Abstract
Tuberculosis (TB) once considered a disease of the developing world is infrequent in the developing world too. Its worldwide prevalence with a huge impact on the healthcare system both in economic and health terms has prompted the World Health Organization to make it a top priority infectious disease. Tuberculous infection of the pulmonary system is the most common form of this disease, however, extrapulmonary TB is being increasingly recognized and more often seen in immunocompromised situations. Gastrointestinal TB is a leading extrapulmonary TB manifestation that can defy diagnosis. Overlap of symptoms with other gastrointestinal diseases and limited accuracy of diagnostic tests demands more awareness of this disease. Untreated gastrointestinal TB can cause significant morbidity leading to prolonged hospitalization and surgery. Prompt diagnosis with early initiation of therapy can avoid this. This timely review discusses the epidemiology, risk factors, pathogenesis, clinical presentation, current diagnostic tools and therapy.
Keywords: Abdominal tuberculosis, clinical features, diagnosis, extrapulmonary tuberculosis, gastrointestinal tuberculosis, pathogenesis, risk factors, treatment, tuberculosis
INTRODUCTION
Tuberculosis, an eighteenth century infectious disease is still relevant in the twenty-first century, despite the emergence of novel pathogens like coronavirus that is currently gripping the whole world. Tuberculosis (TB) is significant because it infects an estimated quarter of the world's population.[1] Though the incidence of TB is the highest in the Asian and African continents, the developed nations too are seeing a surge, mainly in latent TB (LTB) due to immunocompromising diseases, biologic use and migration. The global burden of TB prompted the United Nations to forge a declaration by all member states to curb the disease by 2030.[2] Estimated economic impact due to TB is immense; for example, the annual cost in terms of TB-related deaths in a high burden country like India is approximated around US $30 billion, and to reach its aim, the United Nations needs an annual budget of US $13 billion.[2,3]
Pulmonary TB is the primary manifestation of tuberculous infection and is the focus of the World Health Organization (WHO) strategy to control this disease. However, extra pulmonary TB is increasing recognized with abdominal TB being one of the most common presentations as explained here. Abdominal TB arbitrarily includes infection of gastrointestinal tract, peritoneum, abdominal solid organs, and abdominal lymph nodes. Of all the tuberculous infections of the abdomen, gastrointestinal TB is the most common site of involvement. The clinical presentation of gastrointestinal TB is variable to non-specific making it challenging to diagnose. This leads to a delay in diagnosis with consequential serious morbidity. Given its importance and the need to recognize this old infective foe, we present a timely review of the epidemiology, pathogenesis, clinical features, diagnosis and therapy of gastrointestinal TB.
EPIDEMIOLOGY
Despite a steady decline in TB worldwide, around 10 million people were estimated to be ill with it in 2018 of which more than a million died, making TB the tenth most common cause of death.[1] TB is primarily an infection of the lungs (referred to as pulmonary TB), however, it can affect any organ of the body apart from lungs wherein it is termed extrapulmonary TB (EXPTB). It was globally estimated that 8 to 24% of TB cases were extrapulmonary, making up an average of 15% of the total TB cases notified to WHO.[1] This variation is reflected in the population and region where the study was carried out. In India, the country with the highest burden, 20% of TB cases were EXPTB; of this, 34% were lymphatic TB, 25% pleural, followed by abdominal at nearly 13%.[4,5] From another high burden country, China, EXPTB constituted about 31% (6433/20,534) of all TB cases. Of this 31%, the most common site was, unexpectedly, the skeletal system (41%; 2643/6433), followed by pleura (26%; 1673/6433). The abdominal site was not specifically looked at, and the unclassified “other” site formed 14% (873/6433) of all EXPTB cases.[6] A study from Pakistan, which carries the fifth largest burden of TB, showed that the proportion of EXPTB was nearly 30% (15790/54092) of all the notified TB cases; 21% (3313/15790) of EXPTB cases were abdominal in origin, following pleural (29.6%; 4668/15790) and lymphatic (21.5%; 3581/15790) locations.[7] In countries with medium-sized TB burden, EXPTB constituted 13% (1222/8113) of all TB cases with 9% (105/1222) of these being abdominal TB, making it the sixth most common site.[8] A low TB incidence country like the United States had 20% EXPTB (2412/11088) of all TB cases, with the most common site being the lymphatics (40%; 1012/2412), and abdomen being the fourth most common site at 6% (140/2412).[9] Similarly, in Europe, extrapulmonary location accounted for 17% of all TB cases, with abdomen (3%; 2780/95003) being the sixth most common site[10] [Table 1]. In South Africa, a sub-Saharan country that has high TB and Human Immunodeficiency Virus (HIV) burden, nearly 43% (80/188) of all TB cases presented as EXPTB, and of this, 28% (22/80) involved the abdomen, the third most common site after pleura and lymphatics.[11] In a coinfected population of HIV and TB from a multicenter cohort, EXPTB accounted for 28% (765/2695) of all TB cases, with abdominal TB ranking third (11%; 85/765) among the affected sites.[12]
Table 1.
Various sites involved in extrapulmonary TB from different countries
| Ref. No | Country | n | Abdominal | Lymphatics | Pleural | Bones | Nervous system | Other sites |
|---|---|---|---|---|---|---|---|---|
| 4 | India | 2046 | (13) | (34) | (25) | (9) | (10) | (9) |
| 6 | China | 6433 | - | 333 (5) | 1673 (26) | 2643 (41) | 440 (7) | 873 (14) |
| 7 | Pakistan | 15790 | 3313 (21) | 3581 (23) | 4668 (30) | 1483 (9) | 725 (5) | 2020 (13) |
| 8 | Malaysia | 1222 | 105 (9) | 324 (27) | 227 (19) | 116 (9) | 122 (10) | 328 (27) |
| 9 | United States | 2412 | 140 (6) | 1012 (40) | 393 (16) | 270 (11) | 138 (6) | 448 (18) |
| 10 | Europe | 95003 | 2780 (3) | 28019 (29) | 38029 (40) | 8219 (9) | 3180 (3) | 14779 (16) |
(%) = numbers in parenthesis are percentages; Ref. no=reference number; n=total number of extrapulmonary TB cases
The incidence of TB in general is dropping across the globe; however, the proportion of EXPTB is on the rise. Europe has seen this rise from 16.4 to 22.4% within a decade,[13] and in the United States, the proportion has risen from 15.7% in 1993 to 20.4% in 2018. In contrast, the rates of abdominal TB have remained the same over the years suggesting that it is following the trends of EXPTB.[9,14] A rising trend of EXPTB has been reported from a high-incidence country like India, too.[15] This rising trend may in part be due to an increasing awareness and better diagnostic tools, and additionally may reflect an increase in HIV infections over the years.
RISK FACTORS
Further detailed, studies imply that in general the risk factors associated with EXPTB tend to be the same as those for abdominal TB.[10,16,17] In a population-based study in the USA that looked at the risk factors for EXPTB, both EXPTB and abdominal TB were strongly associated with female gender and end-stage renal disease.[16] Similarly, from national TB surveillance data, it was shown that both EXPTB and abdominal TB were more likely to be related to young age, female gender, Asian ethnicity and Black race.[17] A multistate Malaysian study that collected data on EXPTB from four different states reported similar risk factors for both EXPTB and abdominal TB. Being female (11% vs 7%), an age of <15 years (20% vs 3%, >65 years), being of Indian origin (9.4% vs 8% Malaya), having diabetes (9.4%), being a non-smoker (7% vs 5%), not consuming alcohol (9% vs 6%), and being unemployed (9.7% vs 6.4%) were more likely to have abdominal TB. In comparison, lymphatic TB, the most common EXPTB site was more frequently seen in males (28% vs 25%), middle age group (40% vs 9%), in Malaya (34% vs 27%) and employed (30% vs 24%) patients without any predilection for smokers, alcoholics or diabetics.[8] In Europe, having origins from the Indian subcontinent (adjusted odds ratio [aOR] = 6.36) or Africa (aOR = 4.64), extremes of age (<15 and > 64 years) (aOR = 2.21) and female gender (aOR = 2.02) were strongly associated with both EXPTB and abdominal TB.[10] A nationwide study from Pakistan reported similar risk patterns, i.e., female gender, age <15 years and coming from a specific region (like a tribal area within Pakistan) for both EXPTB and abdominal TB.[7]
One of the important forms of TB to consider in risk factors is LTB. Around 30% of the world's population is estimated to be infected with LTB of which 10-15% may reactivate to active TB.[18] LTB is more of a concern in low-incidence areas of TB such as the United States, where 80% of active TB cases are due to reactivation of LTB.[19] Reactivation of LTB tends to present more in the EXPTB sites, including abdominal TB. England saw a 50% increase in EXPTB with 80% rise in abdominal TB over a 5-year period that was mainly attributed to the reactivation of TB.[20] Hence, factors that increase reactivation of LTB directly influence the risk of abdominal TB. Two such important risk factors are solid organ transplantation and use of anti-tumor necrosis factor (TNF) alpha medications.
