Abstract
Rationale
Although there has been a long-standing interest in the human health effects of vitamin E, a comprehensive analysis of the association between circulating vitamin E and long-term mortality has not been conducted.
Objective
Determine whether serum alpha-tocopherol (the predominant form of vitamin E) is related to long-term overall and cause-specific mortality, and elucidate the dose-response relationships with better quantification of the associations.
Methods and Results
We conducted a biochemical analysis of 29,092 participants in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study that originally tested vitamin E and beta-carotene supplementation. Serum alpha-tocopherol was measured at baseline using high-performance liquid chromatography, and during a 31-year follow-up we identified 23,787 deaths, including deaths from cardiovascular disease (CVD; 9,867), cancer (7,687), respiratory disease (2,161), diabetes mellitus (119), injuries and accidents (1,255), and other causes (2,698). After adjusting for major risk factors, we found that men with higher serum alpha-tocopherol had significantly lower all-cause mortality (hazard ratios=0.83, 0.79, 0.75 and 0.78 for quintile 2 (Q2)-Q5 versus Q1, respectively; Ptrend<0.0001), and significantly decreased mortality from CVD, heart disease, stroke, cancer, respiratory disease, and other causes, with risk reductions from 17%−47% for the highest versus lowest quintile, respectively. The alpha-tocopherol association with overall mortality was similar across subgroups of smoking intensity, years of smoking, alcohol consumption, trial supplementation and duration of follow-up. The association was, however, significantly modified by baseline age and body mass index, with stronger inverse associations for younger men and men with a lower body mass index (Pinteraction≤0.006).
Conclusions and Relevance
In this long-term prospective cohort study, higher baseline serum alpha-tocopherol biochemical status was associated with lower risk of overall mortality and mortality from major causes. Our data support the long-term health benefits of higher serum alpha-tocopherol for overall and chronic disease mortality, and should be replicated in other more diverse populations.
Keywords: serum vitamin E, overall mortality, cause-specific mortality, multivariate analysis, epidemiology
Introduction
Although there has been long-standing interest in the human health effects of vitamin E, a comprehensive analysis of the long-term association between vitamin E status and overall mortality has not been conducted, particularly with respect to cause-specific mortality and characterizations of the dose-response relationship. As an essential fat-soluble vitamin, vitamin E encompasses eight structurally similar compounds, of which alpha-tocopherol is the predominant form in humans that is obtained from vegetable oils, some vegetables and fruits, whole grains, nuts (e.g., almonds), and seeds (e.g., sunflower) as well as supplements.1–3 As a potent chain-breaking antioxidant, vitamin E can act as a peroxyl radical scavenger that prevents low-density lipoprotein (LDL)-cholesterol and lipid peroxidation which have been implicated in chronic disease risk, including cardiovascular disease (CVD) and cancer. In addition to its antioxidant properties, vitamin E has also demonstrated other important functions including enhancement of anti-inflammation activities, regulation of gene expression, improvement of immune response, inhibition of cell proliferation and suppression of tumor angiogenesis.4–9
Population-based studies have shown inconsistent associations between circulating alpha-tocopherol and risk of overall,10–16 cancer,10, 12, 13, 16–18 and CVD mortality.11–13, 16–19 A recent meta-analysis of six studies suggested that higher alpha-tocopherol concentrations were related to lower total mortality (for participants in the highest vs. lowest vitamin E category, relative risk [RR]=0.84, 95% confidence interval [CI]: 0.77, 0.91).20 Another recent meta-analysis showed a small inverse association between alpha-tocopherol status and overall mortality, with a 6% risk reduction for each 5 mg/L increase in alpha-tocopherol concentration.21 Significant between-study heterogeneity was noted for the risk estimates in these meta-analyses, however.20, 21 Moreover, data remain relatively sparse for elucidating the association between vitamin E status and cause-specific mortality risk, for testing a dose-response association, and for examining consistency of the association across population subgroups.
In the present investigation, we examine whether vitamin E biochemical status is prospectively associated with overall and cause-specific mortality. Based on vitamin E’s antioxidant and other beneficial biological properties, we hypothesize that men with higher serum alpha-tocopherol concentrations would experience lower long-term mortality. The analysis is nested within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study, a controlled trial that tested vitamin E and beta-carotene supplementation for 5–8 years. We previously evaluated the same cohort based on 13,380 deaths during 19 years of follow-up and found evidence for an inverse serum alpha-tocopherol-mortality association.13 Here we re-examine the vitamin E associations with overall and cause-specific mortality, including deaths from CVD, heart disease, stroke, cancer, respiratory disease, diabetes mellitus, injuries/accidents, and other causes based on nearly 24,000 deaths and over 31 years of cohort observation. Elucidation of the dose-response relationships and hypothesis-generating analyses of key subgroups (e.g., based on age and body mass index [BMI]) were of particular interest.
Methods
The data and study materials that support the findings will not be available to other researchers for the purposes of reproducing the results because of the cohort data use agreement between the U.S. and Finland (E.U.). However, the analytical methods are available from the corresponding author on appropriate request.
Study participants
Details of the ATBC Study have been published previously.22 In brief, 29,133 male smokers of five or more cigarettes daily, aged 50–69 years, were recruited between 1985 and 1988 from 14 study centers in southwestern Finland. At the first of two baseline visits, self-administered questionnaires were used to collect information on demographic, behavioral and lifestyle characteristics such as age, smoking habits, education, physical activity, medical history (including cardiovascular disease and diabetes) and vitamin supplement use. Height, weight and blood pressure were measured by the trained study nurses, and an overnight fasting blood sample was obtained and stored at −70⁰C until assay. Participants were asked to complete a dietary history questionnaire at home, which collected information on portion size and frequency of consumption of 203 food items and 73 mixed dishes. At the second baseline visit, participants returned the dietary questionnaire (which was checked by the study nurses) and were then randomly assigned to one of the four trial supplementation groups: vitamin E (as dl-alpha-tocopheryl acetate, 50 mg/day), beta-carotene (20 mg/day), both agents or placebo. Intervention continued for 5–8 years (median of 6 years) through the end of the trial (April 30, 1993). Written informed consent was received from all participants, and the study has been approved by the Institutional Review Boards both at the US National Cancer Institute and the Finnish National Public Health Institute.
Laboratory assays
Serum concentrations of alpha-tocopherol, retinol and beta-carotene were measured by high-performance liquid chromatography, and the coefficient of variation of serum alpha-tocopherol was 2.2%.22, 23 Serum concentrations of total and high-density lipoprotein (HDL)-cholesterol were assayed enzymatically (Boehringer Mannheim, Mannheim, Germany). In this study, 29,092 participants were included in the final analysis after excluding subjects with missing data for the serum alpha-tocopherol measurement (n=41).
Cohort follow-up and outcome ascertainment
Participants were followed from their randomization date at baseline until date of death or the end of follow-up (December 31, 2015), whichever occurred first. Mortality in the cohort was determined via linkage to the Causes of Death Registry, Statistics Finland (Online Figure I). The underlying causes of death were based on the following 8th, 9th, and 10th revisions of International Classification of Diseases (ICD-8, -9, -10, respectively) codes: CVD (ICD-8, 390–458; ICD-9, 390–459; ICD10, I00-I99), heart disease (ICD-8 and ICD-9, 390–398, 401–404, 410–429, and 440–448; ICD10, I00-I13, I20-I51, and I70-I78), stroke (ICD-8 and ICD-9, 430–438; ICD10, I60-I69), cancer (ICD-8 and ICD-9, 140–239; ICD10, C00-D48), respiratory disease (pneumonia, influenza, chronic obstructive pulmonary disease, and other related conditions) (ICD-8, 470–474, 480–486, 490–493, and 518; ICD-9, 480–487 and 490–496; ICD-10, J10-J18 and J40-J47), diabetes (ICD-8 and ICD-9, 250; ICD-10, E10-E14), injuries and accidents (ICD-8, 800–978; ICD-9, E800-E978; ICD-10, U01-U02, U03, V01-X59, X60-X84, X85-Y09, Y35, Y85-Y86, Y87.0, Y87.1, and Y89.0), and other causes (cause of death was missing for 47 subjects, and they were included in the “other causes” category).
