Abstract
Background & objectives:
In the context of India's ongoing resurgence of COVID-19 (second wave since mid-February 2021, following the subsiding of the first wave in September 2020), there has been increasing speculation on the possibility of a future third wave of infection, posing a burden on the healthcare system. Using simple mathematical models of the transmission dynamics of SARS-CoV-2, this study examined the conditions under which a serious third wave could occur.
Methods:
Using a deterministic, compartmental model of SARS-CoV-2 transmission, four potential mechanisms for a third wave were examined: (i) waning immunity restores previously exposed individuals to a susceptible state, (ii) emergence of a new viral variant that is capable of escaping immunity to previously circulating strains, (iii) emergence of a new viral variant that is more transmissible than the previously circulating strains, and (iv) release of current lockdowns affording fresh opportunities for transmission.
Results:
Immune-mediated mechanisms (waning immunity, or viral evolution for immune escape) are unlikely to drive a severe third wave if acting on their own, unless such mechanisms lead to a complete loss of protection among those previously exposed. Likewise, a new, more transmissible variant would have to exceed a high threshold (R0>4.5) to cause a third wave on its own. However, plausible mechanisms for a third wave include: (i) a new variant that is more transmissible and at the same time capable of escaping prior immunity, and (ii) lockdowns that are highly effective in limiting transmission and subsequently released. In both cases, any third wave seems unlikely to be as severe as the second wave. Rapid scale-up of vaccination efforts could play an important role in mitigating these and future waves of the disease.
Interpretation & conclusions:
This study demonstrates plausible mechanisms by which a substantial third wave could occur, while also illustrating that it is unlikely for any such resurgence to be as large as the second wave. Model projections are, however, subject to several uncertainties, and it remains important to scale up vaccination coverage to mitigate against any eventuality. Preparedness planning for any potential future wave will benefit by drawing upon the projected numbers based on the present modelling exercise.
Keywords: COVID-19, mathematical modelling, resurgence, SARS-CoV-2, third wave, transmission, variant
The COVID-19 pandemic has taken a serious toll in India, and globally1. Its population dynamics mirror the typical waves of infection displayed in earlier pandemics of other respiratory pathogens, including the influenza pandemics in 1918, and in 20092,3. In particular, India's first wave of SARS-CoV-2 infection began in late January 2020 and lasted for about nine months. A total of 11 million cases and 0.157 million deaths were reported1 from India over this period with a peak attained in mid-September, 2020. This was relatively mild compared to the second wave that followed, from mid-February 2021 onwards, and exhibiting a more explosive spread across the country. A major factor driving this second wave is the emergence of more-infectious variants of SARS-CoV-2, principally B.1.1.7 (Alpha variant) and B.1.617.2 (Delta variant), of which the latter has played a dominant role in recent months4,5. Opening of public places, super-spreading events following mass gatherings after the first wave, and neglect of personal protective measures (correct and consistent use of face masks) due to prevention fatigue also contributed to this surge which set in shortly after the vaccination rollout, which began on January 16, 20216.
There is now increasing speculation about the potential of a third wave of SARS-CoV-2 infection in India7. Third waves have manifested in other country settings, and may be driven by a range of factors. In the UK, for example, the third wave of SARS-CoV-2 occurred during the winter months of 2020, coinciding with their annual influenza season in the northern hemisphere, but also following the relaxation of lockdown restrictions there8,9. Similar factors drove a winter resurgence of COVID-19 in the USA as well10. Also viral evolution remains an ongoing concern about the potential for future resurgence11.
The present study sought to examine the potential drivers of a third wave in the Indian context as such a wave could exert a substantial public health burden. In India, seasonal drivers of transmission are likely to have a more subtle role than countries in temperate regions, as evidenced by the lack of any temporally coherent ‘influenza season’ across the length and breadth of the country12. Thus, four hypotheses were examined as drivers of a third wave in India: (i) waning immunity without any change in the virus, (ii) emergence of a new virus variant that is capable of escaping pre-existing immunity, (iii) emergence of a more transmissible variant without loss of immunity to previously circulating strains and (iv) fresh opportunities for transmission afforded by the relaxation of local restrictive measures (lockdowns) in response to the second wave.
To examine these hypotheses, simple mathematical models of SARS-CoV-2 transmission were employed, and parameterised to be consistent with the current COVID-19 situation in India. The models that are presented here are only illustrative, and not predictive. Overall, the present analysis helps to cast light on data that will be valuable to collect on an ongoing basis, in order to assess the potential for a third wave and to inform preparedness planning. Furthermore, based on prior knowledge acquired from the first and second waves about the proportion of symptomatic cases that would require hospital admission and intensive care support, the current study intends to inform the policy makers regarding potential mitigating measures that need to be ensured for future.
Material & Methods
Model overview: A simple, compartmental deterministic model of SARS-CoV-2 transmission was used (Figure 1A), a detailed description of which is presented under supplementary material (681.7KB, pdf) . The model captures essential features of the natural history of SARS-CoV-2, such as the latent period of infection, as well as asymptomatic, but transmissible states of infection. As described below, uncertainty was incorporated in parameters relating to the natural history of SARS-CoV-2 infection.
Fig. 1.
