Abstract
During postnatal intestinal development, the intestinal epithelium is highly proliferative, and this proliferation is regulated by signaling in the intervillous and crypt regions. This signaling is primarily mediated by Wnt, and requires membrane trafficking. However, the mechanisms by which membrane trafficking regulates signaling during this developmental phase are largely unknown. Endotubin (EDTB, MAMDC4) is an endosomal protein that is highly expressed in the apical endocytic complex (AEC) of villus enterocytes during fetal and postnatal development, and knockout of EDTB results in defective formation of the AEC and giant lysosome. Further, knockout of EDTB in cell lines results in decreased proliferation. However, the role of EDTB in proliferation during the development of the intestine is unknown. Using Villin-CreERT2 in EDTBfl/fl mice, we deleted EDTB in the intestine in the early postnatal period, or in enteroids in vitro after isolation of intervillous cells. Loss of EDTB results in decreased proliferation in the developing intestinal epithelium and decreased ability to form enteroids. EDTB is present in cells that contain the stem cell markers LGR5 and OLFM4, indicating that it is expressed in the proliferative compartment. Further, using immunoblot analysis and TCF/LEF-GFP mice as a reporter of Wnt activity, we find that knockout of EDTB results in decreased Wnt signaling. Our results show that EDTB is essential for normal proliferation during the early stages of intestinal development and suggest that this effect is through modulation of Wnt signaling.
Keywords: Trafficking, Intestinal proliferation, Endotubin, Wnt, MAMDC4
Graphical Abstract
Introduction
The intestinal epithelium is a highly specialized layer that serves as the port of entry for nutrients and cofactors, as well as a selective barrier to macromolecules and pathogens. Disruption of this barrier through damage or inflammation can lead to disease in both neonates and adults (Hackam et al., 2005; Landy et al., 2016; Moore et al., 2016). The adult intestine is comprised of protruding villi that contain differentiated cells that mediate absorption and secretion; and crypts, that harbor proliferative cells as well as cells that are important in innate defense. In the developing intestine, crypts have not yet formed and proliferative cells are restricted to the “intervillous” zone between villi (Korinek et al., 1998). The intestinal epithelium turns over at a remarkable pace, with the entire epithelium being replaced every 4–5 days. Thus, proliferation is a constant feature of intestinal renewal.
Proliferation in the intestine is regulated in large part by Wnt signaling, which is activated during development and remains active throughout life (Fevr et al., 2007; Kim et al., 2007). While Wnt signaling appears to be dispensable for proliferation of the mouse intestinal epithelium in early embryonic development (E13.5–14.5) (Chin et al., 2016), it is detected at day E16, primarily in the villus epithelium (Kim et al., 2007). After birth in the mouse, Wnt signaling is restricted to the intervillous region by postnatal day 3 and is localized to the crypts by postnatal day 8 (Gregorieff et al., 2005; Kim et al., 2007).
We previously identified an endosomal protein, Endotubin (EDTB, also known as MAMDC4), that is highly expressed in the villus enterocytes of developing intestine (Trahair et al., 1995; Wilson et al., 1987, 1991), and found that EDTB modulates cell proliferation and YAP signaling at the endosomal membrane in cultured cells (Cox et al., 2015). EDTB expression in the intestine is developmentally regulated, and primarily localizes to the endosome-rich Apical Endocytic Compartment (AEC) of villus cells. EDTB villus expression decreases after weaning, coinciding with the decrease in endosomes of the AEC (Pasternak et al., 2016; Wilson et al., 1991). Deletion of EDTB in the developing intestine results in loss of the AEC and apical giant lysosome in enterocytes, in addition to impaired pup growth (Cox et al., 2018). Since Wnt signaling is a primary proliferative signal in intestinal stem cells (Fevr et al., 2007; Korinek et al., 1998; Muncan et al., 2006) and may act with YAP within the epithelium (Azzolin et al., 2014; Barry et al., 2013; Deng et al., 2018; Oudhoff et al., 2016), we tested if loss of EDTB could impact signaling or proliferation in the developing intestine. We examined the effects of EDTB deletion on proliferation at ages where Wnt signaling is restricted to the intervillous (P3), when crypts are forming (P8), and after crypt maturation (P11-P15), which are all prior to weaning when EDTB expression levels decrease. Using in vivo and in vitro approaches, we find that EDTB knockout decreases proliferation in the developing intestine. We also show that EDTB is expressed in cells that contain intestinal stem cell markers and that knockout results in decreased OLFM4- and LGR5-positive cells. Reduced proliferation is accompanied by decreased activation of the Wnt signaling components LRP6 and ß-catenin, and decreased TCF/LEF-GFP in the nucleus upon Wnt stimulation. Our results suggest that EDTB modulates Wnt signaling in the proliferative cells of the developing intestine.
Materials and Methods
Mice
The EDTB (MAMDC4) and Villin-CreERT2 mouse colonies were previously described (Cox et al., 2018). MAMDC4fl/+/ Villin-CreERT2 males were mated with MAMDC4fl/fl females. Pregnant females were gavaged with 100μl of 10mg/ml 4-hydroxytamoxifen (4-OHT; cat. T176–50; Sigma) on day 15 of gestation (E15) to induce expression of Cre recombinase. Offspring were collected at postnatal day 3 (P3). For the P8 pups, lactating females were gavaged with 100μl of 10mg/ml of 4-OHT for 5 days starting at P3.
TCF/LEF:H2BGFP (013752) and LGR5-EGFP-IRES-CreERT2 (008875) mice were purchased from Jackson Laboratory and maintained in the experimental mouse resources shared services at the University of Arizona Cancer Center. TCF/LEF:H2BGFP mice were crossed with the MAMDC4fl/fl / Villin-CreERT2 to generate MAMDC4fl/fl / TCF/LEF:H2BGFP females and MAMDC4fl/fl / Villin-CreRTt2 / TCF/LEF:H2BGFP males. Dams were gavaged with 100μl of 10mg/ml 4-OHT for 5 days starting at P5 and enteroids were generated from P11 pups. LGR5-EGFP-IRES-CreERT2 mice were crossed with MAMDC4fl/fl mice to generate MAMDC4fl/+/ LGR5- EGFP-IRES-CreERT2 females and MAMDC4fl/+/ LGR5- EGFP-IRES-CreERT2 males. Dams were gavaged with 100μl of 10mg/ml 4-OHT for 5 days starting at P3 and crypts were isolated at P8 for FACS analysis.
All genotyping was carried out by the University of Arizona Genetics Core. The care, maintenance, and treatment of mice used in these studies was approved by the University of Arizona Institutional Animal Care and Use Committee.
Tissue Labeling
The intestine was processed for histochemical analysis as described (Cox et al., 2018). Antibodies used were: Cyclin D1 (cat. 2978; Cell Signaling), E-cadherin (cat. 3195; Cell Signaling), Ki67 (cat 15580; Abcam), OLFM4 (cat. 39141; Invitrogen), Chromogranin A (Chgr A) (cat. ab15160; abcam), rabbit anti-HES1 (cat. 11988; CST) and anti-EDTB hybridoma supernatant (Wilson et al., 1987). Antigen retrieval with Tris/EDTA pH 9 was used for Cyclin D1 (1:50). Citrate pH 6 antigen retrieval was used for E-cadherin (1:200), Ki67 (1:200), Chgr A (1:200), HES1 (1:200) and OLFM4 (1:400). Signal Boost (cat. 8114S; Cell Signaling Technology) and DAB detection kits (cat. 8059S; Cell Signaling Technology) were used for visualization of Cyclin D1 and HES1. To visualize goblet cells, tissue was labelled with Alcian Blue Solution according to manufacturer’s instructions (cat. IW-3000A; IHCWORLD).
Immunoblot Analysis
Intestine was filleted, lysed in RIPA and analyzed as previously described (Cox et al., 2015). The following antibodies were used: mouse anti-EDTB (Wilson Lab), rabbit anti-actin (cat. 4970; CST), rabbit anti- non-phospho (active) ß-catenin (cat. 19807; CST), rabbit anti-HES1 (cat. 11988; CST), rabbit anti-NICD (cat.4147; CST).
Enteroids
Ileum was isolated from P3-P11 mice, flushed with cold PBS and filleted. Ileum was cut into 5mm segments and transferred to cold PBS containing 5mM EDTA, 1.5mM DTT and Pen/Strep. Tissue was incubated on ice for 30min and shaken every 10min. Ileum segments were allowed to settle by gravity and PBS solution was replaced with cold PBS/EDTA without DTT. Dissociation of villi and intervillous/crypt region was monitored every 10min. Once intervillous segments were visible, the sample was filtered using a 100um cell strainer (cat 22363549; Fisher Scientific) and a 70μm cell strainer (cat 352350; ThermoFisher). Epithelial tissue was pelleted at 300g at 4°C for 5min, washed with Advanced DMEM F12, 10% FBS and Pen/Strep and pelleted at 300g at 4C for 5min. Intervillous/crypt pellets were resuspended in cold IntestiCult (cat 06005; StemCell Technologies), mixed with Matrigel (cat. 356237; Corning) and plated in wells of prewarmed 24 well plate. IntestiCult media (500μl) was added after 30min at 37°C and changed every 2 to 3 days.
