Abstract
To induce bone regeneration there is a complex cascade of growth factors. Growth factors such as recombinant BMP-2, BMP-7, and PDGF are FDA-approved therapies in bone regeneration. Although, BMP shows promising results as being an alternative to autograft, it also has its own downfalls. BMP-2 has many adverse effects such as inflammatory complications such as massive soft-tissue swelling that can compromise a patient’s airway, ectopic bone formation, and tumor formation. BMP-2 may also be advantageous for patients not willing to give up smoking as it shows bone regeneration success with smokers. BMP-7 is no longer an option for bone regeneration as it has withdrawn off the market. PDGF-BB grafts in studies have shown PDGF had similar fusion rates to autologous grafts and fewer adverse effects. There is also an FDA-approved bioactive molecule for bone regeneration, a peptide P-15. P-15 was found to be effective, safe, and have similar outcomes to autograft at 2 years post-op for cervical radiculopathy due to cervical degenerative disc disease. Growth factors and bioactive molecules show some promising results in bone regeneration, although more research is needed to avoid their adverse effects and learn about the long-term effects of these therapies. There is a need of a bone regeneration method of similar quality of an autograft that is osteoconductive, osteoinductive, and osteogenic. This review covers all FDA-approved bone regeneration therapies such as the “gold standard” autografts, allografts, synthetic bone grafts, and the newer growth factors/bioactive molecules. It also covers international bone grafts not yet approved in the United States and upcoming technologies in bone grafts.
1. Introduction
Bone regeneration naturally occurs in most cases, but in cases such as nonunions, malunions, tumors that cause bone defects, and avascular necrosis, bones may not heal independently and there may be a need for surgery and a bone graft to induce bone regeneration. Bone grafting is also used in orthopedic, oncologic, and dental surgeries/procedures. Worldwide there are 2 million bone grafting procedures performed annually (with half a million of these occurring yearly in the United States), making bone the second most transplanted tissue right behind blood transfusions [1–5]. The cost of treating bone defects in the United States is estimated around 5 billion dollars annually [6]. Bone regeneration therapy currently has limitations that cause societal economic burden and reduced quality of life for patients [7]. There is a need for bone regeneration therapies that are affordable and have better clinical outcomes for patients.
Orthopedic procedures using bone grafts were first reported to be used in the 17th century. Dutch surgeon Job Van Meekeren recorded the transplant of a bone from a dog into the cranial defect of a soldier. This was the first known bone graft, but due to orders from the church the graft was ordered to be removed. The graft could not be removed from the soldier’s skull as the bone had already been incorporated into his skull [8, 9]. Experiments with synthetic materials also began in the 19th century with materials such as wood as marble, but the first promising synthetic material was the plaster of Paris (calcium sulfate) in 1892 [8, 10–12].
Luckily, today there is an approval process to make sure bone grafts are safe to use and effective at regenerating bone. The approval process in the United States is through the Food and Drug Administration (FDA) and the type of approval needed varies based on the type of graft or grafting device. The four main approval processes for bone grafts are 510K clearance; Investigational Device Exemption/Premarket Approval (IDE/PMA); human cells, tissues, and cellular and tissue-based products (HTC/P); and Regenerative Medicine Advanced Therapy (RMAT) are described below in table 1. There are also three classes of medical devices through the FDA: class I, II, and III. Class I medical devices are devices that are unlikely to cause bodily harm if a malfunction occurs, such as devices like tongue depressors, crutches, and blood pressure cuffs [13, 14]. Class II medical devices are devices that are unlikely to lead to preposterous bodily harm if a malfunction occurs, such as devices like cardiac monitors and surgical drapes [13, 14]. Class III medical devices are devices that have a risk of serious injury but are intended to significantly modify patient health, such as pacemakers and defibrillators [13, 14].
Table 1:
FDA-approval process for bone grafts, describes 510K, IDE/PMA, and HCTP approval processes.
| FDA-approval Processes: | Process of Approval: |
|---|---|
| 510K | The 510K process is a premarket submission is that the device is at least as safe and effective, that is, substantially equivalent, to a legally marketed device [15]. |
| IDE/PMA | An IDE allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data. This data will then be used to support the Premarket Approval [16]. |
| HCT/P | HCT/P’s are regulated by the Center for Devices and Radiological Health as medical devices and/or are regulated by the Center for Biologics Evaluation and Research [17]. Whether products are regulated by one or both of these agencies depends on the product wanting to be marketed. No premarket approval is needed through the FDA. |
| RMAT | RMAT’s must be made with an existing or new Investigational New Drug (IND) application. This designation includes certain human gene therapies and xenogeneic cell products[18]. |
510K would be used to approve grafts such as demineralized, autologous cellular, and synthetic grafts for use as bone void fillers in applications not intrinsic to stability of the bone. Animal studies or benchwork studies are required for 510k clearance and limited clinical studies are required [19]. IDE/PMA would be used to approve grafts that are classified as class III medical drug-device combinations such as recombinant bone morphogenetic protein-2 (rhBMP-2), rhBMP-7, and P-15, a synthetic amino-acid peptide. To get premarket approval a level I IDE human clinical trial is required [19]. HCTP would be used to approve grafts such as nonstructural allografts and cellular-based allografts. Through the HCTP approval pathway, there is no premarket review by the FDA and limited clinical and pre-clinical studies are required [19]. The RMAT pathway would be used if the drug is a regenerative medicine therapy, which is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products [18].
This review aims to summarize bone grafting options that are currently FDA-approved for bone regeneration including autologous grafts, allografts, and synthetic grafts. It also highlights the growth factors such as BMP-2, BMP-7, PDGF-BB and bioactive marker P-15 that are FDA-approved for bone regeneration. This review also covers bone graft clinical indications and discusses literature related to clinical studies on bone healing.
2. Clinical Indications of Bone Grafts
2.1. Nonunion
Nonunion of bone is when the body cannot heal a fracture. The FDA defines nonunion as a fracture that persists for nine months and has no sign of healing for 3 months [20, 21]. To treat a nonunion depends on the type of nonunion that has occurred. Hypertrophic nonunions occur from excess motion at the fracture site. The fracture still has the biological factors it needs to heal and the reason it cannot heal as the bone cannot bridge due to the excess motion [22]. Hypertrophic non-unions are treated by fixing the mechanical stability by internal fixation[20]. Atrophic non-unions occur due to inadequate biological conditions for healing such as lack of adequate bone stability or blood supply [22]. Atrophic non-unions are treated by fixing the mechanical stability and the biology of the bone. This is achieved by using internal fixation in addition to bone graft or parathyroid hormone therapy [20]. Figure 1 below shows an x-ray of an atrophic nonunion of fracture of the humerus. To diagnosis a nonunion, a physician would see no progress in bone healing or a persistent gap in the fracture on repeat imaging over several months [23].
Figure 1:
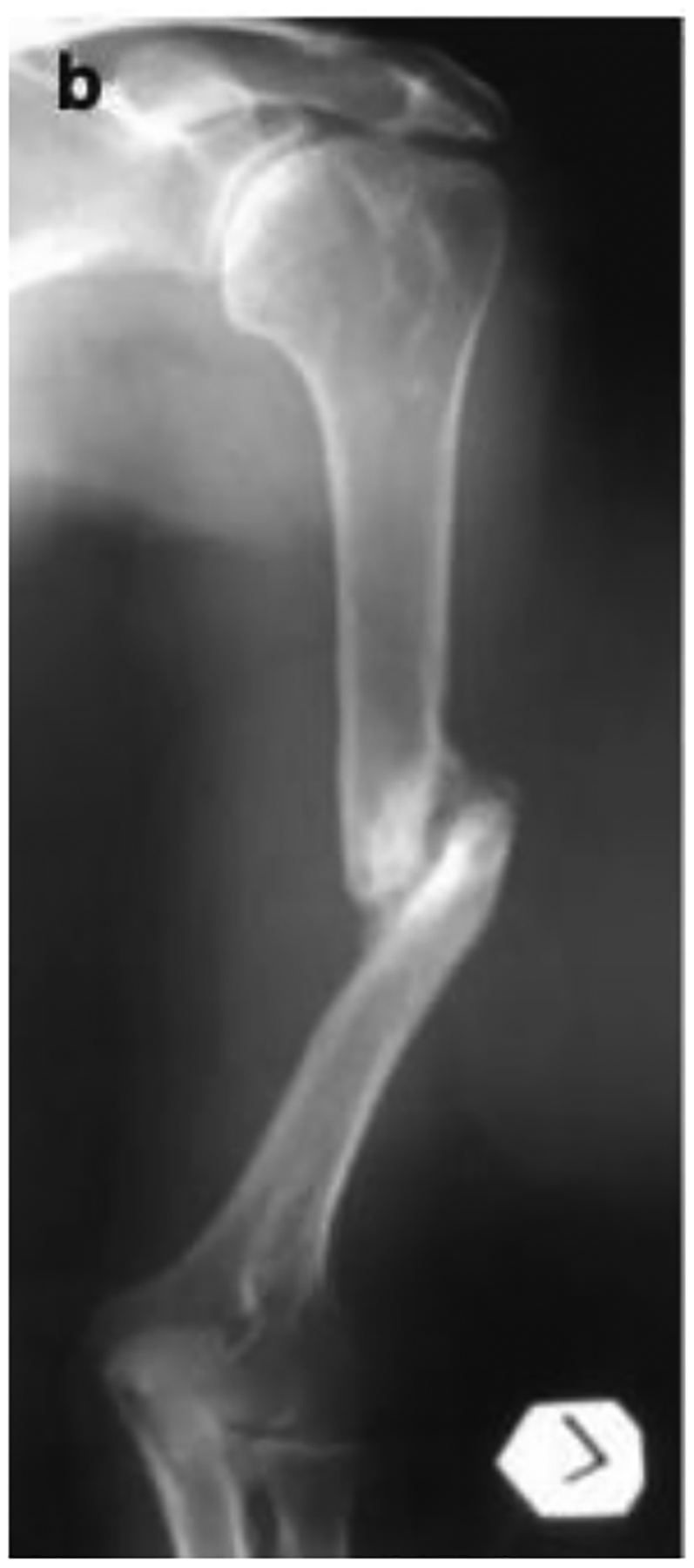
Radiographic image of atrophic nonunion of fracture of the humerus [24].
Reprinted/adapted by permission from [CopyRight Clearance Center]: [Springer Nature]
2.2. Malunion
Malunion of bone occurs when the fracture completely heals, but the bone doesn’t heal in the proper location or orientation. It is defined as an angular or rotational deformity exceeding 5 degrees or a shortening of bone [25]. Malunions can be managed by fixation of bone, osteotomies, and/or bone grafting depending on the case [26]. Around 24 percent of patients treated conservatively for distal radius fracture develop malunions [27]. Figure 2 below shows a severe case of a malunion in the proximal femur.
Figure 2:
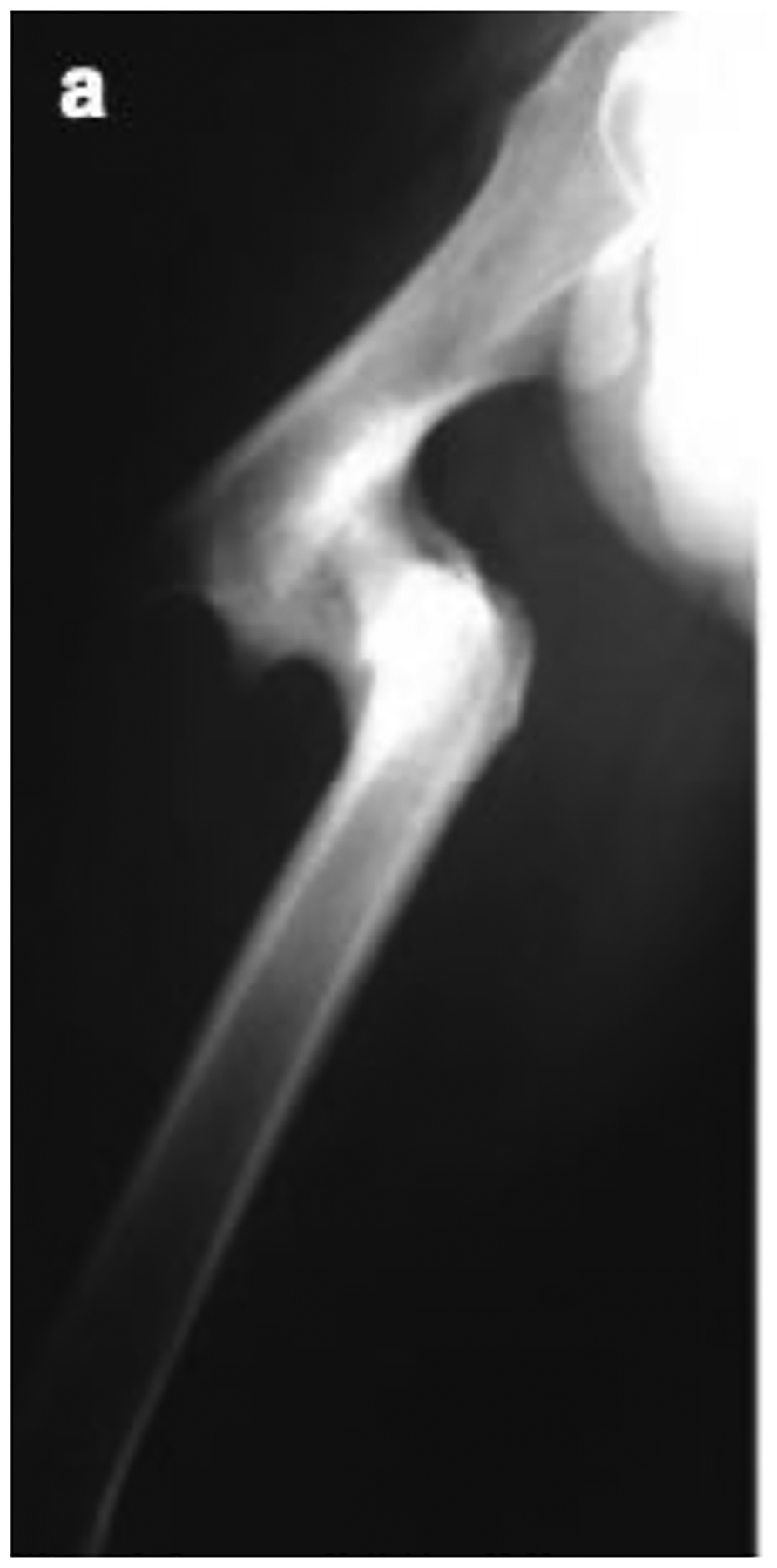
Radiographic image of malunion of the proximal femur [24].
Reprinted/adapted by permission from [CopyRight Clearance Center]: [Springer Nature]
2.3. Tumors that Cause Bone Defects
Another reason to need a bone graft is due a tumor such as a cancer, cyst, or benign tumor causing a bone defect. Multiple myeloma is a possible cause of tumors of the bone or osteolytic lesions, which are a result of the myeloma cells making bone marrow cells remove calcium from bone [28]. Other causes of bone tumors are benign tumors such as osteochondromas and osteoid osteomas; and cancerous tumors such as osteosarcoma, Ewing’s sarcoma, chondrosarcoma, or a secondary bone cancer. A possible treatment plan is to remove the tumor or cyst via curettage and pack the defect with a bone graft to give the region mechanical strength and encourage growth of bone to the area [3, 29]. If the tumor is cancerous, treatment such as radiation or chemotherapy may be needed after removing the tumor. Figure 3 below shows a radiographic image of a bone cyst in the calcaneus.
