Abstract
Hypertensive brainstem hemorrhage (HBSH) is of high morbidity and mortality rate. But many clinical studies were written in Chinese and had not been reviewed. A systemic review of Chinese clinical studies for HBSH was performed. A systemic literature search in PubMed, Web of Science, China National Knowledge Infrastructure, and Weipu database and Wanfang database up to March 2020 was performed. Clinical control studies including a surgical evacuation (SE) group and a conservative management (CM) group were included. The clinical outcome and mortality rate were compared. Ten cohort studies were included, involving 944 participants (304 in the SE group and 640 in the CM group). All included patients were comatose, with the average age ranged from 45 to 65 years old. Among five studies using mRS or GOS as outcome score, a total of 16.6% (89/535) of patients achieve self-maintenance with minor disabilities, including 26.8% (34/127) in the SE group and 13.5% (55/408) in the CM group. The overall mortality rate in the SE group was 27.6%, ranged from 9.3 to 60% among different studies. The overall mortality rate in the CM group was 60.6%, ranged from 18.5 to 100.0%. Elder and comatose HBSH patients are not contraindicated for surgery. The review showed that this group of patients obtained a better outcome and lower mortality rate after surgical treatment. The quality of included studies was relatively low, but a high-level clinical study on HBSH is of great difficulty, as both clinicians and patients faced various sociological issues rather than pure medical problems.
Keywords: Brainstem hemorrhage, Surgery, Outcome, Mortality rate
Introduction
Brainstem hemorrhage (BSH) accounts for 5–10% of intracranial hemorrhage, with overall mortality rate ranged from 25 to 90% [4, 8, 21, 29, 32, 40]. It had been divided into two groups according to different pathophysiologic literature: hypertensive patients and normotensive patients. It was well known that normotensive hemorrhage is commonly caused by a cavernous malformation, which had a significantly better outcome [34, 36]. However, nearly 90% of patients with BSH were hypertensive [6]. Hitherto, there is still no consensus on whether hematoma evacuation improves the outcome of hypertensive brainstem hemorrhage (HBSH) patients after a hundred-year practice. The main reason is the lack of high-quality clinical control studies in the literature. Compared to English publications from other countries, there is a larger amount of clinical studies on HBSH published by Chinese academics. It is very important to share those researches worldwide to help to improve the clinical outcome of patients suffering from HBSH.
Material and methods
Search strategy
A systemic literature search in PubMed, Web of Science, China National Knowledge Infrastructure, and Weipu database and Wanfang database was performed according to Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (PRISMA).[31] The search was conducted in March 2020. The search term in PubMed was (“brainstem”[Title/Abstract] OR “pontine”[Title/Abstract] OR “medullary”[Title/Abstract]) AND (“hematoma”[Title/Abstract] OR “hemorrhage”[Title/Abstract]) AND (“surgery”[Title/Abstract] OR “approach”[Title/Abstract]). The references of included articles were also reviewed.
Study selection
Two authors (ZHENG and SHI) independently conducted the literature search. A study was included in this systematic review when the following criteria were met: (1) type of research: published randomized controlled trials (RCT) or non-randomized concurrent controlled trials (NRCCT) including a surgical evacuation (SE) group and a conservative management (CM) group. Of note, external ventricular drainage (EVD) was regarded as a life-saving procedure in CM; (2) studies carried out by tertiary hospitals in China. Studies with overlapping populations were excluded; (3) outcome and mortality rate in both the treatment group and control group were available. The outcome should be measured with a quantified scale; (4) full texts were available in English or Chinese. Exclusion criteria: studies included patients with a definite diagnosis of vascular malformation (cavernous malformation, etc.). The difference of opinion on study inclusion was solved by discussion. Disagreements were resolved through discussion with the third author (GONG). The search results were merged using Endnote X9, and the duplicate records were removed.
Methodological quality assessment
Two authors (ZHENG and SHI) independently assessed the full text of all included papers. Publication year, hospital level, patients’ baseline information, sample size, and type of surgery, outcome score, mortality rate, and follow-up period were collected. Patients’ outcomes and mortality rates were recorded at the end of follow-up.
The quality of NRCCT was assessed with the Methodological Index for Non-Randomized Studies (MINORS) guidelines [41]. The studies with a total score of ≥ 16 points were regarded as high quality, and vice versa. The quality of randomized controlled trial (RCT) was assessed using the Cochrane collaboration’s tool for assessing the risk of bias.
Various outcome scores were applied in different studies. A good outcome was defined if (1) GOS > 3[12, 17, 19], (2) mRS < 3[24, 39], (3) National Institute of Health stroke scale (NIHSS) decrease 45% after treatment[5, 22, 37], (4) Barthel index > 40[50] or > 60[26].
