Abstract
Introduction:
Cells exfoliated into urine from the bladder can help to diagnose the cancer. The objective of this study was to validate the hypothesis that bladder cancer could be detected noninvasively by a simple and reliable assay targeting genomic VPAC (combined vasoactive intestinal peptide and pituitary adenylate cyclase-activating peptide family of cell surface receptors) receptors expressed on the malignant bladder cancer cells shed in the voided urine.
Methods:
Patients ≥18 years of age with either imaging (ultrasonography/computed tomography [CT])-confirmed bladder tumors or those who have been previously treated for nonmuscle invasive bladder tumors and were visiting the department for check cystoscopy, formed the study group. Freshly voided urine sample was collected from these patients and sent for conventional cytology examination, 5-aminolevulinic acid (ALA) fluorescent urine cytology, and for positivity of VPAC receptors.
Results:
A total of 103 patients were prospectively included in the study. Of these, 65 patients (Group I) presented with image-diagnosed (ultrasonography and/or CT) bladder cancer. The remaining 38 patients (Group II) were previously diagnosed cases of nonmuscle invasive bladder cancer and presented for follow-up and check cystoscopy. The sensitivity for VPAC receptor positivity was 89.23% compared to conventional cytology (63.07%) and 5-ALA fluorescent urine cytology (87.69%). The specificity of VPAC receptor positivity was 100% compared to conventional cytology (100%) and 5-ALA-induced fluorescent cytology (90.47%).
Conclusions:
Our preliminary study shows encouraging results with VPAC receptor positivity studies, which has a high sensitivity when compared to the conventional cytology.
INTRODUCTION
Cancer of the bladder is a global disease, with an estimated incidence of 540,000 cases and 188,000 deaths in the year 2015 worldwide.[1] The bladder as an organ often comes in constant contact with the environment and is therefore sensitive to the environmental carcinogens and inflammation. Tobacco smoke, which is full of aromatic amines, has been implicated in bladder cancer. These amines, when hydroxylated, lead to DNA adduction and damage.[1]
Cystoscopy and biopsy remain the gold standard test for the diagnosis of bladder cancer. Currently, white light cystoscopy remains the standard of care for diagnosing bladder tumor and enables the urologist to identify and resect all the visible tumors.[2,3]
Cells exfoliated into urine from the bladder cancer can reliably diagnose the cancer. Urine cytology has been a standard diagnostic test to aid in the diagnosis of bladder cancer. The reported contemporary sensitivity and specificity of urine cytology for the detection of bladder cancer are 31%–62% and 94%–100%, respectively.[4,5,6] In 2013, Paris Reporting System for Urinary Cytology was first introduced at the International Congress of Cytology to standardize the cytologic interpretation of the urine samples.[7] Several urine-based biomarkers have since been developed as an adjunct to the standard diagnostic modalities. Noninvasive testing with improved sensitivity over urine cytology has been proposed as a desirable alternative to cystoscopy, which is costly and uncomfortable.[1] In this preliminary study, we hypothesized that cells shed in the voided urine of the patients with bladder cancer could be detected under microscope by targeting the VPAC1 receptors with the same peptide labeled with a fluorophore and compared the results with conventional cytology and 5-aminolevulinic acid (ALA)-induced fluorescent urine cytology.
MATERIALS AND METHODS
This prospective study was conducted following clearance obtained from the University/Institutional Ethical Committee KAHER/EC/20–21/001/05. Patients ≥18 years of age with either imaging (ultrasonography/computed tomography [CT])-confirmed bladder tumors or those who had been previously treated for nonmuscle invasive bladder tumors and were visiting the department for check cystoscopy, formed the study group. Male patients with a serum prostate-specific antigen value >1.5 ng/mL were excluded from the study. Freshly voided urine sample was collected from these patients and divided into three samples of 50 cc each. One of these samples was sent for conventional cytology examination, one was sent for 5-ALA fluorescent photodynamic diagnosis, and the last one was sent for fluorescent microscopic examination and identification of VPAC receptors.