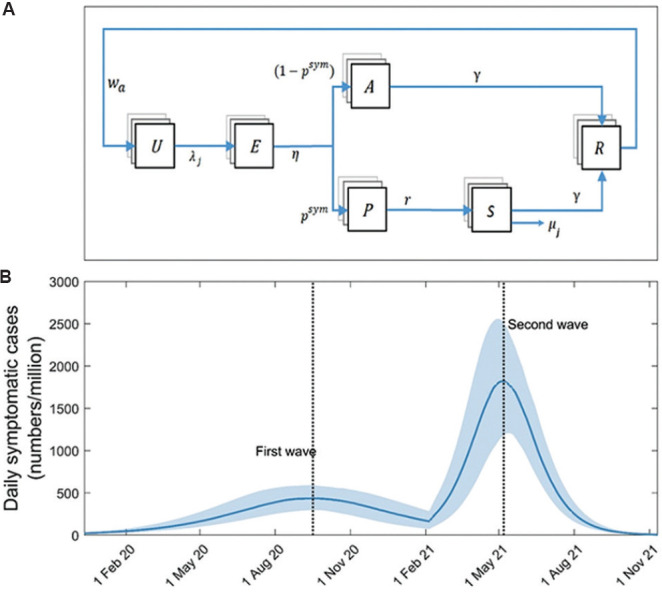
Model structure and approach. (A) Schematic illustration of the model structure. Boxes show states representing different stages in the natural history of SARS-CoV-2 infection, while arrows show flows between these states, as a result of infection, recovery, etc. U, uninfected; E, exposed (latent infection); P, presymptomatic; A, asymptomatic; S, symptomatic; R, recovered and immune. Model parameters are as listed in Table S1. (B) Modelled first and second waves of COVID-19 in India. As described in the main text, these dynamics arise from assuming the emergence of a virus with R0=1.2 in a fully susceptible population (first wave), and then the subsequent emergence of a virus with R0=2.2, to which those with prior exposure remain immune (second wave).
Table S1.
List of state variables
| State symbol | Meaning |
|---|---|
| Uj | Uninfected (j=1, 2, 3 indicating three age groups) |
| Ej | Exposed |
| Aj | Asymptomatic |
| Pj | Pre-symptomatic |
| Sj | Severe symptomatic |
| Rj | Recovered |
Capturing past and current waves of infection: Previous modelling studies have aimed to calibrate formally to the available epidemiological data, taking into account the fact that only a proportion of cases and deaths are likely to have been reported13,14. Given the emphasis of this work on simple qualitative illustration, we did not aim to perform a similar, formal model calibration. Instead, the focus of the current investigation was on ensuring that model projections were reasonably consistent with the available data. Therefore, a two-step process was performed: first, the first wave of infection in India (in 2020) was modelled by assuming a fully susceptible population, and a virus with a basic reproduction number R0(1) chosen to give simulations consistent with the estimated seroprevalence of 7.1 per cent at a country level in early September 202015.
Next, once that first wave diminished, a second wave was simulated by assuming the emergence of a novel, more-infectious virus with a given basic reproduction number R0(2), which was chosen to generate a second wave consistent with the country-level data for the peaks of the first and the second waves: that the latter is four times greater than the former. For simplicity, those infected in the first wave were assumed to remain immune to this novel virus (subject to sensitivity analysis as described below). Figure 1B illustrates the first- and second-wave dynamics arising from these assumptions, using values R0(1)=1.2 and R0(2)=2.2 and exhibiting model behaviour that is qualitatively consistent with the pace and extent of spread in India since end-January 20201. In the analysis below, when incorporating additional mechanisms that affected the dynamics of the second wave, R0(2) was adjusted as necessary to maintain the correct four-fold ratio between the peaks of the second and first waves.
Capturing future waves of infection: Once the peak of the second wave diminished, the following four hypotheses for the emergence of a third wave were considered:
-
(i)
Fully waning immunity: Infection-induced immunity may decay over time, permitting reinfection of those previously exposed, even if the circulating virus remains unchanged. Previous work has shown how partially waning immunity could cause future waves of SARS-CoV-2 to be more benign16 (discussed further below), but the focus here was on the possibility of a third wave that may cause substantial public health burden. Therefore, a scenario where waning immunity results in a full loss of protection, rendering immune individuals susceptible again was considered, and a range of scenarios for the rate of waning were examined.
-
(ii)
Emergence of a full immune escape variant: Even if immunity remains lifelong, it is theoretically possible for a new variant to emerge that is capable of escaping the immunity induced by the previously circulating strains. This was modelled in a simple way, by assuming that this new variant still has R0=2.2, so being equally infectious as the second-wave virus, but (as an illustrative example) is capable of re-infecting 50 per cent of all those previously exposed to SARS-CoV-2. As for fully waning immunity, an extreme scenario of full immune escape was assumed, that there is no residual protection from the past exposure against severe disease. Taking model conditions at the end of the second wave, 50 per cent of those in the ‘recovered’ compartment were moved to the ‘susceptible’ compartment, to simulate the emergence of the full immune escape variant.
-
(iii)
Emergence of a still-more transmissible variant: In the same manner as the second wave being driven by a virus with R0=2.2, the subsequent emergence of a novel virus with still-higher R0, but to which previously-exposed individuals would remain immune was modelled. We examined how high R0 would need to be, for this new variant to generate a substantial third wave.
-
(iv)
Lockdown/release: Despite an intense rate of transmission, State-wide lockdowns in Delhi and Maharashtra may have been impactful in mitigating the second wave in these States. The potential for the lifting of such restrictions to allow a third wave was modelled. In response to the second wave, lockdowns in India varied across the country in both their timing and stringency, the latter ranging from temporary curfews to complete, sustained restriction of movement17. Rather than aiming to capture these measures in a detailed way, focus was laid on their effect in reducing transmission. This effect was modelled in a simple way, by assuming a multiplier k on the rate of transmission (equivalently, on the effective reproduction number). A value of k<1 was adopted for the period of lockdown. Reducing transmission in this way leads to delaying of the epidemic peak, flattening of the epidemic time course, and a reduction in the overall epidemic size. Following the period of lockdown, we subsequently modelled a gradual, linear transition to k=1 over a period of three months.
