Abstract
Background & objectives:
Vaccination against SARS-CoV-2 is a recommendation from the World Health Organization as the foremost preference in the current situation to control the COVID-19 pandemic. BBV152 is one of the approved vaccines against SARS-CoV-2 in India. In this study, we determined SARS-CoV-2–specific antibody levels at day 0 (baseline, before vaccination), day 28 ± 2 post-first dose (month 1) and day 56 ± 2 post-first dose (month 2) of BBV152 whole-virion–inactivated SARS-CoV-2 recipients, and compared the antibody responses of individuals with confirmed pre-vaccination SARS-CoV-2 infection to those individuals without prior evidence of infection.
Methods:
Blood samples were collected from 114 healthcare professionals and frontline workers who received BBV152 vaccine from February to May & June 2021. Prior infection with SARS-CoV-2 was determined at baseline. Serum samples were used to estimate SARS-CoV-2 nucleoprotein-specific IgG [IgG (N)], spike protein-specific IgG [IgG (S)] and neutralizing antibodies (NAb).
Results:
Participants with previous SARS-CoV-2 infection after a single vaccine dose elicited IgG (N) and IgG (S) antibody levels along with NAb binding inhibition responses levels were similar to infection-naïve vaccinated participants who had taken two doses of vaccine.
Interpretation & conclusions:
Our preliminary data suggested that a single dose of BBV152-induced humoral immunity in previously infected individuals was equivalent to two doses of the vaccine in infection-naïve individuals. However, these findings need to be confirmed with large sized cohort studies.
Keywords: BBV152, COVID-19 vaccine, IgG, neutralizing antibody, SARS-CoV-2
The vaccine BBV152 is a whole-virion–inactivated SARS-CoV-2 vaccine adjuvanted with Algel-IMDG [an imidazoquinoline molecule chemisorbed on alum (Algel)]1. Algel-IMDG is a Toll-like receptor 7/8 agonist2,3. BBV152 has been shown to elicit good humoral and cell-mediated immune responses, with an acceptable safety profile in both Phase 1 and Phase II studies4. BBV152 is one of the first vaccines approved for clinical use in India. The shortages in COVID-19 vaccine manufacturing and supply have led experts to recommend the use of a single dose of COVID vaccines in SARS-CoV-2–recovered individuals so that naïve individuals with no prior SARS-CoV-2 infection can be prioritized to complete the two doses5. Various recent studies have shown that individuals who recovered from COVID-19 exhibit protective memory responses in both humoral and cell-mediated arms that last for at least 6-8 months6,7,8. Others reported increased antibody titres and neutralization activity after the first dose of SARS-CoV-2 mRNA (Pfizer and Moderna) vaccines in COVID-19–recovered individuals9,10. Similarly, a single dose of ChAdOx1/AZD1222 vaccine has been shown to elicit increased neutralizing antibody (NAb) and protective immunity in SARS-CoV-2 infection-recovered individuals in comparison to those with no prior exposure11. On this basis, it has been suggested that shifting the present vaccine recommendation to offer only a single dose of vaccine to COVID-19–recovered individuals would free up many immediately needed vaccine doses. However, whether such immune response holds good for BBV152 vaccine, is not known. Therefore, this study was undertaken to examine SARS-CoV-2–specific antibody responses after day 0 (baseline, before vaccination), day 28±2 post-first dose (month 1) and day 56±2 post-first dose (month 2) of BBV152 in a group of healthcare professionals as well as frontline workers, and the antibody responses of individuals with confirmed pre-vaccination SARS-CoV-2 infection were compared with those individuals without prior evidence of infection.
Material & Methods
Study population: The blood specimens were collected from healthcare professionals (individuals working in the research institutes and hospitals) and frontline workers who received BBV152 vaccine [manufactured by Bharat Biotech, Hyderabad, in collaboration with the Indian Council of Medical Research (ICMR), India] at vaccination centres in Chennai, India, during February to May & June 2021. The collected blood samples were transported on the same day to the Immunology laboratory of ICMR, National Institute for Research in Tuberculosis (NIRT), Chennai, India, in a temperature maintained ice-cool box following which samples were centrifuged and serum samples were stored in −80°C freezers. All participants were more than 18 yr of age and from both genders. Blood samples were collected before receiving the first dose of BBV152. Prior infection with SARS-CoV-2 was determined by SARS-CoV-2 IgG positivity at baseline. The demographics of the study population are shown in the Table, and the outline of participant categorization is shown in Fig. 1. The study was approved by the Ethics Committee of ICMR-NIRT (NIRTINo: 2021007). Informed written consent was received from all study individuals.