No meta-analysis was performed to obtain pooled estimates because the studies were heterogenous according to the MINORS and Cochrane evaluations. Statistical analyses of categorical variables were carried out with chi-square tests or Fisher’s exact tests using SPSS 23.0 (Chicago, IL, USA).
Results
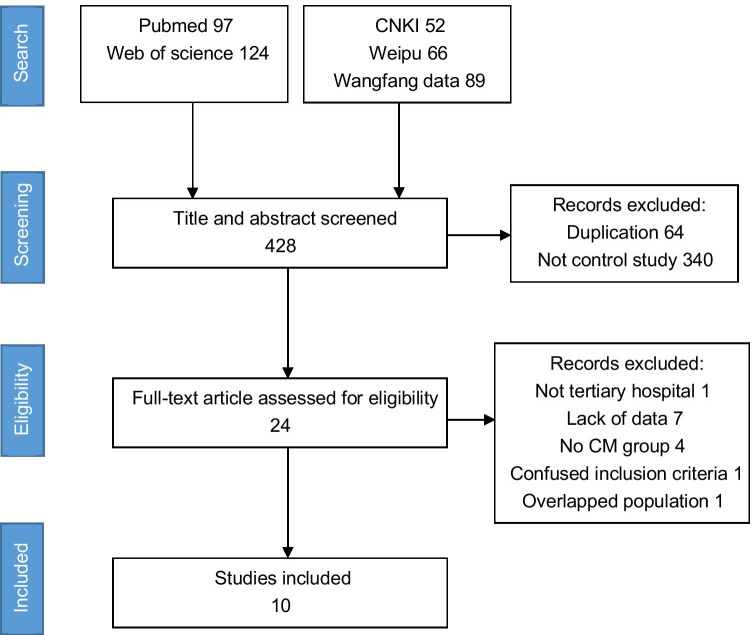
Search results. The initial search obtained 428 articles, including 207 articles in Chinese and 221 articles in other languages. There were 64 studies excluded because of duplication. After reading the title and abstracts, a total of 340 studies were excluded due to inappropriate article types. Fourteen studies were excluded after the full-text review. Reasons for exclusion were as follows: lack of data on outcome score and mortality[7, 15, 16, 18, 40, 48], no CM group[9, 38], not tertiary hospital[25], no enough follow-up duration[46], lack of patient inclusion criteria[3], only external ventricular drainage for treatment group[49, 51], overlapped patients population[10]. The PRISMA flow diagram was described (Fig. 1).
Fig. 1.
The PRISMA flow diagram
Ten studies were selected for systemic analysis, including 2 RCTs[12, 17] and 8 NRCCTs [5, 19, 22, 24, 26, 33, 39, 50] (Table 1). The sample size ranged from 21 to 326, including 304 surgically treated patients and 640 conservatively treat with/without EVD patients, comprising 944 participants. Most included patients were comatose (GCS ≤ 8 in seven studies), with the average age ranging from 45 to 65 years old (average age was not reported in one study). Excluding three studies that did not report hemorrhage location precisely, the remaining seven studies report 164 (20.5%), 584 (73.1%), and 51 (6.4%) cases of mesencephalic, pontine, and medullary HBSH, respectively.
Table 1.
