Abstract
Condylomata acuminata, or genital warts, are proliferative lesions of genital epithelium caused by human papillomavirus (HPV) infection. HPV types 6 and 11 are most often detected in these lesions. Genital lesions consistent with exophytic condylomata acuminata were removed by excision biopsy from 65 patients, 41 of whom were otherwise healthy individuals (control group) and 24 of whom had conditions known to cause immunosuppression. Histologically, the majority of the lesions were typical condylomata acuminata. Three lesions removed from immunosuppressed individuals also contained foci of moderate to severe dysplasia (intraepithelial neoplasia grade II/III). A recently developed PCR and reverse blot strip assay was used to determine the specific HPV types present in the genital lesions. With a set of oligonucleotide primers based on the same primer binding regions used for the MY09 and MY11 primer pair, this PCR assay detects the presence of 27 HPV types known to infect the genital tract. All but two condylomata acuminata contained either HPV type 6 or 11. The predominant type in the lesions from control patients was HPV 6, while lesions from immunosuppressed types most often contained HPV 11. Condylomata acuminata from immunosuppressed patients contained significantly more overall HPV types than lesions from the control group. HPV types associated with an increased risk of dysplasia (high-risk types) were detected in 42 (64.6%) of the total of 65 specimens; 18 (43.9%) specimens were detected in the 41 otherwise healthy individuals, and 24 (100%) specimens were detected in the 24 immunosuppressed patients. HPV 16 was the most common high-risk type detected, found in 21 of 65 (32.3%) specimens. After HPV types 6 and 11, HPV types 53 and 54 were the most frequently detected low-risk HPV types. This study demonstrates that a high percentage of condylomata acuminata lesions contain multiple HPV types, including types associated with a high risk of dysplastic abnormalities. Further studies are needed to determine the influence these additional HPV types have on the epidemiology of genital tract HPV infections and the natural history of condylomata acuminata, especially in immunosuppressed patients.
Approximately one-third of the 90 known human papillomavirus (HPV) types regularly infect the genital tract, causing a range of manifestations from asymptomatic, latent infection to the typical exophytic cauliflower-like growths known as condylomata acuminata to dysplasia and invasive carcinoma of the cervix. Nearly all condylomata acuminata contain HPV type 6 or 11 (4, 9). Using relatively insensitive methods such as dot blot hybridization or Southern blot analysis, additional types have occasionally been detected in genital warts, including HPV types associated with a high risk of dysplasia, such as HPV 16 (1, 2, 14, 17, 18). In a previous study, we analyzed biopsy samples of exophytic condylomata acuminata lesions for HPV DNA by using the hybrid capture assay. Some of the patients in the study had conditions known to depress cell-mediated immunity, such as infection with the human immunodeficiency virus (HIV) or iatrogenic immunosuppression following organ transplantation (3, 5). High-risk HPV types were detected in 55% of the lesions from immunosuppressed individuals but in only 17% of lesions from otherwise healthy patients. In another study, we demonstrated that condylomata acuminata removed from two immunosuppressed patients contained dysplastic abnormalities (8). High-risk genital HPV types were detected in both of these specimens. It is therefore likely that the natural history of genital warts in immunosuppressed individuals is altered by infection with high-risk HPV types.
The exact distribution of specific HPV types in condylomata acuminata is not known, but few studies have used highly sensitive methods such as PCR. Genital lesions consistent with exophytic condylomata acuminata were removed by excision biopsy from 65 patients, 41 of whom were otherwise healthy individuals and 24 of whom had conditions known to cause immunosuppression. Using a recently developed PCR and reverse blot strip assay, the condylomata acuminata lesions were analyzed for the presence of HPV. The PCR assay is a modification of a previously described PCR and reverse blot strip assay with amplimers generated with the MY09 and MY11 primer pair (10, 11, 15).
MATERIALS AND METHODS
Patient populations and excision biopsy.
Patients were evaluated for the presence of condylomata acuminata in a sexually transmitted disease clinic, a hospital-based gynecology outpatient clinic, a hospital-based surgical outpatient clinic, or the Indiana University Transplantation Service. Biopsy was performed if patients had genital lesions consistent with condylomata acuminata of the external genitalia or perianal area. All patients provided informed consent for the excision biopsy procedure. The protocol for biopsy was approved by the Institutional Review Board at the Indiana University School of Medicine. Biopsies of typical exophytic condylomata acuminata were performed on 65 patients as previously described (5). Twenty-two of these patients were included in the original analysis of HPV types in condylomata acuminata performed by hybrid capture (5, 8). Forty-one patients, including 10 males, 23 nonpregnant females, and 8 pregnant females had no known immunosuppressive condition and are referred to as the control group of patients in the present study. Twenty-four patients were immunosuppressed: 8 were organ transplant recipients and 16 were infected with HIV.
