Abstract
Evidence supporting the use of glucagon-like peptide-1 (GLP-1) analogues to pharmacologically treat disorders beyond type 2 diabetes and obesity is increasing. However, little is known about how activation of the GLP-1 receptor (GLP-1R) during pregnancy affects maternal and offspring outcomes. We treated female C57Bl/6J mice prior to conception and throughout gestation with a long-lasting GLP-1R agonist, Exendin-4. While GLP-1R activation has significant effects on food and drug reward, depression, locomotor activity, and cognition in adults, we found few changes in these domains in exendin-4-exposed offspring. Repeated injections of Exendin-4 had minimal effects on the dams and may have enhanced maternal care. Offspring exposed to the drug weighed significantly more than their control counterparts during the preweaning period and demonstrated alterations in anxiety-like outcomes, which indicate a developmental role for GLP-1R modulation in the stress response that may be sex-specific.
Keywords: GLP-1, development, brain, pregnancy, anxiety, developmental programming
Introduction
As is often the case, a healthy pregnancy results in a healthy baby. Disruptions—both physical and chemical—to the uterine milieu can have adverse effects on the offspring. While women are encouraged to abstain from drugs and medication during pregnancy, this is not always feasible. Women that are taking medications for chronic illnesses, such as epilepsy, addiction, diabetes, major depressive disorder, and a number of other neuropsychiatric disorders, are encouraged to continue taking medication during this period (Alexopoulos et al., 2019; Bulletins--Obstetrics, 2008; Committee on Obstetric, 2017; Viale et al., 2015). Agonists for the glucagon-like peptide-1 receptor (GLP-1R) are FDA-approved pharmacotherapies to treat type 2 diabetes mellitus as well as obesity. Gestational diabetes and obesity are strongly associated with negative health outcomes in their offspring including metabolic dysfunction, learning disabilities and elevated neuropsychiatric symptoms (Farahvar et al., 2019; Kong et al., 2020); thus finding effective medications that are safe for the offspring’s development is an important area of investigation.
GLP-1 is an incretin hormone and neuropeptide that plays a role in energy homeostasis and feeding behavior through its cognate receptor. GLP-1 is released from the L-cells of the intestine as well as the nucleus of the solitary tract of the brain following food intake (Drucker, 2006; Hayes et al., 2010), activating receptors throughout the body prior to enzymatic degradation. Modifications of the GLP-1 peptide have produced long-lasting GLP-1R agonists, many of which are FDA-approved for the treatment of type 2 diabetes mellitus as well as obesity (Brown et al., 2018). Currently, few data exist that demonstrate GLP-1R agonists’ safety during pregnancy, either to treat type 2 or gestational diabetes (Comninos et al., 2014); insulin is the preferred method (The Medical Letter On Drugs and Therapeutics, 2019). So even though GLP-1 is secreted during pregnancy (Lencioni et al., 2011; Sukumar et al., 2018), the long-term effects of fetal GLP-1R activation on brain development are not known. A recent study administered a GLP-1R agonist for six days postnatally in mice (roughly equivalent to 3rdtrimester in human) and observed long-lasting reductions in weight gain, fat mass, and protection against diet-induced obesity (Rozo et al., 2017)
The purpose of these experiments was to determine the long-term effects of prenatal GLP-1R activation on behavior. We administered a GLP-1R agonist, exendin-4 (Ex-4), to female mice prior to conception and then throughout the prenatal period. Behavioral testing of offspring commenced in the juvenile period and into young adulthood. We hypothesized that Ex-4 exposure in utero would alter behavioral outputs related to affect and mood, reward, cognition, and motor activity.
Materials and Methods
Animals:
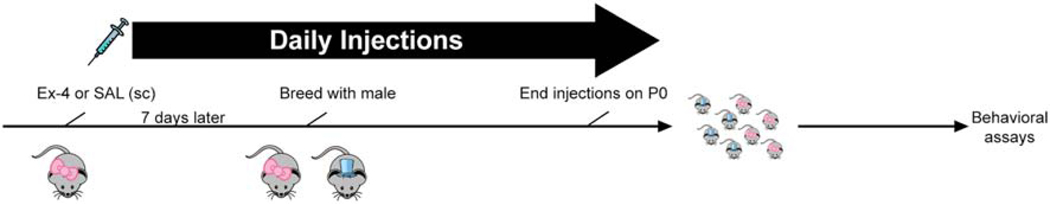
Following habituation to the vivarium for at least 7 days, female C57Bl/6J mice (6–8 weeks; Jackson Laboratories; Bar Harbor, MD) were housed 2 per cage and administered either saline or Ex-4 (10 μg/kg) once daily. Drugs were administered (sc) for approximately one week, at which time females were bred with C57Bl/6J males (6–8 weeks). Females were dosed daily until the day of birth (postnatal day (P)0). We and others have previously used this and higher doses of Ex-4 for behavioral and metabolic studies and have not noted any adverse effects (Graham et al., 2013; Mack et al., 2006; Sedman et al., 2016; Sørensen et al., 2015; Tatarkiewicz et al., 2014; Trammell et al., 2020). Females were weighed daily and observed for any adverse health concerns. Dams were single-housed prior to giving birth and were provided with rodent chow and tap water ad libitum throughout the experiment. Pups were weighed daily, and to minimize time away from the dam as well as removing individual pups over an extended period of time, litters were weighed as a whole and the average weight was calculated per litter. Offspring were weaned ~P21 and housed 2–5/cage until behavioral testing. A schematic of the paradigm is found in Fig. 1. For in situ hybridization assays, timed-pregnant C57Bl/6J mice (Jackson) were used; these mice received no treatments whatsoever.
Fig 1.
Schematic of the experimental design
All mice were housed in a temperature- and humidity-controlled AAALAC-approved facility that is maintained on a 12:12 h light:dark cycle. All experiments were performed during the light cycle, generally starting around 10 AM. All protocols were approved by the local Animal Care and Use Committee, and all studies were performed in accordance with the recommendations in the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
Behavioral assays:
To assess Ex-4’s effect on reproductive outcomes and maternal care, we recorded nest building competency and performed a pup retrieval assay in the early postnatal period to assess maternal care. Starting on ~P30, offspring of both sexes and treatments were divided into two behavioral cohorts for testing. One cohort underwent the following tests: elevated zero maze (EZM), light/dark test (L/D), sucrose preference test (SPT), novelty-induced hypophagia (NIH), locomotor activity (LMA) plus cocaine challenge (20 mg/kg, ip), overnight food intake, cocaine conditioned place preference (CPP), and LMA plus Ex-4 challenge (30 μg/kg, ip). The other cohort underwent the following battery: y-maze, spatial and novel object recognition (SNOR) test, tail suspension test (TST), overnight food intake, cocaine CPP, and LMA plus Ex-4 challenge (30 μg/kg, ip). Only one test was given per day with a minimum of 24 hrs between each assay. In the case of behavioral pharmacology studies (involving cocaine or acute Ex-4 in adulthood), at least one week was given after testing to ensure complete washout. As with previous behavioral batteries performed in our lab, tests were run in the order of least to most stressful to minimize carry-over effects of previous testing (Crawley, 2000; Graham et al., 2018; Graham et al., 2011; Moser, 2011).
Nest building assessment: On P2, we graded the state of the nest for each mouse using a previously published method (Braden et al., 2017). Scores were awarded in 0.5 increments and were as follows: 0, undisturbed; 1, disturbed, no nest; 2, flat nest; 3, cup-shaped nest; 4, incomplete dome shape; and 5, complete dome.
Pup retrieval assay: On P4, dams and pups were moved to a dimly lit testing room (9–13 lux) and habituated for at least 30 min. The dam was then removed briefly from the home cage and placed in a holding cage (~10 min). The pups were gently removed from the nesting material, and 3 pups per litter were placed throughout the cage. The remaining pups were kept in a warmed cage (~37°C) with bedding during the testing period. The dam was then placed back in the cage, and the latency to return the pups to the nesting material was recorded for up to 30 min.
EZM (Frederick et al., 2012; Graham et al., 2015a), L/D (Carpenter et al., 2012), LMA with pharmacological challenges (Frederick et al., 2012; Frederick et al., 2015; Graham et al., 2015a), y-maze (Carpenter et al., 2012; Frederick et al., 2012; Graham et al., 2015a), and TST (Graham et al., 2015b) were performed as previously described. Cocaine CPP was also performed as previously described (Graham et al., 2013) except that two conditioning sessions per day (one with 20 mg/kg cocaine, one with saline, all ip) were performed over the course of 4 days (8 conditioning sessions total), at least 4 hrs apart.
The SNOR test was modified from previously published protocols (Escamilla et al., 2017; Jaramillo et al., 2016) in order to test both spatial memory and novel object recognition. Following ~30 min habituation to the testing room, mice were habituated to the testing chamber, a round plastic tub (~35 cm diameter) with high enough walls to prevent escape and a large ―+‖ sign on side, which was fixed throughout the experiment. This session and all subsequent ones were 5 min in length; chambers and objects were cleaned with 1.6% quatricide between each mouse. The following two days, mice were placed in the chambers again which contained 3 identical objects (50 mL centrifuge tubes filled with white rocks) that were fixed in different areas. On day 4, mice were assessed for spatial memory. Here, one of the objects was moved to a different location within the chamber. On the 5th day, novel object recognition was measured, such that a novel object (a purple plastic toy rocket with a similar surface area as the tubes) replaced one of the stationary tubes; the location of the novel object did not change. Outcomes included time spent exploring each object, defined as the nose being within ~1 cm of the object.
For the SPT, mice were food- and water-deprived overnight. The following morning, mice were moved to a darkened testing room in a new cage. Following a habituation (~60 min), two pre-weighed bottles, one with water and one with 4% sucrose, were placed in the cages. To minimize any side bias, the position of the bottles was alternated between cages. After 60 min, bottles were removed and weighed, and mice were returned to their home cage with free access to chow and water.
Mice were housed singly for at least two days prior to NIH (Dulawa and Hen, 2005). For three consecutive days, water bottles were removed from cages and replaced with a serological pipet containing a premeasured amount of vanilla Ensure™. Latency to drink the milkshake and amount consumed over the 30 min test were measured each day. Mice that did not consume any milkshake during this phase were removed from the test. On the 4th and 5th day, mice tested in their home cage (Familiar) or a new cage and environment (Novel) for these outcomes, with the type of test alternated between groups. The Familiar probe test was identical to the training sessions. For the Novel test, mice were moved to a different testing room, placed in a bare cage without bedding with bright lights over each cage. Latency and consumption between Novel vs. Familiar environments were assessed.
For food intake, mice were weighed and injected with either saline or Ex-4 (30 μg/kg, ip) prior to the dark phase and given a pre-weighed amount of standard rodent chow; water was provided ad libitum. The following morning at the start of the light cycle, the weights of both the mice and the remaining food were measured.
RNAScope:
In situ hybridization was performed using WT C57Bl6/J euthanized by administering a lethal dose of sodium pentobarbital to pregnant dams and removing the fetuses. Fetuses were rapidly removed, decapitated, and frozen in 2-methylbutane cooled over dry ice. Fresh frozen tissue was stored at −80°C until sectioning, upon which they were sliced on a cryostat at 20 μm at −18–20°C and mounted on slides. Slides were again stored at −80°C until experimentation. RNAScope was performed following the guidelines provided by Advanced Cell Diagnostics (Multiplex Fluorescent v2; ACDBio, Newark, CA) and as previously described (Graham et al., 2020). RNAse-free reagents were used in all steps. The probes used for these assays were Mm-Drd1a (dopamine D1 receptor), Mm-Glp1r-C2 (GLP-1R) and Mm-Gad1-C3 (glutamic acid decarboxylase 1) probes. Gad1 and Drd1a mark distinct but somewhat overlapping populations of forebrain neurons at this age. Sections from multiple embryos (N=3–4/age group) were imaged using a Keyence Fluorescence Microscope (BZ-X700; Itasca, IL).
