Abstract
Faithful inheritance of mitochondrial DNA (mtDNA) is crucial for cellular respiration/oxidative phosphorylation and mitochondrial membrane potential. However, how mtDNA is transmitted to progeny is not fully understood. We utilized hypersuppressive mtDNA, a class of respiratory deficient Saccharomyces cerevisiae mtDNA that is preferentially inherited over wild-type mtDNA (rho+), to uncover the factors governing mtDNA inheritance. We found that some regions of rho+ mtDNA persisted while others were lost after a specific hypersuppressive takeover indicating that hypersuppressive preferential inheritance may partially be due to active destruction of rho+ mtDNA. From a multicopy suppression screen, we found that overexpression of putative mitochondrial RNA exonuclease PET127 reduced biased inheritance of a subset of hypersuppressive genomes. This suppression required PET127 binding to the mitochondrial RNA polymerase RPO41 but not PET127 exonuclease activity. A temperature-sensitive allele of RPO41 improved rho+ mtDNA inheritance over a specific hypersuppressive mtDNA at semi-permissive temperatures revealing a previously unknown role for rho+ transcription in promoting hypersuppressive mtDNA inheritance.
Author summary
Functional mitochondrial DNA is important for cells to maintain fitness and too much damaged mitochondrial DNA can cause debilitating diseases in humans. Inheritance of mitochondrial DNA from cell to cell as they divide is still a poorly understood process especially when multiple mitochondrial DNA forms are present. Here, we study a defective yeast mitochondrial DNA with the unusual property that it is inherited almost exclusively when present with functional mitochondrial DNA inside the same cell. We noticed that the functional mitochondrial DNA becomes damaged which suggests the defective mitochondrial DNA somehow promotes the destruction of the functional mitochondrial DNA when both are present; whereas, the current thinking is that the defective mitochondrial DNA is simply made faster than functional mitochondrial DNA. Also, we found that a reduction of functional mitochondrial DNA gene expression protects the functional mitochondrial DNA from destruction by defective mitochondrial DNA revealing a novel role for functional mitochondrial DNA in the preferential inheritance of defective mitochondrial DNA. Both findings suggest there is an interplay between the genomes, either by competition for resources or interaction between the genomes, which has not been previously considered.
Introduction
The mitochondrion is an essential eukaryotic organelle and the site for many critical metabolic reactions such as iron-sulfur cluster metabolism, heme biosynthesis, the TCA cycle, and cellular respiration/oxidative phosphorylation [1–3]. Most mitochondrial proteins are encoded by the nuclear DNA, made in the cytosol and imported into mitochondria [4,5]. However, mitochondria also have their own genome (mtDNA) containing a small number of genes that encode core components of complexes involved in cellular respiration/oxidative phosphorylation and machinery to translate those genes [6,7]. Unlike nuclear DNA, there are many copies of mtDNA per cell and, in Saccharomyces cerevisiae, both linear and circular forms of mtDNA are present [8–10].
In budding yeast, respiration-capable mtDNA (rho+) encodes genes critical for activity of the mitochondrial ribosome (VAR1, 15srRNA, 21srRNA, tRNA), components of mitochondrial electron transport chain (ETC) complexes: complex III (COB), complex IV (COX1, COX2, COX3), and components of the ATP synthase complex (ATP6, ATP8, ATP9) [6,7]. Loss of respiratory function caused either by partial disruption of mtDNA (rho-) or total loss of the mtDNA (rho0) results in impaired cellular respiration/oxidative phosphorylation and a slower colony growth phenotype. This reduced growth occurs even in non-respiratory conditions and is called petiteness [11,12]. Although mtDNA must be faithfully replicated, maintained, and segregated to progeny to maintain cellular fitness, mtDNA inheritance is not fully understood [13–17].
An approach to understand mtDNA inheritance comes from studies of mutant mtDNA in yeast that exhibit extremely biased inheritance. When two yeast cells mate, their cytoplasms fuse and each haploid parent contributes mitochondria with mtDNA to the resulting diploid daughter cell [10]. In most cases, mating between yeast parents containing rho+ respiration competent mtDNA and parents containing respiration incompetent rho- or rho0 mtDNA results in almost entirely respiration competent progeny, indicating strong inheritance of rho+ mtDNA [18]. Some rho- genomes may persist in the progeny after mating rho+ and rho- yeast, and these rho- genomes are called partial suppressives [18,19]. However, a subset of rho- mutants, called hypersuppressive (HS) mtDNA, are extremely biased in their inheritance. When mated with rho+ cells, cells with HS mtDNA result in greater than 95% rho- progeny [18,20]. HS mtDNA mutants display preferential mtDNA inheritance despite giving rise to slow-growing respiration-incompetent cells. Thus, understanding how HS mutants hijack mtDNA inheritance machinery provides insights into how mtDNA inheritance works.
Characterization of HS rho- mutant genomes showed that they consist entirely of short (less than 2.5kbp) tandem repeats of one of three regions of high sequence similarity from the rho+ genome [21–23]. As the HS genomes found in the diploid progeny after mating rho+ and HS rho- are unchanged from those in the HS rho- parent, a prevailing theory is that HS mutants confer a replication advantage over rho+ [19,22,24]. The regions of the rho+ genome present in HS mutants are thought to be origins of replication, having similarities to mammalian mtDNA replication origins [25–27], and are known as ORI or rep regions [23,28–30] despite not being necessary for mtDNA stability [31]. According to the replication advantage theory, HS mtDNAs have a higher density of ORI regions, because of the small tandem repeats, confering a replicative advantage over rho+ mtDNA upon mating. Preferential inheritance of damaged mtDNA has been implicated in the progression of human mtDNA diseases and aging [32,33]. As there is similarity between HS origins in yeast and the heavy strand origin in mammals [26], there is reason to believe that preferential inheritance of mtDNA is similar. So, by understanding the principles of biased mtDNA inheritance in yeast we may gain insights into disease contexts.
One model supporting the replication advantage theory is the RNA priming hypothesis for mtDNA replication. The rho+ genomic regions corresponding to the HS ORIs direct transcription of a ~300bp non-coding RNA that is cleaved and can be used as a primer for in vitro DNA replication [7,29,30]. The rho+ genome has eight regions of ORI homology [34,35], but only the three or four HS ORIs (2, 3, 5 and, in some strain backgrounds, 1) are known to make such an RNA [7,27]. The presence of only RNA producing ORIs in HS mutants suggests that HS genomes are replicated by RNA priming initiation and this mechanism confers their replication advantage over rho+ genomes [7].
There is, however, evidence against the RNA priming hypothesis for mtDNA replication. Certain rho- mtDNA genomes are stably replicated despite lacking either an ORI promoter or the mitochondrial RNA polymerase [31,36,37]. Also, HS mtDNA was shown to still be preferentially inherited without the mitochondrial RNA polymerase [38]; although, there are serious caveats to this experiment. Thus, both replication and HS biased inheritance need not work through ORI RNA or, more broadly, an RNA intermediate. A recombination-based replication model has been proposed to resolve this discrepancy [39,40].
There are some indications that the replication advantage model cannot entirely explain HS biased inheritance. The replication rate of a panel of partially suppressive rho- mutants fails to correlate with the extent of inheritance bias [41]. Also, increasing the pool of available nucleotides reduces the HS inheritance bias [42]. This suggests that alternative models of biased inheritance are partially or wholly responsible for the HS phenotype.
Here, we uncover that a specific HS mtDNA causes DNA damage to rho+ mtDNA. Also, we perform a forward genetic suppressor screen using a high-copy genomic library to look for multicopy suppressors of HS. We find that overexpression of mitochondrial RNA exonuclease PET127 suppresses the HS inheritance bias of certain HS alleles by negatively regulating mitochondrial RNA polymerase RPO41.
Results
Characterization of HS mtDNA
To generate HS mutants, we first used low-level ethidium bromide (EtBr) treatment for short periods of time to generate petite colonies (S1A Fig, [11]). Next, to identify which petite colonies were HS rho-, we mated the newly generated petite strains to rho+ cells and monitored the color of the diploid strains. Monitoring color allowed us to take advantage of the fact that respiration is required for biosynthesis of a red purine analog that turns ade2-1 yeast red [43]. Thus, while rho+ ade2-1 yeast colonies turn red, rho0 or rho- ade2-1 colonies remain white [43]. By definition, HS cells will result predominantly in rho- diploids upon mating with rho+. Thus, we identified HS alleles by looking for white colonies upon mating petite colonies with rho+ (S1B Fig). We validated the potential HS alleles using a quantitative mating assay where we mated the cells, plated the cells to select for diploids, and replica-plated the diploids to medium requiring respiration. We then can assess the fraction of mated cells which retain rho+ mtDNA by dividing the number of cells which grow on respiration requiring medium by the total number of cells on the diploid selection (S1C Fig, [18]).
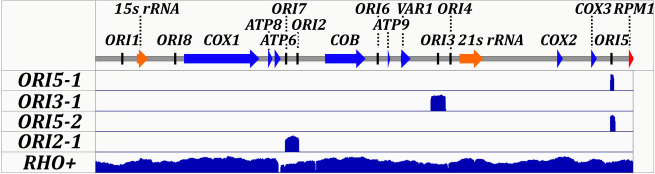
Four of the HS clones were further characterized. We performed sequencing using single molecule real-time (SMRT) sequencing of the mtDNA from each clone and the rho+ parent and mapped the sequenced DNA back to the wild-type reference genome (Fig 1). In accordance with past literature, we found the four clones mapped to the mtDNA at ORI containing regions. We renamed the alleles HS ORI5-1, HS ORI5-2 (spanning all of HS ORI5-1), HS ORI3-1, and HS ORI2-1. The long reads facilitated by SMRT sequencing allowed us to confirm that each of the HS alleles are tandem ORI repeats completely lacking other regions of the rho+ genome.
Fig 1. Generation and Characterization of HS Alleles.
Mitochondria DNA from HS1b (ORI5-1), HS3b (ORI5-2), HS6a (ORI3-1), HS11b (ORI2-1), and rho+ control cells was sequenced using pacific biosciences sequencing technology and mapped back to reference genome. Reads from HS ORI5-1 mapped to base pairs 82,101 to 82,615 of the wild type reference genome. HS ORI3-1 mapped to 53,495 to 55,836. HS ORI5-2 mapped to 82,095 to 82,817. HS ORI2-1 mapped to 30,305 to 32,497. Scale bar ranges are 0 to 1619 for HS ORI5-1, 0 to 1474 for HS ORI5-1, 0 to 1111 for HS ORI3-1, 0 to 660 for HS ORI2-1 and 0 to 305 for rho+.
