ABSTRACT
Alpha-synuclein is a 15 kDa protein associated with neurodegenerative diseases such as Parkinson disease and multiple-system atrophy where pathological forms of alpha-synuclein aggregate and become neurotoxic. Here we describe the nonclinical program to support a first-in-human (FIH) single ascending dose (SAD) study for Lu AF82422, a human recombinant, anti-alpha-synuclein monoclonal antibody (mAb) in development for treatment of synucleinopathies. Alpha-synuclein is primarily expressed in brain, peripheral nerves and in blood cells. A tissue cross-reactivity assessment showed that Lu AF82422 binding was generally restricted to nervous tissues. Flow cytometry analysis did not show extracellular surface binding of Lu AF82422 to human platelets, erythrocytes, granulocytes, or lymphocytes, but to a low fraction of monocytes, without any functional consequences on activation or phagocytic capacity. A single dose pharmacokinetic (PK) study in cynomolgus monkeys with dose levels of 1–30 mg/kg confirmed PK properties in the expected range for a mAb with a soluble target, and target engagement was shown as a decrease in free alpha-synuclein in plasma. Four-week repeat-dose toxicity studies were conducted in rats and cynomolgus monkeys at doses up to 600 mg/kg administered intravenously every 10 days. Results showed no treatment-related adverse findings and the no-observed-adverse-effect-level was the highest dose tested. Target engagement was shown in plasma and cerebrospinal fluid. Taken together, the nonclinical data indicated no safety signal of concern and provided adequate safety margins between observed safe doses in animals and the planned dose levels in the FIH SAD study.
KEYWORDS: Alpha-synuclein, cerebrospinal fluid, monoclonal antibody, nonclinical safety, pharmacokinetics, target engagement, cynomolgus monkeys
Introduction
Alpha-synuclein is a highly soluble cytoplasmic protein of 15 kDa, primarily expressed in brain, peripheral nerves and blood cells. In the brain, alpha-synuclein is located in neuronal presynaptic terminals1 and nuclei2 and has been shown to regulate neurotransmitter vesicle cycling by modulating vesicle exocytosis and endocytosis,3,4 and to facilitate DNA repair.5 Besides in neurons, alpha-synuclein is expressed in astrocytes, microglia, and oligodendroglia.6–8 While normally present as a monomer, alpha-synuclein is post-translationally modified under pathological conditions and present as oligomers and fibrils of phosphorylated, nitrated, oxidized, and truncated forms.9,10 The formation of pathological forms of alpha-synuclein occurs in neurons in Parkinson disease (PD), in the related Lewy body disorders (e.g., dementia with Lewy bodies) and in oligodendrocytes in multiple system atrophy (MSA).11–13 The oligomers can permeabilize membranes by forming a channel that contributes to cellular toxicity by altering membrane potential,14 thereby causing synaptic dysfunction and cell death.15,16 Moreover, the extracellular alpha-synuclein oligomers may trigger or potentiate microglial activation, thereby causing increased neuroinflammation and neuronal cell death, as shown in some experimental models.17 Importantly, alpha-synuclein oligomers and fibrils show prion-like properties.18 Supportive of this is the finding of Lewy bodies in dopaminergic neurons that were grafted into the striatum of PD patients 10–20 years earlier, suggesting that aggregated or misfolded forms of alpha-synuclein can be secreted from the host neurons and subsequently enter grafted neurons, possibly enhancing further aggregation of alpha-synuclein.19Analyses of post mortem tissue from patients with PD indicate that the alpha-synuclein pathology may arise in the peripheral nervous system, possibly in the gastrointestinal tract, transfer to the brain stem via the glossopharyngeal and vagus nerves and spread to the brain at a later stage.20,21 Alternatively, the pathology may originate from the olfactory bulb and the anterior olfactory nucleus spreading from there to the midbrain and the cortex.20,21
In the periphery, alpha-synuclein is primarily expressed in platelets,22 erythrocytes,23 and leucocytes,24,25 where it plays a role in cell differentiation. In platelets, alpha-synuclein is associated with the membranes of secretory α-granules, possibly negatively regulating release of growth factors and coagulation factors.26 In erythrocytes, alpha-synuclein may function as an antioxidant that prevents oxidation of unsaturated membrane lipids.23 Although alpha-synuclein was initially thought to be a purely intracellular protein, it is also detected in extracellular fluids, such as plasma, cerebrospinal fluid (CSF) and brain interstitial fluid.27–30
Lu AF82422 is a human immunoglobulin G1 (IgG1) monoclonal antibody (mAb) that recognizes all major species of alpha-synuclein (monomeric and oligomeric; N- or C-terminal truncated forms). The therapeutic target, extracellular oligomeric alpha-synuclein, is present in the central nervous system (CNS), and possibly in the peripheral nervous system, in patients with PD or MSA.31 Data from in vitro and in vivo models demonstrate that LuAF82422 inhibits the seeding of alpha-synuclein aggregation induced by recombinant alpha-synuclein fibrils or pathological alpha-synuclein from postmortem patient brains extracts (data not shown). The proposed mechanism of action of Lu AF82422 is therefore to bind to extracellular, pathological alpha-synuclein and in that way inhibit seeding and spreading to other cells.
Here, we describe the toxicology program, designed in accordance with current guidelines,32,33 conducted to support a clinical first-in-human (FIH) single-ascending dose (SAD) study with Lu AF82422. The program included assessment of immunotoxicity, a target expression study, a tissue cross-reactivity study, a pharmacokinetic (PK) study in cynomolgus monkeys and repeat-dose toxicity studies in rats and cynomolgus monkeys. These were considered pharmacologically relevant species, as Lu AF82422 showed similar binding to alpha-synuclein from rats and cynomolgus monkeys as to human monomeric alpha-synuclein, with KD values of around 20–40 nM (data not shown). In the presented nonclinical studies, the target was confirmed to be present in high abundance in rats and cynomolgus monkeys with a similar expression profile as in humans. Safety aspects of targeting peripheral and brain alpha-synuclein was therefore considered fully covered. Assessment of target engagement was included both in the PK study and the repeat-dose toxicity studies in cynomolgus monkeys. A dosing interval of 10 days was chosen for the repeat-dose toxicity studies based on toxicokinetics (TK) data from earlier dose-range finding studies and PK simulations made using data from single-dose studies with a tool compound, resulting in an estimated half-life (T½) of approximately 10 days. The PK study, the repeat-dose toxicity studies and the tissue cross-reactivity study were conducted in accordance with Good Laboratory Practice (GLP) regulations.34
Results
Immunotoxicity assessment
Potential binding of Lu AF82422 to the surface of blood cells was assessed in samples of human whole blood using concentrations of compound in the range of those expected in humans after administration of therapeutic dose levels. The results showed no binding of labeled Lu AF82422 to the surface of human erythrocytes or platelets (Figure S1, Table S1). Additionally, no binding of labeled Lu AF82422 to the surface of either resting or thrombin receptor activating peptide-6 (TRAP6)-activated platelets was detected (Table S2). With no significant binding to the surface of platelets, Lu AF82422 is not expected to affect platelet aggregation, platelet plug formation or clotting.
The only observed binding of Lu AF82422 to human leucocytes was to a varying low fraction of monocytes (0–15%; Figure S2, Table S3), and an Fc-null version of Lu AF82422 showed that this binding was not dependent on the Fc portion of Lu AF82422. In human whole blood samples, no effect of Lu AF82422 on monocyte phagocytosis was shown (Table S4 and S5). Additionally, no LuAF82422-induced activation of monocytes was observed (Table S6), and Lu AF82422 had no effect on the monocyte activation induced by interferon-α (IFNα), interleukin-6 (IL-6) or lipopolysaccharide (LPS; Table S7). There was no clear indication of Lu AF82422-induced cytokine release in whole blood samples using soluble or wet-coated presentation (Table S8 and S9). Moreover, the cytokine release profile for Lu AF82422 did not differ from that of the isotype control antibody when using human peripheral blood mononuclear cells (PBMCs) and a wet-coated plate format (Table S10).
The presence of the target was studied in neuronally differentiated induced pluripotent stem (iPS) cells using flow cytometry. It was not possible to use the labeled version of Lu AF82422 in this assay and therefore, a commercially available anti-alphasynuclein antibody was used to demonstrate the presence of the target. There was no binding of the antibody to the intact cell surface. In contrast, binding occurred after permeabilization, as expected. Taken together, the results indicated that alpha-synuclein was not present at the neuronal cell surface, and hence, the risk of surface binding of Lu AF82422 to neurons is considered low (Figure 1).
Figure 1.
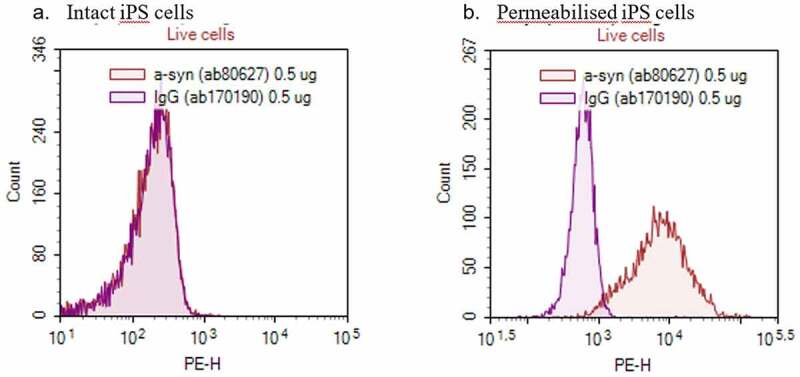
Presence of target on the surface of neuronally differentiated induced pluripotent stem (iPS) cells. Samples of human neuronally differentiated iPS cell suspensions were stained with PE-labelled anti-alpha synuclein antibody (ab80627) or isotype control antibody (ab170190), respectively, before analysis. Flow cytometry histograms of PE-fluorescence counts of (a) intact cells stained to visualize surface-bound alpha-synuclein and (b) permeabilized cells to visualize intracellular alpha-synuclein
Target expression
Tissues from cynomolgus monkeys and rats showed a similar alpha-synuclein tissue expression pattern as in humans. As expected, positive staining was predominantly restricted to central and peripheral nervous tissues (Table 1, Figure S3). Positive staining of peripheral nerve fibers was observed in all species and included myenteric and/or submucosal plexi of the ileum and colon, as well as isolated nerve fibers present within the skin, breast and parotid.
Table 1.