Statistical analysis
The association between serum alpha-tocopherol (quintiles) and mortality risk, including cause-specific mortality, was analyzed using Cox proportional hazard regression models, with attained age as the time metric, to estimate hazard ratios (HR) and 95% confidence intervals (CI). The proportional-hazards assumption was not violated, tested by modeling the interaction of follow-up time with serum alpha-tocopherol. The test for linear trend was examined by entering the median concentration of alpha-tocopherol of each quintile, as a continuous variable, in a separate Cox proportional-hazard regression model. The age-adjusted model adjusted for age at baseline (continuous) and serum total cholesterol (continuous). Multivariate models were additionally adjusted for cigarettes smoked daily (continuous), years of smoking (continuous), intervention assignment (alpha-tocopherol or no alpha-tocopherol, beta-carotene or no beta-carotene), systolic and diastolic blood pressure (continuous), and HDL cholesterol (continuous). History of CVD was further adjusted in the cause-specific mortality models of CVD, heart disease and stroke endpoints. We also assessed the following covariates which were not considered further as they did not materially alter the association between serum alpha-tocopherol and overall and cause-specific mortality: physical activity, BMI (kg/m2), educational status, baseline vitamin E supplement use, history of diabetes mellitus, energy intake, fat, red meat and alcohol. To address the possibility that an inverse association with mortality may be explained by coexisting bioactive nutrients from a vitamin E-rich diet, we further adjusted for consumption of foods having high vitamin E contents, including vegetable oils, eggs, fish, rye, wheat, total fruit, and total vegetables (as continuous variables).
We generated Kaplan-Meier survival curves and used the log-rank test to evaluate survival differences for endpoints of interest across quintiles of serum alpha-tocopherol, in all the subjects and subjects who did not have a history of CVD or diabetes mellitus at baseline. Using cubic-restricted splines, possible non-linear associations between serum alpha-tocopherol concentration (as a continuous variable) and overall and cause-specific mortality were assessed, and four knots were selected at the 5th, 25th, 75th, and 95th percentiles of the serum alpha-tocopherol concentration.
Stratified analyses were conducted based on age at baseline (<54, 54–59, or ≥59 years), daily cigarettes (<16, 16–20, or >20 cigarettes per day), years of smoking (<33, 33–40, or ≥40 years), alcohol consumption (<5.3, 5.3–20.4, or ≥20.4 g per day), BMI (<25, 25–28, or ≥28 kg/m2), intervention group (alpha-tocopherol or no alpha-tocopherol, beta-carotene or no beta-carotene), and years of follow-up (<13, 13–23, or ≥23 years). The test for interaction was assessed through likelihood ratio tests entering the cross-product term for each stratified factor and quintiles of serum alpha-tocopherol. A P value of interaction <0.05 could be due to chance findings as we generated 8 interaction tests for each endpoint of interest. To minimize the influence of possible bias from reverse causation, we excluded the first 5 years of follow-up in a sensitivity lag analysis (excluded subjects n=2,727). We also separately restricted analyses to subjects who did not have a history of CVD or diabetes mellitus at baseline (excluded subjects n=12,482).
Because vitamin E is transported in lipoproteins (primarily, though not exclusively, in the LDL fraction), we conducted a sensitivity analysis that used a “non-HDL cholesterol” variable (total minus HDL cholesterol) which essentially reflected LDL (and to a lesser degree, very low density lipoproteins [VLDL]). Serum alpha-tocopherol concentrations were normalized for this serum LDL-proxy concentration, as well as for serum total cholesterol concentration separately, using the residual method,24 categorized into quintiles, and analyzed through Cox proportional hazards regression models to estimate the HRs for overall and cause-specific mortality. In separate models, we adjusted for the serum LDL-proxy (i.e., instead of using total and HDL cholesterol) in the main analytical model.
In addition to the analyses of serum alpha-tocopherol, we re-examined the ATBC trial alpha-tocopherol supplementation effect on mortality during this 31-year follow-up, overall and stratified by baseline serum alpha-tocopherol quintiles and by less than/greater than the median. Crude and multivariate-adjusted HRs and their 95% CIs were estimated from Cox proportional hazards regression models according to the 2×2 factorial design (i.e., subjects who received alpha-tocopherol versus those who did not).
SAS software (version 9.4; SAS Institute Inc, Cary, NC) and the R statistical language version 3.2.3 (Vienna, Austria) were used for all the analyses.
Results
Mean serum concentration of alpha-tocopherol in the ATBC cohort at baseline was 11.9 mg/L, and the mean value of alpha-tocopherol in the fifth quintile was double that in the first quintile (Table 1). Compared with men in the lowest quintile, men in higher quintiles of serum alpha-tocopherol were slightly younger, were more physically active, had higher BMI, and were more likely to use vitamin supplements and have a history of CVD or diabetes mellitus. Serum alpha-tocopherol was inversely associated with serum HDL cholesterol and alcohol consumption, and positively associated with fruit and vegetable consumption, serum beta-carotene (possibly reflecting fruit and vegetable consumption) and total cholesterol (possibly related to alpha-tocopherol transport in lipoproteins) (Table 1).
Table 1.
| Quintile of serum alpha-tocopherol | |||||
|---|---|---|---|---|---|
| Quintile 1 (n=5,825) | Quintile 2 (n=5,860) | Quintile 3 (n=5,794) | Quintile 4 (n=5,791) | Quintile 5 (n=5,822) | |
| Serum alpha-tocopherol (mg/L) | 8.0 (1.2) | 10.1 (0.4) | 11.5 (0.4) | 13.1 (0.6) | 16.9 (4.0) |
| Age (y) | 57.8 (5.2) | 57.3 (5.1) | 57.0 (5.0) | 57.1 (5.0) | 56.9 (4.9) |
| Cigarettes/d | 21 (9) | 21 (9) | 20 (9) | 20 (9) | 20 (9) |
| Years smoked (y) | 36.8 (8.5) | 36.3 (8.3) | 35.5 (8.5) | 35.5 (8.6) | 35.6 (8.3) |
| Systolic blood pressure (mm Hg) | 142 (20) | 141 (19) | 142 (19) | 142 (19) | 143 (20) |
| Diastolic blood pressure (mm Hg) | 87 (11) | 87 (11) | 88 (11) | 88 (11) | 89 (11) |
| Serum total cholesterol (mmol/L) | 5.2 (0.86) | 5.8 (0.81) | 6.2 (0.85) | 6.6 (0.93) | 7.3 (1.17) |
| Serum HDL cholesterol (mmol/L) | 1.28 (0.35) | 1.23 (0.31) | 1.21 (0.31) | 1.18 (0.30) | 1.09 (0.29) |
| Serum beta-carotene (μg/L) | 150 (117) | 196 (141) | 220 (162) | 241 (202) | 253 (249) |
| BMI (kg/m2) | 25.6 (4.0) | 26.0 (3.8) | 26.2 (3.7) | 26.5 (3.7) | 27.1 (3.6) |
| Education (%, > elementary school) | 21.0 | 21.3 | 19.5 | 20.2 | 23.1 |
| Physically active (%) | 16.5 | 19.6 | 22.6 | 23.5 | 21.8 |
| History of CVD (%) 3 | 39.4 | 38.0 | 38.6 | 42.1 | 49.2 |
| History of diabetes mellitus (%) | 4.2 | 3.4 | 3.6 | 3.7 | 6.4 |
| Vitamin A supplement use (%) | 5.5 | 8.1 | 10.2 | 11.9 | 16.1 |
| Vitamin E supplement use (%) | 5.0 | 7.4 | 9.8 | 11.7 | 16.9 |
| Daily dietary intake | |||||
| Energy (kcal) | 2,708 (779) | 2,716 (774) | 2,710 (745) | 2,674 (733) | 2,638 (734) |
| Fat (triacylglycerol, g) | 106 (38) | 107 (37) | 107 (36) | 105 (35) | 103 (35) |
| Alcohol (g ethanol) | 21.3 (25.2) | 18.1 (21.4) | 17.1 (20.0) | 16.4 (20.1) | 17.1 (20.5) |
| Vitamin E (mg) | 10.2 (4.7) | 11.3 (5.2) | 12.1 (5.5) | 12.8 (5.9) | 13.8 (6.4) |
| Fruit (g) | 115 (96) | 123 (100) | 131 (99) | 136 (102) | 140 (110) |
| Vegetables (g) | 98 (63) | 107 (68) | 115 (70) | 120 (73) | 127 (74) |
| Red meat (g) | 69 (34) | 71 (34) | 72 (34) | 72 (35) | 72 (34) |
Values are means (standard deviation) unless otherwise indicated.
