AtJAT1 could also mediate the entry of JA-Ile into the ER lumen, thereby enabling rapid and efficient collection and import of JA-Ile into the nucleus, given the broad distribution and high surface area of the ER in the cytosol (Figure 2). AtJAT1 shows high but distinct substrate specificity for mediating the nuclear import of JA-Ile (but not JA) and the cellular export of JA (but not JA-Ile). As a half-molecule ABC transporter, AtJAT1 must interact with another half-molecule transporter and form homodimers and/or heterodimers (McFarlane et al., 2010; Le Hir et al., 2013); its interacting partners, including another half-molecule ABC transporter and possibly other unidentified protein(s), may therefore establish its substrate specificity. Furthermore, AtJAT1 mediates the nuclear entry of JA-Ile with higher affinity than cellular export of JA. Therefore, when cytosolic JA/JA-Ile concentration is lower, AtJAT1-mediated nuclear import of JA-Ile predominates to pump JA-Ile into the nucleus and rapidly and efficiently activate JA-Ile signaling. When cytosolic JA/JA-Ile concentration is higher, cellular export of JA predominates to pump JA out of the cytosol and attenuate JA-Ile signaling. The removal of JA-Ile or JA from the cytosol can significantly affect the equilibrium between JA and JA-Ile by modulating the conjugation of JA with Ile (Staswick et al., 2002). Furthermore, the removal of JA-Ile from the perinuclear space can also modulate the hydrolysis (Woldemariam et al., 2012) or hydroxylation of JA-Ile (Koo et al., 2011) that occur in the ER. Thus, the roles of AtJAT1 in regulating the highly dynamic processes of JA biosynthesis, metabolism, and signaling may underlie the close link between nuclear import of JA-Ile and cellular export of JA (Figure 2).
Modulation of JA distribution between the cytosol and apoplasm by transporters
Phytohormones are mostly synthesized locally in particular tissues or cells and transported as short-range signaling molecules to neighboring cells, and they often undergo vasculature-based long-range transmission. Short-distance intercellular transport can occur by apoplastic, symplastic, or transcellular transport, separately or in combination. Cell-to-cell transport is used to load and unload substances from the vasculature and to distribute short-range signals within tissues (Robert and Friml, 2009). Leaf-to-leaf transmission of mobile wound signals in wound-induced systemic response/resistance has been intriguing scientists for decades (Koo et al., 2009; Mielke et al., 2011; Chauvin et al., 2013; Choi et al., 2016; Bozorov et al., 2017; Hilleary and Gilroy, 2018). Stem-stem grafting experiments in tomato (Solanum lycopersicum) (Li et al., 2002; Schilmiller and Howe, 2005), Nicotiana attenuata (Bozorov et al., 2017), and Arabidopsis (Gasperini et al., 2015; Bozorov et al., 2017) have established that, in addition to JA-independent signals such as the rapid electrical signal (Chauvin et al., 2013; Farmer et al., 2014; Gasperini et al., 2015; Nguyen et al., 2018), JAs may be the mobile wound signal transmitted along the phloem, although the particular molecular form remains to be determined. Furthermore, grafting experiments and hormone profiling have revealed that OPDA and its derivatives, but not the bioactive JA-Ile, translocate from wounded shoots to undamaged roots through the phloem (Schulze et al., 2019).
Albeit slower and more energetically demanding, transcellular transport allows more elaborate regulation and integration of various signals at the level of each transporting cell, which typically involve transporters at the PM that facilitate the movement of hormonal molecules between the cytosol and the apoplasm (Robert and Friml, 2009). As mentioned earlier, the cytosol acts as the medium not only for diverse metabolism of JAs but also for the exchange of JAs among intracellular compartments, particularly between the cytosol and the apoplasm. Based on analogy to the well-established ion trapping model for IAA (Robert and Friml, 2009), the equilibrium of JA molecules (pKa ∼4.52) shifts almost entirely in the cytoplasm (pH ∼7.3) to the anionic dissociated form, JA−, which cannot diffuse across the PM. Thus, JA− is trapped in the cytosol unless actively exported by transporters. Cytosolic JA is increased significantly in atjat1 cells (Li et al., 2017), confirming that AtJAT1 is involved in the export of cytosolic JA.
Proton-associated molecules can enter the cell along the JAH gradient by lipophilic diffusion (passive movement) across the PM without the assistance of a carrier protein; however, active transport could greatly facilitate and accelerate cellular import of JA, even against the JAH gradient. Consistent with this supposition, two JAT family members localized at the PM, AtJAT3, and AtJAT4, have been shown to mediate the cellular import of JA (Li et al., 2020). Consistent with the dispensability of cellular JA import under normal conditions, no developmental defect is apparent in atjat3;4 mutants in which the expression of both AtJAT3 and AtJAT4 is knocked out. The atjat3;4 mutants exhibit wound responses in local damaged leaves comparable to those of wild-type plants. However, they exhibit nearly abolished wound-induced systemic resistance/immunity in the distal undamaged leaves, as shown by a wound-induced upsurge in JA/JA-Ile, expression of primary JA-responsive genes, and resistance to the necrotrophic fungal pathogen B. cinerea. AtJAT3/4 are expressed primarily in the phloem parenchyma cells, including the specialized companion cells that connect extensively with and carry out cellular functions of the conductive sieve-tube elements. Thus, AtJAT3/4 may be involved in the collection and loading of JA into sieve-tube elements, which is induced not only in the vascular tissues but also in other leaf cells (Glauser et al., 2009; Gasperini et al., 2015). Rosette-petiole grafting and D5-JA tracing experiments have demonstrated the predominant role of AtJAT3 and AtJAT4 in the modulation of leaf-to-leaf translocation of JA (Li et al., 2020). Thus, a model has been proposed in which homo-/heterodimers of AtJAT3/AtJAT4 mediate the cell-cell transport of JA, driving long-distance, self-propagating transmission of the JA signal (Li et al., 2021). In contrast to importers, exporters (e.g., AtJAT1) may play a more significant role in mediating cell-cell transport of JA and thereby driving long-distance JA transmission. It will be intriguing, however, to validate such a role experimentally in the future.