Enteroids were passaged every 7 to 10 days at a 1:2 to 1:4 ratio. For passaging, media was removed and enteroids were incubated in PBS with 2mM EDTA for 3 min at 4°C. Matrigel/enteroids were mechanically dissociated by pipetting 10 to 20 times and washed with Advanced DMEM F12, 10% FBS and Pen/Strep and pelleted at 300g at 4°C for 5min. They were then plated in a 1:4 mix of Intesticult™:Matrigel
For enteroid growth experiments 0.5μM 4-OHT was added to the media 16hrs after passaging. Media was replaced every 2 days and enteroid growth was assessed on day 6. For proliferation experiments, enteroids were plated in 8 well chamber slides (cat. 155409, ThermoFisher) and treated with 4-OHT 16hrs after plating for 48hrs. Enteroids were labelled with antibodies against pHH3 and CC3. EDTB fl/fl Cre− / TCF/LEF GFP+ and EDTBfl/fl Cre+/ TCF/LEF GFP+ enteroids were treated with 100ng/ml of Wnt3a (cat. 31520; Peprotech) for 6hrs.
For enteroid formation experiments, mothers were gavaged with 4-OHT for 5 days starting at P3. Crypts were isolated from P8 pups and 100 crypts were plated in Matrigel culture and the number of enteroids that formed was quantified from 4 wells for each mouse.
Enteroid Whole Mount Immunohistochemistry
Enteroid media was aspirated, the enteroids were washed with warm PBS and fixed for 10min at 37°C with 4% PFA in PBS. After fixation, they were washed with PBS and incubated with 50mM NH4Cl in water for 30min at RT. They were permeabilized using 0.1% Triton-X 100 in PBS for 15min at RT and blocked in PBS containing 1% FBS (Atlantic Biologicals) and 1% BSA (cat. BP-1605, Fisher Scientific). Antibodies for Phospho-Histone H3 (Ser10) (cat. 9701, Cell Signaling) and CC-3 (cat. 9664, Cell Signaling) were diluted 1:100 and incubated overnight at 4°C followed by washing with 0.1% Tween 20 in PBS. Secondary antibodies were diluted 1:1000 and incubated for 1hr at RT. After washing, they were stained using 1mg/mL DAPI (cat. D9542, Sigma) in PBS for 10min at RT, washed with 0.1% Tween 20 in PBS and stored in PBS at 4°C.
Microscopy and Image Analysis
Brightfield microscopy was conducted on a Leica DMI6000 microscope using Leica LAS-X software. Images were processed and analyzed using Photoshop (Adobe). Immunofluorescence microscopy was performed on a Leica SP5 laser- scanning confocal microscope using a 63X Plan Apo 1.40 NA Leica objective. Confocal z-series were acquired at 1μm z-steps. Images were processed using NIH ImageJ and Photoshop (Adobe). Immunofluorescence microscopy of enteroids was performed on an Olympus FluoView FV1200 laser-scanning confocal microscope using a 20X Apo 0.75 NA Olympus objective. Confocal z-series were acquired at 1.8μm z-steps for the entire height of the enteroid. Images were processed using NIH ImageJ and analyzed using Nikon NIS Elements. Phospho-Histone H3 (pHH3) positivity was determined by nuclear localization of immunostaining and quantified manually. CC-3 positivity was determined by immunostaining and quantified manually. Fold change for each experimental condition was determined by normalizing to total cell number and the manual binary segmentation tool was used to ensure complete separation of volumetric binary masks.
For Ki67 label quantification, the total number of cells in the intervillous or crypt regions was determined by DAPI counterstain. Ki67 positivity was determined by nuclear localization and quantified manually. Analysis was performed on 3–5 tissue sections of 3 control and 3 EDTB KO mice for each age.
For goblet and enteroendocrine cell quantification, the total number of labeled cells per villus was determined. Using NIH image, the area of each villus was used as the area calculation. Data is represented as the cell number per unit area.
For OLFM4 labeling, sections of ileum from P8 control and EDTB KO pups were incubated with antibodies to OLFM4 and imaged using a Leica DMI6000 microscope and Leica LAS-X software. Blinded images from 3 control and 3 EDTB KO animals were analyzed. Channels were separated using NIH image and background intensity was determined by averaging maximum intensity from 4 separate villi of each image. DAPI labeling was used to determine the crypt area and this area was quantified for maximum intensity. Crypt regions were determined to be OLFM4 positive if the maximum intensity was 2X that of the background signal.
Tissue Labeling for FACS analysis
Samples were processed according to the method of (Scurrah et al., 2019). Ileum samples (1mm) of P8 LGR5-EGFP mice were collected and fixed with 4% PFA for 20min at RT. Tissue fragments were washed with PBS, resuspended in ice cold acetone, vortexed and pelleted. For labeling of EDTB, tissue was allowed to dry at RT for 30 seconds and permeabilized for 30 min with 1% saponin, 0.05% Triton X-100 and 0.01% SDS in PBS. Tissue was washed with PBS and blocked with 2.5% Donkey Serum (blocking solution) in PBS for 30min at RT. Tissue was incubated overnight at 4°C in primary antibody (EDTB hybridoma 1:1) diluted in blocking solution, washed with PBS and incubated in Alexa Fluor 647 secondary antibody (cat A21236; Invitrogen) at 1:5000 for 30 min at RT, followed by washing with PBS and post-fixation in 4% PFA for 20 min. Tissue was digested for 1hr at 37°C with 1mg/ml Collagenase (cat. 17018029; ThermoFisher) and 1mg/ml Dispase (cat. D4693; Sigma) in PBS. 4 volumes of PBS with 0.003% Triton X- 100 was added.
Dissociated tissue was passed through a 27gauge needle and filtered through a 40μm filter. Single cells were pelleted at 500g for 5 min and resuspended in PBS for FACS analysis. At least 50,000 singlets were acquired and analyzed (defined using forward scatter height (FSC-H) vs. forward scatter area (FSC-A) density plot) using an LSRII Fortessa (BD Biosciences) and FlowJo software (Tree Star).
LGR5-GFP crypt cell isolation for FACS analysis
Crypts were isolated from ileum as described above and dissociated using TrypLE (cat. 12563029; ThermoFisher) with gentle agitation at 37°C for 5 minutes. Crypt cell solution was filtered using a 40μm mesh and pelleted at 500g for 5 minutes at 4°C. Cells were resuspended in PBS with 7AAD (cat. A1310; ThermoFisher). Singlets were acquired as described above, and 7AAD was used to define live cells. Cells isolated from LGR5-GFP negative ileum were used to define the GFP− population. 5,000 to 10,000 live cells for each animal were used to determine GFP+ cells. GFPhi and GFPlo gates were set as shown in Figure 6.
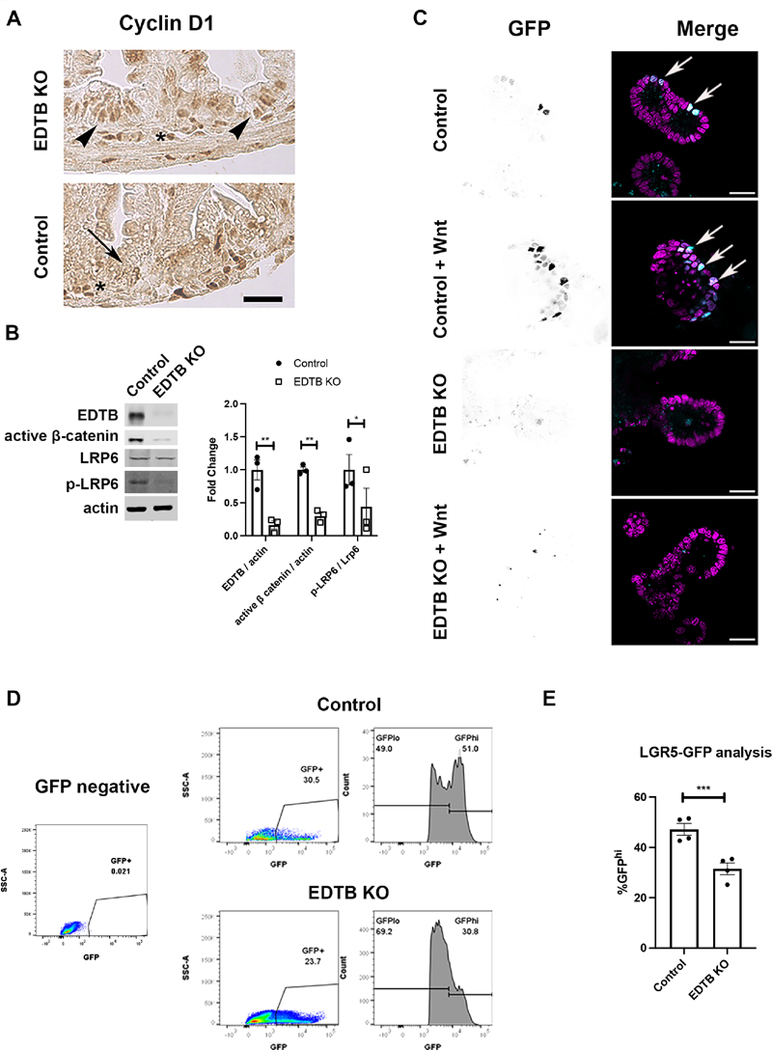
Figure 6. Decreased Wnt signaling in EDTB KO intestinal epithelium.