Based on ANOVA for continuous variable and Chi-square test for categorical variable. All P value<0.0001.
CVD, cardiovascular disease; includes a history of deep vein thrombosis, superficial venous thrombosis, lung infarction or embolus, hypertension, arterial obstruction, stroke, heart arrhythmia, enlarged heart, valvular heart disease, myocardial infarction, coronary heart disease, and heart failure.
In the cohort with 514,087 person-years of follow-up, 23,787 men had died (81.8%), of which 9,867 deaths were from CVD (8,063 heart disease deaths and 1,763 stroke deaths), and 7,687 deaths were due to cancer. In addition, there were 2,161 deaths from respiratory disease, 119 deaths from diabetes, 1,255 deaths from injuries/accidents, and 2,698 deaths from other causes combined. The analysis using age-adjusted models showed that compared with those in the lowest quintile of serum alpha-tocopherol, men with higher quintiles had decreased mortality overall and from CVD, stroke, cancer, respiratory disease, injuries/accidents and other causes, with 10% to 60% risk reductions (all Ptrend≤0.04; Table 2). After multivariable adjustment for potential confounding factors, the inverse associations of serum alpha-tocopherol were observed for mortality from CVD, heart disease, stroke, cancer, respiratory disease, and other causes combined, with risk reductions from 17%−47% in the highest quintile as compared with the lowest quintile (all Ptrend<0.0001; Table 2). Additional adjustment for BMI, alcohol consumption, and educational status did not materially alter the association estimates (Online Table I). The inverse serum alpha-tocopherol associations with mortality were only slightly attenuated by adjustment for vegetable oils, total fruit, total vegetables, eggs, fish, rye and wheat in the multivariate models (Online Table II, Q2–5: 0.85, 0.81, 0.78, 0.82; p-trend <0.0001) compared with the original data in Table 2 (Q2–5: 0.83, 0.79, 0.75, 0.78; p-trend <0.0001). By contrast, we found that serum alpha-tocopherol was not related to risk of mortality from diabetes mellitus or injuries and accidents (multivariable model: Ptrend=0.13 and 0.20, respectively).
Table 2.
Hazard ratios (HRs) and 95% CI for all-cause and cause-specific mortality by quintile of serum alpha-tocopherol *
| Causes of mortality | Serum alpha-tocopherol (mg/L) | P value # | ||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| All-cause | ||||||
| Deaths (n) | 5,066 | 4,824 | 4,661 | 4,582 | 4,654 | |
| Death rate † | 54.75 | 46.68 | 44.07 | 42.81 | 44.15 | |
| Age-adjusted HR (95% CI) ‡ | 1.0 | 0.82 (0.79, 0.85) | 0.77 (0.74, 0.80) | 0.72 (0.69, 0.75) | 0.75 (0.71, 0.78) | <0.0001 |
| Multivariate HR (95%CI) § | 1.0 | 0.83 (0.80, 0.87) | 0.79 (0.76, 0.83) | 0.75 (0.72, 0.78) | 0.78 (0.74, 0.82) | <0.0001 |
| CVD | ||||||
| Deaths (n) | 1,845 | 1,881 | 1,950 | 1,948 | 2,243 | |
| Death rate † | 19.94 | 18.20 | 18.44 | 18.20 | 21.28 | |
| Age-adjusted HR (95% CI) ‡ | 1.0 | 0.86 (0.80, 0.91) | 0.85 (0.79, 0.91) | 0.79 (0.73, 0.84) | 0.90 (0.83, 0.97) | 0.038 |
| Multivariate HR (95%CI) §|| | 1.0 | 0.86 (0.80, 0.91) | 0.83 (0.77, 0.88) | 0.74 (0.69, 0.80) | 0.78 (0.72, 0.85) | <0.0001 |
| Heart disease | ||||||
| Deaths (n) | 1,431 | 1,509 | 1,569 | 1,617 | 1,937 | |
| Death rate † | 15.47 | 14.60 | 14.83 | 15.11 | 18.37 | |
| Age-adjusted HR (95% CI) ‡ | 1.0 | 0.88 (0.82, 0.95) | 0.87 (0.81, 0.94) | 0.83 (0.77, 0.90) | 0.98 (0.90, 1.06) | 0.82 |
| Multivariate HR (95%CI) §|| | 1.0 | 0.88 (0.81, 0.94) | 0.84 (0.78, 0.91) | 0.77 (0.72, 0.84) | 0.83 (0.76, 0.90) | <0.0001 |
| Stroke | ||||||
| Deaths (n) | 407 | 367 | 369 | 319 | 301 | |
| Death rate † | 4.40 | 3.55 | 3.49 | 2.98 | 2.86 | |
| Age-adjusted HR (95% CI) ‡ | 1.0 | 0.78 (0.67, 0.90) | 0.76 (0.66, 0.89) | 0.63 (0.53, 0.74) | 0.62 (0.51, 0.74) | <0.0001 |
| Multivariate HR (95%CI) §|| | 1.0 | 0.81 (0.70, 0.93) | 0.77 (0.66, 0.90) | 0.64 (0.54, 0.75) | 0.60 (0.50, 0.73) | <0.0001 |
| Cancer | ||||||
| Deaths (n) | 1,620 | 1,624 | 1,499 | 1,529 | 1,415 | |
| Death rate † | 17.51 | 15.72 | 14.17 | 14.29 | 13.42 | |
| Age-adjusted HR (95% CI) ‡ | 1.0 | 0.89 (0.83, 0.95) | 0.80 (0.74, 0.86) | 0.79 (0.73, 0.86) | 0.76 (0.70, 0.83) | <0.0001 |
| Multivariate HR (95%CI) § | 1.0 | 0.90 (0.84, 0.97) | 0.83 (0.77, 0.90) | 0.84 (0.77, 0.91) | 0.81 (0.74, 0.89) | <0.0001 |
| Respiratory disease | ||||||
| Deaths (n) | 621 | 501 | 415 | 349 | 275 | |
| Death rate † | 6.71 | 4.85 | 3.92 | 3.26 | 2.61 | |
| Age-adjusted HR (95% CI) ‡ | 1.0 | 0.70 (0.62, 0.79) | 0.58 (0.51, 0.66) | 0.47 (0.40, 0.54) | 0.40 (0.33, 0.47) | <0.0001 |
| Multivariate HR (95%CI) § | 1.0 | 0.76 (0.67, 0.86) | 0.66 (0.58, 0.76) | 0.57 (0.49, 0.67) | 0.53 (0.45, 0.64) | <0.0001 |
| Diabetes mellitus | ||||||
| Deaths (n) | 24 | 19 | 15 | 24 | 37 | |
| Death rate † | 0.26 | 0.18 | 0.14 | 0.22 | 0.35 | |
| Age-adjusted HR (95% CI) ‡ | 1.0 | 0.75 (0.40, 1.38) | 0.61 (0.31, 1.19) | 1.00 (0.53, 1.88) | 1.74 (0.91, 3.31) | 0.016 |
| Multivariate HR (95%CI) § | 1.0 | 0.72 (0.39, 1.34) | 0.56 (0.29, 1.10) | 0.89 (0.47, 1.68) | 1.33 (0.69, 2.57) | 0.13 |
| Injuries and accidents | ||||||
| Deaths (n) | 294 | 275 | 255 | 217 | 214 | |
| Death rate † | 3.18 | 2.66 | 2.41 | 2.03 | 2.03 | |
| Age-adjusted HR (95% CI) ‡ | 1.0 | 0.85 (0.72, 1.01) | 0.79 (0.66, 0.95) | 0.67 (0.55, 0.82) | 0.69 (0.55, 0.85) | 0.0002 |
| Multivariate HR (95%CI) § | 1.0 | 0.92 (0.78, 1.10) | 0.89 (0.74, 1.07) | 0.79 (0.65, 0.97) | 0.89 (0.71, 1.12) | 0.20 |
| Other causes | ||||||
| Deaths (n) | 662 | 524 | 527 | 515 | 470 | |
| Death rate † | 7.15 | 5.07 | 4.98 | 4.81 | 4.46 | |
| Age-adjusted HR (95% CI) ‡ | 1.0 | 0.66 (0.59, 0.74) | 0.66 (0.58, 0.74) | 0.62 (0.54, 0.70) | 0.59 (0.51, 0.68) | <0.0001 |
| Multivariate HR (95%CI) § | 1.0 | 0.68 (0.61, 0.77) | 0.69 (0.61, 0.77) | 0.65 (0.57, 0.74) | 0.64 (0.55, 0.74) | <0.0001 |
Cause of death was missing for 47 subjects.
