Abstract
BACKGROUND:
There is an increased awareness regarding the association between exposure to environmental contaminants and adverse pregnancy outcomes including preterm birth. Whether an individual’s metabolic profile can be utilized during pregnancy to differentiate the subset of patients who are ultimately destined to delivered preterm remains uncertain but could have MEANINGFUL clinical implications.
OBJECTIVE:
We sought to objectively quantify metabolomic profiles of patients at high risk of preterm birth by evaluating midtrimester maternal plasma and to measure whether endogenous metabolites and exogenous environmental substances differ among those who ultimately deliver preterm compared with those who deliver at term.
STUDY DESIGN:
This was a case-control analysis from a prospective cohort of patients carrying a singleton, nonanomalous gestation who were at high risk of spontaneous preterm birth. Subjects with a plasma blood sample drawn at <28 weeks’ gestation and no evidence of preterm labor at the time of enrollment were included. Metabolites were extracted from frozen samples, and metabolomic analysis was performed using liquid chromatography/mass spectrometry. The primary outcome was preterm birth at 16.0 to 36.9 weeks’ gestation.
RESULTS:
A total of 42 patients met the inclusion criteria. Of these, 25 (59.5%) delivered preterm at <37 weeks’ gestation, at a median of 30.14 weeks’ gestation (interquartile range, 28.14–34.14). A total of 812 molecular features differed between preterm birth cases and term controls with a minimum fold change of 1.2 and P<.05. Of these, 570 of 812 (70.1%) were found in higher abundances in preterm birth cases; the other 242 of 812 (29.9%) were in higher abundance in term birth controls. The identity of the small molecule/compound represented by the molecular features differing statistically between preterm birth cases and term controls was identified as ranging from those involved with endogenous metabolic pathways (including lipid catabolism, steroids, and steroid-related molecules) to exogenous exposures (including avocadyne, diosgenin, polycyclic aromatic hydrocarbons, acetaminophen metabolites, aspartame, and caffeine). Random forest analyses evaluating the relative contribution of each of the top 30 compounds in differentiating preterm birth and term controls accurately classified 21 of 25 preterm birth cases (84%).
CONCLUSION:
Both endogenous metabolites and exogenous exposures differ in maternal plasma in the midtrimester among patients who ultimately delivered preterm compared with those who deliver at term.
Keywords: endogenous metabolic pathways, environmental exposures, exogenous exposures, metabolomics, preterm birth
Preterm birth remains the leading cause of neonatal morbidity and mortality among nonanomalous infants in the United States, and it confers significant economic and societal burden.1–4 Although there are multiple established risk factors for preterm birth, including non-Hispanic Black race, short midtrimester cervical length, and a previous pregnancy complicated by prematurity, additional unidentified factors likely contribute to the development of this complex, multifactorial condition.5–8 Furthermore, the mechanisms underlying spontaneous preterm birth remain poorly understood.
There is an increased awareness regarding the association between exposure to environmental contaminants and adverse pregnancy outcomes. Exposure to a variety of pollutants including air pollution,9–11 organochlorine pesticides,12–14 heavy metals,15–17 and perfluoroalkyl substances18,19 has been implicated in preterm birth. Recent scientific advances have enhanced the ability to quantify the simultaneous nontargeted assessment of environmental exposures and physical and chemical stressors through metabolomic analysis techniques. In other fields of medicine, metabolomic analysis of plasma has been fruitful in the identification of aberrant exposures and changes in metabolites among those with and without disease; for example, derangements in lipid and carbohydrate metabolism and oxidative stress and inflammatory pathways are seen in individuals with myocardial ischemia.20,21
Studies investigating the association between metabolomic profiles and preterm birth have evaluated a variety of tissue sources including cervicovaginal fluid, amniotic fluid, placenta, and blood.22–27 A recent study showed that incorporation of acylcarnitine levels and other metabolites from infant newborn screening results at birth accurately classified gestational age with >95% sensitivity and specificity; although not applied during the antenatal period, findings such as these provide “proof of concept” regarding associations between metabolomic profiles and gestational age.28 Whether an individual’s metabolic profile can be utilized during pregnancy to differentiate the subset of patients who are ultimately destined to deliver preterm remains uncertain but could have significant clinical implications.
Thus, the objective of this study was to quantify metabolomic profiles of patients at high risk of preterm birth by evaluating midtrimester maternal plasma and to measure whether endogenous metabolites and exogenous environmental substances differ among those who ultimately deliver preterm compared with those who deliver at term.
Materials and Methods
This was a nested case-control analysis from a prospective cohort of patients carrying a singleton, nonanomalous gestation who were at high risk of spontaneous preterm birth (“University of North Carolina Preterm Birth [UNC PTB] Biobank”). Patients were recruited prospectively at the University of North Carolina at Chapel Hill (Chapel Hill, NC) from 2015 to 2018. For the purposes of this analysis, we included patients from the original cohort who were carrying a singleton pregnancy and had a maternal plasma sample collected before 28 weeks’ gestation. Each participant met at least one of the following criteria placing them at high risk of spontaneous preterm birth: (1) at least 1 previous pregnancy delivering between 16 0/7 and 36 6/7 weeks’ gestation after a spontaneous onset of contractions, preterm prelabor rupture of membranes, or cervical insufficiency; (2) transvaginal cervical length between 16 0/7 and 23 6/7 weeks’ gestation measuring <25 mm; and (3) antepartum hospitalization for threatened preterm labor owing to cervical dilation of ≥2 centimeters or cervical effacement of ≥80% by digital examination, but without symptoms of preterm labor for at least 24 hours before enrollment. Patients carrying a fetus with a major structural anomaly or aneuploidy were excluded. Pregnancies were dated by last menstrual period (if available) and ultrasound using standard American College of Obstetricians and Gynecologists criteria.29 All pregnancy management decisions were made at the discretion of the primary obstetrical provider. The focus of recruitment for the UNC PTB Biobank was individuals with a previous spontaneous preterm birth. Owing to logistic recruiting constraints, not all individuals meeting the eligibility criteria were able to be approached during the study period. If multiple patients with simultaneous clinic appointments met the inclusion criteria for the UNC PTB Biobank, the patient with the most severe preterm birth history was recruited (eg, history of early preterm birth at <28 weeks’ gestation or multiple previous preterm birth). Individuals included in this analysis represent a subset of women from the UNC PTB Biobank who had matched plasma and vaginal swab samples obtained on the same date. Although vaginal swab samples were not used in this analysis, individuals with both sample types available were included in a separate analysis for which a partial plasma aliquot was required.
At enrollment, each woman provided a blood sample by standard venipuncture; samples were collected into an acid-citrate dextrose tube and centrifuged, and plasma was aliquoted and stored at −80°C until future analysis. Clinical data were collected by standardized interviews conducted by trained research assistants and were supplemented by medical record review. All study data were collected and managed using the Research Electronic Data Capture tools, a secure, web-based application designed to support data capture for research studies, hosted at the University of North Carolina at Chapel Hill.30 This study was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill, and all patients provided a written informed consent form before participation.