(A) P3 ileum of control and EDTB KO mice were labeled with antibodies against Cyclin D1. Cyclin D1 is localized to the nucleus in lamina propria in both the control and EDTB KO mice (asterisks), However, Cyclin D1 is absent in EDTB KO epithelium (arrow) compared to control (arrowheads) Scale bar: 50μm. (B) Mothers of EDTB fl/fl Cre− and EDTBfl/fl Cre+ P3 mice were treated with 4-OHT for 5 consecutive days. Ileum lysates from P8 control and EDTB KO pups were analyzed by immunoblot for EDTB and active ß-catenin and LRP6. There is a decrease in active β-catenin and p-LRP6 in EDTB KO ileum. (n=3). (C) EDTBfl/fl Cre− / TCF/LEF GFP+ and EDTBfl/fl Cre+/ TCF/LEF GFP+ P5 mice were treated with 4-OHT for 5 consecutive days. Enteroids were derived from P11 mice, grown in Matrigel and treated for 6hrs with Wnt3a, fixed, and counterstained with DAPI. TCF/LEF-GFP expression (arrows) was visualized by confocal microscopy. TCF/LEF-GFP expression increases with Wnt stimulation in control enteroids, but not in enteroids from EDTB KO mice. Scale bar: 20μm. (D) FACS analysis of LGR5-GFP. Crypts from P8 ileum of the LGR5-EGFP-IRES-CreERT2 control and EDTB KO mice were analyzed by FACS for GFP expression. Crypts isolated from LGR5-EGFP-IRES-CreERT2− mice were used to establish appropriate gating parameters (left panel). Control animals show 47% of the GFP population is GFPhi, with EDTB KO the GFPhi population is reduced. (E) Quantification of GFPhi expression (n=4). Error bars represent mean +/− SEM. *p<0.05, **p<0.01, ***p<0.005.
Statistical Analysis
Statistical comparisons were performed using Prism9 (GraphPad) and analyzed using an unpaired Student t-test. Error bars represent the standard error of the mean.
Results
EDTB is Expressed in Intervillous and Crypt Cells
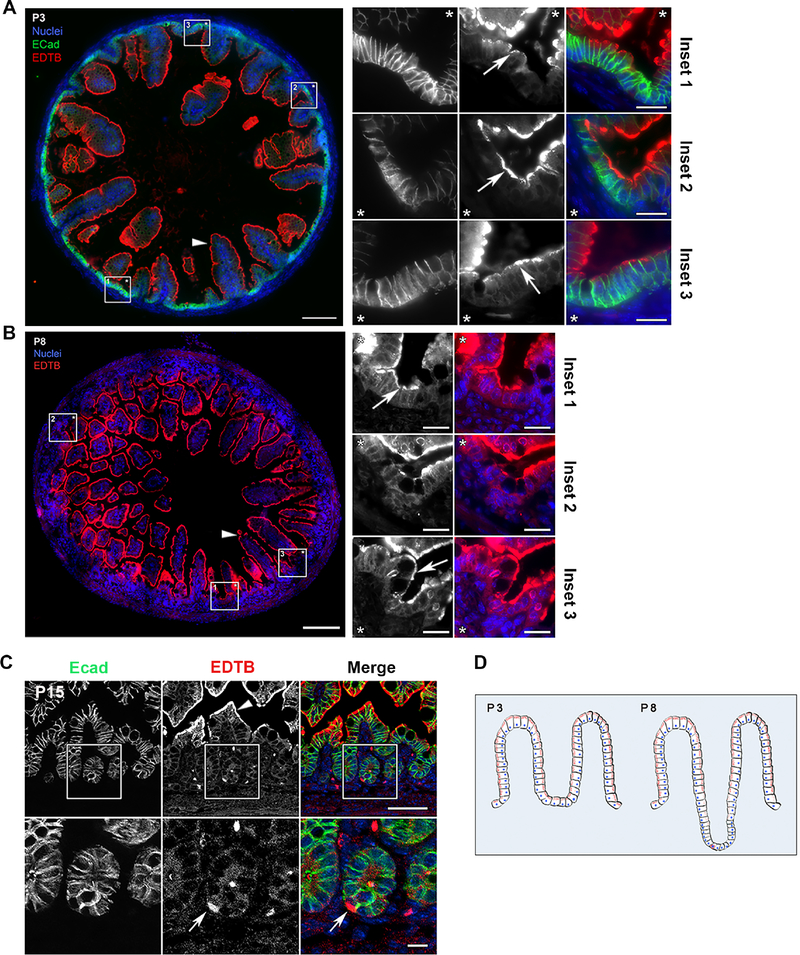
EDTB is an endosomal protein that is highly expressed in villous enterocytes of the developing intestine (Trahair et al., 1995; Wilson et al., 1987). While our previous studies have focused on the role of EDTB on enterocyte differentiation (Cox et al., 2018), experiments in cultured cells have shown that EDTB regulates proliferation through the YAP signal transduction pathway (Cox et al., 2015). Further, knockout of EDTB during development results in defective villus morphogenesis. Since villus formation relies on normal proliferation, we asked whether EDTB is expressed in the proliferative compartment of the developing intestine. During development, the intestine goes through stages in which the proliferative compartment transitions from the intervillous region (P3) to the forming crypts (P8), and then is localized exclusively to crypts (P11-P15) (Korinek et al., 1998). Immunofluorescent labeling of ileum from postnatal days 3, 8, and 15 animals with antibodies against EDTB results in strong labeling of the AEC in the apical cytoplasm of villous enterocytes as previously described (Cox et al., 2018; Wilson et al., 1987, 1991). However, the intervillous and crypt regions, which lack the endosome-rich AEC, also have substantial EDTB labeling at P3 and P8 (Figure 1). In addition, at P15, after crypts are well established, we observe a small number of EDTB positive cells localized to the crypt region (Figure 1). Quantification of P15 ileum found that 6% of crypts contain at least one cell that expresses EDTB. This distribution is summarized in Figure 1D.
Figure 1. EDTB is localized to a subpopulation of cells in the intervillous and crypt regions.
(A-C) Ileum of P3, P8 and P15 mouse pups labeled with antibodies against E-cadherin (A, C, green) and EDTB (A, B, C, red). (A, B) Stitched images of the cross-sections show that EDTB is localized apically (arrowheads) along the length of the villi. Insets can be oriented by the placement of the asterisks. EDTB labels the intervillous region in P3 and P8 ileum (arrows; insets). Scale bar: 100μm, Scale bar inset: 20μm. (C) In P15 ileum, EDTB is localized apically within enterocytes along the villi (arrowhead) as well as in crypt cells (arrow), 6% of crypts contain an EDTB positive cell (n=117). Scale bar: 50μm, Scale bar inset: 10μm. (D). Summary of the distribution of EDTB expression during development. Before crypts form (P3), EDTB (red) is present in villous and intervillous enterocytes. When crypts begin to form, there are fewer EDTB-positive cells in the crypt, but there are occasional strongly labeled cells.