The presence of HS ORI5-1 mtDNA damages rho+ mtDNA
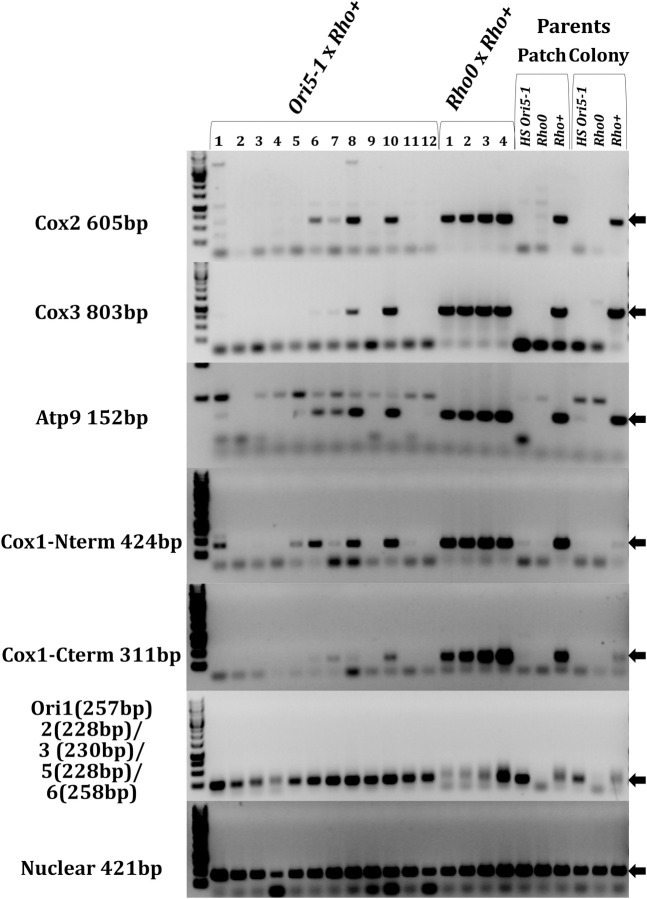
Previous studies showed that HS mtDNA eliminates rho+ mtDNA, however it is unclear how the rho+ mtDNA is eliminated [19,22]. To shed light on the fate of rho+ mtDNA, we asked what happens to different regions of rho+ mtDNA shortly after introducing HS mtDNA to cells. We mated HS ORI5-1 and rho+ parents and used PCR to ask if regions of rho+ mtDNA are still present in the daughter colony. We found three types of respiration incompetent colonies. Five colonies had no observable rho+ regions (Fig 2: Colonies 2, 3, 4, 9, 12), which is expected for the final state of cells after complete takeover of HS mtDNA [19,22]. Four colonies maintained rho+ mtDNA from all regions assayed (Fig 2: Colonies 6, 7, 8, and 10) much like those from the rho0 mated control and the rho+ parent. This was unexpected as respiration incompetent colonies from a rho+ and HS cross were thought to entirely contain HS genomic DNA [24]. Lastly, we observed three colonies that lost some but not all regions of the rho+ genome (Fig 2: Colony 1 losing Cox1-Cterm; Colony 5 losing Cox2, Cox3, and Cox1-Cterm; and Colony 11 losing Cox2, Cox3, Atp9, and Cox1-Cterm). All colonies were assayed for a control nuclear genomic region. Only rho0 parental controls failed to contain any mitochondrial DNA assayed by a nonspecific ORI primer set that detects the ORI5 locus present in both rho+ and HS ORI5-1 mtDNA (Fig 2). The sensitivity of the rho+ detecting primers was assayed by five-fold serial dilutions of rho+ parental DNA into HS ORI5-1 parental DNA (S2 Fig). The most sensitive primers are Cox1-Nterm, Cox3 and Atp9 which were all able to detect 0.64ng of rho+ template. Whereas, Cox1-Cterm and Cox2 failed to detect 3.2 ng of parental DNA. For colony 1, a Cox2 band is present but not a Cox1-Cterminal band and as Cox2 is less sensitive than Cox1 C-terminal that indicates a loss of the Cox1 C-terminal region. For colony 5 and colony 11, Cox3, the most sensitive primer, is missing despite the presence of the Cox1-Nterm bands indicating a loss of Cox3. From these data we conclude that rho+ mtDNA is lost in a piecemeal fashion rather than all at once. This fragmented loss of mtDNA cannot be explained by a replicative advantage, therefore HS mtDNA somehow causes genomic damage to the intact mtDNA.
Fig 2. Colony PCR after HS x rho+ Reveals Remnants of rho+ mtDNA.
The indicated strains were mated and plated on diploid selection medium. The parent strains were plated to single colonies. After two days, individual colonies were scraped off the plates, and DNA was isolated. Genomic DNA was normalized, and PCRs were performed with primer sets amplifying the indicated loci. Reactions were run on agarose gels and stained with ethidium bromide. Arrows indicate the size of the intended product.
PET127 overexpression suppresses the biased inheritance of a subset of HS genomes
To better understand the factors governing mtDNA inheritance, we performed a whole-genome screen to identify suppressors of the biased inheritance of HS mutants. We utilized the HS ORI5-1 allele for our suppression screen as many prior studies of HS mtDNA have focused on ORI5 alleles [39,40,42,44]. Specifically, wild-type rho+ cells were transformed with a high-copy plasmid library [45], mated with the HS ORI5-1 strain, and assayed using the same red/white colony phenotype that we used to isolate HS alleles (Fig 3A) [43]. Most diploid colonies were white due to the dominant inheritance of the HS ORI5-1 mutant. However, four plasmids were identified that repeatedly led to the formation of red rho+ colonies, indicating suppression of the HS biased inheritance. Two colonies contained plasmids bearing a region of chromosome XV with four shared genes: the 3’ region of RTS1, putative gene YOR015W, ERP4, and PET127 (one plasmid fully encompassing PET127, one containing the 5’ 2254 bp out of the 2403 bp coding sequence of PET127) (S3A Fig).
Fig 3. High-copy PET127 is a Suppressor of HS rho- Preferential Inheritance.
A. Rho+ cells were transformed with Yep13 high-copy library [45] and 10,967 colonies were plated. Plasmid containing colonies were mated with lawns of HS Ori5-1, replica plated to diploid selection medium and replica plated again to YEPD with no additional adenine. We identified 154 red colonies initially, however only 5 colonies were red after repetition of the assay. Four of the five colonies showed suppression and were sanger sequenced using Yep13 sequencing primers. B. Quantitative mtDNA inheritance assay validating plasmid 20.4, identified by the screen, and the two candidate genes carried by 20.4. Significance was determined using one-way ANOVA separately on the HS (N = 4) and rho0 (N = 3) crosses with means compared to the vector control and using Dunnett’s multiple comparison’s test. **** indicates adjusted P value less than 0.0001. All other comparisons were not significantly different. C. Quantitative mtDNA inheritance assay as in b testing high-copy PET127 on the other isolated HS alleles. Significance was determined by student’s unpaired T test on the vector and high-copy PET127 pair for each HS or rho0 set (N = 3). * indicates a P value between less than 0.05. ** indicates a P value less than 0.01. *** indicates a P value between 0.001. All other comparisons were not significantly different.
We mapped the gene required for suppression on the plasmids. The region of RTS1 in the plasmids does not contain a functional promoter, and YOR015W is only a putative gene with a start site 45 bp from the end of RTS1, making them less likely candidates. Thus, we focused on ERP4 and PET127 and quantitatively assessed their individual suppressive capabilities (Fig 3B). An empty vector in the rho+ background showed 1.7% ± 1.3 rho+ colonies when mated to HS ORI5-1 but yeast containing the parent plasmid isolated from the screen recovered 42% ± 2.1 rho+ colonies. A plasmid containing either ERP4 or PET127 showed 3.5% ± 3.2 and 43% ± 5.4 rho+ colonies respectively, implicating PET127 as the sole causative suppressive gene. The rho+ colonies resulting from high-copy PET127 suppression grew in a subset region of the original diploid parental colony (S3B Fig). This rho+ subset was not sectored in any discernable pattern, and, as the high-copy plasmid is selected for on the diploid selection plates and should not be lost in large sections of the colony, indicates that there is stochasticity in how high-copy PET127 prevents HS takeover. Mating rho+ yeast containing the suppressive plasmid or a plasmid containing PET127 to the rho0 control did not result in a reduction of rho+ colonies. This indicated that the high-copy PET127 plasmid expression level from its endogenous promoter did not result in the loss of mtDNA in the rho+ haploid parent despite prior literature showing expression of PET127 from the strong ADC1 promoter on multicopy plasmids can result in petite cells [46]. From these data, we conclude that overexpression of PET127 is a suppressor of the preferential inheritance of HS ORI5-1.
Next, we asked if overexpression of PET127 could suppress the phenotype of the other HS rho- mitochondrial genomes using the same approach. We mated rho+ strains containing PET127 with the one clone from each strain in the panel of HS alleles in S1C Fig and determined the number of rho+ diploid colonies (Fig 3C). The suppressive effect of PET127 overexpression was strongest for HS ORI5-1 but also significant in HS2b, HS5b, HS8b, and HS10b. The effect on all other HS alleles was insignificant including HS ORI5-2 which contains the whole region of HS ORI5-1. We genotyped HS alleles using a PCR strategy with primers which have homology to ORI2, ORI3, and ORI5 but have 3’ ends facing each other rather than 5’ ends [47]. As HS alleles are repetitive in nature, this strategy should generate a product spanning the length of a repeat except for the nucleotides between the 3’ primer ends. This strategy did not produce products for all HS alleles, but determined that the PET127 sensitive strain HS10b contains ORI2 DNA indicating that PET127 sensitivity is not due to the features of one particular ORI.