Biopsy specimens were held in normal saline until processing occurred, which was generally within 2 h. A portion of each sample was processed for histological analysis, which was performed by one pathologist (M.S.). The remainder of each specimen was frozen in liquid nitrogen, and DNA was extracted as previously described (5). DNA was quantified by spectrophotometry. The presence of high-molecular-weight DNA was established by agarose gel electrophoresis followed by staining with ethidium bromide. The yields from tissue samples ranged from approximately 20 to 50 μg of DNA.
PCR assay.
A PCR and reverse blot strip assay with degenerate primers for amplifying a conserved region of the L1 open reading frame has been previously described (11). The assay used in the present study has been modified by developing a set of oligonucleotide primer pools, called PGMY09 and PGMY11 (10), based on the same primer binding regions used for the MY09 and MY11 consensus primer PCR assay (15). A set of five upstream oligonucleotides comprising the PGMY11 primer pool was designed as well as a set of nine downstream primers comprising the PGMY09 primer pool (10).
This PCR assay was used to detect 27 HPV types known to infect the genital tract in DNA purified from 65 exophytic condylomata acuminata lesions. Each DNA sample was used in a PCR assay containing the consensus primer pair PGMY09 and PGMY11 (10). The HPV types detected in the assay are types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51–59, 66, 68, MM4, 83 (formerly designated MM7 ;[7;]), MM8, and MM9. To determine specimen adequacy, the GH20/PC04 human β-globin target was coamplified with HPV sequences. Each primer was labeled with a 5′ biotin molecule. For each condylomata acuminata lesion, an individual PCR was performed to potentially amplify any of the 27 HPV types in the immobilized probe array. Each amplification contained 10 mM Tris-HCl (pH 8.5), 50 mM KCl, 4 mM MgCl2, a 200 μM concentration (each) of dCTP, dGTP, and dATP, 600 μM dUTP, 7.5 U of AmpliTaq Gold (Perkin-Elmer, Foster City, Calif.), 2.5 pmol (each) of the β-globin amplification primers BPC04 and BGH20, and 5 to 10 μl (approximately 500 ng) of template DNA. For eventual inclusion of uracil-N-glycosylase to prevent product carryover, dTTP was replaced with dUTP. It has been determined that the dUTP concentration must be increased threefold relative to the other deoxynucleoside triphosphates for efficient strand incorporation by a DNA polymerase (11). Reactions were amplified in a Perkin-Elmer TC9600 thermal cycler by using the following profile: 95°C for 9 min (AmpliTaq Gold activation), 40 cycles of 95°C for 1 min (denaturation), 55°C for 1 min (annealing), 72°C for 1 min (extension), 72°C for 5 min (final extension), and a 15°C hold step. A known positive specimen and a negative (no DNA) specimen were included in each assay as controls.
The general principle of using immobilized probe hybridization has been described elsewhere (11). The HPV-immobilized probe array contains 29 probe lines plus one reference ink line, detecting 27 individual HPV genotypes and two concentrations of the β-globin control probe (11). Bovine serum albumin-conjugated probes for each HPV type are deposited in a single line for each of the HPV types. The high- and low-risk HPV types are visually separated by the β-globin control lines, such that all types between the reference and β-globin control lines are associated with high cancer risk and all types beyond the control lines are associated with low cancer risk. Hybridization and detection of hybridized PCR products to immobilized probes were performed as previously described (11).
Quantitative analysis of PCR products.
To determine the relative abundance of different HPV types in the lesions, developed strips were photographed at a standard magnification with an A2000 digital imaging system (Alpha Innotech, San Leandro, Calif.). The image of the group of strips was scanned by using the one-dimensional multiple-lane-scanning module of the Alphaease software that is an integrated component of the imaging system. The measured density of each band (peak height) was used as the basis of a semiquantitative, 5-point (1 through 5) scoring system for positive bands. The low- and high-concentration β-globin bands served as reference points for this scoring system. The low positive β-globin band was assigned a value of 2, and the high positive band was assigned a value of 4. Bands that were clearly visible with sharp margins extending the full width of the strip but with a peak height that was less than that of the low positive band were assigned a value of 1, while bands with a height greater than the high positive band were given a value of 5. The other values were interpolated between the control values.
To verify that this simple quantitative method correlated with the viral copy number in the lesions, cloned HPV 16 DNA was added to 500 ng of HPV-free human DNA at 10-fold dilutions, beginning at the equivalent of one viral copy per cell and ending at the equivalent of 10−7 viral copies per cell. The PCR and reverse blot strip assay were then performed as for the patient samples. A known positive specimen, a negative (no DNA) specimen, and a viral DNA-free human sample were included in the assay as controls. Quantitative analysis of PCR products was then performed as described above.
RESULTS
Histologic analysis of biopsies.