Conventional cytology
The urine sample was processed using cytocentrifuge (REMI) at 1500 rpm for 10 min. In low cellularity samples, the urine was processed using cytospin (Sakura Cytotec) for 5 min at 1500 rpm. After cytocentrifugation, the supernatant was decanted and the sediment was resuspended to make the required slides and were stained with Papanicolaou stain.[8]
Urine samples and treatment with 5-aminolevulinic acid
The urine sample was centrifuged at 1500 rpm for 5 min, and the supernatant was decanted. The pellet was suspended in minimum essential medium (MEM) with 5-ALA hydrochloride (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany, 2020), and the concentration was adjusted to 200 μg/mL. Then, the suspension was stored in the dark at 37°C for 2 h. After that, the sample was centrifuged again at 1500 rpm for 5 min, and the pellet was resuspended in MEM. Finally, the urine sample was tested for protoporphyrin IX fluorescence using a fluorescent microscope (Nikon ECLIPSE Ni; Nikon Corporation, Tokyo, Japan) at appropriate settings (excitation wavelength of 405 nm and emission wavelength of 600–650 nm). The 5-ALA-induced fluorescent cytology showing no red light or dark red was classified as negative and that showing clear red as positive.[9]
Urine samples and identification of VPAC receptors
Samples were centrifuged at 2000 × g for 10 min and all, but approximately 250 μL of supernatant was discarded. The cells were then suspended, and cytocentrifuged, and fixed in 97% ethyl alcohol. TP4303 solution (0.5 μg) was added to the cells to cover the entire cell area, approximately 1 cm in diameter. The slide was then kept in dark at 22°C for approximately 20 min and was then thoroughly rinsed with deionized water and air-dried. 20 μL of 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Fisher Scientific, PA), which strongly binds to A-T rich region of DNA in the cell nucleus, was then added on the cells. A coverslip was then placed and the slide was observed using a fluorescent microscope. Cells with TP4303 interaction presented themselves with dark orange fluorescence around the nucleus, thereby indicated the presence of VPAC receptor molecules around the cell surface [Figure 1a and b]. In the absence of VPAC receptors, only the DAPI bound cell nucleus was seen in dark blue [Figure 1a and b]. Normal epithelial cells may only have minimal or no expression of VPAC [Figure 2] and therefore do not interact with TP4303 and only show cell nucleus.[10]
Figure 1.
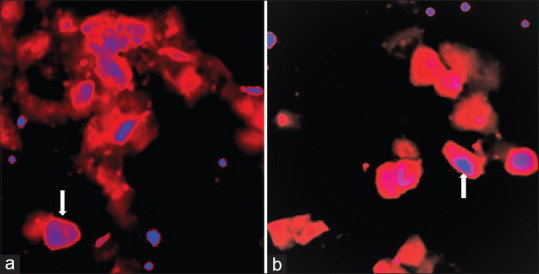
(a and b) Fluorescence imaging of cells prepared from voided urine. Cell morphology with arrow shows cell nucleus stained in blue and cytoplasm with orange fluorescence of TP4303 bound to VPAC receptors expressed on the cell membrane
Figure 2.

Normal epithelial cells stained with blue nucleus that may only have minimal or no expression of VPAC receptors
Comparison with histopathology
All patients with imaging-confirmed bladder tumor underwent cystoscopy/biopsy/transurethral resection of bladder tumor. The surgical specimens were sent for histopathological examination and were reported by the same pathologist. Patients who were undergoing check cystoscopy to identify recurrences, underwent biopsies of the suspected areas. The reports of histopathology were compared to the results of conventional cytology, 5-ALA-induced fluorescent cytology, and fluorescent microscopic examination for VPAC receptors.
Statistical analysis
The data were collected in Microsoft Excel 2019 and compared for specificity and sensitivity. Frequency and percentage are represented for the data collected.
RESULTS
During the period March 2020–February 2021, a total of 103 patients presenting to the urologic-oncology outpatient department (OPD) were prospectively included in the study. Of these, 65 patients (mean age: 59.35 ± 16.72 years) (Group I) presented with image-diagnosed (ultrasonography and/or CT) bladder cancer. The remaining 38 patients (mean age: 55.42 ± 14.37 years) (Group II) were previously diagnosed cases of nonmuscle invasive bladder cancer and were attending the urologic-oncology OPD for follow-up and check cystoscopy. Only 6 of these patients were female and the remaining 97 were male.
Voided urine samples of all these 103 patients (mean age: 57.90 ± 15.9 years) were used to perform all the tests, and the results are shown in Table 1.
Table 1.