Immunosuppression
Solid organ transplants (SOT) with immunosuppressants increase the risk of TB by twenty-fold, and the proportion developing EXPTB ranges between 37 and 67%. This occurs with a corresponding increase in abdominal TB that is much higher than that of the background population.[21] In a meta-analysis of TB in liver-transplanted patients, abdominal TB was the most common site accounting for 35% of all EXPTB cases; similarly, in a multicenter study on TB in renal transplant recipients, abdominal TB was the leading location, comprising 30% of all EXPTB sites.[22,23] LTB plays an important role in SOT patients. Untreated LTB increases the risk of TB with 8.2% developing TB and none in those treated with a course of isoniazid.[22] TB in SOT manifests as two peaks following the transplant. The early peak occurs within the first 2 years and may be related to reactivation of LTB and the late peak after 5 years which is more representative of a new infection.[23] Most TB cases are diagnosed in the early peak (up to 56%), suggesting LTB as the main driver for TB in SOT.[23] However, only around one-third of transplant patients get screened for LTB and with an effective therapy to prevent TB infection, the guidelines recommend active screening for LTB and treatment of positive cases with 9 months of isoniazid.[24]
Another risk factor is the use of anti-tumour nectrotic factor (TNF) alpha medications. TNF alpha is an important armament in the body's immune response to TB infection; decreased activity increases the susceptibility to infection, and hence, use of anti-TNF alpha medications is recognized to reactivate LTB. For this reason, screening for TB prior to starting this medication is a standard recommendation. From surveillance data of the food and drug administration of United States Food and Drug Administration (FDA), the incidence of TB in patients on anti-TNF alpha was fourfold higher than the background rate, and EXPTB accounted for 56% (39/70) of these TB cases. Abdominal TB was the third most common form of EXPTB, a higher than usual proportion, implying an increased association with anti-TNF alpha use.[25] This was further confirmed in a French registry study that reported a similar proportion of 61% (42/69) of EXPTB with abdominal TB being the third most common.[26] Though no formal studies on the risk of TB in developing countries are available, given the high burden of TB in these regions, the risk associated with anti-TNF usage is estimated to be substantially higher, and thus, EXPTB and abdominal TB are estimated to have a higher incidence, too.[27]
Co-infection with HIV
There are more than a quarter of a million deaths due to TB in HIV-positive patients worldwide, with 0.8 million cases of TB linked to HIV infection.[1] HIV-positive patients carry a very high risk of developing TB, estimated to be around twenty-six-fold higher than average, and this risk increases with falling CD4 T cell count. EXPTB, including abdominal TB, is more common in HIV-infected TB patients with low CD4 count.[28] Systemic reviews and meta-analysis have suggested that HIV is more commonly seen in EXPTB than pulmonary TB, more so in patients with a CD4 count of <100, however, most of the studies were cross-sectional in nature.[29,30]
PATHOGENESIS
The mechanism behind EXPTB development is not fully understood. Various factors involved in the manner of pathogen interaction with the host may play a role, however, the dynamics of this is not well-understood.[28] It is now increasingly recognized that the prime route of transmission of TB is through inhalation that causes pulmonary infection, followed by the infection of the other organs by spread from this primary focus [Figure 1]. This happens predominantly through the lymphatic systems and the bloodstream. The initial breach from the pulmonary epithelium to get to the extrapulmonary sites is proposed to occur by one of these four mechanisms (1) by the help of macrophages, (2) direct infection of the epithelial cells, (3) through microfold cells (M cells), a kind of specialized epithelial cells or (4) by assistance of dentritic cells.[31] Genetic lineage of TB may determine the EXPTB site and its clinical presentation with Indian lineage closely associated with EXPTB.[32] Specific lineages of TB may have predilection to cause EXPTB due to their ability to replicate more and invade the macrophages.[33]
Figure 1.

Spread of pulmonary TB to abdomen
Another route of infection postulated is through ingestion of infected sputum from the lungs [Figure 1]; the ingested mycobacterium gains entry into the gut through the intestinal mucosa with the help of M and dendritic cells as explained previously. Macrophages, mainly present in the lymphoid tissue in the intestinal mucosa, ingest these mycobacteria and play an important role in the immune response. Contagious spread from an adjacent organ is a possible mechanism that is not entirely explicit.
CLINICAL FEATURES
Abdominal TB encompasses gastrointestinal, peritoneal, lymph nodal and solid organ involvement; its presentation is usually chronic or acute-on-chronic with non-specific symptoms. The duration of illness before diagnosis varies between weeks and months, some reporting up to 4 years, with an average of around 8 weeks. The symptoms are insidious in onset, and include, starting with the most common, fever, weight loss, night sweats and anorexia. The most frequent symptoms localized to the abdomen, in descending order of reporting, are abdominal pain, abdominal distension, nausea/vomiting, diarrhea and blood in stool. Signs often seen include abdominal tenderness, ascites, abdominal mass, jaundice, hepatomegaly and lymphadenopathy.[34,35,36,37,38,39,39,40,41,42,43],44,45,[46,47]
The most common site of abdominal TB involvement is the gastrointestinal tract, making 43-65% of all abdominal TB cases, followed by peritoneum (20-47%), lymph nodes (4-42%), and then finally solid organs like liver, gall bladder, spleen and pancreas in 1-23% of the cases. Up to 33% of the cases have multiple sites and between 15 and 54% have coexistent pulmonary TB [Table 2].[34,35,36,37,38,39,40,41,42,43,44,45,46,47]
Table 2.
Abdominal TB proportion of different sites
| Ref. No | n | Gastrointestinal | Peritoneal | Lymph nodal | Solid organs | Mixed | Coexist PTB |
|---|---|---|---|---|---|---|---|
| 33 | 139 | 69 (50) | 28 (20) | 7 (5) | 23 (17) | 12 (9) | |
| 34 | 256 | 127 (50) | 106 (41) | 10 (4) | 7 (3) | 58 (23) | |
| 35 | 86 | 38 (44) | 41 (47) | 7 (8) | 28 (33) | 22 (26) | |
| 36 | 17 | 11 (65) | 5 (29) | 1 (6) | |||
| 37 | 59 | 26 (52) | 16 (32) | 8 (16) | (54) | ||
| 38 | 24 | 10 (41) | 14 (58) | 3 (12) | 10 (41) | ||
| 39 | 58 | 25 (43) | 27 (47) | 18 (31) | 2 (8) | 10 (17) | |
| 40 | 57 | 33 (58) | 13 (23) | 24 (42) | 11 (20) | 27 (47) | |
| 41 | 93 | 51 (51) | 33 (33) | 41 (41) | 27 (29) | ||
| 42 | 31 | 15 (48) | 11 (35) | 5 (17) | 11 (35) | ||
| 43 | 46 | 7 (15) | |||||
| 44 | 40 | (60) | (40) | ||||
| 45 | 65 | 23 (35) | 2 (3) | 22 (33) | 20 (31) | ||
| 46 | 209 | 103 (49) | 87 (42) | 9 (4) | 10 (5) | 134 (64) | |
| Total | 1180 | 43-65% | 20-47% | 4-58% | 1-20% | 9-33% | 3-64% |
() = numbers in parenthesis are percentages; Ref. no=reference number; n=total number of abdominal TB cases; Solid organs include liver, spleen and/or pancreas; PTB=pulmonary tuberculosis
Gastrointestinal tuberculosis
This involves the TB of the upper, middle and the lower gastrointestinal tract (GIT) from the esophagus to the anus.
Pathogenesis of gastrointestinal tuberculosis
Gastrointestinal tuberculosis (GITB) infection follows the same pattern described previously for abdominal TB, which includes hematogenous, lymphatic, direct and ingestion route of spread. In addition, ingestion of milk or food infected with bovine mycobacterium was previously a well-recognized route, however, the pasteurization of milk and prevention of bovine mycobacterial infection in livestock has made this rare. Whether GITB occurs as a primary infection, or as a secondary spread from pulmonary focus, is not well-defined; coexisting pulmonary TB and GITB is reported in 13-67% of the cases, with some arguing that this probably is an underestimate, due to unrecognized or asymptomatic primary focus.[48,49,50,51,52,53,54]
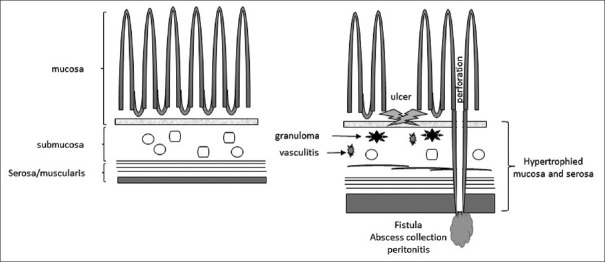
Once the mycobacterium invades into the submucosa through the mucosa of the intestine, it initiates a granulomatous inflammatory response including vasculitis with thickening of the submucosa and serosa; this leads to ulceration that can perforate or heal by fibrosis. Pathological intestinal TB can manifest as ulcerative, hypertrophic, ulcero-hypertrophic or in fibrotic forms. Complications occur as a result of these different forms of enteritis and present as strictures causing obstruction or perforations, including fistulas [Figure 2]. Rarely, hemorrhagic enteritis can occur.[49,55,56]
Figure 2.
Mycobacterium invades the submucosa leading to formation of granulomas, vasculitis and hypertrophy, which in turn cause ulcers, perforation, fistulas and abscess formation
Clinical presentation of gastrointestinal tuberculosis
TB can involve any site of the GIT from the esophagus to the anus, including the perianal region. However, it has a predilection for the ileocecal region, which is the most commonly infected site accounting for 44-84% of all GITB cases.[34,35,36,37,38,39,40,41,42,50,51,52,53] The physiology and anatomic pathology of a constricted ileocecal region, which leads to long contact time, increased absorption and abundant lymphatic tissue, explains the predisposition.[48,49] The other common sites of involvement include caecum, ileum, ascending colon, transverse colon, descending colon, jejunum and rectum.[34,35,36,37,38,39,40,41,42,50,51,52,53] It rarely infects the upper GIT (esophagus, stomach, duodenum) and anus [Table 3].[34,35,36,39,41,52,53]
Table 3.