As described above, in all scenarios the value of R0(2) was adjusted in the second wave to yield the correct, four-fold ratio between the peaks of the first and the second waves. First the effect of each of these mechanisms was examined, in isolation. The following additional scenarios were then modelled
-
(v)
Highly transmissible immune escape variant: Emergence of a variant that can escape immunity, as well as being more transmissible. This scenario represents a combination of mechanisms (ii) and (iii) above, a plausible scenario given the evidence for increased transmission and vaccine escape already shown by the B.1.617.2 (Delta) variant18.
-
(vi)
Highly transmissible immune escape variant, with ramp-up of vaccination: Although vaccination coverage has arguably not yet reached levels sufficient to alter the course of the epidemic in India, it may do so in future. Accordingly, a scenario where 40 per cent of the population have received two doses of vaccine, within three months of the second wave peak was modelled. It was further assumed that the effect of vaccination is (conservatively) to reduce the severity of infection by 60 per cent. For illustration, this scenario was modelled against the background of emergence of an immune escape variant, which is highly transmissible immune escape variant as described in scenario (v) above.
Model uncertainty: There is uncertainty around several parameters relating to natural history, for example, the role of asymptomatic and presymptomatic infection in transmission. To capture this uncertainty in a systematic way, plausible parameter ranges were posed for all such model parameters (Table S2). Two hundred and fifty parameter sets were drawn from these ranges using latin hypercube sampling; model outcomes were simulated independently with each parameter set and, from the resulting ensemble of model outcomes, 95 per cent confidence intervals (CI) were estimated as the interval between the 2.5th and 97.5th percentiles.
Table S2.
Parameters used in the model simulation
| Parameter | Meaning | Values | Source/remarks | ||
|---|---|---|---|---|---|
| β | Transmission rate from symptomatic infection | 0.06 for R0=1.3 (Increases in proportion to R0) | Calculated in order to yield assumed value of R0 for different waves in India, by evaluating spectral radius of next-generation matrix (e.g. as described earlier2) | ||
| η | Amongst those exposed, rate of developing infectiousness | (1/3-1/5)/day | Corresponds to an average latent period of 3-5 days: Together with the period of pre-symptomatic transmission (see r below), corresponds to an overall average incubation period of 4-6 days3 | ||
| p (sym) | Proportion developing symptoms | 1/3-2/3 | Wide variation noted in individual studies and meta-analysis4-6 | ||
| k | Relative infectiousness of asymptomatic versus symptomatic infection | 2/3-1 | |||
| r | Amongst those with pre-symptomatic infection, rate of developing symptoms | 1/day | Assumption, corresponds to mean pre-symptomatic duration of one day | ||
| γ | Recovery rate | 0.2/day | Assumption, corresponds to mean infectious period of five days7 | ||
| wa | Per-capita rate at which post-infection immunity wanes | (1/365-1/120)/day | Assuming mean duration of immunity lasts for four months to one year8 | ||
| Parameter | Meaning | Age groups (yr) | Source/remarks | ||
|
| |||||
| <24 | 24-60 | >60 | |||
| CFRj | Case fatality rate in age Group I | 0.1% | 1.45% | 10.9% | Represents proportion dying amongst those with symptoms. Drawn from a recent study from two Indian States1 |
| µj | Mortality rate for severe cases | 0.0002/day | 0.0029/day | 0.0245/day | Hazard rates of µi are calculated to yield case fatality rates, using: CFRi=µi/(µi+γ) Uncertainty in the mortality hazards are considered +/−25% |
| Nj | Population (India) | 46% | 44.5% | 9.5% | Extrapolated from the Census of India 20119 |
| mij | Connectivity matrix between age Group I with age Group J | 1.37 | 1.43 | 0.05 | Drawn from reference1. Uncertainty in the each element of the contact matrix is considered +/−5% |
| 2.52 | 2.90 | 0.10 | |||
| 0.28 | 0.34 | 0.02 | |||
There remains much uncertainty about parameters relating to SARS-CoV-2 natural history, e.g., infectiousness of asymptomatic people relative to symptomatic ones and, duration of pre-symptomatic period, etc., In this study we adopted a range of parameter values to reflect this uncertainty in our model projections; we sampled uniformly from the parameter ranges shown here.
This modelling was performed using Matlab release 2020 (https://www.mathworks.com/products/matlab.html), through a paid subscription.
Results
Full waning: Figure 2A shows the implications of fully waning immunity in the illustrative example where protective immunity lasts only for 12 months. The Figure illustrates how such waning can generate a third and subsequent waves of symptomatic infection that eventually settle into an endemic equilibrium. Moreover, Figure 2B illustrates that shorter durations of protective immunity tend to hasten the emergence of the third wave, and vice versa. Recapturing some standard behaviour of acute immunizing infections19, these dynamics serve to illustrate how waning immunity can replenish the supply of susceptible individuals, allowing the virus to persist in the population. However, in order to model a third wave that causes a substantial public health burden, it was assumed here that waning immunity would lead to a complete loss of protection. As discussed below, more nuanced (and arguably more realistic) scenarios of waning immunity would be expected to give rise to far milder outcomes and delayed onset than those illustrated here.
Fig. 2.