We wondered whether either the specific HS allele or a background nuclear mutation is responsible for determining which strains are sensitive to high-copy PET127 suppression. To determine which hypothesis is correct, we shuttled the mtDNA of HS ORI5-1 and HS ORI5-2 alleles to the same independently generated rho0 recipient by cytoduction (mating with yeast defective in nuclear fusion to allow for transfer of cytoplasmic factors without changing nuclear genotype) and tested the suppression by quantitatively mating the resulting strains to rho+ cells overexpressing PET127 [48]. This experiment resulted in similar levels of rho+ colonies post-mating (S3C Fig), indicating that differences in the extent of high-copy PET127 suppression are due to features of the mtDNA, not the nuclear DNA of the cells where the HS genomes were isolated. From these data we conclude that high-copy PET127 is a suppressor of a subset of HS genomes, and that PET127 sensitivity depends on the genome but can work on ORI5 or ORI2 based HS alleles.
The Pet127 association with Rpo41 is responsible for the suppression of HS ORI5-1
To gain insights into the molecular mechanism by which high PET127 levels suppress the preferential inheritance of HS mtDNA, we investigated which Pet127 function contributes to this phenotype. PET127 is a nuclear-encoded mitochondrial gene that encodes a putative RNA exonuclease that localizes to the mitochondrial inner membrane [46]. PET127 was discovered as a loss-of-function suppressor of C-terminal PET122 mutations which block translation of COX3 mRNA [49], and, while PET127 deletions fail to cause the petite phenotype implied by the “PET” nomenclature [49], expression of PET127 from the strong ADC1 promoter on high-copy plasmids causes loss of mtDNA [46]. PET127 is homologous to the PD-(D/E)XK exonuclease superfamily [50], and has been proposed to trim the 5’ region of mitochondrial mRNAs prior to translation by the mitochondrial ribosome [46,51]. Thus, we first examined whether the 5’ RNA exonuclease activity of PET127 is required for suppression of the HS phenotype by mutating four conserved exonuclease active site residues to generate a nuclease-dead allele (pet127-nd) [46,49–52]. Deletion of PET127 increases the size of mitochondrial transcripts, presumably by eliminating the nuclease activity required to trim the transcripts to the normal size [46,52]. To confirm that pet127-nd lacks nuclease activity, we performed RNA sequencing and looked for the accumulation of transcripts in regions where the PET127 wild-type does not usually accumulate (S4 Fig). The RNA sequencing of cells harboring pet127-nd showed that the levels of mitochondrial RNA in genic regions was relatively unchanged with respect to PET127 (S4A Fig), but the intergenic regions and non-coding RNAs showed accumulation of RNA that mimics that of pet127Δ (S4B and S4C Fig). These data are consistent with the pet127-nd mutant eliminating nuclease activity. Importantly, the pet127-nd mutant retained full suppression of HS preferential inheritance, as revealed by quantitative mating assay (40% ± 6.7 rho+ colonies) (S5 Fig). Thus, excess RNA exonuclease activity from overexpression of PET127 does not affect HS ORI5-1 mtDNA inheritance.
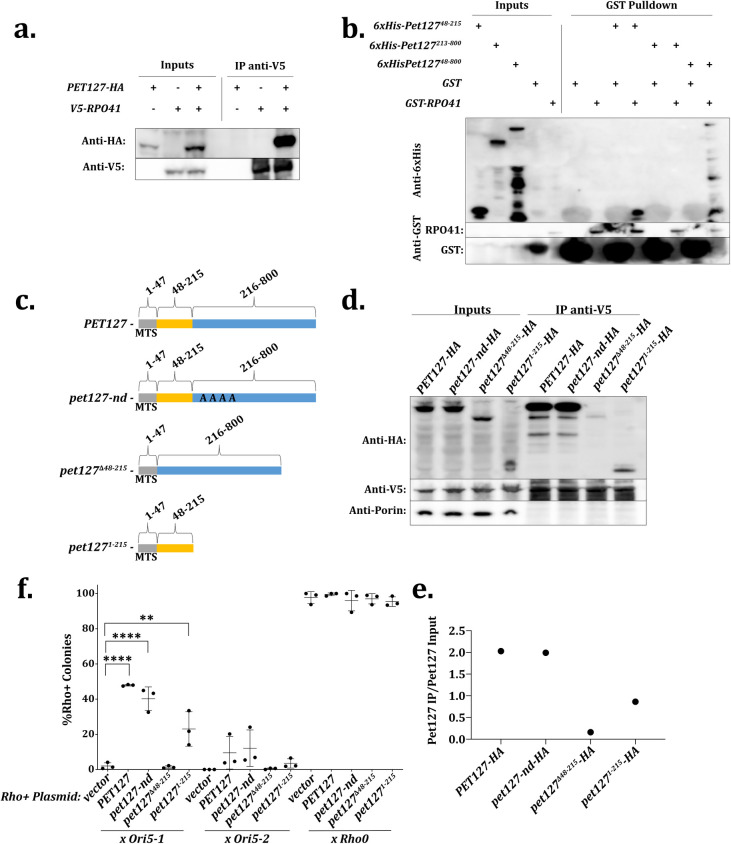
Pet127 also interacts with the sole mitochondrial RNA polymerase, Rpo41, although its role in transcription is unclear [53]. Therefore, we tested whether the association of Pet127 with Rpo41 is required for the observed suppression. First, we verified the association between Pet127 and Rpo41 by co-immunoprecipitation. As specific antibodies are not available, we created epitope-tagged versions of PET127 (PET127-HA) and RPO41 (V5-RPO41). Immunoprecipitation of V5-Rpo41 resulted in co-precipitation of Pet127-HA, indicating that these factors interact in vivo (Fig 4A).
Fig 4. PET127 Binds to RPO41 and the Binding Region is Responsible for Suppression of HS Biased Inheritance.
A. Coimmunoprecipitation of Rpo41 and Pet127. Cells expressing either PET127-HA, V5-RPO41, or PET127-HA and V5-RPO41 were collected, lysed, and V5-Rpo41 was immunoprecipitated using anti-V5 conjugated beads. Samples were immunoblotted as indicated. B. Bacterial expression and lysate mixing of recombinant RPO41 and PET127 alleles. The indicated alleles were expressed in BL21 by the addition of IPTG. Cells were collected and lysed in a French press. For IPs, equal amounts of lysate from Pet127 construct expressing cells was mixed with lysate from either GST-Rpo41 or GST only expressing cells and precipitated with glutathione agarose beads. Samples were immunoblotted as indicated. C. Diagram of PET127 alleles. The predicted mitochondrial targeting sequence is amino acids 1–47 in gray [54]. Amino acids 48–215 contain the Rpo41 binding region in yellow, and amino acids 216–800 contain the region lacking Rpo41 binding activity in blue. pet127-nd is a nuclease dead allele of PET127 with amino acids predicted to be conserved active site residues changed to alanines: E346, D378, D391, and K393 [50]. D. Co-immunoprecipitation of PET127-HA alleles and V5-RPO41 as in a. Samples were immunoblotted as indicated. Anti-porin used as a negative pulldown control. E. Quantitation of IP enrichment in d. F. Quantitative mtDNA inheritance assay with rho+ high copy PET127 alleles x HS ORI5-1, HS ORI5-2, or rho0. Significance was determined using one-way ANOVA separately on each HS and rho0 cross with means compared to the vector control using Dunnett’s multiple comparison’s test (N = 3). **** indicates adjusted P-value less than 0.0001 and ** indicates an adjusted P-value of 0.0025. All other comparisons were not significantly different.
To identify a PET127 allele defective in RPO41 association, we reconstituted the association in vitro using recombinant proteins expressed in bacteria. We expressed full length Pet127 lacking its predicted mitochondrial targeting sequence (aa 1–47) and GST-Rpo41 lacking its mitochondrial targeting sequence and performed a pulldown assay in mixed bacterial lysates [53,54]. The mitochondrial targeting sequences are cleaved in the mitochondria and excluded from the mature forms of Pet127 and Rpo41 in yeast. 6xHis-Pet12748-800 co-precipitated with GST-Rpo41 but not GST alone, indicating a specific and direct interaction between the two proteins (Fig 4B). We then constructed a series of truncation alleles of PET127 from both the N-terminus and the C-terminus (S6 Fig). The smallest PET127 allele that bound Rpo41 contained amino acids 48–215 and the largest allele that failed to bind Rpo41 contained amino acids 213–800 (Fig 4B). Thus, amino acids 48–215 of Pet127 are sufficient for binding to Rpo41.
To test whether PET127 truncations function similarly in yeast, we expressed two of the truncation alleles (including the mitochondrial targeting sequence): pet127Δ48–215, which lacks the binding region, and pet1271-215 which contains the minimal Rpo41 binding region (Fig 4C). The expression level of both truncated proteins was lower than full-length Pet127 (Fig 4D, Inputs); however, both truncated proteins were imported into mitochondria as they were resistant to proteinase K treatment of a mitochondrial enriched preparation (S7 Fig). Yeast expressing the pet1271-215 allele exhibit a second larger species possibly indicative of uncleaved mitochondrial targeting sequence (Fig 4D). Consistent with this hypothesis, the larger band abundance is reduced by proteinase K degradation (S7 Fig). Co-immunoprecipitation showed that Rpo41 binding with Pet127-nd, the allele lacking the RNA exonuclease activity, was unchanged from wild-type and binding with Pet127Δ48–215 was less efficient than with Pet1271-215 (Fig 4D and 4E). Thus, the PET127 truncation mutants are suitable, in yeast, to interrogate whether Rpo41 binding activity is responsible for high-copy PET127 suppression of HS ORI5-1 biased inheritance.
We next used quantitative mating of HS ORI5-1 and rho+ yeast carrying multi-copy plasmids with the truncation alleles to test their ability to suppress HS biased inheritance. In spite its relatively low basal protein abundance, overexpression of the PET127 allele containing the small RPO41 binding region (pet1271-215) was sufficient to suppress HS inheritance (23% ± 9.8 rho+ colonies versus 48% ± 0.44 rho+ colonies for full-length PET127) (Fig 4F). Notably, elimination of the RPO41 binding region completely abolished PET127 suppression, as demonstrated by overexpressing the pet127Δ48–215 truncation (1.4% ± 0.73 rho+ colonies). The suppressive capability of pet1271-215 overexpression is consistent with the observation that plasmid 30.2 isolated from the high copy suppression screen did not contain the full C-terminal region of PET127 (S3A Fig). These data show that the region of PET127 that binds to RPO41 is both necessary and sufficient to suppress HS biased inheritance.
