Abstract
Objective:
To assess the efficacy of cannabidiol (CBD) in the management of crack-cocaine craving and the treatment of frequent withdrawal symptoms.
Methods:
Thirty-one men with a diagnosis of crack-cocaine dependence were enrolled in a randomized, double-blind, placebo-controlled trial. We applied neuropsychological tests and assessed craving intensity, anxiety and depression symptoms, and substance use patterns at baseline and at the end of the trial. The participants were treated with CBD 300 mg/day or placebo for 10 days. During this period, we used a technique to induce craving and assessed the intensity of symptoms before and after the induction procedure.
Results:
Craving levels reduced significantly over the 10 days of the trial, although no differences were found between the CBD and placebo groups. Craving induction was successful in both groups, with no significant differences between them. Indicators of anxiety, depression, and sleep alterations before and after treatment also did not differ across groups.
Conclusion:
Under the conditions of this trial, CBD was unable to interfere with symptoms of crack-cocaine withdrawal. Further studies with larger outpatient samples involving different doses and treatment periods would be desirable and timely to elucidate the potential of CBD to induce reductions in crack-cocaine self-administration.
Keywords: Crack-cocaine, craving, cannabidiol, dependence
Introduction
The use of illicit substances is a major public health problem that affects hundreds of millions people worldwide.1 Exposure to crack-cocaine rapidly induces a pattern of compulsive use and dependence, which is associated with marked personal, social, and economic losses.2,3 In Brazil, the prevalence of crack-cocaine use suggests that the country is amongst the biggest consumer markets of these drugs worldwide.4
Recurrent, severe craving and a high frequency of relapses are remarkable clinical features of crack-cocaine dependence. It should be noted that craving is a critical factor for the onset of compulsive use and dependence, and adds to the failure in remaining abstinent.5,6 Craving measures have increasingly been used as a primary endpoint in clinical trials, as craving severity may predict relapse and treatment outcomes.5,7-9 Different studies have demonstrated that stimuli previously associated with drug use can consistently induce craving in individuals with crack-cocaine dependence,10 leading to the development of craving induction techniques (cue-induced craving) that can be used in the assessment of pharmacological interventions to treat dependence.11,12
Despite unquestionable advances in the understanding of neurobiological alterations associated with drug dependence in recent decades, there is no pharmacological treatment with proven efficacy in the treatment of crack-cocaine dependence.13-15 Different sources of evidence have suggested that the dopaminergic neurotransmission system is involved in the reinforcing effects of cocaine addiction, and may mediate its onset and maintenance.16-18 Furthermore, both preclinical and clinical studies have shown that cocaine withdrawal syndrome is associated with impaired dopamine function.19-22
Different studies have suggested that agonists of cannabinoid CB1 receptors increase the discharge of dopaminergic neurons in the ventral tegmental area, which project to the nucleus accumbens,23 leading to an increase in extracellular dopamine levels in this structure.24-27 This is probably an indirect effect that occurs via inhibition of GABA neurons, which results in tonic inhibition of dopaminergic neurons.28
The use of cannabinoids has been previously highlighted in uncontrolled observations as a strategy for reducing cocaine consumption and to reduce craving,5,29,30 suggesting that these compounds may have a crucial clinical therapeutic role for the treatment of crack-cocaine dependence. However, most of these studies were conducted with cannabis in natura or whole-plant extracts, which limits their reproducibility and raises ethical issues due to the harmful effects of the drug. Moreover, none of these studies tested pure, pharmaceutical-grade cannabidiol (CBD) for the treatment of crack-cocaine dependence.
CBD is a component of Cannabis sativa that is devoid of both psychotomimetic and typical effects of the plant.31-33 CBD inhibits the reuptake and enzymatic hydrolysis of anandamide34 and thus increases the availability of this endogenous CB1 receptor agonist. In animals, CBD reversed dopamine depletion induced by 6-hydroxydopamine in a model of Parkinson’s disease,35 and has been shown to regulate stress response and compulsive behaviors by activating the 5-HT1A serotonergic receptor.36 Moreover, CBD is an allosteric modulator at mu- and delta-receptors in the opioid system.37 Recent articles suggested that CBD may be useful to treat addiction to several substances17,38 including cannabis,39,40 tobacco,41,42 alcohol,43,44 heroin,45 and cocaine.46-49
Furthermore, CBD has demonstrated anxiolytic,38,50-54 antidepressant,55 and neuroprotective properties.56 Although the mechanisms underlying the neuroprotective properties of CBD are still poorly understood, findings from different sources have suggested the involvement of multiple pharmacological targets, including CB1 and 5-HT1A receptors,36,57 antioxidant properties,58 modulation of proinflammatory cytokines,59 and brain-derived neurotrophic factor (BDNF) expression.60 Altogether, findings from preclinical and clinical studies suggest that CBD is involved in different mechanisms related to acquisition of addiction and compulsive drug-seeking behaviors.
The main aim of the present study is to evaluate the efficacy and tolerability of CBD in treatment of crack-cocaine dependence. We hypothesized that CBD would show effectiveness in reducing craving symptoms while having good tolerability.
Methods
This was a randomized, double-blind, placebo-controlled trial. The trial was carried out at a therapeutic community unit specializing in the treatment of substance-related disorders affiliated with Instituto Bairral de Psiquiatria, a hospital that receives patients from the Brazilian public health system (Itapira, state of São Paulo, Brazil).
Subjects
The sample consisted of male subjects aged 18 and older with a DSM-IV diagnosis of crack-cocaine dependence, assessed with the Structured Clinical Interview for DSM-IV axis I Disorders (SCID-CV),61 who had achieved abstinence for a maximum of 30 days (ranging from 8 to 30 days) and agreed to participate by signing an informed consent form.
Patients with current major psychiatric comorbidity (per DSM-IV), chronic infectious diseases, on antidepressants or antipsychotics, with severe or unstable medical conditions (documented episode of acute illness or exacerbation requiring active treatment), a history of brain injury with loss of consciousness, a history of allergies or idiosyncratic reactions to Cannabis sativa, illiteracy, or functional illiteracy were excluded. Of the 65 subjects assessed for eligibility, 31 met the inclusion/exclusion criteria. These participants were randomly assigned to two groups (CBD, n=14; placebo, n=17).