EDTB Knockout Reduces Epithelial Proliferation
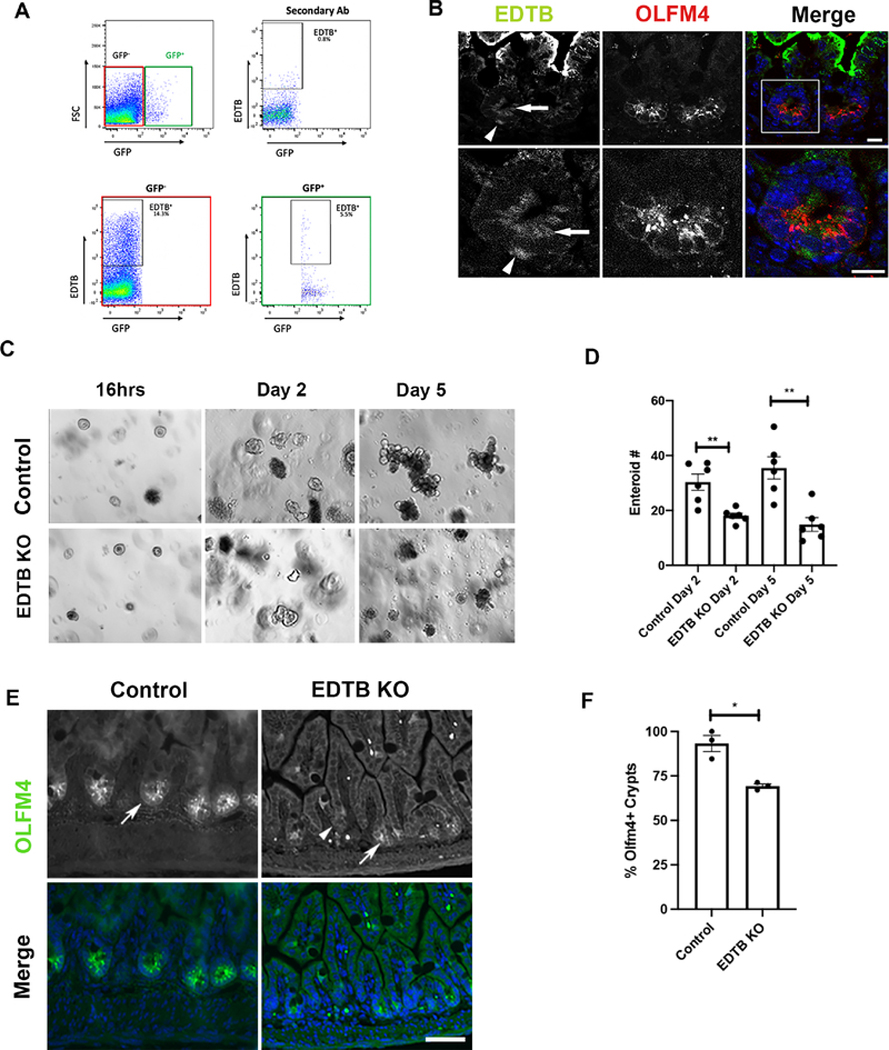
ShRNA-mediated knockdown of EDTB in MDCK cells results in decreased proliferation (Cox et al., 2015). To determine if EDTB expression impacts proliferation in the epithelium of the developing intestine, we induced EDTB KO at embryonic day 15 or postnatal day 3 (P3) through 4-OHT induction of the Cre recombinase under control of the villin promoter in EDTBfl/fl, Cre+ mice, with EDTBfl/fl, Cre− mice as controls (el Marjou et al., 2004). EDTB knockout was confirmed with immunofluorescence and immunoblot (Figure 2). The distal small intestine was collected on P3 and P8 and labeled with the proliferation marker Ki67. As shown in Figure 3, the intervillous and crypt regions of control animals are positive for Ki67, while Ki67 labeling is reduced in EDTBfl/fl, Cre+ animals. We quantified this labeling by comparing Ki67 nuclear labeling to the total number of cells in the intervillous (P3) or crypt (P8) region. This quantification shows that proliferation is decreased after EDTB knockout at all ages examined (Figure 3).
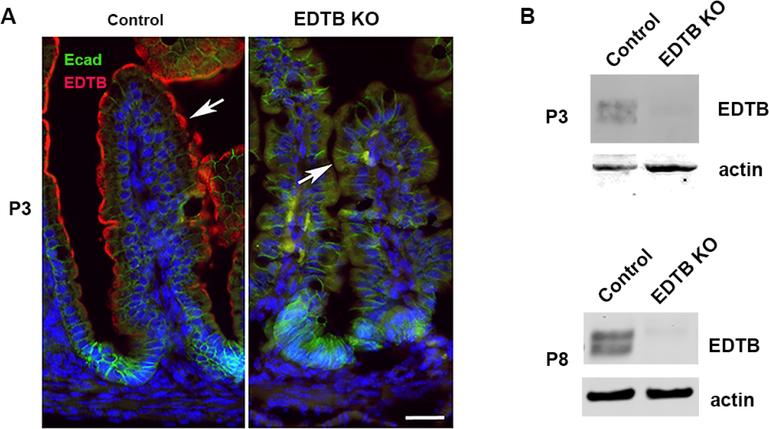
Figure 2. Verification of EDTB KO.
(A) P3 Ileum from EDTBfl/fl Cre− (control) and EDTBfl/fl Cre+ (EDTB KO) mice were labeled with antibodies against E-cadherin (green) and EDTB (red). EDTB is localized to apical endosomes along the length of the villus in control mice and expression is absent after EDTB KO (arrows) Scale bar: 20μm. (B) Lysates from P3 and P8 Ileum from EDTBfl/fl Cre− (control) and EDTBfl/fl Cre+ (EDTB KO) were examined by immunoblot for EDTB expression. EDTB expression is lost in the EDTB KO ileum at P3 and P8.
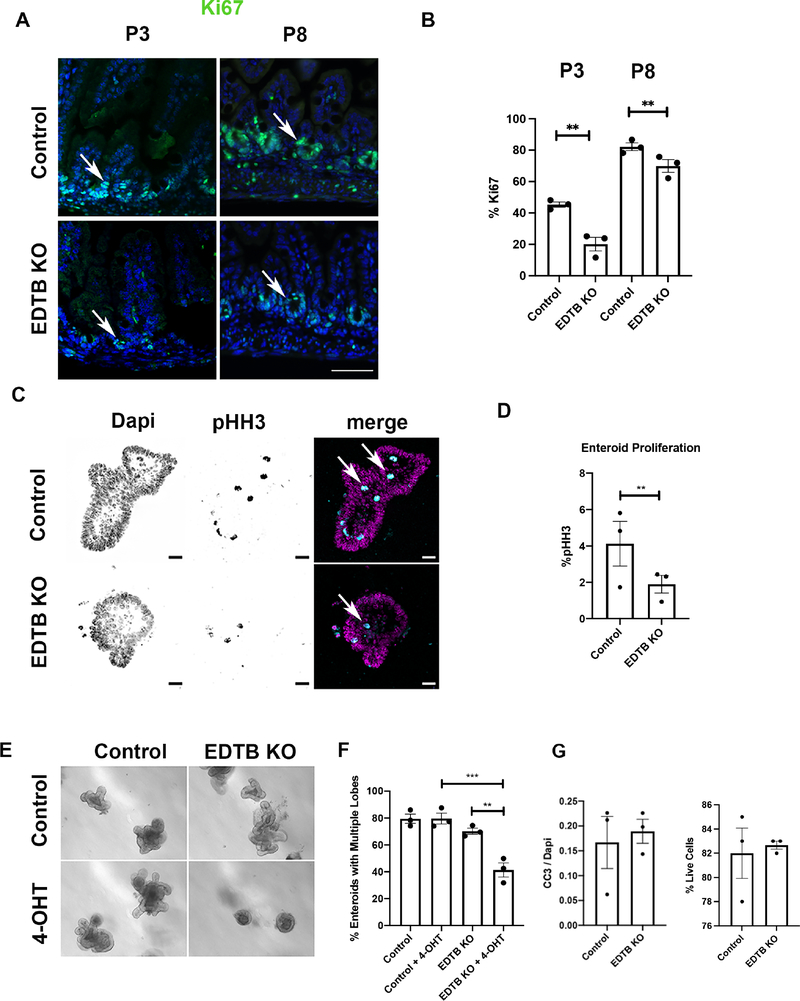
Figure 3. Decreased proliferation after EDTB KO.
(A) P3 and P8 ileum from EDTBfl/fl Cre− (control) and EDTBfl/fl Cre+ (EDTB KO) mice were labeled with antibodies against Ki67 (green) and counterstained with DAPI (blue). Ki67 labels the intervillous in P3 and crypt regions in P8 pups. There is decreased labeling in EDTBfl/fl Cre+ animals (arrows). Scale bar: 50μm. (B) Quantification of Ki67 labeling demonstrates decreased proliferation after EDTB KO (n=3). (C) EDTBfl/fl Cre+ enteroids were grown in the presence or absence of 4-OHT for 48 hours and labeled with antibodies against phospho-histone H3 (pHH3; arrows). pHH3 labels more nuclei in control compared to EDTB KO enteroids. Scale bar: 20μm. (D) Quantification of pHH3 labeling demonstrates decreased proliferation in EDTB KO enteroids (n=3). (E) EDTBfl/fl Cre+ and EDTBfl/fl Cre− enteroids were grown for 6 days in the presence or absence of 4-OHT to induce EDTB KO in vitro. EDTBfl/fl Cre+ enteroids treated with 4-OHT have fewer lobes. There is no reduction in lobes of EDTBfl/fl Cre+ enteroids (Control) without 4-OHT or in EDTBfl/fl Cre− enteroids (KO) treated with 4-OHT. (F) Quantification of enteroid lobe growth (n=3). (G) Enteroids were labeled with antibodies against CC-3 and quantified. There is no difference in CC-3 labeling between control and EDTB KO enteroids (n=3). (H) EDTBfl/fl Cre+ and EDTBfl/fl Cre− enteroids were grown for 48 hours in the presence of 4-OHT, dissociated and incubated with Trypan Blue to assess cell viability. >80% of enteroids in control and EDTB KO samples (n=3). Error bars represent mean +/− SEM. Error bars represent mean +/− SEM, *p<0.05, **p<0.01, ***p<0.005
Whereas Ki67 is expressed throughout the active phases of the cell cycle, phospho-histone H3 (pHH3) expression is restricted to late G2 to late anaphase and serves as a marker of mitosis. To further test the effects of EDTB KO on proliferation and assess the potential impact of cells in the lamina propria, we grew enteroids from EDTBfl/fl, Cre+ and EDTBfl/fl, Cre− mice and induced KO of EDTB in vitro by incubation of the enteroids with 4-OHT, followed by labeling for pHH3 and imaging by confocal microscopy. To ensure counting of all of the labeled cells, a confocal z-series was collected for the entire height of the enteroid, and a 3D volumetric binary mask using DAPI nuclear staining was generated. We observed a decrease in pHH3 labeling in EDTB KO enteroids compared to controls (Figure 3).