Overviews of the ten studies included in the review
| Author | Year | Design | Mean age (years) | History of hypertension | Preoperative angiography | Patient inclusion | Hemorrhage location (n) | Mean HV (ml) | Follow-up (months) | MINORS/Cochrane | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MES | PON | MED | ||||||||||
| ZHANG SW | 2019 | NRCCT | 50.5 | 47 (85.5%) | No |
GCS 3–8 HV > 5 ml |
9 | 45 | 1 | 8.4 ± 1.5 | 3 | 16 |
| LAN ZG | 2019 | NRCCT | 51.5 | All | Yes | GCS 3–8 | 54 | 208 | 24 | 8.7 ± 0.5 | 6 | 20 |
| LV CL | 2019 | NRCCT | NM | All | No | GCS 3–7 | 37 | 71 | 0 | 11.3 | 3 | 16 |
| ZHANG YQ | 2017 | NRCCT | 47.1 ± 8.4 | All | Yes |
GCS 3–8 HV ≥ 5 ml |
43 | 14 | 8 | 8.1 ± 1.4 | 3 | 17 |
| HUANG KY | 2016 | NRCCT | 48.5 ± 5.1 | All | No |
Comatose HV 5–10 ml |
NM | NM | 12 | 15 | ||
| ZONG L | 2016 | NRCCT | 58.9 ± 6.3 | All 8 surgery patients | No |
GCS 3–5 HV 5–20 ml |
40 | 0 | NM | 3 | 13 | |
| CHEN QM | 2015 | NRCCT | 52.5 | All | No | GCS 3–8 | NM | NM | 3 | 15 | ||
| KU HB | 2014 | RCT | 65.2 | 115 (84.6%) | No |
GCS 3–10 HV 5–20 ml |
0 | 136 | 0 | 11.8 | 1 | High risk |
| WANG J | 2014 | NRCCT | 54.5 | All | No | GCS 3–8 | 21 | 89 | 18 | 8.0 | 3 | 16 |
| HUANG JL | 2010 | RCT | 57 | All | No |
GCS 3–8 HV ≥ 7 ml |
0 | 21 | 0 | 11.5 | 6 | High risk |
GOS Glasgow Outcome Scale, GCS Glasgow Coma Scale, HV hematoma volume, MED medullary, MES mesencephalic, NRCCT non-randomized concurrent controlled trials, NM not mention, mRS modified Rankin scale, PON pontine, RCT randomized controlled trials
Characteristics and quality of included studies
Study quality of NRCCTs ranged from 13 to 20 out of 24 on the MINORS Scale. Three studies had a MINORS score less than 16 and were regarded as “low-quality.” Both two RCTs included were regarded as “high risk of bias” according to the Cochrane tool. All included studies stated that the baseline of SE and CM groups were comparable, but only 6 studies provided the statistical valve (P > 0.05). All studies reported adequate follow-up time without patient loss.
As the quality of included articles was low, a meta-analysis was waived.
Overall outcome
The outcome scores were obtained from all ten studies. The result of five studies supported that patients who underwent SE having a better outcome (Table 2). Among five studies using mRS or GOS as outcome score, a total of 16.6% (89/535) of patients achieve self-maintenance with minor disabilities, including 26.8% (34/127) in the SE group and 13.5% (55/408) in the CM group. The follow-up period was 3 to 6 months in those studies.
Table 2.
Patient outcome and complications compared in ten studies
| Author | Year | Treatment | EVD in CM group | Outcome assessment | Good outcome | P value | Mortality rate | P value | Follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Control | Surgery | Control | ||||||||
| ZHANG SW | 2019 | Stereotactic aspiration | Yes | mRS < 3 |
7/19 36.8% |
6/36 16.7% |
0.094 |
4/19 21.1% |
16/36 44.4% |
0.086 | 3 |
| LAN ZG | 2019 | Microscope | No | mRS < 3 |
11/46 23.9% |
45/240 18.8% |
0.419 |
14/46 30.4% |
169/240 70.4% |
< 0.001 | 6 |
| LV CL | 2019 | Stereotactic aspiration | No | NIHSS ↓45% |
25/43 58.1% |
20/65 30.8% |
0.005 |
4/43 9.3% |
12/65 18.5% |
0.190 | 3 |
| ZHANG YQ | 2017 | Aspiration | No | Barthel index > 40 |
8/32 25.0% |
1/33 3.0% |
0.010 |
18/32 56.3% |
27/33 81.8% |
0.026 | 3 |
| HUANG KY | 2016 | Microscope | YES | NIHSS ↓45% |
17/30 56.7% |
12/30 40.0% |
0.196 |
4/30 13.3% |
11/30 36.7% |
0.037 | 12 |
| ZONG L | 2016 | Microscope | No | Barthel index > 60 |
4/8 50.0% |
11/32 34.4% |
0.414 |
2/8 25.0% |
13/32 40.6% |
0.414 | 3 |
| CHEN QM | 2015 | Microscope | No | GOS > 3 |
1/15 6.7% |
0/30 0% |
0.333* |
9/15 60.0% |
29/30 96.7% |
0.001 | 3 |
| KU HB | 2014 | Stereotactic aspiration | Yes | NIHSS ↓45% |
25/64 39.0% |
9/72 12.5% |
< 0.001 |
13/64 30.3% |
42/72 58.3% |
< 0.001 | 1 |
| WANG J | 2014 | Microscope | No | GOS > 3 |
10/37 27.2% |
4/91 4.4% |
< 0.001 |
14/37 37.8% |
58/91 63.7% |
0.007 | 3 |
| HUANG JL | 2010 | Stereotactic aspiration | No | GOS > 3 |
5/10 50.0% |
0/11 0% |
0.012* |
2/10 20.0% |
11/11 100.0% |
< 0.001* | 6 |
CM conservative treatment, EVD external ventricular drainage, GOS Glasgow Outcome Scale, mRS modified Rankin scale, NIHSS National Institute of Health stroke scale, *Fisher’s exact test, bold type indicates significant difference (P < 0.05)
Overall mortality rate
The mortality rates were obtained from all ten studies. The result of seven studies showed a significantly lower mortality rate in the SE group (Table 2). The overall mortality rate for all patients was 50.0% (472/944). The overall mortality rate in the SE group was 27.6%, ranged from 9.3 to 60% among different studies. The overall mortality rate in the CM group was 60.6%, ranged from 18.5 to 100.0%. The follow-up period was 1 to 12 months.
