Short abstract
Content available: Author Interview and Audio Recording
Abbreviations
- 3D
three‐dimensional
- BD
bile duct
- CK19
cytokeratin 19
- CoH
canal of Hering
- CV
central vein
- DAB
3,3′‐diaminobenzidine
- DR
ductular reaction
- H&E
hematoxylin and eosin
- HA
hepatic artery
- K19
keratin 19
- L/I
lymphatics or interstitium
- P.S.
portal space
- PV
portal vein
- THV
terminal hepatic venule
- ThV
terminal hepatic vessel
- TPV
terminal portal vein
Watch the interview with the author.
Listen to an audio presentation of this article.
Although the liver has fascinated physicians since the origins of medicine, and even nonphysicians earlier, it stands apart from most organs in terms of its microscopic and ultramicroscopic complexity. This complexity obscured a precise understanding of liver microanatomy at the outset, which could only be advanced and verified in concordance with refinements in imaging techniques and availability of human liver tissue. Although liver morphology has been studied for more than 350 years, 1 in this essay we explore historical contributions by Frances Glisson, Ewald Hering, Franklin Mall, and Aron M. Rappaport, the champions of liver microanatomy, as well as recent refinements of their discoveries.

Ryan M. Gill, M.D., Ph.D.

Neil D. Theise, M.D.
Much of its anatomic complexity of course stems from the fact that the liver has a dual blood supply, with the portal vein (PV) providing a majority of hepatic blood flow, and in which branches of the PVs, hepatic arteries (HAs), and bile ducts (BDs) ramify together into smaller and smaller limbs of a tripartite tree. However, even this complex model excludes important structures; in reality, five structures must be considered to grow together, once the lymphatic and interstitial spaces are considered, because these structures have more recently been proved to travel in parallel within portal tracts 2 (Fig. 1). The close association between hepatocytes and intrahepatic biliary epithelial cells in the canals of Hering (CoHs; Fig. 2) underlies a common endodermal origin with hepatocytes and suggests a multipotent stem cell reserve. 3 As blood flows through the PVs and HAs, it also eventually traverses the periportal limiting plate (which extends from the porta hepatis) to mix within a labyrinthine sinusoidal space (the “hepatic muralium”), 4 , 5 , 6 , 7 although the precise anatomy of the structural interfaces remains uncertain. 8 Blood transits into terminal hepatic veins and eventually to the inferior vena cava via three large hepatic veins (and accessory veins), which also have a limiting plate of sorts (i.e., the external limiting plate that originates at the liver capsule, extending in a continuous structure along the hepatic veins as an internal perivenular limiting plate) that is also traversed by vascular radicles. 9 The man from Dorsetshire,* Francis Glisson (1597‐1677†; Fig. 3)—whom the famed 17th/18th‐century Dutch physician Herman Boerhaave designated “omnium anatomicorum exactissimus”—described the liver capsule in intricate detail. 10 , 11 , 12 Actually, however, his contemporary, the Dutch anatomist Johannes Waleus (Jan de Wale of Brussels, 1604‐1649) first summarily mentioned, in a 1640 letter to his mentor, the Danish physician, anatomist, mathematician, and theologian Thomas Bartholin, that he had noted a thin membrane (“membrana cingitur tenui”) surrounding the liver. 11 It appears that Glisson’s consummate knowledge of liver anatomy was occasioned by being invited in 1640 to give the 1641 coveted Goulstonian Lecture of the Royal College of Physicians, of which he himself was later its president (1667‐1669), when he raised funds for rebuilding after its complete destruction in the Great Fire in 1666. 10 Glisson weathered the English Civil War (1642‐1651) and also attended the sick of London during the Plague (1665); he was able to avoid the contagion as “he thrust up his nostrils bits of sponge dipped in vinegar.” 10
FIG 1.
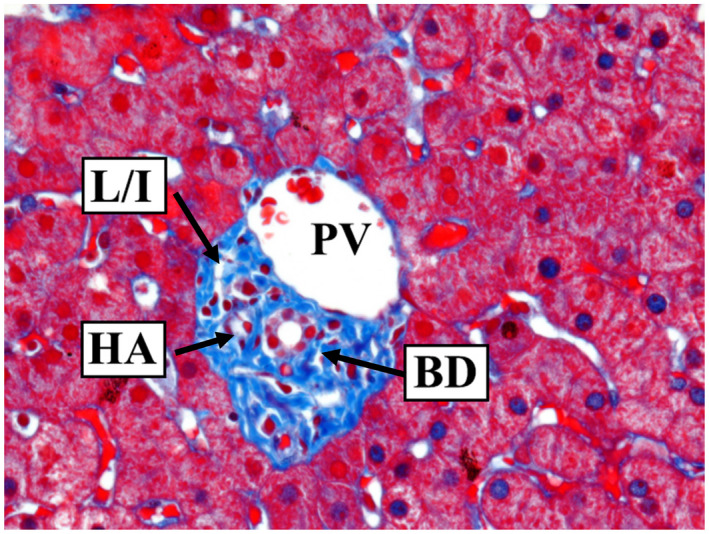
Image of a portal tract with interlobular BD, HA, PV, and spaces representing either L/I. Trichrome stain; original magnification, ×400. Image courtesy of Dr. Neil D. Theise’s lab.
FIG 2.
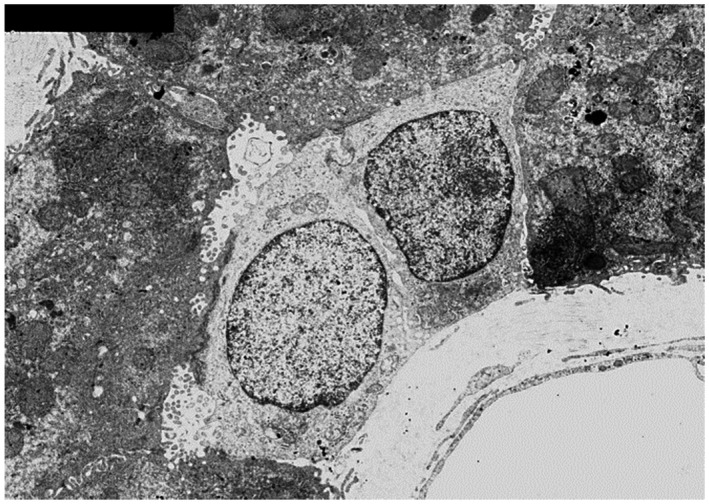
The CoH, lined by two cuboidal, cholangiocyte‐like cells with scant cytoplasmic organelles and by hepatocytes with abundant mitochondria. It is likely that these small cells are functional both as cholangiocytes, involved with bile flow and processing, and as facultative hepatic progenitor cells (electron microscopy). Reproduced with permission from Hepatology. 17 Copyright 2004, American Association for the Study of Liver Diseases.
FIG 3.

Portrait of Francis Glisson located in the Museum of the Royal College of Physicians. ©Royal College of Physicians. It is arguable that this portrait was painted by William Faithorne (ca. 1620 to ca. 1691), who was the artist responsible for the line drawing engraving that is found in the National Portrait Gallery and the Wellcome Institute, because Faithorne was not accustomed to paint in oils.
In the 12 months leading up to his lecture, Glisson prepared assiduously for his daunting speaking assignment, by excarnating (i.e., defleshing) the liver and “delving into the deeper substances” where he made formerly unknown anatomical discoveries that provided the basis of his book on liver anatomy 12 (Fig. 4) and also “the principal subject of his discourse,” as he explained in a handwritten introduction to the reader. 12 Glisson traced the fibrous tissue at the hilum of the liver as a seamless sleeve, or vagina or tunica (coat), that covered the portal vessels outside the liver in a so‐called common capsule (labeled as capsula communis aperta, i.e., common capsule, opened, in Fig. 4, right panel, from chapter 27 of Anatomia Hepatis 12 ), which then enveloped the branches of the PV, HA, and BD, as they divided repeatedly, ever deeper into the substance of the liver. Thus, even though his star pupil, Waleus, had primacy in noticing that the liver has a membranous covering, Master Anatomist Bartholin identified Glisson as the author of the membrane that would bear his name, Capsulam Glissonianam (i.e., Glisson’s capsule).
FIG 4.