Statistical analyses:
All statistical analyses were performed in either GraphPad Prism 8 (GraphPad Software, San Diego, CA) or, for the offspring behavioral testing, in SAS v9.4 (SAS Institute, Cary NC), and, unless otherwise noted, all values are presented as means ± standard error of the means (SEM). Statistical significance was set at p<0.05. Fisher’s exact test was used to determine pregnancy success rate. For those dam-associated or physiological measures analyzed in Graphpad, Student’s t-tests were utilized where applicable and subjected to Sidak’s post hoc analyses, while analysis of variance tests (ANOVAs) were used where applicable in conjunction with Tukey’s post hoc analysis. Main effect variables included Sex, Treatment, and Time, where applicable. Nest building was analyzed via the non-parametric Mann-Whitney U test. For behavioral testing in individual juveniles and adults, we utilized the Proc Mixed feature in SAS. To account for intralitter likeness (―litter effects‖), litter was considered a random, nested effect. We use this approach as a best solution to implementing a litter-based design in which one can still assess valuable interindividual differences among offspring and yet use all the data and pups available (Golub and Sobin, 2020). For each test, if a sex difference was found, males and females were analyzed separately; otherwise, males and females were combined for further analyses. Outliers were removed from analyses if values met a specific criterion (mean ± 2 SD). Samples sizes for the dams were N=4–7/Treatment group; samples sizes for behavioral assays in the offspring are listed in the figure legends.
Results
Glp1r transcript levels within the embryonic brain:
We used fluorescent in situ hybridization (RNAScope) to determine if Glp1r was expressed embryonically. Glp1r expression was broadly throughout the brain, similar to what we and others have previously shown in the adult brain (Alvarez et al., 2005; Graham et al., 2020; Merchenthaler et al., 1999). Transcript was detectable in several regions associated with reward (lateral septum, striatum), energy homeostasis (hypothalamus), and cognition (hippocampus, frontal cortex/forebrain) during the developmental stage used (E18.5). A representative image of Glp1r in the lateral septum and striatum (Fig. 2A), hypothalamus (Fig. 2B), amygdala (Fig. 2C), and hippocampus (Fig. 2D) at E18.5 is shown along with probes against Drd1 (Fig. 2E-H) and Gad1 (Fig. 2I-L). Note the prominent (but not complete) co-expression of Glp1r, Gad1, and Drd1 in the subcortical ventricular zone and ganglionic eminence, but not in the developing cerebral cortex (Fig. 2M). High colocalization of all three markers is also observed in the E18.5 hypothalamus (Fig. 2N), amygdala (Fig. 2O), perirhinal cortex (Fig. 2O), and hippocampus (Fig. 2P). DAPI-counterstaining is shown in panels 2Q-2T for gross neuroanatomical reference.
Fig 2.
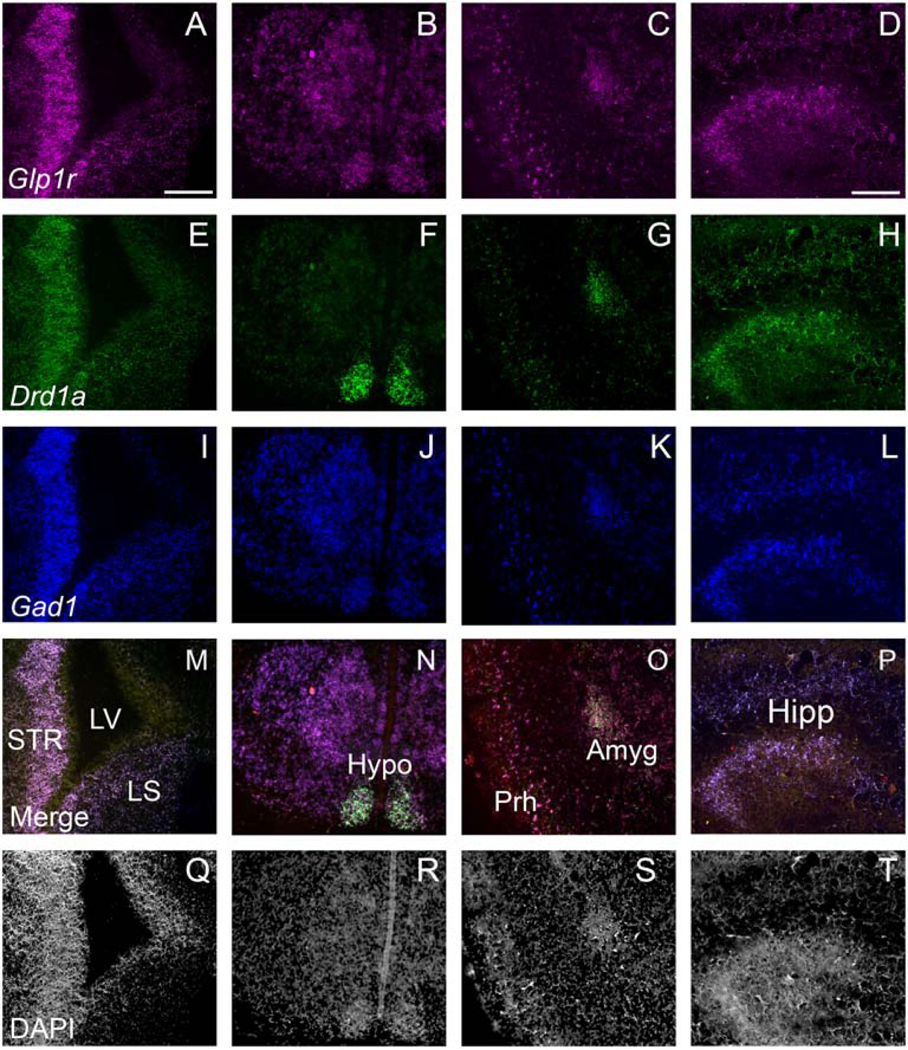
Representative images using in situ hybridization for Glp1r (magenta; A-D), Drd1a (green; E-H), Gad1 (blue; I-L), merged images (M-P), and DAPI (white; Q-T) in the forebrain (A, E, I M, Q), hypothalamus (B, F, J, N, R), amygdala (C, G, K, O, S), and hippocampus (D, H, L, P, T) at E18.5. Scale bars at 10X = 200 μm (A-C, E-G, I-K, M-O, Q-S) and at 20X = 100 μm (D, H, L, P, T). STR = striatum; LS = lateral septum; LV = lateral ventricle; Hypo = hypothalamus; Amyg = amygdala; Prh = perirhinal cortex; Hipp = hippocampus
Physiological measures and behavior of the dams:
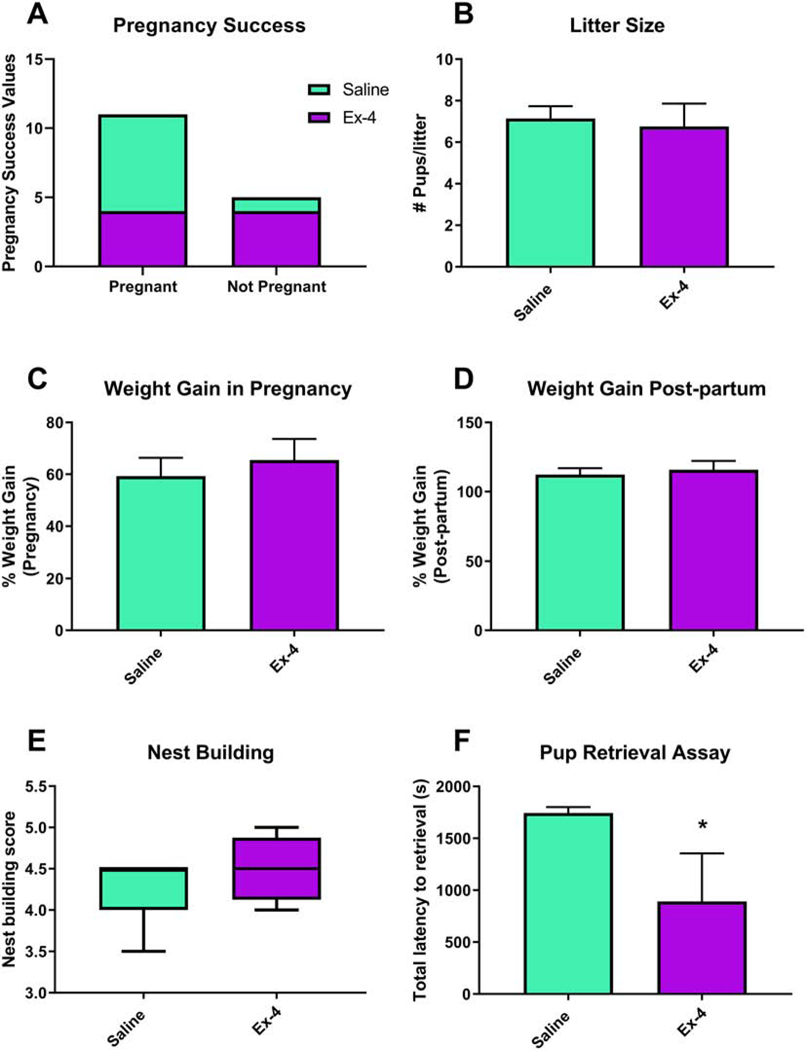
Nulliparous females were administered either saline or Ex-4 (8/treatment) prior to breeding. Only 4 Ex-4-treated dams conceived and gave birth, while 7 of the saline-treated females did so, although this rate was not significant (p=0.2821, Fig. 3A). The size of the litter did not differ between saline (7.143±0.5948) and Ex-4 (6.750±1.109) treatments (t(9)=0.3456, p=0.7376, Fig. 3B). Dams were weighed both prior to mating as well as prior to giving birth. The percent weight gain during pregnancy did not differ between the treatment groups (p=0.5930, Fig. 3C), nor did the post-partum weight change differ (p=0.6633, Fig. 3D).
Fig 3.
Dams treated with Ex-4 (10 μg/kg) throughout gestation did not differ from saline-treated dams in regards to pregnancy success (A) or size of the litter (B). There were also no differences between percent weight gain compared to baseline during (C; Saline: 59.31%±7.053%; Ex-4: 65.52%±8.087%) or after (D; Saline: 112.4%±4.585%; Ex-4: 115.9%±6.390%) pregnancy. While nest building scores did not differ between dams of the two treatment groups (E; Saline: 4.214±0.3934; Ex-4: 4.500±0.2041), Ex-4-treated dams took significantly less time to retrieve all three pups (F; Saline: 1744 sec±55.97 sec; Ex-4: 890.1 sec±464.4 sec). Values are expressed as mean ± SEM. * p<0.05
There were no significant differences in measured aspects of maternal care, as assessed by the nest building (t(9)=1.144, p=0.2821, Fig. 3E). However, Ex-4-treated dams took significantly less time to return all 3 pups to the nest during the pup retrieval assay (t(9)=2.478, p<0.05, Fig. 3F).