Reduced mitochondrial transcription suppresses HS Ori5-1
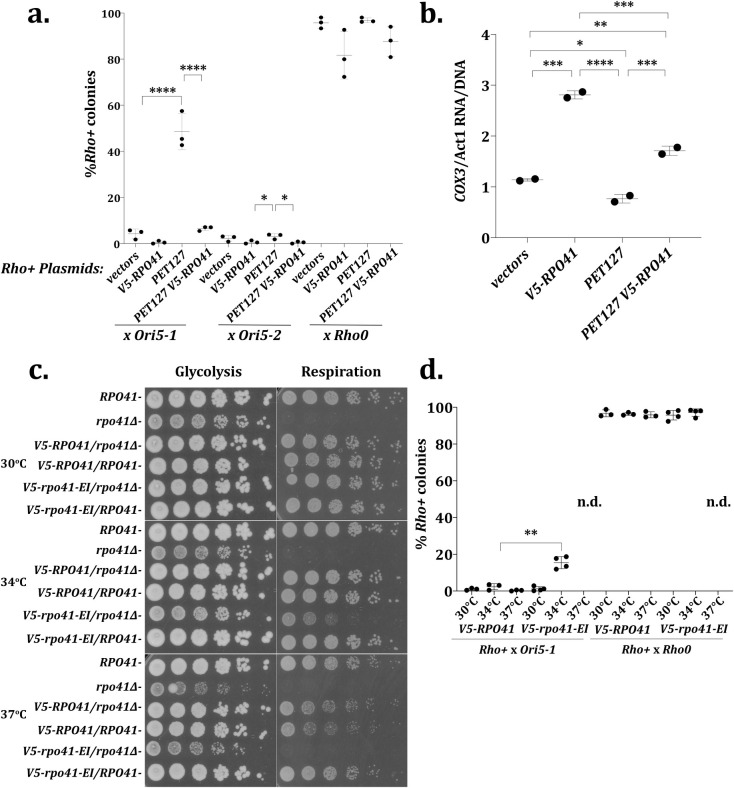
We postulated that Pet127 binding to Rpo41 alters the RNA polymerase activity of Rpo41 and that altered mitochondrial RNA polymerase activity affects HS biased inheritance. To test this hypothesis, we asked whether increasing RPO41 abundance to restore the stoichiometric ratio between Pet127 and Rpo41 amplifies or counteracts the suppressive effect of PET127 overexpression on HS biased inheritance. We assessed the HS biased inheritance on rho+ yeast carrying both a high-copy plasmid expressing V5-RPO41 and a second high-copy plasmid expressing PET127. Quantitative mating with HS ORI5-1 showed that the high-copy PET127 plasmid decreased the inheritance of HS mtDNA (49% ± 7.9 rho+ colonies) relative to control (4.2% ± 2.1 rho+ colonies) (Fig 5A). In contrast, cells with the high-copy V5-RPO41 plasmid showed no significant decrease in rho+ colonies (0.55% ± 0.62 rho+ colonies) relative to control cells (Fig 5A). Interestingly, cells with both V5-RPO41 and PET127 high-copy plasmids significantly abolished the suppression effect of the PET127 plasmid alone (6.6% ± 0.94 rho+ colonies). This finding is consistent with the hypothesis that excess Pet127 binding reduces the polymerase activity of Rpo41.The percent of rho+ colonies after mating rho+ strains carrying V5-RPO41 plasmid or both V5-RPO41 and PET127 plasmids to rho0 cells was not significantly different (82% ± 10 in V5-RPO41, 88% ± 6.6 in PET127 V5-RPO41) from the strains not carrying the V5-RPO41 plasmid (96% ± 2.4 in strain carrying both empty vectors, 97% ± 1.0 in PET127) (Fig 5A). From these data, we conclude that RPO41 and PET127 act in opposition to one another, and, because increasing Pet127 affects HS biased inheritance through Rpo41, there is a strong possibility that Pet127 is an inhibitor of Rpo41 polymerase activity.
Fig 5. Reduction of Transcription from the Mitochondrial RNA pol, RPO41, Suppresses HS Biased Inheritance.
A. Quantitative mtDNA inheritance assay on strains carrying all combinations of high-copy PET127, high-copy V5-RPO41, and their respective empty vector controls. Significance was determined using one-way ANOVA separately on each HS and rho0 cross with means compared to all other samples and using Tukey’s multiple comparison’s test (N = 3). **** indicates adjusted P value less than 0.0001. * indicates adjusted P-value less than 0.05. All other comparisons were not significantly different. B. RT-qPCR of COX3/ACT1 RNA divided by qPCR of COX3/ACT1 DNA on the high-copy PET127 high-copy V5-RPO41 combination strains collected at high cell density to increase respiration. Significance was determined using one-way ANOVA comparing all values with each other using Tukey’s multiple comparison’s test (N = 2). **** indicates adjusted P-value less than 0.0001, *** indicates an adjusted P-value of 0.0008 to 0.0001, ** indicates an adjusted P-value of 0.0057, * indicates an adjusted P-value of 0.0275. C. Five-fold serial dilution of strains containing RPO41 alleles beginning at 1.1 x 107 cells/ml plated on glycolysis (YEPD) or respiration (YEPG) utilizing media. Plates were incubated at either 30°C or 34°C for 2 days or 37°C for 3 days. D. Quantitative mtDNA inheritance assay of rho+ V5-RPO41 x either HS ORI5-1 or rho0 V5-RPO41 and rho+ v5-rpo41-EI x either HS ORI5-1 or rho0 v5-rpo41-EI at 30°C, 34°C, and 37°C. v5-rpo41-EI cross at 37°C was not determined as there was synthetic lethality on the diploid selection plates (see S11 Fig). Significance was determined using student’s T-test on each temperature between V5-RPO41 and v5-rpo41-EI in HS (N = 4) and rho0 (N = 3) cross. ** indicates a P-value of 0.0015. All other comparisons were not significantly different.
As Rpo41 is an RNA polymerase, an increase in Rpo41 abundance should increase mitochondrial transcription, and, if PET127 is an inhibitor of RPO41, increased Pet127 abundance should reduce mitochondrial transcription. Evaluating the mRNA levels of the mitochondrial coding genes COX3, COX2, and ATP9 via RT-qPCR showed that, for all loci assessed, the addition of high-copy V5-RPO41 increases mRNA relative to the control (Figs 5B and S8A). Co-expression of high-copy PET127 and high-copy V5-RPO41 greatly reduced the effect of RPO41, supporting an inhibitory role for Pet127 in transcription (Figs 5B and S8A). Expression of high-copy PET127 alone showed a decreasing trend in mRNA levels, with significant effect on COX3 mRNA, but insignificant effects on ATP9 and COX2 (Figs 5B and S8A). None of the tested conditions affected mtDNA levels (S8B Fig).
To further examine the inhibitory role of Pet127, we maximized its effect on transcription by replacing the wild-type promoter on the high-copy PET127 alleles with the strong galactose-inducible GAL1/10 promoter. In haploid rho+ cells carrying the pGal-PET127 plasmid three hours after galactose addition, COX3 RNA levels were cut in half by qPCR compared to control strains while mtDNA levels were unaffected (S9A Fig). RNA sequencing performed five hours after galactose addition showed a similar trend for all major mitochondrial gene transcripts relative to the time of galactose addition (S9B Fig). Overall, these data indicate that high level of PET127 expression causes a general reduction of mitochondrial transcription, supporting the hypothesis that Pet127 is an inhibitor of Rpo41.
In the RNA priming model for mtDNA replication, transcription from the mtDNA ORI regions is hypothesized to play a role in DNA replication, raising the possibility that the PET127 overexpression effect on the HS phenotype is a consequence of altered ORI transcription. We therefore monitored ORI RNA, utilizing qPCR primers which amplify a transcript, which corresponds to a common area in all “transcription active” ORIs (2, 3, 5, ~230bp) as well as a hypothetical-long transcript, common to ORI1 and ORI6 (~250bp). Surprisingly, ORI RNA levels were increased upon pGAL-PET127 expression in the rho+ background and the HS ORI5-1 background (S9C and S9D Fig). However, this increase was independent of Pet127 binding to Rpo41 as the pGal-pet127Δ48–215 truncation mutant had the same effect as wild-type PET127 (S9C Fig). Expression of the nuclease dead mutant caused ORI RNA to increase above that of the pGal-PET127 allele which suggests that the nuclease activity affects the abundance of ORI transcripts. From these data, we conclude that the levels of the 230 or 250 bp-long ORI transcripts do not play a role in PET127 suppression, but that PET127 expression and nuclease activity can affect levels of those transcripts.
As Pet127 is an inhibitor of Rpo41 when overexpressed, we wanted to know if there are conditions where Pet127 levels change relative to Rpo41 and their inhibitory relationship might be important for cellular life. When Saccharomyces cerevisiae undergo fermentation, a fermentable carbon source such as glucose gets converted into the respiratory carbon source ethanol [55]. In batch cultures, the fermentable carbon source is used up as cell density increases [56]. Saccharomyces cerevisiae can then utilize the ethanol as a carbon source, and this transition is called diauxic shift [57]. As Rpo41 activity is necessary for respiration, we decided to track Pet127 and Rpo41 protein levels as cells undergo diauxic shift. We found that as cell density increases and diauxic shift occurs Pet127 levels decrease relative to Rpo41 (S10A Fig). Conversely, diluting high density cultures into glucose medium increases Pet127 levels relative to Rpo41 (S10A Fig). We wondered whether the observed decrease was specific to ethanol conditions or whether other respiratory carbon sources cause the same effect. To test this, we grew cells in glucose and then split the culture four ways into medium containing either glucose or the respiratory carbon sources glycerol, acetate, or ethanol and waited to let the cells acclimate to the new conditions. We found that while Rpo41 levels remained the same, Pet127 protein levels decreased in all respiratory conditions (S10B Fig). From these data, we conclude that Pet127 protein levels decrease relative to Rpo41 in respiratory conditions.
To directly assess whether biased inheritance of HS can be prevented by inhibiting Rpo41-mediated mitochondrial transcription we attempted to reduce but not eliminate Rpo41 activity in the course of rho+ and HS mating. Since rho+ mtDNA is unstable in the rpo41Δ background [58], we utilized a temperature sensitive allele of RPO41, v5-rpo41-EI [53], at a semi-permissive temperature to assess HS preferential inheritance with a partially functional RNA polymerase. We tested the temperature sensitivity of v5-rpo41-EI at 30°C, 34°C, and 37°C by testing the viability of serial dilutions of exponentially growing yeast in conditions either requiring respiration for growth or allowing for growth using glycolysis (Fig 5C). The v5-rpo41-EI allele permitted haploid cells to grow normally in respiration conditions at 30°C, similar to wild type RPO41, but eliminated growth at 37°C, mimicking rpo41Δ, and caused an intermediate reduction of growth at 34°C (Fig 5C).