Assessment instruments
The instruments used in the sample are listed below.
- Cocaine Craving Questionnaire – Brief (CCQ-Brief): Short version of the original CCQ, a 45-item questionnaire that assess the main dimensions of craving.62,63 The CCQ-Brief comprises only those 10 items related to the desire dimension. Each item is scored on an analog scale from 0 to 7, ranging from fully disagree to fully agree. Higher scores indicate higher levels of craving.64 In this study, we used a Portuguese version of the instrument adapted to assess crack-cocaine craving.65
- Minnesota Cocaine Craving Scale (MCCS): A self-rating scale with five items that measure the intensity, frequency, and duration of craving episodes, as well as the effects of medication on craving intensity. We used a version that was translated and adapted to Portuguese66 and replaced cocaine craving with crack craving.
- Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).67
- Beck Depression Inventory (BDI).68
- Beck Anxiety Inventory (BAI).69
- Visual Analog Sleep Scales (VAS): A self-rating instrument that assesses sleep and wakefulness conditions over the preceding 24 hours. The instrument has 15 items presented as visual analog scales, with each item consisting of two opposite statements located at the two ends of a line.70,71 The VAS is divided into three parts: 1) the sleep disturbance scale, composed of seven items that measure sleep fragmentation and latency; 2) the effectiveness scale, with five items assessing sleep quality and duration; and 3) the supplementation scale, consisting of three items covering additional sleep periods outside the main sleeping time. Different studies have identified dramatic alterations in sleep architecture associated with chronic use of cocaine, suggesting that sleep disturbances could be targetable neurophysiological abnormalities.72-75 In addition, sleep deficits are associated both with clinical disorders and with impairments in cognitive function, which are supposedly associated with relapse.76,77
- UKU Side Effects Rating Scale (UKU-SERS): This is a detailed scale that assesses psychological, neurological, and autonomic effects in drug trials, with each item scored between 0 (absent) and 3 (severe). The UKU-SERS has questions that evaluate causal relationships between the effects observed and the study medication, which are rated as improbable, possible, or probable, in addition to items assessing the interference with and consequences of side effects for the individual’s health.78
Study drug
CBD capsules were prepared with 99.9% pure CBD powder (THC-Pharm, Frankfurt, Germany/STI-Pharm, Brentwood, UK) dissolved in corn oil.54,79 The same amount of oil alone was used to make the placebo capsules. Both CBD (150 mg) and placebo were placed in identical gelatin capsules, and both treatments consisted of two capsules/day. The 300 mg/day dose was chosen on the basis of previous studies in human models of anxiety that show that this dose is within the therapeutic window of the inverted U-shaped dose-response curve of CBD,80,81 as well as on a previous case study of an inpatient with heavy cannabis use and withdrawal symptoms who was successfully treated with this dose regimen.39
Preparation for the study
The staff in charge of the patients were informed about the procedures, objectives, rationale, and inclusion and exclusion criteria of the study. In addition, they received information concerning the use of concomitant medications and possible medical emergencies that might arise involving the study participants.
The research staff received training consisting of study discussions, presentation of the assessment instruments and neuropsychological tests to clarify possible doubts, discussions on adequate and inadequate attitudes by the investigators, and supervised pilot interviews. For the procedure of craving induction, we played a video (approximately 3 minutes long) showing places (areas of drug use known by users), scenes of real crack use, and objects related to crack use (crack rocks, handling of the drug, preparation of the pipe, other instruments involved in the use of crack-cocaine). In order to assess the adequacy of the methods to be used in the trial, we conducted a pilot study with four participants who completed all steps of the data collection: screening, baseline assessment, treatment (placebo), assessment of craving for 10 days, and final assessment.
Procedures
The first 5 days of hospitalization for the treatment of substance use consisted of adaptation and “detoxification” of the patients. Detoxification/abstinence were not confirmed with urine tests because participants were hospitalized throughout the entire period of study. During this period, potentially eligible participants were identified. The patients referred by the staff were invited to participate in the study, and those who provided informed consent to participate were enrolled. After an initial psychiatric interview, participants were assessed with the SCID-IV to confirm diagnosis of crack-cocaine dependence and whether the participant fulfilled the inclusion/exclusion criteria related to the diagnosis of Axis I disorders. At this point, participants included in the study completed the following assessment instruments: ASSIST, CCQ-Brief and MCCS, BDI and BAI, and the VAS.
In the next step, participants were randomly assigned to the treatment groups and started the 10-day treatment period with CBD or placebo. During treatment, crack-cocaine craving was assessed twice daily (before and after craving induction), at about the same time each day (± 1 hour), with the CCQ-Brief and the MCCS. Craving was induced using standardized audiovisual stimuli (cue-induced craving) as described above, before assessment. Any significant events during the treatment period were recorded in the patients’ medical files. After 10 days of treatment, participants underwent a final assessment with the same instruments used at baseline (CCQ-Brief and MCCS, BDI and BAI, VAS, and the UKU-SERS adverse effects assessment). All procedures were conducted individually by an experienced investigator. During the study, participants received group psychotherapy once a week, the standard psychosocial intervention provided by the institution.
Data treatment
Data were analyzed using the intention-to-treat principle, in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines for randomized trials. All analyses were performed in SPSS version 20.0. Statistical significance was set p < 0.05 (two-tailed) for all analyses.
Data were captured for all visits where craving was assessed with the CCQ-Brief and MCCS (baseline and days 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10). First, skewness and kurtosis were calculated to verify normality of data distribution, which was not confirmed. Thus, we performed all subsequent analyses by using nonparametric tests. Between-group comparisons involving clinical data (e.g., severity of anxiety and depression symptoms) were performed with the Mann-Whitney U test. Means (± standard deviation [SD]) were used to summarize typical values for each group. Between-group comparisons involving categorical variables were performed by Fisher’s exact test.
Assessment of crack-cocaine craving was based on the CCQ-Brief and MCCS total scores. Assessments were performed before and after craving induction each day to validate the cue-induced technique. The mean change from baseline to the day 10 endpoint on those scales was used as the primary outcome measure. Using an intention-to-treat sample, defined as all participants with at least one post-baseline craving assessment, efficacy for CBD vs. placebo was tested using a mixed-model repeated-measures analysis of variance (RMANOVA). The mixed-model approach allows for patients with incomplete data to be included, and utilizes the data that is available for all patients. Frequencies of the most common adverse events are reported if present in at least one subject in either of the study groups.