Offspring outcomes:
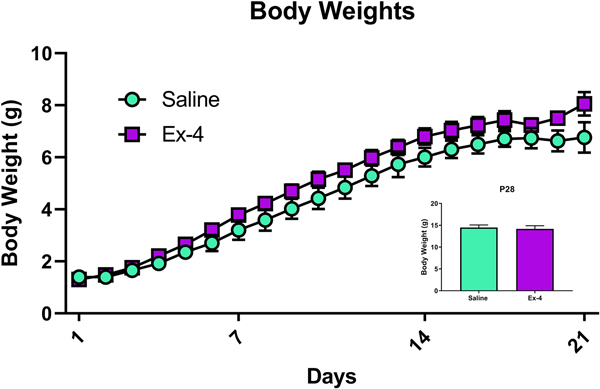
Pups were weighed daily prior to weaning and then weekly thereafter. There was a significant difference between exposed groups prior to weaning, with Ex-4-treated offspring modestly but significantly outweighing their control counterparts (Treatment: F(1, 145)=26.10, p<0.0001, Fig. 4). As expected, weights differed across time (Time: F(19, 145)=70.69, p<0.0001), although there was not a significant Treatment × Time interaction (p=0.9966). After weaning, however, there was no difference in body weight due to prenatal Ex-4 treatment (P28 shown in Fig. 4 inset; F(1, 58)=0.12, p=0.7323; weights were obtained weekly thereafter and no significant differences were observed at any post-weaning age – data not shown).
Fig 4.
Offspring treated in utero with Ex-4 (10 μg/kg administered to dam) weighed significantly more than their control counterparts (Treatment and Time main effects, **** p<0.0001).Ex-4 treatment did not alter weights post-weaning (inset showing mean±SEM on P28; Saline: 14.4927 g±0.5891 g; Ex-4: 14.1714 g±0.7257).
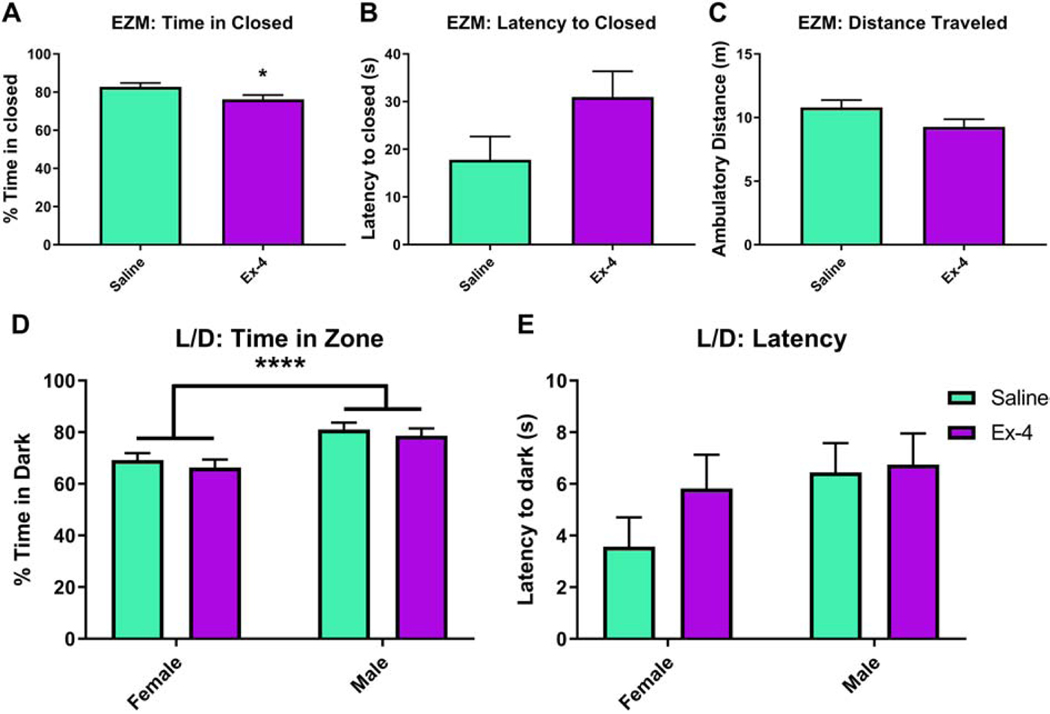
In the EZM, males did not perform differently from females in any of the outcomes examined. Ex-4-exposed mice spent significantly less time in the closed quadrants relative to controls (F(1, 18)=4.69, p=0.044, Fig. 5A). There were trends to increases in the Ex-4-exposed mice in regards to the latency to first enter the closed quadrants (F(1, 19)=3.29, p=0.0855, Fig. 5B) and in total distance traveled (F(1, 18)=3.38, p=0.0827, Fig. 5C), but these did not reach statistical significance. In another test of anxiety-like behavior, L/D, there was a significant effect of Sex, with males spending more time in the dark chamber (F(1, 17)=18.08, p=0.005), but prenatal treatment did not alter this outcome (F(1, 17)=0.89, p=0.3583). Regardless, there were no Treatment × Sex interactions in time spent in the dark chamber (F(1, 17)=0.01, p=0.9293, Fig. 5D). There were no effects of Sex (F(1, 17)=2.51, p=0.1312), Treatment (F(1, 17)=1.13, p=0.3029), or Sex × Treatment (F(1,17)=0.66, p=0.4275) in regards to latency to enter the dark chamber (Fig. 5E).
Fig 5.
Ex-4-exposed offspring spent significantly less time in the closed area (A) of the EZM than saline-treated mice (76.2308%±2.2053% vs. 82.7556%±2.053%, respectively). There were no statistically significant differences in latency to enter the closed areas (B; Saline: 17.8188 sec±4.8501 sec; Ex-4: 30.9615 sec±5.3807 sec) or distance traveled (C; Saline: 10.7958 m±0.5681 m; Ex-4: 9.2638 m±0.6103 m). In the light/dark test, there were no differences between treatment groups in either time spent in the dark (D; Saline: 75.1234%±1.9033%; Ex-4: 72.4347%±2.1178%) or latency to enter the dark side of the chamber (E; Saline: 5.0063 sec±0.8012 sec; Ex-4: 6.2798 sec±0.8915 sec). However, males (79.8328%±1.9701%) did spend more time in the dark area compared to females (67.7253%±2.0558%). N=13–16/Treatment group (EZM); N=6–8/Treatment × Sex group (L/D); * p<0.05, **** p<0.0001
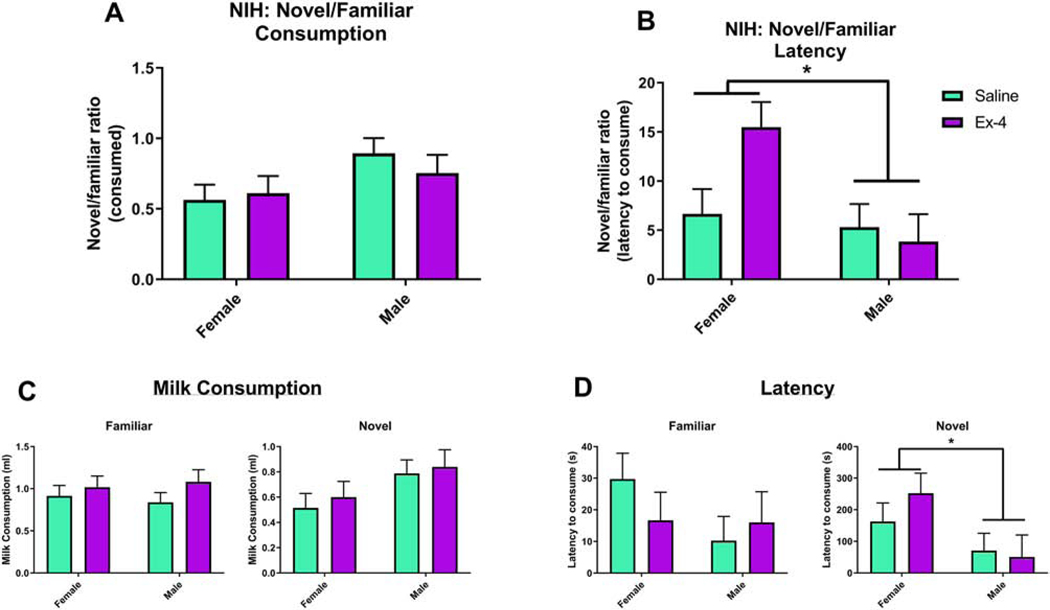
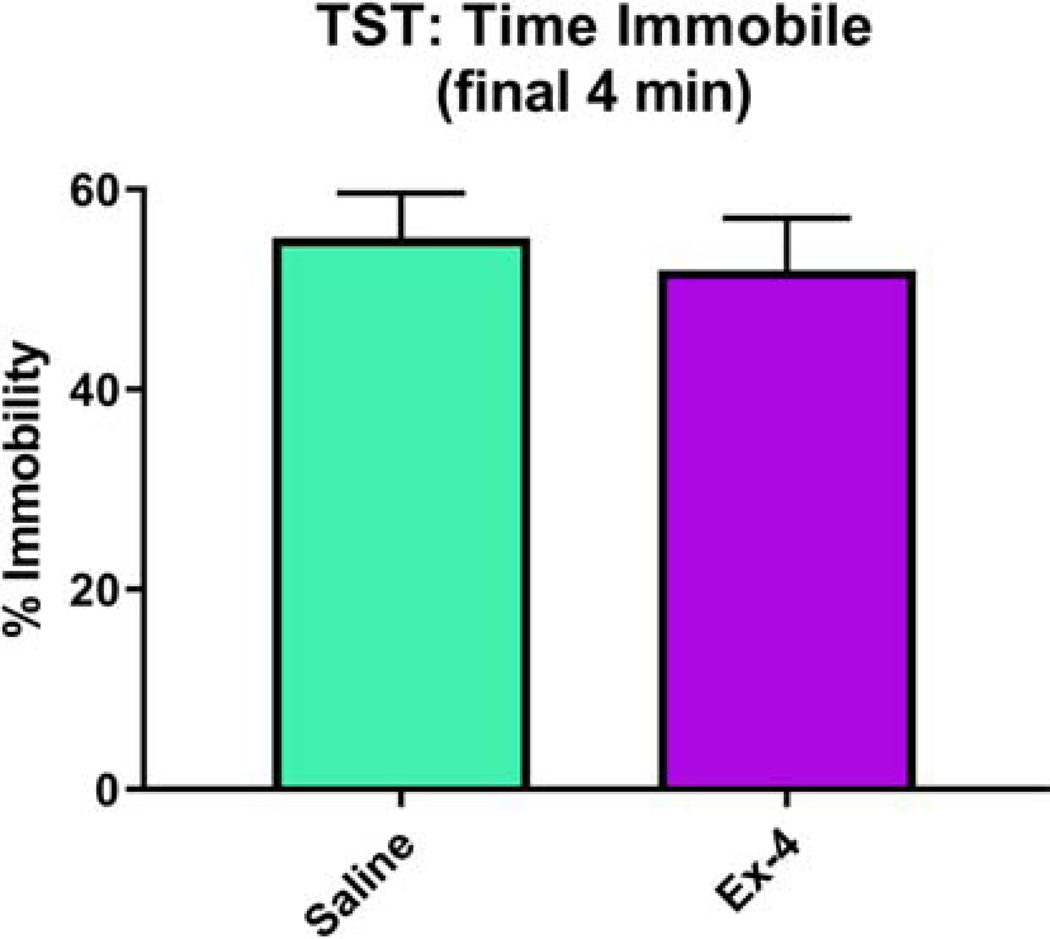
We also performed NIH, which again assesses components of anxiety-like behavior as mice must approach and consume a rewarding substance in a novel, stressful environment. When comparing outcomes from the Novel environment relative to the Familiar one, males consumed more milkshake, albeit not significantly more, than females (Sex: F(1, 13)=4.49, p=0.054; Males: 0.8244±0.08398, Females: 0.5879±0.08108), although there was no significant prenatal treatment (F(1, 13)=0.14, p=0.7125) nor interaction (F(1,13, 0.7, p=0.4172; Saline: 0.729±0.07992; Ex-4: 0.6832±0.09164) effects (Fig. 6A). When assessing the latency to consume the milkshake in the novel vs. the familiar environment, there was a significant effect of Sex (F(1, 12)=6.46, p=0.0259; Males: 4.5746±1.8217, Females: 11.0761±1.7962), such that females took significantly more time in the Novel environment to consume the milkshake than males (Fig. 6B). There was no overall effect of Treatment (F(1,12)=2.08, p=0.1753; Saline: 5.9826±1.7308; Ex-4: 9.6681±1.8838), nor was there a significant Treatment × Sex interaction (F(1, 12)=4.07, p=0.0667),. Outcomes for milkshake consumption and latency in both environments are also shown (Fig. 6C-D, respectively). For the TST, which is an assessment of depressive-like behavior, we analyzed both the full 6 min test as well as the final 4 min phase. Here, we present data from the latter analysis, as the first 2 min of the test are referred to as a habituation period (Sanna et al., 2017). The time spent immobile did not differ between treatment groups (F(1, 18)=0.23, p=0.6387; Fig. 7). We note, however, that the analysis of the full 6 min session also showed no difference between the groups (data not shown)
Fig 6.