Overall complications
Only four studies reported complications in HBSH patients (Table 3). LV et al. observed a higher occurrence rate of pneumonia in the CM group (P = 0.007). Huang et al. showed that hydrocephalus significantly increased in the CM group (P < 0.001).
Table 3.
Major complications reported by four studies
| Author | Year | Pneumonia | P value | Stress ulcer | P value | Renal failure | P value | Others | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Control | Surgery | Control | Surgery | Control | Surgery | Control | |||||
| LAN ZG | 2019 | 14/46 | 89/240 | 0.390 | 12/46 | 57/240 | 0.734 | NM | NM | NM | NM | |
| LV CL | 2019 | 1/43 | 13/65 | 0.007 | 5/43 | 5/65 | 0.754 | 2/43 | 6/65 | 0.607 | NM | NM |
| WANG J | 2014 | 6/128 | 3/128 | 12/128 | Respiratory failure 47/128 | |||||||
| HUANG JL | 2010 | 1/10 | 2/11 | 1.000 | 1/10 | 2/11 | 1.000 | 1/10 | 3/11 | 0.652 |
Hydrocephalus 1/10 Hepatic failure 1/10 MODS 1/10 |
Hydrocephalus 10/11 Hepatic failure 1/11 MODS 1/11 |
MODS multiple organ dysfunction syndrome, NM not mention, bold type indicates significant difference (P < 0.05)
Discussion
Recently, there has been a strong tendency for surgically treating HBSH in China. A total of 20 clinical studies were published in Chinese during the last 3 years. Comparing to worldwide studies in the same period, merely several cases were reported by Japan[14]. Western countries started operating HBSH in the early 1900s[29]. However, due to the low incidence rate, only small single-institution series were available, which were generally with regional and investigator biases[8, 23]. The east and southeast Asian population had a two times higher incidence rate for intracranial hemorrhage compared to other populations.[43] Together with the improvement in medical service in China over the past 20 years, more HBSH patients are willing to pursue better management nowadays.
Chinese reports consist of a larger sample size compared to other countries, but are mostly written in Chinese. This is the reason why we decided to run a systemic review in English for Chinese papers. Another reason is that different health care systems and cultures dictate health care decision-making for both patients’ families and clinicians [35]. A study exclusively for one country can avoid certain non-medical biases.
Surgery or conservative treatment?
This question has been studied for a hundred years, yet a uniform consensus has not been met. Arseni et al. distinct two groups of BSH and its terminology: hematoma and hemorrhage [1]. He defined that a “hemorrhage” was “diffuse and dilaceration,” and a “hematoma” was “localized.” Only patients with “brain stem hematoma” require surgery as the hematoma will evolve into an intracranial space-occupying lesion. Some surgeons believed that there is no role of surgery in primarily comatose patients having HBSH because of uniformly disastrous outcome.[36]
In this review, we focus on clinical studies comparing SE and CM in HBSH patients. The included patients were generally elder, most of them had a history of hypertension. More importantly, all studies only operated on patients who appear comatose (GCS < 10), representing the typical patients whom other countries’ clinicians recommend not to operate on.
Five studies showed that the SE group had a superior outcome to the CM group. Among them, four studies underwent hematoma aspiration and one was microscopic surgery. Another five studies also trended toward surgical treatment but did not achieve statistical significance.
As alluded to earlier, the mortality rate of BSH varies greatly in literature. Our review showed that the mortality in the SE group was approaching the minimum level of literature reported. Most included studies suggested that the survival rate was much higher in the SE group. We also found a slightly lower overall mortality rate in the hematoma aspiration group than the microscopic surgery group (24.4% versus 31.6%, P = 0.162). This indicated that hematoma aspiration may be suitable for older patients in this review because it was of less surgical trauma and shorter operation time. However, the major complications such as rehemorrhage rate were only mentioned in one study.[12]
The mortality rate of the CM group was on the average level reported in the literature. Of note, only three studies claimed that they would perform EVD as a life-saving procedure in the CM group. The mortality rate in these three studies was 36.7%, 44.4%, and 58.3%, which were slightly lower than average mortality in the pool. Absent of EVD when necessary was thought to increase the mortality rate in the CM group.
Patient selection: alert or comatose?