The majority of lesions were histologically condylomata acuminata without any unusual features. The sections prepared from these biopsies did not contain evidence of fused lesions potentially caused by independent HPV types. There were three cases of moderate to severe dysplasia (intraepithelial neoplasia grade II/III). All three of these lesions were removed from immunosuppressed individuals: two from female transplant recipients (patients 45 and 46) and the other from an HIV-infected male (patient 57).
Detection of specific HPV types in condylomata acuminata lesions.
Results of the PCR assay are summarized in Tables 1 and 2. A representative assay is shown in Fig. 1. Amplification of the control DNA (β-globin) was successful in all 65 condylomata acuminata lesions. As expected, HPV DNA was detected in all specimens. There were no apparent differences between otherwise healthy adults and pregnant women, so they were combined (control group) for the analysis of HPV types in the lesions. Condylomata acuminata from the control group contained a mean of 2.1 HPV types per specimen, compared to 4.2 HPV types per specimen from immunosuppressed patients (P < 0.0001, unpaired t test). For high-risk HPV types only, specimens from the control group contained a mean of 0.8 HPV types per specimen, compared to 2.3 HPV types per specimen from immunosuppressed patients (P < 0.0001, unpaired t test). Overall, HPV types associated with an increased risk of dysplasia were detected in 46 of 65 (61.5%) specimens. High-risk HPV types were detected in specimens from 22 of 41 (53.7%) control patients and in all 24 (100%) specimens from immunosuppressed patients (P < 0.0001, Fisher’s exact test).
TABLE 1.
PCR results on condylomata acuminata from control patients
| Patient no. | Gender status (age in yr)a | Strength of signal generated by PCR and reverse blot assay for the following HPV type
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV 16 | HPV 18 | HPV 26 | HPV 31 | HPV 33 | HPV 35 | HPV 39 | HPV 45 | HPV 51 | HPV 52 | HPV 55 | HPV 56 | HPV 58 | HPV 59 | HPV 68 | HPV MM4 | HPV 83 | HPV MM9b | HPV 6c | HPV 11 | HPV 40 | HPV 42 | HPV 53 | HPV 54 | HPV 57 | HPV 66 | HPV MM8 | ||
| 1 | M (23) | 5 | ||||||||||||||||||||||||||
| 2 | M (30) | 4 | 5 | |||||||||||||||||||||||||
| 3 | M (30) | 5 | 5 | |||||||||||||||||||||||||
| 4 | M (62) | 1 | 2 | 5 | ||||||||||||||||||||||||
| 5 | M (21) | 5 | ||||||||||||||||||||||||||
| 6 | M (20) | 5 | 5 | |||||||||||||||||||||||||
| 7 | M (27) | 4 | ||||||||||||||||||||||||||
| 8 | M (23) | 5 | 3 | |||||||||||||||||||||||||
| 9 | M (24) | 5 | ||||||||||||||||||||||||||
| 10 | M (20) | 3 | 2 | 5 | 5 | |||||||||||||||||||||||
| 11 | F (16) | 5 | ||||||||||||||||||||||||||
| 12 | F (22) | 5 | 5 | |||||||||||||||||||||||||
| 13 | F (?) | 1 | 3 | 5 | ||||||||||||||||||||||||
| 14 | F (14) | 3 | 5 | |||||||||||||||||||||||||
| 15 | F (?) | 1 | 5 | 5 | ||||||||||||||||||||||||
| 16 | F (25) | 5 | ||||||||||||||||||||||||||
| 17 | F (15) | 4 | 5 | 5 | 2 | 5 | 5 | |||||||||||||||||||||
| 18 | F (17) | 4 | 4 | |||||||||||||||||||||||||
| 19 | F (26) | 3 | 4 | |||||||||||||||||||||||||
| 20 | F (?) | 5 | ||||||||||||||||||||||||||
| 21 | F (17) | 5 | ||||||||||||||||||||||||||
| 22 | F (17) | 5 | ||||||||||||||||||||||||||
| 23 | F (17) | 1 | 4 | |||||||||||||||||||||||||
| 24 | F (?) | 5 | ||||||||||||||||||||||||||
| 25 | F (17) | 2 | 4 | 4 | 4 | |||||||||||||||||||||||
| 26 | F (19) | 3 | 4 | |||||||||||||||||||||||||
| 27 | F (25) | 1 | 4 | 4 | 5 | |||||||||||||||||||||||
| 28 | F (16) | 4 | 5 | 3 | ||||||||||||||||||||||||
| 29 | F (19) | 2 | 5 | |||||||||||||||||||||||||
| 30 | F (20) | 2 | 5 | |||||||||||||||||||||||||
| 31 | F (24) | 5 | ||||||||||||||||||||||||||
| 32 | F (?) | 5 | 2 | 5 | ||||||||||||||||||||||||
| 33 | F (42) | 5 | 3 | |||||||||||||||||||||||||
| 34 | P (26) | 3 | 5 | 3 | ||||||||||||||||||||||||
| 35 | P (?) | 5 | ||||||||||||||||||||||||||
| 36 | P (20) | 4 | 5 | |||||||||||||||||||||||||
| 37 | P (21) | 2 | 5 | |||||||||||||||||||||||||
| 38 | P (20) | 5 | 2 | |||||||||||||||||||||||||
| 39 | P (16) | 4 | 2 | |||||||||||||||||||||||||
| 40 | P (?) | 5 | ||||||||||||||||||||||||||
| 41 | P (16) | 5 | 5 | 3 | 5 | |||||||||||||||||||||||
M, male; F, female; P, pregnant; ?, age unknown
HPV types in this column and those to the left are high-risk types.