Target expression in human, cynomolgus monkey and rat tissues
| tissue (n = 3) | human |
cynomolgus monkey |
rat |
|||
|---|---|---|---|---|---|---|
| pAb1 | pAb2 | pAb1 | pAb2 | pAb1 | pAb2 | |
| Adrenal | C* | - | C | - | - | - |
| Bone marrow | - | - | - | - | ScC | - |
| Breast | - | - | - | - | NF | - |
| Cerebellum | CB, N | CB, N | WM, GM, N, CB | GM, WM, CB, N | WM, GM, CB, N | CB |
| Cerebral cortex | GM, WM, N | GM, WM, N | WM, GM, N | GM, WM, N | GM, WM, N | GM, WM |
| Colon | MP | - | - | MP | MP | - |
| Heart | - | - | - | - | - | - |
| Ileum | - | - | MP | MP | - | - |
| Kidney | - | - | - | - | - | - |
| Liver | - | - | - | - | - | - |
| Lung | - | - | - | - | PM* | - |
| Lymph node | - | - | - | SC* | - | - |
| Ovary | - | - | - | - | - | - |
| Parotid | - | - | NF | - | - | - |
| Peripheral nerve | NF | NF | NF | NF | NF | NF |
| Pituitary | PN | PN* | PN* | PN | PN, PI** | - |
| Prostate | - | - | - | - | - | - |
| Skeletal muscle | - | - | - | - | - | - |
| Skin | BC* | - | NF | - | NF | - |
| Spinal cord | - | GM* | GM, WM | GM | GM | - |
| Spleen | RP | RP* | RP | - | - | - |
| Tonsil | - | - | - | - | N/A | N/A |
| Testis | - | - | - | - | - | - |
| Uterus (endometrium) | - | - | - | - | - | - |
pAb1- polyclonal anti-alpha-synuclein antibody 1 (ab6162 from Abcam); pAb2- polyclonal anti-alpha-synuclein antibody 2 (AB5038 from Millipore); “-“ – No positive immunostaining; C-chromaffin cells; GM-Grey Matter; WM-white matter; GC- Granule cells; ML-Molecular layer; N- nuclei; CB-Neuronal cell bodies; ScC- Scattered cells; NF- Nerve fibers; MP-Myenteric and submucosal plexi; LP: Lamina propria; PM-Pulmonary alveolar macrophage; SC-Sinus cells; PN-Pars nervosa; PI: Pars intermedia; BC: Basal cells (epidermal); RP-Red pulp; N/A- Not available.
*Staining in one donor only.
** Staining in one donor only, PN and PI not present in tissue sample from the other two donors.
The pars nervosa of the pituitary showed positive staining in most samples where present, and positive staining of the pars intermedia was seen in rats. This is in line with previous reporting of alpha-synuclein expression in the pituitary.35 Other cells with previous reporting of alpha-synuclein expression and showing specific staining with at least one of the two antibodies included chromaffin cells of the adrenal medulla in humans and cynomolgus monkeys (expression reported in the adrenal gland36), scattered cells of the splenic red pulp in humans and cynomolgus monkeys (immunostaining in spleen previously described37), isolated incidences of positive staining in sinus cells of the lymph nodes in cynomolgus monkeys, scattered cells of the bone marrow in rats and basal epidermal cells in human skin (consistent with previous reports of alpha-synuclein expression in skin and bone marrow37). Though the staining observed in non-nervous tissues was not necessarily seen in all species or with both antisera used, the previous reporting of alpha-synuclein expression in these tissues suggest that the observed staining represent expression of alpha-synuclein.
Tissue cross reactivity
Specific staining with Lu AF82422 was restricted to nervous tissues, including cerebellum, cerebral cortex, pituitary (pars nervosa), peripheral nerve, spinal cord, myenteric plexus, optic nerve and retina (Table 2, Figure S4). Staining was generally cytoplasmic in location and was typically distributed diffusely through the tissues listed. Staining intensity, cellular localization and tissue distribution were similar in each of the three human tissue donors tested. In most instances, staining was present at all three concentrations of LuAF82422 tested, and staining intensity showed an association with the concentration (Table 2). While a low level of background staining (also present in negative control sections) was seen in some tissues, there was no unexpected staining identified.
Table 2.
Human tissues showing specific staining with Lu AF82422 in tissue cross reactivity assessment
| Positively1 stained human tissues (n = 3) | Lu AF82442 concentration |
||
|---|---|---|---|
| 0.5 µg/mL | 1 µg/mL | 2 µg/mL | |
| Cerebellum (Molecular Layer, Granular Cell Layer and White Matter) | +, ++ or +++ | +, ++ or +++ | +, ++ or +++ |
| Cerebral Cortex (Gray Matter and White Matter) | + or ++ | +, ++ or +++ | +, ++ or +++ |
| Colon (Myenteric Plexus) | + | + | + |
| Eye (Optic Nerve and Retina) | - or + | + | +, ++ or +++ |
| Ileum (Myenteric Plexus) | - or + | + or ++ | + or ++ |
| Pituitary (Pars Nervosa) | + | + or ++ | ++ |
| Peripheral Nerve | + or ++ | ++ | ++ |
| Spinal Cord (White Matter and Grey Matter) | + | ++ | + or ++ |
Staining: – = negative; + = weakly positive; ++ = moderately positive; +++ = strongly positive
1Only tissues with positive staining are included in the table, a panel of 32 tissues was evaluated in line with FDA guidelines.
PK study in cynomolgus monkeys
After a single intravenous (IV) administration of 1, 3, 10 or 30 mg/kg Lu AF82422 in female cynomolgus monkeys, dose-proportional exposure was observed in plasma and CSF (Figure 2). The volume of distribution (Vz) of 70–80 mL/kg, the clearance (Cl) of 0.16–0.19 mL/h/kg and the terminal T½ of 10–15 days are in the expected range for a mAb IgG1 (Table 3). In CSF, the mean Tmax was 64–88 hours, and the mean CSF to plasma ratio was 0.22–0.29%. The terminal T½ in CSF was estimated to be 15–24 days, suggesting a slightly longer T½ in CSF compared to plasma (Table 4).
Figure 2.
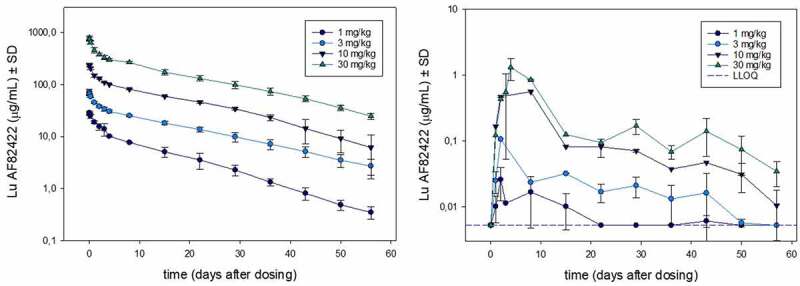
Lu AF82422 exposure in plasma and CSF after single dose IV administration of 1, 3, 10 or 30 mg/kg Lu AF82422 in female cynomolgus monkeys (n=3). Exposure (mean ± SD) shown in plasma (left panel) and in CSF (right panel). The LLOQ for Lu AF82422 measured in plasma (50 ng/mL) is not indicated on the left panel as it is below the lowest point on the y-axis. For CSF, samples with high haemoglobin content were excluded, therefore some data points at time points 3 and 4 days after dosing are missing (see Materials and methods)
Table 3.
Plasma PK parameters after single dose IV administration in cynomolgus monkey
| Dose (mg/kg) | AUC0-inf (h·µg/mL) |
Tmax (h) |
Cmax (µg/mL) | T½ (h) |
Vz (mL/kg) |
Cl (mL/h/kg) |
n |
|---|---|---|---|---|---|---|---|
| 1 | 5,230 | 0.75 | 28.8 | 252 | 69.9 | 0.193 | 2 |
| 3 | 19,100 | 0.83 | 72.2 | 354 | 79.9 | 0.158 | 3 |
| 10 | 60,700 | 0.67 | 237 | 308 | 72.2 | 0.166 | 3 |
| 30 | 189,000 | 0.50 | 733 | 345 | 79.8 | 0.161 | 3 |
AUC0-inf: area under the concentration-time curve from hour 0 to infinity; Tmax: time at maximum concentration; Cmax: maximum observed concentration, T½: terminal half-life, Vz: volume of distribution; Cl: clearance.
Table 4.
CSF PK parameters after single dose IV administration in cynomolgus monkeys
| Dose (mg/kg) | AUC0-inf (h·µg/mL) | Tmax (h) |
Cmax (µg/mL) | T½ (h) |
CSF/plasma ratioa (%) |
n |
|---|---|---|---|---|---|---|
| 1 | 14.5 | 88 | 0.0786 | 479 | 0.221b | 3 |
| 3 | 49.3 | 64 | 0.239 | 569 | 0.264 | 3 |
| 10 | 174 | 64 | 0.704 | 367 | 0.289 | 3 |
| 30 | 437 | 88 | 2.91 | 433 | 0.236 | 3 |
AUC0-inf: area under the concentration-time curve from hour 0 to infinity; Tmax: time at maximum concentration; Cmax: maximum observed concentration, T½: terminal half-life.
aCSF/plasma ratios were calculated from AUC0-inf.
bn = 2
Anti-drug antibodies (ADAs) were detected in 3 of 12 animals, one from each of the groups administered 1, 10, or 30 mg/kg. The ADA formation did not affect PK or the overall interpretation of the study since only one animal at each dose level was affected, and the influence on Lu AF82422 exposure was minor or absent. No further characterization of the detected ADAs was conducted.
The amount of total alpha-synuclein measured in CSF predose (n = 12) was 3.25% of that in plasma with mean ± SD values of 18.3 ± 5.73 ng/mL in plasma and 0.595 ± 0. 601 ng/mL in CSF. In animals dosed with 30 mg/kg, there was a tendency to increased levels of total alpha-synuclein in plasma during the first 2 weeks after the Lu AF82422 administration (Figure 3a). The increase is in accordance with antibody binding prolonging the T½ of the target. The mean ± SD predose level of free alpha-synuclein in plasma was 18.7 ± 5.95 ng/mL (n = 12). A dose-dependent decrease in free alpha-synuclein in plasma was seen within 30 minutes after dosing with Lu AF82422 (Figure 3b). Generally, the measured levels of both total and free alpha-synuclein varied considerably during the study. However, when using the free to total alpha-synuclein ratios, the dose-dependent decrease in free alpha-synuclein was evident throughout the 8-week sampling period (Figure 3c). A dose-dependent correlation was shown between the free to total alpha-synuclein ratios and the plasma concentration of Lu AF82422, and an IC50 value of 5.6 μg/mL Lu AF82422 was obtained fitting an inhibitory Imax model to the data using nonlinear regression (Figure 3d). The base line (E0) and the maximal inhibition (IMAX) were estimated to 0.998 and 0.940, respectively. The R square was 0.928. The CSF levels of total alpha-synuclein varied considerably with no dose-related changes (data not shown). Free alpha-synuclein in CSF was not measured.