It had been replicated by many researchers that patients with a lower conscious level would have a worse outcome, no matter surgery or not [2, 13, 20, 21, 30, 36]. The question of why Chinese surgeons are willing to operate on comatose patients is in fact not a medical but a sociological problem.
As the mean age of patients was 50 to 60 years old in the included studies, the treatment plan was mostly decided by patients’ spouses and children. In traditional Chinese families, the concept of “filial piety” played a unique and positive role in family associations.[42] However, when it comes to the decision on the treatment plan for life-threatening diseases such as HBSH, even though a dismal prognosis was carefully informed, Chinese families were more likely to choose surgery. Because receiving conservative treatment may represent “palliative care” or “giving up.”[27]
Another very important aspect is the tense doctor-patient relationship in China. Chinese doctors are very likely to expose to violent confrontations if the patients and their families were unsatisfied.[44] Under this circumstance, the risk of operating an alert patient obviously overpass to that of a comatose one, especially when there was not an official guideline or a book-written indication for the disease. The surgeon would readily lose in a lawsuit, even he/she did only what the patient’s family asked.
The most “safety” manner is that the surgeons manage all the HBSH patients conservatively. But here they face the policy of the Chinese health care system. Most provinces in China now implement the “Per case for simple illness” or “Per case for complex conditions” payment system[47]. A fixed fee for each disease was set based on the average medical expense in the local city. For example, the “conservative treatment fee” for brainstem hemorrhage is 36612.0 CNY in our distinct, and it doubles to 77,436.0 CNY if the patient underwent surgical treatment. The age of the patient, the location and amount of the hematoma, and the complications followed by the primary disease were not on the consideration of this system. The department must be responsible for its own profits and losses while considering the treatment plan for patients. A patient with HBSH may easily develop pneumonia during conservative treatment, and he/she has to be on a ventilator in an intensive care unit (ICU) for a long period. If so, he/she will definitely cost more than the health care system covered. In this circumstance, the doctor may prefer the surgical treatment, which provides an extra medical fee and a potential possibility for the patient leaving ICU earlier.
As a result, the decision of Chinese surgeon to operate comatose patients was largely economic and sociological considerations, rather than medical necessity. But it gave us the chance to observe the clinical outcome in these patients, and the result was encouraging. Depressed mental status in HBSH patients is attributed to the destruction of the reticular formation and acute obstructing hydrocephalus. The former one acts immediately after the onset, and its delayed perifocal edema may lead to aggravation of neurological deficits. While the neurological damage is irreversible, hematoma evacuation can prevent secondary damage from the hematoma [1, 2]. It showed that 36% of HBSH patients would develop hydrocephalus initially.[24] Acute hydrocephalus is caused by the direct oppression of the hematoma or blockage of intraventricular hemorrhage. It is manageable and reversible but makes patients look worse than they really are. One RCT study[17] showed that 91% of patients in the CM group without EVD eventually developed hydrocephalus, contributing to a 100% mortality in the group. Aggressively managing hydrocephalus will remarkably improve the patient’s outcome.
Barriers in HBSH clinical study
Above we have seen the charming outcome from surgically treated HBSH. In fact, we are still far away from achieving high-quality evidence. It is noteworthy that all included studies strongly supported the same conclusion: the SE group was superior to the CM group over patients’ outcome or mortality rate. This implies a typical publication bias, which is likely attributed to the well-known Cash-per-publication Reward Policy[45] in China. As studies with positive results are much easier to be accepted, they are more profitable. Besides monetary rewards, clinical doctors require a certain amount of papers or impact factors for promotion. Both “fame and gain” lead to inevitable publication bias in Chinese articles. This explains why studies reporting negative results are extremely sparse in China.
Only four studies reported systemic complications between SE and CM groups (Table 3). However, complications vary greatly in HBSH patients. For example, patients having no spontaneous respiration will bear a higher risk of pneumonia on long-term ventilator usage. It is idle to compare complications irrespective of patients’ general condition on admission and hematoma’s location and volume. While the inclusion criteria of the included studies were not uniform, a statistical analysis was not performed.
When referred to the mortality rate, families’ decision on the treatment plan is critical. Only two studies clearly stated that “patients whose families decided giving up treatment were excluded”[17, 50], and one study[24] stated that “patients whose families refused to have surgery were served as the control group.” This resulted in significant selection bias for further analysis because (1) family refused to have surgery may imply that they were unable or unwilling to financially support the advanced treatment; (2) these patients may receive a relatively negative treatment, or “futile treatment”[11], from the aspect of medical staff. These will increase the mortality rate in the CM group.