For the NIH task, there was no significant effect of Sex or Treatment in the novel environment compared to the familiar environment, (A; Female Saline: 0.564±0.1081, Male Saline: 0.8941±0.1081, Female Ex-4: 0.6118±0.1208, Male Ex-4: 0.7547±0.1285). However, females took significantly more time to consume the milkshake in the novel versus the familiar environment compared to males (B; Female Saline: 6.6537±2.5402, Male Saline: 5.3115±2.3517, Female Ex-4: 15.4986±2.5402, Male Ex-4: 3.8377±2.7826). Graphs of the raw values for milkshake consumption (C) and latency (D) in the familiar and novel environments are also shown. N=5–8/Treatment × Sex group; * p<0.05.
Fig 7.
Ex-4 administration in utero (10 μg/kg administered to dam) did not alter immobility time during the tail suspension test (Saline: 55.1968%±4.4074%; Ex-4: 51.9382%±5.2079%). N=13–15/Treatment group
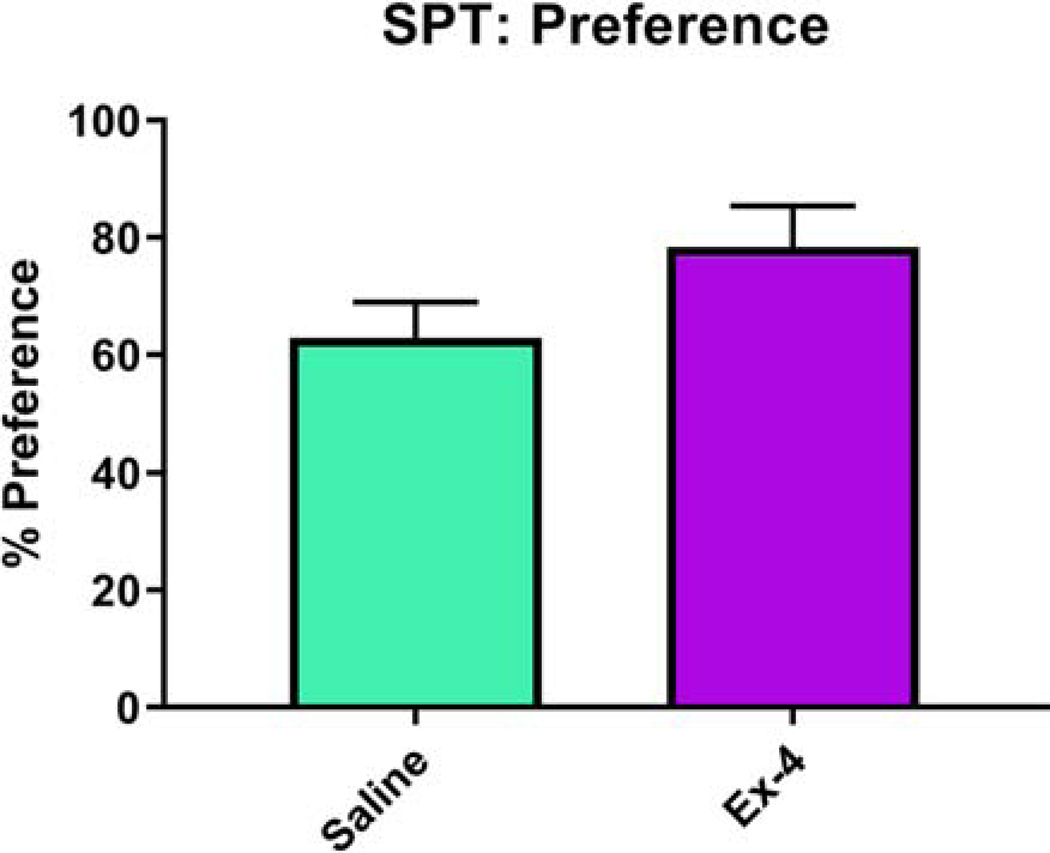
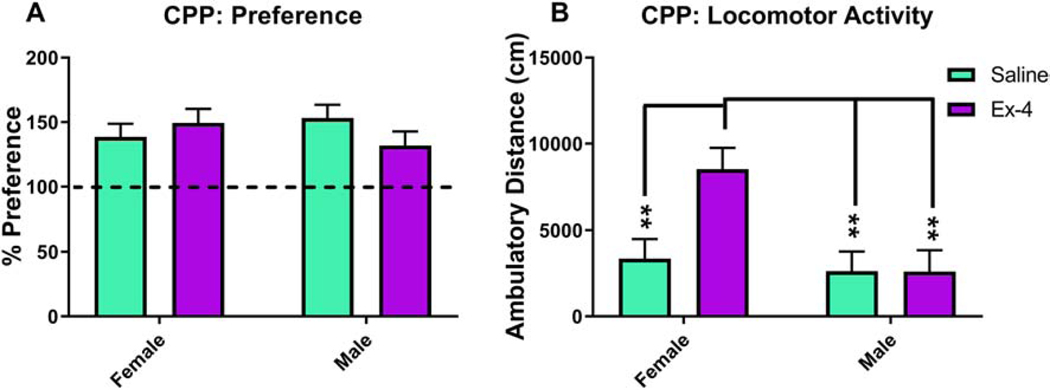
We administered a number of tests of reward-related outcomes and psychostimulant drug responses. Mice exposed to Ex-4 in utero consumed more sucrose compared to controls, but this value did not reach the level of significance (F(1, 18)=2.84, p=0.109, Fig. 8). We have previously demonstrated that in adult mice, Ex-4 administration decreases cocaine reward with little effect on cocaine-induced locomotor activity (Graham et al., 2013). Here, prenatal Ex-4 exposure did not alter place preference in mice (F(1, 20)=0.22, p= 0.6466, Fig. 9A). Interestingly, there was a non-significant effect of Treatment (F(1,18)=4.16, p=0.0562), yet a significant effect of Sex (F(1,18)=8.73, p=0.0085) and a corresponding interaction (F(1,18)=5.32, p=0.0332), in regards to locomotor activity, such that Ex-4-treated females were significantly more hyperactive relative to all other groups during the probe session (Fig. 9B).
Fig 8.
No changes to sucrose preference resulted from prenatal GLP-1R activation (Saline: 62.9129%±6.0131%; Ex-4 (10 μg/kg): 78.3996%±6.9434%). N=12–16/Treatment group
Fig 9.
No alterations in cocaine place preference were observed in Ex-4-exposed mice (10 μg/kg administered to dam) (A; Saline: 145.88%±7.1887%; Ex-4: 140.61%±7.6851%), although Ex-4-treated females (8514.07 cm±1244.56 cm) were significantly more hyperactive than all other groups (B; Ex-4 males: 2603.37 cm±1244.56 cm; Saline females: 3354.8 cm±1139.11 cm; Saline males: 2625.95 cm±1139.11 cm). N=14–16/Treatment group; ** p<0.01
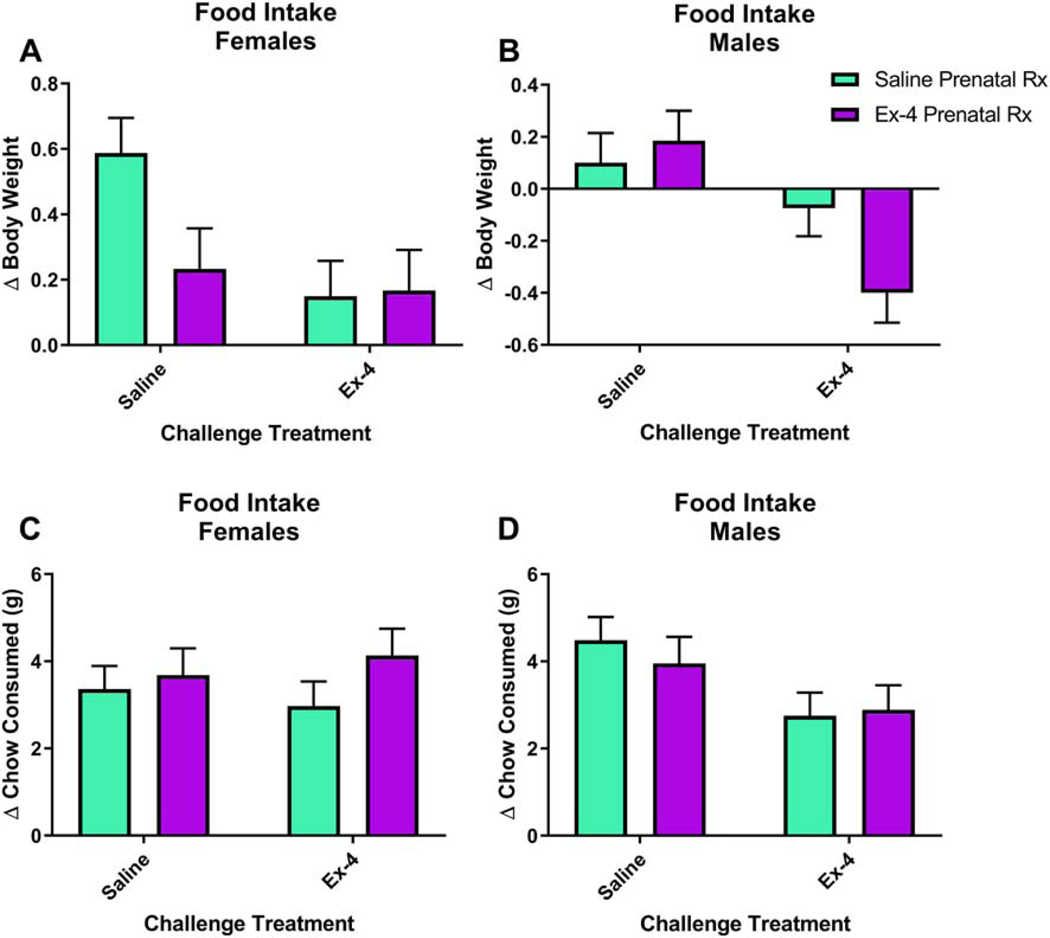
Just as GLP-1R activation decreases drug reward, others have shown that it also decreases food reward and intake (Dickson et al., 2012; Dossat et al., 2011). It is not known if an early developmental exposure to a GLP-1R agonist will alter consumption. We administered a challenge injection of either saline or 30 μg/kg Ex-4 prior to the start of the ―lights out.‖ Body weights and the amount of chow in each cage were measured at the time of the injection and then in the morning (start of ―lights on‖ phase). There were significant alterations in change in body weight based on Sex (F(1,41)=15.26, p=0.0003) and Challenge Treatment (F(1, 41)=16.79, p=0.0002), a non-significant trend related to Prenatal Treatment (F(1, 41)=3.17, p=0.0823), and a corresponding significant interaction of Prenatal Treatment × Sex × Challenge Treatment (F(1, 41)=5.83, p=0.0203; Fig. 10A-B). In females, prenatal Ex-4 treatment and/or the Ex-4 challenge altered body weights relative to saline controls, while males treated with Ex-4 in both situations altered body weights relative to all other groups (See Table 1 for statistically significant comparisons). On the contrary, there were no significant alterations in regards to changes in chow consumed related to Sex (F(1,40)=0, p=0.9621) or Prenatal Treatment (F(1, 40)=0.45, p=0.5082) yet a non-significant trend related to Challenge Treatment (F(1, 40)=2.87, p=0.098; Fig. 10C-D). Values for each group can be found in Table 2.
