Abstract
BACKGROUND:
Non-Hispanic black maternal race is a known risk factor for preterm birth. However, the contribution of paternal race is not as well established.
OBJECTIVE:
We sought to evaluate the risk of preterm birth among non-Hispanic black, non-Hispanic white, and mixed non-Hispanic black and non-Hispanic white dyads.
STUDY DESIGN:
This was a population-based cohort study of all live births in the United States from 2015 to 2017, using live birth records from the National Vital Statistics System. Singleton, nonanomalous infants whose live birth record included maternal and paternal self-reported race as either non-Hispanic white or non-Hispanic black were included. The primary outcome was preterm birth at <37 weeks’ gestation; secondary outcomes included preterm birth at <34 and <28 weeks’ gestation and delivery gestational age (as a continuous variable). Data were analyzed using chi-square, t test, analysis of variance, and logistic regression. A Kaplan-Meier survival curve was also generated.
RESULTS:
There were 11,809,599 live births during the study period; 4,008,622 births met the inclusion criteria. Of included births, 291,647 (7.3%) occurred at <37 weeks’ gestation. Using the convention of maternal race first followed by paternal race, preterm birth at <37 weeks’ gestation was most common among non-Hispanic black and non-Hispanic black dyads (n=70,987 [10.8%]), followed by non-Hispanic black and non-Hispanic white (n=3137 [9.5%]), non-Hispanic white and non-Hispanic black (n=9136 [8.3%]), and non-Hispanic white and non-Hispanic white dyads (n=209,387 [6.5%]; P<.001 for trend). Births at <34 weeks’ (n=74,474) and <28 weeks’ gestation (n=18,474) were also more common among non-Hispanic black and non-Hispanic black dyads. Specifically, 24,351 (3.7%) non-Hispanic black and non-Hispanic black, 1017 (3.1%) non-Hispanic black and non-Hispanic white, 2408 (2.2%) non-Hispanic white and non-Hispanic black, and 46,698 non-Hispanic white and non-Hispanic white dyads delivered at <34 weeks’ gestation, and 7988 non-Hispanic black and non-Hispanic black (1.2%), 313 (1.0%) non-Hispanic black and non-Hispanic white, 584 (0.5%) non-Hispanic white and non-Hispanic black, and 9589 (0.3%) non-Hispanic white and non-Hispanic white dyads delivered at <28 weeks’ gestation. Non-Hispanic white and non-Hispanic white dyads delivered at a mean 38.8± standard deviation of 1.7 weeks’ gestation, although non-Hispanic white and non-Hispanic black, non-Hispanic black and non-Hispanic white, and non-Hispanic black and non-Hispanic black dyads delivered at 38.6±2.0, 38.5±2.3, and 38.3±2.4 weeks’ gestation, respectively (P<.001). Adjusted odds ratios for the association between maternal or paternal race and preterm birth were highest for non-Hispanic black and non-Hispanic black dyads at each gestational age cutoff: adjusted odds ratio, 1.60 (95% confidence interval, 1.11–1.19) (<37 weeks’ gestation); adjusted odds ratio, 2.47 (95% confidence interval, 2.41–2.53) (<34 weeks’ gestation); and adjusted odds ratio, 4.22 (95% confidence interval, 4.04–4.41) (<28 weeks’ gestation) compared with the non-Hispanic white referent group. Models adjusted for insurance status, chronic hypertension, tobacco use during pregnancy, history of previous preterm birth, and male fetus. In the Kaplan-Meier survival analysis, non-Hispanic black and non-Hispanic black dyads delivered the earliest across the range of delivery gestational ages compared with all other combinations of dyads.
CONCLUSION:
Non-Hispanic black paternal race is a risk factor for preterm birth and should be considered when evaluating maternal a priori risk of prematurity. Future research should investigate the mechanisms behind this finding, including determining the contribution of factors, such as racism, maternal and paternal genetics, and epigenetics to an individual’s risk of preterm birth.
Keywords: paternal race, preterm birth, racial disparities, risk assessment
Non-Hispanic black (NHB) women have the highest rates of preterm birth (PTB) in the United States. In 2018, the PTB rate among NHB women was 14.8%—almost 50% higher than non-Hispanic white (NHW) women (9.1)%.1 Racial and ethnic disparities in prematurity have persisted for decades, even during periods when the overall PTB rates were declining nationwide. From the 1980s to 2010s, various theories were investigated to determine whether underlying socioeconomic factors or environmental causes might explain the NHB-NHW disparity in PTB, including neighborhood deprivation and the weathering hypothesis.2−5
Clinically, obstetrical care providers typically assess only maternal race when determining the a priori risk of PTB. The associations among paternal race, maternal or paternal dyads of mixed race, and PTB are not as well established, and previous studies have limitations.5,6 In fact, a 2018 study, which evaluated the contribution of paternal race to birth outcomes, assumed that missing data for paternal race on the birth certificate meant “missingness” of paternal support or involvement in the pregnancy.7 This assumption is a complex and important limitation, as it assigns meaning to unavailable data and makes the study conclusion reliant on a hypothesized interpretation of missing data. Other studies were limited by small sample sizes8 and a focus on unique, local populations9 that reduce the generalizability of their results. In addition, a 2019 study evaluating the association between paternal race and birth outcomes using data from the National Vital Statistics System had a large sample size (N=16.4 million) but only focused on the association between paternal race and multiple adverse obstetrical outcomes; maternal race and, thus, the “pair” of maternal-paternal race were not considered, limiting the conclusions and clinical applicability of the results.10
Thus, our objective was to quantify the risk of PTB based on maternal and paternal self-identified race. Here, we hypothesized that PTB risk is highest for NHB and NHB dyads and lowest for NHW and NHW dyads.