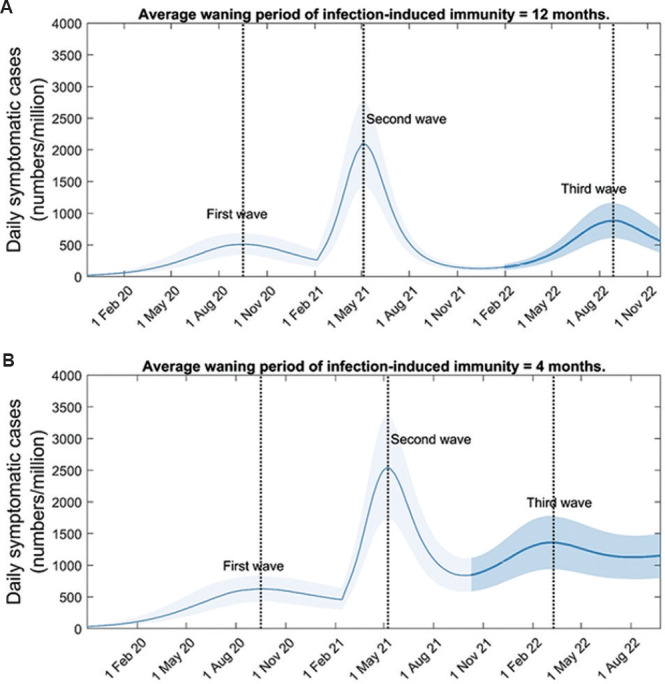
Impact of waning immunity on post-second-wave dynamics. We assumed no change in the virus, but only that any protective immunity to past exposure wanes at a given rate. (A) Potential third-wave dynamics arising from the example of infection-induced immunity fully waning after an average of 12 months. (B) Alternative dynamics for a scenario of shorter-duration immunity (four months). Results illustrate that the size of the third wave is roughly unchanged, but that it occurs more early than in the scenario shown in panel A. In order to maintain the correct ratio of peak heights of first and second waves, here we took R0(2) = 2 in panel A, and R0(2) = 1.8 in panel B.
Full immune escape: Figure 3A shows illustrative dynamics in the event of emergence of a virus, to which 50 per cent of those previously exposed are rendered fully susceptible again. In the example shown here, a third wave would have an attack rate estimated at 1.2 per cent of the population (95% CI, 0.8 - 1.6), with a peak symptomatic incidence of 760 cases per million population per day (95% CI, 506 - 1046). Figure 3B shows third-wave outcomes for a range of scenarios for the degree of immune escape, illustrating that any new virus would need to restore at least 30 per cent of previously-infected individuals to full susceptibility again, in order to cause a severe third wave.
Fig. 3.
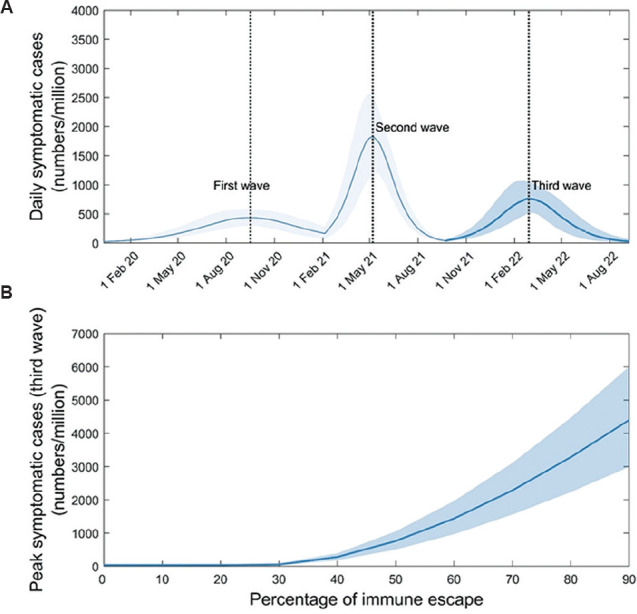
Impact of an immune escape variant on post-second-wave dynamics. In contrast to Fig. 2, here we control for waning by assuming that infection confers lifelong immunity to the infecting strain. Following the second wave, we assume the emergence of a novel virus that is equally infectious (R0(3)=2.2), but that renders a proportion p of those previously exposed as fully susceptible once again. (A) Potential third-wave dynamics arising from the example of 50 per cent immune escape (i.e. P=0.5). (B) How the size of the third wave would vary with the value of P. For the (purely illustrative) parameters assumed here, an immune escape variant would need to render at least 30 per cent of those with prior immunity completely susceptible again, in order to cause a substantial third wave.
Increased transmission: Figure 4A illustrates the potential outcome of emergence of a new virus that has evolved to have about 2.5 times more infectiousness than the virus causing the second wave (i.e. with R0(3)=5.4). In the example shown here, the third wave has a symptomatic attack rate of 0.8 per cent of the population (95% CI, 0.5 - 1.0), and causes a peak symptomatic incidence of 551 cases per million population per day (95% CI, 297 - 854). Figure 4B shows third wave outcomes for a range of scenarios for the transmissibility of the variant, illustrating that any new virus would need to have R0(3) of at least 4.5 to cause an epidemic in the face of pre-existing immunity following the first and the second waves.
Fig. 4.