Figure 3:
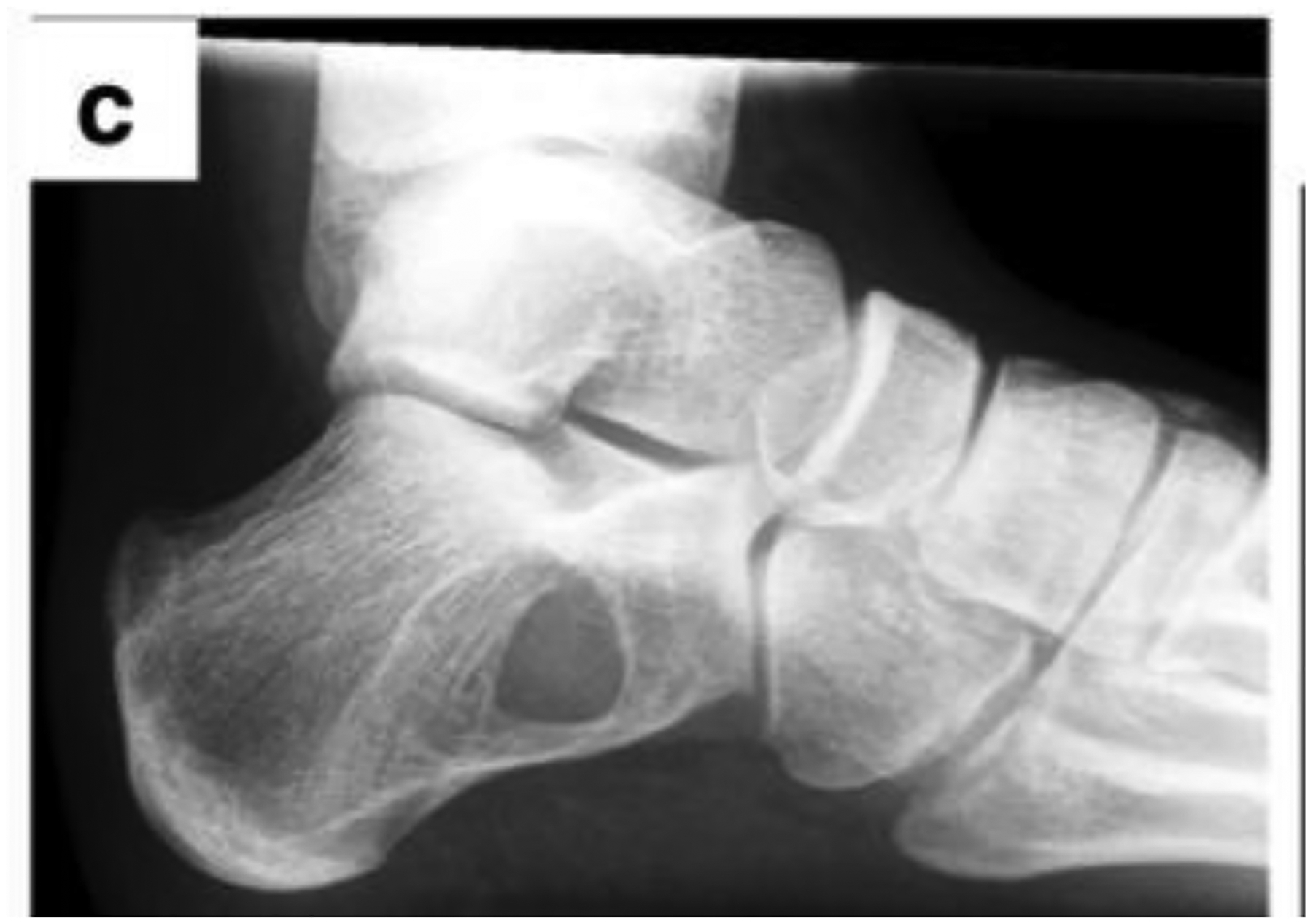
Radiographic image of bone cyst in the calcaneus. Reprinted from [29] with permission from the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
2.4. Avascular Necrosis
Osteonecrosis/avascular necrosis is death of cellular components of bone due to lack of subchondral blood flow. The most common location for osteonecrosis to occur is the femoral head. It is also common to see osteonecrosis occur in the humerus, talus, and knee. Common causes of osteonecrosis are trauma, long-term/ high dose corticosteroid use, alcoholism, sickle cell anemia, and idiopathic [30]. To treat osteonecrosis, a vascular bone graft can be used to help restore viable bone and blood supply to the avascular area [31]. Figure 4 below shows a radiographic of avascular necrosis of the femoral head.
Figure 4:
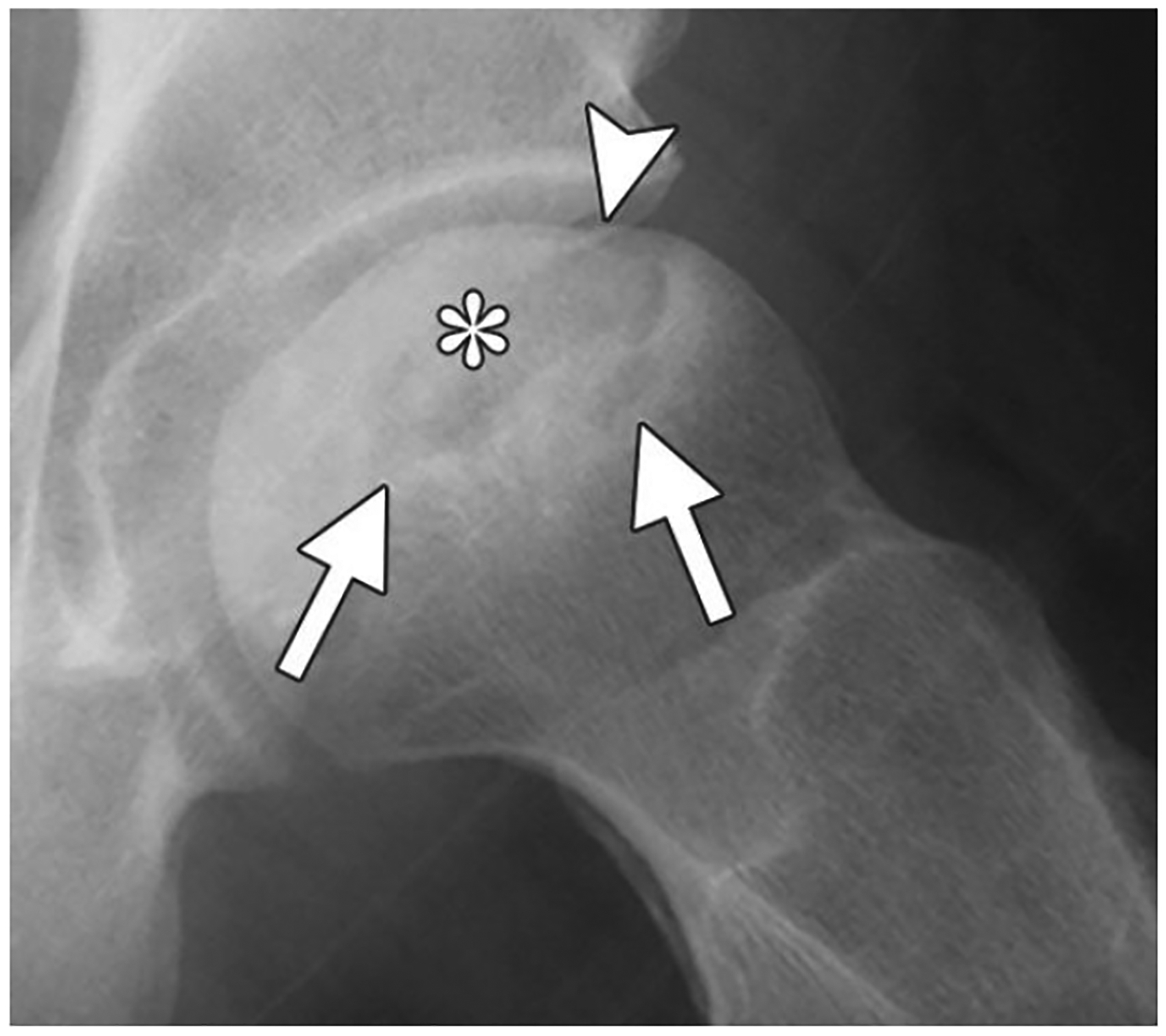
Radiographic image of avascular necrosis of the femoral head. Reprinted from [30] with permission from The Radiological Society of North America.
2.5. Orthopedic Surgery
In addition to correction of nonunions and malunions, other applications of bone grafts in orthopedic surgery include trauma applications, spinal fusion, fracture repair, and revision of endoprostheses [32]. Causes of an orthopedic trauma could be a fall or car accident. A spinal fusion is when a bridge is formed by a bone graft between two vertebrae segments to fuse the vertebrae [33]. The process of a spinal fusion is placing the bone graft between the vertebrae wanting to be fused and then using metal plates or screws to hold the bones together until the bone graft can hold the vertebrae in place.
Indications for a spinal fusion are symptomatic scoliosis, spondylolisthesis, or other spine deformity; low back pain from damage to vertebrae disc or from stretching of the joint capsule; neurogenic claudication; and radiculopathy due to foraminal stenosis [34].
2.6. Other Applications
Bone grafts are also used in dentistry applications such as dental implants. They are also used in cranial and maxillofacial applications such as cranioplasty, alveolar bone grafts, and repairing defects of the jaw. Causes of bone defects in the mouth and cranial region include congenital defects such as cleft lip and palates, trauma, infections, and surgery to remove tumors [35–37].
3. Bone Repair and Healing
3.1. Bone repair process
Bone repair occurs by two different processes: primary and secondary bone healing. Primary bone healing is when the bone cortex directly heals without formation of a callus. Secondary bone healing, as shown in figure 5, is what occurs more commonly and this is the type of bone healing that happens during fracture repair and incorporation of a bone graft. Secondary bone healing involves stages such as inflammation, proliferation, and remodeling. The inflammation stage is when the cascade of growth factors work to stimulate mesenchymal stem cells (MSCs) to differentiate into osteoblasts. This inflammation stage also ends up forming a callus. In the proliferation phase, angiogenesis occurs and granulation tissue is formed. Then woven bone is formed via intramembranous or endochondral ossification. Remodeling then occurs and is when woven bone is removed and replaced by new lamellar bone [39, 40].
Figure 5:
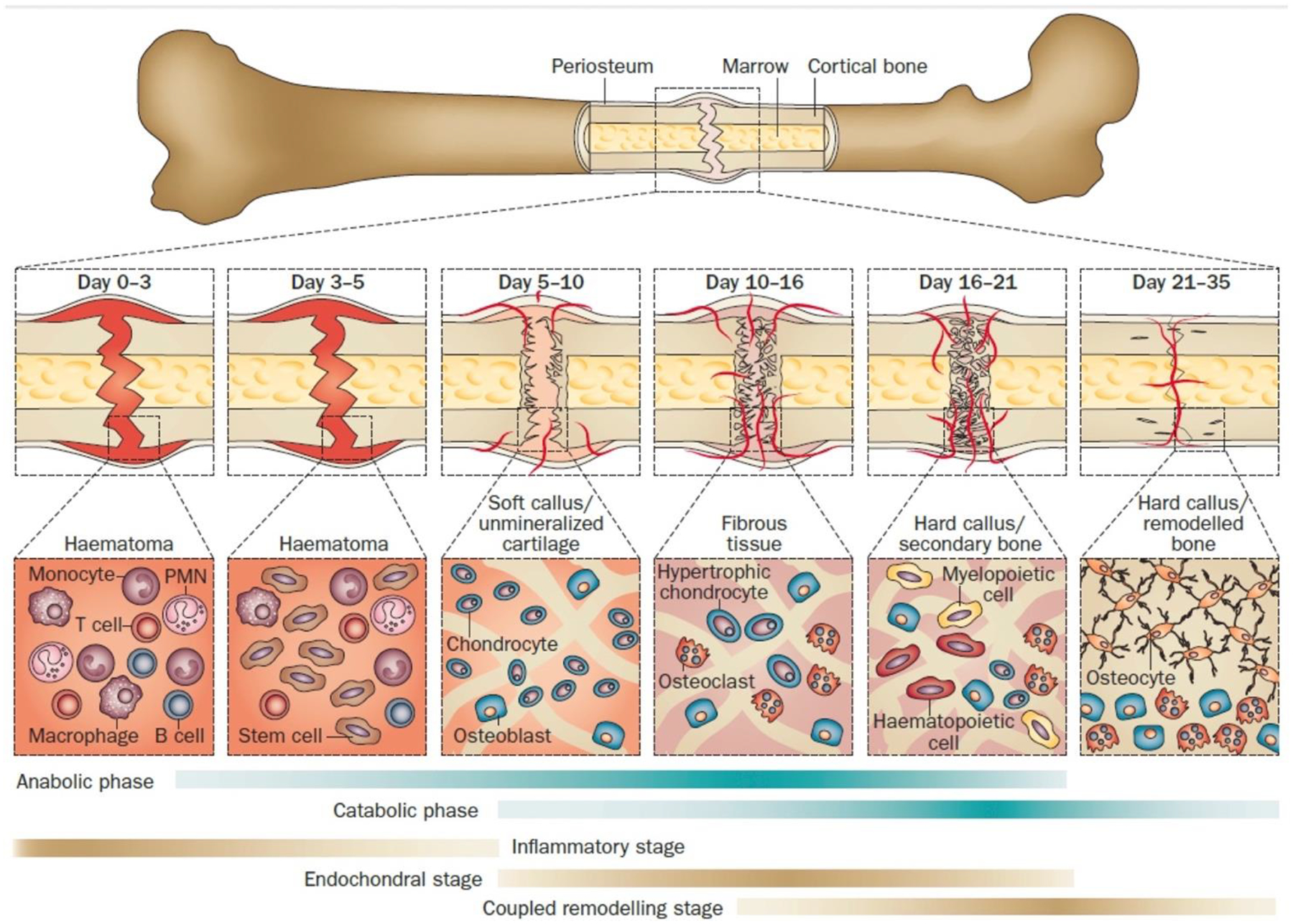
Typical fracture healing process shown at different phases of bone healing with primal cell types found at each stage and time of each stage. Below the fracture healing illustration, metabolic phases (blue bars) and biological stages (brown bars) are shown. The metabolic stages are anabolic and catabolic. The three major biological stages of healing are inflammatory, endochondral bone formation, and coupled remodeling. The time scale of healing is equivalent to a mouse closed femur fracture fixed with an intramedullary rod. Reprinted from [38] with permission from Journal of Bioactive Materials under the Creative Commons Attribution-NonCommercial License (https://creativecommons.org/licenses/by-nc-nd/4.0/).
3.2. Ideal Bone Graft Qualities
The goal for a bone graft is to be osteoconductive, osteoinductive, and osteogenic, while minimizing risks to the patient [1]. Other goals of bone grafts include affordability, decreased risk of infection to patient, biocompatible, and structurally similar to bone with similar porosity and mechanical strength [4, 8]. For fracture healing to occur osteoconduction, osteogenesis, osteoinduction, and mechanical stability, as shown in figure 6, need to all occur as they all play a part in bone regeneration.