Regulatory networks connected by transporter-mediated intra- and intercellular transport of JAS
The occurrence of JA biosynthesis, metabolism, and perception/signaling in diverse subcellular compartments (Figure 1) enables the prompt and delicate regulation of the metabolic flux and signaling of JAs through modulation of their subcellular distribution. At the pH values of various intracellular compartments, OPDA, JA, and JA-Ile exist predominantly in their deprotonated form, and transporters are thus important for modulating the intracellular distributions of these JAs, particularly for the selection and concentration of substrates for reactions in distinct compartments. This has been illustrated in cts mutants in which the conversion from OPDA to JA is impaired, probably owing to the impaired peroxisomal import of OPDA (Theodoulou et al., 2005). In the cts-2 mutant, basal and wound-induced JA levels are greatly decreased relative to wild-type plants, and the OPDA level is significantly elevated, revealing the role of CTS in modulating the metabolic flux between OPDA and JA. In addition, levels of α-ketol and γ-ketol, which are generated from α-LeA in the cytosol, are also significantly elevated in cts mutants (Theodoulou et al., 2005), suggesting that increased OPDA may enhance the efflux of α-LeA from the plastid/chloroplast to the cytosol, where it is then converted to α- and γ-ketol (Figure 1). AtJAT1 shows high substrate specificity at the NE for JA-Ile and the PM for JA, separating and concentrating JA-Ile in the nucleus. Variations in cytosolic JA levels will thus constantly adjust the equilibrium between JA and JA-Ile (and other JA conjugates). In addition, these variations in cytosolic JA also affect the export of JA to the apoplasm and, accordingly, cell-cell transport. Thus, AtJAT1 can integrate and coordinate JA biosynthesis, metabolism, and signaling by regulating the distribution of JA-Ile between the cytosol and nucleus and of JA between the cytosol and the apoplasm (Figure 3A). In addition, AtJAT3 and AtJAT4 are localized at the PM and mediate the cellular import of JA, which also regulates the distribution of JA between the cytosol and apoplasm and thereby regulates the biosynthesis, metabolism, and signaling of JA. How exporters and importers are coordinated to regulate the distribution of JA between the cytosol and the apoplasm remains to be clarified.
Figure 3.
Regulatory networks connected by transporter-mediated intra- and intercellular transport of JAs.
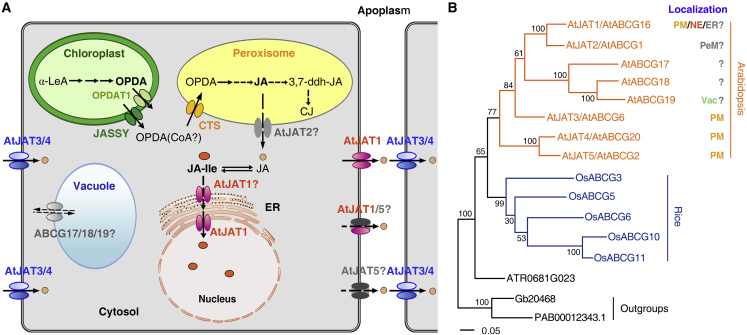
(A) Schematic model showing networks connected by transporter-mediated intracellular and intercellular transport of JAs. JASSY and OPDAT1 channels localized at the membrane of the plastid envelope and the CTS transporter localized at the peroxisomal membrane mediate the plastidial efflux and peroxisomal influx of OPDA, respectively. AtJAT1 localized at both the NE and PM mediates the nuclear entry of JA-Ile and cellular efflux of JA. AtJAT2 localized at the peroxisomal membrane probably participates in the peroxisomal export of JA. AtJAT3, AtJAT4, and AtJAT5 are localized at the PM; AtJAT3/4 mediate JA import, and AtJAT5 may be involved in JA export. The possible vacuolar localization and transport activity of AtABCG17/18/19 in the AtJAT family await evaluation. Question marks indicate putative transporters that remain to be further characterized.
(B) The phylogenies of JAT families in Arabidopsis and rice (O. sativa). The maximum likelihood (ML) tree was constructed with confidence (bootstrap) values shown on the branches, and the scale bar represents the number of amino acid changes per site. The subcellular localizations of AtJAT members (PeM, peroxisomal membrane; Vac, vacuole) are shown. The single JAT members in the genomes of the gymnosperms G. biloba (Gb20468) and P. abies (PAB 00012343.1) (used as the outgroup) and the basal angiosperm A. trichopoda (ATR0681G023) were included in the analysis.
In the AtJAT family, AtJAT2 is localized at the peroxisome (Wang et al., 2019a). Although genetic and biochemical data remain to be collected, it is possible that AtJAT2 may mediate the peroxisomal export of JA, thereby regulating the distribution of JA between the peroxisome and the cytosol, where JA is conjugated with Ile to generate bioactive JA-Ile and then enters the nucleus through the activity of AtJAT1. In the AtJAT family, three ABCG members (ABCG17, ABCG18, and AtABCG19) comprise a well-supported clade (Figure 3), and AtABCG19 appears to localize at the vacuole and participate in kanamycin resistance (Mentewab and Stewart, 2005). It will be intriguing to confirm whether these JAT members are indeed localized at the vacuole and have JAs transport activity and to determine the identity of their transport substrate. In summary, transporters act as nodes in a dynamic network that regulates the biosynthesis, metabolism, and signaling of JAs by modulation of their intracellular distributions. Further characterization of these JATs in future studies will provide new insights into the crucial roles of these transporters in this regulatory network.
Evolutionary studies indicate that the JA/JA-Ile biosynthetic pathway has evolved concurrently with terrestrial conquest by plants, and JA-Ile has exploited the preexistent and functionally conserved COI1-mediated signaling pathway with the JA precursor dn-OPDA as the ligand in M. polymorpha (Monte et al., 2018). Intriguingly, there is only one JAT member in each of the genomes of the gymnosperms Ginkgo biloba and Picea abies and the basal angiosperm Amborella trichopoda, implying that these represent the progenitor JATs in the most recent common ancestor of seed plants. When these JATs were used as the outgroup, JATs in Arabidopsis and rice (Oryza sativa) constituted separate clades (Figure 3B), indicating that they have undergone independent evolution. Although absent in the chlorophyte alga Chlamydomonas reinhardtii and the charophyte algae Spirogloea muscicola, Chara braunii, and Klebsormidium nitens, one to two JAT members have been identified in the genomes of the bryophytes M. polymorpha and Physcomitrella patens and the lycophyte Selaginella moellendorffii. Their functions in the transport of JAs remain to be elucidated. Understanding the evolutionary trajectory in subcellular localizations, substrate specificities, and transport directions of JAT members in land plants will provide new insights into the evolution of JA signaling during terrestrial adaptation, which is also of great economic and ecological importance in agriculture.