Fig 10.
Ex-4 administration, both in utero and acutely in adulthood, alters body weight (A-B), but not food consumption (C-D), in both sexes. For a full list of significant effects and for the values for individual groups, please refer to Tables 1 and 2, respectively. N=6–8/Prenatal treatment × Acute treatment/group. Prenatal treatment consisted of 10 μg/kg Ex-4 administered to dam and acute treatment in adulthood was 30 μg/kg.
Table 1:
Significant interactions in body weight changes following overnight food intake
| Group 1 (Prenatal Rx × Sex × Challenge Rx) | Group 2 (Prenatal Rx × Sex × Challenge Rx) | P-value |
|---|---|---|
| Ex-4 × Female × 30Ex-4 | Ex-4 × Male × 30Ex-4 | 0.0017** |
| Ex-4 × Female × 30Ex-4 | Sal × Female × Sal | 0.014* |
| Sal × Female × 30Ex-4 | Ex-4 × Male × 30Ex-4 | 0.0011** |
| Sal × Female × 30Ex-4 | Sal × Female × Sal | 0.0063** |
| Ex-4 × Male × 30Ex-4 | Sal × Male × 30Ex-4 | 0.045* |
| Ex-4 × Male × 30Ex-4 | Ex-4 × Female × Sal | 0.0005*** |
| Ex-4 × Male × 30Ex-4 | Sal × Female × Sal | <.0001**** |
| Ex-4 × Male × 30Ex-4 | Ex-4 × Male × Sal | 0.0008*** |
| Ex-4 × Male × 30Ex-4 | Sal × Male × Sal | 0.0037** |
| Sal × Male × 30Ex-4 | Sal × Female × Sal | <.0001**** |
| Ex-4 × Female × Sal | Sal × Female × Sal | 0.0367* |
| Sal × Female × Sal | Ex-4 × Male × Sal | 0.0144* |
| Sal × Female × Sal | Sal × Male × Sal | 0.0035** |
p<0.05,
p<0.01,
p<0.001,
p<0.0001
Table 2: Changes in body weight and chow consumed following acute Ex-4 administration.
All values are calculated as (initial value – end value) and are expressed as mean±SEM.
| Prenatal Rx × Sex × Challenge Rx | Δ Body Weight (g) | Δ Chow Consumed (g) |
|---|---|---|
| Sal × Male × Sal | 0.10±0.12 | 4.49±0.53 |
| Sal × Female × Sal | 0.59±0.11 | 3.36±0.53 |
| Sal × Male × 30Ex-4 | −0.08±0.11 | 2.75±0.53 |
| Sal × Female × 30Ex-4 | 0.15±0.11 | 2.97±0.57 |
| Ex-4 × Male × Sal | 0.19±0.12 | 3.95±0.61 |
| Ex-4 × Female × Sal | 0.23±0.12 | 3.68±0.61 |
| Ex-4 × Male × 30Ex-4 | −0.40±0.12 | 2.89±0.57 |
| Ex-4 × Female × 30Ex-4 | 0.17±0.12 | 4.13±0.61 |
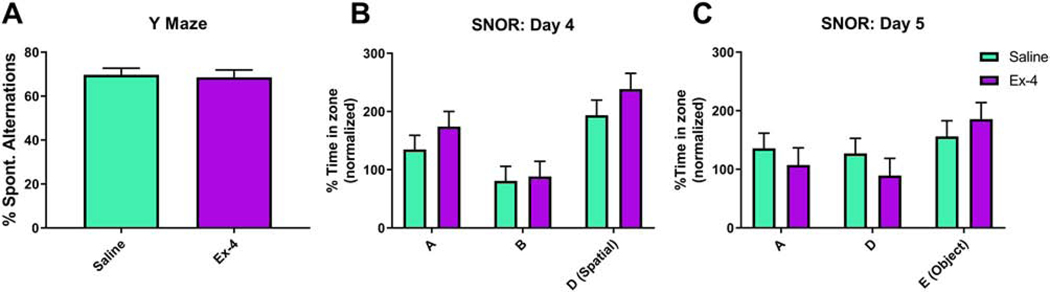
Mice underwent a number of cognitive tests as well. Mice of both treatments performed equally well during the y-maze, such that there was no significant difference in the percent spontaneous alternations (F(1, 18)=0.07, p=0.7993; Fig. 11A). Similar results were found in the SNOR. On Day 4, when one object was moved to a different location (spatial phase), all mice spent more time with the new Location compared to the time spent with that object in the previous location the day before (Object: F(2, 38.5)=15.56, p<0.001; Fig. 11B). Neither a main effect of prenatal Treatment (F(1, 5.68)=1.63, p=0.252) nor a corresponding Treatment × Object interaction (F(2, 38.5)=0.38, p=0.6848) were observed. A similar trend was seen on Day 5 (Object: F(2, 39.2)=4.07, p=0.0247; Treatment: F(1, 6.49)=0.18, p=0.683; Treatment × Object: F(2, 39.2)=1.22, p=0.3072; Fig. 11C; novel object phase).
Fig 11.
Mice exposed to Ex-4 prenatally did not perform differently from their control counterparts in the y-maze, as determined by the percent of spontaneous alternations (A; Saline: 69.6216%±3.1719%; Ex-4: 68.4067%±3.618%). Prenatal GLP-1R activation had no effect in the SNOR test in either the time spent exploring the familiar object in a new location (B) or a new object in a familiar location (C). Data are expressed as a percentage of the time spent exploring the object/location relative to that same location/object from the prior day. N=14–16/Treatment group
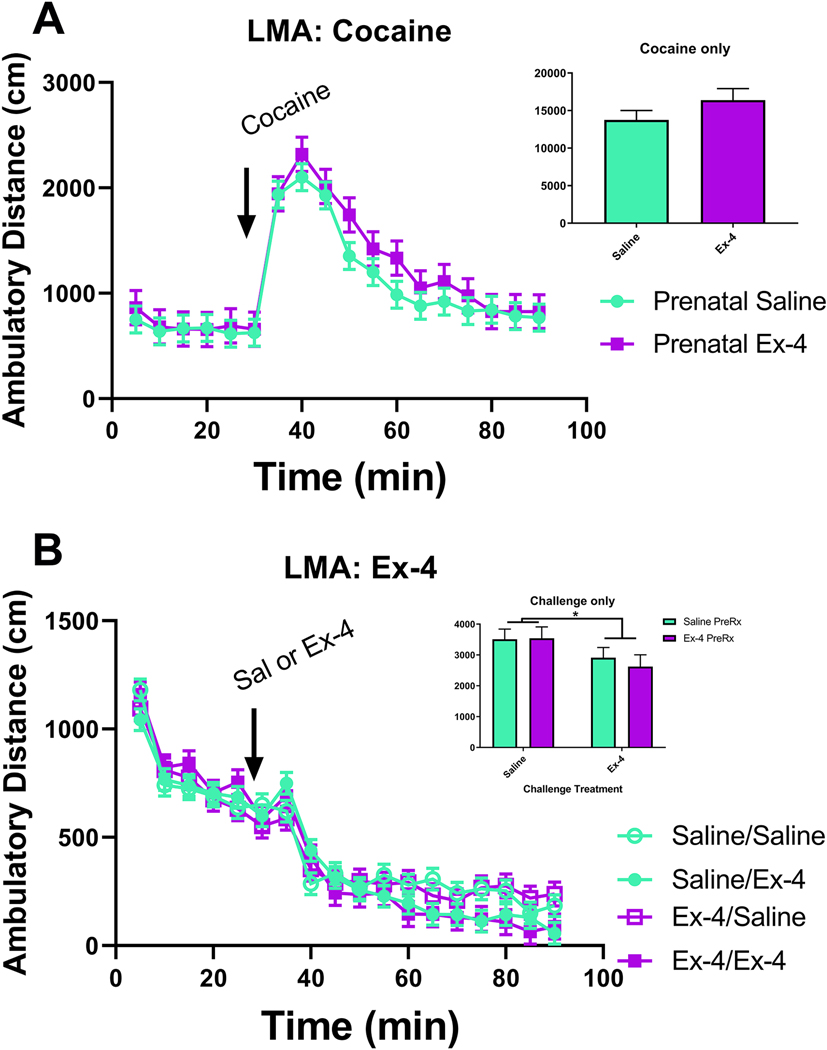
Finally, we tested locomotor activity in response to a pharmacological challenge. We found no differences in cocaine-induced locomotor activity due to the prenatal treatments (F(1, 52.5)=1.26, p=0.2668, Fig. 12A). This finding held true even when taking into account total distance traveled after cocaine administration (F(1, 16)=1.77, p=0.2024; Fig. 12A inset). Similarly, when a different cohort of mice were injected with either saline or Ex-4 (30 μg/kg, ip) in lieu of cocaine, there were no significant effects of either prenatal treatment (F(1, 55.3)=0, p=0.9935), challenge treatment (F(1, 55.3)=1.08, p=0.3026), or an interaction of the two treatments (F(1, 55.3)=0.01, p=0.9399) on overall activity levels (Fig. 12B). However, when examining activity following the challenges, the Ex-4 challenge did appear to induce some hypoactivity (F(1, 45)=4.6, p=0.0373; Fig. 12B inset), with no significant changes resulting from the in utero exposures (F(1, 45)=0.13, p=0.7192) or the Prenatal × Challenge Treatment interaction (F(1, 45)=0.21, p=0.6474).
Fig 12.
