Abstract
Dusky-footed wood rats (Neotoma fuscipes) and Peromyscus sp. mice (P. maniculatus and P. truei) were collected from one site in Placer County, one site in Santa Cruz County, and two sites in Sonoma County in northern California. Serum or plasma samples from 260 rodents were tested for antibodies to the agent of human granulocytic ehrlichiosis. Of these, samples from 25 wood rats (34% of those tested) and 10 (8%) Peromyscus sp. mice were found to be seropositive, but only those from one site. PCR assays targeting the groESL heat shock operon were conducted on all seropositive specimens and a subset of seronegative blood specimens. Ehrlichial DNA was identified in 17 (68%) of the 25 seropositive wood rat blood samples and in 1 of the 10 (10%) Peromyscus sp. specimens. None of 40 seronegative blood samples was PCR positive. Both seropositive and PCR-positive animals were collected during each trapping period. One male tick out of 84 Ixodes pacificus adults collected was PCR positive; samples of Dermacentor occidentalis nymphs and adults were negative. Nucleotide sequences of amplicons from three wood rat blood specimens and from the single PCR-positive tick differed by one and two bases, respectively, from a sequence previously obtained from Ehrlichia equi. At one site in Sonoma County, wood rats had a concurrent high prevalence of seropositivity and PCR positivity, while other sigmodontine rodents collected at the site were only occasionally infected. We suggest that dusky-footed wood rats serve as reservoirs of granulocytic ehrlichial agents in certain areas of northern California. The tick species involved in the transmission of granulocytic ehrlichiae among wood rats remains unknown.
Human granulocytic ehrlichiosis (HGE) has been diagnosed in patients from the northeastern and upper midwestern portions of the United States and in patients from Europe (7, 14). Several cases of human illness due to Ehrlichia spp. have been identified in residents of northern California, most of which were confirmed by serology (10, 26). A seroepidemiologic study in a northern California community indicated infrequent (0.4%) human exposure to granulocytic ehrlichiae (9). The disease is caused by infection with an Ehrlichia sp. that is very closely related (and likely conspecific) to Ehrlichia equi and Ehrlichia phagocytophila (7). Interestingly, E. equi has been known to be a cause of equine disease in this region for at least 3 decades (23).
In the northeastern and upper midwestern regions of the United States, the arthropod vector for granulocytic ehrlichiae is the blacklegged tick, Ixodes scapularis (25). The likely vector for humans and animals in northern California is the western blacklegged tick, Ixodes pacificus. Immature I. pacificus ticks often prefer lizards as hosts but are occasionally found on small rodents (8). This tick is the most common of the four species in this area that may bite humans (20), and Ehrlichia-infected I. pacificus ticks have been identified by PCR assays (2–4). This species has also been shown to be an efficient vector for E. equi in transmission studies with horses (21, 22).
While these studies have suggested a likely vector for humans and horses, the animal reservoir(s) of the infection in northern California has not been identified. While it has been known for some time that granulocytic ehrlichiae can be found in horses in this region (23), additional evidence for the presence of granulocytic ehrlichiae in other animals has been gathered through studies of llamas (4) and wild rodents (18). In the northeastern and upper midwestern parts of the United States, the white-footed mouse (Peromyscus leucopus) has been shown to be a likely reservoir (18, 25). Although this species is not found in California, other Peromyscus species might play a comparable role. Because of the similarities of the geographic distribution of the pathogens in the state, and because of the use of similar vectors, we hypothesized that the natural cycle of granulocytic ehrlichiae might be similar to that of Borrelia burgdorferi, the agent of Lyme borreliosis (6). Because the primary reservoir of B. burgdorferi in California is the dusky-footed wood rat (Neotoma fuscipes), and because we had previously identified several specimens of this species as seropositive for the HGE agent (18), we chose to examine these animals for molecular and serologic evidence of their involvement in the natural maintenance cycle of the agent of HGE and equine ehrlichiosis in northern California.
MATERIALS AND METHODS
Collection sites.