Concluding remarks and future perspectives
The last three decades have seen great achievements in understanding the synthesis, metabolism, and signaling of JAs in plant cells. Here, we focused on the complex intracellular compartmentation of these processes, which may account for their great chemical and metabolic diversity and highly complex and dynamic signaling. Understanding the role of this intracellular partitioning in the modulation of metabolic flux and signaling may also lead to the discovery of new metabolites and metabolic routes of JAs. Although evidence is emerging for the biological activities of OPDA, CJ, and other JAs that are probably COI1 independent, their signaling pathways remain to be elucidated. The identification and characterization of JASSY, CTS, and particularly JAT transporters localized at the endomembrane of various organelles and the PM have established that the coordinated actions of these transporters play crucial roles in modulating the intracellular distribution of OPDA, JA/JA-Ile, and other JAs, thereby connecting and orchestrating the biosynthesis, metabolic flux, and signaling of JAs partitioned in diverse cellular compartments. In particular, the coupling of JA-Ile nuclear entry and JA cellular export by AtJAT1 provides a new avenue to rapidly modulate the metabolic flux between JA and JA-Ile and to integrate JA signaling with its cellular export. Further characterization of the peroxisome- and vacuole-localized JATs may furnish more details of this transporter-based regulatory network. Although AtJATs are probably under positive feedback regulation by JA-Ile signaling, other regulatory mechanisms remain to be identified. In addition, the means by which the substrate specificities, transport directions, and diverse subcellular localizations of these JATs are determined represent the most challenging questions to be clarified. The finding that transporters are localized at various types of endomembranes and the PM highlights the significance of this intra- and intercellular compartmentation for the actions of JAs.
Funding
This work was supported by the Natural Science Foundation of China (NSFC) (no. 31970310, 31470326, and 30870358), the Major Research Plan from the Ministry of Science and Technology of the People's Republic of China (no. 2013CB945100), and the Program for New Century Excellent Talents in University (NECT-08-0529) to P.L.
Author contributions
P.L., G.Y., and M.L. planned the review outline. P.L. wrote the manuscript with contributions from all authors. M.L., G.Y., and C.C. prepared the figures.
Acknowledgments
We appreciate all the work in this field and apologize to colleagues whose work is not cited due to space limitations. The authors declare no competing interests.
Published: August 11, 2021
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
References
- Afitlhile M.M., Fukushige H., Nishimura M., Hildebrand D.F. A defect in glyoxysomal fatty acid beta-oxidation reduces jasmonic acid accumulation in Arabidopsis. Plant Physiol. Biochem. 2005;43:603–609. doi: 10.1016/j.plaphy.2005.03.016. [DOI] [PubMed] [Google Scholar]
- An C., Li L., Zhai Q., You Y., Deng L., Wu F., Chen R., Jiang H., Wang H., Chen Q. Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc. Natl. Acad. Sci. U S A. 2017;114:8930–8939. doi: 10.1073/pnas.1710885114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg G., Martinez M., Hamberg M., Castresana C. Diversity of the enzymatic activity in the lipoxygenase gene family of Arabidopsis thaliana. Lipids. 2009;44:85–95. doi: 10.1007/s11745-008-3245-7. [DOI] [PubMed] [Google Scholar]
- Bannenberg G., Martinez M., Rodriguez M.J., Lopez M.A., Ponce de Leon I., Hamberg M., Castresana C. Functional analysis of alpha-DOX2, an active alpha-dioxygenase critical for normal development in tomato plants. Plant Physiol. 2009;151:1421–1432. doi: 10.1104/pp.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso V., Veneault-Fourrey C. Role of jasmonates in beneficial microbe-root interactions. Methods Mol. Biol. 2020;2085:43–67. doi: 10.1007/978-1-0716-0142-6_4. [DOI] [PubMed] [Google Scholar]
- Bender R.L., Fekete M.L., Klinkenberg P.M., Hampton M., Bauer B., Malecha M., Lindgren K., J A.M., Perera M.A., Nikolau B.J. PIN6 is required for nectary auxin response and short stamen development. Plant J. 2013;74:893–904. doi: 10.1111/tpj.12184. [DOI] [PubMed] [Google Scholar]
- Bhosale R., Jewell J.B., Hollunder J., Koo A.J., Vuylsteke M., Michoel T., Hilson P., Goossens A., Howe G.A., Browse J. Predicting gene function from uncontrolled expression variation among individual wild-type Arabidopsis plants. Plant Cell. 2013;25:2865–2877. doi: 10.1105/tpc.113.112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blee E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002;7:315–322. doi: 10.1016/s1360-1385(02)02290-2. [DOI] [PubMed] [Google Scholar]
- Bozorov T.A., Dinh S.T., Baldwin I.T. JA but not JA-Ile is the cell-nonautonomous signal activating JA mediated systemic defenses to herbivory in Nicotiana attenuata. J. Integr. Plant Biol. 2017;59:552–571. doi: 10.1111/jipb.12545. [DOI] [PubMed] [Google Scholar]
- Brandizzi F. Maintaining the structural and functional homeostasis of the plant endoplasmic reticulum. Dev. Cell. 2021;56:919–932. doi: 10.1016/j.devcel.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 2009;60:183–205. doi: 10.1146/annurev.arplant.043008.092007. [DOI] [PubMed] [Google Scholar]