(Left) The title page of the first edition of Glisson’s Anatomia Hepatis. (Right) Illustration of the division of the PV from the same book (1654).
Connections at the transition point between hepatic parenchyma and portal tract structures represented a microanatomic mystery that was well suited to investigation with emerging dye injection techniques in the 19th century. Enter Karl Ewald Konstantin Hering (1834‐1918; Fig. 5), a German physiologist and psychologist (originally from Saxony) who found professorial fame briefly in Vienna, mostly in the Charles‐Ferdinand University in Prague (1870‐1895), and in his alma mater in Leipzig, where he ended his days. Arguably one of the greatest personalities among contemporary German physiologists, K.E.K. Hering departed briefly from his seminal, and at times adversarial, work on eye movement, sense physiology, and color vision theory to experiment with Prussian blue dye excretion in the liver and first demonstrate a link between hepatic canaliculi and BDs. 13 , 14
FIG 5.

Karl Ewald Konstantin Hering (1834‐1918). Reproduced from the National Library of Medicine (available at: http://resource.nlm.nih.gov/101418303).
Prussian blue, also known as Berlin blue or Paris blue, was the first modern synthetic pigment—likely created in 1706 in Berlin by the Swiss paint maker, Johann Jacob Diesbach—that was stable and relatively lightfast, and subsequently became widely used as the synthetic process for Egyptian blue was lost. The blue color, caused by the serendipitous formation of iron ferricyanide, was the unexpected consequence of contamination with blood of the potash that Diesbach borrowed during his attempted synthesis of red lake cochineal dye. Prussian blue was soon widely favored by artists in Europe and as far afield as Japan (Fig. 6), as well as for dying clothes—from the uniform coats worn by the Prussian infantry and artillery until the outbreak of the First World War, to the isolated blue threads of the fringes of orthodox Jewish prayer shawls—and its inclusion in engineers’ blue and some laundry bluing preparations. To pathologists, however, the histopathology stain for detecting iron is known eponymously as Perls Prussian blue, after Max Perls (1843‐1881), the German pathologist who first described its use in 1867.
FIG 6.

The Great Wave off Kanagawa (Japanese: 神奈川沖浪裏, Kanagawa‐oki Nami Ura, lit. “Under the Wave off Kanagawa”), a woodcut print rich in Prussian blue that is arguably the most famous of the Thirty‐Six Views of Mount Fuji, by the Japanese ukiyo‐e artist Katsushika Hokusai (1760‐1849). Copies of the ca. 1831 print are held in several Western institutions, including the Metropolitan Museum of Art in New York, the British Museum in London, the Art Institute of Chicago, and the National Library of France.
Karl Ewald postulated that a structure linking canaliculi to BDs was lined by both hepatocytes and cholangiocytes (Fig. 7). 13 Although he is perhaps most celebrated for his 1899 paper dealing with hyperacuity and miniature eye movements (which forms the foundation for modern perception research 15 , 16 ), both his theories on color vision (“opponent color theory”) and liver microanatomy represent other major discoveries that were eventually proved correct, once electron microscopy allowed for the ultrastructural evaluation of the hepatic canal that bears his name (Fig. 2). 17 He began and finished his career at Leipzig University, Saxony (which joined the German Empire during this period), but his work on the liver was published while he was Chair of Physiology at the Collegium Medico‐Chirurgicum‐Josephinum in Vienna, Austria. 18
FIG 7.
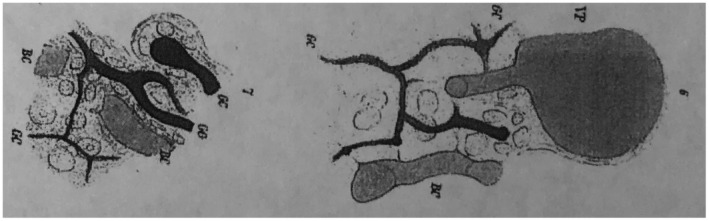
Juncture of the ducts of canaliculi. Reproduced from Math Naturic KL. 13 Copyright 1865.
It seems particularly fitting that Hering, who is perhaps the ultimate student of perception, would establish the connection between liver parenchyma and the biliary tree, but although he established that the hepatobiliary link exists, his sketches did not ultimately reflect the actual anatomy in human livers. Sharp vision was insufficient; a leap from two dimensions to three was required. Following on observations from liver pathologist Michael Gerber (Fig. 8), during his tenure as Chair of Pathology at Tulane University Medical School, attention was brought to small cells with protein expression suggestive of cholangiocytes 19 (Fig. 9) that are present within and beyond the limiting plate in human livers. Because of similarities in staining with fetal hepatoblasts, he suggested that these keratin 19 (K19)–positive cells were related to bipotent progenitor cells in fetal livers: “They may represent remnants of the ductal plate or biliary epithelial cells of canals of Hering. It is tempting to speculate, however, that they are related to hepatic progenitor cells at an early developmental stage.” 19
FIG 8.