Quantitative mating of HS ORI5-1 and rho+ at 37°C, in the V5-RPO41 background showed no changes relative to the other temperatures (0.31% ± 0.29 rho+ colonies), but the v5-rpo41-EI background showed a reduction of diploid colonies on synthetic dropout plates at 37°C despite being a non-respiratory growth condition (S11 Fig). Because the result would be distorted by the synthetic lethal effect present on the diploid selection plate, we did not assess the percent of rho+ colonies in the v5-rpo41-EI at 37°C when mated with either HS ORI5-1 or rho0. However, quantitative mating of HS ORI5-1 and rho+ at 34°C resulted in a small but significant increase in rho+ colonies in the v5-rpo41-EI background (15% ± 3.3) over the V5-RPO41 background (2.3% ± 1.7 rho+ colonies) (Fig 5D). No change was observed when mating rho+ and HS ORI5-1 at 30°C in either the V5-RPO41 background (1.0% ± 0.59) or the v5-rpo41-EI background (1.2% ± 1.2). As partial reduction of mitochondrial RNA polymerase activity is sufficient to suppress the preferential inheritance of HS mtDNA, we conclude that transcription of mitochondrial RNA promotes the HS ORI5-1 preferential inheritance over rho+ mtDNA.
Discussion
HS ORI5-1 mtDNA damages rho+ mtDNA
The main hypothesis regarding the mechanism of HS biased inheritance is that the HS mtDNA is better replicated and outcompetes the rho+ mtDNA. However, we show here that during HS ORI5-1 takeover mtDNA of some regions of rho+ mtDNA are eliminated while other regions remain. If purely a replication competition was responsible for the HS takeover, a uniform loss of the entire rho+ genome would be expected rather than loss of some regions before others. Therefore, the introduction of HS ORI5-1 mtDNA actively eliminates regions of the rho+ genome. The idea that HS biased inheritance is not exclusively due to replicative advantage is consistent with the observation that there is no correlation between replication rates of different partially suppressive rho- mtDNAs and the extent to which they take over the rho+ mtDNA [41].
How does HS ORI5-1 mtDNA cause selective elimination of rho+ mtDNA? HS mtDNA could cause a defect in rho+ replication. Replication defects that could cause selective genome loss include a subset of origins failing or elongation defects preventing complete genome replication. Alternatively, HS mtDNA could directly recombine with rho+ mtDNA with unequal crossovers causing destruction of rho+ regions. A recombination DNA destruction mechanism provides an alternative explanation, besides recombination-based replicative-advantage, as to why recombination mutants are reported to reduce HS biased inheritance [39,40].
The HS phenotype damaging rho+ mtDNA is a harmonious solution to some of the problems of the replicative-advantage model. The replicative advantage model has problems accounting for how there are so few rho+ cell progeny from a HS cross. The replication advantage model proposes that the replication rate of HS genomes is so superior that almost no progeny obtain rho+ mtDNA. The rho+ containing parents then die due to replicative aging. It is known that rho+ mtDNA is present in the progeny of the first division of a zygote [44]. Once it is inherited to cells, the rho+ mtDNA would quickly become a pure population [59]. However, according to the DNA damage model, rho+ mtDNA would have DNA damage which allows it to appear in progeny without a respiration phenotype.
Pet127 is a negative regulator of Rpo41
Although, we show that Pet127 binds and represses Rpo41 function, the molecular mechanism is still unclear. Pet127 binding could be blocking a region required for Rpo41 binding with DNA. Alternatively, Pet127 binding could cause a conformational change in Rpo41 rendering it unable to transcribe RNA or bind to DNA. It is unclear how cells utilize the inhibitory relationship of Pet127 with Rpo41. Pet127 inhibition of Rpo41 could have a buffering role on Rpo41 to allow the cell to respond to fluctuations of Rpo41 levels. Rpo41 activity may be required more in respiratory conditions and Pet127 protein levels dropping in respiratory conditions may be a way cells can regulate Rpo41 activity.
Biased inheritance of ORI5-1 hypersuppressive mtDNA utilizes mitochondrial RNA polymerase activity
We show that transcription is involved in the HS phenotype because modulation of mitochondrial RNA polymerase activity alters the phenotype. Although a role for transcription is clear, the exact mechanism of transcription involvement in HS is not. The theory that mitochondrial RNA is a required intermediate in HS biased inheritance was previously tested by assessing HS biased inheritance in the rpo41Δ background lacking mtRNA [38]. Since rho+ mtDNA is unstable in the rpo41Δ background, a non-preferentially inherited “neutral” rho- mtDNA was used in place of rho+. These studies showed that HS mtDNA was preferentially inherited over neutral rho- mtDNA in rpo41Δ cells. The problem with this experiment is that neutral rho- mtDNA is not an appropriate substitute for rho+ mtDNA, as rho+ mtDNA is inherited far better and has a different transcriptional landscape than the neutral rho- mtDNA. By utilizing the temperature sensitive v5-rpo41-EI allele at semi-permissive temperatures, we were able to maintain the stability of rho+ mtDNA while reducing mitochondrial transcription and show that mitochondrial RNA polymerase, RPO41, plays a role in HS biased inheritance.
How mitochondrial RNA plays a role in HS biased inheritance remains unclear. Prior thinking is that the only transcript in HS alleles is ORI RNA, so transcription must be involved in the HS phenotype through production of ORI RNA. However, we show here that changes in ORI RNA levels do not correlate with changes in HS phenotype across PET127 alleles. Although this finding does not completely rule out a role for ORI RNA in the HS phenotype, it is unlikely that PET127 overexpression reverses HS biased inheritance through ORI RNA effects. Instead, we show that reduction of the HS phenotype occurs in concert with a general reduction of rho+ transcription. How rho+ transcription could be involved in the HS phenotype has not previously been considered. It is possible that a specific rho+ transcript or gene product promotes HS biased inheritance. Alternatively, general rho+ transcription or translation could be required. Given the lack of effects on the ORI transcript, it is unclear which specific transcripts are candidates and how, mechanistically, they would impact inheritance.
There are several possible models for how general transcription reduction could reduce biased inheritance. One possibility is that transcription machinery physically blocks rho+ replication on the same stretch of DNA. If so, then removing RNA polymerase from the genome allows replication machinery access. While it has been shown that transcription can block nuclear replication initiation [60] and replication fork progression [61], it is unclear which, if either, method is responsible for preventing rho+ replication.
Alternatively, a general reduction of transcription could increase the pool of available ribonucleotides. Hyperactivating RNR pathway, which increases nucleotide availability, partially suppresses HS biased inheritance, indicating that nucleotides are limiting for rho+ mtDNA during HS takeover [42]. It is thought that ribonucleotides can substitute for deoxyribonucleotides in the yeast mtDNA during replication because of a promiscuous mtDNA polymerase and an absence of a mitochondrial ribonucleotide excision repair pathway [62]. Thus, reducing rho+ mitochondrial mRNAs could increase the local ribonucleotide pool allowing for elongation utilizing ribonucleotides in a deoxyribonucleotide limited state.
It is puzzling that the methods of HS suppression described here only work on certain HS alleles and not on others. The implication is that the different alleles use different mechanisms for biased inheritance. it could be that the base repeat length of the HS allele determines the method of biased inheritance or that spontaneous mutations in the repeat sequence change the way HS alleles behave.
Materials and methods
Yeast strains and culture conditions
All strains are derivatives of W303 (AA2587) unless noted otherwise. See Table 1 for details. Liquid cultures were grown in YEPD(1% yeast extract, 2% peptone, 2% glucose) with additional adenine (55μg/L), uracil (22.4μg/L), and tryptophan (80μg/L) or SD media [2% glucose, 6.7 g/L yeast nitrogen base w/o amino acids (Difco), 2 g/L complete supplement mixture (without Histidine, without Leucine or without Uracil and Leucine, CSM MP Biomedicals)], to maintain plasmid selection, overnight at room temp (~23°C for rho+ strains) or 30°C (for rho-/0 strains) and diluted back to 3.3 x 106 cells/ml and grown at 30°C.
Table 1. List of Yeast Strains Used in this Study.