Table.
Demographic and clinical characteristics of the study participants
| Charecteristics | Total | Prior infection | No prior infection |
|---|---|---|---|
| Total number of participants | 114 | 30 | 84 |
| Age in yr, median (range) | 35 (23-60) | 39 (23-58) | 34 (23-60) |
| Gender (male/female) | 70/44 | 18/12 | 52/32 |
| Temperature (°F), median | 96.7 | 97.3 | 96.7 |
| Contact with COVID-19 case (%) | 12 (10.5) | 8 (26.6) | 4 (4.7) |
| Tuberculosis | Nil | Nil | Nil |
| Diabetes mellitus (%) | 15 (13.1) | 6 (20) | 9 (10.7) |
| Hypertension (%) | 15 (13.1) | 5 (16.6) | 10 (11.9) |
| BCG scar (%) | 95 (83.3) | 22 (73.3) | 73 (86.9) |
| SARS-CoV-2 IgG N protein (AU/ml) | |||
| Baseline, median (n=114) | 0.93 (0.16-183.5) | 29.3 (11.3-183.5)*** (n=30) | 0.71 (0.16-8.2) (n=84) |
| Month 1, median (n=104) | 6.1 (0.4-168.8) | 78.6 (17.1-168.8)*** (n=27) | 2.4 (0.4-149.9) (n=77) |
| Month 2, median (n=74) | 67 (0.45-157.5) | 95 (38-157.5)* (n=16) | 56.3 (0.45-138.1) (n=58) |
| SARS-CoV-2 IgG S protein (AU/ml) | |||
| Baseline, median (n=61) | 0.62 (0.13-388.6) | 48.8 (16.8-388.6)*** (n=21) | 0.37 (0.13-6.3) (n=40) |
| Month 1, median (n=61) | 9.4 (0.18-401.1) | 167.2 (39.9-401.1)*** (n=21) | 2.3 (0.18-259.7) (n=40) |
| Month 2, median (n=61) | 121.7 (14.9-410) | 211 (43.5-410) (n=21) | 86.7 (14.9-240.3) (n=40) |
| SARS-CoV-2 neutralizing antibody (% inhibition) | |||
| Baseline, median (n=87) | 2.1 (−20.6-97.7) | 74.1 (4.5-97.7)*** (n=21) | −1.43 (−20.61-19.7) (n=66) |
| Month 1, median (n=85) | 13.7 (−14.6-97.8) | 95.8 (68.2-95.2)*** (n=20) | 9.2 (−14.6-97.8) (n=65) |
| Month 2, median (n=65) | 74.3 (1.2-97.5) | 94.5 (72.4-93.3)* (n=14) | 68.9 (1.2-97.5) (n=51) |
AU/ml values represented as log-median values. P*<0.05, ***<0.001 compared to those with no prior infection
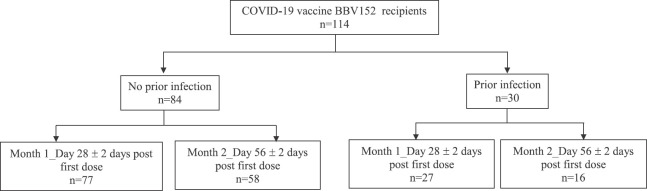
Fig. 1.
Outline of participant categorization. Blood samples were collected from COVID-19 vaccine BBV152 vaccinated individuals (n=114) during day 0 (baseline, before vaccination), day 28±2 post-first dose (month 1) and day 56±2 post-first dose (month 2).