Ethics statement
The study protocol was approved by the Universidade Federal de São Paulo ethics committee (UNIFESP; process 0302/11). All participants agreed to participate by signing an informed consent form.
Results
Sample characteristics
Groups were matched for age, education, days of hospitalization, and frequency of crack-cocaine use. Most subjects reported a frequency of use higher than five times per week; the mean age at first exposure was 20 years (SD = 7.2), and the mean duration of crack-cocaine use was 12 years (SD = 6.7). Participants reported the use of alcohol, marijuana, and crack in the 3 months preceding the trial. Regarding alcohol and marijuana, most participants were not classified as dependent; however, many fell in the category of risky use. Only two subjects (one from each group) used medications during the trial. Both used benzodiazepines “as needed.”
The sociodemographic and clinical characteristics of the sample are summarized in Table 1. The distribution with respect to hospitalization time and pattern of crack use are presented in Table 2. Problems related to the current use of psychoactive substances according to the ASSIST are summarized in Table 3.
Table 1. Sociodemographic characteristics of the study sample.
| Variable | Experimental group (n=14) | Control group (n=17) | Total (n=31) | p-value* |
|---|---|---|---|---|
| Age (mean [SD]) | 32.5 (6.9) | 33.2 (6.9) | 32.9 (6.8) | |
| Marital status | ||||
| Married/stable relationship | 4 (28.6) | 4 (23.5) | 8 (25.8) | 0.99 |
| Single | 7 (50) | 10 (58.8) | 17 (54.8) | |
| Divorced/widowed | 3 (21.4) | 3 (17.7) | 6 (19.4) | |
| Education | ||||
| Primary education (1st-4th grade) | 2 (14.3) | 3 (17.7) | 5 (16.1) | 0.95 |
| Primary education (5th-8th grade) | 4 (28.6) | 5 (29.4) | 9 (29.0) | |
| Higher education (incomplete) | 2 (14.3) | 1 (5.9) | 3 (9.7) | |
| Higher education (complete) | 6 (42.9) | 8 (47.1) | 14 (45.2) | |
| Occupational status | ||||
| Self-employed/employed | 7 (50.0) | 9 (52.9) | 16 (51.6) | 0.99 |
| Unemployed/on leave | 7 (50.0) | 8 (47.1) | 15 (48.4) | |
| Socioeconomic class | ||||
| A | 0.0 | 0.0 | 0.0 | |
| B | 3 (21.4) | 4 (23.5) | 7 (22.6) | 0.15 |
| C | 9 (64.3) | 9 (52.9) | 18 (58.1) | |
| D | 0.0 | 4 (23.5) | 4 (12.9) | |
| E | 2 (14.3) | 0.0 | 2 (6.4) |
Data presented as n (%), unless otherwise specified.
SD = standard deviation.
Mann-Whitney U test.
Table 2. Distribution of the two groups with respect to hospitalization time and pattern of crack use.
| Variable | Experimental group (n=14) | Control group (n=17) | Total (n=31) | p-value* |
|---|---|---|---|---|
| (n = 14) | (n = 17) | (n = 31) | ||
| Days of hospitalization | 15.93 (4.7) | 13.18 (5.2) | 14.42 (5.1) | 0.10 |
| Previous hospitalizations | ||||
| No | 1 (7.1) | 6 (35.3) | 7 (22.6) | 0.99 |
| Yes | 13 (92.9) | 11 (64.7) | 24 (77.4) | |
| Number of hospitalizations | 2.64 (1.9) | 1.71 (3.1) | 2.13 (2.7) | 0.06 |
| Medication use, current | ||||
| No | 13 (92.9) | 15 (88.2) | 28 (90.3) | 0.99 |
| Yes | 1 (7.1) | 2 (11.8) | 3 (9.7) | |
| Medication use, past | ||||
| No | 14 (100.0) | 16 (94.1) | 30 (96.8) | 0.99 |
| Yes | 0 (0.0) | 1 (5.9) | 1 (3.2) | |
| Age at onset of crack use | 23.36 (7.8) | 18.59 (6.0) | 20.74 (7.2) | 0.07 |
| Quantity used (g/week) | 11.64 (8.6) | 12.65 (12.8) | 12.19 (10.9) | 0.99 |
| Frequency of use (times/week) | ||||
| 1-2 | 0 (0.0) | 1 (5.9) | 1 (3.2) | 0.99 |
| 3-4 | 3 (21.4) | 3 (17.7) | 6 (19.4) | |
| > 5 | 11 (78.6) | 13 (76.5) | 24 (77.4) |
Data presented as mean (SD) or n (%).
SD = standard deviation.
Mann-Whitney’s U test.
Table 3. Distribution of problems related to the current use of psychoactive substances in the two groups according to the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).
| Psychoactive substances | Experimental group (n=14) | Control group (n=17) | Total (n=31) | p-value* |
|---|---|---|---|---|
| Tobacco | ||||
| Low risk | 5 (35.7) | 4 (23.5) | 9 (29.0) | 0.36 |
| Moderate risk | 9 (64.3) | 10 (58.8) | 19 (61.3) | |
| High risk/dependence | 0 (0.0) | 3 (17.6) | 3 (9.7) | |
| Alcohol | ||||
| Low risk | 10 (71.4) | 12 (70.6) | 22 (71.0) | 0.99 |
| Moderate risk | 2 (14.3) | 2 (11.8) | 4 (12.9) | |
| High risk/dependence | 2 (14.3) | 3 (17.6) | 5 (16.1) | |
| Marijuana | ||||
| Low risk | 11 (78.6) | 7 (41.2) | 18 (58.1) | 0.07 |
| Moderate risk | 3 (21.4) | 10 (58.8) | 13 (41.9) | |
| Cocaine/crack | ||||
| High risk/dependence | 14 (100.0) | 17 (100.0) | 31 (100.0) |
Data presented as n (%).
Mann-Whitney’s U test.
Effects of treatment
Eleven subjects (79%) in the experimental group and 14 subjects (82%) in the control group completed the trial. The remaining subjects dropped out and abandoned the institution. Table 4 summarizes the results for baseline (before treatment) and final (after treatment) assessments with the BDI, BAI, and VAS.