Preliminary rodent and tick collections were conducted at four sites in northern California in March and May 1997: one site in Placer County, one site in Santa Cruz County, and two localities in Sonoma County (Table 1). Based on preliminary results, one site in Sonoma County (site B) was selected for continued evaluation. Site B is located in a disturbed oak woodland habitat (122°32′30"W, 38°20′35"N) and has an elevation ranging from 840 to 960 ft above sea level. The predominant tree species include live oaks (Quercus agrifolia and Quercus wislizenii), madrone (Arbutus menziesii), and California bay (Umbellularia californica). Coast redwood (Sequoia sempervirens), California buckeye (Aesculus californica), and apple and plum trees remaining from an old orchard were also present. Other vegetation included coyote brush (Baccharis pilularis), poison oak, soap plant, ferns, and wild oats.
TABLE 1.
Serologic and molecular detection of granulocytic ehrlichiae in rodents collected (1997 to 1998) at four sites in northern California
| Site (location) | Collection date | Rodent species | No. collected | IFA
|
PCR
|
||
|---|---|---|---|---|---|---|---|
| No. tested | No. positive (%) | No. tested | No. positive (%) | ||||
| A (Sonoma Co.) | May 1997 | N. fuscipes | 20 | 20 | 0 | 0 | |
| B (Sonoma Co.) | May 1997 | N. fuscipes | 7 | 7 | 4 (57) | 7 | 1 (14) |
| July 1997 | N. fuscipes | 18 | 18 | 6 (33) | 6 | 6 (100) | |
| P. maniculatus | 2 | 2 | 0 | 0 | |||
| P. truei | 6 | 6 | 0 | 0 | |||
| R. megalotis | 1 | 1 | 0 | 0 | |||
| Aug. 1997 | N. fuscipes | 32 | 31 | 11 (35) | 12 | 9 (75) | |
| P. maniculatus | 10 | 10 | 0 | 4 | 0 | ||
| P. truei | 16 | 16 | 0 | 9 | 0 | ||
| Sept. 1997 | N. fuscipes | 28 | 27 | 9 (33) | 9 | 4 (44) | |
| P. maniculatus | 3 | 3 | 0 | 0 | |||
| P. truei | 5 | 5 | 2 (40) | 0 | |||
| M. californicus | 1 | 1 | 0 | 0 | |||
| Sorex sp. | 1 | 0 | |||||
| Oct. 1997 | N. fuscipes | 24 | 24 | 9 (38) | 13 | 5 (38) | |
| P. maniculatus | 3 | 3 | 0 | 0 | |||
| P. truei | 7 | 6 | 1 (17) | 1 | 1 (100) | ||
| R. megalotis | 2 | 2 | 0 | 0 | |||
| May 1998 | N. fuscipes | 11 | 11 | 7 (64) | 7 | 5 (71) | |
| P. maniculatus | 10 | 10 | 2 (20) | 0 | |||
| P. truei | 5 | 5 | 0 | 0 | |||
| June 1998 | N. fuscipes | 25 | 24 | 13 (54) | 9 | 6 (67) | |
| P. maniculatus | 30 | 30 | 3 (10) | 3 | 0 | ||
| P. truei | 13 | 13 | 2 (15) | 2 | 0 | ||
| C (Placer Co.) | May 1997 | N. fuscipes | 30 | 30 | 0 | 0 | |
| P. maniculatus | 1 | 0 | 0 | ||||
| D (Santa Cruz Co.) | Mar. 1997 | P. californicus | 3 | 3 | 0 | 0 | |
| P. truei | 10 | 10 | 0 | 0 | |||
Survey samples.
All animal collections and manipulations were conducted by California Department of Health personnel. The procedures were consistent with the guidelines recommended by the Centers for Disease Control and Prevention (16) as well as other state and federal regulations related to animals. Rodents were collected in Tomahawk traps baited with peanut butter and oats or Sherman traps baited with oats. Trapping stations (n = 35) were established near wood rat huts and monitored for 2 to 3 days each month in July, August, September, and October 1997 and in May and June 1998 (no trapping was conducted in the winter months). Twenty traps were located in brushy areas with little canopy cover, while 15 traps were located in the interface between brushy areas or in a wooded area.
Captured rodents were anesthetized with ether for handling. Blood specimens were collected by cardiocentesis and transferred to EDTA vials for storage and testing. All blood samples were coded and sent to the Centers for Disease Control and Prevention for serologic and molecular evaluation. Ectoparasites were removed from the anesthetized animals with forceps and preserved in ethanol or saline. At each sampling period, questing ticks were collected by dragging a 1-m2 flannel cloth across the ground or vegetation in the areas immediately surrounding the wood rat huts. Additional questing I. pacificus ticks were collected at site E in Sonoma County, a site where rodent collection was not attempted but where cases of equine ehrlichiosis were previously identified. Ticks were stored in 70% ethanol, and later, tick species were determined by standard morphologic keys.