Figure 3.

Alpha-synuclein levels in plasma after single dose IV administration of Lu AF82422 (1, 3, 10 and 30 mg/kg) in female cynomolgus monkeys (n=3). (a). Total alpha-synuclein (mean ± SD) in plasma shown for time points up to Day 15. LLOQ for the assay (0.156 ng/mL) is not shown. (b) Free alpha-synuclein (mean ± SD) in plasma shown for time points up to Day 15. (c) Free to total alpha-synuclein ratios (mean ± SD) shown for time points up to Day 57 (all samples included). (d) Free to total alpha-synuclein ratios versus plasma concentration of Lu AF82422. IC50 determined by nonlinear regression = 5.6 µg/mL
4-week repeat-dose toxicity study in Wistar Han rats
The toxic potential and TK were evaluated after IV administration of 0, 60, 300 or 600 mg/kg every 10 days. Recovery from any effect was evaluated in the high-dose group during a 12-week recovery phase. The maximum concentration (Cmax) and area under the curve from 0 to 240 hours (AUC0-240h) values evaluated in satellite animals are shown in Table 5. All animals except control animals were systemically exposed to LuAF82422. There was a large variability in exposure between animals at several time points on Day 21. Therefore, the TK parameters calculated on Day 21 are associated with some uncertainty. No sex differences in terms of AUC0240h were observed. The accumulation index was 1.1 to 1.5 following the three administrations of Lu AF82422, 10 days apart. The T½ was determined in the recovery animals to a mean of 13.3 and 14.2 days in males and females, respectively. At the end of the treatment period, one treated animal (60 mg/kg dose group) was ADA-positive with a titer of 7. The level of free Lu AF82422 in this animal did not appear to be affected by the presence of ADAs. No further characterization of the detected ADAs was conducted.
Table 5.
4-week IV repeat-dose tox study in Wistar Han rats; mean TK parameters.a.
| Dose |
Tmax |
Cmax |
AUC0-240h |
|||||
|---|---|---|---|---|---|---|---|---|
| Group | (mg/kg) | Day | Sex | (h) | (µg/mL) | (h*µg/mL) | AIobs | Male/female ratio |
| 2 | 60 | 1 | Male | 0.5 | 1,400 | 92,400 | 1.1 | |
| Female | 0.5 | 1,250 | 81,100 | |||||
| 21 | Male | 0.5 | 1,730 | 143,000 | 1.5 | 1.3 | ||
| Female | 0.5 | 1,380 | 113,000 | 1.4 | ||||
| 3 | 300 | 1 | Male | 0.5 | 5,190 | 255,000 | 0.8 | |
| Female | 0.5 | 6,650 | 327,000 | |||||
| 21 | Male | 0.5 | 5,580 | 285,000 | 1.1 | 0.7 | ||
| Female | 0.5 | 7,150 | 403,000 | 1.2 | ||||
| 4 | 600 | 1 | Male | 0.5 | 8,710 | 526,000 | 1.2 | |
| Female | 0.5 | 9,130 | 456,000 | |||||
| 21 | Male | 0.5 | 12,800 | 686,000 | 1.3 | 1.3 | ||
| Female | 0.5 | 11,900 | 533,000 | 1.2 |
Tmax: time at maximum concentration; Cmax: maximum observed concentration; AUC0-240h: area under the concentration-time curve from hour 0 to 240 hours; AIobs: Accumulation index from Day 1 to Day 21.
asparse sampling was applied (3 animals per time point)
No deaths occurred during the study, and no Lu AF8422-related effects were noted on clinical observations, bodyweight, food consumption, body temperature or at the ophthalmic examinations. At the end of treatment, hematology assessment showed a slight and reversible increase in reticulocyte numbers in males and/or females at all dose levels. It was considered non-adverse, as the differences from controls were small and there was no effect on other erythrocyte indices.
The coagulation assessment after 4 weeks revealed slightly increased fibrinogen in males receiving 60 or 300 mg/kg and in males and females receiving 600 mg/kg which showed full recovery by the end of the 12-week recovery period. Clinical chemistry showed a dose-related increase of total cholesterol in females at all dose levels; slightly increased phosphorus in males receiving 600 mg/kg; and reduced albumin and small increases of plasma globulin at all doses and in both sexes leading to decreased albumin to globulin ratios in all treated groups. These changes showed partial recovery. In the absence of correlating histopathological findings, and as they all showed partial or full recovery, none of the findings seen in the coagulation and clinical chemistry parameters were considered adverse.
Urinary sodium and chloride outputs after 4 weeks were slightly increased in animals, particularly females, receiving 600 mg/kg. Full recovery occurred in males, but not in females. In the absence of any evidence of renal toxicity, these changes were considered unlikely to be of any toxicological importance.
Increased spleen weights were observed at all doses and in both sexes, but particularly in males. In males administered 60, 300 or 600 mg/kg, the histopathological examination showed increased extramedullary hematopoiesis in the spleen, which was the likely cause of the increased spleen weight and the macroscopically enlarged spleen observed in some males. As the increased extramedullary hematopoiesis was reversible, confined to males and occurred in the presence of slightly increased reticulocyte numbers but without effect on the other erythrocyte indices, it was considered non-adverse.
With no toxicologically significant changes, the no-observed-adverse-effect-level (NOAEL) was the high dose of 600 mg/kg, corresponding to a Cmax of 12,800 µg/mL (males) and 11,900 µg/mL (females), and AUC0-240h values of 686,000 h x µg/mL (males) and 533,000 h x µg/mL (females) after the third dose (Table 5). The plasma exposure during the recovery phase (measured Day 31–111) decreased from means of 1,320 to 26.3 µg/mL in males and from 710 to 11.1 µg/mL in females. Despite measurable plasma exposure of Lu AF82422 throughout the recovery period, no additional findings were observed in recovery animals, suggesting that Lu AF82422 exposure for up to 111 days was well tolerated.
4-week repeat-dose toxicity studies in cynomolgus monkeys
Dose levels of 0, 30, 100 and 300 mg/kg administered IV every 10 days were initially assessed, followed by a separate study with dose levels of 0 and 600 mg/kg. Recovery from any effect was evaluated in the high-dose group during a 12-week recovery phase. Plasma exposure to Lu AF82422 was demonstrated for all animals treated with Lu AF82422. The plasma Cmax and AUC0-240h values are shown in Table 6. No sex differences were observed after administration of 30–300 mg/kg, whereas after dosing at 600 mg/kg, male monkeys had higher Cmax and AUC 0–240h values than female monkeys. Lu AF82422 accumulated between 1.5 and 1.9-fold following three administrations given 10 days apart. The terminal T½ varied considerably between the animals administered 300 mg/kg, ranging from 8.2 to 22 days. The variability may partially be explained by the presence of ADA during the recovery phase in one of the four animals previously administered 300 mg/kg (see below). Terminal T½ after administration of 600 mg/kg ranged from 10 to 15 days. ADAs were detected in five animals across the two studies: one female dosed with 30 mg/kg (Day 1), two females dosed with 300 mg/kg (in one female at Day 31 and in another female from Day 71 and onwards in the recovery phase), one male dosed with 600 mg/kg (Day 1), and one female dosed with 600 mg/kg (during the recovery phase). The levels of free Lu AF82422 in plasma did not appear to be affected by the presence of ADAs, except for the ADA-positive recovery animal previously dosed with 300 mg/kg, where exposure appeared to decline relatively fast resulting in a short terminal T½. No further characterization of the detected ADAs was conducted.
Table 6.
4-week IV repeat-dose toxicity studies in cynomolgus monkeys; mean TK parameters
| Dose |
Tmax |
Cmax |
AUC0-240h |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Group | n | (mg/kg) | Day | (h) | (µg/mL) | (haµg/mL) | AIobs | Male/female ratio |
| Male | 2 | 3 | 30 | 1 | 0.5 | 842 | 78,400 | ||
| 21 | 0.5 | 1,180 | 146,000 | 1.9 | |||||
| 3 | 3 | 100 | 1 | 0.5 | 2,680 | 266,000 | |||
| 21 | 0.5 | 4,230 | 484,000 | 1.8 | |||||
| 4 | 5 | 300 | 1 | 0.5 | 7,750 | 824,000 | |||
| 21 | 1.6 | 11,300 | 1,350,000 | 1.6 | |||||
| 5a | 5 | 600 | 1 | 7.4 | 23,800 | 2,400,000 | |||
| 21 | 11 | 29,800 | 3,860,000 | 1.6 | |||||
| Female | 2 | 3 | 30b | 1 | 0.5 | 489 | 61,700 | NR | |
| 30 | 21 | 0.5 | 1,060 | 139,000 | NR | NR | |||
| 3 | 3 | 100 | 1 | 0.5 | 2,190 | 250,000 | 1.1 | ||
| 21 | 0.5 | 3,730 | 463,000 | 1.9 | 1.0 | ||||
| 4 | 5 | 300 | 1 | 0.5 | 7,290 | 823,000 | 1.0 | ||
| 21 | 0.5 | 10,900 | 1,360,000 | 1.7 | 1.0 | ||||
| 5a | 5 | 600 | 1 | 0.5 | 14,400 | 1,680,000 | 0.7 | ||
| 21 | 10 | 19,200 | 2,490,000 | 1.5 | 0.6 |
Tmax: time at maximum concentration; Cmax: maximum observed concentration; AUC0-240h: area under the concentration-time curve from hour 0 to 240 hours; AIobs: Accumulation index observed from single dose to Day 21.NR: No result given due to suspected error in the dosing solution
a600 mg/kg was assessed in a separate study
bAn error in dose level was suspected based on dosing solution analysis.