Crude death rate per 1000 person-years.
Adjusted for age and serum total cholesterol.
Adjusted for age, serum total and serum HDL cholesterol, cigarettes smoked per day, years of smoking, intervention assignment, systolic and diastolic blood pressure.
Further adjusted for history of CVD.
P value for trend: based on statistical significance of the coefficient of the quintile variable (median value within each quintile).
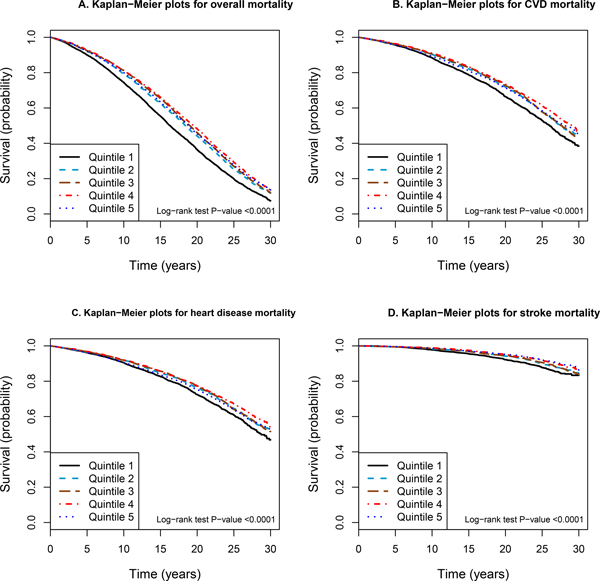
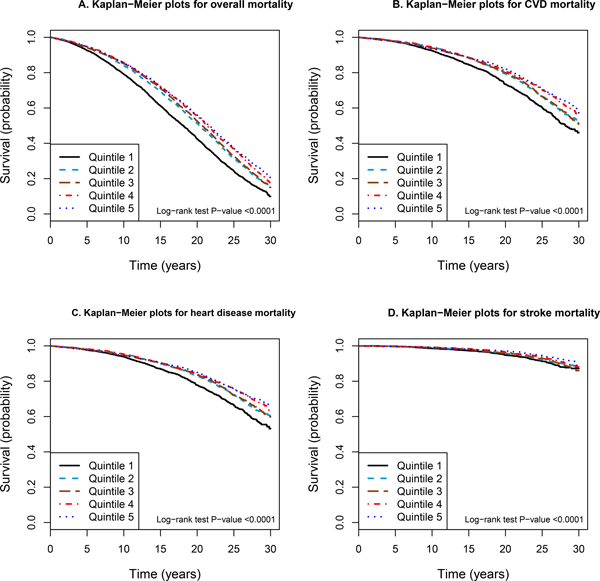
Kaplan-Meier survival plots revealed that men in the lowest quintile of serum alpha-tocopherol experienced a significant excess in overall mortality than those in the higher quintiles (log-rank P value <0.0001; Figure 1A). Similar risk patterns were obtained for cause-specific mortality from CVD, heart disease, stroke, cancer, respiratory disease, and other causes combined (all log-rank P value <0.0001; Figure 1B–1D; Online Figure II). In analyses restricted to subjects who did not have a history of CVD or diabetes mellitus, the Kaplan-Meier survival curves showed stronger reductions in mortality for men in the highest quintile (Figure 2; Online Figure III).
Figure 1.
Kaplan-Meier curves of overall and cause-specific mortality according to quintiles of serum alpha-tocopherol in 29,092 subjects in the ATBC Study. A) Overall mortality. B) Cardiovascular disease (CVD) mortality. C) Heart disease mortality. D) Stroke mortality.
Figure 2.
Kaplan-Meier curves of overall and cause-specific mortality according to quintiles of serum alpha-tocopherol in 16,610 subjects who do not have a history of CVD or diabetes mellitus in the ATBC Study. A) Overall mortality. B) Cardiovascular disease (CVD) mortality. C) Heart disease mortality. D) Stroke mortality.
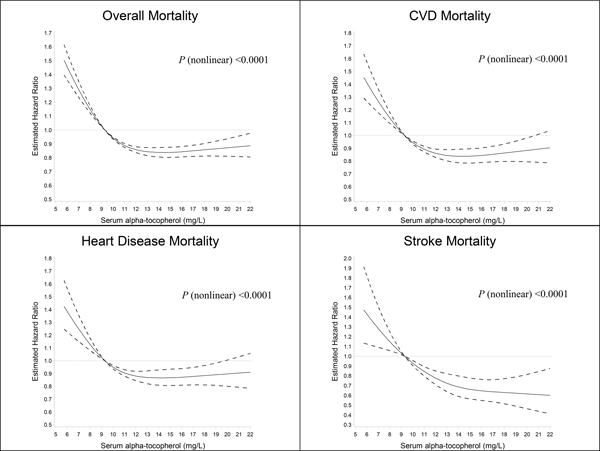
We used a restricted cubic spline regression to determine the dose-response association between serum alpha-tocopherol and mortality, computing serum alpha-tocopherol as a continuous variable. Overall and cause-specific mortality increased when serum alpha-tocopherol concentrations decreased below 9.3 mg/L, the reference value corresponding to the first quintile cut-point (Figure 3). As the serum alpha-tocopherol concentrations increased, overall and cause-specific mortality was lower and relatively stable for serum alpha-tocopherol concentrations above 13 mg/L, with the possible exception of deaths from stroke which declined further (Figure 3; Online Figure IV).
Figure 3.
Cubic spline regression for estimated hazard ratios of overall and cause-specific death according to serum alpha-tocopherol concentrations in the ATBC Study. The reference value (9.3 mg/L; hazard ratio = 1) corresponds to the cutoff value of the first quintile of serum alpha-tocopherol concentration. A) Overall mortality. B) Cardiovascular disease (CVD) mortality. C) Heart disease mortality. D) Stroke mortality. The solid line suggests the hazard ratio for mortality and serum alpha-tocopherol with a 4-knot spline (knots were selected at the 5th, 25th, 75th, and 95th percentiles of the serum alpha-tocopherol); dashed lines denoted the 95% confidence intervals. Total number of participants: 29,092. Event number of overall-, CVD-, heart disease-, stroke death is 23,787, 9,867, 8,063, and 1,763, respectively.
Stratified analyses of baseline factors and duration of follow-up showed similar serum alpha-tocopherol-mortality associations across subgroups of smoking intensity, years of smoking, alcohol consumption, trial intervention and duration of follow-up (Table 3). The association was significantly modified by age and BMI (Pinteraction=0.0059 and <0.0001, respectively; Table 3), however, with a stronger inverse association among younger (<59 years) and leaner men (BMI <25 kg/m2). Analyses of cause-specific mortality yielded similar patterns for the subgroups with the exception of a stronger inverse heart disease mortality association in younger men (Pinteraction=0.0034, Online Table IV), and more prominent inverse associations for cancer, respiratory disease and other causes of mortality during the first 13 years of follow-up (Pinteraction=0.005, 0.0021 and <0.0001, respectively, Online Tables VI–VIII). Also, the inverse association with respiratory disease mortality appeared stronger for older and leaner men (Pinteraction=0.0061 and 0.0038, respectively; Online Table VII).
Table 3.