Antibody measurements: Blood (2 ml) was drawn during day 0 (baseline, before vaccination), day 28±2 post-first dose (month 1) and day 56±2 post-first dose (month 2). Serum samples were used to estimate the SARS-CoV-2 nucleoprotein-specific IgG [IgG (N)], SARS-CoV-2 spike protein-specific IgG [IgG (S)] and SARS-CoV-2 NAb. Serological testing for antibodies targeting the IgG (N) and IgG (S) was performed using YHLO iFlash 1800 Chemiluminescence Immunoassay Analyzer, Shenzhen, China, using iFlash-SARS-CoV-2 IgG (N) and iFlash-SARS-CoV-2 IgG (S). Antibody levels were measured at three time points: on the day of vaccination (baseline), at month one following the first dose and at month two following the first dose. The results were determined via a calibration curve, which is an instrument specifically generated by two-point calibration and a master curve provided via the reagent QR code. The cut-off value for SARS-CoV-2 IgM and IgG was decided according to the manufacturer and the IgM and IgG concentrations ≥10.00 AU/ml were considered as positive and <10.00 AU/ml were considered as non-reactive. Serum samples were used to measure the circulating NAb levels using SARS-CoV-2 Surrogate Virus Neutralization Test Kit, version 2.0 (GenScript, New Jersey, USA), according to the manufacturer's instructions. The positive and negative cut-off values for SARS-CoV-2 NAb detection were interpreted based on the inhibition rate. The result was determined by comparing the inhibition rate using the following calculation:
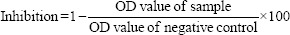
The cut-off values for SARS-CoV-2 NAb detection, according to the manufacturer were ≥20 per cent as positive and <20 per cent as non-reactive.
Statistical analysis: The antibody levels were compared between individuals with and without a prior SARS-CoV-2 infection diagnosis. For comparing between-group proportions, non-parametric Mann–Whitney U test was used. Multiple comparisons were corrected using Holm's correction. Data analyses were done using GraphPad PRISM version 9 (GraphPad Software, Inc., San Diego, CA, USA).
Results & Discussion
There were 114 vaccine recipients, with a mean age of 35 yr, 62 per cent of whom were males (Table). Baseline (pre-vaccine) blood samples for SARS-CoV-2 antibodies were collected from all the 114 vaccine recipients, 30 of whom had prior confirmed SARS-CoV-2 infection-exposure varied between months 1 and 2. One hundred and four vaccine recipients (27 with prior infection) provided blood specimens at month one (wherein they also received the second dose), and 74 recipients (16 with prior infection) provided blood samples at month two. A total of 74 individuals provided blood samples at all three time points. IgG (N) was used to denote prior SARS-CoV-2 exposure.
As shown in Fig 2A, for IgG (N) as anticipated, study participants with previous SARS-CoV-2 infection had elevated antibody levels at all time points: baseline (P<0.001), month 1 (P<0.001) and month 2 (P<0.05) (Table) in comparison with the individuals who did not have a discernible prior infection. Notably, IgG (N) levels were significantly higher in previously infected individuals at baseline in comparison to newly vaccinated participants who received a single vaccine dose [log-median AU/ml (interquartile range), 29.3 (18.2, 65.8) vs. 2.4 (0.93, 17.2), P=0.001)]. Further, among the previously infected individuals after a single vaccine dose, IgG (N) levels were not significantly different in comparison to newly vaccinated participants who had taken two doses [78.6 (59, 122.4) vs. 56.3 (23.2, 92.6)].