To further test the role of EDTB in proliferation in the epithelium, we isolated the intervillous regions from EDTBfl/fl Cre− and EDTBfl/fl, Cre+ mice and placed them into culture. After 16hrs in culture, one half of the control and EDTBfl/fl, Cre+ enteroids were incubated with 4-OHT to induce expression of the Cre recombinase, while others were maintained in normal media. To assess proliferation, the percentage of enteroids with multiple lobes was quantified after 6 days. Multiple lobes were observed in control enteroids incubated with 4-OHT, as well as in both control and EDTBfl/fl, Cre+ incubated without 4-OHT (Figure 3). However, enteroids from EDTBfl/fl, Cre+ mice form lobes at a decreased frequency in the presence of 4-OHT (Figure 3). Taken together, these in vitro results suggest that the effect on proliferation after EDTB KO is due to changes autonomous to the epithelial cells and are not from effects mediated by cells in the lamina propria.
To determine if the decreased cell numbers could be due to increased apoptosis, we measured cleaved caspase-3 (CC-3) in enteroids from EDTBfl/fl, Cre+ and EDTBfl/fl, Cre− animals using immunofluorescence. We observe no differences in CC3 in control and EDTB KO enteroids (Figure 3). We assessed cell viability using EDTBfl/fl, Cre+ and EDTBfl/fl, Cre− enteroids treated with 4-OHT for 2 days. Following 4-OHT treatment, enteroids were dissociated and labeled with trypan blue, and find that EDTB KO does not affect cell viability (Figure 3). Taken together, these results suggest that EDTB impacts signaling pathways that regulate proliferation within epithelial cells.
EDTB is Present in LGR5 and OLFM4 positive cells
To characterize the crypt region EDTB-positive cells, we used mice that were expressing the stem cell marker LGR5-GFP (Barker et al., 2007). For these experiments, ileum tissue was fixed, labeled for EDTB, dissociated, and analyzed by flow cytometry (Manolopoulou et al., 2019; Scurrah et al., 2019). To establish the proper gates to detect GFP positive cells and EDTB labeling, we used GFP− control ileum to define the signal from secondary antibody alone and the GFP− populations. Using these parameters, we found that 14.3% of cells isolated from P8 ileum express EDTB. EDTB labeling of LGR5-GFP+ ileum shows that 5.5% of the GFP+ cells were also positive for EDTB, indicating that EDTB is expressed in a portion of LGR5 positive cells (Figure 4). To further characterize the cell populations in the crypt that express EDTB, we labeled P8 intestine for EDTB and the stem cell marker OLFM4. As previously reported, EDTB is strongly expressed in the endosome-rich AEC in cells on the villus. Cells at the base of the villus do not contain this membrane complex (Wissig and Graney, 1968). However, EDTB was detected in the crypt and co-localized with OLFM4 positive cells (Figure 4). These results show that EDTB is expressed in the stem cell compartment.
Figure 4. EDTB is present in LGR5 and OLFM4 positive cells.
(A) FACS analysis of LGR5-GFP/EDTB cells. Ileum from P8 LGR5-GFP mice was labeled for EDTB and analyzed by FACS as described in the Methods. Tissues not expressing GFP or incubated only in secondary antibody were used to establish appropriate gating parameters (top panels). Approximately 14.3% of the cells were EDTB positive but did not contain LGR5-GFP (bottom left panel), whereas 5.5% of LGR5-GFP cells are EDTB positive (bottom right panel). (B) P8 ileum labeled with antibodies against EDTB (green) and OLFM4 (red) and counterstained with DAPI. EDTB is localized to the basal (arrowhead) and apical cytoplasm (arrow) of OLFM4 positive crypt cells (inset). Scale bar: 10μm. (C) 100 crypts isolated from P8 pups were plated in Matrigel and grown for 5 days. Enteroids from control mice form mature enteroids. Crypts isolated from EDTB KO mice show a decreased ability to form enteroids. (D) Quantification of enteroid formation (n=6). (E) Ileum from control and EDTB KO P8 pups were labeled with antibodies against OLFM4 and counterstained with DAPI. OLFM4 expression is localized to the crypt region in control and EDTB KO ileum (arrows) and absent from a portion of crypts (arrowhead). Scale bar: 50μm (F) Quantification of OLFM4 positive crypts (n=3). Error bars represent mean +/− SEM. *p<0.05, **p<0.01.
Based on our findings of colocalization of EDTB and stem cell markers, we next tested if EDTB is impacting the stem cell population by quantifying the ability of cells derived from EDTBfl/fl, Cre− and EDTBfl/fl, Cre+ mice to form enteroids. Crypts were isolated from the distal intestine of P8 mice whose mothers had been treated with 4-OHT and plated into Matrigel culture. The number of enteroids that formed was quantified after 2 and 5 days. As shown in Figure 4, knockout of EDTB impaired the ability of these cells to form enteroids. To further characterize the stem cell population in control and EDTB KO animals, we labelled P8 ileum with antibodies to OLFM4. Control ileum shows strong OLFM4 labeling in the crypt (Figure 4). EDTB KO crypts show reduced or lack of expression in the crypt regions (Figure 4). The percentage of crypts expressing OLFM4 was quantified and greater than 90% of crypts in control animals contain OLFM4, while fewer than 70% of crypts in KO ileum are OLFM4 positive (Figure 4). As OLFM4 is a marker of the stem cell population (Schuijers et al., 2014; Srivillibhuthur et al., 2018; van der Flier et al., 2009), these data suggest that loss of EDTB results in the loss of cells in this compartment.
EDTB Knockout Does Not Impact Secretory Cell Differentiation
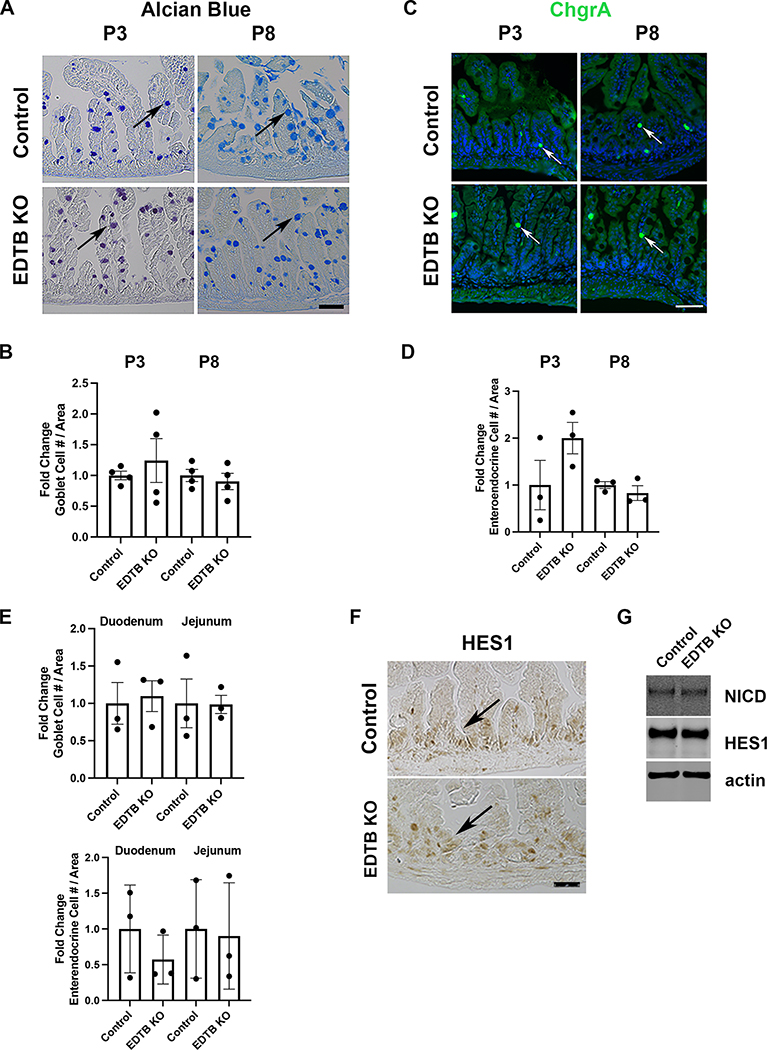
In zebrafish, plasmolipin is localized apically in intestinal epithelial cells where it regulates endocytosis and Notch signaling (Rodriguez-Fraticelli et al., 2015), and differentiation of absorptive and secretory cells in the intestinal epithelium is controlled by Notch (Yang et al., 2001). To determine if EDTB KO impacts Notch signaling, we examined the differentiation of secretory cells after EDTB KO. For these analyses, P3 control and EDTB KO ileum was labelled to identify goblet cells. Alcian Blue staining shows goblet cells along the crypt-villus axis in control and EDTB KO ileum, and quantification did not show a change in goblet cell number (Figure 5). Further, labeling using ChgrA showed no significant change in enteroendocrine cells, although there was a great deal of variability between animals, likely due to the relatively low number of these cells (Figure 5). To assess differentiation of secretory cells along the length of the small intestine, we assessed P8 duodenum, jejunum and ileum for numbers of goblet and enteroendocrine cells. Quantification of these cell types shows no change in either goblet or enteroendocrine cell number in EDTB KO intestine (Figure 5).