Gastrointestinal TB proportion of different infected sites
| Ref. No | n | Ileocecal | Large bowel | Esophagus | Stomach | Duodenum | Small bowel |
|---|---|---|---|---|---|---|---|
| 33 | 69 | 46 (67) | 12 (17) | 3 (4) | 3 (4) | ||
| 34 | 212 | 122 (58) | 6 (3) | ||||
| 35 | 38 | 14 (37) | 6 (16) | 2 (5) | 2 (5) | 4 (36) | |
| 36 | 11 | 5 (45) | 2 (18) | ||||
| 37 | 26 | 20 (40) | 6 (12) | ||||
| 38 | 8 | 3 (38) | 3 (38) | 1 (1) | |||
| 39 | 25 | 14 (56) | 5 (20) | 2 (8) | 1 (4) | 1 (4) | 2 (8) |
| 40 | 33 | 2 (6) | 28 (85) | ||||
| 41 | 51 | 16 (31) | 36 (71) | ||||
| 49 | 85 | 56 (66) | |||||
| 50 | 81 | 46 (84) | |||||
| 51 | 61 | 26 (44) | 16 (26) | 21 (34) | |||
| 52 | 104 | 29 (28) | 6 (6) | ||||
| Total | 719 | 44%-84% | 3%-71% | 4%-8% | 4%-6% | 1%-6% | 8%-85% |
Ref. no=reference number; () = numbers in parenthesis are percentages; n=total number of gastrointestinal TB cases
Gastrointestinal TB of the twenty-first century is a disease of the young, with a mean age ranging between 32 and 40 years, and with a tendency to affect males (ranging from 48% to 66% ).[50,51,52,53,54,55] Patients present late, over weeks and months, and in some instances as late as 8 years.[51] In a multinational study of 104 GITB patients, the median time to initiation of anti-TB treatment was 70 days (range 10 days to 30 months).[53] History of prior TB or a history of family member with TB is discernable in only 5% (4/85) to 19% (20/104) of all cases.[50,53] Symptoms can be broadly classified into systemic and localized; the localized symptoms vary depending on the site involved and the pathological type of GITB. Systemic or constitutional symptoms are common to all sites involved and consist typically of fever, weight loss and night sweats.[50,51,52,53] Fever is of low grade usually between 37.5 and 38.5°C and is commonly reported in studies from high-burden TB countries in up to 73% (50/69) of patients.[50,51] Nevertheless, a study from England recorded fever in only 19% (12/61) of its cases.[52] In addition, 50% (52/104) to 80% (65/81) have weight loss, with associated malnourishment in about half of them with a BMI <18.[50,51,52,53,54,55] Abdominal pain is the most common localized symptom with more than 74% (45/61) reporting it. The pain is chronic, generalized or restricted to umbilical or right lower quadrant; occasionally, pain is acute in cases of intestinal obstruction and peritonitis.[50,51] Nausea and vomiting is present in 31% (19/61) to 44% (46/104) of the patients.[50,52,53] Change in bowel habits could be in the form of diarrhea or constipation, or alternating bowel habits, with diarrhea being described as bowel movements of three or five times a day with soft or watery stools.[51] Blood in stools is infrequent, and seen in less than 15% (10/69) [Figure 3].[51,52,53,54,55]
Figure 3.
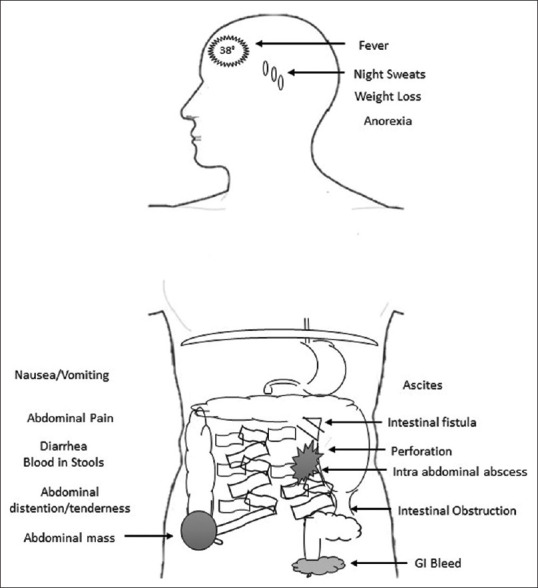
Constitutional and local symptoms/signs due to GITB
On examination, abdominal distention and tenderness are present in more than 50% of the cases.[50,53] A palpable abdominal mass is less often felt than previously thought and is reported in only 6% (5/81) to 19% (20/104) of the patients.[50,51,53,54] Whether the presence of ascites in 10% (7/69) to 35% (28/81) of the cases is a reflection of associated TB of other vicinal organs or primarily due to GITB is unclear.[50,51,53,54] In a study from a high-burden disease center, one-third of the patients presented with acute abdomen and signs of peritonitis.[50] When the adjoining viscera are concurrently involved with TB, lymphadenopathy and abdominal organomegaly may be present infrequently [Figure 350,52] and perianal disease in the form of perianal fistula or abscess is rare.[50,52,53] GITB can lead to serious complications like intestinal obstruction, perforation, intestinal fistula, intra-abdominal collection and GI bleeding. Intestinal obstruction is the most common complication, more often reported from high TB burden countries and from surgical literature, prevalent in up to 50% (127/256) to 75% of GITB cases.[35,45,50] Comparatively, in low TB incidence countries, 2% (2/86) to 10% (6/61) of GITB present with intestinal obstruction as a complication.[36,38,52] Intestinal strictures and adhesions were identified as the main reasons for intestinal obstruction. Similarly, intestinal perforation complicates GITB in nearly 15% (32/212) to 32% (36/110) of cases in developing countries compared to 13% (8/61) of cases in developed ones.[35,45,50,52,54]
Specific site gastrointestinal tuberculosis
Esophageal tuberculosis
Esophageal TB (OTB) is uncommon and present mainly with dysphagia [Figure 4].[34,36,40] It is predominantly secondary to spread from contagious intrathoracic viscera, though primary OTB with isolated esophageal lesions has been described.[48,57,58,59] The lesions are seen predominantly to involve the middle third of the esophagus in the form of ulcers[60,61]; rarely, the lesions may be proliferative appearing as malignancy or submucosal growth.[48,59] Complications in the form of abscesses, stricture, perforation, bleeding and fistulas to adjacent structures like trachea, bronchi, mediastinum and aorta may be the presenting features of OTB.[48,57,58,59,60,61,62]
Figure 4.

Different sites involved in GITB with associated symptoms
Gastroduodenal tuberculosis
Gastroduodenal TB (GDTB), similarly to OTB, is not commonly seen and accounts for around 1-6% of all GITB cases [Figure 4]. In a series of 52 GDTB patients, almost all presented with gastric outlet symptom in the form of vomiting, exclusively due to stricturing lesions in the gastroduodenal tract.[63] The most common form of presentation was by stricturing, mostly duodenal (52%; 12/23), followed by pyloric (9%; 2/23). The other findings were gastric ulcers (13%; 3/23), duodenal ulcers (4%; 1/23) and periampullary ulcers (9%; 2/23).[64] GDTB may present as submucosal gastric tumors with non-specific abdominal pain and distention, but rarely as fistulas to surrounding viscera and peritonitis due to perforation.[65,66,67,68]
Small bowel tuberculosis
Also referred to as tuberculous enteritis, small bowel tuberculosis (SBTB) has a predilection to the ileocecal region as explained previously and other sites with mainly obstructive symptoms[34,35,36,39,41,42,50,51,52,53] [Figure 4]. Presentation of SBTB is rarely described in exclusivity as most studies include small bowel with other parts of GITB.
The main precipitating factors found during surgery have been adhesions in 59% (129/212), strictures in 37% (78/212) as well as mass lesions in 7% (14/212).[35] The pathological morphology, giving rise to the symptoms are predominantly ulcerative in 22% (4/18) to 66% (73/110) of cases, followed by proliferative in 20% (11/55) to 51% (57/110), and less often due to a combination of ulceroproliferative lesions in around 27% (15/55).[50,51,52,53,54,56]
Colonic tuberculosis
The reports of large bowel involvement is variable, ranging from as low as 3% of cases up to 71% of all GITB cases [Figure 4].[36,41,42,50,51,52,53]
Symptoms of presentation are similar to GITB and SBTB, as most of these infections often coexist. Intestinal obstruction, perforation, fistulae and bleeding are generally the presenting complaints.[69,70,71] Anal TB is rare and the main symptoms are pain and discharge, though bleeding and feeling of mass may be other less reported symptoms. A common finding is anal fistula, followed by ulcers, inflammation and mass lesion. More than two-thirds have coexisting pulmonary TB and nearly one-third have associated colonic inflammation.[72,73,74]
DIAGNOSIS
Due to its protean manifestations and non-specific symptoms, diagnosing GITB is a challenge. A singular test may rarely be sufficient in obtaining the diagnosis. Apart from biochemistry, a combination of microbiological, radiological and endoscopic investigations may clinch the diagnosis.
Biochemistry
Generally, a high erythrocyte sedimentation rate (ESR) should raise suspicion as it is present in 69% (72/104) to 87% of GITB cases.[50,51,53] Anemia is also common, seen in 22% (19/85) to 90% (94/104), and is usually mild to moderate.[50,51,53] Other abnormalities are hypoalbuminea reported in around 44% of GITB patients, and leukocytosis in about 16-26%.[50,51,53] A purified protein derivative skin test may be positive in 52% (42/81) to 88% and a interferon-gamma release assay like T-spot test can be positive in 86% (70/81) of GITB patients. While these tests aid in diagnosis, a negative result does not exclude GITB.[50,51,53]
Ascites, when present, is a valuable source for diagnosis. Ascitic fluid is described as straw colored with white cell counts of 500-1,500 per cubic mm that are mainly lymphocytes.[59,75,76] In a series of GITB, the differential count was 60% lymphocytes, 25% neutrophils and 15% monocytes.[44] The protein in the fluid is typically elevated with levels >2.5 g/dL and a serum-ascites albumin gradient of <11 g/L.[59,75,76]
Adenosine deaminase (ADA) is a biomarker increasingly used as an aid in the diagnosis of abdominal TB. A systemic review of over 1,305 patients showed that an ADA level >30 U/L had a sensitivity of 94%.[76] Two meta-analyses were done looking at the ADA diagnostic accuracy: One included four studies using levels between 36 and 40 IU/L with a sensitivity and specificity of 100 and 97%, respectively, and suggested a level of ≥39 IU/L to be most diagnostic,[77] and a later meta-analysis including 17 studies estimated the sensitivity and specificity of 93 and 94%, respectively, however the range of ADA cut-off was between 21 and 40 IU/L.[78]
Radiology
Radiological imaging is a cornerstone in GITB diagnosis. Small bowel follow-through or barium enema are now rarely used due to far better imaging by computerized tomography (CT) and less radiation. The classical signs described on such contrast studies like “Fleischner sign”: wide open ileocecal valve, “conical caecum”: pulled up view of caecum; “purse string sign”: short stricture ileocecal valve with proximal ileal dilatation are seldom seen.[79] The use of ultrasound scan to look for specific features of abdominal TB did not fare well as a diagnostic tool. In a Cochrane review of 11 studies, the overall sensitivity and specificity was 63 and 68%, respectively.[80]
CT imaging is the preferred modality, with the ability to detect extra and intramural changes and complications like obstruction and perforation. Involvement of the peritoneum can be identified by its thickening on CT, that is described either as being smooth or nodular with a smooth smudge pattern being more common.[81,82,83] Ascites can be seen either loculated or free with fibrin strands and has high attenuation.[81,82,83] Lymph node involvement commonly appears as enlarged necrotic with enhancement of periphery and is either discrete or matted, calcification is uncommon. Mesenteric, periportal and peripancreatic are the most frequently involved sites.[81,82,83] Intestinal TB characteristically appears as homogeneous bowel wall thickening that is usually circumferential. Other features include segmental strictures that could be isolated or multiple.[81,82,83] Uncommonly, heterogeneous mass may be noted, caused by the thickening of ileocecal valve, terminal ileum, part of caecum and lymph nodes.[81] In addition, CT scan can identify complications like intestinal obstruction, perforations, fistulae and abscess collection. On review of different studies that used CT as a diagnostic modality, the frequency of the above-mentioned features varied. Ascites was detected in 33% (26/104) to 79% (87/110) of the cases, abdominal lymph nodes in 14% (3/31) to 54% (25/65), bowel wall thickening in 25% (28/110) to 71% (49/69) and intra-abdominal collection was seen less frequently in 4% (3/104) to 18% (11/61).[41,43,44,46,47,52,53,55,81] Apart from imaging, CT can be used to take tissue specimens for histopathological and microbiological analysis.