Michael Gerber, M.D., liver pathologist, Disciple of Hans Popper, Chairman of Pathology at Tulane University School of Medicine, and early pioneer of the concept of liver stem cells in humans. Courtesy of Dr. Gerber’s daughter, Elisa Gerber.
FIG 9.
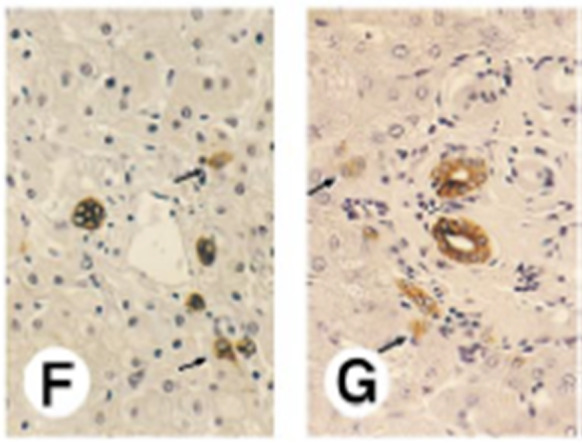
Expression of cytokeratins, vimentin, and Hep‐Par1 antigen in developing human liver. (F) Expression of CK19 in infant liver (3 years old) and (G) adult liver (65 years old). In both livers, small oval epithelial cells expressing CK19 (arrows) are located in the limiting plate. Original magnification, ×1250. Reproduced with permission from Hepatology. 19 Copyright 1996, American Association for the Study of Liver Diseases.
Alas, Gerber did not live long enough to follow up on that temptation, as he and his wife died in a tragic car crash the year after publication of this seminal paper, 19 , 20 and the puzzle of how these single “isolated” cells could function as both hepatocyte and biliary progenitors, given their location, remained unclear. Well positioned in the parenchyma to be hepatocyte precursors, how could these cells migrate through portal tract stroma to reach the biliary tree? In an attempt to find a channel along which these cells could migrate, Theise et al. 3 performed manual three‐dimensional (3D) reconstructions of normal adult human liver and stumbled upon a surprising fact. The CoHs did not lie at the limiting plate, as had been diagramed by Hering or as assumed in the rare images captured by electron microscopy. They extended outward as much as one‐third of the way into the hepatic lobule. The interface of the hepatocyte canalicular system with the biliary tree was not at the limiting plate, but in the periportal parenchyma (Fig. 10). Thus, Gerber’s first hunch was correct: these cells were part of the CoHs, appearing as isolated cells because they are most likely to be seen in cross section.
FIG 10.
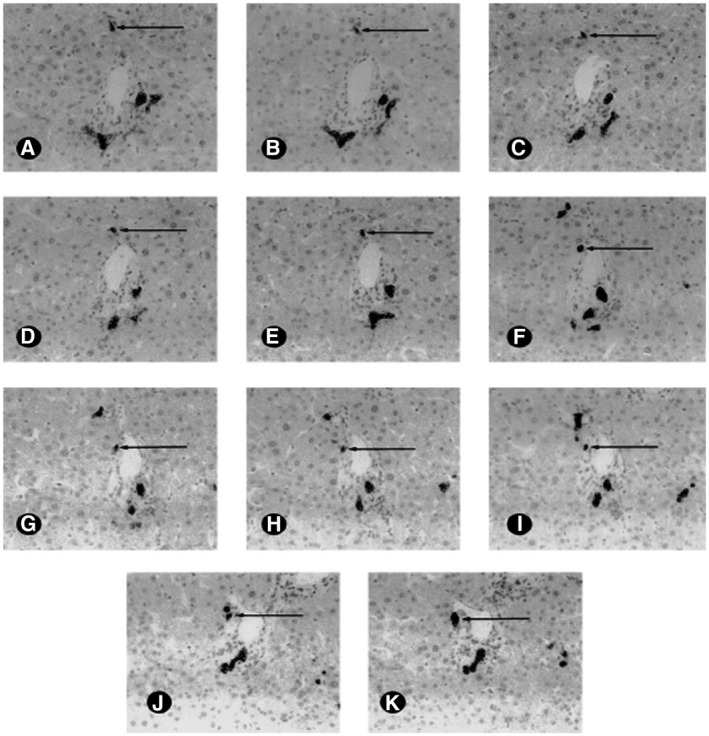
Sample sequential images from a single portal tract in a normal liver. The tissue was immunohistochemically stained for CK19, highlighting the BDs and CoHs profiles distant from the portal tract. Here we follow one CoH (arrows) from its lobular terminus (A) to its BD terminus (K). Note that other CoHs can be seen in these levels (original magnification x400, immunohistochemistry, DAB, Mayer’s hematoxylin). Reproduced with permission from Hepatology. 3 Copyright 1999, American Association for the Study of Liver Diseases.
Gerber’s second intuition that these cells could be hepatobiliary progenitor cells was strongly supported by data in the second part of this study. 3 In cases of fulminant hepatic failure (also known as acute liver failure), “ductular proliferation” was often seen in periportal regions, sometimes reaching deep into the lobule (Fig. 11). These cells variously expressed combinations of hepatocyte and biliary markers, recapitulating Gerber’s findings in hepatoblasts (Fig. 12). Arguments over whether these cells were hepatobiliary progenitors or dying, cholestatic hepatocytes (undergoing biliary metaplasia as they died) persisted for years. Gerber’s paper was an attempt to explore that question. In more complex 3D reconstructions (Fig. 13), these cells were seen to be arborizing expansions from the biliary tree. 21 This paper led to reconsideration of the nomenclature of what we now refer to as ductular reactions (DRs), 17 with the CoHs now understood to be a source for DRs in many diseases. 22 , 23 Interestingly, a robust DR, originating from the CoHs, was clearly visualized in the landmark paper by Yamada, Howe, and Scheuer, 24 which is the first 3D reconstruction of human liver microanatomy by modern means of which we are aware. The association between CoHs and DRs led to an explosion of hepatobiliary stem cell research, although the physiological purpose of this cell population is still debated. 25 , 26 , 27 , 28 , 29 , 30
FIG 11.
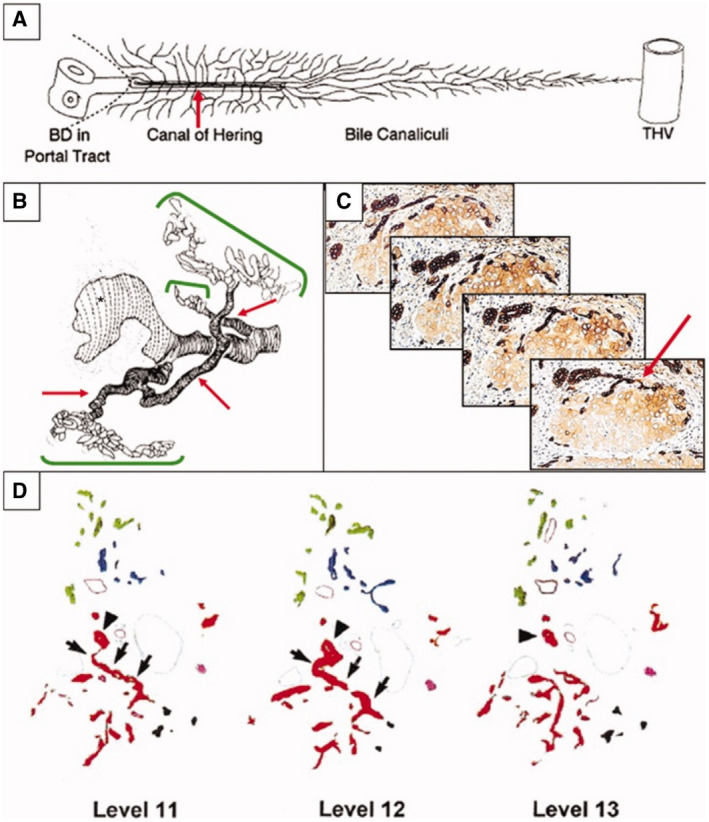
Relationship of DR hepatobiliary cells to the ductule/CoH stem cell niche. DR hepatobiliary cells are largely the “transit‐amplifying progeny” of intrabiliary stem cells. Their primary source is therefore the stem cell niche located in the most proximal branch of the biliary tree, the ductule/CoH unit. This relationship is best seen in 3D representations of DRs. (A) Schematic diagram of normal ductule and CoH (red arrow) structures and their relationship to BD, portal tract stroma, limiting plate, hepatocyte canalicular system (narrow, branching lines), and THV. Reproduced with permission from Hepatology. 3 Copyright 1999, American Association for the Study of Liver Diseases. (B) In primary biliary cholangitis, computer‐generated 3D reconstruction shows a granulomatous duct destructive lesion (large white area with vertical dashed black lines) and DR (green brackets) arising from preexisting ductule/CoH structures. Reproduced with permission from Journal of Pathology. 24 Copyright 1987, Pathological Society of Great Britain and Ireland. (C) Serial 4‐μm sections of hepatitis C virus–related cirrhosis. A small intraseptal hepatocyte nodule links to an interlobular BD via a single intermediate, K19‐positive, CoH‐like structure. The complete link can be appreciated only with examination of the serial sections. Immunostained with DAB; hematoxylin counterstain; original magnification, ×200. Reproduced with permission from Journal of Hepatology. 22 Copyright 2003, Elsevier. (D) Three sample tracings of K19‐positive DRs in sequential levels around a single portal tract. Colors are assigned to indicate contiguity of structures when analyzed in three dimensions. On each level, one BD is marked by an arrowhead. Note in levels 11 and 12 where the red arborizing structure connects via a single ductule/CoH branch to this BD (indicated by arrows). Reproduced with permission from Hepatology. 3 Copyright 1999, American Association for the Study of Liver Diseases.
FIG 12.
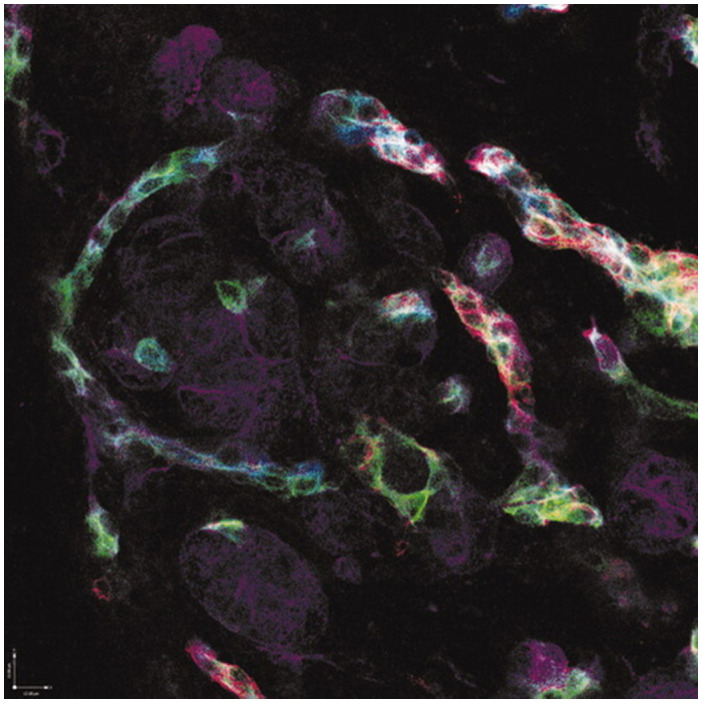
Immunophenotypic diversity within DR hepatobiliary cells. Four‐color immunofluorescence of hepatitis C virus–associated DRs with K19 appearing as pseudocolored blue, K7 appearing as green, CD56 (neural cell adhesion molecule) appearing as red, and K18 (primarily staining hepatocytes) appearing as purple. Original magnification, ×200. Courtesy of E. Prakoso, N. Shackel, and G. McCaughan, University of Sydney, Sydney, Australia.
FIG 13.
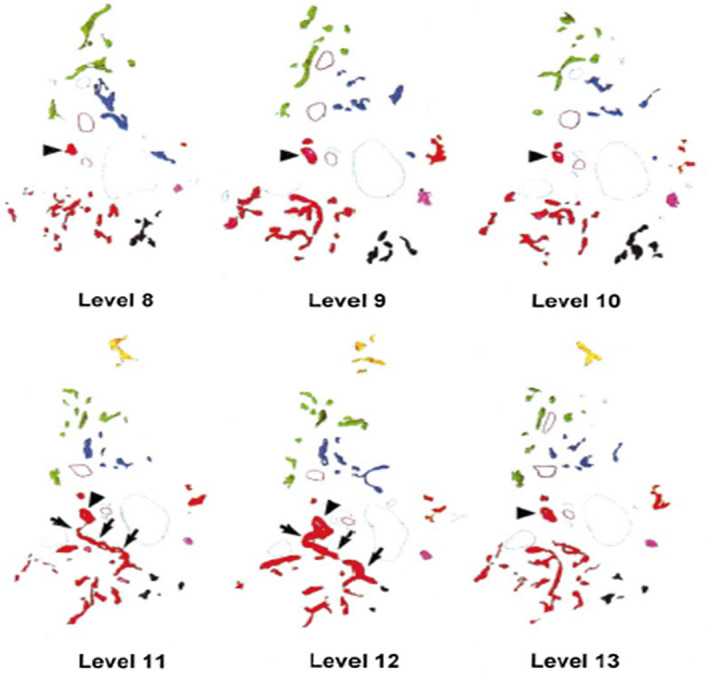
Six sample tracings of CK19‐positive DRs in sequential levels around a single portal tract. Colors are assigned to indicate contiguity of structures when analyzed in three dimensions. On each level, one BD is marked by an arrowhead. Note in levels 11 and 12 that the red arborizing structure connects via a single branch to this BD (arrows). Reproduced with permission from Hepatology. 3 Copyright 1999, American Association for the Study of Liver Diseases.
Even in the 19th century, anatomists grappled with resolving the relationship between structure and function in the liver and aimed to reduce the liver to a single microscopic unit. Early concepts centered around a “hexagonal” (now termed “classic”) lobule concept (Fig. 14), first proposed by Kiernan, 31 ~30 years before Hering published on the liver; later, in the early 20th century, another former student of Leipzig University, Franklin P. Mall (1862‐1917; Fig. 15), proposed a variation to the hexagonal lobule concept with a portal tract at the center of the true lobule and arranged essentially parallel to the central vein (CV; the “portal lobule”). 32 Mall was an American pathologist who greatly impacted the field of anatomy and embryology.
FIG 14.
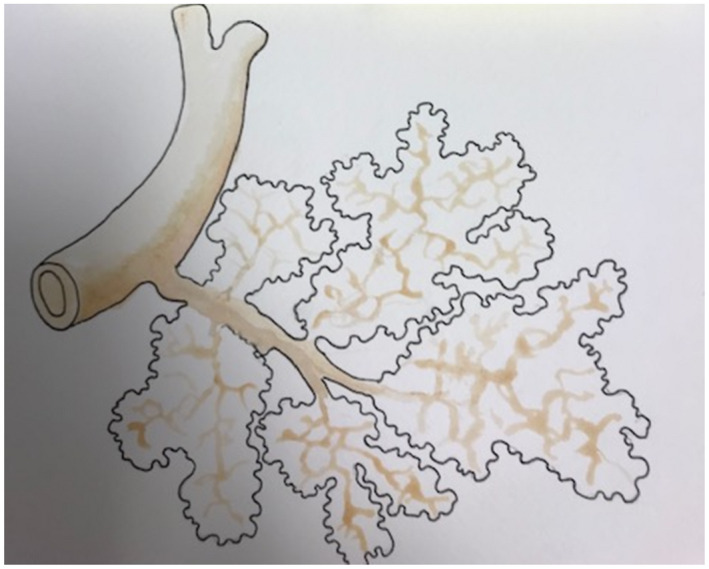
Lobules arranged around the intralobular branches of the hepatic veins, as they are frequently seen at the posterior part of the concave surface of the liver. This arrangement is more distinctly seen in the liver of the sheep than in the human liver. These lobules are parallel to the surface. Adapted from Philosophical Transactions of the Royal Society of London, 31 with drawing by Diana H. Pierce, Pathology Assistant, NYU Langone Medical Center.
FIG 15.