No Lu AF8422-related effects were noted on clinical observations, bodyweight, food consumption, body temperature, neurological, physical, ophthalmic, or cardiovascular examinations, hematology, coagulation, urinalysis, immunophenotyping or cytokine analysis. In animals administered 100 or 300 mg/kg, cytokine analysis revealed a minimal and transient upregulation of monocyte chemoattractant protein-1 (MCP-1), returning to baseline levels within 24 hours. However, as this was a standalone finding, with increases less than 2-fold compared with baseline levels, it was not considered indicative of an inflammatory response or related to treatment with Lu AF82422. There were no Lu AF82422-related clinical chemistry findings, apart from slightly increased serum total protein and significantly increased serum globulin levels in animals treated with 600 mg/kg Lu AF82422. The findings were fully reversible after the recovery phase. The protein and globulin increases were considered a result of the large amount of protein (IgG1) that was administered IV at regular intervals. Since it was not accompanied by any correlating adverse clinical or pathology findings, it was considered non-adverse. There were no treatment-related macroscopic or histopathological findings in any of the study animals.
As no treatment-related adverse findings were observed, the NOAEL was 600 mg/kg, corresponding to mean Cmax values of 29,800 µg/mL (males) and 19,200 µg/mL (females), and AUC0-240h values of 3,860,000 h x µg/mL (males) and 2,490,000 h x µg/mL (females). The plasma exposure during the recovery phase (measured Day 41–111) decreased from means of 3,580 to 108 μg/mL in males and from 2,220 to 51.5 μg/mL in females. Despite measurable plasma exposure of Lu AF82422 until Day 111, no additional findings were observed in recovery animals. Hence, the Lu AF82422 exposure for up to 111 days was well tolerated.
Total and free alpha-synuclein were determined in plasma and CSF after administration of doses of 30–300 mg/kg. In pre-dose samples, total and free alpha-synuclein levels were similar; approximately 30 ng/mL in plasma and 0.3 ng/mL in CSF (Table 7). It was not feasible to reliably determine total alpha-synuclein levels in plasma from animals dosed with Lu AF82422, as most samples had Lu AF82422 concentrations above the drug tolerance level for the total alpha-synuclein assay. Evaluations of free alpha-synuclein in plasma from animals administered 30–300 mg/kg Lu AF82422, showed a rapid and sustained decrease following IV administration at all dose levels. For all animals administered 100 or 300 mg/kg, levels were below the lower limit of quantification (LLOQ) or below 7.5% of pre-dose values throughout the dosing period (data not shown). Alpha-synuclein levels were not determined in plasma after dosing, with 600 mg/kg. Lu AF82422 exposure in CSF was demonstrated in all dosed animals with levels increasing from first to third dose (Table 8). Levels of total alpha-synuclein in CSF varied considerably during the studies, especially in the control group, hence no significant changes in total alpha-synuclein in CSF after dosing with LuAF82422 was seen. When using the ratio of free to total alpha-synuclein, there was a significant decrease in CSF from animals dosed with 100, 300 and 600 mg/kg LuAF82422 at Day 11 and Day 31 compared to pre-dose. No significant changes were observed in control animals or in animals dosed with 30 mg/kg (Figure 4a,b). For both studies, a dose-dependent correlation was shown between the free to total alpha-synuclein ratios and the CSF concentration of LuAF82422 (Figure 4c). When the CSF data were superimposed on the nonlinear regression plot of the plasma data (Figure 3d), a similar slope of the binding curve was seen, suggesting comparable-binding characteristics of LuAF82422 to alpha-synuclein in plasma and CSF (Figure 4d).
Table 7.
Predose concentrations of alpha-synuclein determined in cynomolgus monkeys.a.
| Alpha-synuclein | Sex | No. of animals | In plasma (ng/mL) |
In CSF (ng/mL) |
||
|---|---|---|---|---|---|---|
| Mean ± SD | Range (min-max) |
Mean ± SD | Range (min-max) |
|||
| Total | Males | 16 | 23.3 ± 8.23 | 13.3–44.2 | 0.358 ± 0.560 | 0.101–1.90 |
| Females | 16 | 37.2 ± 18.2 | 11.6–76.3 | 0.164 ± 0.142 | 0.080–0.666 | |
| Free | Males | 16 | 29.6 ± 9.81 | 17.6–50.9 | 0.380 ± 0.534 | 0.139–2.08 |
| Females | 16 | 38.8 ± 19.9 | 12.9–84.1 | 0.229 ± 0.216 | 0.0928–1.01 | |
aMeasured in study with 0, 30, 100 and 300 mg/kg LuAF82422.
Table 8.
LuAF82422 in CSF after IV administration on Days 1, 11 and 21 in cynomolgus monkeys
| Lu AF82422 (mg/kg) |
No. of animals (males + females) |
Day 11 (predose) |
Day 31 |
||
|---|---|---|---|---|---|
| Mean ± SD (ng/mL) |
Range (min-max) (ng/mL) |
Mean ± SD (ng/mL) |
Range (min-max) (ng/mL) |
||
| 30 | 3 + 3 | 159 ± 52.4 | 101–235 | 450 ± 169 | 258–637 |
| 100 | 3 + 3 | 1,569 ± 1,349 | 573–3,350 | 3,402 ± 3,221 | 883–9,470 |
| 300 | 5 + 5 | 3,621 ± 2,075 | 740–6,090 | 6,828 ± 4,956 | 1,520–14,100 |
| 600a | 5 + 5 | 4,855 ± 2,589 | 2,370–11,100 | 8,294 ± 4,573 | 3,060–18,200 |
aThe dose level of 600 mg/kg was assessed in a separate study
Figure 4.
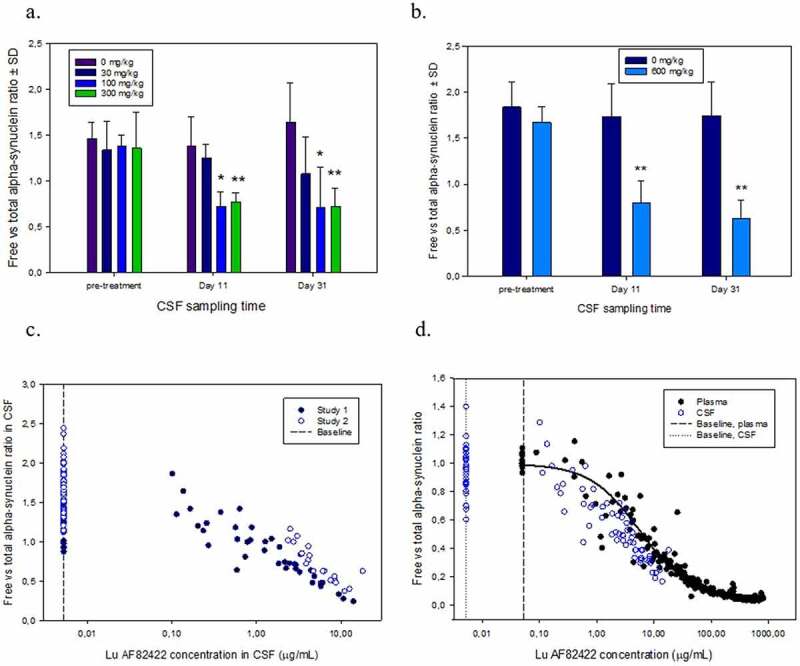
Free to total alpha-synuclein ratios in CSF taken in repeat-dose toxicity studies in cynomolgus monkeys pre-treatment and 10 days after 1st (Day 11) and third (Day 31) IV administration of (a) 0, 30, 100 or 300 mg/kg and (b) 0 and 600 mg/kg LuAF82422. * Significant decrease in free versus total alpha-synuclein compared to pre-treatment values in corresponding group * P<0.01; ** P<0.001 (One-way ANOVA followed by all Pairwise Multiple Comparison Procedures, Holm-Sidak method). (c) Free to total alpha-synuclein ratios versus CSF concentration of LuAF82422 in samples from Study 1 with 0, 30, 100 and 300 mg/kg LuAF82422 and Study 2 with 0 and 600 mg/kg LuAF82422. (d) Free to total alpha-synuclein ratios from CSF were normalized by dividing with the mean baseline ratio for each study and values were superimposed on the nonlinear regression plot of the plasma data (Figure 3d). A similar slope is seen, suggesting similar binding characteristics in plasma and CSF. Baseline free to total alpha-synuclein ratios in samples taken pre-treatment and in control animals receiving vehicle only are shown and marks the LLOQ of the LuAF82422 assay in CSF (5.23 ng/mL) and plasma (50 ng/mL)
Safety pharmacology
Results from the modified Irwin screen in rats showed no neurobehavioral or physiological changes or any effect on body temperature after IV administration of LuAF82422 at doses up to 600 mg/kg. In cynomolgus monkeys, there were no neurobehavioral changes, as assessed by twice daily observations, and no neurological changes or alterations of peripheral and CNS activities observed after treatment with LuAF82422 at doses up to 600 mg/kg.
In cynomolgus monkeys, no changes were observed on respiratory function or heart function, as evaluated as part of the physical examinations by auscultation pre-dose and approximately 0.5 hours after the first and last administration of LuAF82422 up to 600 mg/kg. No effect on qualitative and quantitative electrocardiogram (ECG) data or on blood pressure were observed after IV administration of LuAF82422 up to 600 mg/kg.
Discussion
PK and target engagement
The single-dose PK study in cynomolgus monkeys with IV dose levels from 1 to 30 mg/kg confirmed PK properties of LuAF82422 in the expected range for a human IgG1 mAb with a soluble target exhibiting linear PK with dose-proportional exposure and plasma terminal T½ of 10–15 days, allowing prediction of human PK. Antibody access across the blood-brain-barrier is limited and challenging to quantify. An estimated 0.1–0.2% of circulating antibodies are found in CSF at steady-state.38,39 In line with this, exposure profiles of LuAF82422 in the CSF in terms of AUC0-inf were approximately 0.22 to 0.29% of the exposure in plasma. In the repeat-dose toxicity assessment in cynomolgus monkeys, an increase in LuAF82422 exposure in CSF as well as in plasma was shown between first and third administration. A slightly higher T½ of LuAF82422 was determined in CSF than in plasma.