Table 4. Results for baseline and final assessments with the Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), and the Visual Analog Sleep Scales (VAS) in the two groups.
| Variable/group/time | n | Mean (SD) | p-value* | Difference from baseline to final, mean (SD) | Comparison between EG and CG (p-value) |
|---|---|---|---|---|---|
| Anxiety symptoms | |||||
| EG | |||||
| 1 | 14 | 18.21 (11.6) | 0.02* | -8.64 (9.6) | 0.80 |
| 2 | 11 | 8.45 (6.6) | |||
| CG | |||||
| 1 | 17 | 15.94 (11.8) | < 0.01* | -8 (9.4) | |
| 2 | 14 | 6.57 (8.5) | |||
| Depressive symptoms | |||||
| EG | |||||
| 1 | 14 | 18.64 (6.6) | 0.06 | -5.91 (7.6) | 0.46 |
| 2 | 11 | 13.09 (8.0) | |||
| CG | |||||
| 1 | 17 | 17.06 (10.4) | < 0.01* | -8.5 (7.8) | |
| 2 | 14 | 8.93 (8.4) | |||
| Sleep disturbances | |||||
| EG | |||||
| 1 | 14 | 26.91 (11.6) | 0.99 | -0.73 (15.5) | 0.27 |
| 2 | 11 | 26.18 (18.2) | |||
| CG | |||||
| 1 | 17 | 26.57 (21.1) | 0.27 | -9.93 (21.2) | |
| 2 | 14 | 16.64 (14.0) | |||
| Sleep effectiveness | |||||
| EG | |||||
| 1 | 14 | 9.27 (5.5) | 0.40 | 3.73 (10.6) | 0.37 |
| 2 | 11 | 13.00 (10.2) | |||
| CG | |||||
| 1 | 17 | 9.86 (11.4) | 0.56 | -1 (11.1) | |
| 2 | 14 | 8.86 (9.3) | |||
| Sleep supplementation | |||||
| EG | |||||
| 1 | 14 | 5.64 (7.2) | 0.77 | -0.64 (8) | 0.54 |
| 2 | 11 | 5.00 (6.0) | |||
| CG | |||||
| 1 | 17 | 4.71 (6.3) | 0.07 | -3.5 (6.5) | |
| 2 | 14 | 1.21 (2.0) |
CG = control group; EG = experimental group; SD = standard deviation; time 1 = baseline assessment; time 2 = final assessment.
Statistically significant difference.
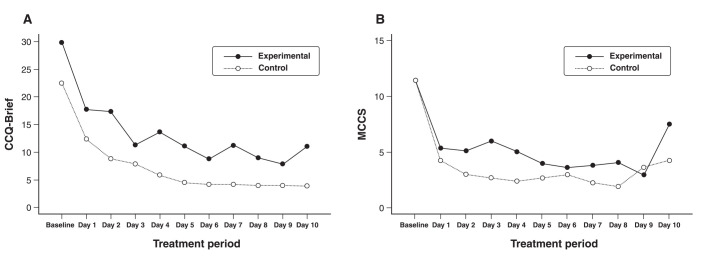
The RMANOVA of CCQ-Brief scores showed a significant effect of time (F10,230 = 16.174; p < 0.001), but not of treatment (F10,230 = 2.663; p = 0.116) or time/treatment interaction (F10,230 = 0.489; p = 0.897). The MCCS also showed a significant effect of time (F10,230 = 16.450; p < 0.001), but not of treatment (F10,230 = 2,460; p = 0.130) or time/treatment interaction (F10,230 = 1,580; p = 0.113). The mean scores of the two scales are shown in Figure 1. We reanalyzed the data including cannabis use (amount) as a covariate. Effects of treatment and time/treatment interaction remained nonsignificant for both scales. There was a significant reduction in severity of symptoms of anxiety and depression in both groups, but improvements in the CBD group did not differ from placebo. Differences in VAS sleep scores were nonsignificant in the two groups.
Figure 1. Craving levels assessed with A) the Cocaine Craving Questionnaire – Brief (CCQ-Brief) and B) the Minnesota Cocaine Craving Scale (MCCS) over the treatment period.
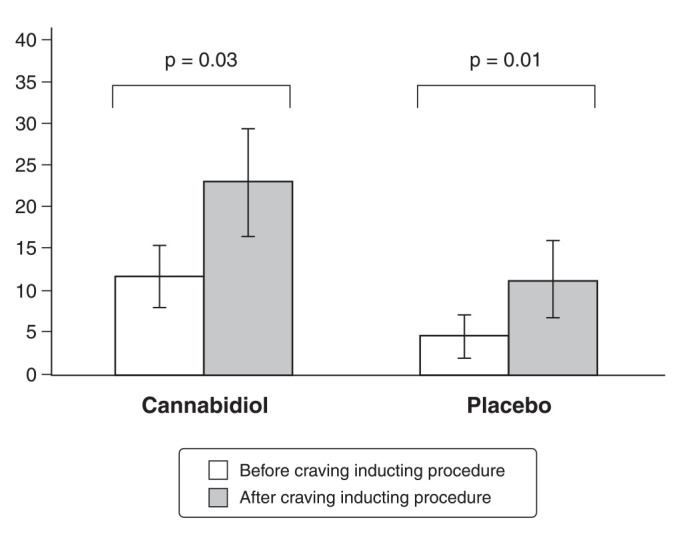
Regarding CCQ-Brief scores, there was a significant effect for phase (F1,21 = 7.792; p = 0.011), but not for treatment (F1,21 = 3.144; p = 0.091) or the phase/treatment interaction (F1,21 = 3.080; p = 0.094). As shown in Figure 2, the procedure was successful in inducing craving in the two groups. However, differences between groups were nonsignificant. Findings for the craving induction procedure are shown in Figure 1.
Figure 2. Cocaine Craving Questionnaire – Brief (CCQ-Brief) scores before and after the craving induction procedure.
The adverse events recorded using the UKU-SERS were sleepiness and increased sleep duration (five subjects in the experimental group and three in the control group; p = 0.45), nausea (two subjects in the experimental group and one in the control group; p = 0.59), and headache (two subjects in the experimental group and one in the control group; p = 0.59). All adverse events were of mild or moderate severity. No serious adverse event occurred during the trial. The frequency of adverse events did not differ between groups (p = 0.34).