MRI is rarely used as a diagnostic tool. Although, it has been used as a supportive tool to further define abnormalities on CT like fistulae, and characterize lymph nodes to differentiate from other causes like lymphoma.[52,84]
Endoscopy
Endoscopy pays an important part in diagnosis by complementing other modalities. In some instances, it may be the initial tool for diagnosis, depending on the type of symptoms and presentation. Apart from detecting typical endoscopic lesions, the additional benefit of endoscopy is the ability to obtain specimens both for histopathological and microbiological analysis.
Lesions seen on endoscopy, both in upper and lower GIT, include inflammation in the form of erythema or erosions, ulcers, nodules, pseudopolyps, strictures and rarely fistulae or mass-like lesions. On colonoscopy, ulceration is a common finding seen in up to 78% of the cases.[85,86,87,88,89,90,91] The ulcers can be of varying sizes and orientation. Typical ulcers are transverse or ring-shaped found in between 27% (13/49) and 73% (71/98) of the cases, while other forms of ulcers seen are aphthous ones in 10% (4/40) to 21% (21/98), and less often longitudinal ulcers seen in 2% (1//4) to 8% (4/53) of cases.[50,51,55,85,86,87,88,89,90,91] Another common feature is a deformed, widely open, ileocecal value noted in 26% (10/39) to 66% (36/55) of cases.[50,51,55,85,86,87,88,89,90,91] The colonic mucosa can be nodular in 22% (15/69) to 56% (24/43) and pseudopolyps can be seen in 16% (4/43) to 78% due to chronic inflammation.[50,51,55,85,86,87,88,89,90,91] Strictures, cobblestone appearance and skip lesions are less frequently seen.
Similarly, as mentioned previously, the most predominant findings on upper endoscopy are strictures, followed by ulcers, erythema and less often mass-like lesions that are either submucosal or mucosal, and fistulae.
Diagnostic laparoscopy
Use of laparoscopy in patients with peritoneal disease has shown to be confirmatory in the diagnosis of abdominal TB. Typical findings on laparoscopy including thickened peritoneum with erythema, whitish nodules and adhesions is accurate enough to diagnose abdominal TB with a sensitivity and specificity ranging between 84 and 100% and 96 and 100%, respectively.[92] Along with the histology of biopsy specimens from the peritoneum, this approached a diagnostic sensitivity and specificity of 93 and 98%, respectively.[76]
Histopathology
Histopathological analysis of the biopsy specimens usually reveals chronic inflammation associated with granulomas. In the GIT, inflammation is non-specific with features of chronicity and alteration of architecture like crypt distortion and cryptitis.[93] Granulomas are found in around 62% (33/53) to 71% (49/69) of specimens analyzed and are typically well-defined, large, mean number per section of 2.5-4.8 with central caseation.[50,85,86,89,90,93] Characteristic granulomas with central necrosis, when found on biopsy specimens taken at laparoscopy, give an estimated sensitivity and specificity of 71-100% and 100%, respectively.[92] The presence of distinctive granulomas in the intestinal biopsy can help establish the diagnosis of GITB with a sensitivity, specificity, positive predictive value and negative predictive value of 28-58%, 69-100%, 64-100% and 41-65%, respectively [Figure 5].[55,94,95,96,97]
Figure 5.
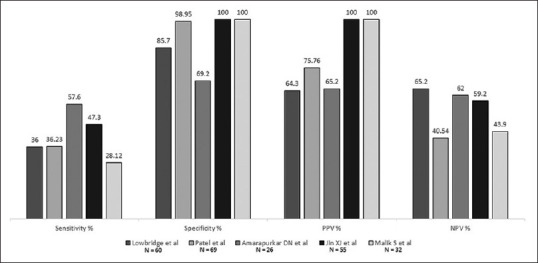
Diagnostic accuracy of histopathology for GITB
Microbiology
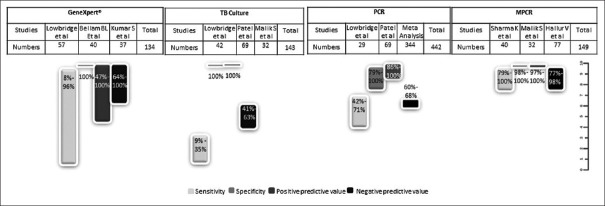
Microbiological analysis of ascitic fluid or biopsy specimen can be carried out for acid-fast bacilli on smear, polymerase chain reaction (PCR) and mycobacterial culture. The yield on ascitic fluid of stain for acid-fast bacilli and cultures is low, with reported sensitivities of 3 and 35%, respectively.[76] Similarly, the smear positivity for laparoscopic biopsy specimens was 3-25%, and for culture was 38-92%.[44,76] Smears on intestinal biopsy are positive for acid-fast bacilli (AFB) in around 20-45% and the cultures are positive in 14-80%.[50,53,98] The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for detection of AFB on biopsy smear are 6-31%, 100%, 100% and 57%, respectively [Figure 6].[97,99,100] Similarly, the sensitivity, specificity, PPV and NPV of AFB culture on biopsy are 9-35%, 100%, 100% and 38-63%, respectively [Figure 7].[55,94,97] PCR on intestinal biopsy shows a high rate of positivity between 50 and 95%.[50,53,55] The sensitivity, specificity, PPV and NPV of PCR on biopsy are 50-71%, 79-100%, 86-100% and 60-68%, respectively.[55,97] Meta-analysis of 10 studies estimated the sensitivity and specificity of PCR for GITB on biopsy to be 42 and 97%, respectively [Figure 7]. Concluding that a positive test is very helpful, however, a negative test is a poor discriminator for excluding GITB.[101] Nevertheless, use of multiple targets in multiple PCR tests improved the sensitivity, specificity, PPV and NPV to 78- 88%, 98-100%, 97-100% and 77-98%, respectively [Figure 7].[94,98,102] The use of other gene amplification techniques like nucleic acid amplification test called GenXpert-MTB/RIF has been used as an alternative to PCR for rapid diagnosis. The reported sensitivities and specificity ranges between 8 and 96%, and 2 and 100%, respectively [Figure 7].[97,103,104]
Figure 6.
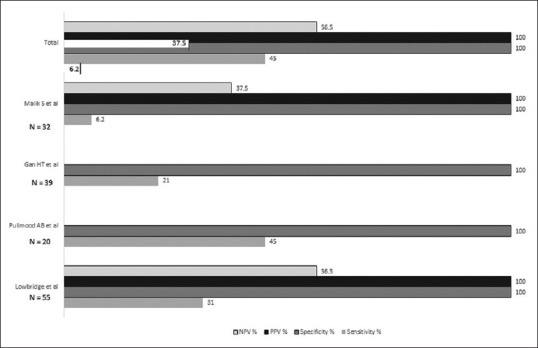
Diagnostic accuracy of AFB on biopsy smear
Figure 7.
Microbiological Diagnostic accuracy for intestinal biopsy
DIFFERENTIAL DIAGNOSIS
Given that abdominal TB manifests in different ways and presents with non-specific symptoms, it can mimic other diseases and needs to be differentiated from intra-abdominal malignancies including lymphoma and other granulomatous inflammatory and infective diseases.
Peritoneal carcinomatosis including ovarian cancer can present with abdominal mass, ascites, and peritoneal involvement similar to abdominal TB.[105,106] These diseases can be difficult to differentiate just on tumor markers like cancer antigen (CA)-125, which is elevated in both diseases and on radiological imaging. Some authors have proposed a composite CA-125 and carcinoembryonic antigen (CEA) score to confirm diagnosis of ovarian cancer.[107] In addition, diligent analysis of ascitic fluid for cytology, and histopathology and culture of appropriate biopsy specimens usually makes the distinction. If it is difficult to obtain biopsy by radiological imaging, frozen section analysis at the time of surgery may clinch the diagnosis and help prevent major resection.[106] GITB can present as a malignant looking mass at endoscopy or imaging, and once again, histopathology and culture of biopsy specimens are key to the diagnosis. Due to similar presentations, distinguishing abdominal TB from primary intestinal lymphoma or abdominal lymph node lymphoma may prove difficult.[108,109] Contrast-enhanced CT or MRI with specific enhancement pattern and anatomical distribution of lymph nodes with histopathology helps to reach the right diagnosis.[84,110]
Crohn's disease needs specific mention due to its rising incidence worldwide and the need for immunosuppressive therapy that can activate LTB. A great deal of literature has been devoted to make a definite distinction between these diseases, however, there are no pathognomonic features that clearly set them apart. Numerous clinical features like patient demographics, clinical presentation, serology, microbiology and radiology have been evaluated to provide more conclusive distinctions.[85,86,87,88,89,90,91,93] Several parameters that incorporate various above features have been used to generate different models to predict the right diagnosis with sensitivities reaching 90% and specificities of 100%.[91,111,112] However, these models are impractical and cumbersome to utilize in daily clinical practice and remain more as research tools. Acid-fast bacilli on smear, positive culture for mycobacteria, histopathology showing definitive granuloma with central caseation and radiology elucidating lymph node with necrosis are the most reliable parameters favoring TB.[93]
Other rare conditions like granulomatous chronic infections including histoplasmosis and cryptococcus and chronic inflammations with granulomas without central caseation like sarcoidosis need to be considered in the differential diagnosis.