Alpha-synuclein levels in plasma and CSF was determined in cynomolgus monkeys to examine target engagement of LuAF82422. A relatively high variability was observed, both between individual animals and in individual animals over time. As erythrocytes express high levels of alpha-synuclein, the variability can be caused by blood contamination of CSF samples40 and hemolysis in plasma samples,41 leading to release of alpha-synuclein from erythrocytes. A rapid and dose-dependent decrease in free alpha-synuclein in plasma was shown after single dose IV administration of dose levels ranging from 1 to 300 mg/kg, suggesting systemic target engagement. Due to the high variability, it was only possible to show a significant decrease in free alpha-synuclein (P < 0.05) in CSF after IV administration of the high LuAF82422 doses of 300 (males) or 600 mg/kg (males and females) in samples taken 10 days after both first and third administration of LuAF82422 in cynomolgus monkeys. However, when normalizing the free alpha-synuclein to the total alpha-synuclein concentration, a significant decrease indicating target engagement in CSF was shown after IV administration of 100–600 mg/kg LuAF82422. This relationship in plasma was described by an inhibitory Imax model with an estimated IC50 value close to that observed in vitro (data not shown). The more variable CSF data was superimposed to the plasma profile, implying the same binding in CSF. It should be noted that, based on the Cmax in CSF in the PK study being reached after 64–88 hours, the results from available samples taken 10 days after administration may not reflect the maximum target engagement at the dose given, and 100 mg/kg may therefore not be the lowest dose needed for CSF target engagement. Moreover, the equilibrium of free and LuAF82422-bound alpha-synuclein is likely to differ between the in vivo situation and the assay conditions. Dilution may shift the equilibrium and in the assay plate, alpha-synuclein is likely to favor binding to the plate-bound LuAF82422 compared to the soluble drug. The free alpha-synuclein measured might therefore be overestimated (and target engagement thereby underestimated) compared to the in vivo situation. Moreover, the CSF exposure does not necessarily reflect exposure in the brain interstitial fluid and finally, the affinity to the therapeutic target is expected to be higher than that to monomeric alpha-synuclein. Hence, the translation of doses with detectable target engagement in CSF in the current study conditions to doses needed for a therapeutic effect is uncertain. However, using a similar modeling approach as used for the anti-synuclein-targeting mAb cinpanemab,42 85–95% of oligomeric alpha-synuclein in CSF is expected to be bound by LuAF82422 at dose levels of 15–60 mg/kg, resulting in a safety factor of 10–40 based on the dose in mg/kg.
Nonclinical safety evaluation
As an IgG1 isotype, LuAF82422 has the potential to exert immunomodulatory functions through Fc-receptor interactions and complement activation. Consequently, an IgG1 antibody can eliminate cells with its target present on the cell surface, either by inducing antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis or complement-dependent cytotoxicity.43 Alpha-synuclein is expressed in platelets, erythrocytes, and leucocytes.22–26 In tissues, the target expression study confirmed that alpha-synuclein is primarily expressed in the brain and peripheral nerves in humans, cynomolgus monkeys and rats. Staining was also seen in some non-nervous tissues (pituitary, adrenal medulla, spleen, lymph node, bone marrow and skin) and considered to represent alpha-synuclein expression based on previous reports,35–37 though staining was not necessarily seen in all species or with both antisera used. The tissue cross reactivity study showed binding of LuAF82422 to brain and peripheral nerves only, and the immunotoxicity assessment was therefore focused on blood cells and neurons.
The nonclinical assessments did not suggest any LuAF82422-related risk of immunotoxicity related to neither blood cells nor neurons. The only extracellular surface binding of LuAF82422 to blood cells was to a low fraction of human monocytes with no observed functional consequences. Hence, no change in function of monocytes expressing target on the cell surface is expected in vivo. Additionally, there were no indications of LuAF82422-mediated cytokine release in analyses performed using human PBMCs or human whole blood. Despite the very high exposure of LuAF82422 in plasma, no treatment-related changes in hematology and coagulation parameters, immunophenotyping or cytokine levels were observed in the repeat-dose toxicity studies. Our results showing no binding of alpha-synuclein antibodies to intact, but to permeabilised, neuronal cells indicate that alpha-synuclein is not present at the neuronal cell surface. However, as alpha-synuclein may be associated with extracellular cell membranes,16,44 a low degree of binding of LuAF82422 to neuronal surfaces cannot be completely excluded. As the target expression study showed similar expression profiles in humans and the toxicity species, issues relating to potential presence of alpha-synuclein on neuronal surfaces would be expected to be revealed in the repeat-dose toxicity studies in rats and monkeys. The comprehensive microscopic examinations of the brain and spinal cord using markers for inflammation and neuronal death as well as the extended histopathological examination of peripheral nervous tissue did not reveal any treatment-related findings, suggesting that even if target was present on neuronal cell surfaces, it did not lead to any immunotoxicity.
While several physiological roles of cellular alpha-synuclein are known, a biological role of the extracellular alpha-synuclein has not been identified. A rapid and sustained decrease in free alpha-synuclein levels in plasma was shown in samples from cynomolgus monkeys administered 30–300 mg/kg LuAF82422. For all animals administered 100 or 300 mg/kg, free alpha-synuclein was below LLOQ or below 7.5% of pre-dose values throughout the dosing period. No functional effects and no safety concerns of the binding of LuAF82422 to the majority of extracellular alpha-synuclein in the blood were identified. Also, there were no safety issues related to the target engagement shown in CSF. When administered IV to rats and cynomolgus monkeys every 10 days during a 30-day period at doses up to 600 mg/kg, LuAF82422 was well tolerated and did not result in any toxicologically significant changes. The NOAEL was the high dose of 600 mg/kg in both species.
Taken together, the nonclinical data indicated no safety signal of concern and provided adequate safety margins between observed safe doses in animals and the planned dose levels in a FIH SAD study in healthy subjects and PD patients to investigate safety, tolerability, PK and target engagement. Based on the dose-dependent target engagement shown in plasma after a single IV administration of 1 to 30 mg/kg in cynomolgus monkeys, a starting dose of 1 mg/kg LuAF82422 was considered appropriate. Though this dose level is not expected to be pharmacologically relevant, it is expected to contribute to the evaluation of the PK/PD response in plasma and hence to the estimation of IC50 in humans. Assuming human PK in the expected range for mAbs, the NOAEL from the 4-week repeat-dose toxicity studies provided at least a 600-fold safety margin to this dose level, based on the dose in mg/kg.
Materials and methods
Materials
LuAF82422 is a human IgG1 with a molecular weight of approximately 150 kDa and was produced in Chinese hamster ovary cells. For the PK and repeat-dose toxicity studies, a formulation similar to that intended for humans was used.
For the blood binding and tissue cross reactivity studies, LuAF82422 was labeled with AlexaFluor 647, AlexaFluor 488 and fluorescein isothiocyanate (FITC), respectively. An isotype control IgG1 (prepared in-house) with no known specificity to mammalian proteins was labeled with the corresponding fluorophore and used to assess the background staining.
Blood sample collection
For the blood cell and platelet-binding assays, blood was collected from healthy humans, cynomolgus monkeys or rats.
Binding to blood cells and platelets
The extracellular surface binding of LuAF82422 to resting human platelets and erythrocytes was assessed by flow cytometry using human whole blood samples from three healthy donors and blood concentrations from 5 to 250 µg/mL of AlexaFluor 647-labeled LuAF82422 compared with AlexaFluor 647-labeled isotype control antibody. Additionally, a study evaluating extracellular surface binding of LuAF82422 to resting and activated human platelets was conducted. Samples prepared from platelet-rich plasma obtained from freshly drawn human blood samples diluted with autologous plasma to obtain a platelet count corresponding to whole blood; 250,000/µL (n = 3) were incubated with or without TRAP6 for activation of the platelets. After activation, AlexaFluor 647-labeled LuAF82422 or isotype control antibody was added in concentrations up to 30 µg/mL and the samples were analyzed by flow cytometry. Activation of the platelets was confirmed using p-selectin (CD62-FITC, Serotec, cat. no. 345815). The concentration of LuAF82422 in this study was chosen to assess specific target-related binding to the platelet surface and was therefore in the concentration range normally used for antibody binding in flow cytometry.
Furthermore, extracellular surface binding of LuAF82422 to leucocytes was investigated in whole blood from humans, rats and cynomolgus monkeys (3 donors for each species). AlexaFluor 647-labeled LuAF82422 or isotype control antibody was added to fresh whole blood samples in concentrations up to 250 µg/mL blood (1, 5, 10 and 50 µg/106 leukocytes). The erythrocytes were then lysed, and the leucocyte populations were analyzed for LuAF82422 binding using flow cytometry. Finally, the dependence on the Fc portion for surface binding to the human leukocyte populations was tested by comparing binding of Alexa Fluor 488-labeled full and Fcnull versions of LuAF82422 after incubation of 7, 35, 70 and 350 µg/mL in whole blood samples from 4 donors.
Phagocytosis capability in human monocytes
The general phagocytosis capability of human monocytes in human whole blood was tested using the PhagoTestTM assay (BD Biosciences, cat. no. 341060) to evaluate whether there was any effect of the observed LuAF82422 binding to human monocytes. Concentrations of LuAF82422 from 10 to 3,000 µg/mL blood was included to cover a dose range reflecting plasma levels of LuAF82422 in humans at therapeutic dose levels and the procedure outlined in the assay kit was followed for the analysis. Two high doses (1,000 and 3,000 µg/mL) were included to cover or exceed the expected Cmax concentrations. Blood samples from four healthy donors were pre-incubated with LuAF82422 for 20 minutes, and the ability of monocytes to phagocytose inactivated FITC-labeled bacteria was then assessed using flow cytometry, after lysis of erythrocytes.
Activation of human monocytes
The effect of LuAF82422 on monocyte activation was determined in human whole blood by flow cytometry using BD PhosFlowTM Monocyte/NK cell activation kit (BD Biosciences, cat. no. 562089) according to the kit procedure.
Blood samples from three healthy human donors were used in each experiment. Monocyte activation was detected using intracellular staining for the phosphorylated activation markers Stat1, Stat3 and p38 MAPK, after lysis of the erythrocytes. The effect of LuAF82422 was tested using up to 1,000 µg/mL added to the samples and incubated for 30 minutes before staining for the intracellular phosphorylation markers. In addition, the propensity of LuAF82422 to affect human monocyte activation following stimulation with IFNα, IL-6 or LPS was studied. The blood samples were pre-incubated with LuAF82422 before incubation with concentrations of IFNα, IL-6 and LPS resulting in either sub-maximal or maximal activation of monocytes followed by staining for Stat1, Stat3 and p38 MAPK, respectively.
Cytokine release assays
The ability of LuAF82422 to induce cytokine release from human leukocytes in whole blood was assessed with LuAF82422 in solution as well as LuAF82422 wet-coated onto plastic using whole blood samples from 10 healthy human donors. The stimulation was performed for 24 hours at concentrations of 20, 200, 2,000 and 10,000 µg/mL in the soluble assay format and 4, 40, 400 and 2,000 µg/mL in the wet-coated immobilized antibody assay format, respectively. After the incubation, samples were analyzed for IL-1β, IL-2, IL-6, IL-10, IL-12(p70), IFN-γ, tumor necrosis factor (TNF), granulocyte colony-stimulating factor (G-CSF) and IL-8 using Luminex assays.