Metabolites were extracted from stored samples by adding 180 μL cold methanol to 20 μL maternal blood plasma. All samples were vortexed for 1 minute, incubated at 4°C for 20 minutes, and then centrifuged at 12,000 rpm for 20 minutes. The resulting supernatant was collected, dried in a SpeedVac evaporator (Thermo Fisher Scientific, Waltham, MA), and resuspended in 30 μL of 98:2 water:acetonitrile (98:2, v/v) upon instrumental analysis. Metabolomic analysis was performed by a liquid chromatography–mass spectroscopy system consisting of a Thermo Vanquish UHPLC coupled to a Thermo Q-Exactive high-resolution mass spectrometer interfaced with a heated electrospray ionization source and a hybrid quadrupole/orbitrap mass analyzer (Thermo Fisher Scientific, Waltham, MA). Mass spectroscopy-1 level full-scan profiling was operated at 70,000 full width at half maximum (FWHM) mass resolution, whereas mass spectroscopy-2 structural analysis was performed in a hybrid mode alternating between full-scan (at 70,000 FWHM) and parallel reaction monitoring (at 17,500 FWHM). Chromatographic separation was conducted using an Acquity ultraperformance liquid chromatography HSS T3 C18 column (2.1 × 100 mm, 1.8 μm) (Waters, Mil-ford, MA). Stringent quality assurance and quality control measures were performed throughout the analysis, including timely mass calibration and intermittent replicate quality control injections. All laboratory personnel were blinded to pregnancy outcome information.
The primary outcome was preterm birth between 16 0/7 and 36 6/7 weeks’ gestation. Bivariate analyses were conducted to compare preterm birth cases with those who delivered at term (≥37 0/7 weeks’ gestation) using Welch’s t test and Fisher’s exact test as appropriate. Bivariate analyses were conducted using STATA MP (version 15.1; StataCorp LLC, College Station, TX). Raw liquid chromatography/mass spectroscopy system data were converted to mzXML format using the Pro-teoWizard MS Convert program and processed in XCMS (Scripps, La Jolla, CA) for peak alignment and gap filling. A nontargeted analysis was used, in which a master list of all detected ion features aligning across all samples was obtained and the ion intensities were compared between preterm birth cases and term controls. Partial least squares discriminant analysis was performed in MetaboAnalyst 4.031 to determine the best linear regression model separating the observed peaks by preterm birth status. Multiple chemical characteristics were used, including accurate mass, isotopic abundance, retention time, and tandem mass spectrometry data to identify ion peaks of interest (P<.05 and fold change of ≥1.2) using MS-FINDER 3.14 (Riken, Wako, Japan). MS-FINDER uses a validated chemoinformatic workflow that incorporates conventional experimental spectral database search results combined with in silico structural dereplication based on bond energies, neutral loss, and hydrogen rearrangement rules. In addition, random forests were used to estimate how well the metabolomic profiles could distinguish preterm birth cases from term controls and to evaluate the relative contribution of each of the top 30 compounds contributing to the differentiation between preterm birth and term controls. Random forest analyses were performed using the random-Forest package of R (version 1.17.30).32 Finally, a MetaMapp network was constructed to obtain an integrated view of all identified compounds in the biochemical and chemical contexts, with clustering based on Kyoto Encyclopedia of Genes and Genomes biochemical pathways and chemical similarity (Tani-moto coefficient, >0.7).33 Post hoc power calculations were not performed because such analyses are often considered misleading by epidemiologists and statisticians.34
Results
From the original UNC PTB Biobank cohort of 271 patients, 42 patients were included in the current analysis. Of the other individuals enrolled in the biobank, 18 were lost to follow-up (missing gestational age at delivery), 12 were twin or triplet gestations, 101 were recruited in active labor, 42 did not have a plasma sample obtained during the study, 16 had their earliest plasma sample obtained at ≥28 weeks’ gestation, and 82 did not have vaginal swabs obtained. Of the 42 included patients, 25 (59.5%) delivered preterm at <37 weeks’ gestation at a median of 301/7 weeks’ gestation (interquartile range [IQR], 281/7–341/7 weeks); all preterm births were spontaneous. The 17 patients delivering at term delivered at a median of 386/7 weeks’ gestation (IQR, 373/7–391/7 weeks). Maternal blood was collected at a median of 191/7 weeks’ gestation (IQR, 151/7–224/7 weeks); the timing of blood collection did not differ by preterm birth status. Demographic and pregnancy characteristics were similar between preterm and term groups and are presented in Table 1.
TABLE 1.
Demographic/baseline/enrollment characteristics, by timing of delivery
| Characteristic | Preterm delivery (<370/7 wk) N=25 | Term delivery (≥370/7 wk) n=17 | P value |
|---|---|---|---|
| Maternal age, y, mean±standard deviation | 31.9±6.2 | 33.2±6.1 | .503 |
| Black race | 7 (28.0) | 6 (37.5) | .524 |
| Married | 15 (60.0) | 10 (58.8) | .939 |
| Smoked during pregnancy | 6 (24.0) | 1 (5.9) | .210 |
| Nulliparous | 5 (20.0) | 4 (23.5) | >.99 |
| Received 17-alpha hydroxyprogesterone caproatea | 12/18 (66.7) | 11/12 (91.7) | .193 |
| Diagnosed as having a short cervical length, <25 mm by endovaginal ultrasound before 24 wk gestationb | 13 (61.9) | 5 (35.7) | .129 |
| Shortest cervical length by endovaginal ultrasound before 24 wk gestation, median mm (interquartile range)b | 14 (3.6–28) | 30 (1.3–37) | .087 |
| Cervical cerclage present during current pregnancy | 19 (76.0) | 8 (50.0) | .087 |
| Received vaginal progesterone | 6 (24.0) | 5 (29.4) | .695 |
| Male fetus | 15 (60.0) | 7 (41.2) | .231 |
Data are expressed as number (percentage), unless specified.
Among 30 patients with at least 1 previous spontaneous preterm birth;
Among 37 patients with at least 1 endovaginal cervical length assessment between 16.0 and 24.0 weeks’ gestation.
A total of 812 molecular features differed between preterm birth cases and term controls with a minimum fold change of 1.2 and P<.05 (Figure 1). Among these altered features, 570 of 812 (70.1%) were found at higher abundances in preterm birth cases, whereas 242 of 812 (29.9%) were in higher abundance in term birth controls. The results derived from partial least square discriminant analysis model are shown in Figure 2. There was a distinct separation between case and control groups on the partial least squares discriminant analysis score plot, demonstrating the predictive capacity of the model to classify preterm birth cases and term controls. The cumulative R2Y (0.980) and Q2Y (0.846) values—metrics of model performance—were both close to the theoretical maximum of 1, indicating high predictive performance of the model. The final partial least squares discriminant analysis model underwent rigorous significance diagnostics to confirm validity, as follows. To ensure that overfitting of the model did not occur, random permutations (n=200) of the model were performed. Significant differences were seen between the final model and random permutation results (P=.005), confirming that the model had not been overfit. Outlier and inertia plot analyses were also evaluated and confirmed model integrity.