Franklin Paine Mall (1862‐1917), born of German immigrant farmers in Belle Plaine, Iowa, was appointed the first Professor of Anatomy at Johns Hopkins University at age 31 years, when he returned from a period of study in Leipzig, Saxony, Germany. From a photograph taken in 1913 by the late Dr. Frederick L. Gates.
While training in Leipzig (1884‐1886), where he was mentored by Wilhelm His and Carl Ludwig, who described Mall as having “remarkable gifts of observation,” 33 he gained experience in imaging blood vessels and lymphatics, by focusing on small intestinal microvasculature. He returned to the United States to train in pathology at Johns Hopkins Medical School (under the mentorship of William Welch, whom he had met while working with Ludwig), 33 where he would eventually lead the Anatomy Department. 33 During his time in Baltimore (and summers at the Woods Hole Biological Laboratory, MA, of which he later was a trustee), his ongoing work on the small intestine perhaps led him from the splanchnic vasculature to the liver, 33 where he also focused, albeit intermittently, on hepatic microanatomy. Mall 32 hypothesized that the “portal lobule” was the primary microscopic unit of the liver. He is also credited with establishing the presence of a reticular organ framework that is independent of cells 33 (which is perhaps most evident in the liver) and with documenting vasomotor innervation of the PV. 33 Finally, Mall is well known for his collection of abnormal human embryos, which greatly informed the field of embryology, and for his interest in medical education, in which he emphasized the importance of a qualified academic medical school faculty who were actively engaged in research. 33
In the “classic” hexagonal lobule model, the terminal hepatic veins are considered the center of the lobule, and thus the adjacent hepatocytes are termed “centrilobular” or “pericentral.” The portal tracts are interspersed regularly along the periphery of the lobule. In the mid‐20th century, in an era of increasing specialization, Aron Moses Rappaport (1904‐1992), Professor of Physiology at the University of Toronto, Canada, then reintroduced the acinar model of hepatic architecture, as first elucidated by Marcello Malpighi 1 in 1666, the year of the Great Fire of London. Aron Rappaport 34 (Fig. 16), who was born in Siret, Bukovina in the Austro‐Hungarian Empire, graduated in Medicine in 1929 from the German University of Prague and trained in surgery in Germany and France; he practiced in Romania during the Second World War. In 1948, Rappaport emigrated to Canada and worked as a research assistant to Charles Best, of insulin fame, but it was his work in experimental cardiovascular surgery that led to a study of the liver’s microcirculation, a Ph.D. in 1952, and a full Professorship in Physiology in 1955. In 1990, the now‐defunct Microcirculation Research Laboratory at Sunnybrook Health Sciences Centre (formerly Sunnybrook Hospital), where he worked as a Senior Research Scientist after retirement, was named in his honor.
FIG 16.