HPV types in this column and those to the right are low-risk types.
TABLE 2.
PCR results on condylomata acuminata from immunosuppressed patients
| Patient no. | Conditiona | Gender (age in yr)b | Strength of signal generated by PCR and reverse blot assay for the following HPV type
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV 16 | HPV 18 | HPV 26 | HPV 31 | HPV 33 | HPV 35 | HPV 39 | HPV 45 | HPV 51 | HPV 52 | HPV 55 | HPV 56 | HPV 58 | HPV 59 | HPV 68 | HPV MM4 | HPV 83 | HPV MM9c | HPV 6d | HPV 11 | HPV 40 | HPV 42 | HPV 53 | HPV 54 | HPV 57 | HPV 66 | HPV MM8 | |||
| 42 | TRA | M (40) | 5 | 5 | 1 | ||||||||||||||||||||||||
| 43 | TRA | M (28) | 5 | 2 | |||||||||||||||||||||||||
| 44 | TRA | F (26) | 2 | 2 | 5 | ||||||||||||||||||||||||
| 45 | TRA | F (27) | 4 | 1 | 1 | 5 | 4 | ||||||||||||||||||||||
| 46 | TRA | F (27) | 4 | 2 | 2 | 4 | |||||||||||||||||||||||
| 47 | TRA | F (17) | 1 | 5 | 4 | 5 | 2 | 4 | 5 | ||||||||||||||||||||
| 48 | TRA | F (25) | 5 | 5 | 5 | 4 | 3 | 3 | |||||||||||||||||||||
| 49 | TRA | F (?) | 2 | 5 | 5 | 3 | 4 | ||||||||||||||||||||||
| 50 | HIV | M (?) | 3 | 2 | 1 | 5 | 5 | 3 | |||||||||||||||||||||
| 51 | HIV | M (27) | 2 | 5 | 1 | 1 | 4 | 5 | 3 | ||||||||||||||||||||
| 52 | HIV | M (?) | 5 | 2 | 5 | 5 | |||||||||||||||||||||||
| 53 | HIV | M (33) | 2 | 5 | 5 | 5 | 5 | ||||||||||||||||||||||
| 54 | HIV | M (?) | 2 | 3 | 5 | 5 | 2 | ||||||||||||||||||||||
| 55 | HIV | M (36) | 3 | 2 | 3 | 3 | |||||||||||||||||||||||
| 56 | HIV | M (38) | 3 | 4 | 5 | ||||||||||||||||||||||||
| 57 | HIV | M (41) | 5 | 5 | 2 | 5 | 5 | ||||||||||||||||||||||
| 58 | HIV | M (31) | 3 | 5 | 5 | ||||||||||||||||||||||||
| 59 | HIV | M (31) | 3 | 2 | 5 | ||||||||||||||||||||||||
| 60 | HIV | M (?) | 5 | 5 | 5 | ||||||||||||||||||||||||
| 61 | HIV | M (25) | 5 | 5 | |||||||||||||||||||||||||
| 62 | HIV | M (?) | 1 | 1 | 1 | 1 | |||||||||||||||||||||||
| 63 | HIV | M (32) | 5 | 3 | 5 | ||||||||||||||||||||||||
| 64 | HIV | F (?) | 3 | 5 | 5 | 5 | 3 | 3 | |||||||||||||||||||||
| 65 | HIV | F (28) | 4 | 5 | |||||||||||||||||||||||||
Condition associated with immunosuppression. TRA, transplant.
M, male; F, female; ?, age unknown
HPV types in this column and those to the left are high-risk types.
HPV types in this column and those to the right are low-risk types.