FIGURE 1. Total ion chromatogram metabolomic cloud plot.
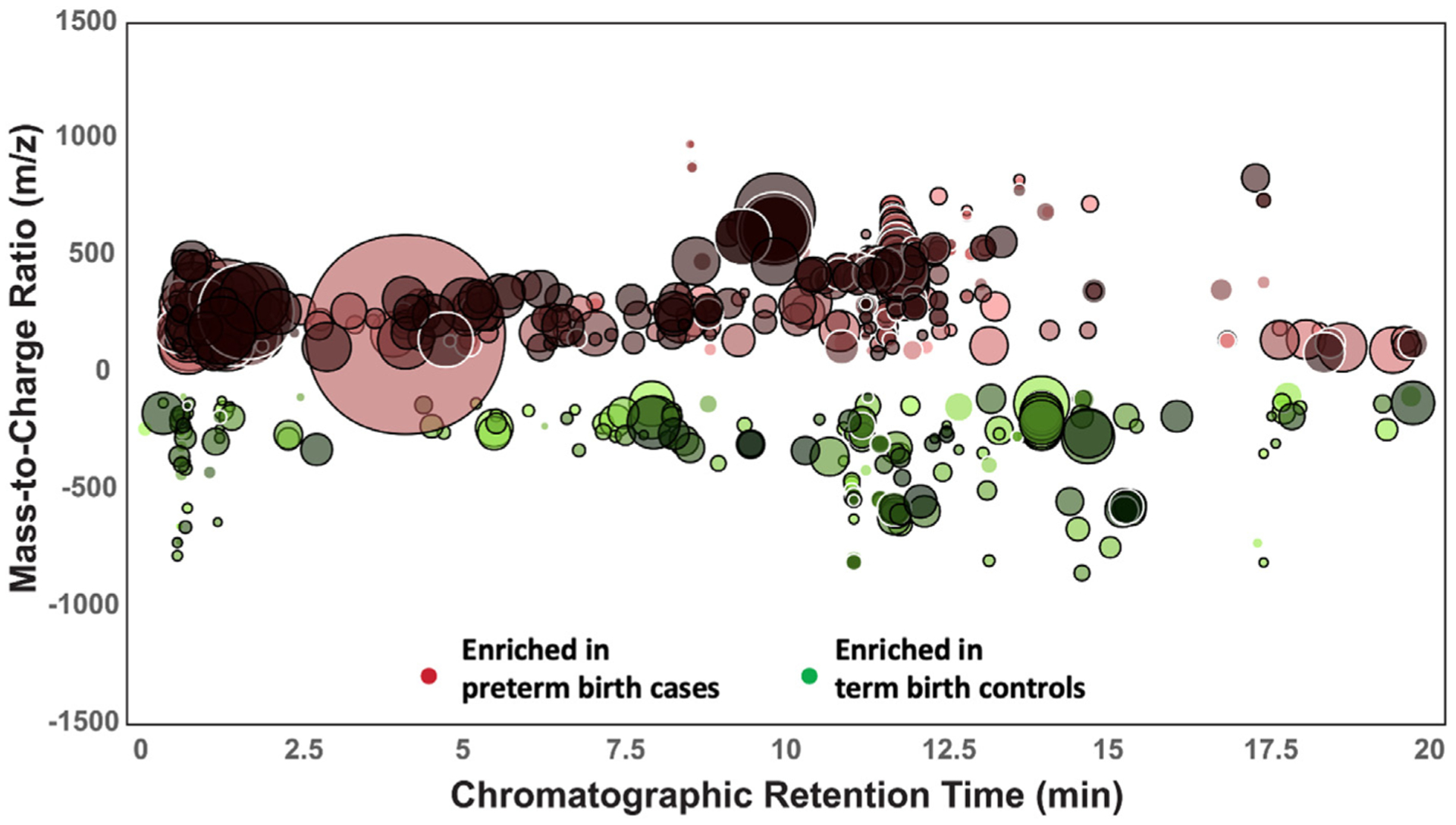
Showing 812 ion features differing in preterm birth cases and term birth controls (fold change, ≥1.2; P<.05). Mass-to-charge ratios (y-axis) are plotted over chromatographic retention time (x-axis). The radius of each circle indicates the relative scale of fold change comparing the 2 groups.
FIGURE 2. Partial least squares discriminant analysis results summary.
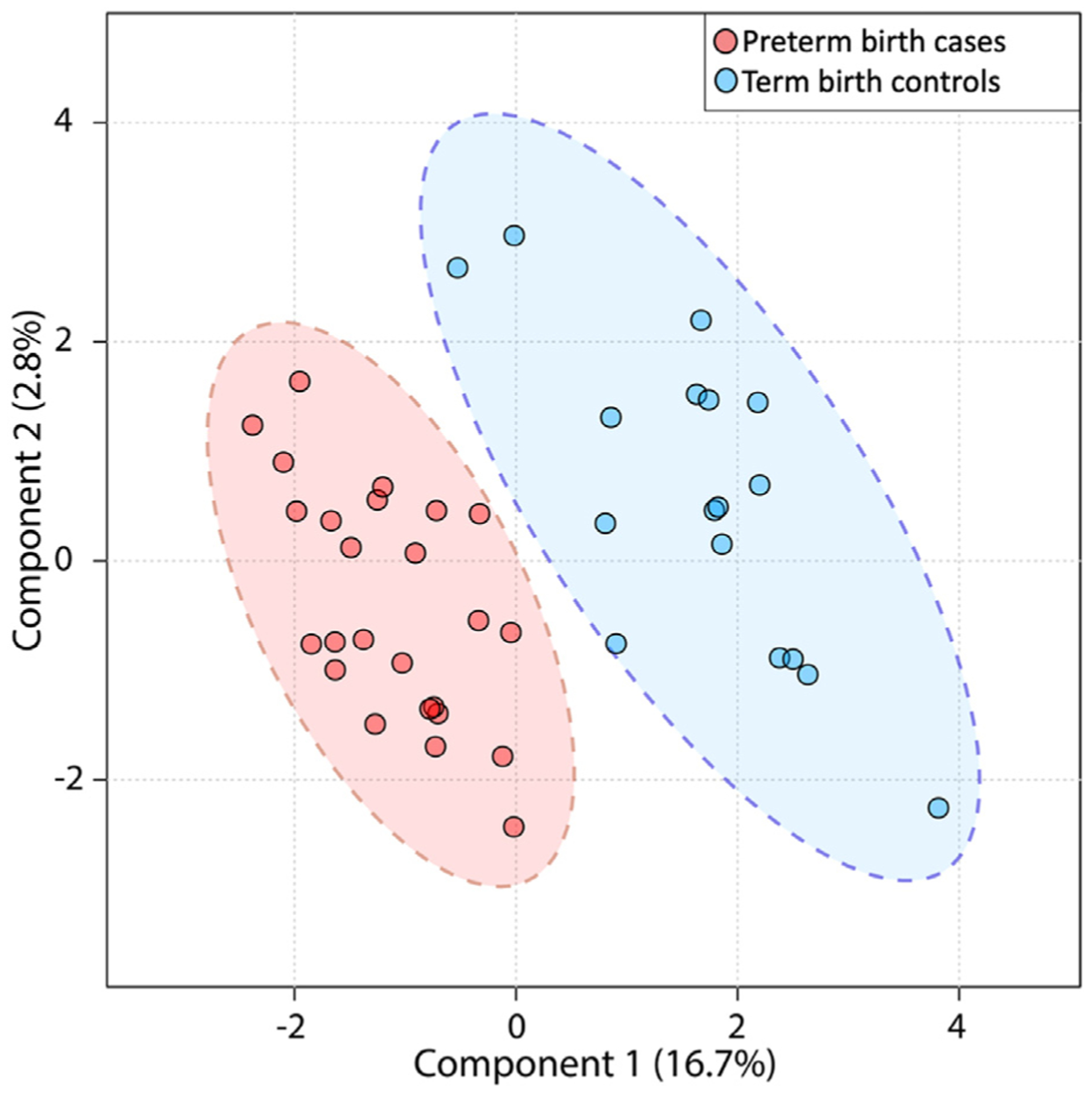
Demonstrating differentiation of preterm birth cases and term controls. Each dot represents 1 subject.