Aron Moses Rappaport. Generous gift from the estate of Prof. Aron M. Rappaport, by his daughter and estate executor, H. Rappaport, M.D.
Rappaport’s major contribution to our current concept of hepatic microarchitecture was his acinar model, which has six acinar units per hexagonal lobule, arranged around a central portal tract that encompasses branches of the PV, HA, and BD. Periportal hepatocytes are thus exposed to the most oxygenated blood and are designated “zone 1.” Oxygenation decreases as part of a gradient (which also includes changes in metabolic functions) in zone 2 and reaches its lowest level in zone 3, around terminal hepatic venules (THVs) (Fig. 17). Of particular interest in this model is recognition of watershed areas, which Rappaport termed the “nodal points of Mall,” in honor of Franklin Mall’s work on the liver lobule 32 (Fig. 18), which represents the point at which opposing portal and hepatic vessels terminate into capillaries (Figs. 17 and 19; Video S1). This “simple” acinus can be conceptualized as a more “complex” acinus, defined as a structure of at least three simple acini, which is exquisitely illustrated by Rappaport’s India ink injections (Figs. 20 and 21). The notion of zonation centered on the portal tracts is sometimes difficult to envisage as long as the memory of the time‐honored classic hepatic vein‐centered lobule devised by Kiernan 31 (Fig. 14) dominates visual perception. It is ironic to imagine that in this perceptual struggle (Fig. 22), 35 one of the greatest liver anatomists, Hering, whom we have described earlier as “the ultimate student of perception,” could have rendered necessary visual guidance. In this context, attention should be directed to an elegant graphic rendition of liver zonation beyond mere oxygenation, embodied in a so‐called Hepatology Snapshot 36 that comprehensively embraces zonation of hepatocyte metabolic functions (i.e., glycolysis, bile acid production, glutamine synthesis, xenobiotic metabolism, gluconeogenesis, ß‐oxidation, cholesterol biosynthesis, ureagenesis, protein secretion, iron homeostasis, and modulation of insulin growth factors), zonation of the function of nonparenchymal cells (i.e., endothelial, stellate, and Kupffer), and even zonated damage in various liver diseases. Although Rappaport’s complex acinar architecture may not completely encompass the true complexity of the liver, it strikes an important balance, which allows for consistent pathological categorization and further study of liver disease. 36
FIG 17.
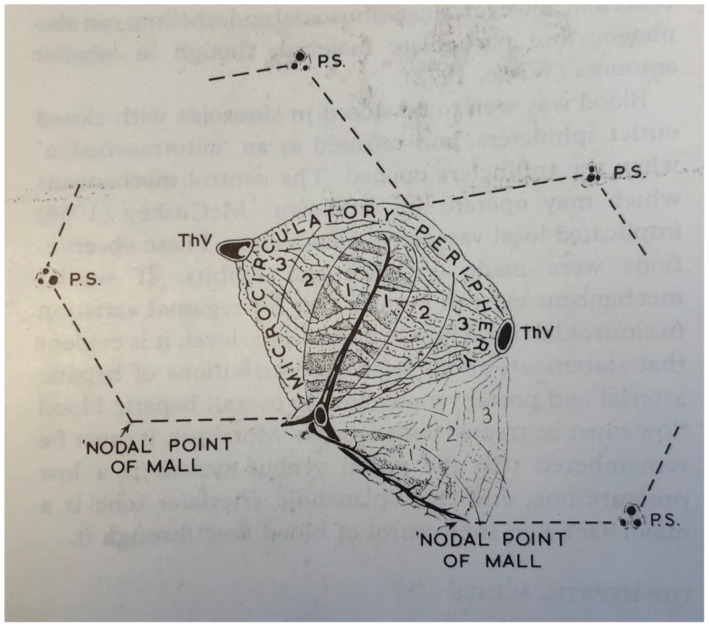
Diagrammatic representation of the simple acinus and the zonal arrangement of hepatocytes. Two neighboring classic lobules are outlined by the discontinuous lines, and the acinus occupies adjacent sectors of these. Although only one channel is shown as forming the central core of the acinus, the latter is arranged round the terminal branches of the PV and HA. Zones 1, 2, and 3 represent areas that receive blood progressively poorer in nutrients and oxygen; zone 3 thus represents the microcirculatory periphery, and the most peripheral portions of zone 3 from adjacent acini form the perivenular area. The nodal points of Mall represent vascular watershed areas where the terminal afferent vessels from neighboring acini meet. ThV, terminal hepatic vein (CV of classic lobule); 1, 2, and 3 represent microcirculatory zones 1, 2, and 3, respectively; 1', 2', and 3' represent microcirculatory zones of neighboring acinus. Outline of classic lobule is shown. Reproduced from Pathology of the Liver.40 Copyright 1979, Churchill Livingston. Reproduced with permission of Publishing Editor.
FIG 18.
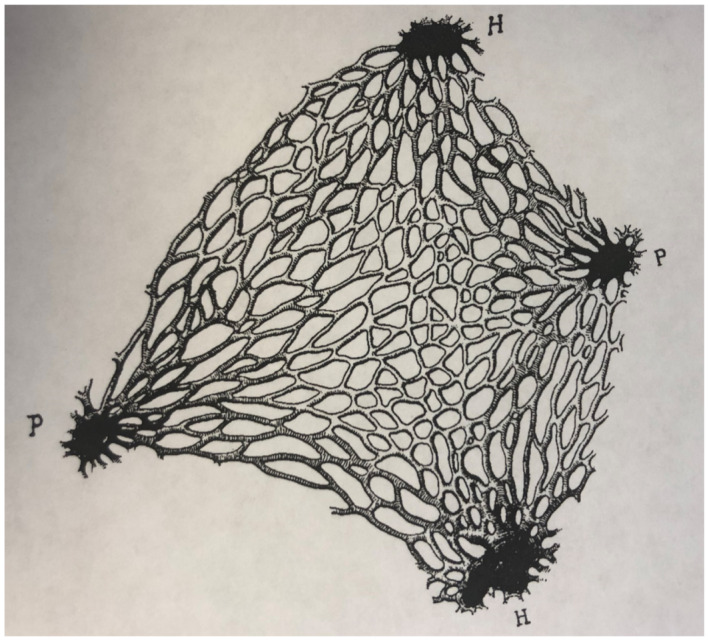
Arrangement of the capillaries at the nodal point of a lobule. Original magnification, ×85. h, hepatic vein; P, portal vein. Reproduced with permission from American Journal of Anatomy. 32 Copyright 1906, American Association of Anatomists.
FIG 19.
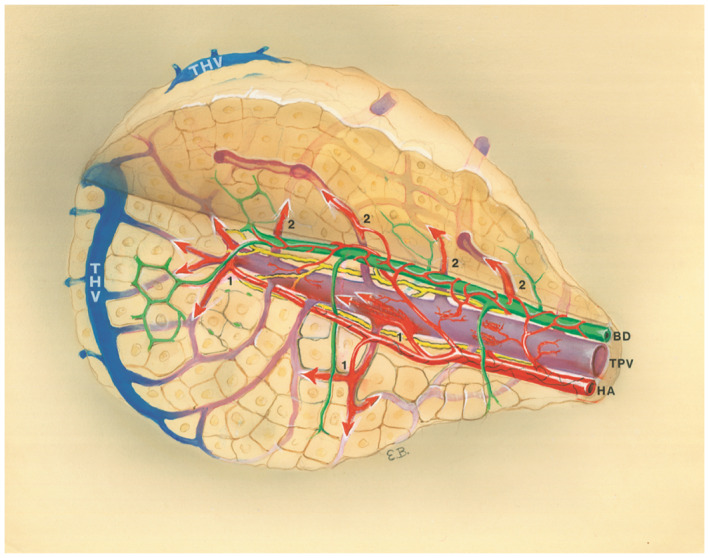
Three‐dimensional depiction of a simple acinus that emphasizes the interconnectedness of hepatic vasculature. Arrows labeled 1 indicate blood flow from the HA; arrows labeled 2 indicate blood flow from TPV. See Video S1. The figure and video are generous gifts from the estate of Prof. Aron Moses Rappaport, by his daughter and estate executor, H. Rappaport, M.D.
FIG 20.
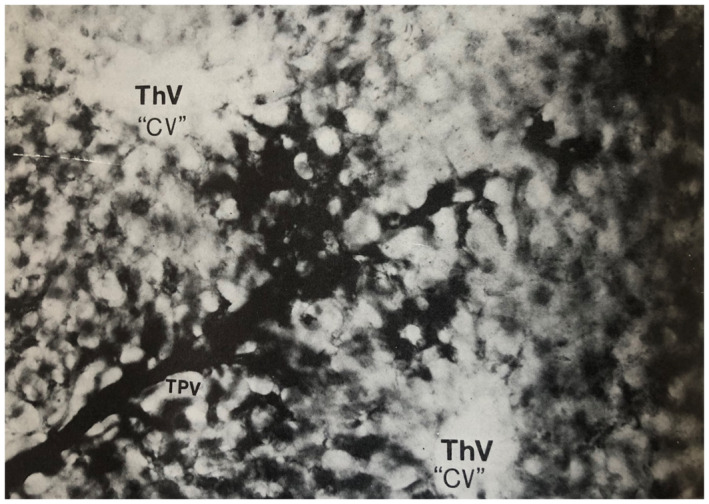
Human liver, simple acinus. The TPV branch of the structural unit is injected with Indian ink and runs perpendicular to the two ThVs or CVs with which it interdigitates. Comparison with figure 1.5 (reproduced as Fig. 17 in this manuscript) will indicate how the acinus occupies sectors of two adjacent classic lobules and extends between the two hepatic venule branches. Thick cleared section original magnification, ×270. Illustration originally by Prof. Aron Moses Rappaport, Toronto, and originally published in Pathology of the Liver. 40 Copyright 1979, Churchill Livingston. Reproduced with permission from the Publishing Editor.
FIG 21.