Figure 5. No change in intestinal secretory lineages with EDTB KO.
Ileum from P3, and P8 control and EDTB KO animals were stained for (A) Alcian blue (goblet cells)(arrows) or labeled with antibodies for (C) chromogranin A (enteroendocrine cells)(arrows) Scale bar: 50μm. (B,D). The total number of Goblet and Enteroendocrine cells per unit area was determined (n=3). There is no change in number of secretory cells in EDTB KO ileum compared to controls. (E) Duodenum and Jejunum from P8 control and EDTB KO were stained for Alcian blue or labeled with antibodies for ChgrA as above. There is no change in the number of secretory cells in the duodenum and jejunum in EDTB KO compared the control (n=3). (F) Ileum from P3 control and EDTB KO were labelled with antibodies for HES1. HES1 is localized to the nucleus in the intervillous region of control and EDTB KO ileum (arrows). (G) Lysates from P8 Ileum from EDTBfl/fl Cre− (control) and EDTBfl/fl Cre+ (EDTB KO) were examined by immunoblot for NICD and HES1 expression. NICD and HES1 expression levels are unchanged in EDTB KO ileum (n=4).
These results suggest that Notch signaling is not impacted in EDTB KO animals. To further assess activation of the Notch pathway prior to crypt formation, we labelled P3 control and EDTB KO ileum with antibodies to HES1. HES1 is expressed throughout the intervillous region in both control and EDTB KO tissue (Figure 5). To confirm these results post-crypt formation, we examined ileum lysates from P8 control and EDTB KO mice by immunoblot for levels of NICD and HES1. NICD and HES1 levels are unchanged in the EDTB KO ileum relative to control samples (Figure 5). Taken together, these results suggest EDTB does not regulate Notch signaling.
EDTB Knockout Results in Decreased Wnt Signaling
Activation of Wnt signaling requires the nuclear localization of ß-catenin, where it acts in cooperation with the TCF/LEF family of transcription factors to regulate gene expression. Elimination of ß-catenin or TCF expression in the intestine results in loss of the proliferative compartment, demonstrating that Wnt signaling is required for intestinal proliferation. (Korinek et al., 1998). One readout of Wnt signaling in the proliferative compartment of the intestinal epithelium is the expression of nuclear Cyclin D1 (Kim et al., 2007). We performed immunohistochemistry for Cyclin D1 on P3 control and EDTB knockout mice. While there was substantial Cyclin D1 labeling of the nuclei in the epithelium in the intervillous region of control tissues, the nuclei of the knockout animals were not labeled (Figure 6). We further tested the activation of ß-catenin in vivo by immunoblotting intestinal lysates for active ß-catenin. For these experiments, we crossed EDTBfl/fl Cre+ males with EDTBfl/fl Cre− females. Mothers were gavaged with 4-OHT from P3 to P7. On P8, ileum lysates were collected and probed for activation of LRP6 and ß-catenin. As shown in Figure 6, phosphorylation of LRP6 and levels of active ß-catenin were decreased in ileum lysates following EDTB KO.
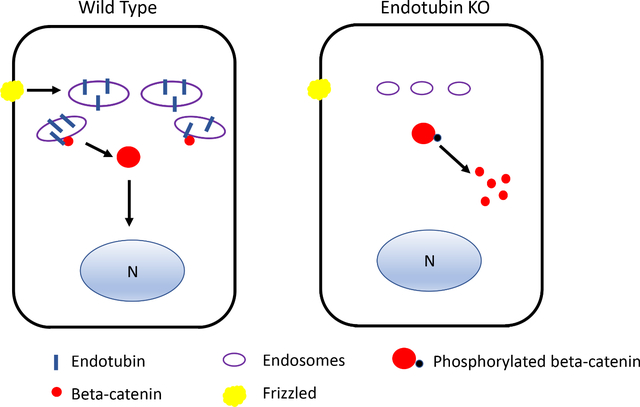
To further test the effects of EDTB knockout on Wnt activity in the epithelium, we crossed EDTB fl/fl, Cre+ mice with mice expressing the Wnt reporter TCF/LEF-GFP. To achieve EDTB knockout, mothers were gavaged with 4-OHT from P5-P10. Crypts were isolated on P11 and placed in enteroid culture. To examine TCF/LEF-GFP activity, we used fluorescence microscopy and imaged cells treated with or without Wnt3a. We found some nuclear TCF/LEF-GFP in control enteroids, but limited TCF/LEF-GFP in EDTB KO enteroids grown in normal growth media (Figure 6). Stimulation of enteroids with Wnt3a results in increased nuclear GFP expression in control cultures (Figure 6), but not in enteroids with EDTB KO. Taken together, these results suggest that EDTB promotes Wnt signaling in a cell autonomous manner in the developing intestine.
Within the stem cell compartment, LGR5 is a direct target of Wnt (Van der Flier et al., 2007; Yamamoto et al., 2003). Previous studies using LGR5-GFP to identify stem cells has shown that there are two GFP-positive populations. GFPhi expressing cells are stem cells and GFPlo cells are in the transit amplifying population (van der Flier et al., 2009). To assess the effects of EDTB KO on Wnt signaling within the stem cell compartment, we crossed the LGR5-EGFP-IRES-CreERT2 mice with EDTBfl/fl mice. Mothers were gavaged with 4-OHT from P3 to P7, and crypts were isolated at P8. Isolated crypts were dissociated and analyzed by flow cytometry for GFP expression. 81% of cells from WT and 83% from EDTB KO crypts excluded 7AAD, indicating no general cell death following EDTB KO. 5,000 to 10,000 live cells were analyzed from each sample. In this analysis, 20 to 40% of crypt cells were GFP positive, and encompassed both GFPlo and GFPhi populations. In control mice, the GFPhi population comprises 47% of GFP-positive cells, whereas EDTB KO reduces the GFPhi population to 31.5% of the total GFP-positive cells (Figure 6). Taken together these results suggest that loss of EDTB results in decreased Wnt activity in the stem cell compartment of the ileum.
Discussion
The mammalian intestine goes through a stage in which it is highly endocytic, with a well-developed apical endosomal complex and extensive lysosomal compartment (Gonnella and Neutra, 1984; Knutton et al., 1974; Pasternak et al., 2016; Trahair et al., 1995; Wilson et al., 1987; Wissig and Graney, 1968). This complex is known to impact the development, patterning, and nutrition of the developing animal, and knockout of endocytic mediators, including EDTB, results in aberrant patterning and morphogenesis (Cox et al., 2018; Park et al., 2019; Rodriguez-Fraticelli et al., 2015; Sobajima et al., 2014). Further, knockout of transcription factors that normally promote the assembly of the endocytic complex also impacts intestinal development and maturation (Gao and Kaestner, 2010; Harper et al., 2011; Muncan et al., 2011). However, our results demonstrate that EDTB is not only a component of the apical endocytic complex on villus epithelial cells, but also plays a role in regulating signaling in the proliferative cells of the intervillous region and crypt during the early postnatal period.
Wnt signaling is developmentally regulated in the intestine. In mice, it is detected at day E16 and is present primarily in the villus epithelium (Kim et al., 2007). After birth, Wnt signaling is restricted to the intervillous region by postnatal day 3 and is localized to the crypts by postnatal day 8 (Gregorieff et al., 2005; Kim et al., 2007). EDTB is also developmentally regulated, with expression increasing at E19 and decreasing after weaning (Pasternak et al., 2016; Wilson et al., 1991). While the highest expression of EDTB is observed on the villi in the endosome-rich AEC, our results both in vivo and in vitro suggest that EDTB levels at the base of the villus, which have no giant lysosome and fewer apical vesicles (Wissig and Graney, 1968) are sufficient to impact signaling in these cells. Our results, including the changes in activation of Wnt pathway components, together with the effect on TCF/LEF localization after EDTB KO, suggest that EDTB is modulating Wnt signaling.