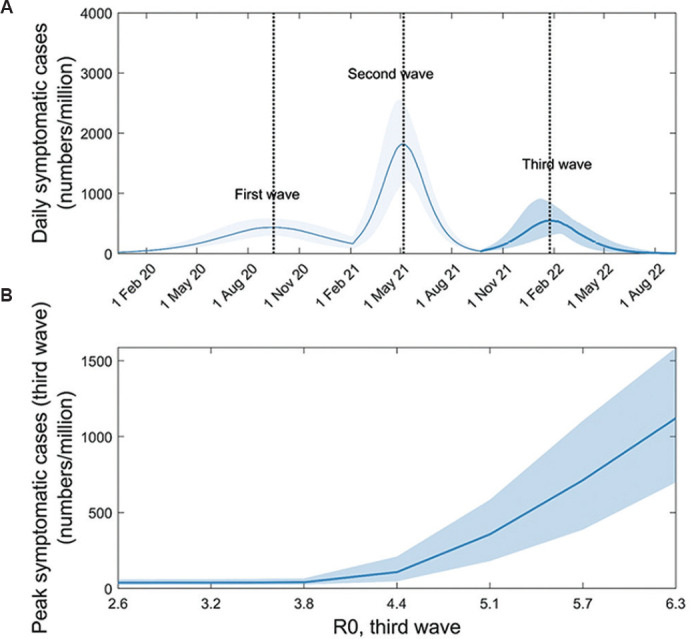
Impact of a more-transmissible variant on post-second-wave dynamics. Following the second wave, we assumed the emergence of a novel virus with basic reproduction number, R0(3)>2.5. Again to control for other factors, we assume that those previously exposed remain immune to this novel virus, and further that prior immunity wanes on a timescale longer than modelled here. (A) Potential third-wave dynamics arising from the example of a novel virus with R0(3)=5.4. (B) How the size of the third wave would vary with R0(3). For the (purely illustrative) background parameters assumed here, a novel, more-transmissible virus would need to have R0(3) at least 4.5, in order to cause a substantial third wave.
Lockdown/release: Figure 5A shows illustrative dynamics for a lockdown that is instated after two months of spread during the second wave, is 50 per cent effective at reducing transmission, and is subsequently released, once the second wave has ended. The Figure illustrates the emergence of a third wave, following the release of the lockdown. Here, a large second wave leaves sufficient numbers of individuals exposed (and immune) to slow the re-emergence of a virus following lifting of lockdown. To capture the range of responses observed in India, Figure 5B shows third wave outcomes under a range of assumptions for lockdown-related reductions in transmission, ranging from zero (essentially, no lockdown) to 90 per cent reduction. The Figure shows that early instated highly effective lockdowns are most at risk of allowing the resurgence of a third wave after being released; these are the lockdowns that minimize exposure most effectively, keeping a sufficient number of vulnerable individuals, thus allowing opportunities for transmission when released.
Fig. 5.
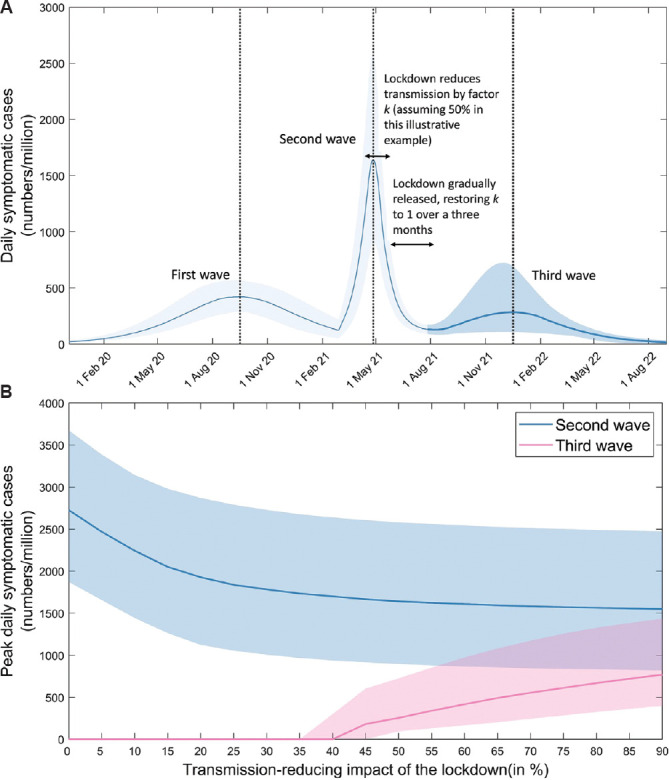
Impact of lockdown-release on post-second-wave dynamics. A basic reproduction number of R0(3)=2.2 was assumed for the second wave. To capture the effect of local lockdowns in response to this wave, we assumed that lockdowns were initiated after seven weeks of epidemic growth, consistent with experience in Delhi, Maharashtra and elsewhere, and further that these lockdowns have the effect of reducing transmission by a given amount. Once the modelled second wave diminished, we then simulated ‘release’ as a gradual restoration of R0(2) to reach its pre-lockdown value over a period of three months. To control for other factors, we assumed no change in the circulating virus, and no waning of infection-induced immunity. (A) Potential third-wave dynamics arising from the example of a lockdown reducing transmission by 50 per cent. In order to maintain the correct ratio of peak heights of first and second waves, here R0(2) = 2.4. (B) How the size of the third wave would vary with different scenarios for the effectiveness of the lockdown, in reducing transmission. The same timings for lockdown and easing were assumed as depicted in panel A. Results illustrate an inverse relationship between the sizes of the second and third waves.
While Figures 2-5 show only mechanisms acting in isolation, it is possible that they may also act in combination. Figure 6 illustrates what is arguably the most likely co-occurrence of mechanisms, the emergence of a new virus that is simultaneously more transmissible (R0=3), and capable of full immune escape from previously circulating strains (here, we assume escaping 25% of prior immunity). The Figure illustrates the potential for a third wave, even if this modelled virus has lower transmissibility than Figure 4, and a lower degree of immune escape than shown in Figure 3 (that is, by combining these mechanisms, the threshold to generate a third wave is lowered).
Fig. 6.