Discussion
The results of this randomized, placebo-controlled trial suggest that, despite excellent safety and tolerability, CBD failed to demonstrate efficacy in the treatment of craving in subjects with crack-cocaine dependence. Despite these negative findings, to our knowledge, this is the first study examining the efficacy of CBD in the treatment of crack-cocaine dependence in humans.
Anxiety symptoms decreased significantly in both groups during the trial. Regarding depressive symptomatology, a significant reduction was found among participants in the control group, while the change in the experimental group only tended towards statistical significance. There were no differences between the experimental and control groups in terms of baseline and final assessments of anxiety and depression symptoms. This finding contrasts with studies reporting the efficacy of CBD in treating anxiety disorders.53,55 However, such evidence mainly comes from preclinical studies in which CBD was administered acutely; evidence from clinical studies investigating chronic CBD use in anxiety disorders is limited.82
The results of the CCQ-Brief and the MCCS showed the presence of moderate to high severity of craving in both groups at first assessment, which decreased significantly over the 10 days of the trial. Between-group comparisons, however, showed no differences, suggesting that CBD treatment did not contribute to the reduction in craving severity. This suggests that the observation that use of cannabis facilitates abstinence from crack cocaine83 cannot be attributed to CBD.
The decrease in craving intensity over the 10 days of the trial in the two groups does not seem to be related to the general treatment measures provided by the institution, since the experiment started, on average, 15 days after admission, and craving scores on the first day of the trial were high for both groups. In addition, decreases in anxiety and depressive symptomatology did not appear to be related to the resolution of withdrawal syndrome, since enrollment in the study occurred at 8 to 30 days of abstinence. Reductions in craving severity and significant decreases in anxiety and depression symptoms might be associated with a placebo effect related to the knowledge that subjects could be receiving a new medication, as well as to the individualized attention that patients received during the study period, with daily application of the assessment instruments. It may also have occurred due to the activities carried out at the facility, the general health care provided by the staff, the impossibility of using the drug during hospitalization, and of being away from the usual environment of drug use.
Different studies have shown that craving is an unstable condition affected by environmental, social, and emotional influences.5,9 Similarly, the duration of abstinence and deprivation of liberty, which makes use and contact with drug-use environments difficult or even impossible, do not favor craving induction.6 It should be noted that conditioned stimuli (such as seeing someone use crack, feeling the smell of the substance, handling paraphernalia, going to typical places of use) have consistently been described as triggers of craving among substance users.4-6
The use of a craving-inducing procedure in the present study increased craving intensity in the two groups, but with no significant differences between them. This finding is in line with a recent double-blind, placebo-controlled functional magnetic resonance imaging study in which CBD did not acutely affect the neural correlates of reward anticipation and feedback in healthy participants.84 However, this observation contrasts with previous animal literature that consistently shows that the administration of CBD reduces self-administration, cocaine-seeking behaviors, and cocaine-induced reward; attenuates the central adaptations induced by cocaine; and changes contextual and emotional memories associated with cocaine, thus decreasing the amount of drug use.47-49,85,86
The main limitation of the present study was related to the treatment setting, since craving and withdrawal symptoms tend to be less severe and to decrease linearly in hospitalized patients, unlike in an outpatient setting where these symptoms tend to be more frequent, intense, and persistent. Additionally, different aspects of the intervention, including the short treatment period and the long interval between cessation of use and the start of the trial, might have contributed to the negative findings. The dose of CBD (300 mg/day) was relatively low, with no uptitration; since the effects of CBD are biphasic,80 the use of a single dose also limits evaluation of the effectiveness of this cannabinoid. For instance, two recent double-blind placebo-controlled trials of CBD in subjects with heroin45 and cannabis40 use disorder, respectively, observed efficacy with the use of higher doses (400 and 800 mg).
Also, the tendency for the CBD group to have more previous hospitalizations, a fact that is usually associated with greater severity and increased substance use-related problems, might also have contributed to the absence of differences between the groups. Moreover, since the rates of psychiatric and infectious comorbidities are high among crack-cocaine users and may have influence treatment outcomes,87 such exclusions in our study would have produced a highly artefactual sample. Thus, the study sample may not represent the general socioclinical profile of crack-cocaine users in general, and in Brazil in particular. Finally, type-II statistical error cannot be ruled out in view of the relatively small overall sample size, and may mean that the study was underpowered for conclusive insights or for adequate testing of interactions with other crucial factors.
Although these preliminary findings failed to show evidence of the efficacy of CBD in reducing craving in subjects with crack-cocaine dependence, further investigations that overcome the limitations described above are necessary. This is particularly true if we consider the excellent tolerability and safety profiles of CBD and the encouraging animal evidence of this cannabinoid as an adjunct therapy for the treatment of crack-cocaine dependence.7,88,89 Therefore, future double-blind, placebo-controlled, randomized clinical trials with larger, less selective samples and using different doses of CBD in outpatients are of particular interest to elucidate the potential of CBD to reduce self-administration of crack cocaine.
Disclosure
JAC is a member of the International Advisory Board of the Australian Centre for Cannabinoid Clinical and Research Excellence (ACRE) – National Health and Medical Research Council (NHMRC). JAC and JEH have received travel support to attend scientific meetings and personal consultation fees from BSPG-Pharm. JAC, JEH, and AWZ are coinventors of the patent “Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023,” Def. US number Reg. 62193296; July 29, 2015; INPI on August 19, 2015 (BR1120150164927; Mechoulam R, Zuardi AW, Kapczinski F, Hallak JEC, Guimarâes FS, Crippa JAS, Breuer A). Universidade de São Paulo (USP) has licensed this patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1) and has an agreement with Prati-Donaduzzi to “develop a pharmaceutical product containing synthetic CBD and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson’s disease, and anxiety disorders.” JAC, JEH, and AWZ are coinventors of the patent “Cannabinoid-containing oral pharmaceutical composition, method for preparing and using same,” INPI on September 16, 2016 (BR 112018005423-2). RAB has received research grants from Janssen; and has participated in speaker bureaus for Janssen and Sanofi-Aventis. ALL has received grants and personal fees from Janssen Pharmaceutical; has received personal fees from Daiichi Sankyo, Cristalia Produtos Químicos e Farmacêuticos, Libbs, Pfizer, Myralis Farma, Aché Laboratórios, Hypera Pharma, and Sanofi-Aventis; and has received grants from Eli Lilly, H. Lundbeck A/S, Servier Laboratories, Hoffman-La Roche, and Forum Pharmaceuticals. The other authors report no conflicts of interest.