Hazard ratios (HRs) and 95% CI for all-cause mortality by quintile of serum alpha-tocopherol, stratified by selected factors
| Quintile of serum alpha-tocopherol | P value † | P for interaction ‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||||||||
| Event | HR | Event | HR (95% CI) * | Event | HR (95% CI) * | Event | HR (95% CI) * | Event | HR (95% CI) * | |||
| Age | ||||||||||||
| <54 y | 1,082 | 1.00 | 1,076 | 0.81 (0.74, 0.88) | 1,130 | 0.77 (0.71, 0.84) | 1,028 | 0.72 (0.65, 0.79) | 1,121 | 0.72 (0.65, 0.81) | <0.0001 | 0.0059 |
| 54–59 y | 1,586 | 1.00 | 1,559 | 0.83 (0.77, 0.89) | 1,515 | 0.74 (0.69, 0.80) | 1,531 | 0.69 (0.64, 0.75) | 1,597 | 0.73 (0.66, 0.79) | <0.0001 | |
| ≥59 y | 2,398 | 1.00 | 2,189 | 0.85 (0.80, 0.90) | 2,016 | 0.83 (0.78, 0.88) | 2,023 | 0.81 (0.75, 0.86) | 1,936 | 0.84 (0.78, 0.90) | <0.0001 | |
| Cigarettes smoked/d | ||||||||||||
| <16 | 1,459 | 1.00 | 1,492 | 0.83 (0.77, 0.89) | 1,516 | 0.79 (0.73, 0.85) | 1,554 | 0.72 (0.67, 0.78) | 1,596 | 0.74 (0.68, 0.81) | <0.0001 | 0.77 |
| 16–20 | 1,692 | 1.00 | 1,670 | 0.86 (0.80, 0.92) | 1,602 | 0.81 (0.76, 0.88) | 1,568 | 0.81 (0.75, 0.88) | 1,522 | 0.82 (0.75, 0.90) | 0.0001 | |
| >20 | 1,915 | 1.00 | 1,662 | 0.82 (0.77, 0.88) | 1,543 | 0.78 (0.73, 0.84) | 1,460 | 0.72 (0.67, 0.78) | 1,536 | 0.78 (0.71, 0.84) | <0.0001 | |
| Years smoked | ||||||||||||
| <33 y | 1,147 | 1.00 | 1,149 | 0.82 (0.76, 0.90) | 1,257 | 0.80 (0.73, 0.87) | 1,177 | 0.69 (0.63, 0.76) | 1,207 | 0.74 (0.67, 0.82) | <0.0001 | 0.017 |
| 33–40 | 1,403 | 1.00 | 1,353 | 0.81 (0.75, 0.88) | 1,362 | 0.73 (0.67, 0.79) | 1,320 | 0.72 (0.66, 0.78) | 1,434 | 0.72 (0.66, 0.79) | <0.0001 | |
| ≥40 y | 2,516 | 1.00 | 2,322 | 0.85 (0.81, 0.91) | 2,042 | 0.83 (0.78, 0.88) | 2,085 | 0.80 (0.75, 0.86) | 2,013 | 0.84 (0.78, 0.90) | <0.0001 | |
| Daily alcohol consumption | ||||||||||||
| <5.3 g | 1,332 | 1.00 | 1,487 | 0.88 (0.81, 0.94) | 1,425 | 0.83 (0.77, 0.90) | 1,506 | 0.82 (0.75, 0.89) | 1,471 | 0.85 (0.77, 0.93) | 0.003 | 0.06 |
| 5.3–20.4 | 1,456 | 1.00 | 1,389 | 0.81 (0.75, 0.88) | 1,488 | 0.79 (0.73, 0.86) | 1,453 | 0.73 (0.67, 0.79) | 1,503 | 0.75 (0.68, 0.82) | <0.0001 | |
| ≥20.4 g | 1,829 | 1.00 | 1,542 | 0.82 (0.77, 0.88) | 1,431 | 0.75 (0.70, 0.81) | 1,323 | 0.70 (0.65, 0.76) | 1,396 | 0.73 (0.67, 0.79) | <0.0001 | |
| BMI (kg/m 2 ) | ||||||||||||
| <25 | 2,478 | 1.00 | 2,069 | 0.79 (0.74, 0.84) | 1,813 | 0.72 (0.68, 0.77) | 1,655 | 0.68 (0.63, 0.73) | 1,301 | 0.68 (0.63, 0.74) | <0.0001 | <0.0001 |
| 25–28 | 1,352 | 1.00 | 1,452 | 0.84 (0.78, 0.90) | 1,512 | 0.81 (0.75, 0.87) | 1,507 | 0.75 (0.69, 0.81) | 1,589 | 0.74 (0.68, 0.81) | <0.0001 | |
| ≥28 | 1,229 | 1.00 | 1,301 | 0.94 (0.87, 1.02) | 1,330 | 0.91 (0.84, 0.98) | 1,420 | 0.89 (0.82, 0.97) | 1,761 | 0.94 (0.86, 1.03) | 0.25 | |
| Trial intervention group | ||||||||||||
| Alpha-tocopherol | 2,525 | 1.00 | 2,418 | 0.87 (0.82, 0.92) | 2,320 | 0.81 (0.76, 0.86) | 2,327 | 0.76 (0.71, 0.81) | 2,361 | 0.80 (0.74, 0.86) | <0.0001 | 0.41 |
| No alpha-tocopherol | 2,541 | 1.00 | 2,406 | 0.80 (0.76, 0.85) | 2,341 | 0.77 (0.73, 0.82) | 2,255 | 0.74 (0.69, 0.79) | 2,293 | 0.75 (0.70, 0.81) | <0.0001 | |
| Beta-carotene | 2,520 | 1.00 | 2,376 | 0.81 (0.76, 0.86) | 2,285 | 0.76 (0.72, 0.81) | 2,327 | 0.73 (0.69, 0.78) | 2,353 | 0.74 (0.69, 0.79) | <0.0001 | 0.18 |
| No beta-carotene | 2,546 | 1.00 | 2,448 | 0.86 (0.82, 0.91) | 2,376 | 0.82 (0.77, 0.87) | 2,255 | 0.76 (0.72, 0.82) | 2,301 | 0.81 (0.76, 0.87) | <0.0001 | |
| Years of follow-up | ||||||||||||
| 0–13 | 2,389 | 1.00 | 1,945 | 0.80 (0.75, 0.85) | 1,739 | 0.72 (0.68, 0.77) | 1,700 | 0.70 (0.65, 0.75) | 1,849 | 0.75 (0.70, 0.81) | <0.0001 | 0.42 |
| 13–23 | 1,913 | 1.00 | 1,949 | 0.84 (0.79, 0.90) | 1,953 | 0.82 (0.76, 0.87) | 1,910 | 0.77 (0.72, 0.83) | 1,866 | 0.80 (0.74, 0.87) | <0.0001 | |
| ≥23 | 764 | 1.00 | 930 | 0.88 (0.80, 0.97) | 969 | 0.87 (0.79, 0.96) | 972 | 0.80 (0.72, 0.89) | 939 | 0.76 (0.68, 0.86) | <0.0001 | |
Adjusted for age, serum total and serum HDL cholesterol, cigarettes smoked per day, years of smoking, intervention assignment, systolic and diastolic blood pressure.
P value for trend: based on statistical significance of the coefficient of the quintile variable (median value within each quintile).
P value for interaction: according to the likelihood test to assess the statistical significance of the cross-product term entered to the Cox proportional hazard regression model.
Our findings were not materially altered by excluding the first 5 years of follow-up (overall mortality in the multivariate model, fifth versus first quintile: HR=0.77, 95% CI: 0.73, 0.81; Online Table IX). The serum alpha-tocopherol associations with all-cause, CVD and heart disease mortality appeared strengthened after excluding the 12,482 subjects with a positive history of CVD or diabetes mellitus (mortality from overall, CVD and heart disease in the multivariate model, fifth versus first quintile: HR=0.69, 0.67 and 0.67, respectively, Ptrend<0.0001), with the results for other causes remaining unchanged (Online Table X).