Human liver, complex acinus. The sinusoids injected with Indian ink are supplied by three TPV branches arising from a single large preterminal vessel. Thick cleared section original magnification, ×80. Illustration originally by Prof. Aron Moses Rappaport, Toronto, and originally published in Pathology of the Liver. 40 Copyright 1979, Churchill Livingston. Reproduced with permission from the Publishing Editor.
FIG 22.
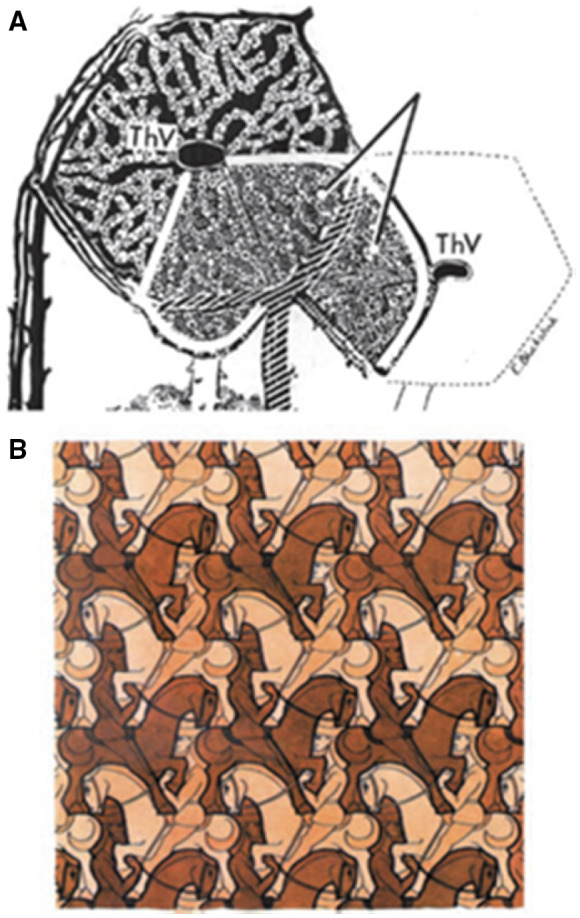
(A) Horizontal section through a crosshatched Rappaport microvascular acinar unit of the liver (light gray, as indicated by the diverging lines), situated between two THVs (i.e., CVs), overlapping with the classic hepatic lobule shown in black. Reproduced with permission from The Anatomical Record. 41 Copyright 1954, American Association for Anatomy. (B) Horsemen: Woodcut in three colours by M.C. Escher, July 1946. M.C. Escher's “Horsemen, 1946” © 2021 The M.C. Escher Company‐The Netherlands. All rights reserved. www.mcescher.com.
But there is another eponymous anatomic structure of Mall beyond those that interweave with Rappaport’s, not only the “nodes of Mall” but also perhaps the better known “space of Mall.” Wikipedia highlights the difference and limitations in reputation. A search for “nodes of Mall” leads to a Wiki page devoted to “shopping malls”; if one inputs “space of Mall,” one gets the appropriate, although quite vague discussion of a “periportal space.” In fact, both structures derive from the body of work reported in his 1906 paper. 32 The space of Mall was defined by pigmented gel injections into the hepatic vascular supply in cat livers. It revealed spaces within the portal tract stroma that were fed by both HAs (cinnabar gelatin) and PVs (Prussian blue gelatin). A prominent feature was indeed a “periportal” space, although the spaces described in the paper include those throughout the portal tract stroma (Fig. 23). He refers to these spaces as “lymphatics” and indicates that they fill from “blue extravasates from the capillaries at the center of the portal unit and invases the connective tissue to reach the beginning of the lymphatics, when of course it is carried rapidly from the liver.” 32 Thus, from this report also derives the concept of these stromal spaces linking to the lymphatics. The confusion in terms of location comes from the fact that cat livers were being studied; they indeed do have a “periportal” space, although humans (and rodents) do not.
FIG 23.
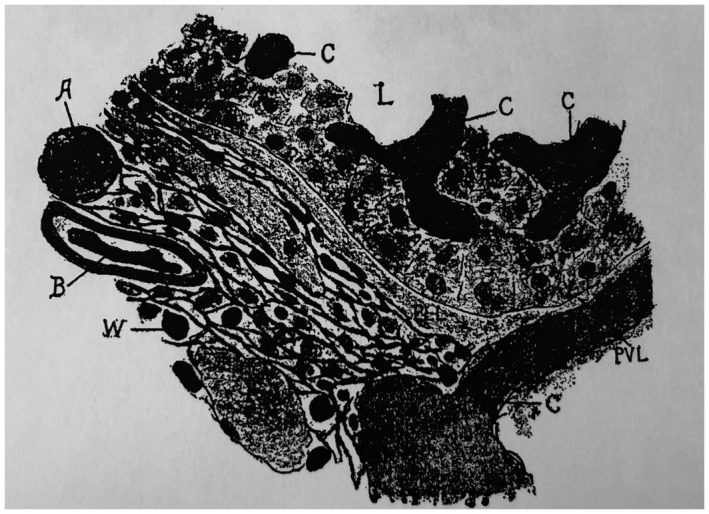
Section through the center of a portal unit of a cat. Original magnification, ×500. Stained by Van Gieson’s method. The HA was injected with cinnabar gelatin and the PV with Prussian blue gelatin. a, artery; c, capillaries; l, lymph vessels; L, lobule of liver; pll, perilobular lymph space; pvl, perivascular lymph space; w, bundles of white fibrous tissue between which are loose connective tissue fibrils and cells. Reproduced with permission from American Journal of Anatomy. 32 Copyright 1906, American Association of Anatomists.
Thus, there is a well‐known “space of Mall” of which little is known! Further specification of the space of Mall, however, arises from, again, new ways of examining tissues. For the CoHs, it took immunostaining and 3D analysis; for the space of Mall, it took visualization of living tissue by in vivo microscopy. The result, from Benias et al., 2 following on visualization of a “reticular pattern” in the submucosa of the extrahepatic biliary tree in humans, is that not only is the extrahepatic and intrahepatic peribiliary stroma a prelymphatic space (i.e., an interstitial space), but the entire portal tract stroma is in this category. The space of Mall was on view all the time, although it was generally mistaken for “cracks” in a presumably very stiff collagen matrix (Fig. 24A). This was wrong: the cracks are physiologically important, fluid‐filled, prelymphatic spaces, rich in hyaluronic acid (Fig. 24B) and glycosaminoglycans. 37 , 38 Ultimately, in that paper, it became clear that submucosae of all visceral organs, fascia of the subcutis, the entire dermis, and perivascular/adventitial connective tissue have the same structure: collagen bundles supporting a fluid‐filled, prelymphatic, interstitial space. Upon its publication, the links to contemporary understandings from fascia scientists 39 brings us all the way around back to Mall’s belief in a reticular connective tissue lattice that forms, shapes, and supports all the structures of the body. 32
FIG 24.
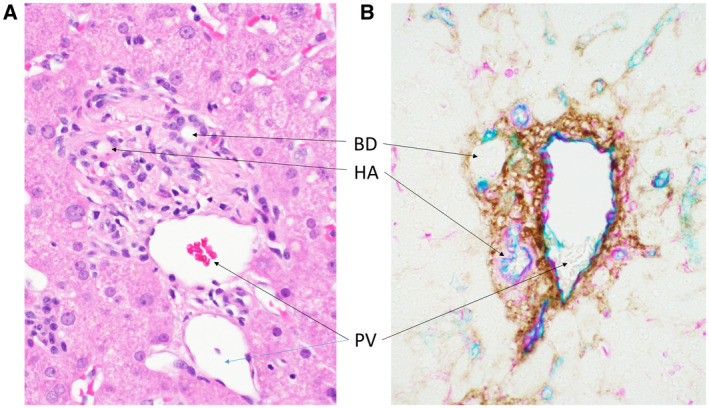
Space of Mall in human liver, hiding in plain sight. (A) White spaces between pink collagen bundles of the portal tract stroma. H&E stain; original magnification, ×400. (B) Immunohistochemical staining of hyaluronic acid (DAB; brown) shows that, between the now‐unstained collagen bundles, these spaces are filled. Vimentin (magenta) and CD34 (teal) highlight vascular structures. Original magnification, ×400.
As hepatopathologists, we owe a great debt to these early investigators because their contributions led to a standard terminology for describing abnormal liver morphology, which formed the basis for our subspecialty. Hans Popper, considered a renowned Founding Father of Hepatology and Hepatopathology, wrote the forward to the first edition of Pathology of the Liver, 40 in which he states: “Specialization entails restriction of knowledge to a circumscribed field to improve acquisition of new data by study in depth. An added justification for this restriction, however, is the application of this initially restricted information to other organ systems,” which has proved prophetic, as advances in classification of liver histopathological findings, now coupled with immunophenotypic, molecular, and in vivo imaging data, continue to build on the early discoveries detailed above and have indeed contributed many times to medicine in general. Mall says it best, “the study of the structure of the liver illustrates beautifully the value of great minds in the study of any subject.” 32
Series Editor’s Postscript
The liver, the second largest organ in the body, has been an enigma since prehistoric times, although it was known, even then, to be a structure full of blood that was considered as the source of life. Hence the seat of the soul was once thought to reside in the liver. After the central role in life of the heart was discovered, hepatocentrism of the soul lost out to cardiocentrism and ultimately to cerebrocentrism. The function of the heart, lungs, and kidneys can be summarized comprehensively and alliteratively as pumping, puffing, and peeing, respectively, which is readily evident to all. In contrast, the function of the liver remains enigmatic to many, especially lay individuals and, truth be told, even among some medical professionals.
With the advent of modern anatomical imaging techniques, however, the liver’s structure no longer epitomizes “a riddle, wrapped in a mystery, inside an enigma,” as Winston Churchill might have characterized it. In contrast, the cut surface of the liver in between the distinctive portal tracts and hepatic venous conduits appears amorphous. Yet if one drills down visually, so to speak, as in a photographic mosaic, a world of distinct structures appears. Not only are these integral hepatobiliary components described elegantly and comprehensively in this essay, and lavishly illustrated, their discovery over the centuries is also consummately traced chronologically by our two contemporary leading anatomist‐pathologists, Ryan M. Gill and Neil D. Theise.
In some instances, the presence of microscopic structures in the liver was predicted by physicians of the Greco‐Roman world, such as the vascular connections between the portal inflow and the hepatic venous outflow that Erasistratus envisioned, Galen ridiculed, and Glisson inferred from the result of his portal perfusion experiment using milky water. The existence of such minute vascular channels was only credible, however, once Marcello Malpighi demonstrated the existence of capillaries. Incidentally, Glisson’s experiment also provided evidence in support of Harvey’s theory of the circulation of the blood.
In this essay that documents the evolution of knowledge about the microscopic and ultramicroscopic anatomy of the liver, the authors cite the contributions of upward of a score of investigators, from whom they selected Glisson, Hering, Mall, and Rappaport for especial acknowledgment of their contributions. The starring order of appearance was chosen by the senior author for the cadence of the title rather than for chronological fidelity. With an abundance of immaculate illustrations, many selected from their own work, the authors guide us through the intricacies of liver microstructure, including the observation that the liver capsule encompasses the portal tracts as they divide ever deeper into the liver; the physiological importance of prelymphatic, lymphatic, and interstitial spaces; the reticular stroma and nonparenchymal liver cells; DRs that are the likely sites of origin of hepatic progenitor cells; and the conceptually difficult visualization of portal‐based versus pericentral zonation in health and disease. One anomaly appears to be that, unlike many examples from the natural world,‡ including the lung bronchi,‖ the branching of the portal tracts and hepatic veins has not been shown yet to follow a Fibonacci sequence or Fibonacci scaling.
Dr. Ryan M. Gill, whose forebears emigrated from the Kingdom of Bavaria to Maryland via Pennsylvania, moved westward with his family to the red (as described by the Spanish) state of Colorado, also nicknamed the Centennial State, because statehood dates from 100 years after the signing of the US Declaration of Independence. After excelling in mountaineering, undergraduate education at Colorado State University in the Choice City of Fort Collins, and M.D./Ph.D. training in the City of Fountains (Kansas City) in the Sunflower or Squatter State of Kansas, he roamed even farther west to the City by the Bay. There, he rose through the ranks to inherit the mantel of Director of Surgical Pathology from the incomparable Linda Ferrell, seen in the photograph below, fourth to the left of the splendid kilt, sporran, and bagpipes, immediately next to which stand bearded and bespectacled Neil Theise and redhead Ryan Gill, respectively. Although it might not occur to you at first glance (see earlier headshot), he is an Elf (see later).