- Caarls L., Elberse J., Awwanah M., Ludwig N.R., de Vries M., Zeilmaker T., Van Wees S.C.M., Schuurink R.C., Van den Ackerveken G. Arabidopsis JASMONATE-INDUCED OXYGENASES down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid. Proc. Natl. Acad. Sci. U S A. 2017;114:6388–6393. doi: 10.1073/pnas.1701101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldelari D., Wang G., Farmer E.E., Dong X. Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol. Biol. 2011;75:25–33. doi: 10.1007/s11103-010-9701-9. [DOI] [PubMed] [Google Scholar]
- Chauvin A., Caldelari D., Wolfender J.L., Farmer E.E. Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: a role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol. 2013;197:566–575. doi: 10.1111/nph.12029. [DOI] [PubMed] [Google Scholar]
- Chen R., Jiang H., Li L., Zhai Q., Qi L., Zhou W., Liu X., Li H., Zheng W., Sun J. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell. 2012;24:2898–2916. doi: 10.1105/tpc.112.098277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y., Shimizu T., Miyakawa S., Kanno Y., Koshiba T., Kamiya Y., Seo M. Identification of Arabidopsis thaliana NRT1/PTR FAMILY (NPF) proteins capable of transporting plant hormones. J. Plant Res. 2015;128:679–686. doi: 10.1007/s10265-015-0710-2. [DOI] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Fernandez G., Adie B., Chico J.M., Lorenzo O., Garcia-Casado G., Lopez-Vidriero I., Lozano F.M., Ponce M.R. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- Chini A., Gimenez-Ibanez S., Goossens A., Solano R. Redundancy and specificity in jasmonate signalling. Curr. Opin. Plant Biol. 2016;33:147–156. doi: 10.1016/j.pbi.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Chini A., Monte I., Zamarreno A.M., Hamberg M., Lassueur S., Reymond P., Weiss S., Stintzi A., Schaller A., Porzel A. An OPR3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis. Nat. Chem. Biol. 2018;14:171–178. doi: 10.1038/nchembio.2540. [DOI] [PubMed] [Google Scholar]
- Choi W.G., Hilleary R., Swanson S.J., Kim S.H., Gilroy S. Rapid, long-distance electrical and calcium signaling in plants. Annu. Rev. Plant Biol. 2016;67:287–307. doi: 10.1146/annurev-arplant-043015-112130. [DOI] [PubMed] [Google Scholar]
- Christensen S.A., Huffaker A., Kaplan F., Sims J., Ziemann S., Doehlemann G., Ji L., Schmitz R.J., Kolomiets M.V., Alborn H.T. Maize death acids, 9-lipoxygenase-derived cyclopente(a)nones, display activity as cytotoxic phytoalexins and transcriptional mediators. Proc. Natl. Acad. Sci. U S A. 2015;112:11407–11412. doi: 10.1073/pnas.1511131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Castillo M., Martinez C., Buchala A., Metraux J.P., Leon J. Gene-specific involvement of beta-oxidation in wound-activated responses in Arabidopsis. Plant Physiol. 2004;135:85–94. doi: 10.1104/pp.104.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska P., Boland W. Iso-OPDA: an early precursor of cis-jasmone in plants? Chembiochem. 2007;8:2281–2285. doi: 10.1002/cbic.200700464. [DOI] [PubMed] [Google Scholar]
- Dave A., Graham I.A. Oxylipin signaling: a distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA) Front. Plant Sci. 2012;3:42. doi: 10.3389/fpls.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito Francisco R., Martinoia E. The vacuolar transportome of plant specialized metabolites. Plant Cell Physiol. 2018;59:1326–1336. doi: 10.1093/pcp/pcy039. [DOI] [PubMed] [Google Scholar]
- Delker C., Zolman B.K., Miersch O., Wasternack C. Jasmonate biosynthesis in Arabidopsis thaliana requires peroxisomal beta-oxidation enzymes–additional proof by properties of pex6 and aim1. Phytochemistry. 2007;68:1642–1650. doi: 10.1016/j.phytochem.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Farmer E.E., Gasperini D., Acosta I.F. The squeeze cell hypothesis for the activation of jasmonate synthesis in response to wounding. New Phytol. 2014;204:282–288. doi: 10.1111/nph.12897. [DOI] [PubMed] [Google Scholar]
- Farmer E.E., Goossens A. Jasmonates: what ALLENE OXIDE SYNTHASE does for plants. J. Exp. Bot. 2019;70:3373–3378. doi: 10.1093/jxb/erz254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Calvo P., Chini A., Fernández-Barbero G., Chico J.M., Gimenez-Ibanez S., Geerinck J., Eeckhout D., Schweizer F., Godoy M., Franco-Zorrilla J.M. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell. 2011;23:701–715. doi: 10.1105/tpc.110.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flokova K., Feussner K., Herrfurth C., Miersch O., Mik V., Tarkowska D., Strnad M., Feussner I., Wasternack C., Novak O. A previously undescribed jasmonate compound in flowering Arabidopsis thaliana–The identification of cis-(+)-OPDA-Ile. Phytochemistry. 2016;122:230–237. doi: 10.1016/j.phytochem.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Fonseca S., Chico J.M., Solano R. The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr. Opin. Plant Biol. 2009;12:539–547. doi: 10.1016/j.pbi.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Gasperini D., Chauvin A., Acosta I.F., Kurenda A., Stolz S., Chetelat A., Wolfender J.L., Farmer E.E. Axial and radial oxylipin transport. Plant Physiol. 2015;169:2244–2254. doi: 10.1104/pp.15.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G., Dubugnon L., Mousavi S.A., Rudaz S., Wolfender J.L., Farmer E.E. Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J. Biol. Chem. 2009;284:34506–34513. doi: 10.1074/jbc.M109.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L., Denkert N., Eisa A., Lehmann M., Sjuts I., Weiberg A., Soll J., Meinecke M., Schwenkert S. JASSY, a chloroplast outer membrane protein required for jasmonate biosynthesis. Proc. Natl. Acad. Sci. U S A. 2019;116:10568–10575. doi: 10.1073/pnas.1900482116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Ponce de Leon I., Rodriguez M.J., Castresana C. Alpha-dioxygenases. Biochem. Biophys. Res. Commun. 2005;338:169–174. doi: 10.1016/j.bbrc.2005.08.117. [DOI] [PubMed] [Google Scholar]