Nonetheless, despite concerted efforts, a confirmed diagnosis of abdominal TB may not be always achievable. In situations where the clinical suspicion remains highly in favor of TB, an empirical course of anti-tubercular treatment is a valid diagnostic and therapeutic option, and remains an established tool in clinical practice.
TREATMENT
The mainstay of a therapeutic approach is medical and is similar to the treatment of pulmonary TB. In addition, surgery may be indicated in patients who do not respond to medical therapy or in cases of complications like obstruction, perforation, abscess collection or fistulae. International guidelines recommend 6-month therapy with standard regimens as for pulmonary TB.[113,114] Despite such recommendations, 9-month therapy is used at times with a view that the therapeutic benefit is difficult to document and to ensure complete eradication of the bacilli. However, a Cochrane systemic review of three randomized controlled trails concluded that 6-month therapy is sufficient to achieve response and any further prolongation did not provide additional benefit.[115]
Response to therapy is usually difficult to assess objectively. Symptomatic improvement either in the form of global or generalized well-being, resolution of fever and/or biomarkers like ESR and C-reactive protein (CRP) are considered as surrogates for therapeutic efficacy. The amelioration of symptoms due to anti-tuberculous treatment (ATT) is usually seen within weeks and majority show full recovery by 2 months. In serial evaluation, 45% (71/157) showed improvement of global symptoms at 1 month, 66% (104/157) at 2, 94% (147/157) at 3 and 99% (155/157) at 6 months.[116] Similarly, 52% (52/101) normalized CRP at 2 months following therapy and more than 93% (94/101) at 6 months post therapy.[117] In addition, resolution of ascites and endoscopic lesions are an objective assessment for response to therapy. Complete mucosal healing of tuberculous lesions on colonoscopy can be seen as early as 2 months, following initiation of ATT in 81% and in 100% (125/125) by 6 months.[116,118] Patients who do not show response by the end of 6 months tend to have a complicated course that may require surgery or an alternative diagnosis.[116,118]
One of the factors leading to poor response to ATT is the presence of intestinal strictures. These strictures show a response varying from 24% (25/106) to 53% (16/30) depending on the location and type of stenosis. Colonic strictures, length >3 cm , and presence of multiple strictures were less likely to respond than other strictures.[119,120]
Endoscopic dilatation is possible for both small bowel and colonic strictures and is shown to be effective in the majority of these cases, however, the strictures that were dilated were mostly short and single.[121,122,123] Likewise, tubercular strictures in the upper GIT respond poorly to ATT with a mere 19% response rate. Furthermore, endoscopic dilatation was feasible and in combination with ATT found to be efficacious.[63]
However, a small proportion of patients may need surgery despite ATT and endoscopic therapy. In a retrospective study from a tertiary center, the main reason for surgery in GITB was obstruction comprising 66% (23/35) of all cases, with the leading cause being intestinal strictures. This was followed by perforation in 29% (10/35) and bleeding in 6% (2/35). The principle form of surgery carried out was ileocecal resection, right hemicolectomy and small bowel resection.[124] In other studies 86% (182/212) to 91% (10/11) of the surgeries in abdominal TB were done in an emergency and only 9% (1/11) to 14% (30/212) were elective.[35,38] The reasons for surgery included adhesions in 27% (6/22) to 59% (124/212), strictures 31% (11/35) to 51% (11/22), perforation 18% (38/212) to 72% (26/35), peritonitis 23% (8/35) to 36% (76/212), ileocecal mass 7% (14/212) to 14% (3/22), and failed ATT in 9% (1/11) to 14% (30/212).[35,38,45,47] The types of surgery included adhesiolysis in 27% to 56% (124/212), followed by segmental resection in 26% (56/212), right hemicolectomy 7% (14/212) to 18%, and rarely stricteroplasty in 1% (1/212).[38,45,48,50]
The role of steroids in GITB is not well-defined. It is used for its anti-inflammatory properties with moderate benefit. A recent systemic review and meta-analysis of three studies does confirm this limited efficacy restricted to peritoneal TB.[125] Nonetheless, international guidelines do not recommend it.
Multiple-drug resistant TB (MDR-TB) is on the rise globally with a 16% increase in 2018 compared to the previous year (WHO TB report). However, only around 51% of TB cases are tested for drug susceptibility. The prevalence of MDR-TB in GITB is not very well-studied because the culture sensitivity is low and testing for drug resistance is not routinely done. The few studies that examined this had a modest study sample size and reported a prevalence of single-drug and multi-drug resistance ranging from 4 to 25% and 3 to 25%, respectively[126,127,128,129,130] [Table 4]. Confirmation of MDR-TB in GITB is important to ensure that the patient gets the most appropriate treatment regimens and to avoid unnecessary delay in the therapy. In addition, where the diagnosis of GITB is difficult and differentiation from Crohn's disease is based on empiric anti-TB therapy, affirming MDR-TB is crucial.
Table 4.
Drug resistance in GITB from different studies
| Reference | n | Pan-susceptible | Single-drug susceptible | Multi-drug resistance |
|---|---|---|---|---|
| Samant H et al. | 12 | 6 (50%) | 3 (25%) | 3 (25%) |
| Ye BD et al. | 74 | 59 (80%) | 13 (18%) | 2 (3%) |
| Lin PY et al. | 26 | - | - | 4 (15%) |
| Sonambekar A et al. | 43 | 33 (77%) | 4 (9%) | 6 (14%) |
| Udgirkar S et al. | 74 | 20 (27%) | 3 (4%) | 8 (10%) |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1. [Last accessed on 2021 Mar 19]. Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 .
- 2.UN. Political Declaration of the UN General Assembly High-Level Meeting. United Nations High-Level Meeting on the Fight Against Tuberculosis. 2018 [Google Scholar]
- 3.Reid MJA, Arinaminpathy N, Bloom A, Bloom BR, Boehme C, Chaisson R, et al. Building a tuberculosis-free world: The Lancet Commission on tuberculosis. Lancet. 2019;393:1331–84. doi: 10.1016/S0140-6736(19)30024-8. [DOI] [PubMed] [Google Scholar]
- 4.Cherian JJ, Lobo I, Sukhlecha A, Chawan U, Kshirsagar NA, Nair BL, et al. Treatment outcome of extrapulmonary tuberculosis under Revised National Tuberculosis Control Programme. Indian J Tuberc. 2017;64:104–8. doi: 10.1016/j.ijtb.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 5. [Last accessed on 2021 Mar 19]. Available from: https://tbcindia.gov.in/WriteReadData/India%20TB%20Report%202019.pdf.In .
- 6.Pang Y, An J, Shu W, Huo F, Chu N, Gao M, et al. Epidemiology of extrapulmonary tuberculosis among inpatients, China, 2008-2017. Emerg Infect Dis. 2019;25:457–64. doi: 10.3201/eid2503.180572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tahseen S, Khanzada FM, Baloch AQ, Abbas Q, Bhutto MM, Alizai AW, et al. Extrapulmonary tuberculosis in Pakistan- A nation-wide multicenter retrospective study. PLoS One. 2020;15:e0232134. doi: 10.1371/journal.pone.0232134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan AH, Sulaiman SAS, Laghari M, Hassali MA, Muttalif AR, Bhatti Z, et al. Treatment outcomes and risk factors of extra-pulmonary tuberculosis in patients with co-morbidities. BMC Infect Dis. 2019;19:691. doi: 10.1186/s12879-019-4312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [Last accessed on 2021 Mar 19]. Available from: https://www.cdc.gov/tb/statistics/reports/2018/table15.htm .
- 10.Sotgiu G, Falzon D, Hollo V, Kodmon C, Lefebvre N, Dadu A, et al. Determinants of site of tuberculosis disease: An analysis of European surveillance data from 2003 to 2014. PLoS One. 2017;12:e0186499. doi: 10.1371/journal.pone.0186499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gounden S, Perumal R, Magula NP. Extrapulmonary tuberculosis in the setting of HIV hyperendemicity at a tertiary hospital in Durban, South Africa. S Afr J Infect Dis. 2018;33:57–64. [Google Scholar]
- 12.Zurcher K, Ballif M, Kiertiburanakul S, Chenal H, Yotebieng M, Grinsztejn B, et al. Diagnosis and clinical outcomes of extrapulmonary tuberculosis in antiretroviral therapy programmes in low- and middle-income countries: A multicohort study. J Int AIDS Soc. 2019;22:e25392. doi: 10.1002/jia2.25392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandgren A, Hollo V, van der Werf MJ. Extrapulmonary tuberculosis in the European Union and European Economic Area, 2002 to 2011. Euro Surveill. 2013;18:20431. [PubMed] [Google Scholar]
- 14. [Last accessed on 2021 Mar 19]. Available from: https://www.cdc.gov/tb/statistics/reports/2018/table7.htm .