Concentrations of serum alpha-tocopherol and LDL proxy-adjusted serum alpha-tocopherol were highly correlated (Pearson correlation coefficient=0.77), as were serum alpha-tocopherol and total cholesterol-adjusted serum alpha-tocopherol (Pearson correlation coefficient=0.79). The mortality associations did not materially change when we used LDL-proxy-adjusted serum alpha-tocopherol or total cholesterol-adjusted serum alpha-tocopherol in the analysis (LDL-proxy-adjusted serum alpha-tocopherol: overall mortality in the multivariate model, fifth versus first quintile, HR=0.83, 95% CI: 0.79, 0.86; Online Table XI; total cholesterol-adjusted serum alpha-tocopherol: overall mortality in the multivariate model, fifth versus first quintile, HR=0.82, 95% CI: 0.78, 0.85; Online Table XII). Our results were also not materially altered when we adjusted for the serum LDL-proxy in the main model (overall mortality in the multivariate model, fifth versus first quintile: HR=0.77, 95% CI: 0.74, 0.81; Online Table XIII).
During the 31 years of observation, there were 11,951 and 11,836 participant deaths in the alpha-tocopherol and no alpha-tocopherol trial supplementation arms, respectively. The HR for overall mortality was 1.009 and 1.008 among alpha-tocopherol recipients compared with non-recipients in crude model and multivariate-adjusted models, respectively (Online Table XIV). Stratified analyses by baseline serum alpha-tocopherol quintiles and by median split showed a consistent lack of an alpha-tocopherol supplementation effect on overall mortality across the serum categories (Online Table XIV).
Discussion
In this large prospective cohort analysis with 30 years follow-up, we observed significant inverse associations between serum alpha-tocopherol and overall and cause-specific mortality, with a 22% reduction in total mortality for men in the highest vs. lowest quintile of serum alpha-tocopherol. The associations were stable throughout the observation period and were stronger among younger and leaner men, as well as those with no history of CVD.
To our knowledge, this is the largest study to examine alpha-tocopherol biochemical status in relation to overall and cause-specific mortality. Consistent with a previous study,25 our findings from the restricted cubic spline analysis support decreasing overall mortality with increasing alpha-tocopherol concentrations, with a mortality nadir for serum values of 13–14 mg/L, and a striking mortality excess for men with concentrations below 10 mg/L. The suggested beneficial role for vitamin E in CVD, heart disease, stroke, cancer, and respiratory disease mortality are supported by recent meta-analyses,20, 21 albeit, the latter data were based on relatively few deaths, insufficient statistical power across studies, population heterogeneity, and inadequate control for important confounders such as serum total cholesterol.21 Vitamin E status has only been sporadically investigated in relation to mortality from stroke, respiratory disease,17 diabetes, injuries/accidents or other causes. The present analysis revealed stronger inverse associations in men under the age of 60, those with a lower BMI and those without a history of CVD or diabetes. The attenuated beneficial association in older men might be explained by age-associated changes in lipoprotein metabolism that may influence the absorption, transport and metabolism of alpha-tocopherol; e.g., decreased lipoprotein lipase activity could result in greater non-chylomicron lipoprotein vitamin E transport in the older men.26–28 The stronger inverse vitamin E-mortality association in leaner men and those without a history of CVD or diabetes could indicate effectiveness of higher alpha-tocopherol status in the setting of reduced metabolic syndrome-related oxidative stress and inflammation. Also, alpha-tocopherol bioavailability may be decreased in adults with metabolic syndrome, possibly due to reduced intestinal absorption and impaired hepatic vitamin E transport.29
The present findings of a strong inverse serum vitamin E-mortality association stand in stark contrast to the results of controlled trials showing a lack of beneficial effects, and possible adverse effects, for vitamin E supplementation, particularly for the highest supplementation dosages (i.e., ≥400 IU/day).25, 30–33 One meta-analysis of antioxidant supplementation trials, for example, showed that vitamin E used alone did not significantly impact overall mortality (RR=1.02, 95% CI: 0.98, 1.05).30 However, half of the trials in the analysis had very short follow-up periods (i.e., <2 years) that did not permit evaluation of long-term mortality related to vitamin E supplementation, and the majority tested vitamin combinations and administered vitamin E dosages substantially above the recommended daily allowances (RDA; 15 mg/day for adults), with only three of 46 trials testing low-dose supplementation within the RDA.30 Similarly, and most directly relevant to the present vitamin E biochemical analysis showing a mortality benefit, is that the parent ATBC trial which administered a relatively low vitamin E supplement dose of 50 IU/day did not impact overall mortality during or after the intervention.32, 33 In addition, the inverse serum alpha-tocopherol-mortality association in the present analysis did not differ by trial supplementation group (P for interaction=0.41). Taken together, the totality of evidence supports a beneficial role in long-term health outcomes for higher diet-based vitamin E status within the physiological range, and particularly, the avoidance of very low serum alpha-tocopherol concentrations, rather than the improvement of vitamin E status through supplementation. The apparent difference in health effects of higher biochemical status (benefit) versus vitamin E supplementation (no benefit or possible harm) could be due to more than one factor. Vitamin E-rich foods contain a wide range of bioactive substances whose combined long-term effects on multiple metabolic pathways may be required to impact mortality. Supplementation trials have generally tested high-dose alpha-tocopherol singly or with some other specific vitamins for relatively short periods, which replicates neither the complex dietary vitamin E intake exposure nor its life-long chronicity; it also introduces potential pharmacological disruption of normal homeostatic metabolism.
Vegetable oils, whole grains, nuts, seeds and some vegetables and fruit are the richest dietary sources of alpha-tocopherol, and studies demonstrating associations between greater consumption of these foods and lower mortality are consistent with the present cohort biochemical findings.34–39 Direct biological actions of alpha-tocopherol and other vitamin E compounds that could impact mortality outcomes are well-known and include antioxidant functions related to suppression of free radicals, lipid peroxidation and oxidative damage to cell membranes, organelles, and DNA that have been related to CVD and carcinogenesis,40, 41 inhibition of protein kinase C in vascular smooth muscle cells and immune/inflammatory responses,5, 40, 42 and reduction of the endothelial platelet aggregation and monocyte adhesion that contribute to atherosclerosis.43–46 Less well known is the dose-dependent nature of these mechanisms in vivo. Beyond such direct effects of vitamin E, serum alpha-tocopherol is also likely a physiological biomarker of a vitamin E-rich diet that reflects greater intake of fiber, minerals, unsaturated fatty acids, other vitamins, phytosterols and other antioxidants.47 These additional bioactive agents have multiple biologic functions including effects on oxidative stress, inflammation, and endothelial function.48–51 Of note, the inverse association for serum alpha-tocopherol persisted when we adjusted for intake of vitamin E-rich, multi-nutrient foods, suggesting that those bioactive substances do not explain the serum alpha-tocopherol-mortality association. We cannot, however, exclude the possibility of residual confounding (e.g., due to dietary measurement error).
Major strengths of this investigation include its prospective design, large sample size and complete follow-up for cause-specific mortality through linkage with national registries for three decades. The large sample size enabled examination with substantial statistical power of the vitamin E association both for the less prevalent causes of mortality and across a range of population subgroups. Serum alpha-tocopherol biochemical concentrations were measured in one institute laboratory through high-quality isocratic high-performance liquid chromatography for the more than 29,000 men, affording a more accurate and objective assessment of vitamin E status as compared to data from a self-administrated dietary questionnaire. Several limitations of the study also deserve mention. First, because a single assay of serum alpha-tocopherol concentration at baseline was used, vitamin E biochemical status (and/or the underlying dietary pattern) could have changed over time. Therefore, bias due to nondifferential misclassification may have led to underestimated associations with mortality. We did, however, observe that serum alpha-tocopherol concentrations at baseline and 3-years after randomization were highly correlated in participants who were not supplemented with vitamin E (Spearman correlation coefficient=0.72, P<0.0001), which suggested its relative stability over time. Second, the homogenous population of Finnish male smokers may limit generalizability of our findings to other populations including women. Third, although we have controlled for several potential confounders, including serum total, HDL, and an LDL cholesterol proxy, we cannot rule out the possibility of residual confounding, such as from unmeasured factors.
In summary, this large prospective cohort analysis with over 30 years of observation provides strong evidence that men with higher vitamin E status (i.e., serum alpha-tocopherol) experience significantly lower overall and cause-specific mortality for CVD, heart disease, stroke, cancer and respiratory disease. The associations observed during this long-term follow-up were independent of several other important mortality risk factors. Our findings should be re-examined in other populations that include women, nonsmokers, and other races/ethnicities.