Figure 6:
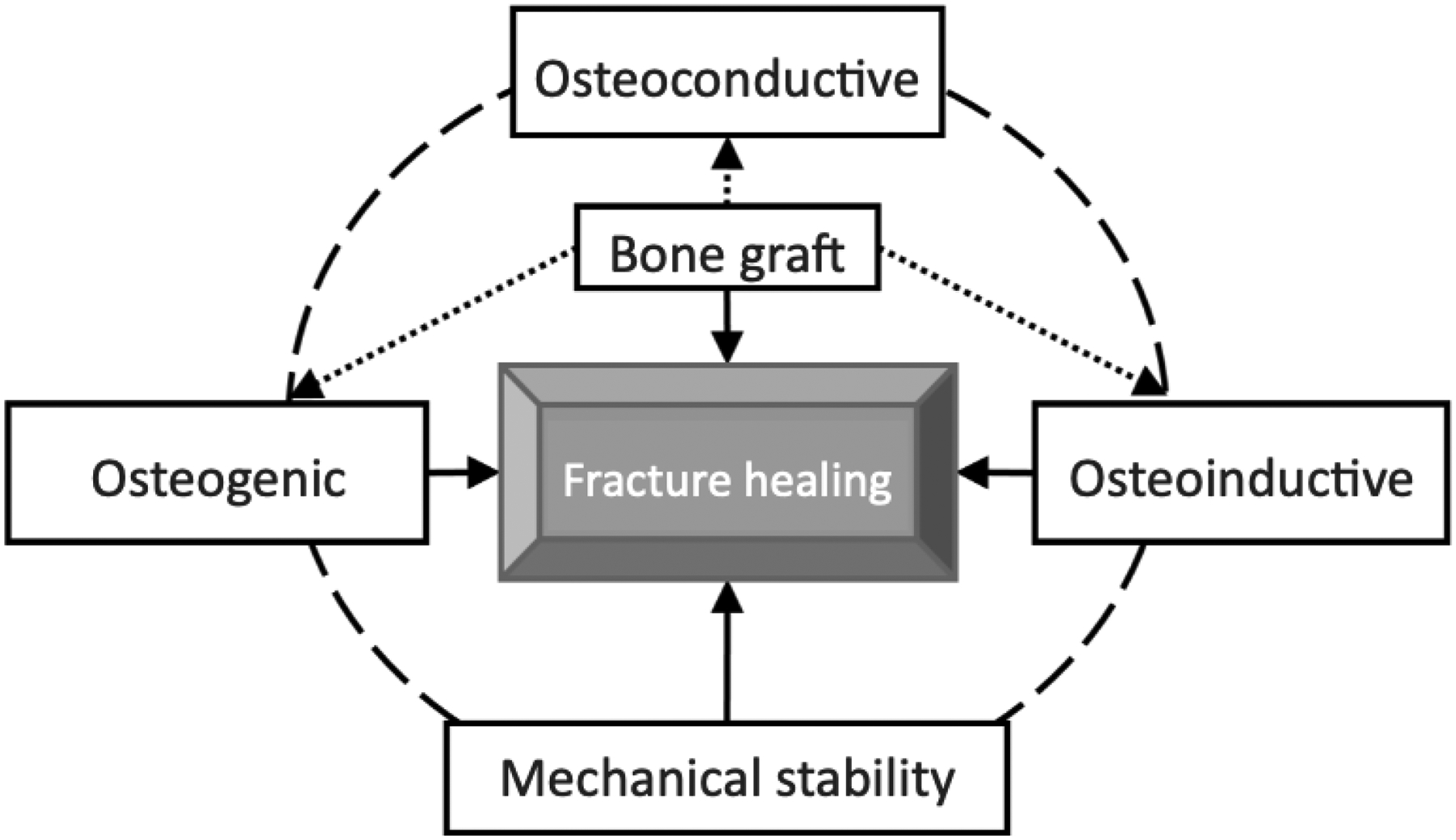
The process of a fractured bone healing is controlled by properties of osteoconduction, osteoinduction, and osteogenesis. Bone graft materials are osteoconductive to allow angiogenesis and cell growth to occur. Then by osteoinduction, recruitment of mesenchymal stem cells occurs. Then osteogenesis releases growth factors to help form new bone. Other important aspects of bone healing include vascularity as adequate blood supply is needed for healing and mechanical stability. Reprinted from [41] with permission from Creative Commons Attribution-NonCommercial License (https://creativecommons.org/licenses/by-nc/4.0/).
Osteoconduction is the process that the new bone is able to grow into the new surface. Bone grafts are ideally osteoconductive so that new bone can grow and flourish in the implanted material surface [42].
Osteoinduction is the process where osteogenesis is induced. It begins by growth factors stimulating MSCs to form osteoblasts and chrondroblasts [22, 42].
Osteogenesis of a bone graft is the process that osteoblasts from the graft help with growth and formation of new bone [36].
3.3. Growth Factors in Bone-Regeneration
There are many growth factors involved in bone-regeneration such as bone morphogenic protein-2 (BMP-2), BMP-4, fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and insulin-growth factor-1 (IGF-1) [43]. In fracture sites, hypoxia regulates osteoblast production of vascular mediators such as growth factors: VEGF, transforming growth factor- β, IGF, and FGF [37, 44]. The action of growth factors in vascularized bone regeneration, is shown below in figure 7.
Figure 7:

Cascade of Growth Factors. Illustration show actions of prevalent growth factors (BMP-2, FGF, PDGF, and VEGF) in vascularized bone regeneration. Reprinted by permission from [CopyRight Clearance Center]: [Elsevier] [45].
BMPs (bone morphogenic proteins) except for BMP-1 are part of the transforming-growth factor-beta superfamily [46]. The action of BMPs are regulated by receptor kinases and transcription factors called Smads [32]. Of the bone morphogenic proteins, BMP-2, 4, 6, 7, and 9 are the inducers of bone growth [47]. BMP-2 is involved in promoting differentiation of MSCs [38, 39]. While, BMP-7 is involved in promoting angiogenesis [38]. Currently there are only 2 recombinant BMPs that are FDA-approved: recombinant BMP-2 (rhBMP-2) and rhBMP-7 [48]. BMP9, also called growth differentiation factor 2 (GDF-2), was first identified in fetal mouse liver cDNA libraries and is currently being researched in its role in bone regeneration [49, 50]. BMP-9 has been proven to promote osteogenic differentiation of mesenchymal stem cells and to be the most osteogenic BMP in vitro and in vivo [50–57].
In a study published in 2019 by Gaihre et al, used a scaffold-based strategy in rats to study the bone forming ability of recombinant BMP-9 combined with VEGF. It was found that BMP-9 enhanced proliferation of human MSCs along the surface of the scaffold. BMP-9 also increased the expression of alkaline phosphatase, collagen 1, and osteocalcin genes [51]. BMP-9 is a poorly characterized member of the BMP family so more research is currently being conducted to learn more about this growth factor, as there is little know on the mechanism of BMP-9 [50].
Fibroblast growth factor has roles in regulating chondrogenesis, osteogenesis, and bone and mineral homeostasis [58]. The fibroblast growth-factor family has 22 members, with FGF-2, FGF-9, and FGF-18 being involved in osteogenesis and possible candidates for bone regeneration [59]. FGF binds the FGF receptor (FGFR) and in gain-of-function mutations of the FGFR genes can cause conditions such as craniosynostosis and dwarfism [59, 60].
VEGF is in a family consisting of VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor [61–63]. VEGF is a regulator of angiogenesis in skeletal growth, embryogenesis, and reproductive functions [63, 64]. VEGF also has a role in proliferation, migration and activation of endothelial cells. Also, VEGF via osteogenic growth factors stimulates osteogenesis [64]. VEGF is important for regulating osteoclasts in the remodeling of bone [61].
The platelet-derived growth factor (PDGF) family is made up of 5 homodimers (AA, AB, BB, CC, and DD). PDGF is secreted from platelet a-granules [65–67]. In bone, PDGF-BB is the most active PDGF and is a key regulatory factor in tissue repair and regeneration [68]. PDGF can also promote angiogenesis [69]. PDGF-BB is unique as it participates in angiogenesis, osteogenesis, and mesengenesis [70]. There are FDA-approved devices on the market using PDGF-BB for bone regeneration.
4. FDA-approved bone grafts and bone graft substitutes
The categories of bone grafts with advantages and disadvantages of each are shown in figure 8 below and will be further discussed throughout the rest of the manuscript. The advantages and disadvantages of all the FDA-approved bone grafts and bone graft devices are shown in table 2.
Figure 8:
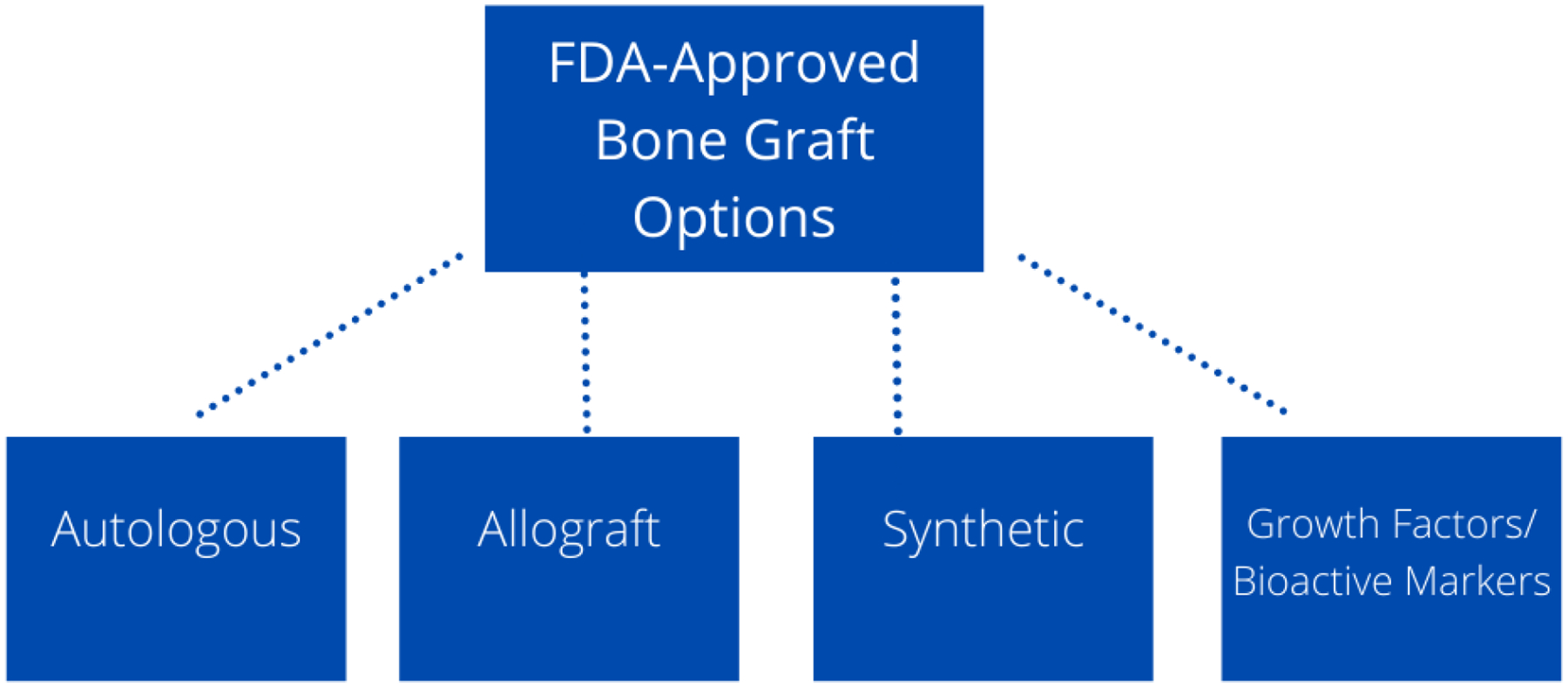
FDA-Approved Bone Graft Options: The four main options of bone grafting include autologous, allograft, synthetic grafts, and growth factors/bioactive markers.
Table 2: Advantages and Disadvantages of Bone Graft Types.
([Ref]= References for column to the left)
| Bone Graft Type | Advantages: | [Ref] | Disadvantages: | [Ref] |
|---|---|---|---|---|
| Autologous | “gold standard” as it is osteoconductive, osteoinductive, and osteogenic | [38, 71] | 2nd surgical site increases risk of infection, limit on amount of graft material available | [22, 74] |
| Autologous: cancellous | Large surface area advantageous for revascularization | [22, 71, 90] | Poor mechanical strength | [22] |
| Autologous: cortical | Structural support and mechanically stable | [38] | Longer to remodel then cancellous graft | [38, 90] |
| Autologous: vascularized cortical | Quick healing time, preserved osteocytes and osteoprogenitor cells in the graft | [22, 71] | Hard to harvest and to implant | [71] |
| Bone Marrow Aspirate | Can be harvested minimally invasively | [22] | Less stem cells in graft then hypothesized | [22, 48, 90] |
| Platelet-rich Plasma | Affordable, easy to obtain, provokes migration of MSCs directly to the site, reduces amount of autograft needed to be harvested | [95, 96] | Variability with individual PRP and preparation methods | [98–100] |
| Allogenic | No second surgical site (decreased risk of infection and no additional pain) | [102] | Risk of disease transmission or unfavorable immune response | [104–113] |
| Allogenic: cancellous | Freeze drying causes graft with low residual moisture and shelf-life of 4–5 years | [114] | Low mechanical strength, hard to incorporate as body incapsulates graft in fibrous tissue | [22, 38, 94] |
| Allogenic: cortical | Strong, can be used in load-bearing areas of body | [115] | Slow healing due to inflammatory response | [22, 90] |
| Allogenic: Demineralized Bone matrix | Contains growth factors making it osteoinductive | [22, 104] | Varying amounts of growth factors from different batches and manufacturers | [118] |
| Synthetic grafts | Many options of graft material, custom scaffolds (3D printed and injectable) | [77, 119–122] | Lack growth factors to encourage bone growth | [123] |
| Calcium Phosphate Ceramics | Similar composition to bone | [124] | Poor mechanical strength, hard to mold | [102] |
| Tricalcium Phosphate | Synthetic graft with most similar composition to bone, “gold standard” of synthetic grafts | [8, 125] | Degrades unpredictably so not good for load-bearing areas | [3, 38] |
| Biphasic Calcium Phosphate | Advantages of both tricalcium phosphate and hydroxyapatite, range of resorption rate and mechanical properties based on mixture | [38, 126, 127] | Limitation of mechanical strength based on mixture of graft | [128] |
| Hydroxyapatite | Very biocompatible, higher compression and tensile strength compared to tricalcium phosphate | [102] | Slow resorption of graft | [8, 129, 130] |
| Calcium Phosphate Cement | Temperature dissolution-precipitation reaction makes them easy to mold | [102, 131] | Poor mechanical strength | [132] |
| Calcium Sulfate | Cheap to make, easy to prepare | [38] | Resorbs quicker than bone dissolution, risk of serous wound drainage, lack of mechanical strength | [22, 102, 133, 134] |
| Bioactive Glass | Forms bonds to bone and tissue, activates genes controlling osteogenesis, antibacterial properties | [135–138] | Small range of SiO2 content for bioactivity of graft | [135, 136] |
| PMMA Bone Cement | Secures orthopedic implants in place | [139] | No intrinsic adhesive properties, heat-sensitive, cement fragmentation and foreign body reaction can wear down and loosen implant, risk of bone cement implantation syndrome | [140–142] |
| Growth factors and bioactive molecules | Have both osteoinductive and osteoconductive properties | [22] | Not studied long-term, inflammatory complications from off-label uses, very expensive | [1, 143] |
| Infuse (BMP-2) | Study results with possible better outcomes then autograft, good bone regeneration abilities in smokers | [144, 145] | Off-label use can cause swelling that can close patients airway, risk of ectopic bone formation | [143, 146–148] |
| OP-1 (BMP-7) | - | - | No longer on the market | [149] |
| PDGF-BB | Less pain then autograft with similar results | [150, 151] | Not studied long term, expensive | [1] |
| iFactor (P-15) | Similar results to autografts in study | [152] | Side effects of axial pain, postoperative radiculopathy, and dysphagia after treatment | [152] |
4.1. Autologous Grafting
The gold standard of bone grafts is autologous grafting [38, 71]. Autologous grafting is harvesting bone from a patient’s body and using it elsewhere as a bone graft in their body. These types of grafts are osteogenic, osteoinductive, and osteoconductive and contain viable bone precursor cells [71]. Since, autologous grafts are fresh and come from the patient they contain growth factors: BMP-2, BMP-4, fibroblast growth factor, VEGF, platelet-derived growth factor, and insulin-growth factor 1 [71]. Benefits of autologous grafts include no issues with rejection and disease-transmission risk as the graft comes from the patient’s body [8, 72, 73].