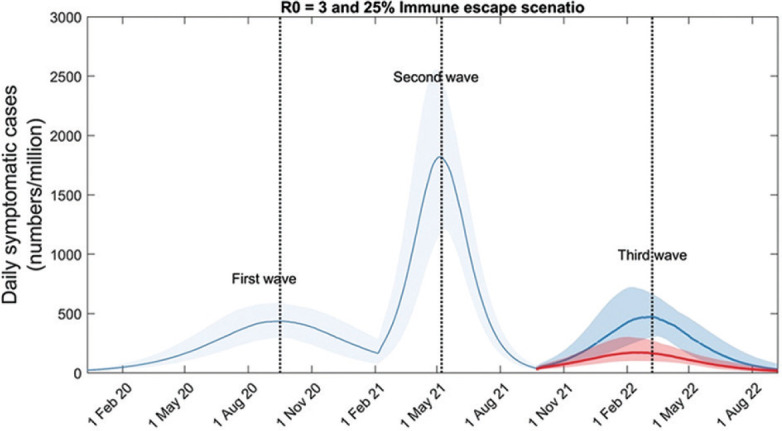
Impact of a ‘highly transmissible immune escape variant’ on post-second-wave dynamics. Here the emergence of a novel variant that combines immune escape with increased transmission potential was assumed, relative to the second-wave virus. In particular, a variant that renders 25 per cent of those previously infected fully susceptible again, and has R0(3)=3. (In order to maintain the correct ratio of peak heights of first and second waves, here R0(2)=2 for the second wave). The dark blue trajectory illustrates a scenario where the combined variant spreads unmitigated. The red trajectory illustrates a scenario where vaccination coverage is rapidly expanded immediately after the second wave, in order for 40 per cent of the population to receive both doses of vaccine within three months. The figure illustrates the substantial reduction in third-wave burden that could result, from such scaled-up vaccination efforts.
However, the emergence of a third wave could be substantially mitigated by the expansion of vaccination (Fig. 6). Shown is an example of the rollout of vaccine, in such a way as to cover 40 per cent of the population with two doses over a period of three months following the end of the second wave. Under the illustrative parameters assumed here, such a rapid vaccination scale-up would reduce symptomatic incidence by around 55 per cent (95% CI, 46 - 73). Figure S1 (1MB, tif) additionally shows model projections for how vaccination may mitigate any third wave arising from a lockdown/release scenario, showing similar impact to that showing in Figure 5B.
In all of the results above, it is assumed - for simplicity and ease of exposition - that the potential for a third wave emerged only after the second wave has resolved. In practice, however, it is equally plausible that a new variant emerges while the second wave is still in progress. Figure S2 (1.3MB, tif) illustrates how the dynamics might appear under these circumstances, in the illustrative example of the emergence of a novel variant that combines immune escape with increased transmissibility (i.e. as illustrated in Figure 6, but assuming an earlier date of emergence). The figure illustrates qualitatively similar dynamics to Figure 6, but with overlapping second and third waves.
Discussion
With ongoing discussion about the potential for a severe third wave in India, it is important to understand the mechanisms by which such a resurgence could occur. Using simple models capturing essential features of SARS-CoV-2 transmission, this study illustrates some possible mechanisms, concentrating on the potential for a wave that causes substantial public health burden. Our findings highlight that: (i) waning immunity could lead to future waves, as a part of the virus becoming endemic in the human population, (ii) a new immune escape variant with the same R0(3) = 2.2 would need to fully escape at least 30 per cent of prior immunity, to cause a third wave, (iii) a more-transmissible variant would need R0(3) of at least 4.5 to cause a third wave, and (iv) lockdown-release mechanisms could be a plausible driver for a third wave in India, depending on how effectively lockdowns have controlled transmission during the second wave particularly when instated at an early stage of the second wave and prior to attainment of peak.
Overall, these results suggest that a third wave, if it should occur, is unlikely to be as severe as the second wave, given the extent of spread that has already taken place. A major reason for this model behaviour is that, given the explosive spread of infection across the country, a substantial proportion of the population is likely to have been exposed by the end of the current second wave, accounting for the effect of lockdowns. Consequently, for a virus to cause a major third wave in the face of this pre-existing immunity, extreme scenarios for the abrogation of that immunity are required, or for that matter, for the transmission fitness of any novel virus.
Largely, our analysis highlights three key considerations in multi-wave dynamics:
-
(i)
Individual behaviour and societal factors: Crowding, use of mask and physical distancing during social interactions are all key factors shaping transmission rate and therefore population-level spread. The reduction in transmission depicted in Figure 5 could equally be applied to other non-pharmaceutical measures such as mask use and physical distancing; their relaxations could likewise contribute to further waves. Moreover, social mixing of individuals between different age groups and within a group also determine the pattern of spread20. These ‘mixing patterns’ have important implications for demographic patterns in the spread of infection, that are typically captured in models (as in the present one) through a ‘mixing matrix’. Further data on these mixing patterns in India will be valuable in refining these models.
-
(ii)
Health systems: Firstly, the effectiveness of any lockdown or other non-pharmaceutical intervention will depend critically on the health system in any given setting. For example, by drawing upon the example of Maharashtra5, the northeastern States of India instated early lockdown in response to the second wave even before attainment of its peak. If these and other similar settings are most vulnerable to re-emergence when restrictions are eased, then such settings may represent priorities for ramped-up vaccination coverage. Secondly, preparedness for any third wave (or indeed future pandemics) is likely to go hand-in-hand with the overall strengthening of the health system, for example, improved tertiary care capacity and enhanced co-ordination throughout India's complex healthcare system. Such developments would have important long-term benefits, not limited only to pandemic preparedness.
-
(iii)
Biological factors: In addition to viral transmission, immunity to SARS-CoV-2 raises several important considerations. First, in relation to virus escape from vaccine-induced protection, evidence from the UK suggests that a single dose of ChAdOx1 nCoV-19 (Oxford AstraZeneca vaccine, Serum Institute of India Limited, Pune) shows reduced efficacy against symptomatic infection with B.1.617.2, but that two doses show high efficacy21,22. As long as current vaccines are similarly effective against any future immune escape variant, ramping up of coverage could substantially reduce the third wave burden arising from any combined virus related factors Figure 6.