| Strain Number | Relevant Genotype | Fig |
|---|---|---|
| AA36030 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 rho+ | S1a |
| AA41772 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS1a | S1c |
| AA41773 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS1b HS ORI5-1 | 1, 2, 3b, 3c, 4f, 5a, S1c, S2, S3b S5, S9d |
| AA41774 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS1c | S1c |
| AA41775 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS2a | S1c |
| AA41776 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS2b | 3c, S1c |
| AA41777 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS2c | S1c |
| AA41778 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS3a | S1c |
| AA41779 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS3b HS ORI5-2 | 1, 3c, 4f, 5a, S1c, S3b, S5, S9d |
| AA41780 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS3c | S1c |
| AA41781 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS4a | S1c |
| AA41782 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS4b | 3c, S1c |
| AA41783 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS4c | S1c |
| AA41784 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS5a | S1c |
| AA41785 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS5b | 3c, S1c |
| AA41786 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS5c | S1c |
| AA41787 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS6a HS ORI3-1 | 1, 3c, S1c |
| AA41788 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS6b | S1c |
| AA41789 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS6c rho0 | 2, 3b, 3c, 4f, 5a, S1c, S3b S5 |
| AA41790 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS7a | S1c |
| AA41791 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS7b | 3c, S1c |
| AA41792 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS7c | S1c |
| AA41793 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS8a | S1c |
| AA41794 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS8b | 3c, S1c |
| AA41795 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS8c | S1c |
| AA41796 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS9a | S1c |
| AA41797 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS9b | 3c, S1c |
| AA41798 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS9c | S1c |
| AA41799 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS10a | S1c |
| AA41800 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS10b | 3c, S1c |
| AA41801 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS10c | S1c |
| AA41802 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS11a | S1c |
| AA41803 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS11b HS ORI2-1 | 1, 3c, S1c |
| AA41804 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 HS Rho- HS11c | S1c |
| AA41805 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-20.4 | S3a |
| AA41806 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-30.2 | S3a |
| AA41807 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-39.1 | S3a |
| AA41808 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-39.4 | S3a |
| AA39745 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13 | 2, 3b, 3c, 4f, S2, S3b, S5 |
| AA41322 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-20.4b (subclone of AA41805) | 3b |
| AA39746 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-PET127 | 3b, 3c, 4f, S3b |
| AA41323 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-ERP4 | 3b |
| AA39257 | MATalpha, ura3-52, lys2-801, ade2-101, his3Del200, trp1Del63, leu2Del1, cyh2, kar1Del15, rho0 | Materials and Methods |
| AA39508 | MATa, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100, HS ORI5-1 rho- | S3c |
| AA40153 | MATa, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100, HS ORI5-2 rho- | S3c |
| AA38266 | MATa, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100, rho0 | S3c |
| AA39535 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13 | S3c |
| AA39534 | MATalpha, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-PET127 | S3c |
| AA39500 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, PET127-6xHA::KANmx6 | 4a |
| AA39479 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3::RPO41preseq-3xV5-RPO41::URA3, rpo41::KANmx6 | 4a |
| AA39607 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3::RPO41preseq-3xV5-RPO41::URA3, rpo41::KANmx6, PET127-6xHA::KANmx6 | 4a, 4d, 4e, S10a, S10b |
| AA2587 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100 | 1, S4a, S4b, S4c |
| AA39672 | MATa, ade2-1, ura3, trp1-1, his3-11,15, can1-100, LEU2::pet127-nd | S4a, S4b, S4c |
| AA39670 | MATa, ade2-1, ura3, trp1-1, his3-11,15, can1-100, pet127::KANmx6, LEU2::pet127-nd | S4a, S4b, S4c |
| AA38088 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, pet127::KANmx6 | S4a, S4b, S4c |
| AA40696 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, pet127-nd-6xHA::HIS3, ura3::RPO41preseq-3xV5-RPO41::URA3, rpo41::KANmx6 | 4d, 4e |
| AA40684 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3::RPO41preseq-3xV5-RPO41::URA3, rpo41::KANmx6, pet127(1–215)-6xHA::HIS3 | 4d, 4e |
| AA40694 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, pet127(Δ48–215)-6xHA::HIS3, ura3::RPO41preseq-3xV5-RPO41::URA3, rpo41::KANmx6 | 4d, 4e |
| AA40287 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3, Yep13-pet127(Δ48–215) | 4f |
| AA40316 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3, Yep13-pet127(1–215) | 4f |
| AA39617 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3, Yep13-pet127-nd | 4f |
| AA40317 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13, pRS426 | 5a, 5b, S8a, S8b |
| AA40319 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-PET127, pRS426 | 5a, 5b, S8a, S8b |
| AA40318 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13, pRS426-MTS-3xV5-RPO41 | 5a, 5b, S8a, S8b |
| AA40320 | MATa, ade2-1, leu2-3, ura3, trp1-1, his3-11,15, can1-100, YEP13-PET127, pRS426-MTS-3xV5-RPO41 | 5a, 5b, S8a, S8b |
| AA36029 | MATa, ade2-1, leu2-3, ura3, trp1-1, HIS3+, can1-100 | 5c, S11 |
| AA40931 | MATa, ade2-1, leu2-3, ura3, trp1-1, can1-100, rpo41::KANmx6, Rho0 | 5c, S11 |
| AA40974 | MATa, ade2-1, trp1-1, leu2-3, can1-100, ura3::RPO41preseq-3xV5-RPO41::URA3, HIS3+ | 5c, S11 |
| AA40937 | MATa, ade2-1, trp1-1, his3-11,15, can1-100, ura3::RPO41preseq-3xV5-RPO41::URA3, rpo41::KANmx6, HIS+ | 5c, S11 |
| AA40972 | MATa, ade2-1, trp1-1, leu2-3, can1-100, ura3::RPO41preseq-3xV5-RPO41-EI::URA3, HIS3+ | 5c, S11 |
| AA40966 | MATa, ade2-1, trp1-1, leu2-3, can1-100, ura3::RPO41preseq-3xV5-RPO41-EI::URA3, rpo41::KANmx6, HIS3+ | 5c, S11 |
| AA41033 | MATa, ade2-1, trp1-1, his3-11,15, can1-100, ura3::RPO41preseq-3xV5-RPO41::URA3, rpo41::KANmx6, LEU2+, Rho- (ORI5-1) | 5d |
| AA41017 | MATa, ade2-1, trp1-1, his3-11,15, can1-100, ura3::RPO41preseq-3xV5-RPO41-EI::URA3, rpo41::KANmx6, LEU2+, Rho- (ORI5-1) | 5d |
| AA40982 | MATa, ade2-1, trp1-1, his3-11,15, can1-100, ura3::RPO41preseq-3xV5-RPO41::URA3, rpo41::KANmx6, LEU2+, Rho0 | 5d S1c |
| AA41006 | MATa, ade2-1, trp1-1, his3-11,15, can1-100, ura3::RPO41preseq-3xV5-RPO41-EI::URA3, rpo41::KANmx6, LEU2+, Rho0 | 5d |
| AA40938 | MATalpha, ade2-1, trp1-1, his3-11,15, can1-100, ura3::RPO41preseq-3xV5-RPO41::URA3, rpo41::KANmx6, HIS+ | 5d. S1c |
| AA40967 | MATalpha, ade2-1, trp1-1, leu2-3, can1-100, ura3::RPO41preseq-3xV5-RPO41-EI::URA3, rpo41::KANmx6, HIS3+ | 5d |
| AA41640 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, Pet127-6xHA::HIS3, TOM70-GFP::KanMX | S7 |
| AA41642 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, Pet127(Δ48–215)-6xHA::HIS3, TOM70-GFP::KanMX | S7 |
| AA41643 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, Pet127(1–215)-6xHA::HIS3, TOM70-GFP::KanMX | S7 |
| AA41809 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3, pYX223-mtGFP | S9a, S9b |
| AA39631 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3, pRS423 | S9a, S9b, S9c |
| AA39838 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3, pRS423-pGal-PET127-3xV5 | S9a, S9b, S9c |
| AA40159 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3, pRS423-pGal-pet127-nd-3xV5 | S9a, S9b, S9c |
| AA40237 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3, pRS423-pGal-pet127Δ(48–215)-3xV5 | S9a, S9b, S9c |
| AA40335 | MATa, ade2-1, leu2-3, trp1-1, his3-11,15, can1-100, ura3, pRS423-pGal-pet127(1–215)-3xV5 | S9a, S9b, S9c |
| AA41810 | MATalpha, ade2-1, leu2-3, ura3, HIS3+, can1-100, trp1-1 LEU2::pGal1-PET127 (Single copy integration) HS ORI5-1 rho- | S9d |
| AA41811 | MATalpha, ade2-1, leu2-3, ura3, HIS3+, can1-100, trp1-1 LEU2::pGal1-PET127 (Single copy integration) HS ORI5-2 rho- | S9d |
For galactose induction, strains were cultured overnight in SD-Histidine medium (rho+ strains) or YEP medium (HS strains) with 2% raffinose instead of 2% glucose. Cells were diluted back to 3.3 x 106 cells/ml in the same medium, allowed to grow one doubling, and the expression of the galactose promoter was induced by the addition of 1% galactose.
EtBr Treatment (Generation of rho0 and HS rho-)
For generation of rho0 cells of a particular strain, cells were grown in YEPD + 10μg/ml ethidium bromide for 3 days, diluted back each morning. After treatment cells were streaked to single colonies and lack of mtDNA was confirmed via DAPI staining at 2μg/ml. Generation of HS alleles was performed by growing log phase rho+ cells in 5μg/ml EtBr for 100 minutes and plated on YEPD plates to ~200 cells per plate.
Screen
Rho+ cells were transformed with Yep13 high-copy library [45] and were plated on plasmid selection media. Colony plates were replica plated to lawns of HS ORI5-1, and further replica plated to diploid selection medium and replica plated again to YEPD with no additional adenine. Identified colonies were selected from the initial transformation plate and the assay was repeated on those colonies. Plasmids from colonies which scored both times were Sanger sequenced using Yep13 sequencing primers. The resulting sequences were identified via NCBI blast [63].
Cytoduction
HS strains were transformed with a matA cassette and a Pringle deletion cassette for matAlpha and mated with rho0 karyogomy defective mtDNA shuttling strain AA39257 and plated on YEPD + 10μg/ml Cycloheximde to select for homozygous cyh2Δ present only in haploid AA39257. The resulting colonies were screened for cells containing mtDNA via DAPI indicating transfer of HS mtDNA from the HS strain to the AA39257 parent. The resulting cells were mated with rho0 recipient strains, and plated on SD-Arg+ 50mg/ml Canavanine to select for homozygous can1-1 allele present only in the haploid recipient strains. The resulting colonies were checked for mtDNA by staining with 2μg/ml DAPI and confirmed to be haploid by mating complementation on minimal media.
Mating assay and temperature shifts
Log phase growing cells were mated by mixing 2.2 x 107 cells from each parent into 1ml YEPD at room temperature for 5 hours. Cells were diluted 1:1000 and plated on diploid selection plates (SD-His-Leu or SD-His-Ura-Leu) for 48hrs at either 30°C, 34°C, or 37°C and then replica plated to YEPG (1% yeast extract, 2% peptone, 3% glycerol) plates and placed at the same temperature for 72 hours. Percent rho+ cells was determined by 100*#colonies growing on YEPG/#colonies growing on diploid selection. A colony was considered growing if any subregion of the colony was growing.
Rpo41-V5-EI temperature sensitivity
Mid-log (~1.1 x 107 cells/ml) cultures were diluted to 1.1 x 107 cells/ml and five-fold serially diluted 5 times and 4 μl was plated on either YEPD, YEPG, or SD-His and placed at either 30°C, 34°C, or 37°C for 2 days.
Cloning and strain construction
Strains were constructed using standard gene integration or tagging strategies [64]. Plasmids were constructed using standard cloning techniques and described in Table 2 [65].