Although there are numerous benefits of autologous grafting, the grafts have to be harvested from the patient resulting in an additional surgical site. This extra surgical site comes with risks to the patient such as: additional pain, increased risk of infection, and risk of donor site morbidity [74]. Common locations to harvest autologous grafts include the iliac crest and intramedullary canal of long bones [75]. Other limitations of this graft material include limited supply of graft material [22]. Another limitation is that autologous grafts can resorb too quickly before new bone growth is complete [76, 77].
In studies conducted on bone graft infections, around 3–5.4 percent of patients ended up with a post-operative recipient graft site infection [78, 79]. Patients with higher risk of infection are those with immunocompromising diseases such as diabetes mellitus. Infections of the bone, require long-term antibiotic use and repetitive irrigation and debridement. Not only can this cost a lot of money for insurances and patients due to prolonged hospital stays and additional procedures needed, it also can lead to serious illness and death in the patient. The mean hospital stay after a bone-allograft procedure for a non-infected patient was 7.82 days, while for an infected patient was 15.10 days [78, 79]. In the United States, health-care acquired infections cost hospitals 28 to 45 billion dollars a year [80]. Orthopedic surgeries have been found to cause pain that lasts at least 2 years at the graft site in 15–39 percent of patients [75, 81]. This is a major problem, since the point of the surgery in the first place was to correct a problem and in a large percentage of patients, we are creating chronic pain. The cost of pain in the United states according to John Hopkins is as 560 to 635 billion dollars a year [82].
In some cases where an autologous bone graft is not possible, another graft type may be chosen. But due to irradiation needed to sterilize, these other types of bone transplants do not have the osteogenic or osteoinductive capabilities of the autologous graft [71, 83–89]. Circumstances in which an autologous graft would not be chosen for the patient would be in a patient is high risk for a second surgical site or has a lack of suitable bone to graft.
Cancellous grafts
The most common type of autologous bone grafts is cancellous. These grafts are very osteogenic due to high concentrations of osteoblasts and osteocytes [22]. Cancellous grafts also have a large trabecular surface, which allows a large surface area for revascularization within 2 days of implantation to occur [22, 71, 90]. After transplant, cancellous grafts take 6–12 months to be completely resorbed and replaced with new bone [38, 91]. Compared to cortical grafts, cancellous grafts have greater osteoconduction. The disadvantage of using cancellous grafts is due to their low density, they have little mechanical support [22].
Cortical grafts
Cortical autologous graft possesses more structural support and mechanical stability than a cancellous graft. Although a cortical graft takes longer to remodel as revascularization and remodeling takes longer to occur due to their dense architecture [38, 90]. After transplant, cortical grafts take years to be completely resorbed and replaced with new bone [38, 90, 92].
Vascularized cortical bone grafts
To harvest a vascularized graft, care must be taken to preserve the vasculature. The main limitation of these grafts is their difficulty to harvest and implant due to needing orthopedic and microvascular skill sets [71]. Vascular grafts are advantageous though if they are able to be harvested as they contain preserved osteocytes and osteoprogenitor cells [22]. The other advantage of an vascularized bone graft is its quick healing time [71]. If adequate vascular anastomosis and stability of the graft is achieved, a study has shown that more than 90 percent of the osteocytes in the graft will survive [71, 93].
4.2. Autologous bone grafts and other biologics
There are also bone substitutes that are enhance with a patient’s biological substances. These bone substitutes are grafts made from bone marrow aspirate or platelet-rich plasma that are combined with autologous bone. This gives the benefits of an autologous graft with added benefit from the growth factors the bone marrow aspirate or platelet-rich plasma contain.
Bone marrow aspirate
The advantage of using a bone marrow aspirate graft is that it can be harvested minimally invasively. The main reason bone marrow aspirate was chosen as to be used as a graft material was due to suspected high number of mesenchymal stem cells in the marrow. The number of stem cells that were found in the marrow were a lot lower than what was initially thought and the number of stem cells found varies from patient to patient [22, 48, 90]. The other hypothesis is that bone marrow aspirate grafts promote fracture healing via stimulating angiogenesis by the endothelial progenitor cells it contains [22, 94].
Platelet-rich plasma
Platelet-rich plasma (PRP) has been used with autologous grafts as it reduces the amount of autologous graft material to harvest from the patient and also is an affordable way to incorporate growth factors into a graft [95]. Administration of PRP is beneficial as it provokes migration of MSCs directly to the site of injury or surgery [95, 96]. PRP also contains growth-factors such as PDGF, TGF-beta, IGF, VEGF and FGF that are involved in the bone regeneration process [97]. PRP is an affordable and easy to obtain material that can extend the amount of autologous graft material. The disadvantage of PRP is that there is lots of variability of each person’s PRP and even the preparation methods can affect each person’s PRP differently [98–100]. It is unclear on how patient comorbidities will affect the composition of their PRP [99, 100]. These challenges need to be addressed so each patient can get the full benefit from PRP in bone regeneration.
4.3. Allogenic bone graft
An allogenic bone graft refers to bone harvested from either a live donor or a cadaver that is then transplanted into the patient needing a bone graft [8, 38, 92]. An allogenic bone graft is considered the best option when an autologous bone graft is not possible, as shown in figure 9 [38]. However, allogenic bone has a higher failure rate then autologous grafts as allogenic grafts are immunogenic and can be rejected by activation of major histocompatibility complex antigens [38, 101]. An advantage is that no additional surgical site is needed on the patient compared to autologous bone grafts [102].
Figure 9:
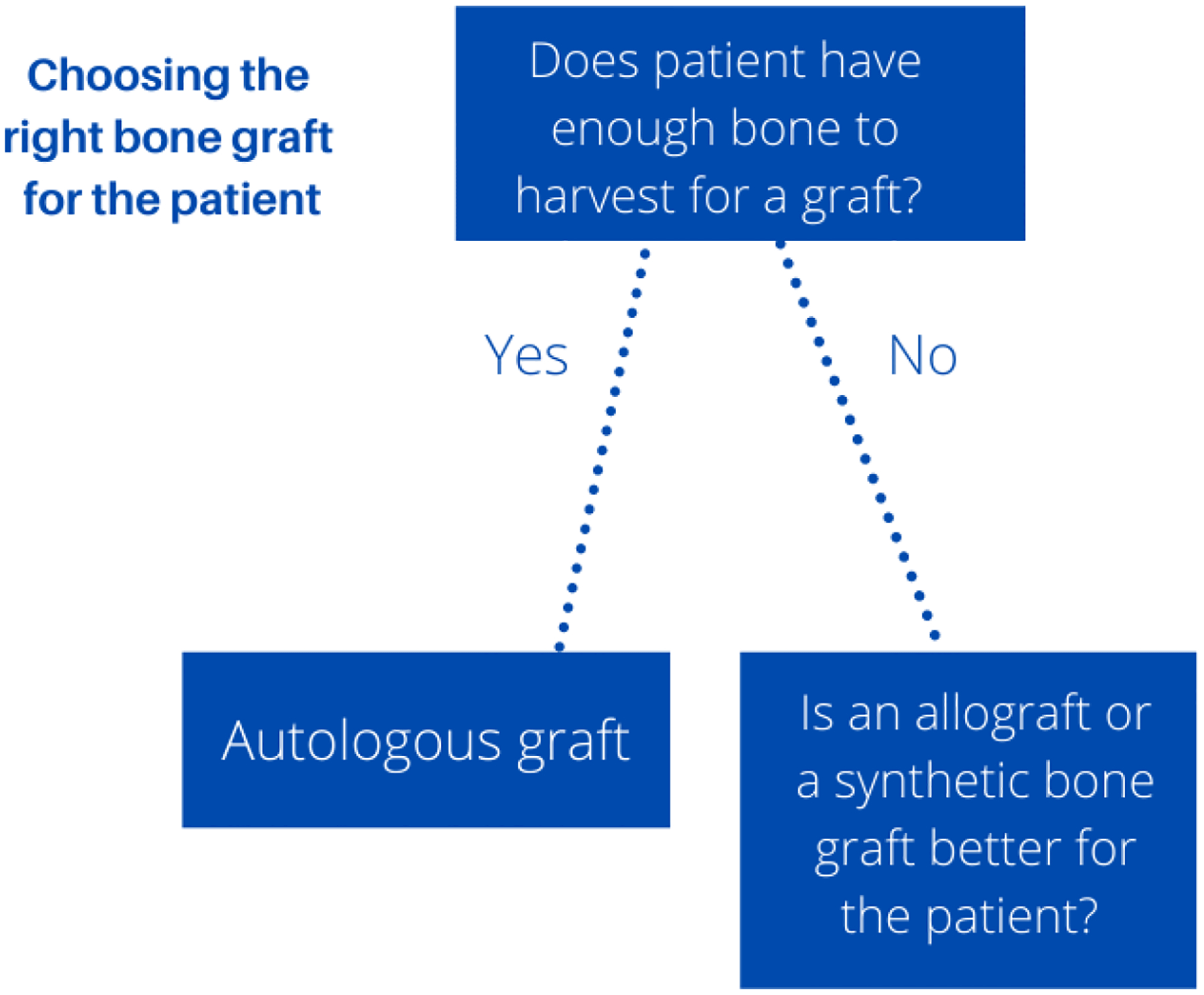
Choosing right bone graft for the patient: the “gold” standard for bone grafting is still using autologous grafts, but the limitation of this method is if there is enough graft to harvest. If there is not enough graft to harvest other bone grafting options such as allografts and synthetic bone grafts will be chosen for the patient.
Allografts can be implanted in 3 different forms: fresh, fresh frozen, and freeze dried. This is a topic of debate of the best processing technique for allografts [48, 103]. An allogenic graft is prepared by removing the soft tissue with ethanol, then is irradiated to sterilize it. The disadvantages of this type of graft are risk of infection or disease due to unsterilized or not properly sterilized graft and possibility of an immune reaction to the graft. The irradiation also comes with disadvantages as it affects the grafts biological properties. The graft before irradiation has osteogenic and osteoinductive properties. After irradiation the graft is also weaker structurally and only has osteoconductive properties [22, 94]. The exception to this is demineralized bone matrix and some fresh/ fresh frozen allografts, which possesses both osteoconduction and osteoinductive qualities. Another disadvantage is that this graft also has a high overall cost then an autograft [22].
Still the biggest risk of allografts is the risk of disease. Although screening does occur for some diseases, not all diseases are tested for and testing is not perfect to detect disease. Diseases that have been documented spread through transplant of allografts include hepatitis C virus and human immunodeficiency virus [104–112]. Although there are not documented cases of prion diseases such as Creutzfeldt-Jakob disease spread through bone grafts, there are still concerns for prion disease transmission when transplanting an allograft [104, 113].
Cancellous allograft
Cancellous allograft is made from cuboid bone chips or “croutons” made from freeze drying samples/lyophilization [22]. A cancellous allograft is used to pack osseous defects and is the most common form of allografts used currently [22, 38]. Advantages of freeze drying are grafts with low residual moisture (3 to 5 percent) and a shelf life of 4 to 5 years [114]. Like its cancellous autograft sister, cancellous allografts lack mechanical strength. However the cancellous autograft has many osteo-properties, while the cancellous allograft only possesses osteoconduction [22]. The disadvantages of this type of graft are its low mechanical strength and it doesn’t promoting secondary healing [38]. Another downside, is the body’s host inflammatory response encapsulates the graft in fibrous tissue, making it more difficult for the graft to integrate [22, 94].
Cortical allograft
A cortical allogenic graft is stronger than the cancellous autograft and can be used in structural or load-bearing areas of the body [115]. They also can be used to fill large defects. Just like the cancellous allograft, cortical grafts heal with an inflammatory response. However, after the initial inflammatory phase, it heals like a cortical autologous graft with creeping substitution [22, 90].
Demineralized bone matrix
To make demineralized bone matrix (DBM), allograft bone goes through an acid extraction with hydrochloric acid or another acid to remove mineral components while leaving the type I collagen, non-collagenous proteins, BMPs and other growth factors [22, 116]. This process enables the natural process of bone formation by increasing the surface area of the implant [48, 117]. This is the only type of allograft with osteoinductive properties as it contains bone morphogenic proteins and other growth factors [22, 104]. The problem with the growth factors is that there is variability of BMP-2, BMP-4, and BMP-9 levels among different manufacturers and even in different batches from the same manufacturer as shown in a study in 2006 by Bae et al [118].
4.4. Synthetic Bone Grafts
When trying to create a synthetic bone graft, it is ideal to make a graft that is biocompatible and will support new bone growth. The synthetic graft should also ideally be structurally similar to cancellous or cortical bone. Synthetic bone grafts are osteoconductive and some possess osteointegrative properties [123]. The ideal synthetic graft will allow for incorporation and proliferation of MSCs [48]. The advantage of synthetic grafts over autografts and allografts is that you know what you are getting. Autografts and allografts graft quality differs based on the individual it is harvested from and since synthetic grafts are manufactured they will always have the same properties. Autografts and allografts there are limits on amount of material to harvest and every graft will not have the same handling properties. Synthetic grafts come in different forms such as moldable, pellets, injectable, and 3D printed. The moldable and pellet forms of grafts are advantageous in trauma or when surgery is not preplanned to 3D print a graft. Injectable forms are a great option as surgery can be performed minimally-invasively [121, 122]. 3D printed scaffolds can be created to perfectly fit the bone defect area. To 3D print a scaffold, a CT scan of the defect is taken that then can be turned into the custom scaffold for the patient [77, 119, 120]. Due to addition processing, this makes the cost of allografts expensive compared to autologous grafts [153]. A concern about 3D printed scaffolds are sterilization and quality control of each implant.
The current methods for sterilization of 3D printed grafts are low temperature sterilization that utilize hydrogen peroxide plasma, often Sterrad or V-PRO machines are used. In a study performed in 2020, there was no increased rate of infection in using 3D printed bone grafts sterilized with Sterrad or V-PRO methods [154]. Of the 114 receiving 3D printed scaffolds, 7 percent ended up with an infection [154]. A FDA-approved brand of 3D printed bone graft on the market today is Advanced Development of Additive Manufacturing’s 3D printed bone graft. Below we will discuss the advantages and disadvantages of the many types of FDA-approved synthetic bone grafts.