Positive results of different diagnostic tests and comparison with histopathology report reports
| Group | HPR | Cytology (%) | 5-ALA (%) | VPAC (%) |
|---|---|---|---|---|
| I | Low-grade TCC (30) | 12 (40) | 24 (80) | 25 (83.33) |
| High-grade TCC (35) | 29 (82.8) | 33 (94.2) | 33 (94.2) | |
| Total | 65 | 41 (63.07) | 57 (87.69) | 58 (89.23) |
| II | Biopsy negative for malignancy (38) | 0 | 0 | 4 (10.52) |
HPR=Histopathology report, 5-ALA=5-Aminolevulinic acid, VPAC=Vasoactive intestinal peptide and pituitary adenylate cyclase-activating peptide, TCC=Transitional cell carcinoma
The histopathology reports confirmed the presence of transitional cell carcinoma (TCC) in all the 65 patients with imaging-confirmed bladder cancer. Thirty of these tumors were of low grade and the remaining 35 were high-grade tumors.
The voided urine studies performed in the 38 patients who were previously diagnosed with TCC bladder and had no evidence of disease on cystoscopy, were also negative for malignant cells on conventional cytology and VPAC receptors [Figure 3]. However, 4 (10.52%) patients showed false-positive results in the 5-ALA-induced fluorescent urine cytology group. The sensitivity of conventional cytology, 5-ALA-induced fluorescent cytology, and VPAC receptor positivity was 63%, 87%, and 89%, respectively [Figure 4]. The specificity of conventional cytology, 5-ALA-induced fluorescent cytology, and VPAC receptor positivity was 100%, 90.47%, and 100%, respectively.
Figure 3.
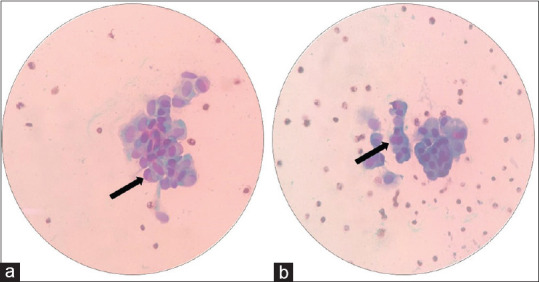
(a and b) Smears with the arrow show clusters of large round pleomorphic urothelial cells showing nuclear atypia (Papanicolaou stain, ×40)
Figure 4.
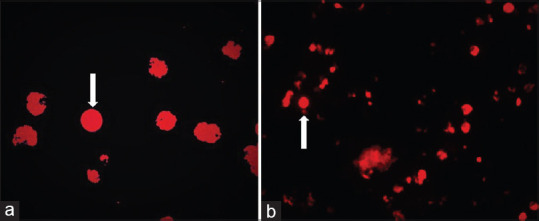
(a) The arrow shows cells with emitting faint red fluorescence suggestive of a nonmalignant cell. (b) The arrow shows cells with emitting intense red fluorescence suggestive of a malignant cell
DISCUSSION
Better understanding of the disease process, both at the cellular and the molecular levels, has paved the way for the development of several unique scientific technologies. One such technology is detecting the circulating tumor cells in blood. This liquid biopsy approach has drawn considerable attention and is advancing into clinical applications. Such approaches are based on collection of body fluids, isolation of the cells, and following up with multiplex genomic profiling and help in the identification of disease-specific fingerprints.[8,11] Molecular profiling has led the investigators to new discoveries in the field of cancer diagnosis and treatment.
The human VPAC1 receptor, named for the combined vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP) family of cell surface receptors, encodes a G-protein-coupled receptor that recognizes both VIP- and PACAP-related peptides with high affinity.[12] These VPAC1 genomic receptors are overexpressed on the surface of malignant cells at the onset of the cancer such as those of the breast, prostate, and lung.[12] Trabulsi et al. targeted VPAC receptors expressed on the malignant prostate cancer cells shed in voided urine with a specific biomolecule, TP4303, developed in their laboratory.[12] They detected VPAC-positive cells in 98.6% of the men with a diagnosis of prostate cancer (141) and none in the 10 patients with benign prostatic hyperplasia. They concluded that their preliminary data were highly encouraging and warranted further evaluation so as to develop a simple and reliable tool to detect prostate cancer noninvasively. We hypothesized that cells shed in the voided urine of patients with bladder cancer could also be imaged optically, by targeting the VPAC1 receptors with the same peptide labeled with a fluorophore.