Testing for changes in cocaine-induced hyperactivity, no changes were observed between either prenatal treatment group over time (A) or when accounting for total distance traveled following cocaine administration (A inset; Saline: 13,766 cm±1247.95 cm; Ex-4: 16,389 cm±1528.42 cm). Repeated prenatal GLP-1R activation (10 μg/kg administered to dam) did not alter locomotor activity following a challenge treatment with 30 μg/kg Ex-4 (B). However, mice treated with the acute challenge of Ex-4 (30 μg/kg) were significantly hypoactive compared to mice receiving the vehicle challenge, regardless of prenatal exposure (B inset). N=12–16/Treatment group (cocaine), N=13–16/Prenatal treatment × Acute Treatment group (Ex-4); * p<0.05
Discussion
GLP-1R activation has long been associated with a number of consequences in clinical and preclinical settings, including insulin secretion, enhanced satiety, the lessening of food and drug reward, enhancing ovarian function in polycystic ovary syndrome (PCOS) (Nylander et al., 2017), protection against traumatic brain injury (Eakin et al., 2013; Rachmany et al., 2013; Tweedie et al., 2013; Tweedie et al., 2016), and potential treating both Alzheimer’s and Parkinson’s diseases (Athauda and Foltynie, 2016; Glotfelty et al., 2020; Holscher, 2018; Kim et al., 2017). However, its role in brain development is not as well delineated. We sought to determine what these long-term functional consequences were following repeated prenatal exposure to a GLP-1R agonist, Ex-4. We selected a dose (10 μg/kg) that has been used by multiple previous groups that produces mild-to-moderate function effects on metabolic functions and CNS modulation of incretin-related phenotypes, including our own group (Graham et al., 2013; Mack et al., 2006; Sedman et al., 2016; Sørensen et al., 2015; Tatarkiewicz et al., 2014). The 30 μg/kg acute dose for adult behavioral pharmacology studies has been used in previous behavioral pharmacology studies by our lab and others for both CPP and locomotor studies, among others (Erreger et al., 2012; Graham et al., 2013; Mack et al., 2006; Sedman et al., 2016; Trammell et al., 2020).
We show here that Glp1r mRNA is expressed embryonically (Fig. 2) in both GABAergic (Gad1+) and dopaminoceptive (Drd1+) populations of developing neurons. Expression Glp1r in the adult brain also often co-localizes with these markers (Graham et al., 2020), however the degree of overlap appears even greater at E18.5. There are also some Glp1r+ cells that do not express these markers, suggesting that the receptor is expressed by diverse subpopulations of developing brain cells. While it is not clear if the GLP-1 peptide is released by the fetus, we do know that adequate maternal GLP-1 secretion is necessary for a healthy pregnancy (Lencioni et al., 2011; Sukumar et al., 2018), so GLP-1 from maternal sources may cross the placenta and play a role in proper fetal development. According to a recent Medical Letter, insulin is usually prescribed to treat type 2 diabetes in pregnant women as it does not cross the placenta (The Medical Letter On Drugs and Therapeutics, 2019). It is unlikely that the endogenous peptide would cross the placenta, as it contains the dipeptidyl peptidase-4 (DPP-4) catalytic enzyme (Kandzija et al., 2019), but there is evidence that other GLP-1R agonists do cross the placenta and can be beneficial (Younes et al., 2020). Embryonic GLP-1R is likely to be functional given that several protein interactors that alter GLP-1R-induced signaling properties have been identified from a fetal brain library (Huang et al., 2013).
Overall, repeated exposure to Ex-4 had minimal effects on pregnancy outcomes. While fewer Ex-4-treated dams successfully conceived, these data were not significant (Fig. 3A). Clinical studies demonstrate that GLP-1R agonists increase the likelihood of pregnancy in women suffering from PCOS or obesity (Liu et al., 2017; Salamun et al., 2018), a mechanism mediated in part by the forkhead box protein O1 (FoxO1) protein (Sun et al., 2020). However, under conditions in which there is no underlying comorbidity, any benefits of treatment may be lost. It is unclear at this time whether GLP-1 analogues have a notable effect on reproductive outcomes (Comninos et al., 2014). Preclinical data suggest that chronic GLP-1, but not Ex-4, synchronizes pubertal onset in female rodents (Outeirino-Iglesias et al., 2015), while male and female GLP-1R knockout mice show delays in pubertal and gonadal development (MacLusky et al., 2000). Our results suggest that Ex-4 administration during pregnancy does not have a significant effect on reproductive success, but given the low number of total litters produced in this initial study, these data are not conclusive. We note, however, that we observed a trend to decreased reproductive success in mice receiving repeated exendin-4 prior to possible conception; we recommend further investigation of this issue in the future.
Body weights of dams did not differ during or after pregnancy, while the offspring prenatally exposed to Ex-4 weighed significantly more than their control counterparts, prior to weaning (Fig. 3C-D, Fig. 4). These findings were somewhat surprising given that GLP-1R activation induces weight loss and is used as an FDA-approved treatment for obesity (Brown et al., 2018). The inability to gain weight or not gain enough weight during pregnancy can be detrimental to maternal health and fetal development (Institute of Medicine and National Research Council Committee to Reexamine I. O. M. Pregnancy Weight Guidelines, 2009), so these data suggest that Ex-4 may be safe to use during this time. However, increased weight gain in offspring—albeit mild—is not ideal given the elevated incidence of obesity in children in the developed world (Kumar and Kelly, 2017). However, this weight gain may not necessarily indicate elevated brown adipose tissue, as GLP-1R activation has been shown to decrease adiposity and increase brown adipose tissue thermogenesis (Lee et al., 2018). Further assessment of muscle and adipose composition in the offspring is necessary to determine the root of the elevated weight. From a behavioral standpoint, dams treated with Ex-4 during pregnancy more rapidly returned the pups to the nest during the pup retrieval test (Fig. 3F). It is not clear why this occurred, perhaps due to enhanced perseverance or increased attention in treated dams. This may also contribute to the higher weights pre-weaning in the Ex-4-exposed offspring, if they were given a greater opportunity to nurse. Regardless, improvement of maternal mental health in the ―fourth trimester‖ has been shown to be enhance outcomes for both the mother and child and may mitigate post-partum depression (Werner et al., 2016).
In the offspring, alterations in neither cognitive function, ingestive behavior, nor reward behavior were found following prenatal GLP-1R activation (Figs. 7–11). We and others have demonstrated that GLP-1R agonists lessen the rewarding effects of drugs of abuse (Egecioglu et al., 2013a, b; Egecioglu et al., 2013c; Graham et al., 2013; Sørensen et al., 2015) and food reward and intake (Dickson et al., 2012; Dossat et al., 2011) in adult animals. Similarly, GLP-1R activation or overexpression significantly improves cognitive function (During et al., 2003; Isacson et al., 2011; Iwai et al., 2014; Iwai et al., 2009; Trammell et al., 2020). Again, this lack of any significant effects demonstrates the safety profile of GLP-1R agonist use during pregnancy. However, the tests used in this study (CPP, sucrose preference, SNOR, y-maze) are general screens to detect blunt changes in reward processing and/or memory; specialized tasks, such as operant conditioning (e.g., self-administration for reward outcomes) or more challenging, cognitively intensive tasks (e.g., 5-choice serial reaction time task/5-choice continuous performance task or pairwise discrimination) may tease out more subtle changes in reward behavior or cognitive function.
We and others have previously shown that GLP-1R activation produces significant modulation of cognitive and reward activities after adult administration (During et al., 2003; Egecioglu et al., 2013a, b; Egecioglu et al., 2013c; Graham et al., 2013; Isacson et al., 2011; Iwai et al., 2014; Iwai et al., 2009; Reddy et al., 2016; Sørensen et al., 2015; Trammell et al., 2020), but few changes were found in these areas during prenatal exposure. However, we did uncover alterations in tests related to mood following exposure during early development. Administration of GLP-1 and its analogues to adult animals is often anxiogenic (Gil-Lozano et al., 2010; Gulec et al., 2010; Kinzig et al., 2003; Moller et al., 2002), a mechanism mediated in part at the central nucleus of the amygdala (Kinzig et al., 2003; Moller et al., 2002). No significant effects were found in the light/dark test (Fig. 5D-E), yet prenatal Ex-4 treatment resulted in differential anxiety-like phenotypes in the EZM, with mice spending less time in the closed areas and an increased latency to enter them (Fig. 5A-C, anxiolytic effect), and NIH, such that Ex-4-treated females took significantly more time to initially consume the milkshake in the novel environment (Fig. 6, anxiogenic effect). The decreased anxiety-like outcome in the EZM is contradictory to what we see in adult animals, but this indicates a GLP-1R-induced change in circuits related to these behaviors. The exaggerated anxiety-like effect in Ex-4-exposed females suggests that GLP-1R activation and downstream signaling are not uniform processes between males and females. It is known that processes related to anxiety differ between the sexes, including within the CeA (Engman et al., 2016; Logrip et al., 2017; Salvatore et al., 2018), where GLP-1R is expressed (Cork et al., 2015; Graham et al., 2020) and serves an epicenter for its anxiogenic effects (Kinzig et al., 2003; Moller et al., 2002). GLP-1 and its receptor also play a role in modulating the stress response via the HPA axis (Ghosal et al., 2013; Ghosal et al., 2017; Kinzig et al., 2003; Zhang et al., 2010). Furthermore, while we noted that Ex-4 treatment had no significant overall effect on food intake (10C-D), Ex-4 treatment at some point during the study (prenatally and/or postnatally) significantly decreased body weights in females (Fig. 10A) in our food intake study. Similarly, males treated both pre- and postnatally with Ex-4 demonstrated a significant decrease in body weight relative to all other male treatment groups (Fig. 10B). Nevertheless, based on previous data from our lab and others, it was surprising that male mice did not show a significant reduction in food intake following acute 30 μg/kg Ex-4 in adulthood. This suggests reduced responsiveness to GLP-1R activation even in the saline-exposed male mice, although this could also reflect a type II error in the current dataset. All mice underwent a mild stressor (i.e., the ip injection) prior to the experiment, suggesting that Ex-4 treatment may produce a stress-induced change in body weight, although we also noted an increase in overall basal body weights following prenatal exposure (Fig. 4). These data as a whole suggest that prenatal GLP-1R activation alters anxiety-like outcomes and may work in a sex-specific manner. There were also significant effects of sex observed in the NIH-assay, with male mice (regardless of prenatal treatment) showing blunted responses as compared to females.
While our paradigm altered anxiety-like behaviors in the offspring, we did not observe any changes in depression-like or anhedonic behaviors as measured by the TST (Fig. 7) and SPT (Fig. 8), respectively. This was contradictory to that seen following adult administration given the role of GLP-1R activation in producing anti-depressant-like phenotypes. In adult naïve (Anderberg et al., 2016; Isacson et al., 2011), diabetic (Komsuoglu Celikyurt et al., 2014), and antipsychotic-treated (Sharma et al., 2015) rodents, GLP-1 analogs decreased immobility time in the forced swim test. GLP-1R activation also induces neurogenesis, specifically in the dentate gyrus of the hippocampus, which is another hallmark of an antidepressant-like phenotype (Belsham et al., 2009; Bertilsson et al., 2008; Hamilton et al., 2011; Hunter and Holscher, 2012; Tweedie et al., 2013). As neural development is a significant period for both neurogenesis and apoptosis (Stiles and Jernigan, 2010), the neurogenic effects of GLP-1R activation may be severely diluted. Further investigation into this concept is needed.
Finally, we noted that prenatal Ex-4 treatment did not alter cocaine-induced locomotion (Fig. 12A), nor did it alter Ex-4-induced locomotor activity (Fig. 12B). A challenge treatment of Ex-4 in adulthood did, however, induce hypoactivity regardless of prenatal exposure, as we and others have shown (Dickson et al., 2012; Erreger et al., 2012; Trammell et al., 2020; Turton et al., 1996) while the inverse is seen following administration of a GLP-1R antagonist (Knauf et al., 2008). We did, however, note a hyperactive response in females prenatally treated with Ex-4 during the final CPP test session (Fig. 9B). Despite its use as a test of drug reward, CPP is in actuality a test of Pavlovian conditioning that depends on the animal’s ability to learn and associate the presentation of a conditioned stimulus (CS, distinct chamber) with an unconditioned (and in this case, pleasurable) stimulus (US, cocaine) (Cunningham et al., 2006). One could hypothesize that when placed into the chamber (CS) in which they had previously associated with cocaine (US), Ex-4-treated females may have learned this association better than the other groups and increased their locomotor activity in response to the anticipatory exposure to the US. As previously mentioned, more complex motivational and learning and memory tests are necessary to understand these Ex-4-induced changes.