Acknowledgements
This study was funded by Instituto Nacional de Ciência e Tecnologia Translacional em Medicina (INCT-TM) – Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2008/09009-2); it was also supported by CNPq/MS/SCTIE/DECIT 26/2014 – Pesquisas sobre Distúrbios Neuropsiquiátricos (466805/2014-4). JAC received a grant from the University Global Partnership Network (UGPN) – Global Priorities in Cannabinoid Research Excellence Program. CMG is a recipient of a Programa Nacional de Pós-Doutorado Institucional – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PNPD-CAPES) fellowship. JAC, JEH, RAB, and AWZ, are recipients of CNPq research fellowships.
Footnotes
How to cite this article: Meneses-Gaya C, Crippa JA, Hallak JE, Miguel AQ, Laranjeira R, Bressan RA, et al. Cannabidiol for the treatment of crack-cocaine craving: an exploratory double-blind study. Braz J Psychiatry. 2021;43:467-476. http://dx.doi.org/10.1590/1516-4446-2020-1416
References
- 1.United Nations Office on Drugs and Crime (UNODC) World drug report 2014. 2014. [cited 2020 Aug 27] http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf.
- 2.Ribeiro M, Dunn J, Laranjeira R, Sesso R. High mortality among young crack cocaine users in Brazil: a 5-year follow-up study. Addiction. 2004;99:1133–5. doi: 10.1111/j.1360-0443.2004.00804.x. [DOI] [PubMed] [Google Scholar]
- 3.Karila L, Seringe E, Benyamina A, Reynaud M. The reliability and validity of the French version of the Cocaine Craving Questionnaire-Brief. Curr Pharm Des. 2011;17:1369–75. doi: 10.2174/138161211796150819. [DOI] [PubMed] [Google Scholar]
- 4.Abdalla RR, Madruga CS, Ribeiro M, Pinsky I, Caetano R, Laranjeira R. Prevalence of cocaine use in Brazil: data from the II Brazilian national alcohol and drugs survey (BNADS) Addict Behav. 2014;39:297–301. doi: 10.1016/j.addbeh.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Chaves TV, Sanchez ZM, Ribeiro LA, Nappo SA. Crack cocaine craving: behaviors and coping strategies among current and former users. Rev Saude Publica. 2011;45:1168–75. doi: 10.1590/s0034-89102011005000066. [DOI] [PubMed] [Google Scholar]
- 6.Balbinot AD, Alves GSL, Amaral AF, Junior, Araujo RB. Associação entre fissura e perfil antropométrico em dependentes de crack. J Bras Psiquiatr. 2011;60:205–19. [Google Scholar]
- 7.Colledge F, Ludyga S, Mücke M, Pühse U, Gerber M. The effects of an acute bout of exercise on neural activity in alcohol and cocaine craving: study protocol for a randomised controlled trial. Trials. 2018;19:713. doi: 10.1186/s13063-018-3062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsden J, Goetz C, Meynen T, Mitcheson L, Stillwell G, Eastwood B, et al. Memory-focused cognitive therapy for cocaine use disorder: theory, procedures and preliminary evidence from an external pilot randomised controlled trial. EBioMedicine. 2018;29:177–89. doi: 10.1016/j.ebiom.2018.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo II, Albet JT, Jiménez-Lerma JM, Landabaso M. [Reliability and validity of the Spanish adaptation of the brief form of the Cocaine Craving Questionnaire-Now (CCQ-N-10)] Adicciones. 2009;21:195–202. doi: 10.20882/adicciones.229. [DOI] [PubMed] [Google Scholar]
- 10.Moscon JA, Conti CL, Nakamura-Palacios EM. Increased electroencephalographic activity in crack-cocaine users visualizing crack cues. J Psychiatr Res. 2016;83:137–9. doi: 10.1016/j.jpsychires.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Johnson MW, Bruner NR, Johnson PS, Silverman K, Berry MS. Randomized controlled trial of d-cycloserine in cocaine dependence: effects on contingency management and cue-induced cocaine craving in a naturalistic setting. Exp Clin Psychopharmacol. 2020;28:157–68. doi: 10.1037/pha0000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vorspan F, Fortias M, Zerdazi EH, Karsinti E, Bloch V, Lépine JP, et al. Self-reported cue-induced physical symptoms of craving as an indicator of cocaine dependence. Am J Addict. 2015;24:740–3. doi: 10.1111/ajad.12303. [DOI] [PubMed] [Google Scholar]
- 13.Fischer B, Blanken P, Da Silveira D, Gallassi A, Goldner EM, Rehm J, et al. Effectiveness of secondary prevention and treatment interventions for crack-cocaine abuse: a comprehensive narrative overview of English-language studies. Int J Drug Policy. 2015;26:352–63. doi: 10.1016/j.drugpo.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Minozzi S, Amato L, Pani PP, Solimini R, Vecchi S, De Crescenzo F, et al. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst Rev. 2015;2015:CD003352. doi: 10.1002/14651858.CD003352.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005;18:265–70. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- 16.Dela Peña I, Gevorkiana R, Shi WX. Psychostimulants affect dopamine transmission through both dopamine transporter-dependent and independent mechanisms. Eur J Pharmacol. 2015;764:562–70. doi: 10.1016/j.ejphar.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prud’homme M, Cata R, Jutras-Aswad D. Cannabidiol as an intervention for addictive behaviors: a systematic review of the evidences. Subst Abuse. 2015;9:33–8. doi: 10.4137/SART.S25081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong P, Liu Y, Hu Y, Wang T, Zhao YP, Liu QS. BDNF interacts with endocannabinoids to regulate cocaine-induced synaptic plasticity in mouse midbrain dopamine neurons. J Neurosci. 2015;35:4469–81. doi: 10.1523/JNEUROSCI.2924-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conrad KL, Ford K, Marinelli M, Wolf ME. Dopamine receptor expression and distribution dynamically change in the rat nucleus accumbens after withdrawal from cocaine self-administration. Neuroscience. 2010;169:182–94. doi: 10.1016/j.neuroscience.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramar CP, Chefer VI, Wise RA, Medina JH, Barbano MF. Dopamine in the dorsal hippocampus impairs the late consolidation of cocaine-associated memory. Neuropsychopharmacology. 2014;39:1645–53. doi: 10.1038/npp.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neisewander JL, Cheung TH, Pentkowski NS. Dopamine D3 and 5-HT1B receptor dysregulation as a result of psychostimulant intake and forced abstinence: implications for medications development. Neuropharmacology. 2014;76 Pt B:301–19. doi: 10.1016/j.neuropharm.