Serologic testing by IFA.
The indirect immunofluorescence assay (IFA) for detecting sigmodontine rodent immunoglobulins reactive with the HGE agent (USG3 isolate) (17) was conducted as previously described (18). Positive and negative control sera were included in all assays. Geometric mean titers (GMT) were calculated for seroreactive samples (reciprocal antibody titers ≥ 16).
DNA extraction.
DNA was extracted from whole-blood specimens (50 μl), blood clots (∼50 μl), and ticks (individually) with QiaAmp tissue kits (Qiagen, Chatsworth, Calif.), and all options for increased yield, according to the manufacturer’s protocol, were employed. Extracted DNA from all sources was eluted in 200 μl of AE buffer. Ticks were removed from the ethanol, air dried, and prepared for extraction as described by Watt et al. (28). To verify that we were obtaining suitable DNA by this method, a random sample of 24 tick DNA extracts was tested for the presence of tick mitochondrial DNA by the method described by Black and Piesman (5).
PCR assay.
The specimens were tested by PCR assays with primers directed against the groESL heat shock operon of Ehrlichia spp. The assay was conducted in a nested format with HS1a and HS6a in the first reaction and HS43 and HSVR in the second reaction. Primers HS1a (5′-AIT GGG CTG GTA ITG AAA T-3′) and HS6a (5′-CCI CCI GGI ACI AIA CCT TC-3′) were modified to include inosine bases from HS1 and HS6 (24) and were used to amplify bacterial DNA in the samples. Primers HS43 (5′-AT[A/T] GC[A/T] AA[G/A] GAA GCA TAG TC-3′) and HSVR (5′-CTC AAC AGC AGC TCT AGT AGC-3′) permitted the amplification of a 1,297-bp region of the heat shock operon that included the end of the groES gene, the entire spacer region, and much of the groEL gene (14). The assay can amplify the region in extracts from granulocytic ehrlichiae and from the monocytic species Ehrlichia chaffeensis.
All PCR assays were prepared with commercial amplification kits (Ready-To-Go PCR; Amersham Pharmacia Biotech, Piscataway, N.J.). One microliter of DNA template was added to 24 μl of a reaction mix containing 100 mM Tris-HCl (pH 8.3), 50 mM KCl, 0.15 mM MgCl2, 0.001% gelatin, 200 μM concentrations of each deoxynucleoside triphosphate, 1 μM concentrations of each primer, and 2.5 U of Taq polymerase. The reaction mix was overlaid with 50 μl of mineral oil and cycled (Thermal Cycler; Perkin-Elmer Cetus, Norwalk, Conn.) for three preliminary cycles (94°C, 1 min; 55°C, 2 min; and 72°C, 1.5 min), followed by 37 amplification cycles (88°C, 1 min; 55°C, 2 min; and 72°C, 1.5 min) and then by an additional extension period of 5 min at 72°C. Nested reactions were conducted for 40 cycles with the primers HS1a and HS6a in the first round followed by 40 cycles with primers HS43 and HSVR in the second round. During the first amplification round, the annealing temperature of the reaction was lowered to 48°C, and the extension temperature was lowered to 68°C. Positive (USG3 DNA) and negative (water) controls were included in each assay. Ten microliters of each PCR product was separated in gels containing 1.2% agarose in 0.04 M Tris-acetate–0.01 M EDTA (TAE) buffer (pH 8.4) by gel electrophoresis conducted for 1 h at 100 V in TAE buffer. The ethidium bromide-stained gels were photographed under shortwave (300 nm) UV illumination.
Sequencing of the PCR products was conducted by purification methods and automated sequencing techniques as previously described (24). Nucleotide sequences from three wood rat blood specimens and one tick were obtained and compared to existing sequences contained in GenBank (Bethesda, Md.) and to unpublished sequences determined in our laboratory. We also sequenced the amplicon of the tick mitochondrial DNA from the Ehrlichia-positive tick by similar methods.
Nucleotide sequence accession number.
The composite granulocytic Ehrlichia sequence obtained from the PCR-positive wood rats has been submitted to GenBank (accession no. AF173988). The ehrlichial sequence obtained from I. pacificus has been submitted to GenBank (accession no. AF173989).