- 15.Arora VK, Gupta R. Trends of EPTB under Revised National TB Control Programme: A study from South Delhi. Indian J Tuberc. 2006;53:77–83. [Google Scholar]
- 16.Qian X, Nguyen DT, Lyu J, Albers AE, Bi X, Graviss EA. Risk factors for extrapulmonary dissemination of tuberculosis and associated mortality during treatment for extrapulmonary tuberculosis. Emerg Microbes Infect. 2018;7:102. doi: 10.1038/s41426-018-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49:1350–7. doi: 10.1086/605559. [DOI] [PubMed] [Google Scholar]
- 18. [Last accessed on 2021 Mar 19]. Available from: https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/
- 19.Schwartz NG, Price SF, Pratt RH, Langer AJ. Tuberculosis-United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:286–9. doi: 10.15585/mmwr.mm6911a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999-2006. Thorax. 2009;64:1090–5. doi: 10.1136/thx.2009.118133. [DOI] [PubMed] [Google Scholar]
- 21.Aguado JM, Herrero JA, Gavalda J, Torre-Cisneros J, Blanes M, Rufi G, et al. Clinical presentation and outcome of tuberculosis in kidney, liver, and heart transplant recipients in Spain. Spanish Transplantation Infection Study Group, GESITRA. Transplantation. 1997;63:1278–86. doi: 10.1097/00007890-199705150-00015. [DOI] [PubMed] [Google Scholar]
- 22.Holty JE, Gould MK, Meinke L, Keeffe EB, Ruoss SJ. Tuberculosis in liver transplant recipients: A systematic review and meta-analysis of individual patient data. Liver Transpl. 2009;15:894–906. doi: 10.1002/lt.21709. [DOI] [PubMed] [Google Scholar]
- 23.Gras J, De Castro N, Montlahuc C, Champion L, Scemla A, Matignon M, et al. Clinical characteristics, risk factors, and outcome of tuberculosis in kidney transplant recipients: A multicentric case-control study in a low-endemic area. Transpl Infect Dis. 2018;20:e12943. doi: 10.1111/tid.12943. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian AK, Theodoropoulos NM. Mycobacterium tuberculosis infections in solid organ transplantation: Guidelines from the infectious diseases community of practice of the American Society of Transplantation. Clin Transplant. 2019;33:e13513. doi: 10.1111/ctr.13513. [DOI] [PubMed] [Google Scholar]
- 25.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 26.Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Breban M, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009;60:1884–94. doi: 10.1002/art.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarra SV, Tang B, Lu L, Lin HY, Mok CC, Asavatanabodee P, et al. Risk of tuberculosis with anti-tumor necrosis factor-alpha therapy: Substantially higher number of patients at risk in Asia. Int J Rheum Dis. 2014;17:291–8. doi: 10.1111/1756-185X.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers. 2016;2:16076. doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 29.Naing C, Mak JW, Maung M, Wong SF, Kassim AI. Meta-analysis: The association between HIV infection and extrapulmonary tuberculosis. Lung. 2013;191:27–34. doi: 10.1007/s00408-012-9440-6. [DOI] [PubMed] [Google Scholar]
- 30.Shivakoti R, Sharma D, Mamoon G, Pham K. Association of HIV infection with extrapulmonary tuberculosis: A systematic review. Infection. 2017;45:11–21. doi: 10.1007/s15010-016-0960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moule MG, Cirillo JD. Mycobacterium tuberculosis dissemination plays a critical role in pathogenesis. Front Cell Infect Microbiol. 2020;10:65. doi: 10.3389/fcimb.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Click ES, Moonan PK, Winston CA, Cowan LS, Oeltmann JE. Relationship between Mycobacterium tuberculosis phylogenetic lineage and clinical site of tuberculosis. Clin Infect Dis. 2012;54:211–9. doi: 10.1093/cid/cir788. [DOI] [PubMed] [Google Scholar]
- 33.Garcia de Viedma D, Lorenzo G, Cardona PJ, Rodriguez NA, Gordillo S, Serrano MJ, et al. Association between the infectivity of Mycobacterium tuberculosis strains and their efficiency for extrarespiratory infection. J Infect Dis. 2005;192:2059–65. doi: 10.1086/498245. [DOI] [PubMed] [Google Scholar]
- 34.Cho JK, Choi YM, Lee SS, Park HK, Cha RR, Kim WS, et al. Clinical features and outcomes of abdominal tuberculosis in southeastern Korea: 12 years of experience. BMC Infect Dis. 2018;18:699. doi: 10.1186/s12879-018-3635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chalya PL, McHembe MD, Mshana SE, Rambau PF, Jaka H, Mabula JB. Clinicopathological profile and surgical treatment of abdominal tuberculosis: A single centre experience in northwestern Tanzania. BMC Infect Dis. 2013;13:270. doi: 10.1186/1471-2334-13-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramesh J, Banait GS, Ormerod LP. Abdominal tuberculosis in a district general hospital: A retrospective review of 86 cases. QJM. 2008;101:189–95. doi: 10.1093/qjmed/hcm125. [DOI] [PubMed] [Google Scholar]
- 37.Mamo JP, Brij SO, Enoch DA. Abdominal tuberculosis: A retrospective review of cases presenting to a UK district hospital. QJM. 2013;106:347–54. doi: 10.1093/qjmed/hct003. [DOI] [PubMed] [Google Scholar]
- 38.Singhal A, Gulati A, Frizell R, Manning AP. Abdominal tuberculosis in Bradford, UK: 1992-2002. Eur J Gastroenterol Hepatol. 2005;17:967–71. doi: 10.1097/00042737-200509000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Chien K, Seemangal J, Batt J, Vozoris NT. Abdominal tuberculosis: A descriptive case series of the experience in a Canadian tuberculosis clinic. Int J Tuberc Lung Dis. 2018;22:681–5. doi: 10.5588/ijtld.17.0685. [DOI] [PubMed] [Google Scholar]
- 40.Singh A, Sahu MK, Panigrahi M, Behera MK, UthanSingh K, Kar C, et al. Abdominal tuberculosis in Indians: Still very pertinent. J Clin Tuberc Other Mycobact Dis. 2019;15:100097. doi: 10.1016/j.jctube.2019.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan KK, Chen K, Sim R. The spectrum of abdominal tuberculosis in a developed country: A single institution's experience over 7 years. J Gastrointest Surg. 2009;13:142–7. doi: 10.1007/s11605-008-0669-6. [DOI] [PubMed] [Google Scholar]
- 42.Mandavdhare HS, Singh H, Dutta U, Sharma V. A real-world experience with 6 months of antitubercular therapy in abdominal tuberculosis. JGH Open. 2019;3:201–5. doi: 10.1002/jgh3.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uygur-Bayramicli O, Dabak G, Dabak R. A clinical dilemma: Abdominal tuberculosis. World J Gastroenterol. 2003;9:1098–101. doi: 10.3748/wjg.v9.i5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muneef MA, Memish Z, Mahmoud SA, Sadoon SA, Bannatyne R, Khan Y. Tuberculosis in the belly: A review of forty-six cases involving the gastrointestinal tract and peritoneum. Scand J Gastroenterol. 2001;36:528–32. doi: 10.1080/003655201750153412. [DOI] [PubMed] [Google Scholar]
- 45.Urabinahatti KA, Singh AK, Nayak A, Gupta R, Jain M, Dubey C, et al. Abdominal tuberculosis: An epidemiological profile and management of 40 cases in a tertiary set up. Int Surg J. 2016;3 doi:10.18203/2349-2902.isj20162737. [Google Scholar]
- 46.Nayagam JS, Mullender C, Cosgrove C, Poullis A. Abdominal tuberculosis: Diagnosis and demographics, a 10-year retrospective review from a single centre. World J Clin Cases. 2016;4:207–12. doi: 10.12998/wjcc.v4.i8.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan R, Abid S, Jafri W, Abbas Z, Hameed K, Ahmad Z. Diagnostic dilemma of abdominal tuberculosis in non-HIV patients: An ongoing challenge for physicians. World J Gastroenterol. 2006;12:6371–5. doi: 10.3748/wjg.v12.i39.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi EH, Coyle WJ. Gastrointestinal tuberculosis. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.TNMI7-0014-2016. doi: 10.1128/microbiolspec.TNMI7-0014-2016. [DOI] [PubMed] [Google Scholar]
- 49.Sheer TA, Coyle WJ. Gastrointestinal tuberculosis. Curr Gastroenterol Rep. 2003;5:273–8. doi: 10.1007/s11894-003-0063-1. [DOI] [PubMed] [Google Scholar]
- 50.Cheng W, Zhang S, Li Y, Wang J, Li J. Intestinal tuberculosis: Clinico-pathological profile and the importance of a high degree of suspicion. Trop Med Int Health. 2019;24:81–90. doi: 10.1111/tmi.13169. [DOI] [PubMed] [Google Scholar]
- 51.Gan H, Mely M, Zhao J, Zhu L. An analysis of the clinical, endoscopic, and pathologic features of intestinal tuberculosis. J Clin Gastroenterol. 2016;50:470–5. doi: 10.1097/MCG.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 52.Kentley J, Ooi JL, Potter J, Tiberi S, O'Shaughnessy T, Langmead L, et al. Intestinal tuberculosis: A diagnostic challenge. Trop Med Int Health. 2017;22:994–9. doi: 10.1111/tmi.12908. [DOI] [PubMed] [Google Scholar]
- 53.Tanoglu A, Erdem H, Friedland JS, Almajid FM, Batirel A, Kulzhanova S, et al. Clinicopathological profile of gastrointestinal tuberculosis: A multinational ID-IRI study. Eur J Clin Microbiol Infect Dis. 2020;39:493–500. doi: 10.1007/s10096-019-03749-y. [DOI] [PubMed] [Google Scholar]
- 54.Tripathi PB, Amarapurkar AD. Morphological spectrum of gastrointestinal tuberculosis. Trop Gastroenterol. 2009;30:35–9. [PubMed] [Google Scholar]