In addition, the ability of wet-coated LuAF82422 to stimulate isolated human PBMCs to secrete cytokines and chemokines was tested. Changes of IFN-γ, IL-2, IL-8, macrophage inflammatory protein-1α (MIP-1α), TNF, IL-1β, IL-6, IL-10, IL-12p40 and IL-17A were measured using Luminex assays after 48 hours incubation with LuAF82422 in concentrations from 0.064 to 200 µg/mL with PBMC samples from 10 human donors.
Presence of target on the surface of neuronally differentiated iPS cells
Binding of a phycoerythrin-labeled anti-alpha-synuclein antibody ([syn211] Abcam, cat. no. ab80627) or isotype control antibody was examined in intact and permeabilised neuronally differentiated iPS cells using flow cytometry. The successful differentiation of the neuronal cells was confirmed using surface staining with an allophycocyanin-conjugated CD56/NCAM antibody (R&D Systems, cat. no. FAB2408A) and isotype control antibody.
Target expression in tissues from rats, cynomolgus monkeys and humans
To compare the target expression profile in human, cynomolgus monkeys and rats, immunohistochemistry was performed on a panel of 24 selected frozen tissues (3 donors per species) using two polyclonal anti-alpha-synuclein antibodies (Abcam cat. no. ab6162 and Millipore cat. no. AB5038). A broad range of tissues both with and without expected alpha-synuclein expression was selected based on anti-alpha-synuclein immunohistochemistry on human tissue arrays (BioChain, cat. no. T8334701-5), published literature on alpha-synuclein expression and data from the human protein atlas.37
Tissue cross reactivity
To determine the cross reactivity of LuAF82422, immunohistochemistry was conducted on a panel of 32 tissues (n = 3) of human origin in line with FDA guidelines45 using FITC-labeled LuAF82422.
For each tissue, the following sections were included from each donor: one section stained with hematoxylin and eosin (HE) to evaluate tissue suitability, two sections stained with mouse anti-vimentin at 0.5 μg/mL or mouse anti-smooth muscle actin at 0.25 μg/mL to demonstrate tissue viability, and three sections incubated with FITC-labeled LuAF82422 at 0.5, 1 or 2 μg/mL. Negative controls included one section incubated with an isotype control IgG1 (2 µg/mL) and one section incubated with buffer only. Brain (cerebellum) was used as positive control tissue. Isotype control IgG1 was negative on the cerebellum sections, confirming specificity.
Tissue sections were examined by light microscopy. An assessment of LuAF82422 binding was made by grading the intensity of staining using a semi-quantitative system, and the pattern of tissue staining, distribution of cells and subcellular localization were recorded where appropriate. Specificity of LuAF82422 staining was confirmed by comparing with the negative control slides to distinguish from nonspecific staining.
Animal welfare
A 4-week repeat-dose toxicity study was conducted in Wistar Han rats (Crl:WI(Han) strain) in a total of 71 males and 71 females. The study was conducted in accordance with the requirements in the applicable sections of the United Kingdom Animals (Scientific Procedures) Act 1986, Amendment Regulations 2012 (the Act).
Purpose-bred cynomolgus monkeys (Macaca fascicularis) of Asian origin were used for a PK study (12 females) and a 4-week repeat-dose toxicity study (16 males and 16 females). An additional 4-week repeat-dose toxicity study with a higher dose level was conducted subsequently (10 males and 10 females). The non-human primate studies were conducted in compliance with the German Animal Welfare Act and are approved by the local Institutional Animal Care and Use Committee. Furthermore, the studies were performed in consideration of “Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes” and “Commission Recommendation 2007/526/EC on guidelines for the accommodation and care of animals used for experimental and other scientific purposes (Appendix A of Convention ETS 123)”.
PK study in cynomolgus monkeys
Twelve female cynomolgus monkeys were assigned to four groups administered a single IV dose of 1, 3, 10, or 30 mg/kg LuAF82422, respectively, with a follow-up period of 56 days. Blood samples were collected for evaluation of PK and total and free alpha-synuclein at time points up to Day 56 (0, 0.5, 1, 2, 6, 24, 48, 72, and 96 hours post-dose and on Days 8, 15, 22, 29, 36, 43, 50, and 56 post-dose). Blood samples for evaluation of ADA were collected pre-dose and on Days 15, 29, 43 and 56. CSF samples were collected by lumbar puncture at pre-dose and 24, 48, 72, and 96 hours post-dose and on Days 8, 15, 22, 29, 36, 43, 50 and 57 for evaluation of PK and total and free alpha-synuclein. The hemoglobin level was determined in the CSF samples as a measure of blood contamination, potentially affecting alpha-synuclein levels and exposure measurements.
4-week repeat-dose toxicity study in Wistar Han rats
Groups of 10 male and 10 female Wistar Han rats received LuAF82422 at dose levels of 0, 60, 300 and 600 mg/kg administered IV on Days 1, 11 and 21 of a 30-day treatment period, at the end of which the animals were sacrificed. The control animals received the vehicle (the formulation buffer) under the same conditions. A recovery phase of 12 weeks was included for the control and high-dose groups with five male and five female rats per group. Blood was collected 0.5, 6, 24, 48, 120 and 240 hours after the first and the last dose in satellite animals for evaluation of toxicokinetic profiles using sparse sampling (3 animals per time point) Additionally, blood samples were collected in main phase animals at termination to confirm exposure and in recovery phase animals to determine T½. Study parameters included ADA assessment, clinical observations, body weight, food consumption, body temperature, ophthalmoscopy, clinical pathology, organ weights, macropathology and histopathology. A microscopic evaluation was also performed on a full tissue panel from the recovery animals. In addition to the standard assessments, histopathology investigations of additional peripheral nervous tissues (dorsal root ganglia and spinal nerves (cervical and lumbar levels), maxillary nerve, parasympathetic ganglia, somatic nerves (tibial, peroneal, sural), and trigeminal ganglion) and a comprehensive histopathological examination of the brain was included. The brains were trimmed according to Bolon et al 2013,46 and besides HE staining, special stains for markers of neuroinflammation (ionized calcium binding adaptor molecule 1 (IBA-1) for microglia and glial fibrillary acidic protein (GFAP) for astrocytes) and neuronal degeneration (Fluoro-Jade B) were included.
4-week repeat-dose toxicity studies in cynomolgus monkeys
Groups of three males and three females received LuAF82422 at dose levels of 0, 30, 100 or 300 mg/kg every 10 days administered IV on Days 1, 11 and 21 of a 30-day treatment period, at the end of which the animals were sacrificed. The control animals received the vehicle (the formulation buffer) under the same conditions. A recovery phase of 12 weeks was included for the control and high-dose groups with two males and two females per group. An additional study was performed to assess the systemic toxic potential of LuAF82422 at 0 and 600 mg/kg using a similar study design.
From all study animals, blood was collected 0.5, 6, 24, 48, 120 and 240 hours after the first and the last dose for evaluation of toxicokinetic profiles, and in recovery animals during the recovery phase. CSF was sampled once prior to initiation of treatment and 240 hours after the first (Day 1) and the last (Day 21) dose (at the time of necropsy for main phase animals) for evaluation of LuAF82422 exposure. Alpha-synuclein levels (total and free) were determined in plasma (in samples from animals dosed at 30–300 mg/kg) and CSF samples to evaluate the target engagement of LuAF82422. Plasma and CSF samples were also analyzed for hemoglobin.
Other study parameters included ADA measurements, clinical observations, body weight, food consumption, body temperature, ophthalmoscopy, clinical pathology, cytokine analysis immunophenotyping, organ weights, macropathology, and histopathology. Cytokines (predose, 6 and 24 hours after first and last dosing) were analyzed in a Luminex 100 using a Milliplex® MAP multiplex kit (Millipore cat. no. HCYTOMAG-60 K). A microscopic evaluation was also performed on a full tissue panel from the recovery animals. Besides the standard list of tissues, additional peripheral nervous tissues were included in the histopathological evaluation: Somatic nerves (tibial, peroneal, sural, and plantar nerves), cranial nerves (trigeminal ganglion and vagus nerve), dorsal root ganglia and spinal nerve roots (cervical, thoracic and lumbar), sympathetic ganglia (thoracic sympathetic ganglion and celiacomesenteric ganglionic plexus) and parasympathetic ganglia (intramural in the digestive system). A comprehensive histopathological examination of the brain was performed. The brains were trimmed according to Bolon et al. 2013,45 and besides HE staining, special stains for markers of neuroinflammation (IBA-1 and GFAP) and neuronal degeneration (Fluoro-Jade C staining) were included.
Safety pharmacology
Safety pharmacology assessments were included in the 4-week repeat-dose toxicity studies.
In rats, a modified Irwin screen for the safety pharmacological evaluation of the CNS was performed pre-dose and on Days 1 and 21 immediately after infusion and 2 hours post dose. During the observations, the animals were systematically evaluated using a modification of the observational assessment originally described by Irwin.47 In cynomolgus monkeys, assessment of the CNS included neurological examinations performed once during the pre-dose phase, on Days 1 and 21 (0.5 hour post dose), and at the end of the recovery phase. The neurological assessments included general sensory aspects, cerebral reflexes (pupillary and orbicularis oculi) and spinal reflexes (patellar, anal and grip reflex). A standard functional observation battery for assessment of peripheral and CNS activities was included to evaluate animals dosed with 600 mg/kg, twice during the pre-dose phase, at 24 hours after first and last administration of LuAF82422 and at the end of the recovery phase.
Heart function was evaluated in cynomolgus monkeys as part of the physical examinations by auscultation pre-dose and approximately 0.5 hours after the first and last administration of LuAF82422. ECG measurements were performed by telemetric cardiovascular investigations (jacketed external telemetry), measured continuously for at least 22 hours pre-dose, after dosing twice during the study (on Days 3 and 23) and once during the last week of the recovery phase. The following parameters were analyzed: heart rate (beats/min), RR and PR-intervals, QRS-duration, QT and corrected QT (QTc) intervals (msec). Blood pressure measurements were performed on temporarily restrained animals once during the pre-dose phase, on Days 1 and 21 (before and 0.5 hours ±15 minutes post-dose) and during the last week of the recovery phase. Systolic-, diastolic-, and mean arterial blood pressures (mmHg) were recorded by a high-definition oscillometry method.48
Respiratory function was evaluated in cynomolgus monkeys by lung auscultation during the physical assessment pre-dose and approximately 0.5 hours after first and last dosing.