Next, using 4 dimensions of analytical information (accurate mass, isotopic ratios, tandem mass spectra, and chromatographic retention time), we sought to identify the compound structures for all molecular features of statistical difference between preterm birth cases and term controls. In total, MS-FINDER was able to identify 131 distinct compounds of the 812 that differed significantly in preterm birth cases compared with term controls. The identified compounds included 60 compounds involved with endogenous metabolic pathways (eg, lipid catabolism) and 67 compounds associated with exogenous exposures (eg, caffeine, food, drugs). An additional 4 compounds could be classified as either endogenous or exogenous. Of these, 84 of 131 molecular features (64%) were detected in higher concentrations among those with preterm birth, with a mean 1.74 fold change (range, 1.21–4.36). Of the 47 molecular features higher among those with term birth, the magnitude of change was similar to those that were higher among patients with preterm birth (mean, 1.74; range, 1.25–2.64).
Of the 60 endogenous metabolites differing by preterm birth case status, 39 (64%) are classified as lipids or are involved with lipid metabolism. Additional significant metabolites included amino acids and steroids or steroid-related molecules. The 15 most statistically significant endogenous metabolites are listed in Table 2 and include estrone and several other lipids. Of the 67 exogenous compounds that differed by preterm birth case status, the 15 most significant exogenous compounds that differed by preterm birth case status are listed in Table 3. These exogenous compounds include avocadyne, diosgenin, and 3 polycyclic aromatic hydrocarbons. Other notable compounds that were outside of the “top 15” but had P<.05 include the artificial sweetener aspartame (fold change, 1.92; P=.03), industrial manufacturing dye 4-nitroaniline-2-sulfonic acid (fold change, 1.71; P=.001), and caffeine (fold change, 1.22; P=.04), all present in higher levels in preterm birth cases. Furthermore, in addition to the polycyclic aromatic hydrocarbon derivative phenanthrene-4,5-dicarboxylate (higher in preterm birth cases) (Table 3), we also noted an association between 2 polycyclic aromatic hydrocarbon metabolites and preterm birth: N-hydroxy-2-acetamidofluorene (higher in preterm birth cases; fold change, 1.48; P=.030) and 3,3,6,6,8a-pentame-thyltetrahydro-1,8-dioxa-4a-azanaph-thalene (present in lower levels in preterm birth cases; fold change, 2.22; P=.03).
TABLE 2.
Top 15 endogenous compounds differing between preterm birth cases and term controls
| Structure | PubChem CID | Description | Fold change | Direction of change for preterm birth cases | P value |
|---|---|---|---|---|---|
| Biliverdin | 5,280,353 | Aryl hydrocarbon receptor ligand | 3.29 | Increased | .0004 |
| Dihydroceramide | 16,755,624 | Lipid - ceramide, sphingolipid metabolism | 1.83 | Decreased | .0008 |
| Gamma-Linoleolenyl carnitine | 53,477,819 | Lipid: acylcarnitine | 2.16 | Increased | .0001 |
| L-Alanine | 5950 | Amino acid and derivative | 1.42 | Increased | .0001 |
| Prolyl-alanine | 418,041 | Amino acid and derivative | 1.49 | Increased | .0002 |
| Estrone | 5870 | Lipid hormone | 1.92 | Increased | .003 |
| 3-hydroxylinoleoylcarnitine | 71,464,556 | Lipid: acylcarnitine | 1.97 | Increased | .003 |
| (11Z,14Z)-Eicosadienoylcarnitine | 71,464,509 | Lipid: acylcarnitine | 2.02 | Increased | .005 |
| 4α-formyl-4β-methyl-5α-cholesta-8,24-dien-3β-ol | 25,200,700 | 3-β-hydroxysterol intermediate in cholesterol synthesis | 1.96 | Increased | .005 |
| Oleoylcarnitine | 46,907,933 | Lipid: acylcarnitine | 1.96 | Increased | .005 |
| 20a,22b-Dihydroxycholesterol | 6,453,841 | Lipid: cholesterol | 1.89 | Increased | .005 |
| trans-Hexadec-2-enoyl carnitine | 53,477,817 | Lipid: acylcarnitine | 1.79 | Increased | .006 |
| S-(3-oxo-3-carboxy-n-propyl)cysteine | 21,252,272 | Cystathionine metabolite | 1.55 | Increased | .006 |
| Linoelaidyl carnitine | 53,477,834 | Lipid: acylcarnitine | 1.92 | Increased | .006 |
CID, Compound identification number.
TABLE 3.