- Harms K., Ramirez I.I., Pena-Cortes H. Inhibition of wound-induced accumulation of allene oxide synthase transcripts in flax leaves by aspirin and salicylic acid. Plant Physiol. 1998;118:1057–1065. doi: 10.1104/pp.118.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B., Stenzel I., Miersch O., Maucher H., Kramell R., Ziegler J., Wasternack C. Tissue-specific oxylipin signature of tomato flowers: allene oxide cyclase is highly expressed in distinct flower organs and vascular bundles. Plant J. 2000;24:113–126. doi: 10.1046/j.1365-313x.2000.00861.x. [DOI] [PubMed] [Google Scholar]
- Hazman M., Sühnel M., Schäfer S., Zumsteg J., Lesot A., Beltran F., Marquis V., Herrgott L., Miesch L., Riemann M. Characterization of jasmonoyl-isoleucine (JA-Ile) hormonal catabolic pathways in rice upon wounding and salt stress. Rice (N Y). 2019;12:45. doi: 10.1186/s12284-019-0303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz T., Smirnova E., Widemann E., Aubert Y., Pinot F., Ménard R. The rise and fall of jasmonate biological activities. Subcellular Biochem. 2016:405–426. doi: 10.1007/978-3-319-25979-6_16. [DOI] [PubMed] [Google Scholar]
- Heitz T., Widemann E., Lugan R., Miesch L., Ullmann P., Desaubry L., Holder E., Grausem B., Kandel S., Miesch M. Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone jasmonoyl-isoleucine for catabolic turnover. J. Biol. Chem. 2012;287:6296–6306. doi: 10.1074/jbc.M111.316364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleary R., Gilroy S. Systemic signaling in response to wounding and pathogens. Curr. Opin. Plant Biol. 2018;43:57–62. doi: 10.1016/j.pbi.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Howe G.A. Plant hormones: metabolic end run to jasmonate. Nat. Chem. Biol. 2018;14:109–110. doi: 10.1038/nchembio.2553. [DOI] [PubMed] [Google Scholar]
- Howe G.A., Major I.T., Koo A.J. Modularity in jasmonate signaling for multistress resilience. Annu. Rev. Plant Biol. 2018;69:387–415. doi: 10.1146/annurev-arplant-042817-040047. [DOI] [PubMed] [Google Scholar]
- Hsieh H.L., Okamoto H., Wang M.L., Ang L.H., Matsui M., Goodman H., Deng X.W. FIN219, an auxin-regulated gene, defines a link between phytochrome A and the downstream regulator COP1 in light control of Arabidopsis development. Genes Dev. 2000;14:1958–1970. [PMC free article] [PubMed] [Google Scholar]
- Huang H., Liu B., Liu L., Song S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017;68:1349–1359. doi: 10.1093/jxb/erw495. [DOI] [PubMed] [Google Scholar]
- Jimenez-Aleman G.H., Almeida-Trapp M., Fernandez-Barbero G., Gimenez-Ibanez S., Reichelt M., Vadassery J., Mithofer A., Caballero J., Boland W., Solano R. Omega hydroxylated JA-Ile is an endogenous bioactive jasmonate that signals through the canonical jasmonate signaling pathway. Biochim. Biophys. Acta Mol. Cell Biol Lipids. 2019;1864:158520. doi: 10.1016/j.bbalip.2019.158520. [DOI] [PubMed] [Google Scholar]
- Koch T., Bandemer K., Boland W. Biosynthesis of cis-jasmone: a pathway for the inactivation and the disposal of the plant stress hormone jasmonic acid to the gas phase? Helv. Chim. Acta. 1997;80:838–850. [Google Scholar]
- Koo A.J. Metabolism of the plant hormone jasmonate: a sentinel for tissue damage and master regulator of stress response. Phytochem. Rev. 2018;17:51–80. [Google Scholar]
- Koo A.J., Chung H.S., Kobayashi Y., Howe G.A. Identification of a peroxisomal acyl-activating enzyme involved in the biosynthesis of jasmonic acid in Arabidopsis. J. Biol. Chem. 2006;281:33511–33520. doi: 10.1074/jbc.M607854200. [DOI] [PubMed] [Google Scholar]
- Koo A.J., Cooke T.F., Howe G.A. Cytochrome P450 CYP94B3 mediates catabolism and inactivation of the plant hormone jasmonoyl-L-isoleucine. Proc. Natl. Acad. Sci. U S A. 2011;108:9298–9303. doi: 10.1073/pnas.1103542108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo A.J., Gao X., Jones A.D., Howe G.A. A rapid wound signal activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J. 2009;59:974–986. doi: 10.1111/j.1365-313X.2009.03924.x. [DOI] [PubMed] [Google Scholar]
- Koo A.J., Thireault C., Zemelis S., Poudel A.N., Zhang T., Kitaoka N., Brandizzi F., Matsuura H., Howe G.A. Endoplasmic reticulum-associated inactivation of the hormone jasmonoyl-L-isoleucine by multiple members of the cytochrome P450 94 family in Arabidopsis. J. Biol. Chem. 2014;289:29728–29738. doi: 10.1074/jbc.M114.603084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir R., Sorin C., Chakraborti D., Moritz T., Schaller H., Tellier F., Robert S., Morin H., Bako L., Bellini C. ABCG9, ABCG11 and ABCG14 ABC transporters are required for vascular development in Arabidopsis. Plant J. 2013;76:811–824. doi: 10.1111/tpj.12334. [DOI] [PubMed] [Google Scholar]
- Li L., Li C., Lee G.I., Howe G.A. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc. Natl. Acad. Sci. U S A. 2002;99:6416–6421. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wang F., Li S., Yu G., Wang L., Li Q., Zhu X., Li Z., Yuan L., Liu P. Importers drive leaf-to-leaf jasmonic acid transmission in wound-induced systemic immunity. Mol. Plant. 2020;13:1485–1498. doi: 10.1016/j.molp.2020.08.017. [DOI] [PubMed] [Google Scholar]
- Li M., Yu G., Ma J., Liu P. Interactions of importers in long-distance transmission of wound-induced jasmonate. Plant Signal. Behav. 2021;16:1886490. doi: 10.1080/15592324.2021.1886490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zheng J., Li S., Huang G., Skilling S.J., Wang L., Li L., Li M., Yuan L., Liu P. Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Mol. Plant. 2017;10:695–708. doi: 10.1016/j.molp.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang C., Yang J., Yu N., Wang E. Hormone modulation of legume-rhizobial symbiosis. J. Integr. Plant Biol. 2018;60:632–648. doi: 10.1111/jipb.12653. [DOI] [PubMed] [Google Scholar]