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Schlussman SD, Rabkin J, Butelman ER, Ho A, Kreek MJ. Chronic escalating cocaine exposure, abstinence/withdrawal, and chronic re-exposure: effects on striatal dopamine and opioid systems in C57BL/6J mice. Neuropharmacology. 2013;67:259–66. doi: 10.1016/j.neuropharm.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 24.Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–62. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- 25.Fanarioti E, Mavrikaki M, Panagis G, Mitsacos A, Nomikos GG, Giompres P. Behavioral and neurochemical changes in mesostriatal dopaminergic regions of the rat after chronic administration of the cannabinoid receptor agonist WIN55,212-2. Int J Neuropsychopharmacol. 2015;18:pyu097. doi: 10.1093/ijnp/pyu097. Published online 2015 Jan 8. http://10.1093/ijnp/pyu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 27.Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperlágh B, Windisch K, Andó RD, E Vizi ES. Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens. Neurochem Int. 2009;54:452–7. doi: 10.1016/j.neuint.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Gonçalves JR, Nappo SA. Factors that lead to the use of crack cocaine in combination with marijuana in Brazil: a qualitative study. BMC Public Health. 2015;15:706. doi: 10.1186/s12889-015-2063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Socías ME, Kerr T, Wood E, Dong H, Lake S, Hayashi K, et al. Intentional cannabis use to reduce crack cocaine use in a Canadian setting: a longitudinal analysis. Addict Behav. 2017;72:138–43. doi: 10.1016/j.addbeh.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grlie L. A comparative study on some chemical and biological characteristics of various samples of cannabis resin. Bull Narcot. 1976;14:37–46. [Google Scholar]
- 32.Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta ? 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76:245–50. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
- 33.Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6:237–49. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 34.Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–52. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol Dis. 2005;19:96–107. doi: 10.1016/j.nbd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–43. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 37.Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:354–61. doi: 10.1007/s00210-006-0033-x. [DOI] [PubMed] [Google Scholar]
- 38.Crippa JA, Guimarães FS, Campos AC, Zuardi AW. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009. doi: 10.3389/fimmu.2018.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crippa JA, Hallak JE, Machado-de-Sousa JP, Queiroz RH, Bergamaschi M, Chagas MH, et al. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J Clin Pharm Ther. 2013;38:162–4. doi: 10.1111/jcpt.12018. [DOI] [PubMed] [Google Scholar]
- 40.Freeman TP, Hindocha C, Baio G, Shaban ND, Thomas EM, Astbury D, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. 2020;7:865–74. doi: 10.1016/S2215-0366(20)30290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2003;38:2433–6. doi: 10.1016/j.addbeh.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Hindocha C, Freeman TP, Grabski M, Crudgington H, Davies AC, Stroud JB, et al. The effects of cannabidiol on impulsivity and memory during abstinence in cigarette dependent smokers. Sci Rep. 2018;8:7568. doi: 10.1038/s41598-018-25846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Ternay J, Naassila M, Nourredine M, Louvet A, Bailly F, Sescousse G, et al. Therapeutic prospects of cannabidiol for alcohol use disorder and alcohol-related damages on the liver and the brain. Front Pharmacol. 2019;10:627. doi: 10.3389/fphar.2019.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turna J, Syan SK, Frey BN, Rush B, Costello MJ, Weiss M, et al. Cannabidiol as a novel candidate alcohol use disorder pharmacotherapy: a systematic review. Alcohol Clin Exp Res. 2019;43:550–63. doi: 10.1111/acer.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176:911–22. doi: 10.1176/appi.ajp.2019.18101191. [DOI] [PubMed] [Google Scholar]
- 46.Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R. Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology (Berl) 2004;175:360–6. doi: 10.1007/s00213-004-1825-7. [DOI] [PubMed] [Google Scholar]
- 47.Luján MÁ, Castro-Zavala A, Alegre-Zurano L, Valverde O. Repeated cannabidiol treatment reduces cocaine intake and modulates neural proliferation and CB1R expression in the mouse hippocampus. Neuropharmacology. 2018;143:163–75. doi: 10.1016/j.neuropharm.2018.09.043. [DOI] [PubMed] [Google Scholar]
- 48.Luján MÁ, Cantacorps L, Valverde O. The pharmacological reduction of hippocampal neurogenesis attenuates the protective effects of cannabidiol on cocaine voluntary intake. Addict Biol. 2020;25:e12778. doi: 10.1111/adb.12778. [DOI] [PubMed] [Google Scholar]
- 49.Galaj E, Bi GH, Yang HJ, Xi ZX. Cannabidiol attenuates the rewarding effects of cocaine in rats by CB2, 5-HT1A and TRPV1 receptor mechanisms. Neuropharmacology. 2020;167:107740. doi: 10.1016/j.neuropharm.2019.107740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guimarães FS, Chiaretti TM, Graeff FG, Zuardi AW. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 1990;100:558–9. doi: 10.1007/BF02244012. [DOI] [PubMed] [Google Scholar]
- 51.Zuardi AW, Cosme RA, Graeff FG, Guimarães FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7(1 Suppl):82–8. doi: 10.1177/026988119300700112. [DOI] [PubMed] [Google Scholar]
- 52.Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29:417–26. doi: 10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- 53.Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121–30. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- 54.Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36:1219–26. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, Machado S, Arias-Carrión O, Crippa JA, et al. Antidepressant-like and anxyolitic-like effects of cannabidiol: a chemical compound of cannabis sativa. CNS Neurol Disord Drug Targets. 2014;13:953–60. doi: 10.2174/1871527313666140612114838. [DOI] [PubMed] [Google Scholar]