Fifteen most statistically significant exogenous compounds differing between preterm birth cases and term controls
| Structure | PubChem CID | Description | Fold change | Direction of change for preterm birth cases | P value |
|---|---|---|---|---|---|
| 4-(3-Methoxy-4-hydroxy)phenyl-3-methyl-3-buten-2-one | 68,254,945 | Plants: bark of Machilus wangchiana | 1.28 | Increased | .0002 |
| Avocadyne | 3,015,189 | Food: avocado | 1.72 | Decreased | .0004 |
| (−)-Fumigaclavine B | 12,309,937 | Microbe: bacterial metabolite Mycotoxin from Aspergillus fumigatus and Rhizopus arrhizus | 2.38 | Increased | .0004 |
| Paracetamol sulfate | 83,939 | Drug: acetaminophen (sulfate metabolite formed in liver) | 2.68 | Increased | .0006 |
| (S)-Annocherine A | 22,297,560 | Food: fruits | 2.35 | Increased | .001 |
| Nigragillin | 15,939,563 | Microbe: fungal metabolite Aspergillus niger group, black mold | 1.87 | Increased | .002 |
| Camelledionol | 131,751,856 | Food: fats and oils | 2.13 | Increased | .002 |
| Tigecycline | 54,686,904 | Glycylcycline antibiotics | 1.35 | Increased | .002 |
| (E)-2-Octenal | 16,900 | Food: flavoring ingredients | 1.33 | Increased | .002 |
| Piperidine | 8082 | Food: barley, black pepper | 1.36 | Increased | .002 |
| Diosgenin | 99,474 | Food: yam | 1.42 | Decreased | .003 |
| (3R,3′R,6′R,9-cis) β, epsilon-carotene-3,3′-diol | 53,477,744 | aka lutein, food-derived pigment chemical commonly detected in human blood | 1.99 | Decreased | .003 |
| Phenanthrene-4,5-dicarboxylate | 95,075 | Polycyclic aromatic hydrocarbon derivative | 2.87 | Increased | .005 |
| (+)-Broussonetine P | 10,472,623 | Plant metabolite | 1.52 | Decreased | .005 |
| Canthaxanthin | 5,281,227 | Natural food metabolite | 2.06 | Decreased | .007 |
CID, Compound identification number.
In random forest analyses evaluating the relative contribution of each of the top 30 compounds in differentiating preterm birth and term controls, biliverdin, diosgenin, prolyl-alanine, (E)-2-octenal, and canthaxanthin were the most important compounds contributing to preterm birth classification, Figure 3. The random forest model accurately classified 21 of 25 preterm birth cases (84.0%) and 12 of 17 term controls (70.6%). Finally, the MetaMapp network analysis revealed that the significant metabolic features differing between preterm birth cases and term controls clustered within common groups, either by biochemical pathways or chemical similarity; these include lipids, steroids, benzene derivatives, and amino acid derivatives (Figure 4).
FIGURE 3. Random forest variable importance plot with top 30 compounds (y-axis).
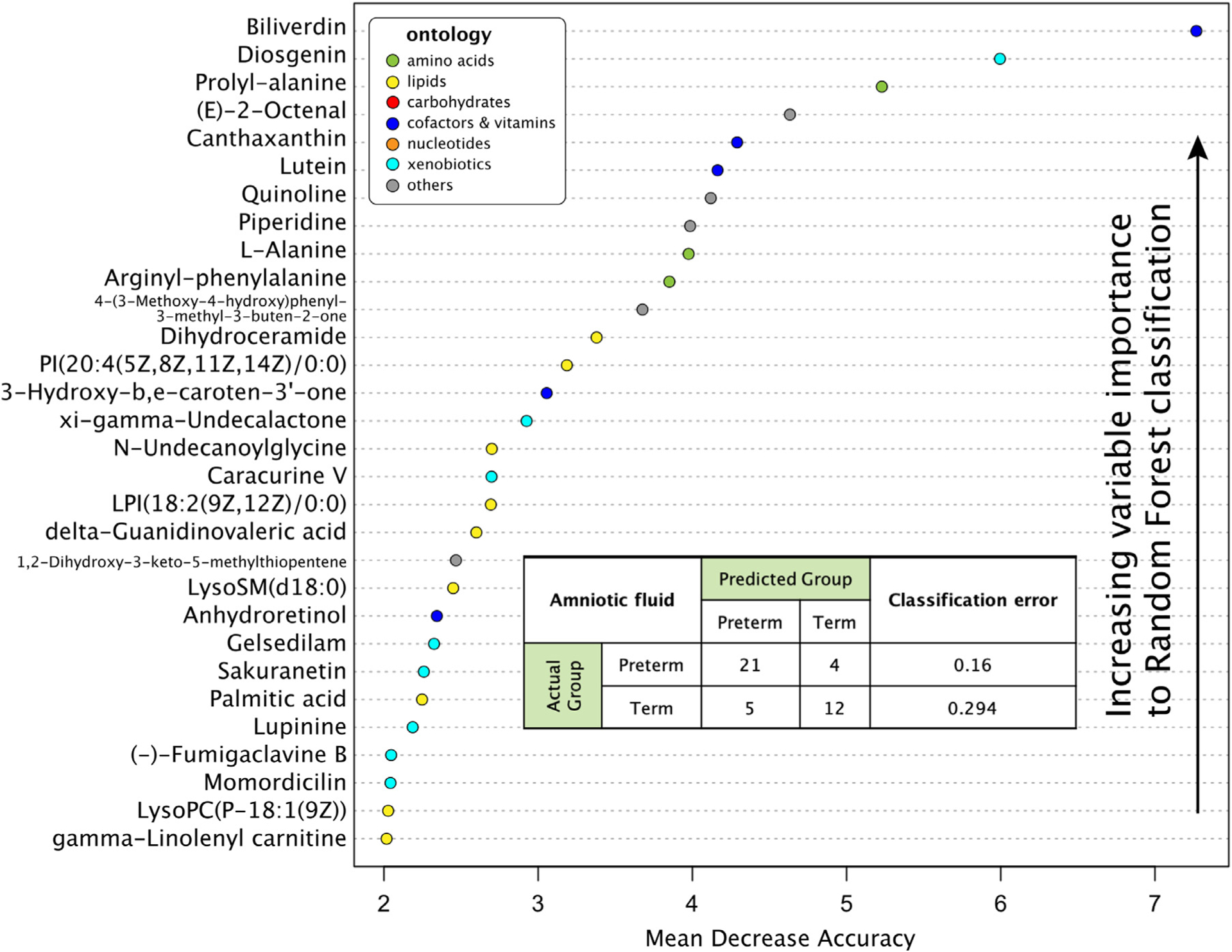
Contributing most to the accuracy of the random forest prediction model.
FIGURE 4. MetaMapp network.