Materials and Methods
We conducted a retrospective, population-based cohort study of all live births in the United States from 2015 to 2017, using live birth certificate data from the National Vital Statistics System (NVSS), of the Centers for Disease Control and Prevention’s National Center for Health Statistics. This publicly available database contains more than 99% of all live births that occur within the 50 states and the District of Columbia. The NVSS includes birth certificates for infants born at ≥20 weeks’ gestation in addition to some previable births at <20 weeks’ gestation. For this analysis, we included all live births of a singleton, nonanomalous infant, with complete data on delivery gestational age and self-reported maternal and paternal race. We only included live births with maternal and paternal race identified as either NHW or NHB. Births with missing paternal race or delivery before 16 weeks’ or greater than 43 weeks’ gestation were excluded. The “best obstetrical estimate” variable in the live birth record was used for the gestational age at delivery. The primary outcome was PTB, defined as delivery between 16 weeks’ gestation and 36 6/7 weeks’ gestation. Secondary outcomes were delivery gestational age (considered a continuous variable), PTB at <34 weeks’ gestation, and PTB at <28 weeks’ gestation.
Demographics, pervious pregnancy, medical history, and antenatal characteristics were compared between women with and without PTB at <37 weeks’ gestation using chi-square, analysis of variance, and the Student t test, as appropriate. All tests were 2-tailed, and P value of <.001 was used to define statistical significance given the large sample size of the dataset. The Pearson correlation was used to evaluate correlations among characteristics that were statistically significant in bivariable analysis. Multivariable logistic regression modeled the association between maternal and paternal race and PTB at <37 weeks’ gestation. Initial models considered several covariates identified a priori from the literature as being associated with PTB, including maternal marital status, insurance status, chronic hypertension, tobacco use during pregnancy, history of previous PTB, and fetal sex. Variables were retained in the final regression models if P values were <.20. Nulliparous women were considered as not having a previous PTB for the purposes of regression models. Kaplan-Meier curves were generated to evaluate time to delivery based on maternal-paternal race combinations; the survival curves were compared using the logrank test. Finally, because pre- and periviable deliveries are significantly more common among NHB women, we conducted a sensitivity analysis excluding deliveries before 24 weeks’ gestation.
All statistical analyses were performed using STATA/SE (version 15.1; StataCorp, Inc, College Station, TX). This project was reviewed by the institutional review board at the University of North Carolina at Chapel Hill; following administrative review, this research was deemed exempt from oversight because the analysis was performed using a publicly available, deidentified dataset and thus did not meet the criteria for human subject research.
Results
In 2015–2017, there were 11,809,599 live births in the United States as recorded in the NVSS; 4,008,622 births met the inclusion criteria (Figure 1). Of 4,008,622 live births, 291,647 (7.3%) were delivered preterm at <37 weeks’ gestation, 74,474 (1.9%) at <34 weeks’ gestation, and 18,474 (0.5%) at <28 weeks’ gestation. Using the maternal or paternal convention, 659,876 (16.5%) births were to NHB and NHB dyads, 32,921 (0.8%) to NHB and NHW dyads, 110,771 (2.8%) to NHW and NHB dyads, and 3,205,054 (80.0) to NHW and NHW dyads. For all analyses, NHW and NHW served as the referent group.
FIGURE 1. Study enrollment.