- 56.Campos AC, Fogaça MV, Scarante FF, Joca SRL, Sales AJ, Gomes FV, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol. 2017;8:269. doi: 10.3389/fphar.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kreutz S, Koch M, Bottger C, Ghadban C, Korf HW, Dehghani F. 2-Arachidonoylglycerol elicits neuroprotective effects on excitotoxically lesioned dentate gyrus granule cells via abnormal-cannabidiol-sensitive receptors on microglial cells. Glia. 2009;57:286–94. doi: 10.1002/glia.20756. [DOI] [PubMed] [Google Scholar]
- 58.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–73. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2012;367:3364–78. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez-Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, et al. Cannabidiol for neurodegenerative disorders:important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75:323–33. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Del-Ben CM, Vilela JA, Crippa JA, Hallaka JE, Labatec CM, Zuardi AW. Confiabilidade da “Entrevista Clínica Estruturada para o DSM-IV – Versão Clínica” traduzida para o português. Rev Bras Psiquiatr. 2001;23:156–9. [Google Scholar]
- 62.Bonfiglio NS, Renati R, Agus M, Penna MP. Validation of a Substance Craving Questionnaire (SCQ) in Italian population. Addict Behav Rep. 2019;9:100172. doi: 10.1016/j.abrep.2019.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.da Silveira DX, Fernandes M, Silveira ED, Jorge MR. Cocaine craving questionnaire: assessing craving among cocaine users in Brazil. Psychiatry Res. 2006;142:257–9. doi: 10.1016/j.psychres.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83:233–7. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 65.Araujo RB, Pedroso RS, de Castro MG. Adaptação transcultural para o idioma português do Cocaine Craving Questionnaire – Brief. Rev Psiquiatr Clin. 2010;37:195–8. [Google Scholar]
- 66.Focchi GR, Leite MC, Scivoletto S. Use of the dopaminergic agonist pergolide in the treatment of cocaine craving. Rev Bras Psiquiatr. 2001;23:188–94. [Google Scholar]
- 67.WHO ASSIST Working Group. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–94. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 68.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 69.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 70.Snyder-Halpern R, Verran JA. Instrumentation to describe subjective sleep characteristics in healthy subjects. Res Nurs Health. 1987;10:155–63. doi: 10.1002/nur.4770100307. [DOI] [PubMed] [Google Scholar]
- 71.Verran JA, Snyder-Halpern R. Do patients sleep in the hospital? Appl Nurs Res. 1988;1:95. doi: 10.1016/s0897-1897(88)80010-7. [DOI] [PubMed] [Google Scholar]
- 72.Angarita GA, Canavan SV, Forselius E, et al. Correlates of polysomnographic sleep changes in cocaine dependence: self-administration and clinical outcomes. Drug Alcohol Depend. 2014;143:173–80. doi: 10.1016/j.drugalcdep.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Angarita GA, Canavan SV, Forselius E, Bessette A, Morgan PT. Abstinence-related changes in sleep during treatment for cocaine dependence. Drug Alcohol Depend. 2014;134:343–7. doi: 10.1016/j.drugalcdep.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morgan PT, Malison RT. Pilot study of lorazepam and tiagabine effects on sleep, motor learning, and impulsivity in cocaine abstinence. Am J Drug Alcohol Abuse. 2008;34:692–702. doi: 10.1080/00952990802308221. [DOI] [PubMed] [Google Scholar]
- 75.Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. Normalizing effects of modafinil on sleep in chronic cocaine users. Am J Psychiatry. 2010;167:331–40. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bellesi M, Riedner BA, Garcia-Molina GN, Cirelli C, Tonon G. Enhancement of sleep slow waves: underlying mechanisms and practical consequences. Front Syst Neurosci. 2014;8:208. doi: 10.3389/fnsys.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roth T, Benca RM, Erman M. An introduction to the clinical correlates of disrupted slow-wave sleep. J Clin Psychiatry. 2010;71:e09. doi: 10.4088/JCP.9007tx1cc. [DOI] [PubMed] [Google Scholar]
- 78.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 79.Zuardi AW, Morais SL, Guimarães FS, Mechoulam R. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56:485–6. [PubMed] [Google Scholar]
- 80.Linares IM, Zuardi AW, Pereira LC, Queiroz RH, Mechoulam R, Guimarães FS, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry. 2019;41:9–14. doi: 10.1590/1516-4446-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuardi AW, Rodrigues NP, Silva AL, Bernardo SA, Hallak JE, Guimarães FS, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol. 2017;8:259. doi: 10.3389/fphar.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12:825–36. doi: 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Oliveira LG, Nappo SA. [Characterization of the crack cocaine culture in the city of Sao Paulo: a controlled pattern of use] Rev Saude Publica. 2008;42:664–71. doi: 10.1590/s0034-89102008005000039. [DOI] [PubMed] [Google Scholar]
- 84.Lawn W, Hill J, Hindocha C, Yim J, Yamamori Y, Jones G, et al. The acute effects of cannabidiol on the neural correlates of reward anticipation and feedback in healthy volunteers. J Psychopharmacol. 2020 Aug 5:269881120944148. doi: 10.1177/0269881120944148. Available from: http://10.1177/0269881120944148. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonzalez-Cuevas G, Martin-Fardon R, Kerr TM, Stouffer DG, Parsons LH, Hammell DC, et al. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacology. 2018;43:2036–45. doi: 10.1038/s41386-018-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Carvalho CR, Takahashi RN. Cannabidiol disrupts the reconsolidation of contextual drug-associated memories in Wistar rats. Addict Biol. 2017;22:742–51. doi: 10.1111/adb.12366. [DOI] [PubMed] [Google Scholar]
- 87.Fischer B, Murphy Y, Rudzinski K, MacPherson D. Illicit drug use and harms, and related interventions and policy in Canada: a narrative review of select key indicators and developments since 2000. Int J Drug Policy. 2016;27:23–35. doi: 10.1016/j.drugpo.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 88.Campos AC, Fogaça MV, Sonego AB, et al. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–27. doi: 10.1016/j.phrs.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 89.Dos Santos RG, Guimarães FS, Crippa JA, Hallak JE, Rossi GN, Rocha JM, et al. Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol. 2020;16:517–26. doi: 10.1080/17425255.2020.1754793. [DOI] [PubMed] [Google Scholar]