Increased attention has also been recently focused on the use of photodynamic technology using 5-ALA as a photosensitizer to identify cancer cells in voided urine samples. The feasibility of diagnosing bladder cancer using 5-ALA was first reported in 1994[13] by Kriegmair et al. who instilled 5-ALA intravesically in 68 patients, followed by fluorescence cystoscopy with violet light from a krypton ion laser that produced fluorescence excitation. Nakai et al.[14] reported on the 5-ALA staining of urine specimens, showed that 5-ALA-induced fluorescent urine cytology was more sensitive than the conventional cytology for the detection of bladder tumors (82% vs. 49%, respectively), particularly for the low-grade and low-stage tumors, and had comparable specificity (80% vs. 100%, respectively).
Our study shows that both 5-ALA-induced fluorescent cytology and VPAC receptor positivity are highly sensitive tests to diagnose bladder cancer and show a significant difference, especially for the detection of low-grade bladder cancer, when compared to the conventional cytology. Probably, this study could be the first report on VPAC receptor positivity for the detection of bladder cancer cells in the voided urine samples, whereas, 5-ALA-induced fluorescent urine cytology has already been described.[14,15,16,17] Pytel and Schmeller[15] detected ALA-induced fluorescence in 34 of the 38 cases. Miyake et al.[16] used three different modalities of 5-ALA-induced fluorescent urine cytology to diagnose bladder cancer. They reported the overall sensitivities of conventional cytology, fluorescence cytology, fluorescent spectrophotometric assay, and cellular fluorescence analysis unit assay as 48%, 86%, 86%, and 87%, respectively. Yamamichi et al.[17] reported that the sensitivity of 5-ALA-induced fluorescent urine cytology was significantly higher than that of the conventional urine cytology (86.9% vs. 69.4%; P = 0.0002), and the specificity was equivalently high (96.2% vs. 95.6%; P = 1.0).
The VPAC genomic biomarker is known to belong to the superfamily of G-protein-coupled surface receptors, which are expressed in high density (104–105/cell) at the onset of oncogenesis, prior to the alterations in cell morphology.[18,19,20] VPAC receptors are also known to be expressed in breast and lung cancers.[18] VPAC1 receptors are minimally present on stroma, normal cells, and benign masses.[21,22,23,24] The VPAC1 receptor-specific peptide covalently binds to a fluorophore (TP4303) that allows us to optically image cancer cells but not the normal epithelial cells shed in voided urine. Our study has several limitations that include a small sample size and a single-center design. Our results need further validation so as to confirm the high diagnostic efficacy of VPAC receptor positivity for the detection of bladder cancer.
CONCLUSIONS
Our study shows that the VPAC receptors on the malignant cells can be targeted to diagnose bladder cancer, and our preliminary results confirm that the sensitivity is higher as compared to the conventional cytology.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of Interest: Madhukar L. Thakur and Leonard Gomella hold patients/have applied for patents for the product/TP4303 molecule reported in this study.
REFERENCES
- 1.Kates M, Bivalacqua TJ. Tumors of the bladder. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, editors. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia: Elsevier; 2021. p. 3073. [Google Scholar]
- 2.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Naito S, Algaba F, Babjuk M, Bryan RT, Sun YH, Valiquette L, et al. The Clinical Research Office of the Endourological Society (CROES) multicentre randomised trial of narrow band imaging–assisted transurethral resection of bladder tumour (TURBT) versus conventional white light imaging-assisted TURBT in primary non-muscle-invasive bladder cancer patients: Trial protocol and 1-year results. Eur Urol. 2016;70:506–15. doi: 10.1016/j.eururo.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 4.Planz B, Jochims E, Deix T, Caspers HP, Jakse G, Boecking A. The role of urinary cytology for detection of bladder cancer. Eur J Surg Oncol. 2005;31:304–8. doi: 10.1016/j.ejso.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Raitanen MP, Aine R, Rintala E, Kallio J, Rajala P, Juusela H, et al. Differences between local and review urinary cytology in diagnosis of bladder cancer.An interobserver multicenter analysis. Eur Urol. 2002;41:284–9. doi: 10.1016/s0302-2838(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 6.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: A systematic review. Eur Urol. 2005;47:736–48. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal DL, Wojcik EM, Kurtycz DF. The Paris System for Reporting Urinary Cytology. Cham, Switzerland: Springer International Publishing; 2016. [Google Scholar]