The ideal approach is to conduct a multi-center RCT, but one will face an ethical issue as the 2016 International ethical guidelines for health‐related research involving humans states had pointed out: “clinical trials in contexts of high‐mortality diseases are morally suspect because equipoise does not exist between a standard of care that offers little prospect of clinical benefit and an unvalidated medical intervention that might offer some clinical advantage”[28]. This perfectly describes the situation in HBSH. As the patients in severe hemorrhage would ultimately die in conservative treatment, surgery which has successful reports is worth attempting.
Limitations
The limitations of this study are massive. The quality of the included studies was low-moderate, and the follow-up period of seven studies was less than 6 months, which hinder the production of reliable results.
Although most of the patients are proven to have hypertension in admission, only two included studies performed angiography prior to the treatment. A total of 60 patients (6.4%) in the pooled data neither have a history of hypertension nor underwent angiography. There is no guarantee that some cases of brainstem cavernous malformation would mix in the review.
BSH had been divided into four groups based on different morphology and outcome, namely “massive type,” “bilateral tegmental type,” “basal tegmental type,” and “small unilateral tegmental type.” Among them, only small unilateral tegmental type has a superior survival rate than other types (94.1% versus 18.2%) [4]. It was a pity that none of the studies carried out such a classification system. All ten studies classified hemorrhage by anatomical location (medullary, pontine and mesencephalic, etc.), and not all of them reported the outcome and mortality accordingly. This makes it impossible to furtherly discuss with different subgroups.
Conclusion
Our findings ran contrary to the theory that “old, hypertensive, and comatose” patients were not the candidates for surgery. A high-level clinical study on HBSH is of great difficulty, as patients’ outcome greatly depends on the location and volume of the hematoma, while excessive grouping makes it hard to achieve enough sample size for analysis. The sociological problems both the clinicians and patients’ families faced also impede us to conduct an objective and strict clinical research. Also, based on the policies for doctor promotion and publication reward, this kind of long-period and delicate study is unlikely to appear in China nowadays.
Author contribution
Zheng and Shi independently assessed the full text of all included papers. Gong made the final decision for which study would be included. Zheng composed the paper.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
This study is approved by the Beijing Tiantan Hospital Institutional Review Board.
Consent to participate
All clinical data are collected in the cited literatures.
Consent to publication
All authors are consent to publish in Neurosurgical Review.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arseni C, Ionescu S, Maretsis M, Ghitescu M. Primary intraparenchymatous hematomas. J Neurosurg. 1967;27(3):207–215. doi: 10.3171/jns.1967.27.3.0207. [DOI] [PubMed] [Google Scholar]
- 2.Balci K, Asil T, Kerimoglu M, Celik Y, Utku U. Clinical and neuroradiological predictors of mortality in patients with primary pontine hemorrhage. Clin Neurol Neurosurg. 2005;108(1):36–39. doi: 10.1016/j.clineuro.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Chao M, Xianhou Y, Pucha J, Weiguo C, Tao W (2004) Surgical treatment in brainstem hemorrhage. Cent Chin Med J 28(04):255–256
- 4.Chung CS, Park CH. Primary pontine hemorrhage: a new CT classification. Neurology. 1992;42(4):830–834. doi: 10.1212/wnl.42.4.830. [DOI] [PubMed] [Google Scholar]
- 5.Chunlei L, Deyun D, Lijun S, Zhaofeng H. Stereotactic aspiration by supratentorial approach for patients having brainstem hemorrhage. China Health Care Nutr. 2019;29(5):260–261. doi: 10.3969/j.issn.1004-7484.2019.05.338. [DOI] [Google Scholar]
- 6.Cole FM, Yates PO. The occurrence and significance of intracerebral micro-aneurysms. J Pathol Bacteriol. 1967;93(2):393–411. doi: 10.1002/path.1700930202. [DOI] [PubMed] [Google Scholar]