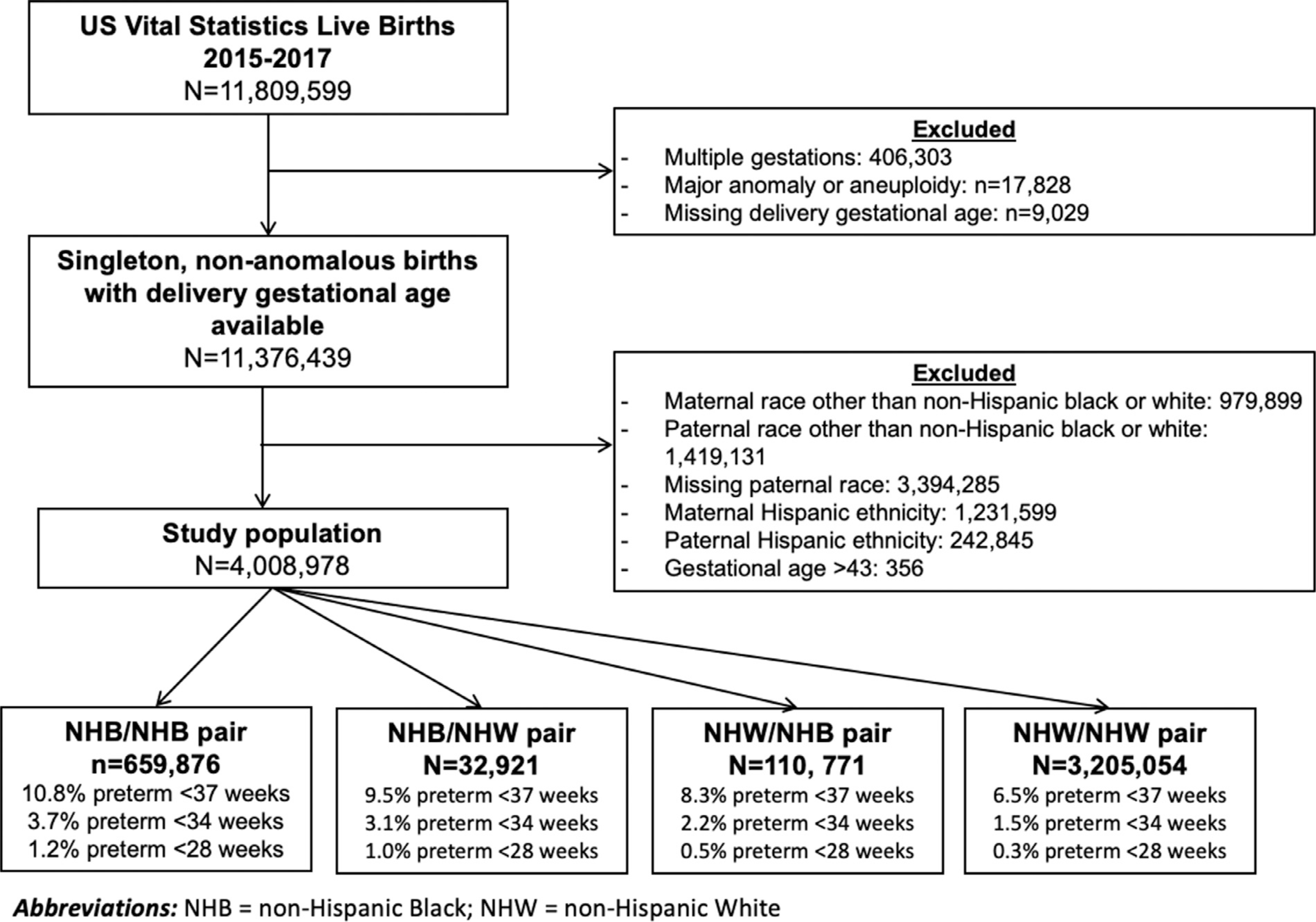
Parental race is listed as maternal or paternal.
NHB, non-Hispanic black; NHW, non-Hispanic white.
Green. Parental race and risk of preterm birth. Am J Obstet Gynecol MFM 2021.
Overall, women in this cohort were predominantly NHW, married, nonsmokers, and privately insured. Among other factors, tobacco use, Medicaid insurance, and a diagnosis of chronic hypertension were more common among women delivering preterm compared with those delivering at term (Table 1). Of the 17,373 multiparous women who delivered preterm, 29,403 had a previous preterm birth (17.2%).
TABLE 1.
Demographic and medical characteristics of the study cohort by preterm birth at <37 weeks’ gestation
| Characteristic | Preterm birth at <37 wk gestation (n=291,647) | Term birth at ≥37 wk gestation (n=3,716,975) |
|---|---|---|
| Prepregnancy body mass index (kg/m2) median (IQR)a | 25.7 (21.9–31.5) | 24.9 (21.8–29.9) |
| Medicaid or self-pay was payment source for delivery | 118,953 (41.1) | 1,259,026 (34.1) |
| Mother with less than a high school education | 25,528 (8.8) | 246,078 (6.7) |
| History of preterm birth (multiparous women) | 29,403/17,373 (17.2) | 87,252/2,284,727 (3.8) |
| Married | 188,352 (64.6) | 2,691,717 (72.4) |
| Chronic hypertension | 14,267 (4.9) | 59,046 (1.6) |
| Tobacco use during pregnancy | 32,472 (11.1) | 282,673 (7.6) |
| Gestational diabetes mellitus | 21,832/287,560 (7.6) | 182,787/3,666,653 (5.0) |
| Male fetus | 156,807 (53.8) | 1,898,325 (51.1) |
| Preeclampsia | 3556 (1.2) | 6221 (0.2) |
Data are presented as number (percentage) unless otherwise specified. All P values are <.001.
IQR, interquartile range.
Prepregnancy body mass index available for 3,856,588 women
Green. Parental race and risk of preterm birth. Am J Obstet Gynecol MFM 2021.