RESULTS
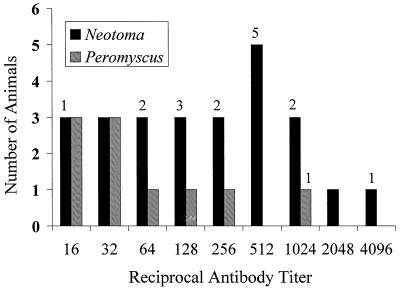
In this study, we examined blood specimens from N. fuscipes wood rats and Peromyscus sp. mice for molecular and serologic evidence of infection with granulocytic ehrlichiae. Of the four collection sites visited in March and May of 1997, seropositive animals were obtained only at site B. Four of seven N. fuscipes wood rats were positive, while no positive samples were obtained from a total of 50 N. fuscipes wood rats and 14 Peromyscus sp. mice at the other sites (Table 1). In several additional sampling periods from May 1997 through June 1998, 187 rodents of five species were trapped at site B (Table 1). Antibodies reactive with the HGE agent were identified in 25 of 74 (68%) wood rats, 5 of 51 (10%) Peromyscus truei, and 5 of 58 (9%) Peromyscus maniculatus. The seroprevalence among wood rats ranged from 25 to 67% over the seven trapping periods. Reciprocal endpoint titers (Fig. 1) of the seropositive wood rats ranged from 16 to 4,096 (GMT = 218), while the titers for P. truei ranged from 64 to 1,024 and those for P. maniculatus ranged from 16 to 64 (GMT for the genus = 56). All specimens from the wood rats, P. truei, and Peromyscus californicus collected at the other sites (A, C, and D), as well as specimens of Reithrodontomys megalotis and Microtus californicus at site B, were seronegative (Table 1).
FIG. 1.
Frequency of IFA antibody titers in seropositive Peromyscus sp. and N. fuscipes specimens collected (1997 to 1998) at one site in Sonoma County, Calif. The number over the bar represents the number of animals that were also PCR positive at each titer.
DNA amplification by PCR yielded clear bands of the expected size for granulocytic ehrlichiae (1,297 bp) in 18 of 35 specimens with antibody titers of ≥16. Of the 25 seropositive wood rats, 17 (68%) had amplifiable DNA of ehrlichiae in their blood. There were no significant differences between the GMT of PCR-positive and PCR-negative wood rats (one-way analysis of variance, P = 0.258), and PCR-positive wood rats were identified on the basis of samples with a wide range of antibody titers (Fig. 1). Ehrlichial DNA was demonstrated in one of five seropositive P. truei specimens and none of the five seropositive P. maniculatus specimens collected at site B. None of the seronegative blood specimens tested (31 wood rats, 7 P. truei mice, and 2 P. maniculatus mice) produced products in the PCR assays (Table 1). Seronegative specimens were chosen at several time periods to ensure representative sampling.
The nucleotide sequences of a 1,256-bp region (after primer sequences were removed) amplified from each of three PCR-positive wood rat specimens were identical. These sequences differed from the sequence previously determined for E. equi originating in California (GenBank U96727) at only one base position. However, this base difference did not affect the deduced amino acid sequence of the gene product encoded by this region.
Adult and nymphal Dermacentor occidentalis and I. pacificus ticks were collected at site B and an additional site (Table 2). Only ticks tested by PCR are included in this report. PCR assays of 158 individual ticks yielded evidence of ehrlichial DNA in one I. pacificus male. Only 10 ticks collected directly from the wood rats were tested, and all were negative by our PCR assay. The ticks varied in their degree of engorgement, and the individual wood rats hosting these ticks were PCR negative. All 24 of the tick extracts tested contained tick mitochondrial DNA, determined by the amplification of a 460-bp region of the tick mitochondrial DNA. The nucleotide sequence of the tick mitochondrial DNA was determined for the Ehrlichia-positive tick and confirmed the identity of this specimen as I. pacificus (data not shown).
TABLE 2.