Second, in relation to virus escape from infection-induced immunity, the immunological scenarios we considered were deliberately exaggerated (e.g., waning immunity leading to complete loss of protection). In practice, even if a previously-exposed individual becomes reinfected, it is likely that the repeat infection would show substantially less severity than the original exposure. For example, even if neutralising antibodies are rendered ineffective either through waning or viral evolution, the T-cell response can continue to play an important role in moderating the clinical course of repeat infection23,24,25. To the extent that such partial loss of immunity is a more realistic scenario than complete loss of protection, one might expect any immune-mediated third wave to be a mild one, without the severe public health challenge posed by the preceding second wave. The previous modelling analysis has shown how such mild reinfections could mediate the establishment of SARS-CoV-2 as an endemic virus in the human population, as benign as the already-circulating coronaviruses26.
Any modelling analysis must make a careful balance between realism and simplicity, with the correct choice for that balance being determined by the modelling question being addressed. Our analysis is intended to be illustrative and not predictive, accordingly, we did not aim to perform a formal model calibration to existing data, instead choosing values of R0 in the first and the second waves to give epidemiological dynamics that are broadly consistent with what has been observed in India. Given our emphasis on simple, illustrative models, our approach also neglects some features relevant to the mechanisms. First, there is evidence to suggest that the rate at which immunity wanes can depend on the severity of infection27,28. In the present approach, we considered essentially a uniform waning rate over the spectrum of severity. Second, the basic reproduction number (equivalently, the rate-of-transmission) was assumed to remain constant during each wave. This value may, however, be modified by non-pharmaceutical interventions such as movement restrictions and mask use, all of which have changed over time, since the first wave. Other modelling analysis has aimed to capture such dynamic phenomena14. Since the main approach of the present analysis was simplicity, one might expect the basic, qualitative insights from this analysis to apply more generally and at the same time to programme planning for preparedness. Third, we have assumed immune escape or more-transmissible variants to emerge at the end of the second wave, although an equally plausible scenario is for such variants to begin spreading much earlier. Modelling such possibilities in a mechanistic way requires our transmission model to be linked with an evolutionary model, although the data to inform the latter data sparse. Still, it is suggested that early emergence of new variants (i.e. while the second wave is still ongoing) would not qualitatively change our overall findings [Fig. S1 (1MB, tif) ].
In the present study, other mechanisms known to provide wave-like behaviour in compartmental models were also not considered. One example is population turnover, where for perfectly immunizing infections such as measles, a buildup of fully susceptible newborns in a population can cause repeated cycles of infection29,30. Seasonal drivers of transmission, such as those that drive the annual season of influenza and other respiratory viruses in temperate regions, were also not considered, given the lack of any coherent influenza season across India12. It seems unlikely for seasonal drivers to play an important role in a country-wide, synchronised third wave of COVID-19. Another possible mechanism, not modelled here, is spatial heterogeneity in spread, that is, areas that are relatively untouched in one wave but are then disproportionately affected in a subsequent wave.
Despite aforementioned limitations, mathematical models can be helpful tools for understanding how underlying mechanisms can act, to generate important epidemiological behaviour such as the emergence of a third wave of COVID-19. These can also help inform programme planning for preparedness, and resource allocation by projecting reasonable as well as worst case scenarios.
Supplementary Materials
Illustrative results for the impact of vaccination on a third wave in a lockdown/release scenario. Using the same parameters as for Fig. 5A in the main text, the red curve shows a vaccine ramp-up scenario where 40 per cent of the population has received two doses within three months of the second wave peak, and further that the effect of vaccination is (conservatively) to reduce severity of to infection by 60 per cent. Results illustrate how vaccination could substantially reduce the overall burden during the third wave.
Illustrative implications for early emergence of a variant. In the main text we assume, for simplicity and ease of exposition, that any variant causing a third wave would emerge only after the second wave has fully resolved. Here we show illustrative examples where emergence occurs while infection levels are still high, during the second wave. (A) illustrates early emergence of an immune escape variant (analogous to Fig. 3), (B) illustrates early emergence of a more-transmissible variant (analogous to Fig. 4). Both cases show the potential for overlapping second and third waves.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. [accessed on May 31, 2021]. Available from: https://covid19.who.int/region/searo/country/in .
- 2.Taubenberger JK, Morens DM. 1918 Influenza: The mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorigatti I, Cauchemez S, Ferguson NM. Increased transmissibility explains the third wave of infection by the 2009 H1N1 pandemic virus in England. Proc Natl Acad Sci U S A. 2013;110:13422–7. doi: 10.1073/pnas.1303117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunday Guardian, Two variants responsible for second wave. [accessed on May 20, 2021]. https://www.sundayguardianlive.com/news/two-variants-responsible-second-wave.
- 5.The Times of India. 20 cases of new Delta-plus variant in India, 8 in Maharashtra. [accessed on May 20, 2021]. Available from: https://timesofindia.indiatimes.com/city/mumbai/20-cases-of-new-delta-plus-variant-in-india-8-in-maharashtra/articleshow/83700399.cms .
- 6.Ministry of Health and Family Welfare Press release 16 Jan 2021. [accessed on May 30, 2021]. Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1689018 .