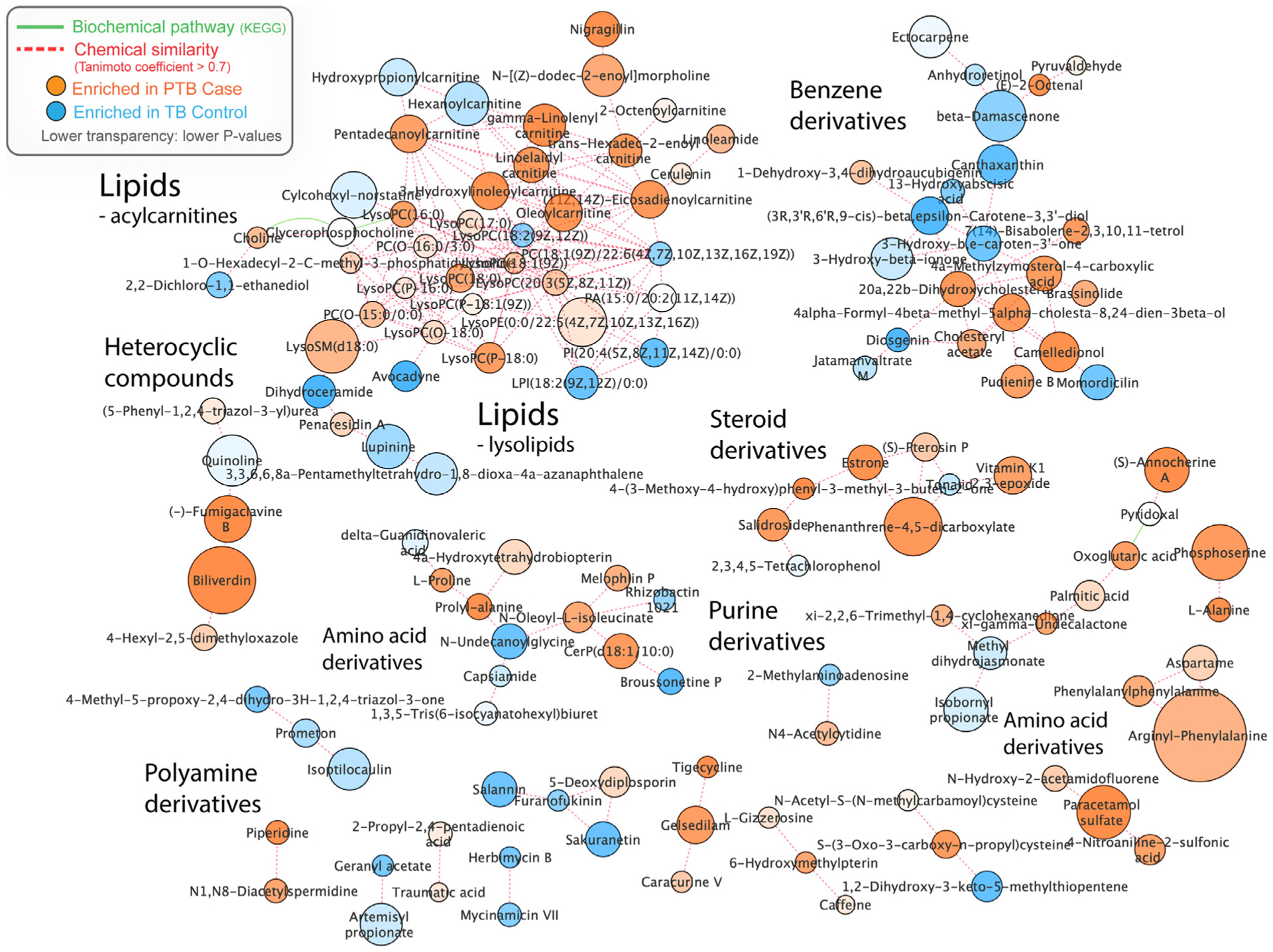
As an integrated view of significant group-differentiating compounds as clustered in biochemical and chemical contexts.
Discussion
Principal findings
We found multiple endogenous metabolites and exogenous exposures that differed in maternal plasma in the midtrimester among patients who ultimately delivered preterm compared with those who delivered at term. Importantly, these data highlight that both an individual’s intrinsic factors and external environmental exposures are associated with ultimate delivery gestational age.
Results in the context of previous literature
Although metabolomic findings are tissue specific,35 our findings are supportive of—and build upon—the current literature in this area. Several studies of amniotic fluid metabolites from patients undergoing amniocentesis to evaluate for intraamniotic infection in the setting of spontaneous preterm labor with intact membranes and those undergoing genetic amniocentesis have found that metabolites related to amino acids, liver function, and fatty acid metabolism display differential abundance from preterm birth cases and term controls.25,26,36,37 In one of the largest studies of maternal plasma metabolites and preterm birth, Lizewska et al38 evaluated metabolomic features in 57 patients with spontaneous preterm birth (idiopathic preterm labor or preterm prelabor rupture of membranes), 49 with threatened preterm labor but who delivered at term, and 25 who presented with spontaneous labor at term. Similar to our findings, they also detected significant differences in amino acids and those metabolites related to fatty acids when they stratified by gestational age at delivery (term vs preterm), although their cohort included only patients with plasma drawn later in pregnancy, at 24 to 36 weeks’ gestation (mean, 30–31 weeks for those with spontaneous preterm birth or threatened preterm labor). Our results detecting higher levels of biliverdin and lipids in those with preterm birth at <37 weeks’ gestation are consistent with the findings of this previous study.38 Our prospective study design expands upon those findings and differs in that we included patients with blood draws at an earlier and more consistent gestational age and only included those without clinical symptoms of preterm labor. It is important to note that patients enrolled in this cohort carried an extremely high a priori risk of preterm birth and 64% had a cervical cerclage placed during pregnancy. Cervical insufficiency lies on the continuum of the preterm birth spectrum, because many individuals with cervical insufficiency eventually develop evidence of overt spontaneous preterm labor. Ideally, in the future, analyses can be performed where metabolites from individuals with and without specific preterm birth phenotypes can be evaluated independently (eg, preterm premature rupture of the membranes, abruption, cervical insufficiency).
Research implications
Several of the endogenous metabolites we detected have biologic plausibility in the multifactorial process underlying spontaneous preterm birth or have previously been associated with the disorder. For example, levels of estrone in blood are known to increase before parturition in primate models, indicating an increase in fetal adrenal activity.39 Numerous other studies have found an association between maternal lipid profile and risk of preterm birth; most suggest dyslipidemia confers an elevated risk.40,41 In one recent population-based study from California, researchers found an 1.49 adjusted odds of preterm birth (95% confidence interval, 1.39–1.59) among patients with dyslipidemia.40 These are areas that warrant further study with additional basic science and translational studies to elucidate potential mechanisms by which dyslipidemia may impact prematurity risk.