Tick characteristics and PCR assay results for ixodid ticks collected in Sonoma County, Calif., March 1997
| Site | Tick species | Life stage (gender) | Collection method | PCR
|
|
|---|---|---|---|---|---|
| No. tested | No. positive (%) | ||||
| B | D. occidentalis | Nymph | Removed from host | 10 | 0 |
| Adult (male) | Flagging | 3 | 0 | ||
| Adult (female) | Flagging | 6 | 0 | ||
| I. pacificus | Nymph | Flagging | 2 | 0 | |
| Adult (male) | Flagging | 45 | 1 (2) | ||
| Adult (female) | Flagging | 39 | 0 | ||
| E | I. pacificus | Adult (male) | Flagging | 28 | 0 |
| Adult (female) | Flagging | 25 | 0 | ||
| Total | 158 | 1 (0.6) | |||
The nucleotide sequence of the overlapping 1,256-bp region amplified from the I. pacificus tick differed by only one base position from the sequence previously determined for E. equi originating in California (GenBank U96727) and by two bases from the wood rat sequences. Again, these base differences did not affect the deduced amino acid sequence of the gene product encoded by this region.
DISCUSSION
This study provides evidence supporting the role of dusky-footed wood rats in the maintenance cycle of granulocytic ehrlichiae in northern California. Although the distribution of infected wood rats is undetermined, our data indicate that high levels of infection can occur at certain foci. A seroprevalence of >30% and the concomitant demonstration of ehrlichial DNA in the blood of a majority of seropositive wood rats indicates either a high incidence of infection or a prolonged period of bacteremia. These data suggest that arthropods feeding on wood rats at site B would be likely to encounter granulocytic ehrlichiae, although our analyses of individual ticks could not confirm a proportionately high level of infection among the tick species we sampled.
Although seropositive Peromyscus sp. mice have been found in several areas of the United States (18) and P. leucopus has been identified as a likely reservoir in other parts of the country (25), the present study does not support the idea that Peromyscus mice can serve as major reservoirs in this region of California. Five P. truei (10%) and five P. maniculatus (9%) mice were seropositive at the site of high seroprevalence in wood rats, but only one mouse was positive by PCR. No seropositive mice or wood rats were found at the three other locations. We cannot rule out the possibility that piñon mice (P. truei) and deer mice (P. maniculatus) may play a role in providing infectious bloodmeals to feeding ticks, but these species certainly played a lesser role than the wood rat at this site. The distribution of antibody titers in the wood rats (Fig. 1) paralleled that seen in Peromyscus spp. in a larger survey (18). Ehrlichial DNA was identified in coded whole-blood specimens with antibody titers of ≥16. The concurrent detection of ehrlichial DNA and circulating antibodies in wood rat samples suggests that seropositivity alone is not an accurate marker of immunity in this species. In our experience, this finding suggests that Neotoma may respond to infection with granulocytic ehrlichiae in a manner or degree that differs from that of Peromyscus. Attempts to identify ehrlichial DNA from seropositive or seronegative Peromyscus blood specimens by PCR methods have, to date, been generally unrewarding (12, 18, 27).
Our study indicates that, at several points in the season, wood rats have a high prevalence of ehrlichiae in their bloodstreams, as detected by PCR assay. The level of ehrlichial DNA in the blood is not known, but it was detectable in only 50 μl of blood in our analysis. A high prevalence of bacteremia would facilitate the uptake of the pathogen by feeding arthropods, which then might serve as vectors of the agent to humans and other animals. Adult I. pacificus ticks have been found to be efficient experimental vectors of E. equi for horses (21, 22), and a few infected adult ticks have been previously collected in the field (3, 4). Our finding of only a single PCR-positive I. pacificus tick fails to provide convincing support that this tick species contributes to the high levels of infection among wood rats. These results may reflect the lower prevalence of immature I. pacificus ticks feeding on wood rats, but they also suggest that another arthropod may be involved. We suspect that Ixodes spinipalpis (= Ixodes neotomae [19]) is a likely vector of the agent among wood rats, but no samples were available for testing. Ticks of this nidicolous species at all life stages feed on wood rats, and they have also been collected from jackrabbits (Lepus californicus), cottontails (Sylvilagus spp.), and occasionally mice (P. maniculatus, Peromyscus mexicana, and Peromyscus boylii) (6, 8, 19).
We had previously identified seropositive wood rats from California (N. fuscipes and Neotoma lepida) and Colorado (Neotoma albigula and Neotoma mexicana) (18). It is possible that other wood rat species in other parts of the country might also maintain enzootic cycles of granulocytic ehrlichiae similar to those seen for B. burgdorferi in California and Colorado (6, 15).