Fig. 2.
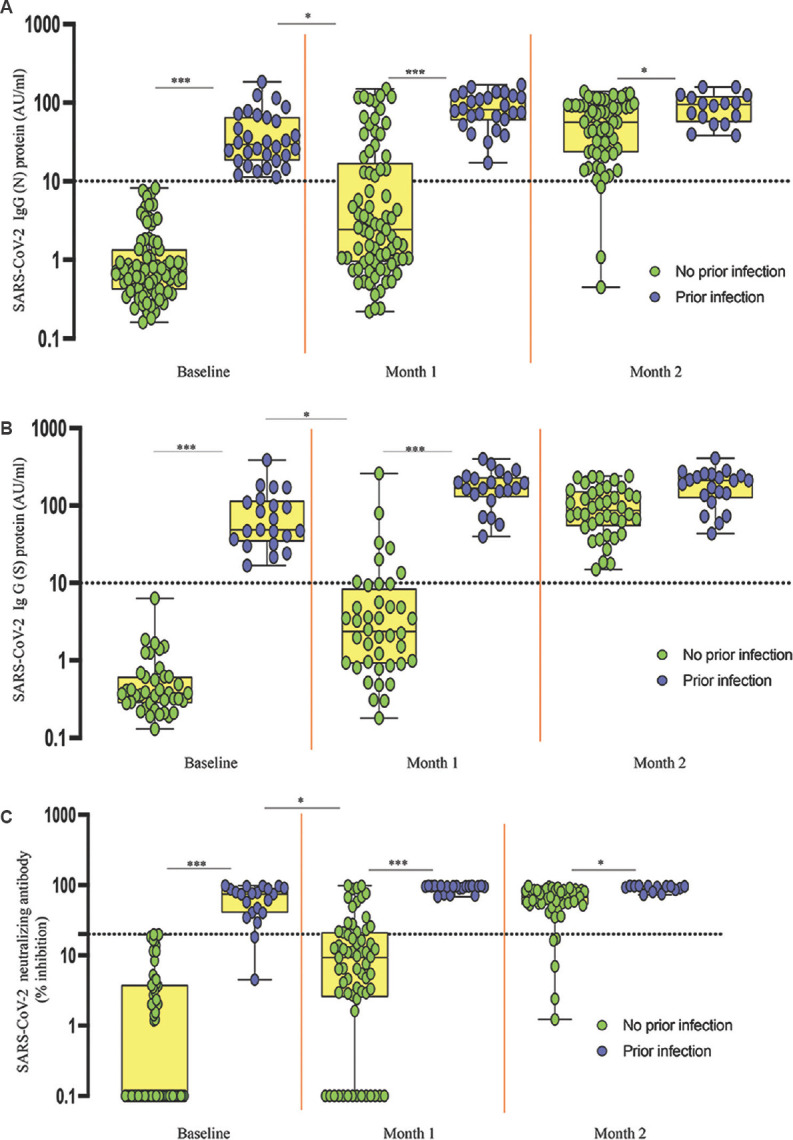
SARS-CoV-2 antibody response to whole-virion–inactivated BBV152 vaccine in individuals with and without prior SARS-CoV-2 infection. The serum levels of (A) SARS-CoV-2 (N) protein, (B) SARS-CoV-2 (S) protein and (C) SARS-CoV-2 neutralizing antibodies (NAb) were measured in no prior infection (n=84) and prior infection (n=30) individuals. Box plots display the median values with the interquartile range (lower and upper hinge). The data are also represented as scatter plots with each circle representing a single individual. The values plotted on y-axis represent log-median values. P *<0.05, ***<0.001 compared to those with no prior infection.
As shown in Fig 2B, for IgG (S), study participants with previous SARS-CoV-2 infection had elevated antibody levels at baseline (P<0.001) and month 1 (P<0.001) whereas no differences were observed between infected and naïve individuals at month 2 (Table). IgG (S) levels were significantly higher in previously infected individuals at baseline in comparison to newly vaccinated participants who received a single vaccine dose [log-median AU/ml (interquartile range), 48.8 (128.4, 233.3) vs. 2.3 (0.91, 8.4), P=0.001]. Further, among the previously infected individuals after a single dose, IgG (S) levels were not significantly different in comparison to newly vaccinated participants who had taken two doses [167 (128.4, 233.3) vs. 86.7 (53.86, 154.2)].
For the SARS-CoV-2 NAb measurement, as shown in Fig 2C participants with previous SARS-CoV-2 infection had elevated neutralization capacity at baseline (P<0.001), month 1 (P<0.001) and month 2 (P<0.05) (Table). SARS-CoV-2 NAb titres were significantly higher in previously infected individuals at baseline in comparison to newly vaccinated participants who received a single vaccine dose [log-median AU/ml (interquartile range, 74.1 (40.3, 89.5) vs. 9.2 (2.5, 21.3), P<0.001]. In addition, among the previously infected individuals after a single dose, SARS-CoV-2 NAb titres were not significantly different in comparison to newly vaccinated participants who had taken two doses [95.8 (89.7, 97) vs. 68.9 (52.3, 83.7)].
It was observed that 71 of the 74 individuals who had received both vaccine doses and provided blood specimens at month two exhibited positive IgG (N) responses, all 61 individuals exhibited positive IgG (S) responses and 60 of the 65 individuals exhibited positive NAb responses.
To further evaluate vaccine responses in individuals with no prior infection (n=84) and prior infection (n=30), the presence of SARS-CoV-2 IgG (S), IgG (N) and NAb was measured at baseline (pre-vaccinated) versus month 1 (28±2 days post-first dose) and baseline versus month 2 (56±2 days post-first dose). At months 1 and 2, there was a significant increase in the SARS-CoV-2 IgG (S), IgG (N) and NAb compared to baseline, indicating that humoral immune responses were boosted in both the groups after vaccination.