- 8.Layfield LJ, Elsheikh TM, Fili A, Nayar R, Shidham V Papanicolaou Society of Cytopathology. Review of the state of the art and recommendations of the Papanicolaou Society of Cytopathology for urinary cytology procedures and reporting: The Papanicolaou Society of Cytopathology Practice Guidelines Task Force. Diagn Cytopathol. 2004;30:24–30. doi: 10.1002/dc.10401. [DOI] [PubMed] [Google Scholar]
- 9.Nerli RB, Shadab R, Saziya B, Ghagane SC, Chandra S. Urinary cytology in the diagnosis of bladder cancer. Int J Cancer Sci Ther. 2021;2:5–5. doi: 10.1007/s13193-021-01340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakur ML, Zhang K, Berger A, Cavanaugh B, Kim S, Channappa C, et al. VPAC1 receptors for imaging breast cancer: A feasibility study. J Nucl Med. 2013;54:1019–25. doi: 10.2967/jnumed.112.114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hessels D, Schalken A. Urinary biomarkers for prostate cancer: A review. Asian J Androl. 2013;15:333–9. doi: 10.1038/aja.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trabulsi EJ, Tripathi SK, Gomella L, Solomides C, Wickstrom E, Thakur ML. Development of a voided urine assay for detecting prostate cancer non-invasively: A pilot study. BJU Int. 2017;119:885–95. doi: 10.1111/bju.13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–30. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 14.Kriegmair M, Baumgartner R, Knuechel R, Steinbach P, Ehsan A, Lumper W, et al. Fluorescence photodetection of neoplastic urothelial lesions following intravesical instillation of 5-aminolevulinic acid. Urology. 1994;44:836–41. doi: 10.1016/s0090-4295(94)80167-3. [DOI] [PubMed] [Google Scholar]
- 15.Pytel A, Schmeller N. New aspect of photodynamic diagnosis of bladder tumors: Fluorescence cytology. Urology. 2002;59:216–9. doi: 10.1016/s0090-4295(01)01528-x. [DOI] [PubMed] [Google Scholar]
- 16.Miyake M, Nakai Y, Anai S, Tatsumi Y, Kuwada M, Onishi S, et al. Diagnostic approach for cancer cells in urine sediments by 5-aminolevulinic acid-based photodynamic detection in bladder cancer. Cancer Sci. 2014;105:616–22. doi: 10.1111/cas.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamichi G, Nakata W, Tani M, Tsujimura G, Tsujimoto Y, Nin M, et al. High diagnostic efficacy of 5-aminolevulinic acid-induced fluorescent urine cytology for urothelial carcinoma. Int J Clin Oncol. 2019;24:1075–80. doi: 10.1007/s10147-019-01447-5. [DOI] [PubMed] [Google Scholar]
- 18.Nakai Y, Anai S, Onishi S, Masaomi K, Tatsumi Y, Miyake M, et al. Protoporphyrin IX induced by 5-aminolevulinic acid in bladder cancer cells in voided urine can be extracorporeally quantified using a spectrophotometer. Photodiagn Photodyn Ther. 2015;12:282–8. doi: 10.1016/j.pdpdt.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Reubi JC, Laderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res. 2000;60:3105–12. [PubMed] [Google Scholar]
- 20.Lelievre V, Pineau N, Waschek J. The biological significance of PACAP and PACAP receptors in human tumors from cell lines to cancer. Pituitary Adenylate Cyclase-Activating Polypeptide. In: Vaudry H, Arimura A, editors. Endocrine Updates. Vol. 20. New York, NY: Springer- Verlag; 2003. pp. 361–99. [Google Scholar]
- 21.Zia H, Hida T, Jakowlew S, Birrer M, Gozes Y, Reubi JC, et al. Breast cancer growth is inhibited by vasoactive intestinal peptide, (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res. 1996;56:3486–9. [PubMed] [Google Scholar]
- 22.Leyton J, Gozes Y, Pisegna J, Coy D, Purdom S, Casibang M, et al. PACAP(6-38) is a PACAP receptor antagonist for breast cancer cells. Breast Cancer Res Treat. 1999;56:177–86. doi: 10.1023/a:1006262611290. [DOI] [PubMed] [Google Scholar]
- 23.Valdehita A, Bajo AM, Fernandez-Martinez AB, Arenas MI, Vacas E, Valenzuela P, et al. Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cancer. Peptides. 2010;31:2035–45. doi: 10.1016/j.peptides.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Shadab R, Nerli RB, Saziya BR, Ghagane SC, Shreya C. 5-ALA-Induced Fluorescent Cytology in the Diagnosis of Bladder Cancer-a Preliminary Report. Indian Journal of Surgical Oncology. 2021 Jun;12(2):415–420. doi: 10.1007/s13193-021-01340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