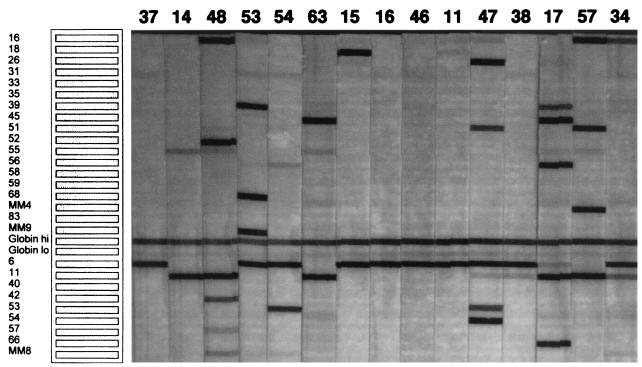
FIG. 1.
A representative PCR and reverse blot assay was performed on condylomata acuminata specimens from 15 patients. The numbers at the top of the figure represent the patients identified in Tables 1 and 2. Shown on the left side of the figure is a template identifying the probe specific for each HPV type in the assay, as well as the β-globin controls.
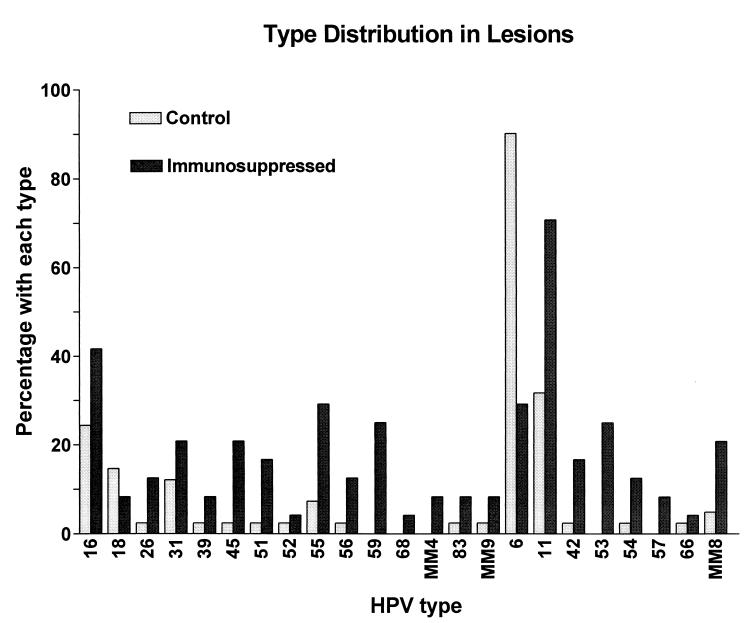
The overall type distribution for condylomata acuminata lesions is shown in Fig. 2. HPV type 6 or 11 was detected in 63 of 65 (96.9%) specimens. All but two specimens (both from immunosuppressed patients) contained either HPV 6 (44 [67.7%] specimens) or HPV 11 (30 [46.2%] specimens). All specimens from control patients contained either HPV 6 (37 of 41 [90.2%]) or HPV 11 (13 of 41 [31.7%]), including 9 (22%) specimens from 41 patients that contained both HPV types 6 and 11. In contrast to specimens from control patients, specimens from immunosuppressed patients contained HPV 6 in only 7 of 24 (29.2%) specimens and HPV 11 in 17 of 24 (70.8%) specimens. Both HPV types 6 and 11 were detected in 2 of 24 (8.3%) specimens from immunosuppressed patients. The difference in detection of HPV type 6 or 11 between the two patient groups was determined to be significant (P = 0.0002) by a two-sided Fisher’s exact test. Specimens with no detectable HPV other than HPV type 6 or 11 were common in control patients (18 of 41 [43.9%]), but no specimen from immunosuppressed patients contained only HPV type 6 or 11 (P < 0.0001, Fisher’s exact test).
FIG. 2.
Bar graph illustrating the type distribution of HPV types in condylomata acuminata lesions. Bars indicate the percentages of lesions from control and immunosuppressed patients containing particular HPV types.
Other low-risk types in addition to HPV types 6 and 11 were commonly detected in specimens from immunosuppressed patients but were uncommon in those from control patients. For all specimens, HPV types 53 and 54 were the most frequently detected low-risk HPV types after HPV types 6 and 11. HPV 53 was especially common in lesions removed from immunosuppressed individuals, being detected in 6 of 24 (25%) specimens but in none of the 41 specimens from control patients (P = 0.002, Fisher’s exact test). HPV types 42, 54, and MM8 also appeared to be more prevalent in lesions from immunosuppressed patients.
For high-risk types, HPV 16 was the most common type detected, being present in 21 of 65 (32.3%) specimens. HPV 16 was especially prevalent in specimens from organ transplant recipients, being detected in five of eight (62.5%) specimens. After HPV 16, the next most frequently detected high-risk type was HPV 59, present in 6 of 24 (25%) specimens from immunosuppressed patients but in no specimen from control patients. HPV 18 was detected in 6 of 41 (14.6%) specimens from control patients and in only 2 of 24 (8.3%) specimens from immunosuppressed patients. HPV 55 was detected in 6 of 16 (37.5%) specimens from HIV-positive patients but less often in specimens from control patients or transplant recipients.