PTB at <37 weeks’ gestation was most common among NHB and NHB dyads (n=70,987 [10.8%]), followed by NHB and NHW (n=3137 [9.5%]), NHW and NHB (n=9136 [8.3%]), and NHW and NHW dyads (n=209,387 [6.5%]) (P<.001). Rates of PTB at <34 and <28 weeks’ gestation showed similar trends. Specifically, 24,351 (3.7%) NHB and NHB, 1017 (3.1%) NHB and NHW, 2408 (2.2%) NHW and NHB, and 46,698 NHW and NHW dyads delivered at <34 weeks’ gestation, and 7988 NHB and NHB (1.2%), 313 (1.0%) NHB and NHW, 584 (0.5%) NHW and NHB, and 9589 (0.3%) NHW and NHW dyads delivered at <28 weeks’ gestation (0.3%). The mean ± standard deviation delivery gestational age also followed these results; the gestational age at delivery was 38.3±2.4 weeks’ gestation for NHB and NHB dyads, 38.5± 2.3 weeks’ gestation for NHB and NHW, 38.6±2.0 weeks’ gestation for NHW and NHB, and 38.8±1.7 weeks’ gestation for NHW and NHW. Characteristics of parental dyads are shown in Table 2.
TABLE 2.
Antenatal characteristics and pregnancy outcomes race of couple
| Characteristic | Race of couple (mother and father) | |||
|---|---|---|---|---|
| NHW and NHW (n=3,205,054) | NHW and NHB (n=110,771) | NHB and NHW (n=32,921) | NHB and NHB (n=659,876) | |
| Primiparous | 1,266,987 (39.5) | 40,170 (36.3) | 13,827 (42.0) | 229,702 (34.8) |
| Mother with less than a high school education | 182,640 (5.7) | 12,487 (11.3) | 2162 (6.6) | 74,317 (11.4) |
| Married | 2,542,073 (79.3) | 44,511 (40.2) | 18,968 (57.6) | 274,517 (41.6) |
| Medicaid or self-pay was payment source for delivery | 889,658 (27.9) | 58,109 (52.8) | 14,633 (44.7) | 415,579 (63.3) |
| History of preterm birth (multiparous women) | 85,842 (2.7) | 3912 (3.5) | 1105 (3.4) | 25,796 (3.9) |
| Prepregnancy body mass index (kg/m2), median (IQR)a | 24.5 (21.6–29.2) | 27.4 (23.2–33.2) | 25.6 (22.1–30.7) | 27.4 (23.2–32.6) |
| Chronic hypertension | 47,638 (1.5) | 2280 (2.1) | 952 (2.9) | 22,443 (3.4) |
| No prenatal care | 21,110 (0.7) | 1488 (1.4) | 397 (1.3) | 12,166 (1.9) |
| Tobacco use during pregnancy | 268,141 (8.4) | 17,766 (16.0) | 2262 (6.9) | 26,976 (4.1) |
| Sexually transmitted infection | 24,202 (0.8) | 3926 (3.5) | 604 (1.8) | 23,642 (3.6) |
| Gestational diabetes mellitus | 163,572 (5.2) | 5943 (5.5) | 1678 (5.3) | 33,426 (5.1) |
| Pregnancy outcomes | ||||
| Gestational hypertension or preeclampsia | 192,929 (6.0) | 6838 (6.2) | 2221 (6.8) | 46,307 (7.0) |
| Diagnosed with chorioamnionitis | 37,235 (1.2) | 1348 (1.2) | 477 (1.5) | 9424 (1.4) |
| Male fetus | 1,647,041 (51.4) | 55,714 (50.3) | 17,090 (51.9) | 335,287 (50.8) |
| Birthweight (g), median (IQR)b | 3410 (3093–3730) | 3345 (3010–3666) | 3265 (2940–3590) | 3180 (2860–3505) |
| Delivered at <37 wk gestation | 208,387 (6.5) | 9136 (8.3) | 3137 (9.5) | 70,987 (10.8) |
| Delivered at <34 wk gestation | 46,698 (1.5) | 2408 (2.2) | 1017 (3.1) | 24,351 (3.7) |
| Delivered at <28 wk gestation | 9589 (0.3) | 584 (0.5) | 313 (1.0) | 7988 (1.2) |
| Delivery gestational age (wk), mean±SD | 38.8±1.7 | 38.6±2.0 | 38.5±2.9 | 38.3±2.4 |
Data are presented as number (percentage) unless otherwise specified. All P values are <.001.
IQR, interquartile range; NHB, non-Hispanic black; NHW, non-Hispanic white; SD, standard deviation.
Prepregnancy body mass index available for 3,856,588 women.
4,005,505 neonates with birthweight available.
Green. Parental race and risk of preterm birth. Am J Obstet Gynecol MFM 2021.
In regression models, maternal marital status was not included in initial models because of colinearity with insurance status. After controlling for insurance status, history of PTB, chronic hypertension, tobacco use during pregnancy, and male fetus, NHB and NHB dyads had the highest odds of delivery at <37, <34, and <28 weeks’ gestation with the most pronounced associations at the earliest gestational ages compared with all other parental race dyads. The full model for PTB at <37 weeks’ gestation is shown in Table 3, PTB at <34 weeks’ gestation in Table 4, and PTB at <28 weeks’ gestation in Table 5. Notably, the odds of PTB when at least 1 biologic parent was NHB were most substantial for the earliest deliveries. For deliveries at <34 weeks’ gestation, compared with NHW and NHW dyads, the increased odds of early delivery was 2.47 (95% confidence interval [CI], 2.41–2.53) for NHB and NHB dyads, 2.18 (95% CI, 2.00–2.38) for NHB and NHW dyads, and 1.29 (95% CI, 1.21–1.39) NHW and NHB dyads (Table 4). For PTB at <28 weeks’ gestation, the increased odds of PTB at <28 weeks’ gestation was 4.22 (95% CI, 4.04–4.41) for NHB and NHB dyads, 3.42 (95% CI, 2.94–4.0) NHB and NHW dyads, and 1.64 (95% CI, 1.43 –1.90) for NHW and NHB dyads (Table 5).