Calcium phosphate ceramics
Calcium phosphate ceramics are synthetic mineral salts or highly-crystalline materials that are made by sintering them at high temperatures above 1000 °C [38, 155]. The disadvantages of ceramic materials are they have poor mechanical strength and are difficult to mold into a desired shape [102]. Calcium phosphate ceramics include tricalcium phosphate, hydroxyapatite, and biphasic calcium phosphate.
Tricalcium Phosphate
Tricalcium phosphate ceramics are considered the “gold standard” of synthetic bone grafts [8, 125]. The material of this graft is the most similar in chemical composition to human bone [102]. Tricalcium phosphate is osteoconductive [102] and is resorbed in 13–20 weeks after implantation [8, 156, 157]. Due to its mechanical strength being comparable to cancellous bone, tricalcium phosphate is not used in regions of body undergoing a mechanical load [102, 158]. Tricalcium phosphate unfortunately degrades unpredictably so that is why it is not the best choice for areas of body undergoing a load. It is best used for applications such as bone defects caused by trauma or tumors [3, 38]. This material was first documented as a bone graft material in 1920 by F.H. Albee [159].
Biphasic calcium phosphate
Biphasic calcium phosphate is produced by mixing hydroxyapatite with tricalcium phosphate to get the advantages of both these materials [38, 126]. Adjusting the hydroxyapatite to tricalcium phosphate mixture allows a range of resorption time and mechanical properties [38, 127]. This mixture of resorption time and mechanical stability makes sure the material is stable and also can promote bone growth [126, 128, 160]. Because of these properties, a biphasic calcium phosphate bone graft can be used in large defects and in load bearing areas [128, 160–162].
Hydroxyapatite
Hydroxyapatite is used as a bone graft material due to its similarities in composition to bone, which also makes it very biocompatible. Hydroxyapatite is osteoconductive and depending on the conditions can be osteoinductive [163]. Resorption of hydroxyapatite is very slow, and the graft material will remain up to 3 years after implantation [8, 129, 130]. This slow resorption impedes the bone remodeling process, making the bone mechanically vulnerable. When comparing hydroxyapatite to tricalcium phosphate, hydroxyapatite has much higher compression and tensile strength [102].
To overcome some of the disadvantages of hydroxyapatite, nanocrystalline hydroxyapatite has been developed. It has a higher surface to volume ratio, allowing faster resorption [38, 163]. Nanocrystalline hydroxyapatite is also easier to produce as it requires a lower sintering temperature in production [163].
Calcium phosphate cements
Calcium phosphate cements (CPC) are made by mixing a calcium phosphate powder with a liquid phase that form a paste. This mixture will then be molded and placed into the bone defect to then harden in situ. CPC bone grafts have the ability to be molded as they undergo a body-temperature dissolution-precipitation reaction [102, 131]. Calcium phosphate cements are also available in injectable and 3D printed forms for minimally invasive and custom applications. Due to CPCs unique body-temperature dissolution-precipitation reaction, allows it to possibility be a carrier for drugs and biological molecules [131, 164, 165].
Calcium phosphate cements are osteoconductive as they gradually absorbed in the bone remodeling process[102]. Calcium phosphate cements handle mechanically like ceramics, so to improve their mechanical strength an additive such as chitosan or Vicryl meshes can be added [132].
Calcium sulfate
Calcium sulfate, also called the plaster of Paris, was the first used in 1892 [40]. Calcium sulfate bone grafts are synthetic ceramic bone grafts [166]. The material degrades rapidly in 4–12 weeks after insertion making this graft the most rapidly dissolving synthetic bone graft [22, 40]. The problem with their rapid dissolution is that they resorb faster than bone dissolution [22, 133]. The grafts are slightly osteoconductive as the graft has lack of porosity. Calcium sulfate grafts are rarely used as calcium phosphate grafts are favored over it [102, 158]. It loses mechanical properties when degrading, so is not compatible to be grafted in locations that bare a load [102]. The advantages of a calcium sulfate graft is its low cost and its ease of preparation [38]. Another disadvantage of the material is a complication of serous wound drainage after implantation [134]. Compared to calcium phosphate, calcium sulfate is not often used as calcium phosphate grafts are preferred over it [102].
Bioactive glass
Bioactive glass or bioglass is a silica-based biomaterial used for synthetic bone grafts. Compared to other synthetic bone grafts, bioactive glass is different as it can form chemical bonds to bone and soft-tissue. For bioactive glass to have proper bioactivity, it is critical that is SiO2 content is below 60 percent of the bioactive glass’ weight. Bioactive glass with percent weight of SiO2 45–52 will form bonds to bone and soft tissue. While, bioactive glass with percent weight of SiO2 55–60 will only form bonds to bone [135, 136].
The reason bioactive glass is named bioactive is, the material stimulates the patient’s body regeneration capabilities. Within 48 hours of implantation, the bioactive glass will begin to dissolve: activating genes controlling osteogenesis [137].
An advantage of bioactive glass over hydroxyapatite, is that bioglass can bond to both hard and soft tissues while the synthetic hydroxyapatite binds to only hard tissues [137, 167]. Another advantage of bioglass is its antibacterial properties due to its alkaline composition [137, 138].
PMMA bone cement
PMMA, polymethylmethacrylate, or bone cement is an acrylic-based resin often used to secure orthopedic prosthetics and fill craniofacial defects [139]. PMMA is mixed by combining a white powder of pre-polymerized PMMA and liquid of monomer of methyl methacrylate in a 2:1 ratio, which polymerizes and forms the PMMA cement [141, 142].
PMMA does not have intrinsic adhesive properties [140]. Bone cements are also heat sensitive, so there must be care in temperature regulation to insure proper handling characteristics and setting times during implantation [140].
Disadvantages of PMMA are cement fragmentation and foreign body reaction that wears down the implant. This can result in loosening of prosthetic and osteolysis in some cases. Other disadvantages is the material is not osteoinductive or osteoconductive, so does not promote growth of new bone in the area [141].
Adverse effects associated with PMMA are hypotension episodes and cardiac arrest during cement insertion. Another concern is the risk of bone cement implantation syndrome (BCIS), this syndrome can present with clinical features such as hypotension, cardiac arrythmias, or cardiac arrest [141, 142].
4.5. Growth factors and bioactive molecules in bone repair
Recently, grafts with recombinant growth factors such as BMP and platelet-derived growth factor incorporated have become FDA-approved and have been used in patients. There are many concerns as adverse reactions or side effects occur due to use of these products.
There is debate on whether BMP products are carcinogenic. There have been 2 meta-analyses found a weak increase in patients receiving rhBMP-2, but not to a level that was statistically significant [168, 169]. There is no evidence of increased cancer risk of BMP products at this time. Once there is more data available on BMP products, their long-term effects can be determined.
BMP products are reported to have inflammatory complications from benign seromas to cervical spine swelling which can be life-threatening. Other complications of BMP products are risk of tumor formation as BMP can upregulate tumor cell proliferation and invasion. Ectopic bone formation can also occur if the BMP leaks outside of the implant site [143]. In 70.1 percent of patients implanted with rhBMP-2 had ectopic bone formation, compared to 12.9 percent patients with an ectopic bone complication that were not administered rhBMP-2 [143, 146].
These types of grafts are not approved to be used in children, pregnant women, or women that want to get pregnant in the future. In 2015, the FDA put out a warning letter to surgeons to not use BMP products in the pediatric population, out of concern for insufficient data to demonstrate long-term efficacy or safety in children [168]. Another disadvantage of these types of grafts are there high cost [1]. An advantage of using growth factors and bioactive molecules in bone repair is their osteoconductive and osteoinductive properties [22]. Since they are relatively new, they have not been studied to know their long-term effects. FDA-approved growth factor and bioactive molecules application(s) approved for and FDA-approval date are shown in Table 3.
Table 3: FDA-approval dates and applications approved for in bone regeneration for growth factors and bioactive molecules (*= was withdrawn from the market in 2014 [149]).
([Ref]= references for FDA Approval Date and Application Approved For)
| Device (Growth Factor/ Bioactive Molecules) | FDA Approval Date | Application Approved For | [Ref] |
|---|---|---|---|
| Infuse (BMP-2) Device | 2002 | anterior lumbar interbody fusion | [147] |
| 2004 | tibial nonunion | [143, 170] | |
| 2007 | alternative to autogenous bone graft for sinus augmentations, and for localized alveolar ridge augmentations for defects associated with extraction sockets. | [143, 170, 171] | |
| OP-1 (BMP-7) Device* | 2001 | alternative to autograft in recalcitrant long bone nonunions where use of autograft is unfeasible and alternative treatments have failed | [172] |
| 2004 | revision posterolateral lumbar fusion | [173] | |
| Augment (PDGF-BB) | 2015 | surgical fusion of ankle (tibiotalar joint) and/or hindfoot (including subtalar, talonavicular, and calcaneocuboid joints) | [174] |
| GEM 21S (PDGF-BB) | 2005 | Periodontal defects | [136] |
| iFactor (P-15) | 2015 | reconstruction of a degenerated cervical disc at one level from C3-C4 to C6- C7 following single-level discectomy for intractable radiculopathy after conservative treatment failure | [175] |
Infuse (BMP-2) device
Infuse, a graft containing recombinant human bone morphogenetic protein-2 (rh-BMP-2) and an absorbable collagen sponge, was FDA-approved for anterior lumbar interbody fusion in 2002 [147]. It was then FDA-approved for tibial nonunion use in 2004 [143, 170]. Infuse is also FDA approved since 2007 for maxillofacial reconstructions such as sinus augmentation and socket preservation [171]. This graft is also widely used off-label for cervical, thoracic, and lumbar spine procedures [176]. To use the Infuse graft, the collagen sponge is opened in the sterile field and reconstituted BMP-2 is distributed into the sponge. This is allowed to sit for at least 15 minutes before implanting into the patient. The advantage of this product over other synthetic grafts on the market, is that Infuse is osteoinductive [177].
There have been complications when using this type of graft many in its off-label uses. The FDA has issued a caution letter in using Infuse off-label in anterior cervical fusions as it can cause massive soft-tissue swelling that can compromise a patient’s airway [147, 148].
In a study published by Boden et al, the fusion rates for posterolateral lumbar spine fusion with rhBMP-2 ceramic composites were assessed with and without instrumentation and autografts fusion rate with instrumentation were also assessed. Of the 25 patients in the study, 5 patients were in the autograft/ pedicle screw instrumentation arm, 11 patients received rhBMP-2/ pedicle screw instrumentation, and 9 patients received rhBMP-2 only without internal fixation. The average follow-up time was 17 months later. The autograft/pedicle screw fusion had 40 percent fusion (2/5), while both the rhBMP-2/ pedicle screw instrumentation and rhBMP-2 only without internal fixation had 100 percent fusion (20/20) [144]. Although this study has many limitations due to small sample size, it shows promise of rhBMP-2 having better outcomes then autografts.
Although rhBMP-2 has many adverse effects, it may be a good option for patients not willing to give up smoking and in need of a product for bone regeneration. In a study published by Glassman et al in 2007, the fusion rate of posterolateral lumbar fusion was looked at in smokers with rhBMP-2 matrix compared to smokers receiving iliac crest bone graft. At 2 years post-operative, fusion rate in the rhBMP-2 group was 100 percent (all 55 smokers in the group obtained fusion) and fusion rate in the iliac crest bone graft was 76.2 percent (16 out of 21 smokers obtained fusion)[145].
OP-1 (BMP-7) device
BMP-7 is also known as the osteogenic protein-1 (OP-1) gene. It was first FDA-approved in 2001 for alternative to autograft in recalcitrant long bone nonunions where use of autograft is unfeasible and alternative treatments have also failed [172]. It was FDA-approved for revision posterolateral lumbar fusion in 2004 [173]. The BMP-7 graft was rhBMP-7 was bound to a collagen carrier and was on the market for a short period of time, however it was removed from the market worldwide [149, 178].
PDGF-BB
In a randomized controlled (2:1) noninferiority trial conducted by DiGiovanni et al, patients needing a hind foot or ankle arthrodesis received either an autologous graft or PDGF-BB combined with tricalcium phosphate. 137 patients received autologous grafts and 260 patients received PDGF-BB, combined with a β-tricalcium phosphate matrix. The hypothesis was that the PDGF graft would be as safe and effective as the “gold standard”, an autologous graft. It was shown that the PDGF graft had less pain then the autologous grafting group. The investigators also concluded that PDGF had similar fusion rates to autologous grafts and fewer adverse effects [150, 151].
Augment bone graft is a combination of recombinant human platelet-derived growth factor B homodimer (rhPDGF-BB) and beta-tricalcium phosphate. It was FDA-approved in 2015 for surgical fusion of ankle (tibiotalar joint) and/or hindfoot (including subtalar, talonavicular, and calcaneocuboid joints) [174].
There are other rhPDGF-BB bone grafting products on the market that are used for dental applications such as GEM 21S. GEM 21S is also a combination rhPDGF-Bb and tricalcium phosphate graft. It was FDA-approved in 2005 for periodontal defects such as: 1) intrabony periodontal defects 2) furcation periodontal defects 3) gingival recession associated with periodontal defects [136].
iFactor (P-15)
Peptides have also been looked into to determine their role in bone formation. A peptide P-15 has been found to be 4500 times more potent for cell binding than other peptides. In 1996, research was published showing that this 15 amino-acid peptide (P-15) attached to calcium-phosphate bone mineral led to a dramatic increases in cellular response in culture [179]. P-15, a synthetic amino-acid peptide mimics the cell-binding domain of type I collagen [152, 180, 181].
iFactor is a combination of P-15 absorbed onto inorganic bone mineral and suspended in a hydrogel carrier [152]. It is FDA-approved since November 2015 for use in reconstruction of a degenerated cervical disc at one level from C3-C4 to C6- C7 following single-level discectomy for intractable radiculopathy (arm pain and/or a neurological deficit) after conservative treatment fails. It also must be used inside an allograft bone ring and with supplemental anterior plate fixation [175]. Since iFactor has not been on the market very long, there is limitations of knowledge of its long-term effects.
In a study published in 2017 by Arnold et al, iFactor is compared to autograft in anterior cervical discectomy and fusion. It was a randomized single-blinded study with 319 patients. Adverse effects that occurred at almost equal rates among iFactor and autografts were axial pain, postoperative radiculopathy, and dysphagia. There were 6 cases of superficial infection in iFactor patients and none in autograft patients. Other complications include 2 cases of chronic lymphatic leukemia in autograft patients and with iFactor, 1 patient developed bone hemangioma in the lumbar spine and 1 patient developed renal cancer. The study showed safety and effectiveness at the 2 year mark for iFactor used for symptomatic radiculopathy due to single-level cervical degenerative disc disease. In iFactor subjects, at the 2 year follow-up 144 out of 148 patients had fusion success (97.30 percent). In the autograft subjects, at the 2 year follow-up 136 out of 144 patients had fusion success (94.44 percent). Overall, iFactor was found to be effective, safe, and have similar outcomes to autograft at 2 years post-op for cervical radiculopathy due to cervical degenerative disc disease [152].