Supplementary Material
Flow Chart for Study of Serum Alpha-Tocopherol and Overall and Cause-Specific Mortality: A 30-Year Prospective Cohort Analysis in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study
Kaplan-Meier curve of cause-specific mortality by quintiles of serum alpha-tocopherol in the ATBC Study. A) Cancer mortality. B) Respiratory disease mortality. C) Other cause mortality.
Kaplan-Meier curve of cause-specific mortality by quintiles of serum alpha-tocopherol in 16,610 subjects who do not have a history of CVD or diabetes mellitus in the ATBC Study. A) Cancer mortality. B) Respiratory disease mortality. C) Other cause mortality.
Cubic spline regression for estimated hazard ratios of cause-specific mortality according to serum alpha-tocopherol concentrations in the ATBC Study. The reference value (9.3 ml/L; hazard ratio = 1) corresponds to the cutoff value of the first quintile of serum alpha-tocopherol concentration. A) Cancer mortality. B) Respiratory disease mortality. C) Other cause mortality. The solid line denoted the hazard ratio for mortality and serum alpha-tocopherol with a 4-knot spline (knots were selected at the 5th, 25th, 75th, and 95th percentiles of the serum alpha-tocopherol); dashed lines denoted the 95% confidence intervals. Total number of participants: 29,092. Event number of cancer-, respiratory disease-, other cause mortality is 7,687, 2,161, and 2,698, respectively.
Novelty and Significance.
What is Known?
Vitamin E is an essential fat-soluble vitamin encompassing eight structurally similar compounds, of which alpha-tocopherol is the predominant form in humans, that is obtained from vegetable oils, some vegetables and fruits, whole grains, nuts (e.g., almonds), and seeds (e.g., sunflower) as well as supplements.
As a potent chain-breaking antioxidant, vitamin E can act as a peroxyl radical scavenger that prevents LDL cholesterol and lipid peroxidation, which have been implicated in chronic disease risk, including cardiovascular disease (CVD) and cancer. In addition to its antioxidant properties, vitamin E has also demonstrated other important functions including enhancement of anti-inflammation activities, regulation of gene expression, improvement of immune response, inhibition of cell proliferation and suppression of tumor angiogenesis.
Higher serum alpha-tocopherol (vitamin E) and greater consumption of vitamin E-rich foods have been related to lower overall mortality and chronic disease risk, whereas some controlled trials demonstrated no beneficial effects, and possible adverse effects, for vitamin E supplementation (particularly for the highest supplementation dosages).
What New Information Does This Article Contribute?
During 30 years of cohort follow-up, men in higher quintiles of baseline serum alpha-tocopherol had decreased mortality overall and from CVD, heart disease, stroke, cancer and respiratory disease, with risk reductions of 17%−47% as compared with men in the lowest quintile.
The associations between serum vitamin E and mortality appeared to be dose-response, were not materially attenuated after adjustment for other important risk factors or for consumption of vitamin E-rich foods, and were stronger among younger and leaner men, as well as those with no history of CVD.
Vitamin E biochemical status has been inversely associated with overall mortality in some studies, but the precise magnitude of the relationship and a comprehensive delineation of cause-specific mortality are lacking. We found significant inverse, dose-response associations between baseline serum vitamin E (alpha-tocopherol) concentrations and overall, CVD, heart disease, stroke, cancer and respiratory disease mortality in a large, prospective cohort of over 29,000 men during a 30-year period, findings that were not confounded by other risk factors including diet. The associations were stable throughout follow-up and were stronger among younger and leaner men, as well as those with no history of CVD. Our findings support a role for greater dietary vitamin E intake in promoting longevity, and they should be re-examined in other studies that include women, nonsmokers and more diverse racial-ethnic groups.
Acknowledgements
We appreciate participants in the ATBC Study for their contributions to this research.
Funding
The ATBC Study is supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, U.S. Public Health Service, Department of Health and Human Services.
Non-standard Abbreviations and Acronyms
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- HR
hazard ratios
- ICD
International Classification of Diseases
- LDL
low density lipoproteins
- RR
relative risk
- VLDL
very low density lipoproteins
Footnotes
Competing interests: none.
References
- 1.Traber MG. Vitamin e regulatory mechanisms. Annu Rev Nutr. 2007;27:347–362 [DOI] [PubMed] [Google Scholar]
- 2.Jiang Q Natural forms of vitamin e: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waniek S, di Giuseppe R, Esatbeyoglu T, Plachta-Danielzik S, Ratjen I, Jacobs G, Nothlings U, Koch M, Schlesinger S, Rimbach G, Lieb W. Vitamin e (alpha- and gamma-tocopherol) levels in the community: Distribution, clinical and biochemical correlates, and association with dietary patterns. Nutrients. 2017;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzi A, Stocker A. Vitamin e: Non-antioxidant roles. Prog Lipid Res. 2000;39:231–255 [DOI] [PubMed] [Google Scholar]
- 5.Pfluger P, Kluth D, Landes N, Bumke-Vogt C, Brigelius-Flohe R. Vitamin e: Underestimated as an antioxidant. Redox Rep. 2004;9:249–254 [DOI] [PubMed] [Google Scholar]
- 6.Azzi A, Breyer I, Feher M, Pastori M, Ricciarelli R, Spycher S, Staffieri M, Stocker A, Zimmer S, Zingg JM. Specific cellular responses to alpha-tocopherol. J Nutr. 2000;130:1649–1652 [DOI] [PubMed] [Google Scholar]
- 7.Shklar G, Schwartz JL. Vitamin e inhibits experimental carcinogenesis and tumour angiogenesis. Eur J Cancer B Oral Oncol. 1996;32B:114–119 [DOI] [PubMed] [Google Scholar]
- 8.Sigounas G, Anagnostou A, Steiner M. Dl-alpha-tocopherol induces apoptosis in erythroleukemia, prostate, and breast cancer cells. Nutr Cancer. 1997;28:30–35 [DOI] [PubMed] [Google Scholar]
- 9.Meydani SN, Beharka AA. Recent developments in vitamin e and immune response. Nutr Rev. 1998;56:S49–58 [DOI] [PubMed] [Google Scholar]
- 10.Sahyoun NR, Jacques PF, Russell RM. Carotenoids, vitamins c and e, and mortality in an elderly population. Am J Epidemiol. 1996;144:501–511 [DOI] [PubMed] [Google Scholar]
- 11.Fletcher AE, Breeze E, Shetty PS. Antioxidant vitamins and mortality in older persons: Findings from the nutrition add-on study to the medical research council trial of assessment and management of older people in the community. Am J Clin Nutr. 2003;78:999–1010 [DOI] [PubMed] [Google Scholar]
- 12.Goyal A, Terry MB, Siegel AB. Serum antioxidant nutrients, vitamin a, and mortality in u.S. Adults. Cancer Epidemiol Biomarkers Prev. 2013;22:2202–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright ME, Lawson KA, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Higher baseline serum concentrations of vitamin e are associated with lower total and cause-specific mortality in the alpha-tocopherol, beta-carotene cancer prevention study. Am J Clin Nutr. 2006;84:1200–1207 [DOI] [PubMed] [Google Scholar]
- 14.De Waart FG, Schouten EG, Stalenhoef AF, Kok FJ. Serum carotenoids, alpha-tocopherol and mortality risk in a prospective study among dutch elderly. Int J Epidemiol. 2001;30:136–143 [DOI] [PubMed] [Google Scholar]
- 15.Huerta JM, Gonzalez S, Fernandez S, Patterson AM, Lasheras C. Lipid peroxidation, antioxidant status and survival in institutionalised elderly: A five-year longitudinal study. Free Radic Res. 2006;40:571–578 [DOI] [PubMed] [Google Scholar]
- 16.Buijsse B, Feskens EJ, Schlettwein-Gsell D, Ferry M, Kok FJ, Kromhout D, de Groot LC. Plasma carotene and alpha-tocopherol in relation to 10-y all-cause and cause-specific mortality in european elderly: The survey in europe on nutrition and the elderly, a concerted action (seneca). Am J Clin Nutr. 2005;82:879–886 [DOI] [PubMed] [Google Scholar]