- Lumba S., Cutler S., McCourt P. Plant nuclear hormone receptors: a role for small molecules in protein-protein interactions. Annu. Rev. Cell Dev. Biol. 2010;26:445–469. doi: 10.1146/annurev-cellbio-100109-103956. [DOI] [PubMed] [Google Scholar]
- Maynard D., Groger H., Dierks T., Dietz K.J. The function of the oxylipin 12-oxophytodienoic acid in cell signaling, stress acclimation, and development. J. Exp. Bot. 2018;69:5341–5354. doi: 10.1093/jxb/ery316. [DOI] [PubMed] [Google Scholar]
- McFarlane H.E., Shin J.J., Bird D.A., Samuels A.L. Arabidopsis ABCG transporters, which are required for export of diverse cuticular lipids, dimerize in different combinations. Plant Cell. 2010;22:3066–3075. doi: 10.1105/tpc.110.077974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentewab A., Stewart C.N., Jr. Overexpression of an Arabidopsis thaliana ABC transporter confers kanamycin resistance to transgenic plants. Nat. Biotechnol. 2005;23:1177–1180. doi: 10.1038/nbt1134. [DOI] [PubMed] [Google Scholar]
- Mielke K., Forner S., Kramell R., Conrad U., Hause B. Cell-specific visualization of jasmonates in wounded tomato and Arabidopsis leaves using jasmonate-specific antibodies. New Phytol. 2011;190:1069–1080. doi: 10.1111/j.1469-8137.2010.03638.x. [DOI] [PubMed] [Google Scholar]
- Monte I., Franco-Zorrilla J.M., García-Casado G., Zamarreño A.M., García-Mina J.M., Nishihama R., Kohchi T., Solano R. A single JAZ repressor controls the jasmonate pathway in Marchantia polymorpha. Mol. Plant. 2019;12:185–198. doi: 10.1016/j.molp.2018.12.017. [DOI] [PubMed] [Google Scholar]
- Monte I., Ishida S., Zamarreno A.M., Hamberg M., Franco-Zorrilla J.M., Garcia-Casado G., Gouhier-Darimont C., Reymond P., Takahashi K., Garcia-Mina J.M. Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nat. Chem. Biol. 2018;14:480–488. doi: 10.1038/s41589-018-0033-4. [DOI] [PubMed] [Google Scholar]
- Monte I., Kneeshaw S., Franco-Zorrilla J.M., Chini A., Zamarreño A.M., García-Mina J.M., Solano R. An ancient COI1-independent function for reactive electrophilic oxylipins in thermotolerance. Curr. Biol. 2020;30:962–971.e3. doi: 10.1016/j.cub.2020.01.023. [DOI] [PubMed] [Google Scholar]
- Mravec J., Skupa P., Bailly A., Hoyerova K., Krecek P., Bielach A., Petrasek J., Zhang J., Gaykova V., Stierhof Y.D. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature. 2009;459:1136–1140. doi: 10.1038/nature08066. [DOI] [PubMed] [Google Scholar]
- Nguyen C.T., Kurenda A., Stolz S., Chetelat A., Farmer E.E. Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. U S A. 2018;115:10178–10183. doi: 10.1073/pnas.1807049115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkama-Ohtsu N., Sasaki-Sekimoto Y., Oikawa A., Jikumaru Y., Shinoda S., Inoue E., Kamide Y., Yokoyama T., Hirai M.Y., Shirasu K. 12-oxo-phytodienoic acid-glutathione conjugate is transported into the vacuole in Arabidopsis. Plant Cell Physiol. 2011;52:205–209. doi: 10.1093/pcp/pcq181. [DOI] [PubMed] [Google Scholar]
- Park J., Lee Y., Martinoia E., Geisler M. Plant hormone transporters: what we know and what we would like to know. BMC Biol. 2017;15:93. doi: 10.1186/s12915-017-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.W., Li W., Viehhauser A., He B., Kim S., Nilsson A.K., Andersson M.X., Kittle J.D., Ambavaram M.M., Luan S. Cyclophilin 20-3 relays a 12-oxo-phytodienoic acid signal during stress responsive regulation of cellular redox homeostasis. Proc. Natl. Acad. Sci. U S A. 2013;110:9559–9564. doi: 10.1073/pnas.1218872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinfield-Wells H., Rylott E.L., Gilday A.D., Graham S., Job K., Larson T.R., Graham I.A. Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J. 2005;43:861–872. doi: 10.1111/j.1365-313X.2005.02498.x. [DOI] [PubMed] [Google Scholar]
- Poudel A.N., Holtsclaw R.E., Kimberlin A., Sen S., Zeng S., Joshi T., Lei Z., Sumner L.W., Singh K., Matsuura H. 12-Hydroxy-jasmonoyl-l-isoleucine is an active jasmonate that signals through CORONATINE INSENSITIVE 1 and contributes to the wound response in Arabidopsis. Plant Cell Physiol. 2019;60:2152–2166. doi: 10.1093/pcp/pcz109. [DOI] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M., Pawlik-Pachucka E., Owczarz M., Budzinska M., Polosak J. Small-molecule hormones: molecular mechanisms of action. Int. J. Endocrinol. 2013;2013:601246. doi: 10.1155/2013/601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T., Huang H., Song S., Xie D. Regulation of jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in Arabidopsis. Plant Cell. 2015;27:1620–1633. doi: 10.1105/tpc.15.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T., Song S., Ren Q., Wu D., Huang H., Chen Y., Fan M., Peng W., Ren C., Xie D. The jasmonate-ZIM-domain proteins interact with the WD-repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell. 2011;23:1795–1814. doi: 10.1105/tpc.111.083261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranocha P., Dima O., Nagy R., Felten J., Corratge-Faillie C., Novak O., Morreel K., Lacombe B., Martinez Y., Pfrunder S. Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat. Commun. 2013;4:2625. doi: 10.1038/ncomms3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert H.S., Friml J. Auxin and other signals on the move in plants. Nat. Chem. Biol. 2009;5:325–332. doi: 10.1038/nchembio.170. [DOI] [PubMed] [Google Scholar]