Several HPV types were detected exclusively in lesions from immunosuppressed patients. These included both low-risk types (HPV types 53 and 57) and high-risk types (HPV types 59, 68, and MM4). In contrast, no HPV types were detected exclusively in specimens from control patients. HPV types 33, 35, and 58 (high risk) and HPV 40 (low risk) were not detected in any specimen.
Quantitative analysis of PCR products.
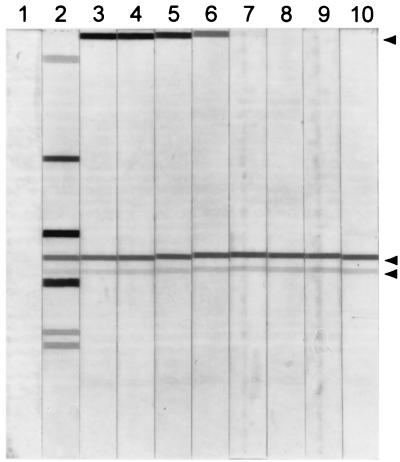
Signals generated in the PCR and reverse blot strip assay of the HPV 16 dilutions were quantified by scanning densitometry (Fig. 3). At one viral copy per cell, HPV 16 was detected at a signal strength of 5. Dilutions of viral DNA up to 100 viral copies per cell also generated signals of 5, indicating that HPV types in amounts greater than a single copy would not be accurately quantified (data not shown). At 10−4 viral copies per cell, a signal strength of 1 was generated. This amount of viral DNA corresponded to approximately 30 genomic viral copies in the 500-ng human DNA sample. At 10−5 viral copies per cell, or the equivalent of three genomic viral copies in the 500-ng human DNA sample, no signal was generated.
FIG. 3.
Quantitative analysis of PCR products by using cloned HPV 16 DNA added to HPV-free human DNA. Lane 1, PCR containing no template; lane 2, PCR containing 500 ng of DNA from a clinical specimen known to contain six different HPV types. The remaining lanes contain strips hybridized with serial 10-fold dilutions of cloned HPV 16 DNA made in 500 ng of HPV-negative human DNA and subjected to PCR. Lane 3, the equivalent of 100 viral copies per cell; lane 4, 10−1 viral copies per cell; lane 5, 10−2 viral copies per cell; lane 6, 10−3 viral copies per cell; lane 7, 10−4 viral copies per cell; lane 8, 10−5 viral copies per cell; lane 9, 10−6 viral copies per cell; lane 10, 10−7 viral copies per cell. The upper arrow on the right side of the figure shows the position of the HPV 16 band, the first HPV type represented on these strips. The two bands just below the center of the strips are the high- and low-quantity β-globin amplification controls, respectively.
Signals generated in the PCR and reverse blot strip assay of patient samples were quantified by scanning densitometry. Signals from 1 to 5 were obtained. As indicated in Table 1, HPV types 6 and 11 were generally detected in abundance (4 or 5) in condylomata acuminata lesions from all patients. Many specimens from all patient groups contained high levels of HPV types in addition to HPV type 6 or 11. For low-risk types other than HPV type 6 or 11, amplimers at level 5 were detected in 3 of 41 (7.3%) specimens from control patients, compared to 6 of 24 (25%) specimens from immunosuppressed patients (P = 0.066, Fisher’s exact test). High-risk types were detected in abundance (5 on a scale of 1 to 5) in 7 of 41 (17.1%) specimens from control patients, compared to 14 of 24 (58.3%) specimens from immunosuppressed patients (P = 0.001, Fisher’s exact test).
DISCUSSION
This study demonstrates that most condylomata acuminata lesions contain multiple HPV types, including types associated with dysplastic epithelial abnormalities. Using PCR, high-risk HPV types were detected in more than half of the condylomata acuminata lesions from otherwise healthy individuals and in 100% of the specimens from immunosuppressed patients. High-risk HPV types that were especially common in specimens from immunosuppressed patients included HPV types 16, 55, and 59.
In addition, the distribution of low-risk HPV types differed between specimens from the healthy patient groups and those from the immunosuppressed patients. HPV 6 is reported to be the most commonly detected type in condylomata acuminata lesions (4, 9). This was the case in the present study in the specimens from control patients. Unexpectedly, the distribution of HPV 6 and HPV 11 in specimens from immunocompetent and immunosuppressed patients differed markedly. In specimens from control patients, HPV 6 was detected more often than HPV 11; this trend was reversed in specimens from immunosuppressed patients. This statistically significant finding is not easily explained, as these two HPV types are closely related. Other low-risk types were commonly detected in specimens from immunosuppressed patients. For example, HPV 53 was commonly detected in specimens from HIV-infected patients (5 of 16 lesions).