Table 2. List of Plasmids Used in this Study.
| Plasmid Number | Description | Fig |
|---|---|---|
| pAA44 | YEp13 | 2, 3b, 3c, 4f, 5a, 5b, S2, S3b, S3c, S5, S8a, S8b |
| pAA2582 | YEp13-Erp4 | 3b |
| pAA2581 | YEp13-Pet127 | 3b, 3c, 4f, 5a, 5b, S3b, S3c, S5, S8a, S8b |
| 20.4 | YEp13-[3’RTS1, YOR015W, ERP4, PET127, 5’ROD1] | 3b, S3a |
| 30.2 | YEp13-[3’RTS1, YOR015W, ERP4, 5’PET127] | S3a |
| 39.1 | YEp13-[3’CHS1, ARS1414, DUG3, YNL190W, SRP1] | S3a |
| 39.4 | YEp13-[3’SEC27, MRM2, RPL1b, 5’PCL10] | S3a |
| pAA2758 | pGEX-6P-1-V5-Rpo41 | 4b |
| pAA2778 | pET15b-Pet127-6xHis (Derived from pET15b Novagen (EMD Millipore)) | 4b |
| pAA2058 | pGEX-6P-1 (Amersham) | 4b |
| pAA2794 | pET15b-Pet127-N504-6xHis | 4b |
| pAA2806 | pET15b-Pet127-C1764-6xHis | 4b |
| pAA2703 | pRS306-Rpo41-internal-3xV5 | 4a, 4d, 5c, 5d, S10a, S10b, S11 |
| pAA2782 | pNH605-Pet127-nd-3xV5 | 4d, S7 |
| pAA2832 | pNH605-Pet127 Δ48-215 -3xV5 | 4d, S7 |
| pAA2842 | pNH605-Pet127 1-215 -6xHA-His3 | 4d, S7 |
| pAA2713 | YEp13-Pet127-nd | 4f |
| pAA2833 | YEp13-Pet127 Δ48–215 | 4f |
| pAA2835 | YEp13-Pet127 1-215 | 4f |
| pAA2837 | pRS426-RPO41-internal-3xV5 | 5a, 5b, S8a, S8b |
| pAA2924 | pRS306-Rpo41-EI-internal-3xV5 | 5c, 5d, S11 |
DNA isolation
DNA for qPCR and colony PCRs was isolated using a modified smash and grab protocol from [66] with RNAse treatment. The mtDNA isolation for SMRT sequencing was done similarly using two phenol chloroform extractions on pellets enriched for mitochondria using the mitochondrial isolation technique below.
SMRT sequencing
mtDNA isolated as above was sequenced using SMRT sequencing technology on Pacific Biosciences Sequel II [67]. 10kb insert size was selected for on 0.75% agarose Blue Pippin cassettes obtaining 12-13kb mean length inserts and mean read length of 6-7kb. Long reads were computationally divided into 50nt fragments and mapped using BWA-mem onto SGD sacCer3 genome wildtype reference and viewed on IGV [68].
Colony PCR
Strains were mated as in the quantitative mating assay. Whole individual diploid colonies were scraped off plates and DNA was isolated from them using the DNA isolation protocol. DNA was normalized across samples and added to PCR reactions using the colony PCR primer sets in Table 3. PCRs were run on either 1% or 2% (ATP9) agarose gel made with 1x TAE (40mM Tris base, 20mM Acetic Acid, 1mM EDTA) and 0.1μg/ml EtBr for 25 minutes at 130V constant voltage in 1 x TAE.
Table 3. List of Primers Used in this Study.
| Primer Set | Forward Primer | Reverse Primer |
|---|---|---|
| Yep13 Backbone | TTCGCTACTTGGAGCCACTAT | ATCGGTGATGTCGGCGATATA |
| ACT1 qPCR set | GTACCACCATGTTCCCAGGTATT | CAAGATAGAACCACCAATCCAGA |
| COX3 qPCR set | TCCATTCAGCTATGAGTCCTGA | CTGCGATTAAGGCATGATGA |
| COX2 qPCR set | GTGGTGAAACTGTTGAATTTGAATC | AGCAGCTGTTACAACGAATCTA |
| ATP9 qPCR set | ATTGGAGCAGGTATCTCAACAA | GCTTCTGATAAGGCGAAACC |
| ORI qPCR set | ATAGGGGGAGGGGGTGGGTGAT | GGGACCCGGATATCTTCTTGTTTATC |
| Nuclear Colony PCR Set (PET127 locus) | GCGCGTTTCCGTCAATGCC | TTTCAGTAGATTAATCGCCTTGTCC |
| ORI Colony PCR set | ATAGGGGGAGGGGGTGGGTGAT | GGGACCCGGATATCTTCTTGTTTATC |
| COX2 Colony PCR set | CAGCAACACCAAATCAAGAAGG | ATGACCTGTCCCACACAAC |
| COX3 Colony PCR set | GACACATTTAGAAAGAAGTAGACATCAAC | GACTCCTCATCAGTAGAAGACTACG |
| ATP9 Colony PCR set | ATTGGAGCAGGTATCTCAACAA | GCTTCTGATAAGGCGAAACC |
| COX1 Nterm Colony PCR set | TAGCTGCACCTGGTTCACAA | CCTCTTTCAGTTGATCCCTCAC |
| COX1 Cterm Colony PCR set | ACTTTCTTCCCCTCCGAATC | CCTGCGGATTGTCCATACTT |
RNA Isolation and qPCR
RNA was isolated from 4.4 x 107 cells using acid phenol and purified using an RNeasy kit (Qiagen) with on column DNAse treatment (Qiagen). Reverse transcription was performed on 750ng of RNA using SuperScript III First-Strand Synthesis SuperMix (Life Technologies) and qPCR was performed using SYBR Premix Ex Taq (Tli RNaseH Plus) or the equivalent TB Green Premix Ex Taq (Tli RNaseH Plus) from TaKaRa. Signals were normalized to ACT1 levels and normalized to the control average. Primers are described in Table 3.
RNA sequencing
Isolated total yeast RNA as above were processed using Ribozero rRNA removal for Yeast (Illumina). For pet127-nd RNA profiling, sequencing was performed on NextSeq500 with 75 + 75 bases pair-end run with 6 + 6 nucleotide indexes. Pair end sequencing reads were mapped to sacCer3 reference genome using star/2.5.3a [69]. The regions of interest were defined using the bed file format. The coverage sub-command of bedtools/2.26.0 was applied to calculate the number of sequences mapped to the specific regions of the reference genome using alignment bam files. The counts of all samples were merged to a matrix with each sample per column and each location per row using MIT IGB in house tool. The regions were defined by the boundaries in Table 4 modified from [7].
Table 4. Bin Boundaries for pet127-nd RNA Sequencing.
| Description | Start | Start |
|---|---|---|
| RPM1 to UGG Proline tRNA | 1 | 730 |
| UGG Proline tRNA | 731 | 802 |
| UGG Proline tRNA to ORI1 | 803 | 4011 |
| ORI1 | 4012 | 4312 |
| ORI1 to 15S rRNA | 4313 | 6545 |
| 15S rRNA | 6546 | 8194 |
| 15S rRNA to UCA Tryptophan tRNA | 8195 | 9373 |
| UCA tryptophan tRNA | 9374 | 9444 |
| UCA tryptophan tRNA to ORI8 | 9445 | 12509 |
| ORI8 | 12510 | 12780 |
| ORI8 to COX1 | 12781 | 13817 |
| COX1 | 13818 | 26701 |
| COX1 to ATP8 | 26702 | 27665 |
| ATP8 | 27666 | 27812 |
| ATP8 to ATP6 | 27813 | 28486 |
| ATP6 | 28487 | 29266 |
| ATP6 to ORI7 | 29267 | 30219 |
| ORI7 | 30220 | 30594 |
| ORI7 to ORI2 | 30595 | 32230 |
| ORI2 | 32231 | 32501 |
| ORI2 to UUC glutamate tRNA | 32502 | 35372 |
| UUC glutamate tRNA | 35373 | 35444 |
| UUC glutamate tRNA to COB | 35445 | 36539 |
| COB | 36540 | 43647 |
| COB to ORI6 | 43648 | 44888 |
| ORI6 | 44889 | 45225 |
| ORI6 to ATP9 | 45226 | 46722 |
| ATP9 | 46723 | 46953 |
| ATP9 to UGA serine tRNA | 46954 | 48200 |
| UGA serine tRNA | 48201 | 48290 |
| UGA serine tRNA to VAR1 | 48291 | 48900 |
| VAR1 | 48901 | 50097 |
| VAR1 to ORI3 | 50098 | 54566 |
| ORI3 | 54567 | 54840 |
| ORI3 to ORI4 | 54841 | 56566 |
| ORI4 | 56567 | 56832 |
| ORI4 to 21S rRNA | 56833 | 58008 |
| 21S rRNA | 58009 | 62447 |
| 21S rRNA to UGU threonine tRNA | 62448 | 63861 |
| UGU threonine tRNA | 63862 | 63934 |
| UGU threonine to GCA cysteine tRNA | 63935 | 64414 |
| GCA cysteine tRNA | 64415 | 64487 |
| GCA cysteine tRNA to GUG histidine tRNA | 64488 | 64596 |
| GUG histidine tRNA | 64597 | 64667 |
| GUG histidine tRNA to UAA leucine tRNA | 64668 | 66094 |
| UAA leucine tRNA | 66095 | 66176 |
| UAA leucine tRNA to UUG glutamine tRNA | 66177 | 66209 |
| UUG glutamine tRNA | 66210 | 66282 |
| UUG glutamine tRNA to UUU lysine tRNA | 66283 | 67060 |
| UUU lysine tRNA | 67061 | 67132 |
| UUU lysine tRNA to UCU arginine tRNA | 67133 | 67308 |
| UCU arginine tRNA | 67309 | 67381 |
| UCU arginine tRNA to UCC glycine tRNA | 67382 | 67467 |
| UCC glycine tRNA | 67468 | 67539 |
| UCC glycine tRNA to GUC aspartate tRNA | 67540 | 68321 |
| GUC aspartate tRNA | 68322 | 68393 |
| GUC aspartate tRNA to GCU Serine tRNA | 68394 | 69202 |
| GCU Serine tRNA | 69203 | 69285 |
| GCU Serine tRNA to ACG arginine tRNA | 69286 | 69288 |
| ACG arginine tRNA | 69289 | 69359 |
| ACG arginine tRNA to UGC alanine tRNA | 69360 | 69845 |
| UGC alanine tRNA | 69846 | 69918 |
| UGC alanine tRNA to GAU Isoleucine tRNA | 69919 | 70161 |
| GAU Isoleucine tRNA | 70162 | 70234 |
| GAU Isoleucine tRNA to GUA tyrosine tRNA | 70235 | 70823 |
| GUA tyrosine tRNA | 70824 | 70908 |
| GUA tyrosine tRNA to GUU Asparagine tRNA | 70909 | 71432 |
| GUU Asparagine tRNA | 71433 | 71504 |
| GUU Asparagine tRNA to CAU methionine tRNA 1 | 71505 | 72631 |
| CAU methionine tRNA 1 | 72632 | 72705 |
| CAU methionine tRNA 1 to COX2 | 72706 | 73757 |
| COX2 | 73758 | 74513 |
| COX2 to GAA phenylalanine tRNA | 74514 | 77430 |
| GAA phenylalanine tRNA | 77431 | 77502 |
| GAA phenylalanine tRNA to UAG threonine tRNA | 77503 | 78088 |
| UAG threonine tRNA | 78089 | 78162 |
| UAG threonine tRNA to UAC valine tRNA | 78163 | 78532 |
| UAC valine tRNA | 78533 | 78605 |
| UAC valine tRNA to COX3 | 78606 | 79212 |
| COX3 | 79213 | 80022 |
| COX3 to ORI5 | 80023 | 82328 |
| ORI5 | 82329 | 82600 |
| ORI5 to CAU methionine tRNA 2 | 82601 | 85034 |
| CAU methionine tRNA 2 | 85035 | 85107 |
| CAU methionine tRNA 2 to RPM1 | 85108 | 85294 |
| RPM1 | 85295 | 85777 |
| RPM1 to UGG Proline tRNA | 85778 | 85779 |
For the pGal-PET127 RNA sequencing experiment, sequencing was performed on HiSeq2000 with 40 bases single end run with 8 + 8 nucleotide indexes [70]. Single end sequencing reads were mapped to sacCer3 reference genome using star/2.5.3a. rsem/1.3.0 was applied for gene level counting, fpkm and tpm calculation. The raw counts, fpkm, and tpm values of each gene in all sample were merged into three corresponding matrices using IGB in house tools. The matrices were formatted as each sample per column and each gene per row. Hierarchical clustering was performed using TIBCO Spotfire 7.11.1 based on log2(fpkm+1) of the expressed coding genes. Differential expression comparisons between 5 hour and 0 hour under different conditions were carried out using Deseq2 1.10.1 under r/3.2.3. with raw counts as input.