4.6. Bone Growth Factors in Pre-Clinical Development
As there is still not a perfect manufactured bone graft on the market and the autologous graft has its own limitations, there is a hunt for new bone-grafting options for patients. Some of the many growth factors being researched for bone regeneration are BMP-9 and VEGF. BMP-9 is getting attention in research due to its osteogenic potential.
In a study published in 2016 by Sreekumar et al, investigated the effect of rhBMP-7 and rhBMP-9 on 110 donor primary human osteoblasts and osteoclasts and also identified the gene specific expression from patterns from the BMPs. This data was then compared to data collected on rhBMP-2. rhBMP-7 treatment correlated with expression of genes AMBI, SOST, Noggin, Smad4, and RANKL, while rhBMP-9 was correlated with expression of genes Alk6, Endoglin, Smurf1, Smurf2, SOST and RANKL. When rhBMP-9 was compared to rhBMP-2 and rhBMP-7, it showed increased osteogenic activity (AP activity and Smad nuclear translocation) over rhBMP-2 and −7. Further research should be conducted on BMP-9 to determine their effectiveness and safety in bone regeneration.
5. Cost of Bone Grafts
Cost is very important as majority of patients needing bone grafts are using insurance to cover the cost of the surgery and graft. If a grafting material is much more expensive than other grafting options on the market, insurance will not want to cover it. Table 4 covers the cost and common brand names of bone grafts. The table only covers the cost per unit of bone graft, and does not cover any additional operating fees, hospital stay time, and cost of complications for the bone grafts.
Table 4:
Cost and Brand Names of Bone Graft Types on the Market in the United States (price varies per area of graft needed)
| Bone Graft Type | Product Name (Manufactor): | Average Cost: |
|---|---|---|
| Autologous | - | $338–1000 depending on the size of area needed to be grafted |
| Bone Marrow Aspirate | - | $135 for the needle |
| Platelet-rich Plasma | - | $250–5000 |
| Allogenic: cancellous | MinerOss Cancellous (BioHorizons) Osteocel (NuVasive): $472/cc |
$464/cc |
| Allogenic: cortical | MinerOss Cortical (BioHorizons) | - |
| Allogenic: Demineralized Bone matrix | DBX (Synthes) Dynagraft |
$200–4500 |
| Tricalcium Phosphate | Allogram-R (Biocomposites) Cellplex Wright Medical Technology) Cerasorb M (Ascension Orthopaedics) Chron OS (Synthes) Conduit (DePuy) TheiLok (Therics) Vitoss (Orthovita) |
Chron OS (Synthes)= $475 for 5 cc |
| Biphasic Calcium Phosphate | BoneCeramic (Straumann) NovaBone (Osteogenics) |
NovaBone (Osteogenics)= $410/cc |
| Hydroxyapatite | Pro Osteon (Biomet) | - |
| Calcium Sulfate | Osteoset (Wright Medical Technology) BonePlast (Biomet) OsteoMax (Orthofix) Stimulan (Biocomposites) |
- |
| Bioactive Glass | Altapore Shape (Baxter International) Signafuse (bioventus surgical) |
- |
| PMMA Bone Cement | Cemex (Exactech) C-ment (Leader Biomedical) |
- |
| BMP-2 | Infuse (Medtronic) | $3,500 per box (5.6 cc) |
| BMP-7 | OP-1 (Osigraft) | $3,664.20 (as of 2009),makes reconstituted amount of 4 cc [180] |
| PDGF-BB | Augment (Wright) | - |
| P-15 | iFactor (Cerapedics) | - |
6. Non-FDA Approved Bone Grafts
In Europe and internationally, there are many products that are used that are not approved within the United States. In Europe approved products are called Conformité Européenne, meaning European Conformity. One product that is approved in Europe and not in the US is Geistlich’s Bio-Oss product. It is a xenograft made from bovine bone that goes through processing to remove the organic parts of the bone. It looks very promising as a bone substitute, however long term clinical studies with histological evaluation are needed to further evaluate [182]. There are concerns about using bovine xenografts and the risk of mad cow disease. However there are no reports on mad cow disease, Transmissible Spongiform Encephalopathies (TSE) and Bovine Spongiform Encephalopathy (BSE) risk with these bovine grafts[183]. Another Conformité Européenne graft that isn’t used in the United States is A-OSS a deproteinized bovine bone graft. In Europe there also approved equine bone grafts, such as Biotek’s Bio-gen. There is no disease risk concern as with equine grafts, as there are no known disease transmitted from horses to humans. Another European approved product that is not approved yet in the United States is Stryker’s Hydroset which has clinically proven its worth in osteoporotic bone[184, 185]. There should be attention to the new products getting approved in Europe as they may be soon approved in the United States based on the efficacy and safety of each graft.
7. Future Technologies of Bone Grafts
Bone grafts are constantly evolving to better meet the needs of the patient populations. Some of the current needs is for custom bone grafts for patients and synthetic grafts that have all the qualities of autologous grafts. One patient population in need of a bone graft with growth factors incorporated is patients with vascular impairment. A bone graft technology that is currently being researched is hydrogels that are osteogenic and angiogenic. To create these grafts, current hydrogels are being formulated with growth factors and peptides [186, 187]. Another similar trending topic in bone graft research currently is implants that modulate growth factor or antibiotic release. Finally, stem cell/tissued engineered therapies where the stem cells are being cultured are being researched.
8. Conclusion
Currently the “gold standard” for bone grafting is an autograft. If an autograft isn’t a good option for the patient, the next best option is normally an allograft. Due to additional surgical site needed for the autograft and risk of disease transmission from the allograft, there is a search for a synthetic bone graft that would be as good or better as an autograft or allograft. To obtain this the synthetic material needs to be biocompatible, osteoconductive, osteoinductive, osteogenic, minimize side-effects for patient (be safe), and be cost-effective. Other options that are FDA-approved include growth factors: rhBMP-2, rhBMP-7, and PDGF-BB and bioactive molecule P-15. Recombinant bone-morphogenic protein that at this time have serious concerns of safety due to risk of compromising the patient’s airway in off-label use and other adverse effects. These newer growth factors and bioactive molecules look promising but there is not long-term data on safety and effectiveness at the moment. The advantages and disadvantages ultimately need to be weighted for each patient by them and their surgeon to determine the best bone grafting option.
Highlights.
Three main FDA approval processes for bone grafts and bone graft substitutes.
Bone grafts are required to heal nonunions, malunions, and bone defects.
FDA-approved bioactive molecule for bone regeneration is a peptide P-15.
Acknowledgments
The authors would like to acknowledge funding support from the National Institutes of Health (Grant number: R01DE023356) and the University of Toledo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, Lattanzi W, and Logroscino G, Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med, 2014. 25(10): p. 2445–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN, and American I. Academy of Orthopaedic Surgeons. The Committee on Biological, Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am, 2001. 83-A Suppl 2 Pt 2: p. 98–103. [DOI] [PubMed] [Google Scholar]
- 3.Finkemeier CG, Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am, 2002. 84(3): p. 454–64. [DOI] [PubMed] [Google Scholar]
- 4.Faour O, Dimitriou R, Cousins CA, and Giannoudis PV, The use of bone graft substitutes in large cancellous voids: any specific needs? Injury, 2011. 42 Suppl 2: p. S87–90. [DOI] [PubMed] [Google Scholar]
- 5.Van Heest A and Swiontkowski M, Bone-graft substitutes. The Lancet, 1999. 353: p. S28–S29. [DOI] [PubMed] [Google Scholar]
- 6.Perez JR, Kouroupis D, Li DJ, Best TM, Kaplan L, and Correa D, Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front Bioeng Biotechnol, 2018. 6: p. 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart SK, Fracture Non-Union: A Review of Clinical Challenges and Future Research Needs. Malays Orthop J, 2019. 13(2): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez de Grado G, Keller L, Idoux-Gillet Y, Wagner Q, Musset AM, Benkirane-Jessel N, Bornert F, and Offner D, Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng, 2018. 9: p. 2041731418776819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall BK, in Bones and Cartilage: Developmental and Evolutionary Skeletal Biology, Elsevier, Editor. 2005. [Google Scholar]
- 10.Dressman H, Uber knochenplombierung. Beitr Klin Chir, 1892. 9: p. 804–810. [Google Scholar]
- 11.Pietrzak WS, Musculoskeletal tissue regeneration: biological materials and methods. 2008: Springer. [Google Scholar]
- 12.Peltier LF, Bickel EY, Lillo R, and Thein MS, The use of plaster of paris to fill defects in bone. Ann Surg, 1957. 146(1): p. 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta SK, Medical Device Regulations: A Current Perspective. Journal of Young Pharmacists, 2016. 8(1). [Google Scholar]
- 14.Schlauderaff A and Boyer KC, An Overview of Food and Drug Administration Medical Device Legislation and Interplay with Current Medical Practices. Cureus, 2019. 11(5): p. e4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S.F.a.D. Adminstration. Premarket Notification 510(k). 2020. July 2, 2020]; Available from: https://www.fda.gov/medical-devices/premarket-submissions/premarket-notification-510k.
- 16.U.S.F.a.D. Adminstration. Investigational Device Exemption (IDE). 2019. July 2, 2020]; Available from: https://www.fda.gov/medical-devices/how-study-and-market-your-device/investigational-device-exemption-ide.
- 17.U.S.F.a.D. Administration. FDA Regulation of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/P’s) Product List. 2018. July 2, 2020]; Available from: https://www.fda.gov/vaccines-blood-biologics/tissue-tissue-products/fda-regulation-human-cells-tissues-and-cellular-and-tissue-based-products-hctps-product-list.
- 18.Vaggelas A and Seimetz D, Expediting Drug Development: FDA’s New Regenerative Medicine Advanced Therapy Designation. Ther Innov Regul Sci, 2019. 53(3): p. 364–373. [DOI] [PubMed] [Google Scholar]
- 19.Abjornson C, Brecevich A, Callanan T, Dowe C, Cammisa FP, and Lorio MP, ISASS Recommendations and Coverage Criteria for Bone Graft Substitutes used in Spinal Surgery. Int J Spine Surg, 2018. 12(6): p. 757–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas JD and Kehoe JL, Bone Nonunion, in StatPearls. 2020: Treasure Island (FL). [PubMed] [Google Scholar]
- 21.Cunningham BP, Brazina S, Morshed S, and Miclau T 3rd, Fracture healing: A review of clinical, imaging and laboratory diagnostic options. Injury, 2017. 48 Suppl 1: p. S69–S75. [DOI] [PubMed] [Google Scholar]
- 22.Roberts TT and Rosenbaum AJ, Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis, 2012. 8(4): p. 114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart SK, Fracture Non-Union: A Review of Clinical Challenges and Future Research Needs. Malaysian orthopaedic journal, 2019. 13(2): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpin G and Shtarker H, Management of Delayed Union, Non-Union and Mal-Union of Long Bone Fractures, in European Surgical Orthopaedics and Traumatology: The EFORT Textbook, Bentley G, Editor. 2014, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 241–266. [Google Scholar]
- 25.Kyro A, Malunion after intramedullary nailing of tibial shaft fractures. Ann Chir Gynaecol, 1997. 86(1): p. 56–64. [PubMed] [Google Scholar]
- 26.Tripathy SK, Goyal T, and Sen RK, Nonunions and malunions of the pelvis. European Journal of Trauma and Emergency Surgery, 2015. 41(4): p. 335–342. [DOI] [PubMed] [Google Scholar]
- 27.Disseldorp DJ, Poeze M, Hannemann PF, and Brink PR, Is Bone Grafting Necessary in the Treatment of Malunited Distal Radius Fractures? J Wrist Surg, 2015. 4(3): p. 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miceli TS, Colson K, Faiman BM, Miller K, Tariman JD, and B. International Myeloma Foundation Nurse Leadership, Maintaining bone health in patients with multiple myeloma: survivorship care plan of the International Myeloma Foundation Nurse Leadership Board. Clin J Oncol Nurs, 2011. 15 Suppl: p. 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiba H, Kobayashi M, Waguri-Nagaya Y, Goto H, Mizutani J, Yamada S, Okamoto H, Nozaki M, Mitsui H, Miwa S, Kobayashi M, Endo K, Saito S, Goto T, and Otsuka T, Treatment of simple bone cysts using endoscopic curettage: a case series analysis. J Orthop Surg Res, 2018. 13(1): p. 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphey MD, Foreman KL, Klassen-Fischer MK, Fox MG, Chung EM, and Kransdorf MJ, From the Radiologic Pathology Archives Imaging of Osteonecrosis: Radiologic-Pathologic Correlation. RadioGraphics, 2014. 34(4): p. 1003–1028. [DOI] [PubMed] [Google Scholar]
- 31.Millikan PD, Karas V, and Wellman SS, Treatment of osteonecrosis of the femoral head with vascularized bone grafting. Curr Rev Musculoskelet Med, 2015. 8(3): p. 252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shegarfi H and Reikeras O, Review article: bone transplantation and immune response. J Orthop Surg (Hong Kong), 2009. 17(2): p. 206–11. [DOI] [PubMed] [Google Scholar]
- 33.Gupta A, Kukkar N, Sharif K, Main BJ, Albers CE, and El-Amin Iii SF, Bone graft substitutes for spine fusion: A brief review. World J Orthop, 2015. 6(6): p. 449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mobbs RJ, Phan K, Malham G, Seex K, and Rao PJ, Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. Journal of spine surgery (Hong Kong), 2015. 1(1): p. 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coots BK, Alveolar bone grafting: past, present, and new horizons. Semin Plast Surg, 2012. 26(4): p. 178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar P, Vinitha B, and Fathima G, Bone grafts in dentistry. J Pharm Bioallied Sci, 2013. 5(Suppl 1): p. S125–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elsalanty ME and Genecov DG, Bone grafts in craniofacial surgery. Craniomaxillofac Trauma Reconstr, 2009. 2(3): p. 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W and Yeung KWK, Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater, 2017. 2(4): p. 224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheen JR and Garla VV, Fracture Healing Overview, in StatPearls. 2020: Treasure Island (FL). [PubMed] [Google Scholar]
- 40.Y. F and J. J, Bone grafts and their substitutes. The Bone & Joint Journal, 2016. 98-B(1_Supple_A): p. 6–9. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad Jabir R, Sugeng S, Aldo Fransiskus M, Pancar Muhammad P, and Bambang S, The potential of carbonate apatite as an alternative bone substitute material. Medical Journal of Indonesia, 2019. 28(1). [Google Scholar]