- 7.The Indian Express. Third Covid wave inevitable, didn't foresee current ferocity: Scientific Advisor to PM. [accessed on May 6, 2021]. Available from: https://indianexpress.com/article/coronavirus/india-coronavirus-pandemic-third-wave-hospitals-oxygen-shortage-7303234/
- 8.Han E, Tan MM, Turk E, Sridhar D, Leung GM, Shibuya K, et al. Lessons learnt from easing COVID-19 restrictions: An analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396:1525–34. doi: 10.1016/S0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Prettner K, Kuhn M, Geldsetzer P, Wang C, Bärnighausen T, et al. Climate and the spread of COVID-19. Sci Rep. 2021;11:9042. doi: 10.1038/s41598-021-87692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes. The real cause of America's third wave of Covid-19: What is driving this third wave? [accessed on May 31, 2021]. Available from: https://www.forbes.com/sites/johndrake/2020/12/07/the-real-cause-of-americas-third-wave-of-covid-19/?sh=16e60b7112fd .
- 11.Vaidyanathan G. Coronavirus variants are spreading in India - what scientists know so far. Nature. 2021;593:321–2. doi: 10.1038/d41586-021-01274-7. [DOI] [PubMed] [Google Scholar]
- 12.Chadha MS, Potdar VA, Saha S, Koul PA, Broor S, Dar L, et al. Dynamics of influenza seasonality at sub-regional levels in India and implications for vaccination timing. PLoS One. 2015;10:e0124122. doi: 10.1371/journal.pone.0124122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pons-Salort M, John J, Watson OJ, Brazeau NF, Verity R, Kang G, et al. Reconstructing the COVID-19 epidemic in Delhi, India: Infection attack rate and reporting of deaths. medRxiv. 2021 doi: 10.3201/eid2804.210879. doi: 10.1101/2021.03.23.21254092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazra DK, Pujari BS, Shekatkar SM, Mozaffer F. The INDSCI-SIM model for COVID-19 in India. medRxiv. 2021 doi: 10.1101/2021.06.02.21258203. [Google Scholar]
- 15.Murhekar MV, Bhatnagar T, Selvaraju S, Saravanakumar V, Thangaraj JW, Shah N, et al. SARS-CoV-2 antibody seroprevalence in India, August-September, 2020: Findings from the second nationwide household serosurvey. Lancet Glob Health. 2021;9:e257–66. doi: 10.1016/S2214-109X(20)30544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandal S, Das H, Deo S, Arinaminpathy N. Combining serology with case-detection, to allow the easing of restrictions against SARS-CoV-2: A modelling-based study in India. Sci Rep. 2021;11:1835. doi: 10.1038/s41598-021-81405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of Home Affairs, Government of India. No.40-3/2020-DM-I (A), 29th April, 2021. [accessed on May 31, 2021]. Available from: https://static.mygov.in/rest/s3fs-public/mygov_16197↰451307401.pdf#page=3 .
- 18.Dhar MS, Marwal R, Radhakrishnan VS, Ponnusamy K, Jolly B, Bhoyar RC, et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 Variant in Delhi, India. medRxiv. 2021 doi: 10.1126/science.abj9932. doi: 10.1101/2021.06.02.21258076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson RM, May RM. Infectious diseases of humans: Dynamics and control. Great Britain, Burgess Hill, West Sussex, Oxford: Oxford University Press; 1992. [Google Scholar]
- 20.Laxminarayan R, Wahl B, Dudala SR, Gopak K, Mohan B Chandra, Neelima S, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020;370:691–7. doi: 10.1126/science.abd7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of infectious SARS-CoV-2 variant B.1.617.2 to monoclonal antibodies and sera from convalescent and vaccinated individuals. bioRxiv. 2021 doi: 10.1101/2021.05.26.445838. [Google Scholar]
- 22.Bernal JL, Andrews N, Gower C, Gallogher E, Simmous R, Thetwall S, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021 doi: 10.1056/NEJMoa2108891. doi: 10.1101/2021.05.22.21257658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan Y, Liu F, Xu X, Ling Y, Huang W, Zhu Z, et al. Durability of neutralizing antibodies and T-cell response post SARS-CoV-2 infection. Front Med. 2020;14:746–51. doi: 10.1007/s11684-020-0822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson AC, Humbert M, Buggert M. The known unknowns of T cell immunity to COVID-19. Sci Immunol. 2020;53 doi: 10.1126/sciimmunol.abe8063. eabe8063. [DOI] [PubMed] [Google Scholar]
- 25.Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase ½clinical trial. Nat Med. 2021;27:270–8. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 26.Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371:741–5. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJ, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang AT, Garcia-Carreras B, Hitchings MD, Yang B, Katzelnick LC, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rota PA, Moss WJ, Takeda M, de Swart RL, Thompson KM, Goodson JL. Measles. Nat Rev Dis Primers. 2016;216049 doi: 10.1038/nrdp.2016.49. [DOI] [PubMed] [Google Scholar]
- 30.Kang HJ, Han YW, Kim SJ, Kim YJ, Kim AR, Kim JA, et al. An increasing, potentially measles-susceptible population over time after vaccination in Korea. Vaccine. 2017;35:4126–32. doi: 10.1016/j.vaccine.2017.06.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Illustrative results for the impact of vaccination on a third wave in a lockdown/release scenario. Using the same parameters as for Fig. 5A in the main text, the red curve shows a vaccine ramp-up scenario where 40 per cent of the population has received two doses within three months of the second wave peak, and further that the effect of vaccination is (conservatively) to reduce severity of to infection by 60 per cent. Results illustrate how vaccination could substantially reduce the overall burden during the third wave.
Illustrative implications for early emergence of a variant. In the main text we assume, for simplicity and ease of exposition, that any variant causing a third wave would emerge only after the second wave has fully resolved. Here we show illustrative examples where emergence occurs while infection levels are still high, during the second wave. (A) illustrates early emergence of an immune escape variant (analogous to Fig. 3), (B) illustrates early emergence of a more-transmissible variant (analogous to Fig. 4). Both cases show the potential for overlapping second and third waves.