Photograph was taken at the symposium held at UCSF in honor of Dr. Ferrell’s retirement as Vice‐Chair of the Department of Pathology. Photo was reproduced with permission from Department of Pathology, USCF (available at: https://pathology.ucsf.edu/node/1616; accessed March 17, 2021).
In the case of our senior author of this essay, Dr. Neil D. Theise, the theme of ancestral migration from Europe to the United States continues, in this instance because of severe adversity as opposed to a quest for lifestyle improvement. In Neil’s genealogy, the name Bach, German for “brook” or “stream,” flows through the family on both sides. His ancestral maternal Bach was not the composer but an acronym of the title of a scholarly legal document written by a learned 17th‐century, rabbinic progenitor. On his father’s side, the Theisebachs came from the tiny German village of Hatzbach, in Hesse, where heavy rains turned a nearby field into a bach (i.e., a brook). His mother’s family came to England before the First World War, prospered, and upgraded from London’s poor immigrant East End to the lush and green affluence of Hampstead in NW London,¶ and thence to the wartime safety of their large country home, “Marglen,” in Northampton, where the Bachs lavishly entertained US servicemen.

Neil D. Theise’s mother's childhood home in Hampstead, NW London. Photo courtesy of Dr. Neil D. Theise.

Neil D. Theise’s grandparent's World War II country home “Marglen” in Northampton, in the East Midlands region of England. Photo courtesy Dr. Neil D. Theise.

One of several groups of US servicemen whom the Bachs senior entertained at their country home during World War II. Photo courtesy of Dr. Neil D. Theise.
Six days before the outbreak of World War II, Neil’s father escaped the Nazis at age 13 years, via the Kindertransport to England, and serendipitously wound up in Northampton, eventually to journey to the United States, where the Theisebachs, who had emigrated between the 1860s and the 1940s to the United States, became Theises—as did he. The maternal Bach grandparents also migrated from England to the United States.
In the style of a romantic novel, the two young Northampton expatriates ultimately met, by chance, or was it beshert, in New York, where Alfred Theise and Sarah Bach—Neil’s parents to‐be—married.
Coincidentally, but much later, for 6 months in 1989, Neil learned liver pathology at Hampstead’s Royal Free Hospital from the famed histopathologist Peter Scheuer (1928‐2006), himself a refugee from Nazi Austria. It was during his sojourn at the Royal Free Hospital that Neil became acquainted with Gnomes (of Zurich),§ a 15‐strong cohort of like‐minded senior European hepatopathologists who shared a passion for the liver and their mutual camaraderie (see, too, the description of this august collection of pathology friends, in the essay by Albert Czaja on autoimmune hepatitis in Clinical Liver Disease [2020;15(suppl 1):S72‐S81]). And so, the would‐be junior Gnomes, the self‐styled International Liver Pathology Study Group, the Elves# (see photograph below) was begotten, whose mission of intense rowdy liver pathology case discussions and lively dinners replete with good food and better wines was modeled on that of their senior mentors, the Gnomes. Naturally, Neil Theise is a founding member of the Elves.