TABLE 3.
Logistic regression results for preterm birth at <37 weeks’ gestation
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Race of couple (mother and father) | |||
| NHW and NHW | 1.0 (referent) | — | — |
| NHW and NHB | 1.15 | 1.11–1.19 | <.001 |
| NHB and NHW | 1.49 | 1.42–1.57 | <.001 |
| NHB and NHB | 1.60 | 1.58–1.63 | <.001 |
| Medicaid or self-pay was payment source for delivery | 1.04 | 1.03–1.05 | <.001 |
| Previous preterm birth at <37 wk gestation | 4.49 | 4.41–4.57 | <.001 |
| Chronic hypertension | 2.88 | 2.81–2.95 | <.001 |
| Smoked during pregnancy | 1.57 | 1.54–1.60 | <.001 |
| Male fetus | 1.14 | 1.13–1.15 | <.001 |
CI, confidence interval; NHB, non-Hispanic black; NHW, non-Hispanic white; OR, odds ratio.
Green. Parental race and risk of preterm birth. Am J Obstet Gynecol MFM 2021.
TABLE 4.
Logistic regression results for preterm birth at <34 weeks’ gestation
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Race of couple (mother and father) | |||
| NHW and NHW | 1.0 | — | — |
| NHW and NHB | 1.39 | 1.34–1.46 | <.001 |
| NHB and NHW | 2.08 | 1.96–2.22 | <.001 |
| NHB and NHB | 2.50 | 2.46–2.54 | <.001 |
| Previous preterm birth at <37 wk gestation | 3.78 | 3.69–3.87 | <.001 |
| Chronic hypertension | 3.00 | 2.92–3.10 | <.001 |
| Smoked during pregnancy | 1.57 | 1.54–1.61 | <.001 |
| Male fetus | 1.12 | 1.10–1.13 | <.001 |
Medicaid or self-pay as payment source for delivery was considered in the initial model but removed here because P>.20.
CI, confidence interval; NHB, non-Hispanic black; NHW, non-Hispanic white; OR, odds ratio.
Green. Parental race and risk of preterm birth. Am J Obstet Gynecol MFM 2021.
TABLE 5.
Logistic regression results for preterm birth at <28 weeks’ gestation
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Race of couple (mother and father) | |||
| NHW and NHW | 1.0 (referent) | — | — |
| NHW and NHB | 1.66 | 1.45–1.92 | <.001 |
| NHB and NHW | 3.41 | 2.93–3.97 | <.001 |
| NHB and NHB | 4.22 | 4.04–4.41 | <.001 |
| Previous preterm birth at <37 wk gestation | 3.18 | 2.97–3.39 | <.001 |
| Chronic hypertension | 2.91 | 2.69–3.16 | <.001 |
| Smoked during pregnancy | 1.62 | 1.49–1.76 | <.001 |
| Male fetus | 1.10 | 1.06–1.15 | <.001 |
Medicaid or self-pay as payment source for delivery was considered in the initial model but removed here because P>.20.
CI, confidence interval; NHB, non-Hispanic black; NHW, non-Hispanic white; OR, odds ratio.
Green. Parental race and risk of preterm birth. Am J Obstet Gynecol MFM 2021.
Finally, in our sensitivity analysis excluding 5667 women with PTB at <24 weeks’ gestation, results were similar. For deliveries at <37 weeks’ gestation, compared with NHW and NHW dyads, the increased odds of early delivery was 1.56 (95% CI, 1.53–1.58) for NHB and NHB dyads, 1.45 (95% CI, 1.38–1.52) for NHB and NHW dyads, and 1.14 (95% CI, 1.10–1.19) for NHW and NHB dyads. Data were similar for preterm births at <34 and <24 weeks’ gestation.
In the Kaplan-Meier survival analysis, after adjusting for insurance status, history of PTB, chronic hypertension, tobacco use during pregnancy, and male fetus, NHB and NHB dyads delivered the earliest across the range of delivery gestational ages compared with all other parental race dyads (Figure 2) (log-rank P<.001).
FIGURE 2. Kaplan-Meier survival curve, censored at delivery at term (≥37 weeks’ gestation).