- 7.Derong X, Yuanfu T, Zhongyong D. The factors influencing prognosis on patients with hypertensive brainstem hemorrhage. Acta Medicinae Sinica. 2019;03:38–42. [Google Scholar]
- 8.Haines SJ, Mollman HD. Primary pontine hemorrhagic events. Hemorrhage or hematoma? Surgical or conservative management? Neurosurg Clin N Am. 1993;4(3):481–495. doi: 10.1016/S1042-3680(18)30572-2. [DOI] [PubMed] [Google Scholar]
- 9.Haixiao G, Weimin Z, Zhensheng X (2015) Comparsion of subtemporal approach and suboccipital approach under stereotactic navigation for brainstem hemorrhage. J Hebei Med Univ 36(5):579–581
- 10.Hao L (2013) The treatment opinion in hypertensive brainstem hemorrhage. Chin J Neurosurg 29(4):339–341
- 11.Hardwig J. Families and futility: forestalling demands for futile treatment. J Clin Ethics. 2005;16(4):335–344. [PubMed] [Google Scholar]
- 12.Hongbin K (2014) Application of temporal approach stereotactic surgery in elderly severe pontine hemorrhage patients. Chin J Geriatr Heart Brain Vessel Dis 16(03):291–294
- 13.Huang K, Ji Z, Sun L, Gao X, Lin S, Liu T, Xie S, Zhang Q, Xian W, Zhou S, Gu Y, Wu Y, Wang S, Lin Z, Pan S. Development and validation of a grading scale for primary pontine hemorrhage. Stroke. 2017;48(1):63–69. doi: 10.1161/STROKEAHA.116.015326. [DOI] [PubMed] [Google Scholar]
- 14.Ichimura S, Bertalanffy H, Nakaya M, Mochizuki Y, Moriwaki G, Sakamoto R, Fukuchi M, Fujii K. Surgical treatment for primary brainstem hemorrhage to improve postoperative functional outcomes. World Neurosurg. 2018;120:e1289–e1294. doi: 10.1016/j.wneu.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 15.Jang JH, Song YG, Kim YZ. Predictors of 30-day mortality and 90-day functional recovery after primary pontine hemorrhage. J Korean Med Sci. 2011;26(1):100–107. doi: 10.3346/jkms.2011.26.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jian L, Jing Z (2017) Microsurgery technique in hypertensive brainstem hemorrhage. Chin J Neurosurg 33(2):184–185
- 17.Jianlong H, Yunhui L, Zhongping L, Dan J, Haibiao L, Weichao W, Weiye L, Dongxiang Z (2010) Microscopic surgery under stereotactic navigation for intensive brainstem hemorrhage. J Guangzhou Univ Tradit Chinese Med 27(05):445–448
- 18.Jinpeng G (2016) The outcome of surgically treated hypertensive pontine hemorrhage. Contemp Med Symp 14(08):139–140
- 19.Jun W (2010) Surgical treatment for hypertensive brainstem hemorrhage for 37 cases. Chin J Stereotact Funct Neurosur 23(3):171–172
- 20.Jung DS, Jeon BC, Park YS, Oh HS, Chun TS, Kim NK. The predictors of survival and functional outcome in patients with pontine hemorrhage. J Korean Neurosurg Soc. 2007;41(2):82–87. [Google Scholar]
- 21.Komiyama M, Boo YE, Yagura H, Yasui T, Baba M, Hakuba A, Nishimura S. A clinical analysis of 32 brain-stem hemorrhages - with special reference to surviving but severely disabled cases. Acta Neurochir. 1989;101(1–2):46–51. doi: 10.1007/bf01410068. [DOI] [PubMed] [Google Scholar]
- 22.Kunyun H, Weitai T, Haowei M (2016) Clinical study of microsurgery for hypertensive brainstem hemorrhage. China Med Pharm 6(04):130–132
- 23.Kushner MJ, Bressman SB. The clinical manifestations of pontine hemorrhage. Neurology. 1985;35(5):637–643. doi: 10.1212/wnl.35.5.637. [DOI] [PubMed] [Google Scholar]
- 24.Lan ZG, Richard SA, Hao L, Chen MJ, You C. Spontaneous hypertensive brainstem hemorrhage: does surgery benefit the severe cases? Interdiscip Neurosurg. 2019;15:66–70. doi: 10.1016/j.inat.2018.10.015. [DOI] [Google Scholar]
- 25.Lei Z. The surgical treatment in hypertension related brainstem hemorrhage. J Aerosp Med. 2015;26(5):2. [Google Scholar]
- 26.Lei Z, Bing R, Laizhao C. Experience of microsurgical in treatment of brainstem hemorrhage. China Mod Dr. 2016;30:36–38. [Google Scholar]
- 27.Lin CP, Evans CJ, Koffman J, Sheu SJ, Hsu SH, Harding R (2019) What influences patients’ decisions regarding palliative care in advance care planning discussions? Perspectives from a qualitative study conducted with advanced cancer patients, families and healthcare professionals. Palliat Med 33(10):1299–1309 [DOI] [PubMed]
- 28.London AJ. Social value, clinical equipoise, and research in a public health emergency. Bioethics. 2019;33(3):326–334. doi: 10.1111/bioe.12467. [DOI] [PubMed] [Google Scholar]