- 42.Albrektsson T and Johansson C, Osteoinduction, osteoconduction and osseointegration. Eur Spine J, 2001. 10 Suppl 2: p. S96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solheim E, Growth factors in bone. Int Orthop, 1998. 22(6): p. 410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Gittes GK, and Longaker MT, Hypoxia increases insulinlike growth factor gene expression in rat osteoblasts. Ann Plast Surg, 2000. 44(5): p. 529–34; discussion 534–5. [DOI] [PubMed] [Google Scholar]
- 45.Bayer EA, Gottardi R, Fedorchak MV, and Little SR, The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. Journal of Controlled Release, 2015. 219: p. 129–140. [DOI] [PubMed] [Google Scholar]
- 46.Chen G, Deng C, and Li YP, TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci, 2012. 8(2): p. 272–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Bialy I, Jiskoot W, and Reza Nejadnik M, Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm Res, 2017. 34(6): p. 1152–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldwin P, Li DJ, Auston DA, Mir HS, Yoon RS, and Koval KJ, Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J Orthop Trauma, 2019. 33(4): p. 203–213. [DOI] [PubMed] [Google Scholar]
- 49.Liu W, Deng Z, Zeng Z, Fan J, Feng Y, Wang X, Cao D, Zhang B, Yang L, Liu B, Pakvasa M, Wagstaff W, Wu X, Luo H, Zhang J, Zhang M, He F, Mao Y, Ding H, Zhang Y, Niu C, Haydon RC, Luu HH, Wolf JM, Lee MJ, Huang W, He TC, and Zou Y, Highly expressed BMP9/GDF2 in postnatal mouse liver and lungs may account for its pleiotropic effects on stem cell differentiation, angiogenesis, tumor growth and metabolism. Genes Dis, 2020. 7(2): p. 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, Li R, Shui W, Zhang H, Kim SH, Zhang W, Zhang J, Kong Y, Denduluri S, Rogers MR, Pratt A, Haydon RC, Luu HH, Angeles J, Shi LL, and He TC, BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng, 2013. 6(8A): p. 32–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaihre B, Unagolla JM, Liu J, Ebraheim NA, and Jayasuriya AC, Thermoresponsive Injectable Microparticle–Gel Composites with Recombinant BMP-9 and VEGF Enhance Bone Formation in Rats. ACS Biomaterials Science & Engineering, 2019. 5(9): p. 4587–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, and He TC, Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther, 2004. 11(17): p. 1312–20. [DOI] [PubMed] [Google Scholar]
- 53.Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC, and He TC, Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res, 2007. 25(5): p. 665–77. [DOI] [PubMed] [Google Scholar]
- 54.Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu BA, Luu HH, Park JK, Li X, Luo J, Montag AG, Haydon RC, and He TC, Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem, 2004. 279(31): p. 32941–9. [DOI] [PubMed] [Google Scholar]
- 55.Peng Y, Kang Q, Cheng H, Li X, Sun MH, Jiang W, Luu HH, Park JY, Haydon RC, and He TC, Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem, 2003. 90(6): p. 1149–65. [DOI] [PubMed] [Google Scholar]
- 56.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, and He TC, Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem, 2004. 279(53): p. 55958–68. [DOI] [PubMed] [Google Scholar]
- 57.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, and He TC, Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am, 2003. 85(8): p. 1544–52. [DOI] [PubMed] [Google Scholar]
- 58.Ornitz DM and Marie PJ, Fibroblast growth factor signaling in skeletal development and disease. Genes Dev, 2015. 29(14): p. 1463–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Charoenlarp P, Rajendran AK, and Iseki S, Role of fibroblast growth factors in bone regeneration. Inflamm Regen, 2017. 37: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilkie AO, Bad bones, absent smell, selfish testes: the pleiotropic consequences of human FGF receptor mutations. Cytokine Growth Factor Rev, 2005. 16(2): p. 187–203. [DOI] [PubMed] [Google Scholar]
- 61.Hu K and Olsen BR, The roles of vascular endothelial growth factor in bone repair and regeneration. Bone, 2016. 91: p. 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cross MJ, Dixelius J, Matsumoto T, and Claesson-Welsh L, VEGF-receptor signal transduction. Trends in biochemical sciences, 2003. 28(9): p. 488–494. [DOI] [PubMed] [Google Scholar]
- 63.Ferrara N, Gerber HP, and LeCouter J, The biology of VEGF and its receptors. Nat Med, 2003. 9(6): p. 669–76. [DOI] [PubMed] [Google Scholar]
- 64.Ogilvie CM, Lu C, Marcucio R, Lee M, Thompson Z, Hu D, Helms JA, and Miclau T, Vascular endothelial growth factor improves bone repair in a murine nonunion model. Iowa Orthop J, 2012. 32: p. 90–4. [PMC free article] [PubMed] [Google Scholar]
- 65.Chou LB, Mann RA, Coughlin MJ, III WTM, and Mizel MS, Stress fracture as a complication of autogenous bone graft harvest from the distal tibia. Foot & ankle international, 2007. 28(2): p. 199–201. [DOI] [PubMed] [Google Scholar]
- 66.Khoshkam V, Chan HL, Lin GH, Mailoa J, Giannobile WV, Wang HL, and Oh TJ, Outcomes of regenerative treatment with rh PDGF-BB and rh FGF-2 for periodontal intra-bony defects: a systematic review and meta-analysis. Journal of Clinical Periodontology, 2015. 42(3): p. 272–280. [DOI] [PubMed] [Google Scholar]
- 67.Sun H, Lu PP, Zhou PH, Sun SW, Zhang HT, Liu YJ, Yang X, Shen XF, and Yang HL, Recombinant human platelet-derived growth factor-BB versus autologous bone graft in foot and ankle fusion: A systematic review and meta-analysis. Foot Ankle Surg, 2017. 23(1): p. 32–39. [DOI] [PubMed] [Google Scholar]
- 68.Phipps MC, Xu Y, and Bellis SL, Delivery of platelet-derived growth factor as a chemotactic factor for mesenchymal stem cells by bone-mimetic electrospun scaffolds. PloS one, 2012. 7(7): p. e40831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M, Yu W, Niibe K, Zhang W, Egusa H, Tang T, and Jiang X, The Effects of Platelet-Derived Growth Factor-BB on Bone Marrow Stromal Cell-Mediated Vascularized Bone Regeneration. Stem Cells International, 2018. 2018: p. 3272098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caplan AI and Correa D, PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res, 2011. 29(12): p. 1795–803. [DOI] [PubMed] [Google Scholar]
- 71.Pape HC, Evans A, and Kobbe P, Autologous bone graft: properties and techniques. J Orthop Trauma, 2010. 24 Suppl 1: p. S36–40. [DOI] [PubMed] [Google Scholar]
- 72.Saikia KC, Bhattacharya TD, Bhuyan SK, Talukdar DJ, Saikia SP, and Jitesh P, Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J Orthop, 2008. 42(2): p. 169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tilkeridis K, Touzopoulos P, Ververidis A, Christodoulou S, Kazakos K, and Drosos GI, Use of demineralized bone matrix in spinal fusion. World J Orthop, 2014. 5(1): p. 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kloss FR, Offermanns V, and Kloss-Brandstatter A, CEComparison of allogeneic and autogenous bone grafts for augmentation of alveolar ridge defects-A 12-month retrospective radiographic evaluation. Clin Oral Implants Res, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, and Giannoudis PV, Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury, 2011. 42 Suppl 2: p. S3–15. [DOI] [PubMed] [Google Scholar]
- 76.Giannoudis PV, Dinopoulos H, and Tsiridis E, Bone substitutes: an update. Injury, 2005. 36(3): p. S20–S27. [DOI] [PubMed] [Google Scholar]
- 77.Bouler JM, Pilet P, Gauthier O, and Verron E, Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater, 2017. 53: p. 1–12. [DOI] [PubMed] [Google Scholar]
- 78.Lee F-H, Shen P-C, Jou IM, Li C-Y, and Hsieh J-L, A Population-Based 16-Year Study on the Risk Factors of Surgical Site Infection in Patients after Bone Grafting: A Cross-Sectional Study in Taiwan. Medicine, 2015. 94(47): p. e2034–e2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delloye C, van Cauter M, Dufrane D, Francq BG, Docquier PL, and Cornu O, Local complications of massive bone allografts: an appraisal of their prevalence in 128 patients. Acta Orthop Belg, 2014. 80(2): p. 196–204. [PubMed] [Google Scholar]
- 80.Stone PW, Economic burden of healthcare-associated infections: an American perspective. Expert review of pharmacoeconomics & outcomes research, 2009. 9(5): p. 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sasso RC, LeHuec JC, Shaffrey C, and Spine Interbody Research G, Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech, 2005. 18 Suppl: p. S77–81. [DOI] [PubMed] [Google Scholar]
- 82.Gaskin DJ and Richard P, The Economic Costs of Pain in the United States. The Journal of Pain, 2012. 13(8): p. 715–724. [DOI] [PubMed] [Google Scholar]
- 83.Bolano L and Kopta JA, The immunology of bone and cartilage transplantation. Orthopedics, 1991. 14(9): p. 987–96. [DOI] [PubMed] [Google Scholar]
- 84.Horowitz MC and Friedlaender GE, Immunologic aspects of bone transplantation. A rationale for future studies. Orthop Clin North Am, 1987. 18(2): p. 227–33. [PubMed] [Google Scholar]
- 85.Lane JM and Sandhu HS, Current approaches to experimental bone grafting. Orthop Clin North Am, 1987. 18(2): p. 213–25. [PubMed] [Google Scholar]
- 86.Reddi AH, Wientroub S, and Muthukumaran N, Biologic principles of bone induction. Orthop Clin North Am, 1987. 18(2): p. 207–12. [PubMed] [Google Scholar]
- 87.Keating JF and McQueen MM, Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Br, 2001. 83(1): p. 3–8. [DOI] [PubMed] [Google Scholar]
- 88.Palmer SH, Gibbons CL, and Athanasou NA, The pathology of bone allograft. J Bone Joint Surg Br, 1999. 81(2): p. 333–5. [DOI] [PubMed] [Google Scholar]
- 89.Pelker RR and Friedlaender GE, Biomechanical aspects of bone autografts and allografts. Orthop Clin North Am, 1987. 18(2): p. 235–9. [PubMed] [Google Scholar]
- 90.Khan SN, Cammisa FP Jr., Sandhu HS, Diwan AD, Girardi FP, and Lane JM, The biology of bone grafting. J Am Acad Orthop Surg, 2005. 13(1): p. 77–86. [PubMed] [Google Scholar]
- 91.Burchardt H, Biology of bone transplantation. Orthop Clin North Am, 1987. 18(2): p. 187–96. [PubMed] [Google Scholar]
- 92.Goldberg V and Akhavan S, Bone regeneration and repair: biology and clinical applications. 2005.
- 93.Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, and Owen M, Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res, 1980(151): p. 294–307. [PubMed] [Google Scholar]
- 94.A.A.o.O. Surgeons and Flynn JM, Orthopaedic knowledge update 10. 2011: American Academy of Orthopaedic Surgeons. [Google Scholar]
- 95.Roffi A, Filardo G, Kon E, and Marcacci M, Does PRP enhance bone integration with grafts, graft substitutes, or implants? A systematic review. BMC Musculoskelet Disord, 2013. 14: p. 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zarate-Kalfopulos B and Reyes-Sanchez A, [Bone grafts in orthopedic surgery]. Cir Cir, 2006. 74(3): p. 217–22. [PubMed] [Google Scholar]
- 97.Pavlovic V, Ciric M, Jovanovic V, and Stojanovic P, Platelet Rich Plasma: a short overview of certain bioactive components. Open Med (Wars), 2016. 11(1): p. 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dhillon RS, Schwarz EM, and Maloney MD, Platelet-rich plasma therapy - future or trend? Arthritis Res Ther, 2012. 14(4): p. 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Russell RP, Apostolakos J, Hirose T, Cote MP, and Mazzocca AD, Variability of platelet-rich plasma preparations. Sports Med Arthrosc Rev, 2013. 21(4): p. 186–90. [DOI] [PubMed] [Google Scholar]
- 100.Fernandes G and Yang S, Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res, 2016. 4: p. 16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stevenson S and Horowitz M, The response to bone allografts. J Bone Joint Surg Am, 1992. 74(6): p. 939–50. [PubMed] [Google Scholar]
- 102.Sohn HS and Oh JK, Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res, 2019. 23: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Costain DJ and Crawford RW, Fresh-frozen vs. irradiated allograft bone in orthopaedic reconstructive surgery. Injury, 2009. 40(12): p. 1260–1264. [DOI] [PubMed] [Google Scholar]
- 104.C. D, O. C, V. D, and O. B, Bone allografts. The Journal of Bone and Joint Surgery. British volume, 2007. 89-B(5): p. 574–580. [DOI] [PubMed] [Google Scholar]
- 105.EASTLUND T and STRONG DM, Infectious disease transmission through tissue transplantation, in Advances in tissue banking. 2004, World Scientific. p. 51–131. [Google Scholar]
- 106.Delloye C, Tissue allografts and health risks. Acta orthopaedica Belgica, 1994. 60: p. 62–67. [PubMed] [Google Scholar]
- 107.Tomford WW, Transmission of disease through transplantation of musculoskeletal allografts. JBJS, 1995. 77(11): p. 1742–1754. [DOI] [PubMed] [Google Scholar]
- 108.Pereira BJ, Milford EL, Kirkman RL, and Levey AS, Transmission of hepatitis C virus by organ transplantation. New England Journal of Medicine, 1991. 325(7): p. 454–460. [DOI] [PubMed] [Google Scholar]
- 109.Roth D, Fernandez JA, Babischkin S, De Mattos A, Buck B, Quan S, Olson L, Burke GW, Nery JR, and Esquenazi V, Detection of hepatitis C virus infection among cadaver organ donors: evidence for low transmission of disease. Annals of internal medicine, 1992. 117(6): p. 470–475. [DOI] [PubMed] [Google Scholar]
- 110.Conrad EU, Gretch DR, Obermeyer KR, Moogk MS, Sayers M, Wilson JJ, and Strong DM, Transmission of the hepatitis-C virus by tissue transplantation. Journal of Bone and Joint Surgery-A-American Volumes, 1995. 77(2): p. 214–224. [DOI] [PubMed] [Google Scholar]
- 111.Carlson ER, Marx RE, and Buck B, The potential for HIV transmission through allogeneic bone A review of risks and safety. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 1995. 80(1): p. 17–23. [DOI] [PubMed] [Google Scholar]
- 112.Nyberg M, Suni J, and Haltia M, Isolation of human immunodeficiency virus (HIV) at autopsy one to six days postmortem. American Journal of Clinical Pathology, 1990. 94(4): p. 422–425. [DOI] [PubMed] [Google Scholar]
- 113.Pauli G, Tissue safety in view of CJD and variant CJD. Cell and tissue banking, 2005. 6(3): p. 191–200. [DOI] [PubMed] [Google Scholar]
- 114.Conrad E, Ericksen D, Tencer A, Strong DM, and Mackenzie A, The Effects of Freeze-Drying and Rehydration on Cancellous Bone. Clinical Orthopaedics and Related Research, 1993. 290. [PubMed] [Google Scholar]
- 115.Eagan MJ and McAllister DR, Biology of Allograft Incorporation. Clinics in Sports Medicine, 2009. 28(2): p. 203–214. [DOI] [PubMed] [Google Scholar]
- 116.Zhang H, Yang L, Yang XG, Wang F, Feng JT, Hua KC, Li Q, and Hu YC, Demineralized Bone Matrix Carriers and their Clinical Applications: An Overview. Orthop Surg, 2019. 11(5): p. 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peterson B, Whang PG, Iglesias R, Wang JC, and Lieberman JR, Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am, 2004. 86(10): p. 2243–50. [DOI] [PubMed] [Google Scholar]