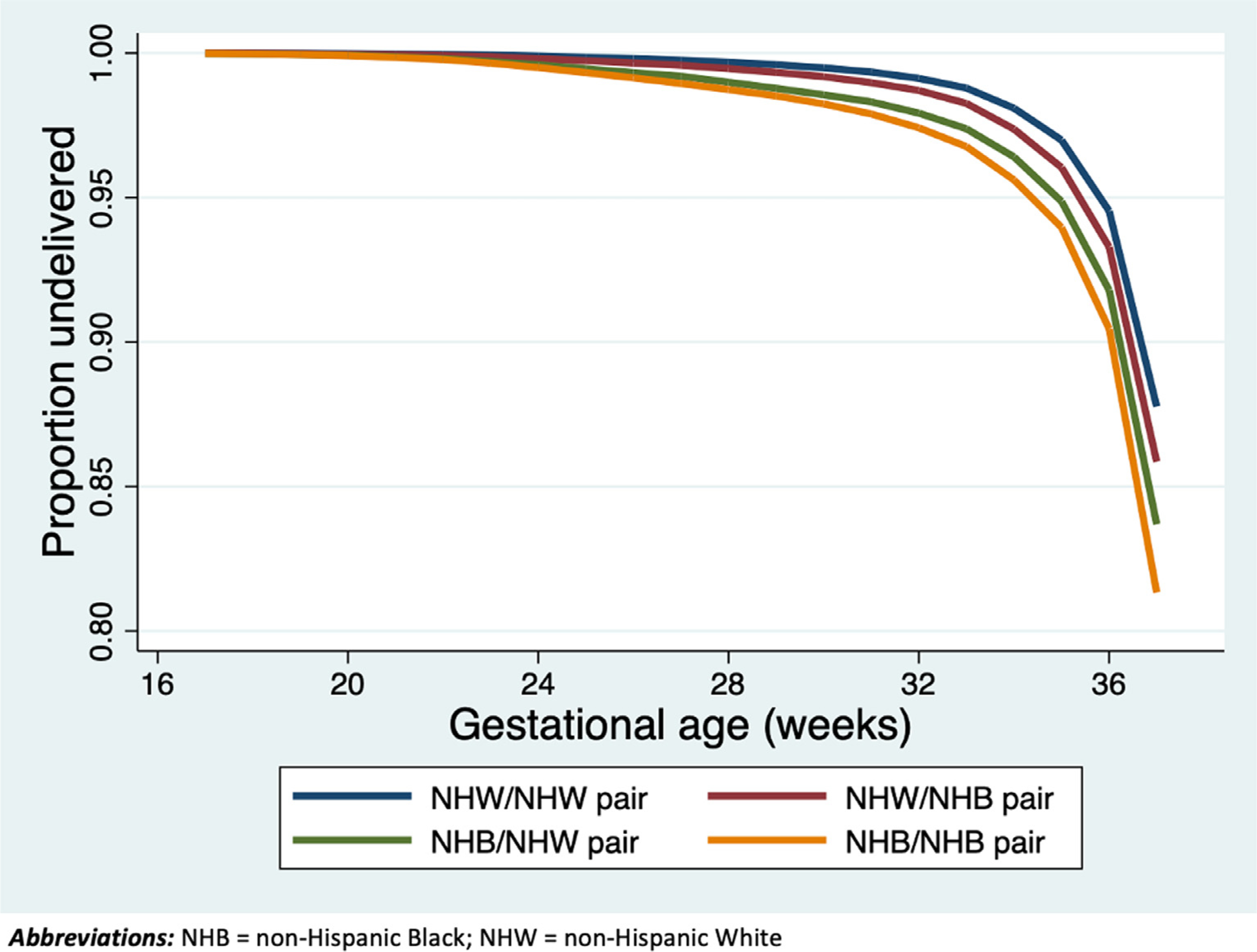
Parental race is listed as maternal or paternal.
NHB, non-Hispanic black; NHW, non-Hispanic white.
Green. Parental race and risk of preterm birth. Am J Obstet Gynecol MFM 2021.
Comment
Principal findings
We found that the adjusted odds ratios for PTB at <37, <34, and <28 weeks’ gestation were highest for NHB and NHB dyads, high for NHB and NHW dyads, and modestly elevated for NHW and NHB dyads compared with NHW and NHW dyads. The increased risk of PTB when at least 1 partner was NHB was most significant for deliveries at the earliest gestational ages. When delivery gestational age was considered a continuous measure, these findings persisted, even after controlling for key covariates. Ultimately, NHB maternal race— regardless of paternal race—conferred the highest risk of PTB. However, NHB women had a lower risk of PTB if their partner was NHW, emphasizing the importance of considering paternal race when determining a woman’s a priori risk of PTB. These risks are significant; for the earliest delivery gestational age cutoff (<28 weeks’ gestation), both NHB and NHW and NHB and NHB dyads had odds of PTB at <28 weeks’ gestation that were 3.41 to 4.22 times higher than NHW and NHW dyads. These odds ratios for maternal and paternal race dyads were greater than the odds ratio conferred by a history of previous PTB (3.18)—a more traditional and established prematurity risk factor.
Results in context of the literature
We recognized and acknowledged, as others have, the inexact definition of race: how a person self-identifies is a complicated topic. Furthermore, and perhaps even more salient in this instance, the identity an individual is expected to hold and, thus, how they are perceived may be based on features, such as their neighborhood of residence or physical complexion, and ultimately, these factors impact health disparities.