Clinical implications
We also found that certain exogenous metabolites were lower among those who ultimately delivered preterm, and that many of these metabolites are related to substances consumed regularly by pregnant women (eg, foods and medications) and common environmental exposures. One of the strongest associations was with avocadyne (Table 3). Derived from avocado, avocadyne is an antioxidant compound rich in monosaturated fat and contains more than 20 vitamins including B vitamins, vitamin K, potassium, copper, vitamin E, vitamin C, and folate—nutrients that may positively influence pregnancy outcomes.42 Another notable finding was diosgenin, which was also identified to be present at lower levels in those delivering preterm (Table 3). Diosgenin originates from yams and is used for the commercial synthesis of steroids including cortisone, pregneno-lone, and progesterone and may also have antioxidant properties.43 It is unclear whether reduced “positive” or “healthy” exposures such as these have a direct effect on increasing the preterm birth risk or whether they are merely surrogates of other health behaviors. Nonetheless, these data support the findings of other studies that have reported that maternal diet—and intake of antioxidant rich foods—may be associated with a reduced risk of preterm birth.44,45 Finally, 3 significant findings were classified as polycyclic aromatic hydrocarbons; exposure to these substances occurs primarily through the ingestion of charbroiled foods and the inhalation of air contaminated after combustion of coal, oil, and gas; increased exposure to this class of pollutant has recently been associated with an elevated risk of preterm birth in several studies.9,11,46
Exposure to various drugs was also an important exogenous metabolite finding. Paracetamol sulfate is the sulfite metabolite of acetaminophen, formed in the liver, and was present at higher levels in patients who delivered preterm (fold change, 2.68; P=.00058). Acetaminophen is the most common analgesic used in pregnancy and in general is considered “low risk” to the pregnancy and fetus.47 However, these data add to other emerging data suggesting an associated between acetaminophen exposure and adverse birth outcomes. For example, in a cohort of 1200 patients in Ontario, self-reported acetaminophen use was associated with small for gestational age and low birthweight.48 Similarly, among 63,833 patients in the Danish National Birth Cohort with data regarding acetaminophen use during pregnancy, the use of acetaminophen was associated with an increased risk of preeclampsia and severe preeclampsia.49 However, it is unclear whether the exposure to acetaminophen is “causative” or whether the reason the pregnant woman took the acetaminophen (eg, for prodromal symptoms of preterm labor) may better explain these findings. From a clinical standpoint, if these findings are confirmed in future studies, they may provide key information regarding dietary, lifestyle, and medication recommendations to reduce the risk of prematurity.
Strengths and limitations
These data provide a robust, simultaneous assessment of both endogenous and exogenous compounds in midpregnancy. The evaluation of maternal blood in midpregnancy is simple and noninvasive and provides a “snapshot” of current metabolites and exposures. Our untargeted analysis was novel in that it permitted us to simultaneously evaluate both endogenous and exogenous metabolites and extend our evaluation beyond “typical” prematurity candidate exposures.
Nonetheless, these data should be interpreted with caution. “Standard” or “normal” levels for the majority of the metabolites studied have not been established. Furthermore, the findings of elevated levels in preterm birth cases compared with term controls were limited by the small sample size of the study and were likely cohort dependent, which may reduce generalizability of these results. Furthermore, these data provide a “snapshot” of metabolites present at a single time point, and it is unclear how much variation might occur with diet variation or medication use, including exogenous progestogen supplementation. This also limited our ability to evaluate the magnitude of the risk of preterm birth associated with each exposure. In addition, we are unable to determine whether the endogenous metabolites are evidence of a separate risk factor for preterm birth or alternatively and whether their detection resulted directly from exposure to one of the detected environmental exposures. Furthermore, levels of some endogenous metabolites—particularly those related to lipids—may vary with diet and the timing of the last meal in relation to the blood draw; unfortunately, dietary information and fasting status were not available. In addition, many of the features that differed on mass spectrometry were unable to be identified or resolved by current analytical techniques. As chemoinformatics and compound identification pipeline continue to improve, the proportion of compounds that are able to be accurately identified should increase in future analyses.
Conclusions
The identification of these endogenous metabolites and exogenous substances provides broad insight into the complex biologic and chemical milieu that pregnant patients are exposed to and has potential for important future clinical relevance. Validation of and further refinement of these data may provide the basis for future recommendations regarding avoidance of specific environmental contaminants or consumption and/or avoidance of specific foods during pregnancy to optimize outcomes. Although some exposures (eg, air pollution) may be difficult to modify, improvement in lipid profile through lifestyle changes and dietary modifications favoring antioxidant consumption is generally considered a minimal risk and can be easily implemented, providing a potentially novel therapeutic approach to prematurity prevention.
AJOG MFM at a Glance.
Why was this study conducted?
Exposure to environmental contaminants may be associated with preterm birth, but the substances that incur the greatest risks have not yet been elucidated. The objective of this study was to measure which endogenous metabolites and exogenous environmental substances differ in midtrimester maternal blood among patients who ultimately deliver preterm compared with those who deliver at term.
Key findings
A total of 812 molecular features differed between preterm birth cases and term controls. Small molecules or compounds represented by the molecular features differing between preterm birth cases and term controls ranged from those involved with endogenous metabolic pathways (including lipid catabolism, steroids, and steroid-related molecules) to exogenous exposures (including avocadyne, diosgenin, polycyclic aromatic hydrocarbons, acetaminophen metabolites, aspartame, and caffeine).
What does this add to what is known?
These data highlight that both an individual’s intrinsic factors and external environmental exposures are associated with ultimate delivery gestational age.
Acknowledgments
This study was funded, in part, by grant numbers R01-MD011609 and K24-ES031131 from the National Institute of Environmental Health Sciences.
Footnotes
The authors report no conflict of interest.
This study was presented, in part, in a poster format at the 66th annual scientific meeting of the Society for Reproductive Investigation, Paris, France, March 15, 2019.