The taxonomic distinction between E. equi and the HGE agent is uncertain. The 16S rRNA gene sequences of E. equi from horses in the United States have shown differences of only one or two bases from the HGE agent (1, 7, 11). Ehrlichiae from horses in Sweden have 16S rRNA gene sequences identical to that of the HGE agent (13). The heat shock operon of granulocytic ehrlichiae was chosen as a target for our PCR analysis because it has been shown to exhibit more variation than does the 16S rRNA gene (24). The heat shock operon sequences amplified from three wood rats were identical. Not surprisingly, the sequences most closely resembled, and differed by only one base from, the sequence of E. equi from California (GenBank U96727). In addition, the sequence obtained from the tick differed by two bases (0.16%) from the wood rat sequences and by one base (0.08%) from the equine sequence, over a 1,256-bp region. Although some variations in antigenic and pathogenic properties have been noted, all genes examined to date have failed to provide convincing evidence of divergence among the granulocytic ehrlichiae. We believe that multiple ehrlichial taxa represent strains of a single species (E. phagocytophila), which would include the ehrlichiae in the wood rats.
In this study, we present data supporting wood rats as a likely reservoir of the agent(s) of HGE and equine ehrlichiosis in northern California. Further studies should examine the persistence of ehrlichiae in wood rats and the transmission of ehrlichiae by I. spinipalpis and other wood rat-associated arthropods.
ACKNOWLEDGMENTS
We thank Aquila Biopharmaceuticals for the antigen used in these assays and the Core Biotechnology Facility of the Centers for Disease Control and Prevention for the production of the oligonucleotide primers.
We appreciate the technical assistance of Kristina Loop and Andy Comer in some of the IFA assays. The field collections of rodents were made through a collaboration with the Vector-Borne Disease Section of the California Department of Health Services (CDHS), including Ken Townzen, Bill Pitcher, Mac Thompson, Eric Ghilarducci, Jim Tucker, Joshua Ogawa, Lucia Hui, Charles Smith, and Barbara Wilson. We also enjoyed the cooperation of the Marin-Sonoma Mosquito and Vector Control District, including Ron Keith, Piper Kimball, Charles Battaglia, Ed Meehan, and Mary Janssen. We thank Curtis Fritz of CDHS for his review of the manuscript.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlough J E, Madigan J E, DeRock E, Bigornia L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus) Vet Parasitol. 1996;63:319–329. doi: 10.1016/0304-4017(95)00904-3. [DOI] [PubMed] [Google Scholar]
- 3.Barlough J E, Madigan J E, Kramer V L, Clover J R, Hui L T, Webb J P, Vredevoe L K. Ehrlichia phagocytophila genogroup rickettsiae in ixodid ticks from California collected in 1995 and 1996. J Clin Microbiol. 1997;35:2018–2021. doi: 10.1128/jcm.35.8.2018-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlough J E, Madigan J E, Turoff D R, Clover J R, Shelly S M, Dumler J S. An Ehrlichia strain from a llama (Lama glama) and llama-associated ticks (Ixodes pacificus) J Clin Microbiol. 1997;35:1005–1007. doi: 10.1128/jcm.35.4.1005-1007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black W C, IV, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rRNA sequences. Proc Natl Acad Sci USA. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown R N, Lane R S. Lyme disease in California: a novel enzootic transmission cycle of Borrelia burgdorferi. Science. 1992;256:1439–1442. doi: 10.1126/science.1604318. [DOI] [PubMed] [Google Scholar]
- 7.Chen S-M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durden L A, Keirans J E. Thomas Say publications in entomology: monographs. Lanham, Md: Entomological Society of America; 1996. Nymphs of the genus Ixodes (Acari: Ixodidae) of the United States: taxonomy, identification key, distribution, hosts, and medical/veterinary importance. [Google Scholar]
- 9.Fritz C L, Kjemtrup A M, Conrad P A, Flores G R, Campbell G L, Schriefer M E, Gallo D, Vugia D J. Seroepidemiology of emerging tickborne infectious diseases in a northern California community. J Infect Dis. 1997;175:1432–1439. doi: 10.1086/516476. [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz A S, Cornbleet P J, Vugia D J, Traver C, Niederhuber J, Kolbert C P, Persing D H. Human granulocytic ehrlichiosis: report of a case in northern California. Clin Infect Dis. 1996;23:653–654. doi: 10.1093/clinids/23.3.653. [DOI] [PubMed] [Google Scholar]