- 118.Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, and Dawson EG, Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976), 2006. 31(12): p. 1299–306; discussion 1307–8. [DOI] [PubMed] [Google Scholar]
- 119.Marques CF, Perera FH, Marote A, Ferreira S, Vieira SI, Olhero S, Miranda P, and Ferreira JM, Biphasic calcium phosphate scaffolds fabricated by direct write assembly: Mechanical, anti-microbial and osteoblastic properties. Journal of the European Ceramic Society, 2017. 37(1): p. 359–368. [Google Scholar]
- 120.Detsch R, Schaefer S, Deisinger U, Ziegler G, Seitz H, and Leukers B, In vitro-osteoclastic activity studies on surfaces of 3D printed calcium phosphate scaffolds. Journal of biomaterials applications, 2011. 26(3): p. 359–380. [DOI] [PubMed] [Google Scholar]
- 121.Xu HH, Weir MD, Burguera EF, and Fraser AM, Injectable and macroporous calcium phosphate cement scaffold. Biomaterials, 2006. 27(24): p. 4279–87. [DOI] [PubMed] [Google Scholar]
- 122.Burguera EF, Xu HH, and Weir MD, Injectable and rapid-setting calcium phosphate bone cement with dicalcium phosphate dihydrate. J Biomed Mater Res B Appl Biomater, 2006. 77(1): p. 126–34. [DOI] [PubMed] [Google Scholar]
- 123.Moore WR, Graves SE, and Bain GI, Synthetic bone graft substitutes. ANZ J Surg, 2001. 71(6): p. 354–61. [PubMed] [Google Scholar]
- 124.Montoro DT, Wan DC, and Longaker MT, Chapter 60 - Skeletal Tissue Engineering, in Principles of Tissue Engineering (Fourth Edition), Lanza R, Langer R, and Vacanti J, Editors. 2014, Academic Press: Boston. p. 1289–1302. [Google Scholar]
- 125.Galois L, Mainard D, and Delagoutte JP, Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int Orthop, 2002. 26(2): p. 109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Daculsi G, Legeros RZ, Nery E, Lynch K, and Kerebel B, Transformation of biphasic calcium phosphate ceramics in vivo: Ultrastructural and physicochemical characterization. Journal of Biomedical Materials Research, 1989. 23(8): p. 883–894. [DOI] [PubMed] [Google Scholar]
- 127.Williams DF, There is no such thing as a biocompatible material. Biomaterials, 2014. 35(38): p. 10009–14. [DOI] [PubMed] [Google Scholar]
- 128.Lobo SE and Arinzeh TL, Biphasic Calcium Phosphate Ceramics for Bone Regeneration and Tissue Engineering Applications. Materials, 2010. 3(2): p. 815–826. [Google Scholar]
- 129.Koshino T, Murase T, Takagi T, and Saito T, New bone formation around porous hydroxyapatite wedge implanted in opening wedge high tibial osteotomy in patients with osteoarthritis. Biomaterials, 2001. 22(12): p. 1579–82. [DOI] [PubMed] [Google Scholar]
- 130.Spivak JM and Hasharoni A, Use of hydroxyapatite in spine surgery. Eur Spine J, 2001. 10 Suppl 2: p. S197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ginebra MP, Canal C, Espanol M, Pastorino D, and Montufar EB, Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev, 2012. 64(12): p. 1090–110. [DOI] [PubMed] [Google Scholar]
- 132.Yokoyama A, Yamamoto S, Kawasaki T, Kohgo T, and Nakasu M, Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials, 2002. 23(4): p. 1091–101. [DOI] [PubMed] [Google Scholar]
- 133.Hak DJ, The use of osteoconductive bone graft substitutes in orthopaedic trauma. J Am Acad Orthop Surg, 2007. 15(9): p. 525–36. [DOI] [PubMed] [Google Scholar]
- 134.Beuerlein MJ and McKee MD, Calcium sulfates: what is the evidence? J Orthop Trauma, 2010. 24 Suppl 1: p. S46–51. [DOI] [PubMed] [Google Scholar]
- 135.Valimaki VV and Aro HT, Molecular basis for action of bioactive glasses as bone graft substitute. Scand J Surg, 2006. 95(2): p. 95–102. [DOI] [PubMed] [Google Scholar]
- 136.Hench LL and West JK, Biological applications of bioactive glasses. 1996: Harwood Academic Publishers. [Google Scholar]
- 137.Krishnan V and Lakshmi T, Bioglass: A novel biocompatible innovation. J Adv Pharm Technol Res, 2013. 4(2): p. 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang D, Leppäranta O, Munukka E, Ylänen H, Viljanen MK, Eerola E, Hupa M, and Hupa L, Antibacterial effects and dissolution behavior of six bioactive glasses. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 2010. 93(2): p. 475–483. [DOI] [PubMed] [Google Scholar]
- 139.Pryor LS, Gage E, Langevin CJ, Herrera F, Breithaupt AD, Gordon CR, Afifi AM, Zins JE, Meltzer H, Gosman A, Cohen SR, and Holmes R, Review of bone substitutes. Craniomaxillofac Trauma Reconstr, 2009. 2(3): p. 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vaishya R, Chauhan M, and Vaish A, Bone cement. J Clin Orthop Trauma, 2013. 4(4): p. 157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rajesh Kumar Ranjan MK, Kumar Rakesh, and Farman Ali Md, Bone cement. International Journal of Orthopaedics Sciences, 2017. 3(4): p. 79–82. [Google Scholar]
- 142.Hines CB, Understanding Bone Cement Implantation Syndrome. AANA J, 2018. 86(6): p. 433–441. [PubMed] [Google Scholar]
- 143.James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, and Soo C, A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev, 2016. 22(4): p. 284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boden SD, Kang J, Sandhu H, and Heller JG, Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976), 2002. 27(23): p. 2662–73. [DOI] [PubMed] [Google Scholar]
- 145.Glassman SD, Dimar JR 3rd, Burkus K, Hardacker JW, Pryor PW, Boden SD, and Carreon LY, The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine (Phila Pa 1976), 2007. 32(15): p. 1693–8. [DOI] [PubMed] [Google Scholar]
- 146.Carragee EJ, Hurwitz EL, and Weiner BK, A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J, 2011. 11(6): p. 471–91. [DOI] [PubMed] [Google Scholar]
- 147.Carreira AC, Lojudice FH, Halcsik E, Navarro RD, Sogayar MC, and Granjeiro JM, Bone Morphogenetic Proteins:Facts, Challenges, and Future Perspectives. Journal of Dental Research, 2014. 93(4): p. 335–345. [DOI] [PubMed] [Google Scholar]
- 148.Devine JG, Dettori JR, France JC, Brodt E, and McGuire RA, The use of rhBMP in spine surgery: is there a cancer risk? Evid Based Spine Care J, 2012. 3(2): p. 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marx J and Lorio M, Class III Spine Grafts. 2019.
- 150.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, and Yin S, Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am, 2001. 83-A Suppl 1(Pt 2): p. S151–8. [PMC free article] [PubMed] [Google Scholar]
- 151.Einhorn TA and Gerstenfeld LC, Fracture healing: mechanisms and interventions. Nat Rev Rheumatol, 2015. 11(1): p. 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arnold PM, Sasso RC, Janssen ME, Fehlings MG, Heary RF, Vaccaro AR, and Kopjar B, i-Factor Bone Graft vs Autograft in Anterior Cervical Discectomy and Fusion: 2-Year Follow-up of the Randomized Single-Blinded Food and Drug Administration Investigational Device Exemption Study. Neurosurgery, 2018. 83(3): p. 377–384. [DOI] [PubMed] [Google Scholar]
- 153.Kinaci A, Neuhaus V, and Ring DC, Trends in bone graft use in the United States. Orthopedics, 2014. 37(9): p. e783–8. [DOI] [PubMed] [Google Scholar]
- 154.Shea GK-H, Wu KL-K, Li IW-S, Leung M-F, Ko AL-P, Tse L, Pang SS-Y, Kwan KY-H, Wong T-M, Leung FK-L, and Fang CX, A review of the manufacturing process and infection rate of 3D-printed models and guides sterilized by hydrogen peroxide plasma and utilized intra-operatively. 3D Printing in Medicine, 2020. 6(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Browner BD, Jupiter JB, Krettek C, and Anderson PA, Skeletal Trauma. 2014: Elsevier Health Sciences. [Google Scholar]
- 156.Bohner M, Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury, 2000. 31 Suppl 4: p. 37–47. [DOI] [PubMed] [Google Scholar]
- 157.Chazono M, Tanaka T, Komaki H, and Fujii K, Bone formation and bioresorption after implantation of injectable beta-tricalcium phosphate granules-hyaluronate complex in rabbit bone defects. J Biomed Mater Res A, 2004. 70(4): p. 542–9. [DOI] [PubMed] [Google Scholar]
- 158.Zwingenberger S, Nich C, Valladares RD, Yao Z, Stiehler M, and Goodman SB, Recommendations and considerations for the use of biologics in orthopedic surgery. BioDrugs, 2012. 26(4): p. 245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Albee FH, Studies in bone growth: triple calcium phosphate as a stimulus to osteogenesis. Annals of surgery, 1920. 71(1): p. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Daculsi G, LeGeros RZ, Heughebaert M, and Barbieux I, Formation of carbonate-apatite crystals after implantation of calcium phosphate ceramics. Calcif Tissue Int, 1990. 46(1): p. 20–7. [DOI] [PubMed] [Google Scholar]
- 161.de Oliveira RS, Brigato R, Madureira JF, Cruz AA, de Mello Filho FV, Alonso N, and Machado HR, Reconstruction of a large complex skull defect in a child: a case report and literature review. Childs Nerv Syst, 2007. 23(10): p. 1097–102. [DOI] [PubMed] [Google Scholar]
- 162.Hunt J, Bone engineering-JE Davies; Em Squared Inc., pp. 656+ xiv, ISBN 0 9686980-0-X. Biomaterials, 2002. 18(23): p. 3914–3915. [Google Scholar]
- 163.Kattimani VS, Kondaka S, and Lingamaneni KP, Hydroxyapatite–-Past, Present, and Future in Bone Regeneration. Bone and Tissue Regeneration Insights, 2016. 7: p. BTRI.S36138. [Google Scholar]
- 164.Ginebra MP, Traykova T, and Planell JA, Calcium phosphate cements as bone drug delivery systems: A review. Journal of Controlled Release, 2006. 113(2): p. 102–110. [DOI] [PubMed] [Google Scholar]
- 165.Xu HHK, Wang P, Wang L, Bao C, Chen Q, Weir MD, Chow LC, Zhao L, Zhou X, and Reynolds MA, Calcium phosphate cements for bone engineering and their biological properties. Bone Research, 2017. 5(1): p. 17056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kumar CY, K BN, Menon J, Patro DK, and B HB, Calcium sulfate as bone graft substitute in the treatment of osseous bone defects, a prospective study. J Clin Diagn Res, 2013. 7(12): p. 2926–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Padrines M, Rohanizadeh R, Damiens C, Heymann D, and Fortun Y, Inhibition of apatite formation by vitronectin. Connect Tissue Res, 2000. 41(2): p. 101–8. [DOI] [PubMed] [Google Scholar]
- 168.Liang F, Leland H, Jedrzejewski B, Auslander A, Maniskas S, Swanson J, Urata M, Hammoudeh J, and Magee W 3rd, Alternatives to Autologous Bone Graft in Alveolar Cleft Reconstruction: The State of Alveolar Tissue Engineering. J Craniofac Surg, 2018. 29(3): p. 584–593. [DOI] [PubMed] [Google Scholar]
- 169.Cahill KS, Chi JH, Groff MW, McGuire K, Afendulis CC, and Claus EB, Outcomes for single-level lumbar fusion: the role of bone morphogenetic protein. Spine (Phila Pa 1976), 2011. 36(26): p. 2354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rengachary SS, Bone morphogenetic proteins: basic concepts. Neurosurg Focus, 2002. 13(6): p. e2. [DOI] [PubMed] [Google Scholar]
- 171.Suarez-Lopez Del Amo F, Monje A, Padial-Molina M, Tang Z, and Wang HL, Biologic Agents for Periodontal Regeneration and Implant Site Development. Biomed Res Int, 2015. 2015: p. 957518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, and Glassman SD, Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine (Phila Pa 1976), 2010. 35(19): p. 1794–800. [DOI] [PubMed] [Google Scholar]
- 173.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, and McKee MD, Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop, 2007. 31(6): p. 735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Augment Bone Graft Summary of Safety and Effectiveness Data (SSED). June 26, 2020; Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf10/p100006b.pdf.
- 175.iFactor Summary of Safety and Effectiveness Data. June 26, 2020]; Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140019B.pdf.
- 176.Epstein NE, Pros, cons, and costs of INFUSE in spinal surgery. Surg Neurol Int, 2011. 2: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McKay WF, Peckham SM, and Badura JM, A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop, 2007. 31(6): p. 729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sreekumar V, Aspera-Werz RH, Tendulkar G, Reumann MK, Freude T, Breitkopf-Heinlein K, Dooley S, Pscherer S, Ochs BG, Flesch I, Hofmann V, Nussler AK, and Ehnert S, BMP9 a possible alternative drug for the recently withdrawn BMP7? New perspectives for (re-)implementation by personalized medicine. Archives of Toxicology, 2017. 91(3): p. 1353–1366. [DOI] [PubMed] [Google Scholar]
- 179.Qian JJ and Bhatnagar RS, Enhanced cell attachment to anorganic bone mineral in the presence of a synthetic peptide related to collagen. J Biomed Mater Res, 1996. 31(4): p. 545–54. [DOI] [PubMed] [Google Scholar]
- 180.Hanks T and Atkinson BL, Comparison of cell viability on anorganic bone matrix with or without P-15 cell binding peptide. Biomaterials, 2004. 25(19): p. 4831–6. [DOI] [PubMed] [Google Scholar]
- 181.Bhatnagar RS, Qian JJ, and Gough CA, The role in cell binding of a beta-bend within the triple helical region in collagen alpha 1 (I) chain: structural and biological evidence for conformational tautomerism on fiber surface. J Biomol Struct Dyn, 1997. 14(5): p. 547–60. [DOI] [PubMed] [Google Scholar]
- 182.Gokhale ST and Dwarakanath CD, The use of a natural osteoconductive porous bone mineral (Bio-Oss) in infrabony periodontal defects. J Indian Soc Periodontol, 2012. 16(2): p. 247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim Y, Nowzari H, and Rich SK, Risk of Prion Disease Transmission through Bovine-Derived Bone Substitutes: A Systematic Review. Clinical Implant Dentistry and Related Research, 2013. 15(5): p. 645–653. [DOI] [PubMed] [Google Scholar]
- 184.Moroni A, Hoang-Kim A, Lio V, and Giannini S, Current augmentation fixation techniques for the osteoporotic patient. Scand J Surg, 2006. 95(2): p. 103–9. [DOI] [PubMed] [Google Scholar]
- 185.Sallent I, Capella-Monsonís H, Procter P, Bozo IY, Deev RV, Zubov D, Vasyliev R, Perale G, Pertici G, Baker J, Gingras P, Bayon Y, and Zeugolis DI, The Few Who Made It: Commercially and Clinically Successful Innovative Bone Grafts. Frontiers in Bioengineering and Biotechnology, 2020. 8(952). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Qiao Y, Liu X, Zhou X, Zhang H, Zhang W, Xiao W, Pan G, Cui W, Santos HA, and Shi Q, Osteogenic Hydrogels: Gelatin Templated Polypeptide Co-Cross-Linked Hydrogel for Bone Regeneration (Adv. Healthcare Mater. 1/2020). Advanced Healthcare Materials, 2020. 9(1): p. 2070001. [DOI] [PubMed] [Google Scholar]
- 187.Park SH, Kwon JS, Lee BS, Park JH, Lee BK, Yun J-H, Lee BY, Kim JH, Min BH, Yoo TH, and Kim MS, BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells. Scientific Reports, 2017. 7(1): p. 6603. [DOI] [PMC free article] [PubMed] [Google Scholar]