Overall, good vaccine-induced antibody responses were seen in prior SARS-CoV-2–infected individuals, except in two, who received a single dose of BBV152 vaccine that was similar to antibody responses seen after a two-dose vaccination course administered to infection-naïve individuals. Our results in a varied group of healthcare professionals and frontline workers lend support to the previous studies (albeit mainly focused on mRNA vaccines) that increased levels of SARS-CoV-2 binding and neutralizing antibodies are present after a single vaccine dose in previously infected individuals and are comparable to the levels seen after two doses in those without prior infection9,12. Our study also delineated the binding and neutralizing antibody response following two doses of BBV152 and demonstrated that the whole-virion–inactivated vaccine induced a robust humoral immune response against SARS-CoV-2 antigens at one month following the two-dose regimen in most of the vaccinated individuals, with all individuals mounting significant IgG (S) and most individuals mounting significant IgG (N) and NAb responses. These findings suggested that a single dose of BBV152 vaccine administered to individuals with prior SARS-CoV-2 infection could not only serve as an adequate stimulus for anti-N and anti-S antibody titres but also for SARS-CoV-2 NAb response generation, indicating the potential neutralizing capability of the elicited antibodies. However, both vaccine doses would be ideal even for previously infected individuals in the long run due to the occurrence of variants of concern.
Characterization of the T cell immune responses can reveal additional information on how a single dose versus a double dose of vaccine could be appropriate for boosting T cell memory in previously infected individuals13. More research studies are required to define the window period for vaccination to further increase the efficacy as well as safety in prior infected individuals. Limitations of the present study included a smaller sample size and failure to examine the NAb capacity against the SARS-CoV-2 variants of concern. In addition, the present study could not determine the efficacy of a single dose of BBV152 vaccine in immunocompromised individuals and in those recovered from COVID-19 but lacked antibody response, like the two individuals in our study. This group needs to be studied further to address the effect of single or booster dose of BBV152 vaccine. Furthermore, larger-sized cohort studies need to be executed with the appropriate statistical power to measure differences in the wider population and clinical subgroups that are known to display variation in antibody responses after vaccination14,15. Some alterations in the antibody response might also be associated with heterogeneity within prior infected individuals which includes timing and severity of prior illness.
In conclusion, almost all participants with prior COVID-19 infection, except two, had detectable antibodies at the time of vaccination. This study offers evidence in support of public health-oriented and immunologically sustained vaccine strategies. If our preliminary findings are confirmed in large population studies, a single dose of BBV152 vaccine may be recommended to previously confirmed SARS-CoV-2 infected individuals so that the naïve individuals could attain the larger benefit of a limited vaccine supply.
Acknowledgment:
Authors thank the staff of the departments of Clinical Research, Immunology and ICER, ICMR-NIRT, Chennai for the timely help.
Footnotes
Financial support & sponsorship: This work was supported by the ICMR.
Conflicts of Interest: None.
References
- 1.Ganneru B, Jogdand H, Daram VK, Das D, Molugu NR, Prasad SD, et al. Th1 skewed immune response of whole virion inactivated SARS-CoV-2 vaccine and its safety evaluation. iScience. 2021;24:102298. doi: 10.1016/j.isci.2021.102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philbin VJ, Dowling DJ, Gallington LC, Cortés G, Tan Z, Suter EE, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130:195–204.e9. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shukla NM, Salunke DB, Balakrishna R, Mutz CA, Malladi SS, David SA. Potent adjuvanticity of a pure TLR7-agonistic imidazoquinoline dendrimer. PLoS One. 2012;7:e43612. doi: 10.1371/journal.pone.0043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637–46. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frieman M, Harris AD, Herati RS, Krammer F, Mantovani A, Rescigno M, et al. SARS-CoV-2 vaccines for all but a single dose for COVID-19 survivors. EBioMedicine. 2021;68:103401. doi: 10.1016/j.ebiom.2021.103401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ripperger TJ, Uhrlaub JL, Watanabe M, Wong R, Castaneda Y, Pizzato HA, et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925–33.e4. doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Bert N, Tan AT, Kunasegaran K, Tham CY, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–62. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 9.Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384:1372–4. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–22. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasikala M, Shashidhar J, Deepika G, Ravikanth V, Krishna VV, Sadhana Y, et al. Immunological memory and neutralizing activity to a single dose of COVID-19 vaccine in previously infected individuals. Int J Infect Dis. 2021;108:183–6. doi: 10.1016/j.ijid.2021.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–8. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–81. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–83. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021 doi: 10.2807/1560-7917.ES.2021.26.6.2100096. 262100096. [DOI] [PMC free article] [PubMed] [Google Scholar]