Bioanalytical method (exposure)
The bioanalytical method used to quantify the exposure of LuAF82422 (i.e., PK and TK) was a sandwich-format electrochemiluminescence immunoassay (ECLIA) using two different inhibitory anti-idiotypic antibodies (custom made, BioRad) for capture and detection. As both the capture and detection agents were inhibitory anti-idiotypic antibodies, LuAF82422 was only detected in the assay when free at the target-binding sites. LuAF82422 may be bound i.e., by antibodies at other parts of LuAF82422 than at the target-binding sites and still be detected in the assay. Therefore, the term “free LuAF82422” is here defined as LuAF82422 that is free at the target-binding sites.
The assay was validated for quantification of LuAF82422 in rat plasma, cynomolgus monkey plasma and cynomolgus monkey CSF in accordance with relevant guidelines49,50 and conducted in accordance with GLP.34
The validated ranges were 50–10,000 ng/mL in plasma and 5–320 ng/mL in CSF. The validation parameters were precision, accuracy, integrity of dilution (for plasma only), dilutional linearity, prozone effect, selectivity and stability (for plasma only). In addition, alpha-synuclein interference was assessed to confirm detection of free drug.
Method used for ADA determination
Assessment of immunogenicity in rat and cynomolgus monkey plasma was carried out using a tiered approach including a screening step, a confirmatory step and a titration step. To detect anti-LuAF82422 antibodies of different isotypes and affinities across species and to avoid false negatives, a bridging ECLIA method with a very low limit of detection and a single wash step was used for screening. Samples screened positive were subsequently assessed in a confirmatory assay using the same bridging ECLIA method but including excess amount of LuAF82422 to evaluate whether the binding could be inhibited by LuAF82422 and hence confirmed. Finally, confirmed positive samples were titrated using the same ECLIA method used for screening.
Considering the relatively high levels of LuAF82422 in plasma following dosing in rat and cynomolgus monkey, an acid dissociation step was included in the assay to obtain a sufficient drug tolerance. The obtained drug tolerance levels were 1.25 mg/mL and 0.625 mg/mL for rat and cynomolgus monkey plasma, respectively.
Polyclonal positive control antibodies were generated by immunizing goats with the Fab fragment of LuAF82422. The resulting serum was affinity purified against the Fab fragment followed by adsorption to polyclonal human IgG. Using this positive control, the sensitivity of the assay was determined to 20 and 5 ng/mL for rat and cynomolgus monkey plasma, respectively.
The bridging ECLIA method was validated for screening, confirmation and titration of anti-LuAF82422 antibodies in rat and cynomolgus monkey plasma according to regulatory guidance51–54 and in accordance with GLP.
The validations included determination of cutpoints, precision of relative response, precision of scoring, sensitivity, prozone effect, precision of titers, drug tolerance, target interference (for monkey only) matrix variability and freeze-thaw stability
Methods used for alpha-synuclein determination (total and free)
Two methods were developed and scientifically validated to measure the relative levels of free and total alpha-synuclein in cynomolgus monkey plasma and CSF. The term “free alpha-synuclein” was defined here as alpha-synuclein that is not bound to LuAF82422. Alpha-synuclein may be bound by other molecules at other sites than the site bound by LuAF82422 and still be detected in the assay. The term “total alpha-synuclein” was here defined as alpha-synuclein whether bound by LuAF82422 or not. It does not mean that all isoforms and truncated versions of alpha-synuclein were detected.
Both methods used a commercially available alpha-synuclein sandwich ECLIA kit (MSD K151TGD-2/K151WKK-4) with specific modifications.
For the relative measurement of total alpha-synuclein in cynomolgus monkey plasma, an acid dissociation step was included to reduce drug interference in the samples whereas this was found not to be necessary for the relative measurement of total alpha-synuclein in cynomolgus monkey CSF. The validated ranges for total alpha-synuclein were 78–10,000 pg/mL in plasma and 80–5,000 pg/mL in CSF. The validation parameters were precision, parallelism, drug tolerance and freeze/thaw stability (plasma only).
For the detection of free alpha-synuclein in plasma and CSF, the capturing agent of the kit was replaced by biotinylated LuAF82422 excluding the capture of alpha-synuclein already engaged by LuAF82422 in the sample, thereby measuring free alpha-synuclein. The validated ranges for free alpha-synuclein were 600–50,000 pg/mL in plasma and 50–4,000 pg/mL in CSF. The validation parameters were precision, parallelism, drug inhibition and freeze/thaw stability.
Hemoglobin enzyme-linked immunosorbent assay (ELISA)
Hemoglobin analysis of monkey CSF samples was performed by an enzyme-linked immunosorbent assay (Human Hemoglobin ELISA kit, Abcam cat. no. ab157707) according to the manufacturer´s instructions.
Data evaluation
The PK/TK interpretation was performed by non-compartmental analysis in Phoenix WinNonlin, version 6.3, on plasma or CSF concentrations of LuAF82422.
In the PK study in cynomolgus monkeys, all plasma samples (including pre-dose) from one animal dosed with 1 mg/kg showed a very high signal during bioanalysis, and no cause was identified. The LuAF82422 concentration in CSF samples from this animal were in the same range as in the other animals of the group. Together with the high signal in the pre-dose plasma sample, this suggested that the high plasma signal did not reflect actual exposure. The plasma samples from this animal were therefore excluded from the PK calculations.
Increased hemoglobin levels in CSF indicate blood contamination, which could affect alpha-synuclein concentrations (total and free) because alpha-synuclein is highly expressed in erythrocytes. Blood contamination could also affect the CSF levels of LuAF82422, as the concentration in blood is around 1000 times higher than in CSF.56,57 For each of the three studies that included collection of CSF samples, a cut point was determined, below which there was no/minimal linear correlation between the concentration of hemoglobin and total alpha-synuclein. CSF samples with a hemoglobin concentration above the cut point were excluded from the data evaluation of alpha-synuclein concentration (free and total) in the CSF. In the PK study in cynomolgus monkeys, a cut point of 5000 ng/mL was determined, leading to exclusion of 63 out of 156 samples. In the repeat-dose toxicity study in cynomolgus monkeys with dose levels of 30–300 mg/kg, the determined cut point of 8000 ng/mL led to exclusion of five samples. In the repeat-dose toxicity study with 600 mg/kg, no CSF samples were excluded, as no linear correlation was shown between hemoglobin and alpha-synuclein.
Evaluation of target engagement of LuAF82422 was based on changes in alpha-synuclein levels (total and free) in plasma or CSF. For plasma, a direct inhibitory Imax model was fitted to the data. The IC50 value in plasma was calculated by nonlinear regression, plotting the free to total alpha-synuclein ratios against the time-matched plasma concentration of LuAF82422 using SigmaPlot version 11. The model was selected based on the plot of the data and was of the form: I = E0-IMAX*C/(IC50 + C), where E0 is the base line, IMAX is the maximal inhibition, and the IC50 is the plasma concentration (C) of LuAF82422 that provides 50% of IMAX.
For the CSF samples collected in the 4-week toxicity studies in cynomolgus monkeys, there were no differences in alpha-synuclein levels between males and females, and the results were therefore pooled. Mean free to total alpha-synuclein ratios before and after dosing were compared by Kruskal-Wallis One Way Analysis of Variance on Ranks followed by All Pairwise Multiple Comparison Procedures (Holm-Sidak method) using SigmaPlot version 11. For comparison of the binding properties in CSF and plasma, the free-to-total alpha-synuclein ratios from CSF were normalized by dividing with the mean baseline ratio for each study and superimposed on the plot with the plasma data.
Supplementary Material
Acknowledgments
The authors thank Tina Charlotte Stummann, Dept. Cell Biology, H. Lundbeck for providing the neuronally differentiated iPS cells.
Funding Statement
The research was funded by H. Lundbeck A/S, Copenhagen, Denmark.
Abbreviations
| ADAs | anti-drug antibodies |
| AUC | area under the concentration-time curve |
| Cl | clearance |
| Cmax | maximum concentration |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| ECG | electrocardiogram |
| ECLIA | electrochemiluminescence immunoassay |
| ELISA | enzyme-linked immunosorbent assay |
| FDA | U.S. Food and Drug Administration |
| FIH | first-in-human |
| FITC | fluorescein isothiocyanate |
| G-CSF | granulocyte colony-stimulating factor |
| GFAP | glial fibrillary acidic protein |
| GLP | good laboratory practice |
| HE | hematoxylin and eosin |
| IBA-1 | ionized calcium binding adaptor molecule-1 |
| IFN | interferon |
| IL | interleukin |
| LPS | lipopolysaccharide |
| IgG | immunoglobulin G |
| iPS cells | induced pluripotent stem cells |
| IV | intravenous |
| mAb | monoclonal antibody |
| MIP-1α | macrophage inflammatory protein-1α |
| MSA | multiple system atrophy |
| NOAEL | no-observed-adverse-effect-level |
| SAD | single ascending dose |
| SD | standard error |
| PBMCs | peripheral blood mononuclear cells |
| PD | Parkinson´s disease |
| PK | pharmacokinetic |
| T½ | terminal half-life |
| TK | toxicokinetic |
| TRAP-6 | thrombin receptor activating peptide-6 |
| Vz | volume of distribution |
Disclosure statement
Dorte Kornerup Ditlevsen, Annemette Thougaard, Karen Malene Wegener, Joan Christiansen, Frank Larsen, Lise Maj Schrøder-Hansen and Marianne Kaarde are full-time employees of H. Lundbeck A/S. Lone Fjord-Larsen was a full-time employee of H. Lundbeck A/S at the time of this work. The research was funded by H. Lundbeck A/S, Copenhagen, Denmark.