- Saito H., Oikawa T., Hamamoto S., Ishimaru Y., Kanamori-Sato M., Sasaki-Sekimoto Y., Utsumi T., Chen J., Kanno Y., Masuda S. The jasmonate-responsive GTR1 transporter is required for gibberellin-mediated stamen development in Arabidopsis. Nat. Commun. 2015;6:6095. doi: 10.1038/ncomms7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P.M., Lee P.Y., Biesgen C., Boone J.D., Beals T.P., Weiler E.W., Goldberg R.B. The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell. 2000;12:1041–1061. doi: 10.1105/tpc.12.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner A., Estelle M. Recent advances and emerging trends in plant hormone signalling. Nature. 2009;459:1071–1078. doi: 10.1038/nature08122. [DOI] [PubMed] [Google Scholar]
- Schaller F., Weiler E.W. Molecular cloning and characterization of 12-oxophytodienoate reductase, an enzyme of the octadecanoid signaling pathway from Arabidopsis thaliana. Structural and functional relationship to yeast old yellow enzyme. J. Biol. Chem. 1997;272:28066–28072. doi: 10.1074/jbc.272.44.28066. [DOI] [PubMed] [Google Scholar]
- Schaller F., Zerbe P., Reinbothe S., Reinbothe C., Hofmann E., Pollmann S. The allene oxide cyclase family of Arabidopsis thaliana: localization and cyclization. FEBS J. 2008;275:2428–2441. doi: 10.1111/j.1742-4658.2008.06388.x. [DOI] [PubMed] [Google Scholar]
- Schilmiller A.L., Howe G.A. Systemic signaling in the wound response. Curr. Opin. Plant Biol. 2005;8:369–377. doi: 10.1016/j.pbi.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Schilmiller A.L., Koo A.J., Howe G.A. Functional diversification of acyl-coenzyme A oxidases in jasmonic acid biosynthesis and action. Plant Physiol. 2007;143:812–824. doi: 10.1104/pp.106.092916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze A., Zimmer M., Mielke S., Stellmach H., Melnyk C.W., Hause B., Gasperini D. Wound-induced shoot-to-root relocation of JA-Ile precursors coordinates Arabidopsis growth. Mol. Plant. 2019;10:1383–1394. doi: 10.1016/j.molp.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Shan X., Yan J., Xie D. Comparison of phytohormone signaling mechanisms. Curr. Opin. Plant Biol. 2012;15:84–91. doi: 10.1016/j.pbi.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Sheard L.B., Tan X., Mao H., Withers J., Ben-Nissan G., Hinds T.R., Kobayashi Y., Hsu F.F., Sharon M., Browse J. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010;468:400–405. doi: 10.1038/nature09430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyama T., Tominaga A., Arima S., Sakai T., Inada S., Jikumaru Y., Kamiya Y., Uchiumi T., Abe M., Hashiguchi M. Additional cause for reduced JA-Ile in the root of a Lotus japonicus phyB mutant. Plant Signaling Behav. 2012;7:1–3. doi: 10.4161/psb.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalicky V., Kubes M., Napier R., Novak O. Auxins and cytokinins-the role of subcellular organization on homeostasis. Int. J. Mol. Sci. 2018;19:1–21. doi: 10.3390/ijms19103115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E., Marquis V., Poirier L., Aubert Y., Zumsteg J., Ménard R., Miesch L., Heitz T. Jasmonic acid oxidase 2 hydroxylates jasmonic acid and represses basal defense and resistance responses against Botrytis cinerea infection. Mol. Plant. 2017;10:1159–1173. doi: 10.1016/j.molp.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Song W.C., Brash A.R. Purification of an allene oxide synthase and identification of the enzyme as a cytochrome P-450. Science. 1991;253:781–784. doi: 10.1126/science.1876834. [DOI] [PubMed] [Google Scholar]
- Song W.C., Funk C.D., Brash A.R. Molecular cloning of an allene oxide synthase: a cytochrome P450 specialized for the metabolism of fatty acid hydroperoxides. Proc. Natl. Acad. Sci. U S A. 1993;90:8519–8523. doi: 10.1073/pnas.90.18.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Wang M., Zeng R., Groten K., Baldwin I.T. Priming and filtering of antiherbivore defences among Nicotiana attenuata plants connected by mycorrhizal networks. Plant Cell Environ. 2019;42:2945–2961. doi: 10.1111/pce.13626. [DOI] [PubMed] [Google Scholar]
- Staehelin L. In: Biochemistry and Molecular Biology of Plants. 2nd edn. Buchanan B., Gruissem W., Jones R., editors. John Wiley & Sons; West Sussex: 2015. Membrane structure and membranous organelles; pp. 2–44. [Google Scholar]
- Staswick P.E. The tryptophan conjugates of jasmonic and indole-3-acetic acids are endogenous auxin inhibitors. Plant Physiol. 2009;150:1310–1321. doi: 10.1104/pp.109.138529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P.E., Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P.E., Tiryaki I., Rowe M.L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel I., Otto M., Delker C., Kirmse N., Schmidt D., Miersch O., Hause B., Wasternack C. ALLENE OXIDE CYCLASE (AOC) gene family members of Arabidopsis thaliana: tissue- and organ-specific promoter activities and in vivo heteromerization. J. Exp. Bot. 2012;63:6125–6138. doi: 10.1093/jxb/ers261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A., Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. U S A. 2000;97:10625–10630. doi: 10.1073/pnas.190264497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassner J., Schaller F., Frick U.B., Howe G.A., Weiler E.W., Amrhein N., Macheroux P., Schaller A. Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J. 2002;32:585–601. doi: 10.1046/j.1365-313x.2002.01449.x. [DOI] [PubMed] [Google Scholar]
- Suza W.P., Staswick P.E. The role of JAR1 in jasmonoyl-L- isoleucine production during Arabidopsis wound response. Planta. 2008;227:1221–1232. doi: 10.1007/s00425-008-0694-4. [DOI] [PubMed] [Google Scholar]
- Takeuchi J., Fukui K., Seto Y., Takaoka Y., Okamoto M. Ligand-receptor interactions in plant hormone signaling. Plant J. 2021;105:290–306. doi: 10.1111/tpj.15115. [DOI] [PubMed] [Google Scholar]