- 11.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 12.Hofmeister E K, Kolbert C P, Abdulkarim A S, Magera J M H, Hopkins M K, Uhl J R, Ambyaye A, Telford III S R, Cockerill III F R, Persing D H. Cosegregation of a novel Bartonella species with Borrelia burgdorferi and Babesia microti in Peromyscus leucopus. J Infect Dis. 1998;177:409–416. doi: 10.1086/514201. [DOI] [PubMed] [Google Scholar]
- 13.Johansson K-E, Pettersson B, Uhlen M, Gunnarsson A, Malmqvist M, Olsson E. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horses by direct solid phase sequencing of PCR products from the 16S rRNA gene. Res Vet Sci. 1995;58:109–112. doi: 10.1016/0034-5288(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 14.Lotric-Furlan S, Petrovec M, Zupanc T A, Nicholson W L, Sumner J W, Childs J E, Strle F. Human granulocytic ehrlichiosis in Europe: clinical and laboratory findings for four patients from Slovenia. Clin Infect Dis. 1998;27:424–428. doi: 10.1086/514683. [DOI] [PubMed] [Google Scholar]
- 15.Maupin G O, Gage K L, Piesman J, Montenieri J, Sviat S L, VanderZanden L, Happ C M, Dolan M, Johnson B J B. Discovery of an enzootic cycle of Borrelia burgdorferi in Neotoma mexicana and Ixodes spinipalpis from northern Colorado, an area where Lyme disease is nonendemic. J Infect Dis. 1994;170:636–643. doi: 10.1093/infdis/170.3.636. [DOI] [PubMed] [Google Scholar]
- 16.Mills J N, Childs J E, Ksiazek T G, Peters C J, Velleca W M. Methods for trapping and sampling small mammals for virologic testing. U.S. Atlanta, Ga: Department of Health and Human Services; 1995. [Google Scholar]
- 17.Nicholson W L, Comer J A, Sumner J W, Gingrich-Baker C, Coughlin R T, Magnarelli L A, Olson J G, Childs J E. An indirect immunofluorescence assay using a cell culture-derived antigen for detection of antibodies to the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1510–1516. doi: 10.1128/jcm.35.6.1510-1516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson W L, Muir S, Sumner J W, Childs J E. Serologic evidence of infection with Ehrlichia spp. in wild rodents (Muridae: Sigmodontinae) in the United States. J Clin Microbiol. 1998;36:695–700. doi: 10.1128/jcm.36.3.695-700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norris D E, Klompen J S H, Keirans J E, Lane R S, Piesman J, Black W C., IV Taxonomic status of Ixodes neotomae and Ixodes spinipalpis (Acari: Ixodidae) based on mitochondrial DNA evidence. J Med Entomol. 1997;34:696–703. doi: 10.1093/jmedent/34.6.696. [DOI] [PubMed] [Google Scholar]
- 20.Peavey C A, Lane R S, Kleinjan J E. Role of small mammals in the ecology of Borrelia burgdorferi in a peri-urban park in north coastal California. Exp Appl Acarol. 1997;21:569–584. doi: 10.1023/a:1018448416618. [DOI] [PubMed] [Google Scholar]
- 21.Reubel G H, Kimsey R B, Barlough J E, Madigan J E. Experimental transmission of Ehrlichia equi to horses through naturally infected ticks (Ixodes pacificus) from northern California. J Clin Microbiol. 1998;36:2131–2134. doi: 10.1128/jcm.36.7.2131-2134.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter P J, Jr, Kimsey R B, Madigan J E, Barlough J E, Dumler J S, Brooks D L. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae) J Med Entomol. 1996;33:1–5. doi: 10.1093/jmedent/33.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telford S R, III, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vugia D J, Holmberg E, Steffe E M, Ascher M S, Gallo D. A human case of monocytic ehrlichiosis with adult respiratory distress syndrome in northern California. West J Med. 1996;164:525–528. [PMC free article] [PubMed] [Google Scholar]
- 27.Walls J J, Greig B, Neitzel D F, Dumler J S. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:853–855. doi: 10.1128/jcm.35.4.853-855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watt D, Sparagano O, Brown C G D, Walker A R. Use of the polymerase chain reaction for identification and quantification of Theileria parva protozoa in Rhipicephalus appendiculatus ticks. Parasitol Res. 1997;83:359–363. doi: 10.1007/s004360050262. [DOI] [PubMed] [Google Scholar]