Our outcomes were similar to those reported in the largest previous study to investigate the role of paternal race on birth outcomes (N=90.7 million), where mixed race and ethnicity dyads of Hispanic and non-Hispanic origins had higher rates of PTB than the NHW and NHW referent groups. In that study by Li et al,7 NVSS data from 1989 to 2013 were evaluated, and within each maternal race and ethnicity group, women whose partner’s race was “missing" on the birth certificate had the highest risk of PTB; those who reported their partner was NHB had the second highest PTB risk. Li et al7 surmised that missing paternal race data implied a lack of paternal involvement in the pregnancy, hypothesizing that the lack of paternal social support increased the risk of PTB.11,12 However, the conclusions of the Li et al7 study are limited by these assumptions, and some of these data are more than 30 years old. In this study, we excluded records without available data on paternal race because our focus was on PTB risk among pregnancies with known maternal-paternal race and ethnicity. Ideally, we would also have the ability to capture the level of paternal involvement in the pregnancy, in addition to parental race and ethnicity, to disentangle the effects of race and paternal involvement on the risk of PTB. Other studies evaluating PTB among mixed race dyads have been limited to study populations in smaller geographic locations, such as Minnesota (data from 1989 to 1997)13 and New York City (data from 2000 to 2010).9 Our contemporary study with a larger, national cohort reports similar findings as in these smaller studies, which each showed that infants born to NHB dyads overall and to NHB fathers regardless of maternal race and ethnicity, had higher rates of PTB than the NHW referent groups. Our study increased the generalizability of the findings given the nationwide cohort used for this analysis.
A 2019 study by Palatnik et al10 investigated the association between paternal race and multiple adverse birth outcomes, including gestational diabetes, hypertensive disorders of pregnancy, and PTB at <37 weeks’ gestation using data from the 2013 to 2017 NVSS. All paternal racial and ethnic groups within the NVSS dataset were included; similar to our study, records with missing paternal race data were excluded. However, maternal race and, specifically, maternal-paternal dyads were not evaluated. The analysis found that NHB paternal race was most strongly associated with low birthweight and PTB. In a separate study evaluating the intergenerational risk of preterm birth, researchers found that women who themselves were born preterm were more likely to deliver their firstborn preterm and that these effects were most pronounced for black women. However, in that study, no information was available regarding paternal race.14 We extend the Palatnik findings by including an analysis of maternal and paternal dyads.
Clinical implications
Our study offered additional perspective regarding how racism because of self-identified maternal and paternal race may influence a mother’s a priori risk of PTB. Public acknowledgment of disparate rates of prematurity should be validated and acknowledged throughout a patient’s pregnancy care. Interventions providing robust social support preconception and during pregnancy that may decrease systemic racism—for example, collaborations with local and federal policymakers, social workers, doulas, and other community organizations may improve outcomes for black pregnant women. Bolstering these ancillary services while providing high-quality antenatal care will over time help change standards of care to address the system factors that contribute to disparate birth outcomes. Finally, incorporating paternal race information into routine obstetrical intake data obtained at the first prenatal visit could be easily implemented. This has the potential to improve counseling regarding an individual’s a priori PTB risk and provides a mechanism by which providers could further risk stratify which women are at higher risk of PTB.
Research implications
Future studies should further elucidate the social and biologic mechanisms underlying these findings, to determine whether improvements in long-standing systemic marginalization of NHB men and women in the United States, genetic and/or epigenetic factors, or other etiologies may reduce these disparities. In addition, evaluation of whether similar differences apply to couples of other mixed races and ethnicities would provide key information regarding specific risk for other individuals. Throughout these investigations, it is imperative to remember that race remains a social construct; acknowledging this will better elucidate the generational and pervasive effects of this social categorization on the health of communities of color and aid in future research.
Strengths and limitations
Our study has several strengths; the large, US population-wide sample size enhances the generalizability of these findings. Furthermore, the study is pragmatic in that it relies on information provided by women at the time of delivery. It is reasonable to assume that each woman would provide similar information regarding paternal race earlier in pregnancy if she were asked. Our study should be interpreted in the context of its limitations, including the reliability and validity of reported birth certificate data. It is unclear whether similar associations would be appreciated among women carrying multiple gestations. However, many of the variables in our study—including patient demographics (race, maternal age), insurance status, and maternal tobacco use in pregnancy—have been previously validated in other studies.15–17 In addition, some factors known to be associated with PTB, for example, cervical length, are unavailable in this dataset. The large number of records with missing paternal ethnicity and race data (29.8%) limited our study findings. Because paternal race information was required to complete the primary analysis, birth records with missing paternal race were excluded. Missing paternal race data may imply a lack of paternal involvement in the pregnancy or social support and/or serve as a surrogate for other risk factors for PTB.18 Although we took a different approach than Li et al7 in the utilization and meaning assigned to “missingness,” the underlying reason behind this missing information and their real-world implications regarding maternal support and pregnancy outcomes was unknown and important to investigate. Because of the nature of our dataset, we could not reliably discern the etiologies of the premature deliveries (eg, spontaneous vs medically indicated). Important differences in underlying PTB etiologies by race may exit, but we are unable to address these questions in the current analysis. Finally, our decision to include only NHB and NHW dyads was made because of the vast discrepancies in rates of prematurity between these groups, and it is uncertain if similar differences might be appreciated in dyads, including individuals of other racial and/or ethnic backgrounds.