- Taki N., Sasaki-Sekimoto Y., Obayashi T., Kikuta A., Kobayashi K., Ainai T., Yagi K., Sakurai N., Suzuki H., Masuda T. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005;139:1268–1283. doi: 10.1104/pp.105.067058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J.R. Signalling through nuclear receptors. Nat. Rev. Mol. Cell Biol. 2002;3:702–710. doi: 10.1038/nrm914. [DOI] [PubMed] [Google Scholar]
- Theodoulou F.L., Job K., Slocombe S.P., Footitt S., Holdsworth M., Baker A., Larson T.R., Graham I.A. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 2005;137:835–840. doi: 10.1104/pp.105.059352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- Ueda M., Kaji T., Kozaki W. Recent advances in plant chemical biology of jasmonates. Int. J. Mol. Sci. 2020;21:1124. doi: 10.3390/ijms21031124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene T., Delwiche C.F., Rensing S.A., Friml J. Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci. 2013;18:5–10. doi: 10.1016/j.tplants.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Wang F., Yu G., Liu P. Transporter-mediated subcellular distribution in the metabolism and signaling of jasmonates. Front. Plant Sci. 2019;10:390. doi: 10.3389/fpls.2019.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li S., Li Y., Xu Y., Wang Y., Zhang R., Sun W., Chen Q., Wang X.J., Li C. MED25 connects enhancer-promoter looping and MYC2-dependent activation of jasmonate signalling. Nat. Plants. 2019;5:616–625. doi: 10.1038/s41477-019-0441-9. [DOI] [PubMed] [Google Scholar]
- Wasternack C. In: Plant Hormone Signaling. Thomas S., Hedden P., editors. Blackwell Publishing Ltd; Oxford: 2006. Oxylipins: biosynthesis, signal transduction and action; pp. 185–228. [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C., Feussner I. The oxylipin pathways: biochemistry and function. Annu. Rev. Plant Biol. 2018;69:363–386. doi: 10.1146/annurev-arplant-042817-040440. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Forner S., Strnad M., Hause B. Jasmonates in flower and seed development. Biochimie. 2013;95:79–85. doi: 10.1016/j.biochi.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. Ann Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C., Hause B. OPDA-Ile - a new JA-Ile-independent signal? Plant Signal. Behav. 2016;11:e1253646. doi: 10.1080/15592324.2016.1253646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C., Hause B. A bypass in jasmonate biosynthesis–the OPR3-independent formation. Trends Plant Sci. 2018;23:276–279. doi: 10.1016/j.tplants.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Song S. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017;68:1303–1321. doi: 10.1093/jxb/erw443. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Strnad M. Jasmonates: news on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int. J. Mol. Sci. 2018;19:1–26. doi: 10.3390/ijms19092539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall C.S., Zubieta C., Herrmann J., Kapp U., Nanao M.H., Jez J.M. Structural basis for prereceptor modulation of plant hormones by GH3 proteins. Science. 2012;336:1708–1711. doi: 10.1126/science.1221863. [DOI] [PubMed] [Google Scholar]
- Widemann E., Miesch L., Lugan R., Holder E., Heinrich C., Aubert Y., Miesch M., Pinot F., Heitz T. The amidohydrolases IAR3 and ILL6 contribute to jasmonoyl-isoleucine hormone turnover and generate 12-hydroxyjasmonic acid upon wounding in Arabidopsis leaves. J. Biol. Chem. 2013;288:31701–31714. doi: 10.1074/jbc.M113.499228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers J., Yao J., Mecey C., Howe G.A., Melotto M., He S.Y. Transcription factor-dependent nuclear localization of a transcriptional repressor in jasmonate hormone signaling. Proc. Natl. Acad. Sci. U S A. 2012;109:20148–20153. doi: 10.1073/pnas.1210054109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldemariam M.G., Onkokesung N., Baldwin I.T., Galis I. Jasmonoyl-L-isoleucine hydrolase 1 (JIH1) regulates jasmonoyl-L-isoleucine levels and attenuates plant defenses against herbivores. Plant J. 2012;72:758–767. doi: 10.1111/j.1365-313X.2012.05117.x. [DOI] [PubMed] [Google Scholar]
- Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- Yan J., Li S., Gu M., Yao R., Li Y., Chen J., Yang M., Tong J., Xiao L., Nan F. Endogenous bioactive jasmonate is composed of a set of (+)-7-iso-JA-amino acid conjugates. Plant Physiol. 2016;172:2154–2164. doi: 10.1104/pp.16.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q., Deng L., Li C. Mediator subunit MED25: at the nexus of jasmonate signaling. Curr. Opin. Plant Biol. 2020;57:78–86. doi: 10.1016/j.pbi.2020.06.006. [DOI] [PubMed] [Google Scholar]
- Zhang T., Poudel A.N., Jewell J.B., Kitaoka N., Staswick P., Matsuura H., Koo A.J. Hormone crosstalk in wound stress response: wound-inducible amidohydrolases can simultaneously regulate jasmonate and auxin homeostasis in Arabidopsis thaliana. J. Exp. Bot. 2016;67:2107–2120. doi: 10.1093/jxb/erv521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.M., Xue J.Y., Liu L.W., Sun X.Q., Zhou G.C., Chen M., Shao Z.Q., Hang Y.Y. Divergence and conservative evolution of XTNX genes in land plants. Front. Plant Sci. 2017;8:1844. doi: 10.3389/fpls.2017.01844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Li N., Song Q., Li X., Meng H., Luo K. OPDAT1, a plastid envelope protein involved in 12-oxo-phytodienoic acid export for jasmonic acid biosynthesis in Populus. Tree Physiol. 2021:11–20. doi: 10.1093/treephys/tpab037. [DOI] [PubMed] [Google Scholar]
- Ziegler J., Stenzel I., Hause B., Maucher H., Hamberg M., Grimm R., Ganal M., Wasternack C. Molecular cloning of allene oxide cyclase. The enzyme establishing the stereochemistry of octadecanoids and jasmonates. J. Biol. Chem. 2000;275:19132–19138. doi: 10.1074/jbc.M002133200. [DOI] [PubMed] [Google Scholar]