References
- 1.Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013;10 (Suppl1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marret S, Ancel PY, Marpeau L, et al. Neonatal and 5-year outcomes after birth at 30–34 weeks of gestation. Obstet Gynecol 2007;110:72–80. [DOI] [PubMed] [Google Scholar]
- 3.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 2002;288:728–37. [DOI] [PubMed] [Google Scholar]
- 4.Wolke D, Eryigit-Madzwamuse S, Gutbrod T. Very preterm/very low birthweight infants’ attachment: infant and maternal characteristics. Arch Dis Child Fetal Neonatal Ed 2014;99:F70–5. [DOI] [PubMed] [Google Scholar]
- 5.Gotsch F, Gotsch F, Romero R, et al. The preterm parturition syndrome and its implications for understanding the biology, risk assessment, diagnosis, treatment and prevention of preterm birth. J Matern Fetal Neonatal Med 2009;22(Suppl2):5–23. [DOI] [PubMed] [Google Scholar]
- 6.Kramer MS, Papageorghiou A, Culhane J, et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol 2012;206:108–12. [DOI] [PubMed] [Google Scholar]
- 7.Schaaf JM, Liem SM, Mol BW, Abu-Hanna A, Ravelli AC. Ethnic and racial disparities in the risk of preterm birth: a systematic review and meta-analysis. Am J Perinatol 2013;30:433–50. [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep 2017;66:1. [PubMed] [Google Scholar]
- 9.Choi H, Rauh V, Garfinkel R, Tu Y, Perera FP. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect 2008;116:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padula AM, Yang W, Lurmann FW, Balmes J, Hammond SK, Shaw GM. Prenatal exposure to air pollution, maternal diabetes and preterm birth. Environ Res 2019;170:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B. Traffic-related air toxics and preterm birth: a population-based case-control study in los Angeles County, California. Environ Health 2011;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porpora MG, Resta S, Fuggetta E. Organochlorine pesticides exposure & preterm birth. Indian J Med Res 2016;143:685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyagi V, Mustafa MD, Sharma T, et al. Association of organochlorine pesticides with the mRNA expression of tumour necrosis factor-alpha (TNF-α) & cyclooxygenase-2 (COX-2) genes in idiopathic preterm birth. Indian J Med Res 2016;143:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling C, Liew Z, von Ehrenstein OS, et al. Prenatal exposure to ambient pesticides and preterm birth and term low birthweight in agricultural regions of California. Toxics 2018;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wai KM, Mar O, Kosaka S, Umemura M, Watanabe C. Prenatal heavy metal exposure and adverse birth outcomes in Myanmar: a birth-cohort study. Int J Environ Res Public Health 2017;14:1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Huo W, Zhang B, et al. Maternal urinary cadmium concentrations in relation to preterm birth in the Healthy Baby Cohort Study in China. Environ Int 2016;94:300–6. [DOI] [PubMed] [Google Scholar]
- 17.Kim SS, Meeker JD, Carroll R, et al. Urinary trace metals individually and in mixtures in association with preterm birth. Environ Int 2018;121:582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National birth cohort. Int J Environ Res Public Health 2018;15:1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagiv SK, Rifas-Shiman SL, Fleisch AF, et al. Early-pregnancy plasma concentrations of perfluoroalkyl substances and birth outcomes in project viva: confounded by pregnancy hemodynamics? Am J Epidemiol 2018; 187:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabatine MS, Liu E, Morrow DA, et al. Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation 2005;112: 3868–75. [DOI] [PubMed] [Google Scholar]
- 21.Tzoulaki I, Castagn[notdef]e R, Boulange[notdef] CL, et al. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur Heart J 2019;40:2883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghartey J, Bastek JA, Brown AG, Anglim L, Elovitz MA. Women with preterm birth have a distinct cervicovaginal metabolome. Am J Obstet Gynecol 2015;212. 776.e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghartey J, Anglim L, Romero J, Brown A, Elovitz MA. Women with symptomatic preterm birth have a distinct cervicovaginal metabolome. Am J Perinatol 2017;34:1078–83. [DOI] [PubMed] [Google Scholar]
- 24.Thomas MM, Sulek K, McKenzie EJ, et al. Metabolite profile of cervicovaginal fluids from early pregnancy is not predictive of spontaneous preterm birth. Int J Mol Sci 2015;16:27741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baraldi E, Giordano G, Stocchero M, et al. Untargeted metabolomic analysis of amniotic fluid in the prediction of preterm delivery and bronchopulmonary dysplasia. PLoS One 2016; 11:e0164211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon R, Jones J, Gunst PR, et al. Amniotic fluid metabolomic analysis in spontaneous preterm birth. Reprod Sci 2014;21:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virgiliou C, Gika HG, Witting M, et al. Amniotic fluid and maternal serum metabolic signatures in the second trimester associated with preterm delivery. J Proteome Res 2017;16:898–910. [DOI] [PubMed] [Google Scholar]
- 28.Jelliffe-Pawlowski LL, Norton ME, Baer RJ, Santos N, Rutherford GW. Gestational dating by metabolic profile at birth: a California cohort study. Am J Obstet Gynecol 2016;214. 511.e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Committee opinion no 611: method for estimating due date. Obstet Gynecol 2014; 124:863–6. [DOI] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr Protocols Bioinformatics 2019;68:e86. [DOI] [PubMed] [Google Scholar]
- 32.Liaw A, Wiener M. Classification and Regression by random Forest. R News 2002;2:18–22. [Google Scholar]
- 33.Barupal DK, Haldiya PK, Wohlgemuth G, et al. MetaMapp: mapping and visualizing metabolomic data by integrating information from biochemical pathways and chemical and mass spectral similarity. BMC Bioinformatics 2012;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine M, Ensom MH. Post hoc power analysis: an idea whose time has passed? Pharmacotherapy 2001;21:405–9. [DOI] [PubMed] [Google Scholar]
- 35.Fanos V, Atzori L, Makarenko K, Melis GB, Ferrazzi E. Metabolomics application in maternal-fetal medicine. BioMed Res Int 2013;2013 :720514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero R, Mazaki-Tovi S, Vaisbuch E, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med 2010;23: 1344–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Considine EC, Khashan AS, Kenny LC. Screening for preterm birth: potential for a metabolomics biomarker panel. Metabolites 2019;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lizewska B, Teul J, Kuc P, et al. Maternal plasma metabolomic profiles in spontaneous preterm birth: preliminary results. Mediators Inflamm 2018;2018:9362820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh SW, Stanczyk FZ, Novy MJ. Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab 1984;58:629–39. [DOI] [PubMed] [Google Scholar]
- 40.Smith CJ, Baer RJ, Oltman SP, et al. Maternal dyslipidemia and risk for preterm birth. PLoS One 2018;13:e0209579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang S, Jiang J, Xu H, et al. Maternal dyslipidemia during pregnancy may increase the risk of preterm birth: a meta-analysis. Taiwan J Obstet Gynecol 2017;56:9–15. [DOI] [PubMed] [Google Scholar]
- 42.Comerford KB, Ayoob KT, Murray RD, Atkinson SA. The role of avocados in maternal diets during the periconceptional period, pregnancy, and lactation. Nutrients 2016;8:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hua S, Li Y, Su L, Liu X. Diosgenin ameliorates gestational diabetes through inhibition of sterol regulatory element-binding protein-1. Biomed Pharmacother 2016;84:1460–5. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Zhao D, Mao X, Xia Y, Baker PN, Zhang H. Maternal dietary patterns and pregnancy outcome. Nutrients 2016;8:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin CL, Sotres-Alvarez D, Siega-Riz AM. Maternal dietary patterns during the second trimester are associated with preterm birth. J Nutr 2015;145:1857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vassilev ZP, Robson MG, Klotz JB. Associations of polycyclic organic matter in outdoor air with decreased birth weight: a pilot cross-sectional analysis. J Toxicol Environ Health A 2001;64:595–605. [DOI] [PubMed] [Google Scholar]
- 47.Black E, Khor KE, Kennedy D, et al. Medication use and pain management in pregnancy: a critical review. Pain Pract 2019;19:875–99. [DOI] [PubMed] [Google Scholar]
- 48.Arneja J, Hung RJ, Seeto RA, et al. Association between maternal acetaminophen use and adverse birth outcomes in a pregnancy and birth cohort. Pediatr Res 2020;87:1263–9. [DOI] [PubMed] [Google Scholar]
- 49.Rebordosa C, Zelop CM, Kogevinas M, Sørensen HT, Olsen J. Use of acetaminophen during pregnancy and risk of preeclampsia, hypertensive and vascular disorders: a birth cohort study. J Matern Fetal Neonatal Med 2010;23:371–8. [DOI] [PubMed] [Google Scholar]