- 29.Mangiardi JR, Epstein FJ. Brain-stem hematomas - review of the literature and presentation of 5 new cases. J Neurol Neurosurg Psychiatry. 1988;51(7):966–976. doi: 10.1136/jnnp.51.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meguro T, Kuwahara K, Tomita Y, Okuma Y, Tanabe T, Muraoka K, Terada K, Hirotsune N, Nishino S. Primary pontine hemorrhage in the acute stage: clinical features and a proposed new simple scoring system. J Stroke Cerebrovasc Dis. 2015;24(4):860–865. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097–e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima K. Clinicopathological study of pontine hemorrhage. Stroke. 1983;14(4):485–493. doi: 10.1161/01.str.14.4.485. [DOI] [PubMed] [Google Scholar]
- 33.Qingming C, Bangqing Y, Xianqun W, Li L, Guang Y (2015) The clinical outcome of microscopic surgery for intensive brainstem hemorrhage. Chin J Stereotact Funct Neurosur 28(03):173–175
- 34.Rabinstein AA, Tisch SH, McClelland RL, Wijdicks EFM. Cause is the main predictor of outcome in patients with pontine hemorrhage. Cerebrovasc Dis. 2004;17(1):66–71. doi: 10.1159/000073900. [DOI] [PubMed] [Google Scholar]
- 35.Rice T. The behavioral economics of health and health care. Annu Rev Public Health. 2013;34:431–447. doi: 10.1146/annurev-publhealth-031912-114353. [DOI] [PubMed] [Google Scholar]
- 36.Rohde V, Berns E, Rohde I, Gilsbach JM, Ryang YM. Experiences in the management of brainstem hematomas. Neurosurg Rev. 2007;30(3):219–223. doi: 10.1007/s10143-007-0081-9. [DOI] [PubMed] [Google Scholar]
- 37.The 4th national cerebrovascular academic seminar (1996) Assessment of neurological defect for patients with cerebrovascular disease (1995). Chin J Neurol 06:2
- 38.Shangming Z, Mingchao S, Lin Z, Liangfeng W, Bangqing Y, Shoulin W. Clinical efficacy observation and reconsideration of spontaneous hypertension pontine hemorrhage via microsurgery. Chin J Neuromed. 2019;18(10):996–1000. doi: 10.3760/cma.j.issn.1671-8925.2019.10.005. [DOI] [Google Scholar]
- 39.Shaowei Z, Guangming N, Junhui Y, Yansong L, Chaofeng D (2019) Comparison of clinical effects of stereotactic operation and conservative treatment for brain stem hemorrhage. Chinese J Pract Nerv Dis 22(08):853–858
- 40.Shitamichi M, Nakamura J, Sasaki T, Suematsu K, Tokuda S. Computed-tomography guided stereotaxic aspiration of pontine hemorrhages. Stereotact Funct Neurosurg. 1990;54–5:453–456. doi: 10.1159/000100252. [DOI] [PubMed] [Google Scholar]
- 41.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 42.Sun P, Fan X, Sun Y, Jiang H, Wang L. Relations between dual filial piety and life satisfaction: the mediating roles of individuating autonomy and relating autonomy. Front Psychol. 2019;10:2549. doi: 10.3389/fpsyg.2019.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. The Lancet Neurology. 2010;9(2):167–176. doi: 10.1016/s1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 44.Wang XQ, Wang XT, Zheng JJ. How to end violence against doctors in China. Lancet. 2012;380(9842):647–648. doi: 10.1016/s0140-6736(12)61367-1. [DOI] [PubMed] [Google Scholar]
- 45.Wei Quan BC, Shu F. Publish or impoverish: an investigation of the monetary reward system of science in China (1999–2016) Aslib J Inf Manag. 2017;69(5):16. [Google Scholar]
- 46.WU Z (2017) Microscopic surgery for primary hypertensive hemorrhage. Mod Med Health Res 1(09):49
- 47.Yan J, Lin H-H, Zhao D, Hu Y, Shao R. China’s new policy for healthcare cost-control based on global budget: a survey of 110 clinicians in hospitals. BMC Health Serv Res. 2019;19(1):84. doi: 10.1186/s12913-019-3921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang W. Clinical outcome of microsurgical treatment for brainstem hemorrhage. China Health Care Nutr. 2014;24(2):974–975. [Google Scholar]
- 49.Yinjuan C, Chunmei H (2017) The treatment strategy and outcome of spontaneous brainstem hemorrhage. Chin J Clinical Rational Drug Use 10(28):118–120
- 50.Yinqing Z (2017) Minimally invasive aspiration for 32 cases of intensive hypertensive brainstem hemorrhage. Chin J Minim Invasive Neurosurg 22(12):562–563
- 51.Zhang B. Treatment in 30 cases of primary pontine hemorrhage. Chin Urban Rural Enterp Health. 2012;03:17–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.