While these data demonstrate that developmental GLP-1R activation alters some long-term phenotypes, there are caveats. Our paradigm utilized prenatal exposure only, which corresponds to approximately first and part of the second trimester of human brain development (Clancy et al., 2001; Clancy et al., 2007a; Clancy et al., 2007b; Rice and Barone, 2000; Ross et al., 2015); therefore, exposure covered only very early brain development and the entirety of human gestation was not examined. However, inclusion of the early postnatal period in rodents for drug exposure omits the transplacental transfer that is inherent in human fetal drug exposure. It is not yet clear if there is a critical period(s) of exposure in regards to developmental GLP-1R activation – there likely are one of more sensitive periods for GLP-1R modulation of developing neural circuits and resultant behaviors and future studies should examine this important issue. For example, a recent paper showed that Ex-4 administration in the early postnatal period (P0–6, equivalent to late 2nd-early 3rd trimester brain development) alters long-term metabolic outcomes, including resistance to obesity in adulthood and altering the fiber density of orexigenic (decreased) and anorexigenic (increased) peptides within the hypothalamus (Rozo et al., 2017). Interestingly, it is suggested that there is a biphasic increase in Glp1r expression in the mouse brain, with increases in the late embryonic period and again around P14, per the Allen Brain Atlas. While our model encompasses the first of these expression periods, it is not known if the outcomes we tested are appropriate for this period or if a later exposure (P14–21, for instance) may show more latent alterations in behavior. Next, once daily injections of Ex-4 produce peaks and valleys in exposure, as compared to the more consistent steady-state blood concentrations induced by human clinical treatment protocols. Additional studies using longer-lasting analogues or mini-pump style exposures would be helpful in establishing the physiological relevance of our observed changes. An additional caveat results from our use of a behavioral test battery approach, in that test order and previous behavioral experience can affect behavioral outcomes. We progressed through tasks in an order used by our laboratory previously that is designed to move from least to most invasive or stressful paradigms to minimize the likelihood of interference, but we cannot eliminate a contribution of order effects in our current data set. Future studies defining dose-response relationships and testing additional GLP-1R agonists with different metabolism and pharmacokinetic profiles would also be very helpful.
Clinical studies on reproductive outcomes following GLP-1R agonist exposure have focused almost exclusively on women that suffer from PCOS and obesity, only sometimes in conjunction with diabetes [see review (Jensterle et al., 2019)]. Oftentimes, any positive effects on reproductive success was correlated with weight loss; effects of GLP-1 directly on reproductive organs or other physiological systems are unknown. It is unclear how activation of GLP-1R would alter reproductive outcomes in women without one of these underlying conditions. This is especially prudent, as clinical trials utilizing GLP-1R agonists to treat other unrelated disorders (e.g., addiction, Parkinson’s and Alzheimer’s diseases, cardiovascular disease, COPD, arthritis, depression/cognition, etc.) are currently underway. Regardless, these data indicate that prenatal GLP-1R activation via Ex-4 has minimal effects on ―normal‖ female mice and their offspring, indicating that use of GLP-1R agonists during pregnancy may be expanded to other populations.
Highlights.
GLP-1R activation during pregnancy had no negative effects on maternal outcomes
Prenatal GLP-1 agonist exposure did not affect cognition or locomotor activity
Anxiety-like behaviors were altered differentially after in utero GLP-1R activation
These data highlight the potential safety of GLP-1R agonist use during pregnancy
Acknowledgements
We thank Dr. Pradeep Bhide and Deirdre McCarthy for assistance with confocal microscopy as well as Dr. Michael T. Williams for additional advice on statistical modeling. This work was supported by NIH grant R03 MH110749 (DLG), the FSU Council on Research & Creativity (DLG), and the FSU College of Medicine. The authors declare no conflict of interest or any competing financial or commercial interests.
Abbreviations:
- Ex-4
Exendin-4
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- HPA axis
hypothalamus-pituitary-adrenal axis
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos AS, Blair R, Peters AL, 2019. Management of Preexisting Diabetes in Pregnancy: A Review. JAMA 321(18), 1811–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, Sanz C, Vazquez P, Maldonado A, de Caceres J, Desco M, Pozo MA, Blazquez E, 2005. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 92(4), 798–806. [DOI] [PubMed] [Google Scholar]
- Anderberg RH, Richard JE, Hansson C, Nissbrandt H, Bergquist F, Skibicka KP, 2016. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 65, 54–66. [DOI] [PubMed] [Google Scholar]
- Athauda D, Foltynie T, 2016. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today 21(5), 802–818. [DOI] [PubMed] [Google Scholar]
- Belsham DD, Fick LJ, Dalvi PS, Centeno ML, Chalmers JA, Lee PK, Wang Y, Drucker DJ, Koletar MM, 2009. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J. 23(12), 4256–4265. [DOI] [PubMed] [Google Scholar]
- Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Ronnholm H, Wikstrom L, 2008. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J. Neurosci. Res. 86(2), 326–338. [DOI] [PubMed] [Google Scholar]
- Braden GC, Rasmussen S, Monette S, Tolwani RJ, 2017. Effects of Breeding Configuration on Maternal and Weanling Behavior in Laboratory Mice. J Am Assoc Lab Anim Sci 56(4), 369–376. [PMC free article] [PubMed] [Google Scholar]
- Brown E, Cuthbertson DJ, Wilding JP, 2018. Newer GLP-1 receptor agonists and obesity-diabetes. Peptides 100, 61–67. [DOI] [PubMed] [Google Scholar]
- Bulletins--Obstetrics A.C.o.P., 2008. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet. Gynecol 111(4), 1001–1020. [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Saborido TP, Stanwood GD, 2012. Development of hyperactivity and anxiety responses in dopamine transporter-deficient mice. Dev. Neurosci 34(2–3), 250–257. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL, 2001. Translating developmental time across mammalian species. Neuroscience 105(1), 7–17. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ, 2007a. Extrapolating brain development from experimental species to humans. Neurotoxicology 28(5), 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL, 2007b. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics 5(1), 79–94. [DOI] [PubMed] [Google Scholar]
- Committee on Obstetric P, 2017. Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstet. Gynecol 130(2), e81–e94. [DOI] [PubMed] [Google Scholar]
- Comninos AN, Jayasena CN, Dhillo WS, 2014. The relationship between gut and adipose hormones, and reproduction. Hum. Reprod. Update 20(2), 153–174. [DOI] [PubMed] [Google Scholar]
- Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S, 2015. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab 4(10), 718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, 2000. What’s Wrong with My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley-Liss, New York. [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA, 2006. Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1(4), 1662–1670. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP, 2012. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J. Neurosci 32(14), 4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Lilly N, Kay K, Williams DL, 2011. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J. Neurosci 31(41), 14453–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, 2006. The biology of incretin hormones. Cell Metab 3(3), 153–165. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R, 2005. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci. Biobehav. Rev 29(4–5), 771–783. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN, 2003. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med 9(9), 1173–1179. [DOI] [PubMed] [Google Scholar]
- Eakin K, Li Y, Chiang YH, Hoffer BJ, Rosenheim H, Greig NH, Miller JP, 2013. Exendin-4 ameliorates traumatic brain injury-induced cognitive impairment in rats. PLoS One 8(12), e82016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E, 2013a. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One 8(10), e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E, 2013b. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One 8(7), e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E, 2013c. The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38(8), 1259–1270. [DOI] [PubMed] [Google Scholar]
- Engman J, Linnman C, Van Dijk KR, Milad MR, 2016. Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology 63, 34–42. [DOI] [PubMed] [Google Scholar]
- Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A, 2012. Exendin-4 decreases amphetamine-induced locomotor activity. Physiol. Behav 106(4), 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamilla CO, Filonova I, Walker AK, Xuan ZX, Holehonnur R, Espinosa F, Liu S, Thyme SB, Lopez-Garcia IA, Mendoza DB, Usui N, Ellegood J, Eisch AJ, Konopka G, Lerch JP, Schier AF, Speed HE, Powell CM, 2017. Kctd13 deletion reduces synaptic transmission via increased RhoA. Nature 551(7679), 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahvar S, Walfisch A, Sheiner E, 2019. Gestational diabetes risk factors and long-term consequences for both mother and offspring: a literature review. Expert Rev Endocrinol Metab 14(1), 63–74. [DOI] [PubMed] [Google Scholar]
- Frederick AL, Saborido TP, Stanwood GD, 2012. Neurobehavioral phenotyping of G(alphaq) knockout mice reveals impairments in motor functions and spatial working memory without changes in anxiety or behavioral despair. Front Behav Neurosci 6, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Meszaros J, Urizar E, Sibley DR, Kellendonk C, Sonntag KC, Graham DL, Colbran RJ, Stanwood GD, Javitch JA, 2015. Evidence against dopamine D1/D2 receptor heteromers. Mol. Psychiatry 20(11), 1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Myers B, Herman JP, 2013. Role of central glucagon-like peptide-1 in stress regulation. Physiol. Behav 122, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Packard AE, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D’Alessio DA, Herman JP, 2017. Disruption of Glucagon-Like Peptide 1 Signaling in Sim1 Neurons Reduces Physiological and Behavioral Reactivity to Acute and Chronic Stress. J. Neurosci 37(1), 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Lozano M, Perez-Tilve D, Alvarez-Crespo M, Martis A, Fernandez AM, Catalina PA, Gonzalez-Matias LC, Mallo F, 2010. GLP-1(7–36)-amide and Exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology 151(6), 2629–2640. [DOI] [PubMed] [Google Scholar]
- Glotfelty EJ, Olson L, Karlsson TE, Li Y, Greig NH, 2020. Glucagon-like peptide-1 (GLP-1)-based receptor agonists as a treatment for Parkinson’s disease. Expert Opin Investig Drugs 29(6), 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Sobin CA, 2020. Statistical modeling with litter as a random effect in mixed models to manage “intralitter likeness”. Neurotoxicol. Teratol 77, 106841. [DOI] [PubMed] [Google Scholar]
- Graham DL, Buendia MA, Chapman MA, Durai HH, Stanwood GD, 2015a. Deletion of Galphaq in the telencephalon alters specific neurobehavioral outcomes. Synapse 69(9), 434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Durai HH, Garden JD, Cohen EL, Echevarria FD, Stanwood GD, 2015b. Loss of dopamine d2 receptors increases parvalbumin-positive interneurons in the anterior cingulate cortex. ACS chemical neuroscience 6(2), 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Durai HH, Trammell T, Noble BL, Mortlock DP, Galli A, Stanwood GD, 2020. A novel mouse model of glucagon-like peptide-1 receptor expression: a look at the brain. J. Comp. Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Erreger K, Galli A, Stanwood GD, 2013. GLP-1 analog attenuates cocaine reward. Mol. Psychiatry 18(9), 961–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Meyer JS, Stanwood GD, 2018. Behavioral Phenotyping in Developmental Neurotoxicology—Simple Approaches Using Unconditioned Behaviors in Rodents, in: Slikker W, Paule MG, Wang C. (Eds.), Handbook of Developmental Neurotoxicology. Academic Press, pp. 287–308. [Google Scholar]