It is well established that HPV types 6 and 11 are the major etiologic agents of condylomata acuminata lesions. The significance of additional HPV types in these lesions has not been established, although foci of high-grade dysplasia have been identified in lesions removed from immunosuppressed patients (8). The presence of multiple HPV types in a large percentage of condylomata acuminata lesions suggests that many individuals acquire additional HPV types at the time of infection with HPV type 6 or 11. High-risk HPV types and additional low-risk HPV types may be retained at very low quantities in condylomata acuminata lesions in healthy people but may begin to replicate if immunosuppression occurs. While reactivation of latent infection appears to be the likely mechanism for detection of high-risk and additional low-risk types in these lesions, it is also possible that some patients have a large number of sexual partners and are exposed to multiple HPV types during their lives. Additional HPV types could therefore be accumulated over time.
Another possibility is that low-risk types are more difficult for the cellular immune system of genital epithelium to control than are high-risk types. This hypothesis could explain the observation that low-risk types were found more often in nearly all condylomata acuminata lesions, while high-risk HPV types were detected most often in immunosuppressed patients.
Other studies using less sensitive and less comprehensive HPV detection methods have shown that more than one HPV type may be present in condylomata acuminata lesions. Bergeron et al. found evidence of more than one HPV type in 17% of lesions analyzed by Southern blot hybridization (2). Wickenden et al. analyzed condylomata acuminata and cervical cells by a dot hybridization assay for the presence of HPV (cited in reference 2). They found by using whole genomic probes that 32% of the DNA contained more than one HPV type, including HPV types 6, 11, 16, and 18. Langenberg et al. found evidence of more than one HPV type in 6.3% of condylomata acuminata samples by the Southern blot method (14). Wilbur et al. analyzed 180 condylomata acuminata by RNA in situ hybridization (18). Two cases of coinfection with HPV types 6 and 16 were found. In contrast to these studies, Beckman et al. analyzed 33 condylomata acuminata by a variety of methods and found no cases containing more than one HPV type (1).
A few studies have utilized PCR to detect HPV types in genital tract lesions. Hildesheim et al. identified mixed HPV infections in 43% of patients with typable HPV detected by consensus primer PCR (12). The samples analyzed in that study were cervical lavages and thus were obtained from a large anatomic area, unlike our samples, which were biopsies of external skin containing only abnormal cells by histology.
A recent study used the degenerate MY09 and MY11 primer pair in a PCR to amplify the conserved portion of the L1 open reading frame (16). In that study, 47 condylomata acuminata lesions were shown to contain a single infection with HPV type 6 or 11 in 45 specimens and a double infection with a high-risk type in only two cases. No information was given regarding the immune status of the patient population. Our analysis differs markedly. A possible explanation is that the PCR assay in our study amplifies and detects HPV in genital samples with better sensitivity than other assays. The PCR and reverse blot strip assay employed in our study used different primer pools than the MY09 and MY11 primer pair used in prior studies. In addition, prior studies utilized a generic probe for detection rather than the individual probes used in our study.
The quantitative assay devised for analysis of condylomata acuminata lesions showed that a wide range of viral copy numbers was present in the lesions. HPV types 6 and/or 11 were detected in abundance in nearly all samples (level 4 or 5 in the quantitative assay). Although many additional HPV types were detected in relatively large quantities, the amount of HPV type 6 or 11 in most specimens was probably outside the linear range for quantification in this assay.
Confirmation of multiple HPV types in these lesions by additional tests would be desirable but difficult with current methods other than PCR. Methods such as DNA in situ hybridization lack the combination of high sensitivity, high specificity, and simplicity afforded by PCR. Although HPV cannot be grown in cell culture, certain HPV types have been propagated in the athymic mouse xenograft system (13). We have confirmed the presence of three high-risk HPV types (HPV types 18, 59, and 83) in lesions from three patients (patients 60, 43, and 64, respectively) by producing extracts, infecting human foreskin fragments, and propagating infectious stocks of these HPVs (6, 7a).
In conclusion, multiple HPV types were detected in most exophytic condylomata acuminata specimens. HPV types associated with a high risk of dysplasia were detected frequently. Certain HPV types were detected only in specimens from immunosuppressed patients. These immunosuppression-related types include several that are largely uncharacterized in terms of epidemiology and pathogenesis. Further studies are needed to determine the influence that multiple HPV type infections have on the natural history of condylomata acuminata, especially in immunosuppressed individuals. As patients with conditions that alter immune functions benefit from new therapies and experience extended life spans, it is possible that coinfection of genital epithelium with low- and high-risk HPV types will be manifested as dysplastic disease.
ACKNOWLEDGMENTS
This study was funded in part by a cooperative agreement AI31494 from the National Institute of Allergy and Infectious Diseases.