- 55.Patel B, Yagnik VD. Clinical and laboratory features of intestinal tuberculosis. Clin Exp Gastroenterol. 2018;11:97–103. doi: 10.2147/CEG.S154235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dasgupta A, Singh N, Bhatia A. Abdominal tuberculosis: A histopathological study with special reference to intestinal perforation and mesenteric vasculopathy. J Lab Physicians. 2009;1:56–61. doi: 10.4103/0974-2727.59700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogbomo H, Thiesen A, Zepeda-Gomez S, Kohansal-Vajargah A. Primary esophageal tuberculosis without dysphagia or odynophagia in a patient without HIV. ACG Case Rep J. 2020;7:e00323. doi: 10.14309/crj.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan MS, Maan MHA, Sohail AH, Memon WA. Primary esophageal tuberculosis mimicking esophageal carcinoma on computed tomography: A case report. World J Gastrointest Surg. 2019;11:373–80. doi: 10.4240/wjgs.v11.i9.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malikowski T, Mahmood M, Smyrk T, Raffals L, Nehra V. Tuberculosis of the gastrointestinal tract and associated viscera. J Clin Tuberc Other Mycobact Dis. 2018;12:1–8. doi: 10.1016/j.jctube.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puri R, Khaliq A, Kumar M, Sud R, Vasdev N. Esophageal tuberculosis: Role of endoscopic ultrasound in diagnosis. Dis Esophagus. 2012;25:102–6. doi: 10.1111/j.1442-2050.2011.01223.x. [DOI] [PubMed] [Google Scholar]
- 61.Dahale AS, Kumar A, Srivastava S, Varakanahalli S, Sachdeva S, Puri AS. Esophageal tuberculosis: Uncommon of common. JGH Open. 2018;2:34–8. doi: 10.1002/jgh3.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Desai P, Mayenkar P, Northrup TF, Mallela V. Bronchoesophageal fistula due to esophageal tuberculosis. Case Rep Infect Dis. 2019;2019:6537437. doi: 10.1155/2019/6537437. doi: 10.1155/2019/6537437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalal A, Puri AS, Sachdeva S, Sakuja P. Nonsurgical management of gastroduodenal tuberculosis: Nine-year experience from a tertiary referral center. Endosc Int Open. 2019;7:E1248–52. doi: 10.1055/a-0957-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao YG, Pande GK, Sahni P, Chattopadhyay TK. Gastroduodenal tuberculosis management guidelines, based on a large experience and a review of the literature. Can J Surg. 2004;47:364–8. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu R, Zhou Y, Wang H, Di L, Zhao K, Tuo B, et al. Gastric tuberculosis mimicking submucosal tumor: A case series. BMC Gastroenterol. 2020;20:23. doi: 10.1186/s12876-020-1175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lv M, Tang K, Meng Y, Tian C, Wang M. Primary isolated asymptomatic gastric tuberculosis of the cardia mimicking gastric stromal tumor: A rare case report and literature review. BMC Gastroenterol. 2020;20:108. doi: 10.1186/s12876-020-01242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Udgirkar S, Surude R, Zanwar V, Chandnani S, Contractor Q, Rathi P. Gastroduodenal tuberculosis: A case series and review of literature. Clin Med Insights Gastroenterol. 2018;11:1179552218790566. doi: 10.1177/1179552218790566. doi:10.1177/1179552218790566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geo SK, Harikumar R, Varghese T, Rajan P, Aravindan KP. Isolated tuberculosis of gastric cardia presenting as perforation peritonitis. Indian J Gastroenterol. 2005;24:227–8. [PubMed] [Google Scholar]
- 69.Michalopoulos A, Papadopoulos VN, Panidis S, Papavramidis TS, Chiotis A, Basdanis G. Cecal obstruction due to primary intestinal tuberculosis: A case series. J Med Case Rep. 2011;5:128. doi: 10.1186/1752-1947-5-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joshi MA, Balsarkar D, Abhyankar A, Pereira DG, Avasare N, Pradhan C, et al. Massive rectal bleeding due to jejunal and colonic tuberculosis. Trop Gastroenterol. 1998;19:168–70. [PubMed] [Google Scholar]
- 71.Cömert FB, Cömert M, Külah C, Taşcilar O, Numanoğlu G, Aydemir S. Colonic tuberculosis mimicking tumor perforation: A case report and review of the literature. Dig Dis Sci. 2006;51:1039–42. doi: 10.1007/s10620-006-8002-2. [DOI] [PubMed] [Google Scholar]
- 72.Tai WC, Hu TH, Lee CH, Chen HH, Huang CC, Chuah SK. Ano-perianal tuberculosis: 15 years of clinical experiences in Southern Taiwan. Colorectal Dis. 2010;12:e114–20. doi: 10.1111/j.1463-1318.2009.02057.x. [DOI] [PubMed] [Google Scholar]
- 73.Choi YS, Kim DS, Lee JB, Kim JK, Jung HJ, Lee SD, et al. Clinical features of tuberculous versus Crohn's anal fistulas, in Korea. J Crohns Colitis. 2015;9:1132–7. doi: 10.1093/ecco-jcc/jjv164. [DOI] [PubMed] [Google Scholar]
- 74.Luquín N, Masiá M, Noguera R, Gutiérrez F. Anal tuberculosis complicating anti-TNFα therapy. BMJ Case Rep 2014. 2014 doi: 10.1136/bcr-2014-206976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaid U, Kane GC. Tuberculous Peritonitis. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.TNMI7-0006-2016. doi: 10.1128/microbiolspec.TNMI7-0006-2016. [DOI] [PubMed] [Google Scholar]
- 76.Sanai FM, Bzeizi KI. Systematic review: Tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 77.Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, et al. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: A meta-analysis. J Clin Gastroenterol. 2006;40:705–10. doi: 10.1097/00004836-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Tao L, Ning HJ, Nie HM, Guo XY, Qin SY, Jiang HX. Diagnostic value of adenosine deaminase in ascites for tuberculosis ascites: A meta-analysis. Diagn Microbiol Infect Dis. 2014;79:102–7. doi: 10.1016/j.diagmicrobio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 79.Gupta P, Kumar S, Sharma V, Mandavdhare H, Dhaka N, Sinha SK, et al. Common and uncommon imaging features of abdominal tuberculosis. J Med Imaging Radiat Oncol. 2019;63:329–39. doi: 10.1111/1754-9485.12874. [DOI] [PubMed] [Google Scholar]
- 80.Van Hoving DJ, Griesel R, Meintjes G, Takwoingi Y, Maartens G, Ochodo EA. Abdominal ultrasound for diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV-positive individuals. Cochrane Database Syst Rev. 2019;9:Cd012777. doi: 10.1002/14651858.CD012777.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee WK, Van Tonder F, Tartaglia CJ, Dagia C, Cazzato RL, Duddalwar VA, et al. CT appearances of abdominal tuberculosis. Clin Radiol. 2012;67:596–604. doi: 10.1016/j.crad.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Pereira JM, Madureira AJ, Vieira A, Ramos I. Abdominal tuberculosis: Imaging features. Eur J Radiol. 2005;55:173–80. doi: 10.1016/j.ejrad.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 83.Deshpande SS, Joshi AR, Deshpande SS, Phajlani SA. Computed tomographic features of abdominal tuberculosis: Unmask the impersonator! Abdom Radiol (NY) 2019;44:11–21. doi: 10.1007/s00261-018-1700-3. [DOI] [PubMed] [Google Scholar]
- 84.Shao H, Yang ZG, Deng W, Chen J, Tang SS, Wen LY. Tuberculosis versus lymphoma in the abdominal lymph nodes: A comparative study using contrast-enhanced MRI. Eur J Radiol. 2012;81:2513–7. doi: 10.1016/j.ejrad.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 85.Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005;37:351–6. doi: 10.1055/s-2005-861116. [DOI] [PubMed] [Google Scholar]
- 86.Yu H, Liu Y, Wang Y, Peng L, Li A, Zhang Y. Clinical, endoscopic and histological differentiations between Crohn's disease and intestinal tuberculosis. Digestion. 2012;85:202–9. doi: 10.1159/000335431. [DOI] [PubMed] [Google Scholar]
- 87.Li X, Liu X, Zou Y, Ouyang C, Wu X, Zhou M, et al. Predictors of clinical and endoscopic findings in differentiating Crohn's disease from intestinal tuberculosis. Dig Dis Sci. 2011;56:188–96. doi: 10.1007/s10620-010-1231-4. [DOI] [PubMed] [Google Scholar]
- 88.Lee YJ, Yang SK, Byeon JS, Myung SJ, Chang HS, Hong SS, et al. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn's disease. Endoscopy. 2006;38:592–7. doi: 10.1055/s-2006-924996. [DOI] [PubMed] [Google Scholar]
- 89.Jung Y, Hwangbo Y, Yoon SM, Koo HS, Shin HD, Shin JE, et al. Predictive Factors for Differentiating Between Crohn's Disease and Intestinal Tuberculosis in Koreans. Am J Gastroenterol. 2016;111:1156–64. doi: 10.1038/ajg.2016.212. [DOI] [PubMed] [Google Scholar]
- 90.Makharia GK, Srivastava S, Das P, Goswami P, Singh U, Tripathi M, et al. Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol. 2010;105:642–51. doi: 10.1038/ajg.2009.585. [DOI] [PubMed] [Google Scholar]