- Graham DL, Schaefer TL, Vorhees CV, 2011. Neurobehavioral testing for developmental toxicity, in: Hood RD (Ed.) Developmental and Reproductive Toxicology: A Practical Approach. CRC Press, Boca Raton, pp. 346–387. [Google Scholar]
- Gulec G, Isbil-Buyukcoskun N, Kahveci N, 2010. Effects of centrally-injected glucagon-like peptide-1 on pilocarpine-induced seizures, anxiety and locomotor and exploratory activity in rat. Neuropeptides 44(4), 285–291. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Patterson S, Porter D, Gault VA, Holscher C, 2011. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J. Neurosci. Res 89(4), 481–489. [DOI] [PubMed] [Google Scholar]
- Hayes MR, De Jonghe BC, Kanoski SE, 2010. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol. Behav 100(5), 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C, 2018. Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer’s and Parkinson’s disease models. Neuropharmacology 136(Pt B), 251–259. [DOI] [PubMed] [Google Scholar]
- Huang X, Dai FF, Gaisano G, Giglou K, Han J, Zhang M, Kittanakom S, Wong V, Wei L, Showalter AD, Sloop KW, Stagljar I, Wheeler MB, 2013. The identification of novel proteins that interact with the GLP-1 receptor and restrain its activity. Mol. Endocrinol 27(9), 1550–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K, Holscher C, 2012. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine, National Research Council Committee to Reexamine I. O. M. Pregnancy Weight Guidelines, 2009. The National Academies Collection: Reports funded by National Institutes of Health, in: Rasmussen KM, Yaktine AL (Eds.), Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press (US) Copyright © 2009, National Academy of Sciences., Washington (DC). [Google Scholar]
- Isacson R, Nielsen E, Dannaeus K, Bertilsson G, Patrone C, Zachrisson O, Wikstrom L, 2011. The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur. J. Pharmacol 650(1), 249–255. [DOI] [PubMed] [Google Scholar]
- Iwai T, Sawabe T, Tanimitsu K, Suzuki M, Sasaki-Hamada S, Oka J, 2014. Glucagon-like peptide-1 protects synaptic and learning functions from neuroinflammation in rodents. J. Neurosci. Res 92(4), 446–454. [DOI] [PubMed] [Google Scholar]
- Iwai T, Suzuki M, Kobayashi K, Mori K, Mogi Y, Oka J, 2009. The influences of juvenile diabetes on memory and hippocampal plasticity in rats: improving effects of glucagon-like peptide-1. Neurosci. Res 64(1), 67–74. [DOI] [PubMed] [Google Scholar]
- Jaramillo TC, Speed HE, Xuan Z, Reimers JM, Liu S, Powell CM, 2016. Altered Striatal Synaptic Function and Abnormal Behaviour in Shank3 Exon4–9 Deletion Mouse Model of Autism. Autism Res 9(3), 350–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensterle M, Janez A, Fliers E, DeVries JH, Vrtacnik-Bokal E, Siegelaar SE, 2019. The role of glucagon-like peptide-1 in reproduction: from physiology to therapeutic perspective. Hum. Reprod. Update 25(4), 504–517. [DOI] [PubMed] [Google Scholar]
- Kandzija N, Zhang W, Motta-Mejia C, Mhlomi V, McGowan-Downey J, James T, Cerdeira AS, Tannetta D, Sargent I, Redman CW, Bastie CC, Vatish M, 2019. Placental extracellular vesicles express active dipeptidyl peptidase IV; levels are increased in gestational diabetes mellitus. J Extracell Vesicles 8(1), 1617000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH, 2017. A New Treatment Strategy for Parkinson’s Disease through the Gut-Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway. Cell Transplant. 26(9), 1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ, 2003. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J. Neurosci 23(15), 6163–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C, Colom A, Uldry M, Rastrelli S, Sabatier E, Godet N, Waget A, Penicaud L, Valet P, Burcelin R, 2008. Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology 149(10), 4768–4777. [DOI] [PubMed] [Google Scholar]
- Komsuoglu Celikyurt I, Mutlu O, Ulak G, Uyar E, Bektas E, Yildiz Akar F, Erden F, Tarkun I, 2014. Exenatide treatment exerts anxiolytic- and antidepressant-like effects and reverses neuropathy in a mouse model of type-2 diabetes. Medical science monitor basic research 20, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Nilsson IAK, Brismar K, Gissler M, Lavebratt C, 2020. Associations of Different Types of Maternal Diabetes and Body Mass Index With Offspring Psychiatric Disorders. JAMA Netw Open 3(2), e1920787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kelly AS, 2017. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc 92(2), 251–265. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Sanchez-Watts G, Krieger JP, Pignalosa A, Norell PN, Cortella A, Pettersen KG, Vrdoljak D, Hayes MR, Kanoski SE, Langhans W, Watts AG, 2018. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Mol Metab 11, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencioni C, Resi V, Romero F, Lupi R, Volpe L, Bertolotto A, Ghio A, Del Prato S, Marchetti P, Di Cianni G, 2011. Glucagon-like peptide-1 secretion in women with gestational diabetes mellitus during and after pregnancy. J. Endocrinol. Invest 34(9), e287–290. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Zheng SY, Lin R, Xie YJ, Chen H, Zheng YX, Liu E, Chen L, Yan JH, Xu W, Mai TT, Gong Y, 2017. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin. Endocrinol. (Oxf). 87(6), 767–774. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Oleata C, Roberto M, 2017. Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology 114, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, Roan JL, Vu C, Laugero KD, Parkes DG, Young AA, 2006. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 30(9), 1332–1340. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Cook S, Scrocchi L, Shin J, Kim J, Vaccarino F, Asa SL, Drucker DJ, 2000. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology 141(2), 752–762. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P, 1999. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol 403(2), 261–280. [DOI] [PubMed] [Google Scholar]
- Moller C, Sommer W, Thorsell A, Rimondini R, Heilig M, 2002. Anxiogenic-like action of centrally administered glucagon-like peptide-1 in a punished drinking test. Prog. Neuropsychopharmacol. Biol. Psychiatry 26(1), 119–122. [DOI] [PubMed] [Google Scholar]
- Moser VC, 2011. Functional assays for neurotoxicity testing. Toxicol. Pathol 39(1), 36–45. [DOI] [PubMed] [Google Scholar]
- Nylander M, Frossing S, Clausen HV, Kistorp C, Faber J, Skouby SO, 2017. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: a randomized clinical trial. Reprod Biomed Online 35(1), 121–127. [DOI] [PubMed] [Google Scholar]
- Outeirino-Iglesias V, Romani-Perez M, Gonzalez-Matias LC, Vigo E, Mallo F, 2015. GLP1 Increases Preovulatory LH Source and the Number of Mature Follicles, As Well As Synchronizing the Onset of Puberty in Female Rats. Endocrinology 156(11), 4226–4237. [DOI] [PubMed] [Google Scholar]
- Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, Miller J, Hoffer BJ, Greig NH, Pick CG, 2013. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age 35(5), 1621–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy IA, Pino JA, Weikop P, Osses N, Sorensen G, Bering T, Valle C, Bluett RJ, Erreger K, Wortwein G, Reyes JG, Graham D, Stanwood GD, Hackett TA, Patel S, Fink-Jensen A, Torres GE, Galli A, 2016. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl Psychiatry 6, e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S Jr., 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect 108 Suppl 3, 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EJ, Graham DL, Money KM, Stanwood GD, 2015. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology 40(1), 61–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozo AV, Babu DA, Suen PA, Groff DN, Seeley RJ, Simmons RA, Seale P, Ahima RS, Stoffers DA, 2017. Neonatal GLP1R activation limits adult adiposity by durably altering hypothalamic architecture. Mol Metab 6(7), 748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamun V, Jensterle M, Janez A, Vrtacnik Bokal E, 2018. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur. J. Endocrinol 179(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Salvatore M, Wiersielis KR, Luz S, Waxler DE, Bhatnagar S, Bangasser DA, 2018. Sex differences in circuits activated by corticotropin releasing factor in rats. Horm. Behav 97, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna MD, Ghelardini C, Thurmond RL, Masini E, Galeotti N, 2017. Behavioural phenotype of histamine H4 receptor knockout mice: Focus on central neuronal functions. Neuropharmacology 114, 48–57. [DOI] [PubMed] [Google Scholar]
- Sedman T, Runkorg K, Krass M, Luuk H, Plaas M, Vasar E, Volke V, 2016. Exenatide Is an Effective Antihyperglycaemic Agent in a Mouse Model of Wolfram Syndrome 1. J Diabetes Res 2016, 9239530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AN, Ligade SS, Sharma JN, Shukla P, Elased KM, Lucot JB, 2015. GLP-1 receptor agonist liraglutide reverses long-term atypical antipsychotic treatment associated behavioral depression and metabolic abnormalities in rats. Metab. Brain Dis 30(2), 519–527. [DOI] [PubMed] [Google Scholar]
- Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, Galli A, Fink-Jensen A, 2015. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol. Behav 149, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL, 2010. The basics of brain development. Neuropsychol. Rev 20(4), 327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar N, Bagias C, Goljan I, Weldeselassie Y, Gharanei S, Tan BK, Holst JJ, Saravanan P, 2018. Reduced GLP-1 Secretion at 30 Minutes After a 75-g Oral Glucose Load Is Observed in Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes 67(12), 2650–2656. [DOI] [PubMed] [Google Scholar]
- Sun Z, Li P, Wang X, Lai S, Qiu H, Chen Z, Hu S, Yao J, Shen J, 2020. GLP-1/GLP-1R Signaling Regulates Ovarian PCOS-Associated Granulosa Cells Proliferation and Antiapoptosis by Modification of Forkhead Box Protein O1 Phosphorylation Sites. Int J Endocrinol 2020, 1484321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarkiewicz K, Sablan EJ, Polizzi CJ, Villescaz C, Parkes DG, 2014. Long-term metabolic benefits of exenatide in mice are mediated solely via the known glucagon-like peptide 1 receptor. Am J Physiol Regul Integr Comp Physiol 306(7), R490–498. [DOI] [PubMed] [Google Scholar]
- The Medical Letter On Drugs and Therapeutics, 2019. Drugs for type 2 diabetes. Med. Lett. Drugs Ther 61(1584), 169–178. [PubMed] [Google Scholar]
- Trammell TS, Henderson NL, Madkour HS, Stanwood GD, Graham DL, 2020. GLP-1R activation alters performance in cognitive tasks in a sex-dependent manner. Neurol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR, 1996. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379(6560), 69–72. [DOI] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, Perez E, Miller J, Hoffer BJ, Greig NH, Pick CG, 2013. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp. Neurol 239, 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Rubovitch V, Li Y, Holloway HW, Lehrmann E, Zhang Y, Becker KG, Perez E, Hoffer BJ, Pick CG, Greig NH, 2016. Blast traumatic brain injury-induced cognitive deficits are attenuated by preinjury or postinjury treatment with the glucagon-like peptide-1 receptor agonist, exendin-4. Alzheimers Dement 12(1), 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale L, Allotey J, Cheong-See F, Arroyo-Manzano D, McCorry D, Bagary M, Mignini L, Khan KS, Zamora J, Thangaratinam S, Collaboration EC, 2015. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet 386(10006), 1845–1852. [DOI] [PubMed] [Google Scholar]
- Werner EA, Gustafsson HC, Lee S, Feng T, Jiang N, Desai P, Monk C, 2016. PREPP: postpartum depression prevention through the mother-infant dyad. Arch Womens Ment Health 19(2), 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes ST, Maeda KJ, Sasser J, Ryan MJ, 2020. The glucagon-like peptide 1 receptor agonist liraglutide attenuates placental ischemia-induced hypertension. Am J Physiol Heart Circ Physiol 318(1), H72–H77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP, 2010. Role of glucocorticoids in tuning hindbrain stress integration. J. Neurosci 30(44), 14907–14914. [DOI] [PMC free article] [PubMed] [Google Scholar]