Contributions
Lone Fjord-Larsen, Dorte Kornerup Ditlevsen, Annemette Thougaard and Karen Malene Wegener for designing; Joan Christiansen for ex vivo studies of leukocyte binding and monocyte effects; Frank Larsen for PKPD modelling; Lise Maj Schrøder-Hansen for immunohistochemistry on human tissue arrays; Marianne Kaarde for hemoglobin ELISA analysis; Lone Fjord-Larsen, Dorte Kornerup Ditlevsen, Annemette Thougaard and Karen Malene Wegener for final data analysis and writing up.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Maroteaux L, Campanelli JT, Scheller RH.. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8(8):2804–16. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu S, Li X, Liu G, Han J, Zhang C, Li Y, Xu S, Liu C, Gao G, Yang G, et al. Extensive nuclear localization of α-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody. Neuroscience. 2007;145(2):539–55. doi: 10.1016/j.neuroscience.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Sudhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol. 2011; 3(12) doi: 10.1101/cshperspect.a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendor JT, Logan TP, Edwards RH. The function of alpha-synuclein. Neuron. 2013;79:1044–66. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaser AJ, Osterberg VR, Dent SE, Stackhouse TL, Wakeham CM, Boutros SW, Weston LJ, Owen N, Weissman TA, Luna E, et al. Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders. Sci Rep. 2019;9(1):10919. doi: 10.1038/s41598-019-47227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin S, Floden AM, Murphy EJ, Combs CK. a-synuclein expression modulates microglial activation phenotype. J Neursci. 2006;26(41):10558–63. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- 8.Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM. alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res. 2000;62(1):9–14. doi:. PMID: 11002283 [DOI] [PubMed] [Google Scholar]
- 9.Pajarilloa E, Rizora A, Leeb J, Aschner M, Lee E. The role of posttranslational modifications of α-synuclein and LRRK2 in Parkinson’s disease: potential contributions of environmental factors. Biochim Biophys Acta Mol Basis Dis. 2019. August 01;1865(8):1992–2000. doi: 10.1016/j.bbadis.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Li X, Li J-D. The roles of post-translational modifications on α-synuclein in the pathogenesis of Parkinson’s diseases. Front Neurosci. April 18 2019. doi: 10.3389/fnins.2019.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefanis L. α-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;4:a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998. Sep;44(3):415–22. doi: 10.1002/ana.410440324. PMID: 9749615. [DOI] [PubMed] [Google Scholar]
- 13.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998. Jul 31;251(3):205–08. PMID: 9726379. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 14.Pacheco C, Aguayo LG, Opazo C. An extracellular mechanism that can explain the neurotoxic effects of α-synuclein aggregates in the brain. Front Physiol. 2012. July 26;3. doi: 10.3389/fphys.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C, Lee MK. Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo. J Neurosci. 2012. Mar 7;32(10):3301–05. doi: 10.1523/JNEUROSCI.5368-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diógenes MJ, Dias RB, Rombo DM, Vicente Miranda H, Maiolino F, Guerreiro P, Näsström T, Franquelim HG, Oliveira LM, Castanho MA, et al. Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J Neurosci. 2012. Aug 22;32(34):11750–62. doi: 10.1523/JNEUROSCI.0234-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4(1):1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarutani A, Arai T, Murayama S, Hisanaga SI, Hasegawa M. Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods. Acta Neuropathol Commun. 2018. Apr 18;6(1):29. PMID: 29669601; PMCID: PMC5907316. doi: 10.1186/s40478-018-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008. May;14(5):501–03. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003. Mar-Apr;24(2):197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 21.McCann H, Cartwright H, Halliday GM. Neuropathology of α-synuclein propagation and braak hypothesis. Mov Disord. 201. 6;31(2):152-60. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto M, Yoshimoto M, Sisk A, Hsu LJ, Sundsmo M, Kittel A, Saitoh T, Miller A, Masliah E. NACP, a synaptic protein involved in Alzheimer’s disease, is differentially regulated during megakaryocyte differentiation. Biochem Biophys Res Commun. 1997. Aug 28;237(3):611–16. PMID: 9299413. doi: 10.1006/bbrc.1997.6978. [DOI] [PubMed] [Google Scholar]
- 23.Nakai M, Fujita M, Waragai M, Sugama S, Wei J, Akatsu H Ohtaka-Maruyama C, Okado H, Hashimoto M. Expression of alpha-synuclein, a presynaptic protein implicated in Parkinson’s disease, in erythropoietic lineage. Biochem Biophys Res Commun. 2007;358(1):104–10. doi: 10.1016/j.bbrc.2007.04.108. [DOI] [PubMed] [Google Scholar]
- 24.Shameli A, Xiao W, Zheng Y, Shyu S, Sumodi J, Meyerson HJ, Harding CV, Maitta RW. A critical role for alpha-synuclein in development and function of T lymphocytes. Immunobiology. 2016;221(2):333–40. doi: 10.1016/j.imbio.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin EC, Cho SE, Lee DK, Hur MW, Paik SR, Park JH, Kim J. Expression patterns of alpha-synuclein in human hematopoietic cells and in Drosophila at different developmental stages. Mol Cells. 2000. Feb 29;10(1):65–70. PMID: 10774749. doi: 10.1007/s10059-000-0065-x. [DOI] [PubMed] [Google Scholar]
- 26.Park SM, Jung HY, Kim HO, Rhim H, Paik SR, Chung KC Park JH, Kim J. Evidence that alpha-synuclein functions as a negative regulator of Ca(++)-dependent alpha-granule release from human platelets. Blood. 2002;100(7):2506–14. doi: 10.1182/blood.V100.7.2506. [DOI] [PubMed] [Google Scholar]
- 27. El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S et al. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17(13):1–1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 28.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005. Jun 22;25(25):6016–24. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, Abbruzzese G, Tabaton M. Full length α-synuclein is present in cerebrospinal fluid from Parkinson’s disease and normal subjects. Neurosci Lett. 2000;287(1):65–67. doi: 10.1016/S0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- 30.Emmanouilidou E, Elenis D, Papasilekas T, Stranjalis G, Gerozissis K, Ioannou PC, Vekrellis K. Assessment of α-synuclein secretion in mouse and human brain parenchyma. PLoS One. 2011;6(7):e22225. doi: 10.1371/journal.pone.0022225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miraglia F, Betti L, Palego L, Giannaccini G. Parkinson’s disease and alpha-synucleinopathies: from arising pathways to therapeutic challenge. Cent Nerv Syst Agents Med Chem. 2015;15(2):109–16. doi: 10.2174/1871524915666150421114338. PMID: 25896035 [DOI] [PubMed] [Google Scholar]
- 32.ICH Guideline S6 (R1) Preclinical safety evaluation of biotechnology-derived pharmaceuticals (2011) and subsequent addendum; 2012.
- 33.ICH M3 (R2) Non-clinical safety studies for the conduct of human clinical trials for pharmaceuticals. CPMP/ICH/286/95, 2013. Feb 11.
- 34.Organisation for Economic Co-operation and Development (OECD): principles of GLP, ENV/MC/CHEM (98) 17
- 35.Homma T, Mochizuki Y, Mizutani T. Phosphorylated α-synuclein immunoreactivity in the posterior pituitary lobe. Neuropathology 2012; 32(4): 385–89. doi: 10.1111/j.1440-1789.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- 36.Fumimura Y, Ikemura M, Saito Y, Sengoku R, Kanemaru K, Arai T, Ito G, Iwatsubo T, Fukayama M, Mizusawa H, et al. Analysis of the adrenal gland is useful for evaluating pathology of the peripheral autonomic nervous system in Lewy body disease. J Neuropathol Exp Neurol. 2007. May;66(5):354–62. doi: 10.1097/nen.0b013e3180517454. [DOI] [PubMed] [Google Scholar]
- 37.Protein Atlas. http://www.proteinatlas.org/ENSG00000145335-SNCA/tissue Avaiable from:
- 38.Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. 2013;10(3):459–72. doi: 10.1007/s13311-013-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 40.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain. 2010. Mar;133(Pt 3):713–26. doi: 10.1093/brain/awq008. Epub 2010 Feb 15. PMID: 20157014; PMCID: PMC2842513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5(2):55–59. doi: 10.1159/000112832. Epub 2008 Jan 4. PMID: 18182779 [DOI] [PubMed] [Google Scholar]
- 42.Kuchimanchi M, Monine M, Kandadi Muralidharan K, Woodward C, Penner N. Phase II dose selection for alpha synuclein-targeting antibody cinpanemab (BIIB054) based on target protein binding levels in the brain. CPT Pharmacometrics Syst Pharmacol. 2020. Sep;9(9):515–22. PMID: 32613752; PMCID: PMC7499191. doi: 10.1002/psp4.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011. Feb;10(2):101–11. doi: 10.1038/nrd3365. PMID: 21283105. [DOI] [PubMed] [Google Scholar]
- 44.Masaracchia C, Hnida M, Gerhardt E, Lopes da Fonseca T, Villar-Pique A, Branco T, Stahlberg MA, Dean C, Fernández CO, Milosevic I, Outeiro TF. Membrane binding, internalization, and sorting of alpha-synuclein in the cell. Acta Neuropathol Commun. 2018;6(1):79. doi: 10.1186/s40478-018-0578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. FDA guideline: points to consider in the manufacture and testing of monoclonal antibody products for human use. doi: 10.1097/00002371-199705000-00007. [DOI] [PubMed]
- 46.Bolon B, Garman RH, Pardo ID, Jensen K, Sills RC, Roulois A Radovsky A, Bradley A, Andrews-Jones L, Butt M, et al. STP position paper: recommended practices for sampling and processing the nervous system (brain, spinal cord, nerve, and eye) during nonclinical general toxicity studies. Toxicol Pathol. 2013;41(7):1028–48. doi: 10.1177/0192623312474865. [DOI] [PubMed] [Google Scholar]
- 47.Irwin S. Comprehensive observational assessment: la. A systematic quantitative procedure for assessing the behavioural and physiologic state of the mouse. Psychopharmacologia. 1968;13(3):222–57. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 48.Schmelting B, Niehoff M, Egner B, Korte S, Weinbauer G. High definition oscillometry: a novel technique for non-invasive blood pressure monitoring in the cynomolgus monkey (Macaca fascicularis). J Med Primatol. 2009;38(5):293–301. doi: 10.1111/j.1600-0684.2009.00344.x. [DOI] [PubMed] [Google Scholar]
- 49.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM) . Guidance for industry - bioanalytical method validation. 2001. [Google Scholar]
- 50.European Medicines Agency . Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009; 2012. [DOI] [PubMed]
- 51.EMEA/CHMP/114720/2009 . Concept paper on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use; 2009.
- 52.EMEA/CHMP/BMWP/14327/2006 Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins (2007).
- 53.Neyer L, Hiller J, Gish K, Keller S, Caras I. Confirming human antibody responses to a therapeutic monoclonal antibody using a statistical approach. J Immunol Methods. 2006. Aug 31;315(1–2):80–87. doi: 10.1016/j.jim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Shankar G, Devanarayan V, Amaravadi L. Barrett YC, Bowsher R, Finco-Kent D, Fiscella M, Gorovits B, Kirschner S, Moxness M, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008. Dec 15;48(5):1267–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