- 17.Bates CJ, Hamer M, Mishra GD. Redox-modulatory vitamins and minerals that prospectively predict mortality in older british people: The national diet and nutrition survey of people aged 65 years and over. Br J Nutr. 2011;105:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilander L, Berglund L, Boberg M, Vessby B, Lithell H. Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25-year follow-up of swedish 50-year-old men. Int J Epidemiol. 2001;30:1119–1126 [DOI] [PubMed] [Google Scholar]
- 19.Marniemi J, Jarvisalo J, Toikka T, Raiha I, Ahotupa M, Sourander L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int J Epidemiol. 1998;27:799–807 [DOI] [PubMed] [Google Scholar]
- 20.Jayedi A, Rashidy-Pour A, Parohan M, Zargar MS, Shab-Bidar S. Dietary antioxidants, circulating antioxidant concentrations, total antioxidant capacity, and risk of all-cause mortality: A systematic review and dose-response meta-analysis of prospective observational studies. Adv Nutr. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2018;108:1069–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: Design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4:1–10 [DOI] [PubMed] [Google Scholar]
- 23.Milne DB, Botnen J. Retinol, alpha-tocopherol, lycopene, and alpha- and beta-carotene simultaneously determined in plasma by isocratic liquid chromatography. Clin Chem. 1986;32:874–876 [PubMed] [Google Scholar]
- 24.Willett W Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 25.Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage vitamin e supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46 [DOI] [PubMed] [Google Scholar]
- 26.Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res. 1988;29:469–479 [PubMed] [Google Scholar]
- 27.Krasinski SD, Cohn JS, Schaefer EJ, Russell RM. Postprandial plasma retinyl ester response is greater in older subjects compared with younger subjects. Evidence for delayed plasma clearance of intestinal lipoproteins. J Clin Invest. 1990;85:883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borel P, Mekki N, Boirie Y, Partier A, Grolier P, Alexandre-Gouabau MC, Beaufrere B, Armand M, Lairon D, Azais-Braesco V. Postprandial chylomicron and plasma vitamin e responses in healthy older subjects compared with younger ones. Eur J Clin Invest. 1997;27:812–821 [DOI] [PubMed] [Google Scholar]
- 29.Mah E, Sapper TN, Chitchumroonchokchai C, Failla ML, Schill KE, Clinton SK, Bobe G, Traber MG, Bruno RS. Alpha-tocopherol bioavailability is lower in adults with metabolic syndrome regardless of dairy fat co-ingestion: A randomized, double-blind, crossover trial. Am J Clin Nutr. 2015;102:1070–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjelakovic G, Nikolova D, Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin a, and vitamin e singly or in different combinations on all-cause mortality: Do we have evidence for lack of harm? PLoS One. 2013;8:e74558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjelakovic G, Nikolova D, Gluud C. Antioxidant supplements to prevent mortality. JAMA. 2013;310:1178–1179 [DOI] [PubMed] [Google Scholar]
- 32.Alpha-Tocopherol BCCPSG. The effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035 [DOI] [PubMed] [Google Scholar]
- 33.Virtamo J, Taylor PR, Kontto J, Mannisto S, Utriainen M, Weinstein SJ, Huttunen J, Albanes D. Effects of alpha-tocopherol and beta-carotene supplementation on cancer incidence and mortality: 18-year postintervention follow-up of the alpha-tocopherol, beta-carotene cancer prevention study. Int J Cancer. 2014;135:178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369:2001–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eslamparast T, Sharafkhah M, Poustchi H, Hashemian M, Dawsey SM, Freedman ND, Boffetta P, Abnet CC, Etemadi A, Pourshams A, Malekshah AF, Islami F, Kamangar F, Merat S, Brennan P, Hekmatdoost A, Malekzadeh R. Nut consumption and total and cause-specific mortality: Results from the golestan cohort study. Int J Epidemiol. 2017;46:75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luu HN, Blot WJ, Xiang YB, Cai H, Hargreaves MK, Li H, Yang G, Signorello L, Gao YT, Zheng W, Shu XO. Prospective evaluation of the association of nut/peanut consumption with total and cause-specific mortality. JAMA Intern Med. 2015;175:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Brandt PA, Schouten LJ. Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: A cohort study and meta-analysis. Int J Epidemiol. 2015;44:1038–1049 [DOI] [PubMed] [Google Scholar]
- 38.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricciarelli R, Zingg JM, Azzi A. Vitamin e: Protective role of a janus molecule. FASEB J. 2001;15:2314–2325 [DOI] [PubMed] [Google Scholar]
- 41.Cardenas E, Ghosh R. Vitamin e: A dark horse at the crossroad of cancer management. Biochem Pharmacol. 2013;86:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azzi A, Gysin R, Kempna P, Munteanu A, Villacorta L, Visarius T, Zingg JM. Regulation of gene expression by alpha-tocopherol. Biol Chem. 2004;385:585–591 [DOI] [PubMed] [Google Scholar]
- 43.Rashidi B, Hoseini Z, Sahebkar A, Mirzaei H. Anti-atherosclerotic effects of vitamins d and e in suppression of atherogenesis. J Cell Physiol. 2017;232:2968–2976 [DOI] [PubMed] [Google Scholar]
- 44.Devaraj S, Hugou I, Jialal I. Alpha-tocopherol decreases cd36 expression in human monocyte-derived macrophages. J Lipid Res. 2001;42:521–527 [PubMed] [Google Scholar]
- 45.Devaraj S, Jialal I. Alpha-tocopherol decreases interleukin-1 beta release from activated human monocytes by inhibition of 5-lipoxygenase. Arterioscler Thromb Vasc Biol. 1999;19:1125–1133 [DOI] [PubMed] [Google Scholar]
- 46.Ricciarelli R, Zingg JM, Azzi A. Vitamin e reduces the uptake of oxidized ldl by inhibiting cd36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation. 2000;102:82–87 [DOI] [PubMed] [Google Scholar]
- 47.Kris-Etherton PM, Hu FB, Ros E, Sabate J. The role of tree nuts and peanuts in the prevention of coronary heart disease: Multiple potential mechanisms. J Nutr. 2008;138:1746S–1751S [DOI] [PubMed] [Google Scholar]
- 48.Jenkins DJ, Kendall CW, Josse AR, Salvatore S, Brighenti F, Augustin LS, Ellis PR, Vidgen E, Rao AV. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006;136:2987–2992 [DOI] [PubMed] [Google Scholar]
- 49.Torabian S, Haddad E, Rajaram S, Banta J, Sabate J. Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J Hum Nutr Diet. 2009;22:64–71 [DOI] [PubMed] [Google Scholar]
- 50.Jiang R, Jacobs DR Jr., Mayer-Davis E, Szklo M, Herrington D, Jenny NS, Kronmal R, Barr RG. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:222–231 [DOI] [PubMed] [Google Scholar]
- 51.Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: A randomized controlled crossover trial. Diabetes Care. 2010;33:227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow Chart for Study of Serum Alpha-Tocopherol and Overall and Cause-Specific Mortality: A 30-Year Prospective Cohort Analysis in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study
Kaplan-Meier curve of cause-specific mortality by quintiles of serum alpha-tocopherol in the ATBC Study. A) Cancer mortality. B) Respiratory disease mortality. C) Other cause mortality.
Kaplan-Meier curve of cause-specific mortality by quintiles of serum alpha-tocopherol in 16,610 subjects who do not have a history of CVD or diabetes mellitus in the ATBC Study. A) Cancer mortality. B) Respiratory disease mortality. C) Other cause mortality.
Cubic spline regression for estimated hazard ratios of cause-specific mortality according to serum alpha-tocopherol concentrations in the ATBC Study. The reference value (9.3 ml/L; hazard ratio = 1) corresponds to the cutoff value of the first quintile of serum alpha-tocopherol concentration. A) Cancer mortality. B) Respiratory disease mortality. C) Other cause mortality. The solid line denoted the hazard ratio for mortality and serum alpha-tocopherol with a 4-knot spline (knots were selected at the 5th, 25th, 75th, and 95th percentiles of the serum alpha-tocopherol); dashed lines denoted the 95% confidence intervals. Total number of participants: 29,092. Event number of cancer-, respiratory disease-, other cause mortality is 7,687, 2,161, and 2,698, respectively.