We thank Patti Gravitt and Raymond J. Apple (Roche Molecular Systems, Inc.) for providing the PCR and reverse blot assay and for helpful advice.
REFERENCES
- 1.Beckmann A M, Sherman K J, Myerson D, Daling J R, McDougall J K, Galloway D A. Comparative virologic studies of condylomata acuminata reveal a lack of dual infections with human papillomavirus. J Infect Dis. 1991;163:393–396. doi: 10.1093/infdis/163.2.393. [DOI] [PubMed] [Google Scholar]
- 2.Bergeron C, Ferenczy A, Shah K V, Naghashfar Z. Multicentric human papillomavirus infections of the female genital tract: correlation of viral types with abnormal mitotic figures, colposcopic presentation, and location. Obstet Gynecol. 1987;69:736–742. [PubMed] [Google Scholar]
- 3.Brachman D G. Molecular biology of head and neck cancer. Semin Oncol. 1994;21:320–329. [PubMed] [Google Scholar]
- 4.Brown D R, Bryan J T, Cramer H, Fife K H. Analysis of human papillomavirus types in exophytic condylomata acuminata by hybrid capture and Southern blot techniques. J Clin Microbiol. 1993;31:2667–2673. doi: 10.1128/jcm.31.10.2667-2673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D R, Bryan J T, Cramer H, Katz B P, Handy V, Fife K H. Detection of multiple human papillomavirus types in condylomata acuminata from immunosuppressed patients. J Infect Dis. 1994;170:759–765. doi: 10.1093/infdis/170.4.759. [DOI] [PubMed] [Google Scholar]
- 6.Brown D R, McClowry T L, Bryan J T, Stoler M, Schroeder-Diedrich J M, Fife K H. A human papillomavirus related to human papillomavirus MM7/LVX82 produces distinct histological abnormalities in human foreskin implants grown as athymic mouse xenografts. Virology. 1998;249:150–159. doi: 10.1006/viro.1998.9294. [DOI] [PubMed] [Google Scholar]
- 7.Brown D R, McClowry T L, Woods K, Fife K H. Nucleotide sequence and characterization of human papillomavirus type 83, a novel genital papillomavirus. Virology. 1999;260:165–172. doi: 10.1006/viro.1999.9822. [DOI] [PubMed] [Google Scholar]
- 7a.Brown, D. R., et al. Unpublished observations.
- 8.Bryan J T, Stoler M H, Tyring S K, McClowry T, Fife K H, Brown D R. High-grade dysplasia in genital warts from two patients infected with the human immunodeficiency virus. J Med Virol. 1998;54:69–73. [PubMed] [Google Scholar]
- 9.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnurch H G, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci USA. 1983;80:560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravitt, P. E., C. Peyton, T. Alessi, C. Wheeler, F. Coultee, A. Hildesheim, and R. Apple. Unpublished data.
- 11.Gravitt P E, Peyton C L, Apple R J, Wheeler C M. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–3027. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildesheim A, Schiffman M H, Gravitt P E, Glass A G, Greer C E, Zhang T, Scott D R, Rush B B, Lawler P, Sherman M E, Kurman R J, Manos M M. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–240. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 13.Kreider J W, Howlett M K, Lill N L, Bartlett G L, Zaino R J, Sedlacek T V, Mortel R. In vivo transformation of human skin with human papillomavirus type 11 from condylomata acuminata. J Virol. 1986;59:369–376. doi: 10.1128/jvi.59.2.369-376.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langenberg A, Cone R, McDougall J, Kiviat N, Corey L. Dual infection with human papillomavirus in a population with overt genital condylomas. J Am Acad Dermatol. 1993;28:434–442. doi: 10.1016/0190-9622(93)70064-z. [DOI] [PubMed] [Google Scholar]
- 15.Manos M M, Ting Y, Wright D K, Lewis A J, Broker T R, Wolinsky S M. Use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells. 1989;7:209–214. [Google Scholar]
- 16.Meyer T, Arndt R, Christophers E, Beckmann E R, Schroder S, Gissmann L, Stockfleth E. Association of rare human papillomavirus types with genital premalignant and malignant lesions. J Infect Dis. 1998;178:252–255. doi: 10.1086/517447. [DOI] [PubMed] [Google Scholar]
- 17.Nuovo G J, Darfler M M, Impraim C C, Bromley S E. Occurrence of multiple types of human papillomavirus in genital tract lesions. Am J Pathol. 1991;138:53–58. [PMC free article] [PubMed] [Google Scholar]
- 18.Wilbur D C, Reichman R C, Stoler M H. Detection of infection by human papillomavirus in genital condylomata. A comparison study using immunocytochemistry and in situ nucleic acid hybridization. Am J Clin Pathol. 1988;89:505–510. doi: 10.1093/ajcp/89.4.505. [DOI] [PubMed] [Google Scholar]