Conclusions
We found that paternal race is an important factor to consider when determining a priori risk of PTB. As the United States becomes increasingly ethnically and racially diverse, these findings are particularly clinically relevant.
AJOG MFM at a Glance.
Why was this study conducted?
Non-Hispanic black (NHB) maternal race is a known risk factor for preterm birth (PTB). The contribution of both the maternal and paternal race to risk of PTB is not well described.
Key findings
The adjusted odd ratios for PTB at <37, <34, and <28 weeks’ gestation were highest for NHB and NHB dyads, high for NHB and non-Hispanic white (NHW) dyads, and modestly elevated for NHW and NHB dyads compared with NHW and NHW dyads. The increased risk of PTB when at least 1 partner was NHB was most significant for deliveries at the earliest gestational ages (<34 and <28 weeks’ gestation).
What does this add to what is known?
These data demonstrated the importance of considering paternal race when evaluating maternal a priori risk of PTB.
Acknowledgments
This study was supported, in part, by grant numbers R01-MD011609 (T.A.M.) and K24-ES031131 (T.A.M.).
Footnotes
The authors report no conflict of interest.
Presented in poster format at the 40th Annual Society for Maternal-Fetal Medicine Meeting, Gaylord, TX, Feb. 6, 2020 (final abstract identification number 303).
Contributor Information
Celeste A. Green, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill, NC.
Jasmine D. Johnson, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill, NC.
Catherine J. Vladutiu, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill, NC.
Tracy A. Manuck, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill, NC; Institute for Environmental Health Solutions, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC.
References
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep 2019;68:1–47. [PubMed] [Google Scholar]
- 2.O’Campo P, Burke JG, Culhane J, et al. Neighborhood deprivation and preterm birth among non-Hispanic black and white women in eight geographic areas in the United States. Am J Epidemiol 2008;167:155–63. [DOI] [PubMed] [Google Scholar]
- 3.O’Campo P, Schetter CD, Guardino CM, et al. Explaining racial and ethnic inequalities in postpartum allostatic load: results from a multisite study of low to middle income women. SSM Popul Health 2016;2:850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer MR, Hogue CJ, Dunlop AL, Menon R. Preconceptional stress and racial disparities in preterm birth: an overview. Acta Obstet Gynecol Scand 2011;90:1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giurgescu C, Misra DP. Psychosocial factors and preterm birth among black mothers and fathers. MCN Am J Matern Child Nurs 2018;43:245–51. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasjois RM, Shah S, Shah PS. Knowledge Synthesis Group on Determinants of Preterm/ LBW Births. Biracial couples and adverse birth outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand 2012;91:1134–46. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Luo Z, Holzman C, Liu H, Margerison CE. Paternal race/ethnicity and risk of adverse birth outcomes in the United States, 1989–2013. AIMS Public Health 2018;5:312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shachar BZ, Mayo JA, Lyell DJ, Stevenson DK, Shaw GM, Blumenfeld YJ. Risk for spontaneous preterm birth among inter-racial/ethnic couples. J Matern Fetal Neonatal Med 2018;31:633–9. [DOI] [PubMed] [Google Scholar]
- 9.Borrell LN, Rodriguez-Alvarez E, Savitz DA, Baquero MC. Parental race/ethnicity and adverse birth outcomes in New York City: 2000–2010. Am J Public Health 2016;106:1491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palatnik A, Garacci E, Walker RJ, Ozieh MN, Williams JS, Egede LE. The association of paternal race and ethnicity with adverse pregnancy outcomes in a contemporary U.S. Cohort. Am J Perinatol 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan H, Wen SW, Walker M, Demissie K. Missing paternal demographics: a novel indicator for identifying high risk population of adverse pregnancy outcomes. BMC Pregnancy Childbirth 2004;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surkan PJ, Dong L, Ji Y, et al. Paternal involvement and support and risk of preterm birth: findings from the Boston birth cohort. J Psychosom Obstet Gynaecol 2019;40:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palomar L, DeFranco EA, Lee KA, Allsworth JE, Muglia LJ. Paternal race is a risk factor for preterm birth. Am J Obstet Gynecol 2007;197:152.e1–7. [DOI] [PubMed] [Google Scholar]
- 14.Smid MC, Lee JH, Grant JH, et al. Maternal race and intergenerational preterm birth recurrence. Am J Obstet Gynecol 2017;217:480. e1–9. [DOI] [PubMed] [Google Scholar]
- 15.Northam S, Knapp TR. The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs 2006;35:3–12. [DOI] [PubMed] [Google Scholar]
- 16.Howland RE, Mulready-Ward C, Madsen AM, et al. Reliability of reported maternal smoking: comparing the birth certificate to maternal worksheets and prenatal and hospital medical records, New York City and Vermont, 2009. Matern Child Health J 2015;19:1916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zollinger TW, Przybylski MJ, Gamache RE. Reliability of Indiana birth certificate data compared to medical records. Ann Epidemiol 2006;16:1–10. [DOI] [PubMed] [Google Scholar]
- 18.Manuck TA. Racial and ethnic differences in preterm birth: a complex, multifactorial problem. Semin Perinatol 2017;41:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