Aging Elves and their spouses in Bordeaux in 2018, including Neil D. Theise (front row) and Ryan M. Gill (back row). Courtesy of Prof. Paulette Bioulac‐Sage, University of Bordeaux.
In addition to his vital role in the gestation of the Elves and as Professor of Pathology at New York University School of Medicine and Co‐Service Chief of Gastrointestinal and Liver Pathology, Dr. Neil Theise’s academic curriculum vitae documents many awards, honors, and keynote lectureships to learned societies that included the Association for Comprehensive Energy Psychology. It should come as no surprise that he is the recipient of several teaching awards, as he is a lucid, informative, thought‐provoking, and challenging speaker, who has been invited scores of times to give lectures and seminars, not only on hepatic microstructure but also including a presentation at the 2018 Conference on Science and Phenomenology in Tantric Buddhism at the Victoria and Albert Museum in London, UK, and other arcane topics.
Lest I forget to end with a reckoning of his original peer‐reviewed reports, invited reviews, books and chapters, and non‐peer‐reviewed articles, at last count there have been 234 of these collectively…and still counting.
Supporting information
Video S1
Potential conflict of interest: Nothing to report.
Footnotes
Or was he born in the City of Bristol and not in the village of Rampisham?
Or was he born in 1598 or 1599?
Boman BM, Dinh T‐N, Decker K, et al. Why do Fibonacci numbers appear in patterns of growth in nature? A model for tissue renewal based on asymmetric cell division. The Fibonacci Quarterly 2017;55:30‐41.
Goldberger AL, West BJ, Dresselhaus T, et al. Bronchial asymmetry and Fibonacci scaling. Experienta 1985;41:1537‐1538.
All family photographs were supplied by Dr. Neil D. Theise.
Torbenson M, Desmet V, Denk H, et al. Fifty years of impact on liver pathology: a history of the Gnomes. Virchows Arch 2021;478:191‐200.
Crawford JM, Alves V, Balabaud C, et al. Strategies for a successful anatomic pathology subspecialty working group: the 26‐year collaboration of “the Elves.” Acad Pathol 2016;3:1‐7.
References
- 1. Malpighi M. De viscerum structura exercitatio anatomica. Bologna: Jacopo Monti; 1666. [Google Scholar]
- 2. Benias PC, Wells RG, Sackey‐Aboagye B, et al. Structure and distribution of an unrecognized interstitium in human tissues. Sci Rep 2018;8:4947‐4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Theise ND, Saxena R, Portmann BC, et al. The canals of Hering and hepatic stem cells in humans. Hepatology 1999;30:1425‐1433. [DOI] [PubMed] [Google Scholar]
- 4. Elias H. A re‐examination of the structure of the mammalian liver; parenchymal architecture. Am J Anatomy 1949;84:311‐333. [DOI] [PubMed] [Google Scholar]
- 5. Elias H. A re‐examination of the structure of the mammalian liver. II. The hepatic lobule and its relation to the vascular and biliary systems. Am J Anat 1949;85:379‐456. [DOI] [PubMed] [Google Scholar]
- 6. Grisham J, Nopanitaya W, Compagno J. Scanning electron microscopy of the liver: a review of methods and results. Prog Liver Dis 1976;5:1‐23. [PubMed] [Google Scholar]
- 7. Grisham J, Nopanitaya W, Compagno J, et al. Scanning electron microscopy of normal rat liver: the surface structure of its cells and tissue components. Am J Anat 1975;144:295‐321. [DOI] [PubMed] [Google Scholar]
- 8. Saxena R, Theise ND, Crawford JM. Microanatomy of the human liver—exploring the hidden interfaces. Hepatology 1999;30:1339‐1346. [DOI] [PubMed] [Google Scholar]
- 9. Elias H. Anatomy of the liver. In: Rouiller C, ed. The Liver, Morphology, Biochemistry, Physiology. New York: Academic Press, Inc.; 1963:41‐59. [Google Scholar]
- 10. Walker RM. Glisson and his capsule. Ann R Coll Surg Engl 1966;38:71‐91. [PMC free article] [PubMed] [Google Scholar]
- 11. Helling TS, McCleary SP. The tunics of Glisson. Surgery 2016;160:94‐99. [DOI] [PubMed] [Google Scholar]
- 12. Glisson F. Anatomia hepatis, cui praemittuntur quaedam ad rem anatomicam universe spectantia et ad calcem operis subjiciuntur nonnulla de lymphae ductibus nuper repertis. London: DuGardianis for Pulleyn; 1654. [Google Scholar]
- 13. Hering E. Über den Bau der Wirbeltierleber, Sitzber. Akad Wiss Wien, Math Naturic KL 1865;54:496‐515. [Google Scholar]
- 14. Hering E. Üeber den Bau der Wirbelthierleber. Archiv für mikroskopische Anatomie 1867;3:88‐114. [Google Scholar]
- 15. Hering E. Über die Grenzen der Sehschärfe Ber. d. math,‐phys. Classe der Königl. Sächs. Ges. der Wissensch. zu Leipzig 1899;51:16‐24. [Google Scholar]
- 16. Strasburger H, Huber J, Rose D . Ewald Hering’s (1899) On the Limits of Visual Acuity: A Translation and Commentary: With a Supplement on Alfred Volkmann’s (1863) Physiological Investigations in the Field of Optics. i‐Perception 2018;9:2041669518763675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 2004;39:1739‐1745. [DOI] [PubMed] [Google Scholar]
- 18. Bosmia AN, Binello E, Griessenauer CJ, et al. Karl Ewald Konstantin Hering (1834‐1918), Heinrich Ewald Hering (1866‐1948), and the namesake for the Hering‐Breuer reflex. Childs Nerv Syst 2016;32:1561‐1565. [DOI] [PubMed] [Google Scholar]
- 19. Haruna Y, Saito K, Spaulding S, et al. Identification of bipotential progenitor cells in human liver development. Hepatology 1996;23:476‐481. [DOI] [PubMed] [Google Scholar]
- 20. Tulane University Health Sciences Center . The Dr. and Mrs. Michael A. Gerber Memorial Lecture Series 2019. Available at: https://medicine.tulane.edu/departments/pathology‐laboratory‐medicine/annual‐lecture‐series. Accessed February 15, 2021.
- 21. Theise ND. Liver stem cells. Cyto Tech Rev 2003;139:139‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falkowski O, An HJ, Ianus IA, et al. Regeneration of hepatocyte 'buds' in cirrhosis from intrabiliary stem cells. J Hepatol 2003;39:357‐364. [DOI] [PubMed] [Google Scholar]
- 23. Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology 2011;54:1853‐1863. [DOI] [PubMed] [Google Scholar]
- 24. Yamada S, Howe S, Scheuer PJ. Three‐dimensional reconstruction of biliary pathways in primary biliary cirrhosis: a computer‐assisted study. J Pathol 1987;152:317‐323. [DOI] [PubMed] [Google Scholar]
- 25. Clerbaux L‐A, Manco R, Van Hul N, et al. Invasive ductular reaction operates hepatobiliary junctions upon hepatocellular injury in rodents and humans. Am J Pathol 2019;189:1569‐1581. [DOI] [PubMed] [Google Scholar]
- 26. Theise ND. Liver stem cells: the fall and rise of tissue biology. Hepatology 2003;38:804‐806. [DOI] [PubMed] [Google Scholar]
- 27. Theise ND, Dollé L, Kuwahara R. Low hepatocyte repopulation from stem cells: a matter of hepatobiliary linkage not massive production. Gastroenterology 2013;145:253‐254. [DOI] [PubMed] [Google Scholar]
- 28. Yoon S‐M, Gerasimidou D, Kuwahara R, et al. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 2011;53:964‐973. [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Theise N, Chua M, et al. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology 2008;48:1598‐1607. [DOI] [PubMed] [Google Scholar]
- 30. Kuwahara R, Kofman AV, Landis CS, et al. The hepatic stem cell niche: identification by label‐retaining cell assay. Hepatology 2008;47:1994‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiernan F. The anatomy and physiology of the liver. Philos Trans R Soc Lond 1833;123:711‐770. [Google Scholar]
- 32. Mall FP. A study of the structural unit of the liver. Am J Anat 1906;5:227‐308. [Google Scholar]
- 33. Sabin FR. Third Memoir. In: Biographical Memoir of Franklin Paine Mall, 1862‐1917. Washington, DC: National Academy of Sciences; 1934:16. [Google Scholar]
- 34. University of Toronto Discover Archives . Aron M. Rappaport: Professor, research scientist and a specialist in diseases of the liver. Available at: https://discoverarchives.library.utoronto.ca/index.php/rappaport‐aron‐m. Accessed February 15, 2021.
- 35. Reuben A. Now you see it, now you don’t. Hepatology 2003;38:781‐784. [DOI] [PubMed] [Google Scholar]
- 36. Manco R, Itzcovitz S. Liver zonation. J Hepatol 2012;74:466‐468. [DOI] [PubMed] [Google Scholar]
- 37. Khandekar G, Llewellyn J, Kriegermeier A, et al. Coordinated development of the mouse extrahepatic bile duct: implications for neonatal susceptibility to biliary injury. J Hepatol 2020;72:135‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cenaj O, Allison DHR, Imam R, et al. Evidence for continuity of interstitial spaces across tissue and organ boundaries in humans. Commun Biol 2021;4:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stecco C, Adstrum S, Hedley G, et al. Update on fascial nomenclature. J Bodyw Mov Ther 2018;22:354. [DOI] [PubMed] [Google Scholar]
- 40. MacSween RNM, Anthony PP, Scheuer P, eds. Pathology of the Liver. Edinburgh: Churchill Livingston; 1979. [Google Scholar]
- 41. Rappaport AM, Borowy ZJ, Lougheed WM, et al. Subdivision of hexagonal liver lobules into a structural and functional unit. Role in hepatic physiology and pathology. Anat Rec 1954;119:11‐33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1