Proliferation during intestinal development appears to require distinct signaling and cell populations. For example, before E14.5, proliferation takes place throughout the pseudostratified epithelium and is Wnt independent (Chin et al., 2016). However, later in development, proliferation becomes Wnt dependent. Notch signaling relies on membrane trafficking and regulates stem cell proliferation and homeostasis in the adult intestine (Fre et al., 2011; VanDussen et al., 2012). In the embryonic intestine, ectopic expression of NICD blocks proliferation in the in the intervillus region. However, in adults, gain of function experiments result in decreased of secretory cells. (Stanger et al., 2005; van Es et al., 2005). We find no change in NICD or secretory populations with EDTB KO, suggesting that EDTB is not impacting Notch signaling. Other pathways that regulate proliferation in the developing and adult intestine include EGF/Neuregulin1 and AKT (Berlanga-Acosta et al., 2001; Bongers et al., 2012; Guo et al., 2021; Holloway et al., 2021; Jarde et al., 2020; Kitchen et al., 2005; Kotani et al., 2020; Ulshen et al., 1986; Zhang et al., 2020). However, our studies of EDTB in cultured cells suggest that it does not affect the Erk and AKT pathways (Cox et al., 2015). YAP signaling does impact regeneration in the intestine, and can be a downstream effector of Wnt (Azzolin et al., 2014; Guillermin et al., 2021). We have found that EDTB modulates YAP signaling in cultured cells, and these pathways may function together during development.
Wnt signaling and membrane trafficking intersect at many points (Demir et al., 2013; Johansson et al., 2019; Saito-Diaz et al., 2018; Vinyoles et al., 2014). Transduction of the Wnt signal involves internalization of the receptor complex (Blitzer and Nusse, 2006; Seto and Bellen, 2006), although recent results suggest this is not always the case (Rim et al., 2020). In colorectal cancer, the transmembrane protein TMEM9 regulates vacuolar acidification and promotes degradation of APC, promoting ß-catenin translocation to the nucleus (Jung et al., 2018). EDTB is essential for formation of lysosomes during intestinal morphogenesis (Cox et al., 2018), and it is possible that it plays a role in promotion of degradation of some of these inhibitory components and in its absence these inhibitory components dampen Wnt signaling.
While EDTB has been identified as regulator of apical membrane trafficking and barrier function in epithelial cells and differentiated enterocytes (Cox et al., 2018; Cox et al., 2015; McCarter et al., 2010), the results reported here imply an additional role for EDTB. Given its role as an endocytic factor, EDTB could play a role in signalosome recycling. Furthermore, since we find that EDTB is expressed OLFM and LGR5-positive stem cells, it may have a specialized role in these cells. Recent work has identified a “revival” stem cell type that utilizes YAP signaling for intestinal regeneration (Ayyaz et al., 2019). Since EDTB has also been shown to impact YAP signaling (Cox et al., 2015), it could be that EDTB plays a role in this newly identified stem cell population that is particularly responsive to intestinal epithelial injury. Further characterization of these cells will be necessary to establish their role in this process.
Highlights.
Endosomal regulation of proliferation
Endotubin expression in stem cell compartment
Endotubin regulation of Wnt signaling
Membrane trafficking regulates Wnt signaling
Acknowledgements
These studies were supported by grants from the NIDDK RO1 DK109701(J.M.W.); R00 DK103126 (C.T.), NIDKK 5RO1 DK109711(P.K.). Support was also provided for the Experimental Mouse Shared Resource and Imaging Core via the National Cancer Institute Cancer Center Support Grant P30 CA020374. We are also grateful to Dr. Gregory Rogers for his comments on the manuscript.
Grant Support:
These studies were supported by grants from the NIDDK RO1 DK109701(J.M.W.); R00 DK103126 (C.T.), NIDKK 5RO1 DK109711(P.K.).
Experimental Mouse Shared Resource and Imaging Core are supported through the National Cancer Institute Cancer Center Support Grant P30 CA020374.
Abbreviations:
- AEC
apical endocytic complex
- APC
Adenomatous polyposis coli
- Caco2BBE
human colon adenoma carcinoma cell line
- CC-3
cleaved caspase-3
- Cre
cre recombinase
- EDTB
Endotubin
- EDTB KO
Endotubin knockout
- GFP
green fluorescent protein
- LRP6
LDL receptor related protein 6
- LGR5
Leucine-rich repeat-containing G-protein Coupled Receptor 5
- pHH3
Phospho-Histone H3
- P3
postnatal day 3
- TCF/LEF
T-cell factor/lymphoid enhancer-binding factor
- 4-OHT
4-hydroxytamoxifen
- YAP
Yes-associated protein
Footnotes
Conflict of interest: No conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, Fink M, Barutcu S, Trcka D, Shen J, Chan K, Wrana JL, Gregorieff A, 2019. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569, 121–125. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S, 2014. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H, 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD, 2013. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga-Acosta J, Playford RJ, Mandir N, Goodlad RA, 2001. Gastrointestinal cell proliferation and crypt fission are separate but complementary means of increasing tissue mass following infusion of epidermal growth factor in rats. Gut 48, 803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer JT, Nusse R, 2006. A critical role for endocytosis in Wnt signaling. BMC Cell Biol 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers G, Muniz LR, Pacer ME, Iuga AC, Thirunarayanan N, Slinger E, Smit MJ, Reddy EP, Mayer L, Furtado GC, Harpaz N, Lira SA, 2012. A role for the epidermal growth factor receptor signaling in development of intestinal serrated polyps in mice and humans. Gastroenterology 143, 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AM, Tsai YH, Finkbeiner SR, Nagy MS, Walker EM, Ethen NJ, Williams BO, Battle MA, Spence JR, 2016. A Dynamic WNT/beta-CATENIN Signaling Environment Leads to WNT-Independent and WNT-Dependent Proliferation of Embryonic Intestinal Progenitor Cells. Stem Cell Reports 7, 826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CM, Lu R, Salcin K, Wilson JM, 2018. The Endosomal Protein Endotubin Is Required for Enterocyte Differentiation. Cell Mol Gastroenterol Hepatol 5, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CM, Mandell EK, Stewart L, Lu R, Johnson DL, McCarter SD, Tavares A, Runyan R, Ghosh S, Wilson JM, 2015. Endosomal regulation of contact inhibition through the AMOT:YAP pathway. Mol Biol Cell 26, 2673–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir K, Kirsch N, Beretta CA, Erdmann G, Ingelfinger D, Moro E, Argenton F, Carl M, Niehrs C, Boutros M, 2013. RAB8B is required for activity and caveolar endocytosis of LRP6. Cell Rep 4, 1224–1234. [DOI] [PubMed] [Google Scholar]
- Deng F, Peng L, Li Z, Tan G, Liang E, Chen S, Zhao X, Zhi F, 2018. YAP triggers the Wnt/beta-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis 9, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S, 2004. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193. [DOI] [PubMed] [Google Scholar]
- Fevr T, Robine S, Louvard D, Huelsken J, 2007. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 27, 7551–7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Hannezo E, Sale S, Huyghe M, Lafkas D, Kissel H, Louvi A, Greve J, Louvard D, Artavanis-Tsakonas S, 2011. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS One 6, e25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Kaestner KH, 2010. Cdx2 regulates endo-lysosomal function and epithelial cell polarity. Genes Dev 24, 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnella PA, Neutra MR, 1984. Membrane-bound and fluid-phase macromolecules enter separate prelysosomal compartments in absorptive cells of suckling rat ileum. J Cell Biol 99, 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H, 2005. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129, 626–638. [DOI] [PubMed] [Google Scholar]
- Guillermin O, Angelis N, Sidor CM, Ridgway R, Baulies A, Kucharska A, Antas P, Rose MR, Cordero J, Sansom O, Li VSW, Thompson BJ, 2021. Wnt and Src signals converge on YAP-TEAD to drive intestinal regeneration. EMBO J 40, e105770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Gao J, Qian Y, Wang H, Liu J, Peng Q, Zhou Y, Wang K, 2021. miR-125b-5p inhibits cell proliferation by targeting ASCT2 and regulating the PI3K/AKT/mTOR pathway in an LPS-induced intestinal mucosa cell injury model. Exp Ther Med 22, 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam DJ, Upperman JS, Grishin A, Ford HR, 2005. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg 14, 49–57. [DOI] [PubMed] [Google Scholar]
- Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ, 2011. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci U S A 108, 10585–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway EM, Czerwinski M, Tsai YH, Wu JH, Wu A, Childs CJ, Walton KD, Sweet CW, Yu Q, Glass I, Treutlein B, Camp JG, Spence JR, 2021. Mapping Development of the Human Intestinal Niche at Single-Cell Resolution. Cell Stem Cell 28, 568–580 e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarde T, Chan WH, Rossello FJ, Kaur Kahlon T, Theocharous M, Kurian Arackal T, Flores T, Giraud M, Richards E, Chan E, Kerr G, Engel RM, Prasko M, Donoghue JF, Abe SI, Phesse TJ, Nefzger CM, McMurrick PJ, Powell DR, Daly RJ, Polo JM, Abud HE, 2020. Mesenchymal Niche-Derived Neuregulin-1 Drives Intestinal Stem Cell Proliferation and Regeneration of Damaged Epithelium. Cell Stem Cell 27, 646–662 e647. [DOI] [PubMed] [Google Scholar]
- Johansson J, Naszai M, Hodder MC, Pickering KA, Miller BW, Ridgway RA, Yu Y, Peschard P, Brachmann S, Campbell AD, Cordero JB, Sansom OJ, 2019. RAL GTPases Drive Intestinal Stem Cell Function and Regeneration through Internalization of WNT Signalosomes. Cell Stem Cell 24, 592–607 e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YS, Jun S, Kim MJ, Lee SH, Suh HN, Lien EM, Jung HY, Lee S, Zhang J, Yang JI, Ji H, Wu JY, Wang W, Miller RK, Chen J, McCrea PD, Kopetz S, Park JI, 2018. TMEM9 promotes intestinal tumorigenesis through vacuolar-ATPase-activated Wnt/beta-catenin signalling. Nat Cell Biol 20, 1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BM, Mao J, Taketo MM, Shivdasani RA, 2007. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology 133, 529–538. [DOI] [PubMed] [Google Scholar]
- Kitchen PA, Goodlad RA, FitzGerald AJ, Mandir N, Ghatei MA, Bloom SR, Berlanga-Acosta J, Playford RJ, Forbes A, Walters JR, 2005. Intestinal growth in parenterally-fed rats induced by the combined effects of glucagon-like peptide 2 and epidermal growth factor. JPEN J Parenter Enteral Nutr 29, 248–254. [DOI] [PubMed] [Google Scholar]
- Knutton S, Limbrick AR, Robertson JD, 1974. Regular structures in membranes. I. Membranes in the endocytic complex of ileal epithelial cells. J Cell Biol 62, 679–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H, 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19, 379–383. [DOI] [PubMed] [Google Scholar]
- Kotani T, Setiawan J, Konno T, Ihara N, Okamoto S, Saito Y, Murata Y, Noda T, Matozaki T, 2020. Regulation of colonic epithelial cell homeostasis by mTORC1. Sci Rep 10, 13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, Ciclitira PJ, Al-Hassi HO, 2016. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol 22, 3117–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulou M, Matlock BK, Nlandu-Khodo S, Simmons AJ, Lau KS, Phillips-Mignemi M, Ivanova A, Alford CE, Flaherty DK, Gewin LS, 2019. Novel kidney dissociation protocol and image-based flow cytometry facilitate improved analysis of injured proximal tubules. Am J Physiol Renal Physiol 316, F847–F855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter SD, Johnson DL, Kitt KN, Donohue C, Adams A, Wilson JM, 2010. Regulation of tight junction assembly and epithelial polarity by a resident protein of apical endosomes. Traffic 11, 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Nighot P, Reyes C, Rawat M, McKee J, Lemon D, Hanson J, Ma TY, 2016. Intestinal barrier dysfunction in human necrotizing enterocolitis. Journal of pediatric surgery 51, 1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncan V, Heijmans J, Krasinski SD, Buller NV, Wildenberg ME, Meisner S, Radonjic M, Stapleton KA, Lamers WH, Biemond I, van den Bergh Weerman MA, O’Carroll D, Hardwick JC, Hommes DW, van den Brink GR, 2011. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun 2, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncan V, Sansom OJ, Tertoolen L, Phesse TJ, Begthel H, Sancho E, Cole AM, Gregorieff A, de Alboran IM, Clevers H, Clarke AR, 2006. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol Cell Biol 26, 8418–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudhoff MJ, Braam MJS, Freeman SA, Wong D, Rattray DG, Wang J, Antignano F, Snyder K, Refaeli I, Hughes MR, McNagny KM, Gold MR, Arrowsmith CH, Sato T, Rossi FMV, Tatlock JH, Owen DR, Brown PJ, Zaph C, 2016. SETD7 Controls Intestinal Regeneration and Tumorigenesis by Regulating Wnt/beta-Catenin and Hippo/YAP Signaling. Dev Cell 37, 47–57. [DOI] [PubMed] [Google Scholar]
- Park J, Levic DS, Sumigray KD, Bagwell J, Eroglu O, Block CL, Eroglu C, Barry R, Lickwar CR, Rawls JF, Watts SA, Lechler T, Bagnat M, 2019. Lysosome-Rich Enterocytes Mediate Protein Absorption in the Vertebrate Gut. Dev Cell 51, 7–20 e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak AJ, Hamonic GM, Van Kessel A, Wilson HL, 2016. Postnatal regulation of MAMDC4 in the porcine intestinal epithelium is influenced by bacterial colonization. Physiol Rep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim EY, Kinney LK, Nusse R, 2020. β-catenin-mediated Wnt signal transduction proceeds through an endocytosis-independent mechanism. Mol Biol Cell 31, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli AE, Bagwell J, Bosch-Fortea M, Boncompain G, Reglero-Real N, Garcia-Leon MJ, Andres G, Toribio ML, Alonso MA, Millan J, Perez F, Bagnat M, Martin-Belmonte F, 2015. Developmental regulation of apical endocytosis controls epithelial patterning in vertebrate tubular organs. Nat Cell Biol 17, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito-Diaz K, Benchabane H, Tiwari A, Tian A, Li B, Thompson JJ, Hyde AS, Sawyer LM, Jodoin JN, Santos E, Lee LA, Coffey RJ, Beauchamp RD, Williams CS, Kenworthy AK, Robbins DJ, Ahmed Y, Lee E, 2018. APC Inhibits Ligand-Independent Wnt Signaling by the Clathrin Endocytic Pathway. Dev Cell 44, 566–581 e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijers J, van der Flier LG, van Es J, Clevers H, 2014. Robust cre-mediated recombination in small intestinal stem cells utilizing the olfm4 locus. Stem Cell Reports 3, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scurrah CR, Simmons AJ, Lau KS, 2019. Single-Cell Mass Cytometry of Archived Human Epithelial Tissue for Decoding Cancer Signaling Pathways. Methods Mol Biol 1884, 215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ, 2006. Internalization is required for proper Wingless signaling in Drosophila melanogaster. J Cell Biol 173, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobajima T, Yoshimura S, Iwano T, Kunii M, Watanabe M, Atik N, Mushiake S, Morii E, Koyama Y, Miyoshi E, Harada A, 2014. Rab11a is required for apical protein localisation in the intestine. Biol Open 4, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivillibhuthur M, Warder BN, Toke NH, Shah PP, Feng Q, Gao N, Bonder EM, Verzi MP, 2018. TFAM is required for maturation of the fetal and adult intestinal epithelium. Dev Biol 439, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ, Datar R, Murtaugh LC, Melton DA, 2005. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A 102, 12443–12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahair JF, Wilson JM, Neutra MR, 1995. Identification of a marker antigen for the endocytic stage of intestinal development in rat, sheep, and human. J Pediatr Gastroenterol Nutr 21, 277–287. [DOI] [PubMed] [Google Scholar]
- Ulshen MH, Lyn-Cook LE, Raasch RH, 1986. Effects of intraluminal epidermal growth factor on mucosal proliferation in the small intestine of adult rats. Gastroenterology 91, 1134–1140. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H, 2009. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 137, 15–17. [DOI] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H, 2007. The Intestinal Wnt/TCF Signature. Gastroenterology 132, 628–632. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H, 2005. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963. [DOI] [PubMed] [Google Scholar]
- VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud A, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC, 2012. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139, 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinyoles M, Del Valle-Perez B, Curto J, Vinas-Castells R, Alba-Castellon L, Garcia de Herreros A, Dunach M, 2014. Multivesicular GSK3 sequestration upon Wnt signaling is controlled by p120-catenin/cadherin interaction with LRP5/6. Mol Cell 53, 444–457. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Whitney JA, Neutra MR, 1987. Identification of an endosomal antigen specific to absorptive cells of suckling rat ileum. J Cell Biol 105, 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Whitney JA, Neutra MR, 1991. Biogenesis of the apical endosome-lysosome complex during differentiation of absorptive epithelial cells in rat ileum. J Cell Sci 100 ( Pt 1), 133–143. [DOI] [PubMed] [Google Scholar]
- Wissig SL, Graney DO, 1968. Membrane modifications in the apical endocytic complex of ileal epithelial cells. J Cell Biol 39, 564–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Sakamoto M, Fujii G, Tsuiji H, Kenetaka K, Asaka M, Hirohashi S, 2003. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology 37, 528–533. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY, 2001. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bandyopadhyay S, Araujo LP, Tong K, Flores J, Laubitz D, Zhao Y, Yap G, Wang J, Zou Q, Ferraris R, Zhang L, Hu W, Bonder EM, Kiela PR, Coffey R, Verzi MP, Ivanov II, Gao N, 2020. Elevating EGFR-MAPK program by a nonconventional Cdc42 enhances intestinal epithelial survival and regeneration. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]