- 91.Bae JH, Park SH, Ye BD, Kim SO, Cho YK, Youn EJ, et al. Development and validation of a novel prediction model for differential diagnosis between Crohn's disease and intestinal tuberculosis. Inflamm Bowel Dis. 2017;23:1614–23. doi: 10.1097/MIB.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 92.Chow KM, Chow VC, Szeto CC. Indication for peritoneal biopsy in tuberculous peritonitis. Am J Surg. 2003;185:567–3. doi: 10.1016/s0002-9610(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 93.Kedia S, Das P, Madhusudhan KS, Dattagupta S, Sharma R, Sahni P, et al. Differentiating Crohn's disease from intestinal tuberculosis. World J Gastroenterol. 2019;25:418–32. doi: 10.3748/wjg.v25.i4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malik S, Sharma K, Vaiphei K, Dhaka N, Berry N, Gupta P, et al. Multiplex polymerase chain reaction for diagnosis of gastrointestinal tuberculosis. JGH Open. 2019;3:32–7. doi: 10.1002/jgh3.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn's disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14:741–6. doi: 10.3748/wjg.14.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin XJ, Kim JM, Kim HK, Kim L, Choi SJ, Park IS, et al. Histopathology and TB-PCR kit analysis in differentiating the diagnosis of intestinal tuberculosis and Crohn's disease. World J Gastroenterol. 2010;16:2496–503. doi: 10.3748/wjg.v16.i20.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lowbridge C, Fadhil SAM, Krishnan GD, Schimann E, Karuppan RM, Sriram N, et al. How can gastro-intestinal tuberculosis diagnosis be improved? A prospective cohort study. BMC Infect Dis. 2020;20:255. doi: 10.1186/s12879-020-04983-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hallur V, Sharma M, Sethi S, Sharma K, Mewara A, Dhatwalia S, et al. Development and evaluation of multiplex PCR in rapid diagnosis of abdominal tuberculosis. Diagn Microbiol Infect Dis. 2013;76:51–5. doi: 10.1016/j.diagmicrobio.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 99.Pulimood AB, Peter S, Rook GW, Donoghue HD. In situ PCR for Mycobacterium tuberculosis in endoscopic mucosal biopsy specimens of intestinal tuberculosis and Crohn disease. Am J Clin Pathol. 2008;129:846–51. doi: 10.1309/DKKECWQWMG4J23E3. [DOI] [PubMed] [Google Scholar]
- 100.Gan HT, Chen YQ, Ouyang Q, Bu H, Yang XY. Differentiation between intestinal tuberculosis and Crohn's disease in endoscopic biopsy specimens by polymerase chain reaction. Am J Gastroenterol. 2002;97:1446–51. doi: 10.1111/j.1572-0241.2002.05686.x. [DOI] [PubMed] [Google Scholar]
- 101.Jin T, Fei B, Zhang Y, He X. The diagnostic value of polymerase chain reaction for Mycobacterium tuberculosis to distinguish intestinal tuberculosis from crohn's disease: A meta-analysis. Saudi J Gastroenterol. 2017;23:3–10. doi: 10.4103/1319-3767.199135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma K, Sinha SK, Sharma A, Nada R, Prasad KK, Goyal K, et al. Multiplex PCR for rapid diagnosis of gastrointestinal tuberculosis. J Glob Infect Dis. 2013;5:49–53. doi: 10.4103/0974-777X.112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bellam BL, Mandavdhare HS, Sharma K, Shukla S, Soni H, Kumar MP, et al. Utility of tissue Xpert-Mtb/Rif for the diagnosis of intestinal tuberculosis in patients with ileocolonic ulcers. Ther Adv Infect Dis. 2019;6:2049936119863939. doi: 10.1177/2049936119863939. doi: 10.1177/2049936119863939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar S, Bopanna S, Kedia S, Mouli P, Dhingra R, Padhan R, et al. Evaluation of Xpert MTB/RIF assay performance in the diagnosis of abdominal tuberculosis. Intest Res. 2017;15:187–94. doi: 10.5217/ir.2017.15.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharma JB, Jain SK, Pushparaj M, Roy KK, Malhotra N, Zutshi V, et al. Abdomino-peritoneal tuberculosis masquerading as ovarian cancer: A retrospective study of 26 cases. Arch Gynecol Obstet. 2010;282:643–8. doi: 10.1007/s00404-009-1295-6. [DOI] [PubMed] [Google Scholar]
- 106.Ertas IE, Gungorduk K, Ozdemir A, Emirdar V, Gokcu M, Dogan A, et al. Pelvic tuberculosis, echinococcosis, and actinomycosis: Great imitators of ovarian cancer. Aust N Z J Obstet Gynaecol. 2014;54:166–71. doi: 10.1111/ajo.12191. [DOI] [PubMed] [Google Scholar]
- 107.Tong H, Tai Y, Ye C, Wu H, Zhang LH, Gao JH, et al. Carbohydrate antigen 125 and carcinoembryonic antigen in the differentiation of tuberculous peritonitis and peritonitis carcinomatosa. Oncotarget. 2017;8:78068–75. doi: 10.18632/oncotarget.17355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu QQ, Zhu WR, Wu JT, Chen WX, Wang SA. Comparative study of intestinal tuberculosis and primary small intestinal lymphoma. World J Gastroenterol. 2014;20:4446–52. doi: 10.3748/wjg.v20.i15.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu YY, Chen MK, Cao Z, Liu SZ, Ding BJ. Differential diagnosis of intestinal tuberculosis from Crohn's disease and primary intestinal lymphoma in China. Saudi J Gastroenterol. 2014;20:241–7. doi: 10.4103/1319-3767.136979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pongpornsup S, Eksamutchai P, Teerasamit W. Differentiating between abdominal tuberculous lymphadenopathy and lymphoma using multidetector computed tomography (MDCT) J Med Assoc Thai. 2013;96:1175–82. [PubMed] [Google Scholar]
- 111.Limsrivilai J, Shreiner AB, Pongpaibul A, Laohapand C, Boonanuwat R, Pausawasdi N, et al. Meta-analytic Bayesian model for differentiating intestinal tuberculosis from Crohn's disease. Am J Gastroenterol. 2017;112:415–27. doi: 10.1038/ajg.2016.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He Y, Zhu Z, Chen Y, Chen F, Wang Y, Ouyang C, et al. Development and validation of a novel diagnostic nomogram to differentiate between intestinal tuberculosis and Crohn's disease: A 6-year prospective multicenter study. Am J Gastroenterol. 2019;114:490–9. doi: 10.14309/ajg.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 113.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 114.World Health Organization. Guidelines for Treatment of Tuberculosis. (4th ed) 2010 [PubMed] [Google Scholar]
- 115.Jullien S, Jain S, Ryan H, Ahuja V. Six-month therapy for abdominal tuberculosis. Cochrane Database Syst Rev. 2016;11:Cd012163. doi: 10.1002/14651858.CD012163.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pratap Mouli V, Munot K, Ananthakrishnan A, Kedia S, Addagalla S, Garg SK, et al. Endoscopic and clinical responses to anti-tubercular therapy can differentiate intestinal tuberculosis from Crohn's disease. Aliment Pharmacol Ther. 2017;45:27–36. doi: 10.1111/apt.13840. [DOI] [PubMed] [Google Scholar]
- 117.Sharma V, Mandavdhare HS, Lamoria S, Singh H, Kumar A. Serial C-reactive protein measurements in patients treated for suspected abdominal tuberculosis. Dig Liver Dis. 2018;50:559–62. doi: 10.1016/j.dld.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 118.Sharma V, Mandavdhare HS, Dutta U. Letter: Mucosal response in discriminating intestinal tuberculosis from Crohn's disease-when to look for it? Aliment Pharmacol Ther. 2018;47:859–60. doi: 10.1111/apt.14495. [DOI] [PubMed] [Google Scholar]
- 119.Aggarwal P, Kedia S, Sharma R, Bopanna S, Madhusudhan KS, Yadav DP, et al. Tubercular intestinal strictures show a poor response to anti-tuberculous therapy. Dig Dis Sci. 2017;62:2847–56. doi: 10.1007/s10620-017-4727-3. [DOI] [PubMed] [Google Scholar]
- 120.Mukewar S, Mukewar S, Ravi R, Prasad A, Dua KS. Colon tuberculosis: Endoscopic features and prospective endoscopic follow-up after anti-tuberculosis treatment. Clin Transl Gastroenterol. 2012;3:e24. doi: 10.1038/ctg.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsuboi A, Oka S, Tanaka S, Iio S, Otani I, Kunihara S, et al. Experience with balloon dilatation in Crohn's and non-Crohn's benign small-bowel strictures: Is there a difference? Gastroenterol Res Pract. 2019;2019:1262595. doi: 10.1155/2019/1262595. doi: 10.1155/2019/1262595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh Rana S, Kumar Bhasin D, Rao C, Singh K. Tubercular versus Crohn's ileal strictures: Role of endoscopic balloon dilatation without fluoroscopy. Ann Gastroenterol. 2013;26:141–5. [PMC free article] [PubMed] [Google Scholar]
- 123.Misra SP, Misra V, Dwivedi M, Arora JS, Kunwar BK. Tuberculous colonic strictures: Impact of dilation on diagnosis. Endoscopy. 2004;36:1099–103. doi: 10.1055/s-2004-826046. [DOI] [PubMed] [Google Scholar]
- 124.Singh H, Krishnamurthy G, Rajendran J, Sharma V, Mandavdhare H, Kumar H, et al. Surgery for abdominal tuberculosis in the present era: Experience from a tertiary-care center. Surg Infect (Larchmt) 2018;19:640–5. doi: 10.1089/sur.2018.077. [DOI] [PubMed] [Google Scholar]
- 125.Soni H, Bellam BL, Rao RK, Kumar PM, Mandavdhare HS, Singh H, et al. Use of steroids for abdominal tuberculosis: A systematic review and meta-analysis. Infection. 2019;47:387–94. doi: 10.1007/s15010-018-1235-0. [DOI] [PubMed] [Google Scholar]
- 126.Samant H, Desai D, Abraham P, Joshi A, Gupta T, Rodrigues C, et al. Acid-fast bacilli culture positivity and drug resistance in abdominal tuberculosis in Mumbai, India. Indian J Gastroenterol. 2014;33:414–9. doi: 10.1007/s12664-014-0467-x. [DOI] [PubMed] [Google Scholar]
- 127.Sonambekar A, Desai D, Abraham P, Mehta V, Samant H, Joshi A, et al. Drug resistance in intestinal tuberculosis: A reason to worry? JGH Open. 2017;1:22–4. doi: 10.1002/jgh3.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Udgirkar S, Jain S, Pawar S, Chandnani S, Contractor Q, Rathi P. Clinical profile, drug resistance pattern and treatment outcomes of abdominal tuberculosis patients in Western India. Arq Gastroenterol. 2019;56:178–83. doi: 10.1590/S0004-2803.201900000-35. [DOI] [PubMed] [Google Scholar]
- 129.Lin PY, Wang JY, Hsueh PR, Lee LN, Hsiao CH, Yu CJ, et al. Lower gastrointestinal tract tuberculosis: An important but neglected disease. Int J Colorectal Dis. 2009;24:1175–80. doi: 10.1007/s00384-009-0721-3. [DOI] [PubMed] [Google Scholar]
- 130.Ye BD, Yang SK, Kim D, Shim TS, Kim SH, Kim MN, et al. Diagnostic sensitivity of culture and drug resistance patterns in Korean patients with intestinal tuberculosis. Int J Tuberc Lung Dis. 2012;16:799–804. doi: 10.5588/ijtld.11.0252. [DOI] [PubMed] [Google Scholar]