Mitochondria isolation
Mitochondrial enrichment protocol modified from [71]. Cells were grown into logarithmic phase growth, transferred into DTT buffer (0.1 M Tris pH 9.4, 10 mM DTT) to shake for 20 minutes at 30°C, and transferred into zymolyase buffer (1.2M sorbitol, 20mM K2HPO4 pH 7.4) with the addition of 1% zymolyase 100T or equivalent for 1 hour at 30°C to digest the cell wall. Cells were lysed by dounce homogenization 20 strokes in homogenization buffer (0.6M sorbitol, 10mM Tris pH 7.4, 1mM EDTA, 0.2% BSA no fatty acid, 1mM PMSF). Mitochondria were isolated by differential centrifugation 5 minutes at 1200g, the resulting supernatant was spun for 5 minutes at 2000g, and the resulting supernatant was spun at 17500g for 15 minutes all at 4°C. The resulting pellet was resuspended in SEM buffer (0.25M sucrose, 10mM MOPS KOH pH 7.2, and 1mM EDTA). For assessing whether proteins are mitochondrial, 3μg of enriched mitochondria was treated with 50μg/ml proteinase K for 5mins at 37°C. The reaction was stopped by addition of TCA to 12.5%.
Coimmunoprecipitation assay
Cells were grown in YEPD to 1.1 x 107 cells /ml. 1.1 x 109 cells were collected and frozen. Cells were lysed with a FastPrep-24 Classic (MP Biomedicals, Speed 6.5, 60s, 10 cycles) with 200μl NP40 buffer [50mM Tris pH7.5, 150mM NaCl, 1% Np40 (IGEPAL) and Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific)]. Lysates were brought up to 1.5ml with NP40 buffer and were pelleted by centrifugation at 20,000g for 10 minutes at 4C. 1% of sample was taken for Input and boiled with 10μl of 3x SDS sample buffer, and the rest of the lysates were brought to 0.2% BSA. Clarified sample was added to 20μl of Anti-V5 agarose affinity gel antibody beads (Sigma) and incubated for 2 hours at 4C. Beads were washed 3 times with Np40 buffer with Protease inhibitor and 2 times in NP40 buffer without inhibitors. To elute, 30μl of 1.5x SDS sample buffer was added to the beads and samples were boiled for 5 minutes.
Immunoblotting
Samples in SDS sample buffer were run on 8% polyacrylamide gels in SDS running buffer (0.1918M glycine, 0.0248M Tris base, 0.1%SDS), or gradient gels in MES running buffer (2.5mM MES, 2.5 mM Tris Base, 0.005% SDS, 0.05mM EDTA pH7.3), transferred to PVDF membranes via semi-dry transfer, washed with PBST (10mM, 2.7mM potassium chloride, 137mM sodium chloride, and 1.76mM potassium phosphate. pH 7.4 + 0.1% Tween-20), blocked with PBST and 3% milk for 1 hour and probed overnight in 1xPBS with 1% milk, 1%BSA, 0.1% Sodium Azide and with one of the following primary antibody dilutions: Mouse Anti-HA (1:2000; HA.11, Biolegend), Mouse Anti-V5 (1:2000; R960-25, Thermo-Fisher Scientific), Mouse Anti-VDAC/Porin (1:1000; 16G9E6BC4/ab110326, Abcam), Mouse Anti-6xHis (1:5000, ab18184, Abcam), Mouse Anti-GST (1:5000, ab19256, Abcam), Mouse Anti-PGK (1:5000; 22C5D8, Thermo-Fisher Scientific), Mouse Anti-COX4 (1:1000; ab110272, Abcam), Mouse Anti-GFP (1:1000; JL-8, Clontech). Ponceau S (Abcam) was applied before blocking for 15 minutes and destained for 30 minutes with distilled water. Blots were washed three times with PBST and probed with secondary antibodies diluted in PBS with 1% milk and 1% BSA for one hour at 4°C. Secondary antibody dilutions were Anti-mouse HRP 1:10,000 and Anti-mouse HRP TrueBlot 1:1000 (for coimmunoprecipitation experiments). Membranes were washed three times in PBST and blots were imaged using the ECL Plus system (GE healthcare).
Bacterial expression and binding and truncations
BL21 Bacteria containing expression plasmids, described in Table 5, were grown in LB+AMP (1% Difco Bacto Tryptone, 0.5% Difco Yeast Extract, 1% NaCl, 10mM Tris pH 7.5, Amp 0.1 mg/ml) overnight at 37°C. Cells were diluted to 2.66 x 108 cells/ml in LB + AMP and grown at 37°C for 1h, IPTG was added to 0.5 mM final concentration and cells were allowed to express for 5h at 37°C. Cells were spun at 22K g for 20 minutes at 4°C and resuspended to 1.33 x 1010 cells/ml in PBS + PI [cOmplete, mini, EDTA-free Protease Inhibitor Cocktail (Millipore-Sigma) 1/50mls] + 0.3% BSA. Cells were lysed via French press. GST expressing samples were added to Glutathione beads (pre-washed in PBS+PI). Beads were incubated for 2h at 4°C and spun down at 500 rcf for 2 minutes at 4°C. Beads were washed 3 times with cold NP40 buffer + PI + BSA 0.3%. Equal amounts of non-GST expressing samples were added to the beads. Beads were incubated for 2h at 4°C and spun down at 500 rcf for 2 minutes at 4°C. Beads were washed 3 times with cold NP40 buffer + PI +BSA 0.3% and washed 2 times with NP40 buffer. Protein was eluted by adding 2x SDS Sample buffer equal in volume to beads and boiling for 5 minutes.
Table 5. List of Bacterial Strains Used in this Study.
| Strain number | Relevant Genotype | Fig |
|---|---|---|
| AAE568 | BL21 pGEX-6P-1-V5-Rpo41 (pAA2758) | 4b |
| AAE569 | BL21 pET15b-PET127-6xHIS (pAA2778) | 4b |
| AAE570 | BL21 pGEX-6P-1 (pAA2058) | 4b |
| AAE571 | BL21 pET15b-PET127N504-6xHis (pAA2794) | 4b |
| AAE572 | BL21 pET15b-Pet127-C1764-6xHis (pAA2806) | 4b |
Diauxic shift experiments
For the glucose to ethanol shift, cells were grown overnight in YEPD medium and diluted back to 3.3 x 107 cells/ml in YEPD. Cells were grown for 12 hours with 2.2 x 107 cells collected every 2 hours. For the ethanol to glucose shift, A 2.2 x 107 cells from an overnight culture were collected and then the culture was diluted back to 3.3 x 106 cells/ml in YEPD. 2.2 x 107 cells were collected 3h, 5h, and 7h later.
Medium swap experiment
Cells were grown overnight in YEPD medium and diluted back to 1.1x107 cells/ml and grown until 1.1x107 cells/ml. 2.2 x 107 cells were collected and then equal amounts of cells were collected using filters (Supor membrane disc filters, Pall laboratory, 47mm, 0.8μM, VWR) and released into YEPD, YEPG (2% Glycerol), YEPA (1% yeast extract, 2% peptone, 2% Potassium Acetate). YEPE (1% yeast extract, 2% peptone, 2% ethanol) at 1.1x107 cells/ml. Cells were grown for 4 hours and 2.2 x 107 cells were collected and immunoblotted.
Supporting information
A. 5 μg/ml EtBr was added to rho+ cells and cells were collected every 30 minutes for 180 minutes. Colonies were assessed for respiration. Percent rho- or rho0 colonies was calculated by the formula 100 x [1- (respiratory colonies/total colonies)]. B. Diagram of screen for HS alleles. Rho+ cells were treated with 5μg/ml EtBr for 100 minutes to create a mixed mtDNA population, plated to single colonies on YEPD (1% yeast extract, 2% peptone, 2% glucose), mated with lawns of rho+ yeast on YEPD, selected for diploids on SD-His-Leu, and plated on YEPD plates with no added adenine. Red colonies on the low adenine YEPD plate are rho+ and white colonies are rho- or rho0. White colonies were taken for further analysis. C. HS candidates tested for mtDNA inheritance bias by quantitative mating assay. HS candidates or a rho0 control were mated with a rho+ tester and selected for diploids colonies which were assessed for rho+ mtDNA.
(TIF)
Genomic DNA was isolated from rho+ or HS ORI5-1 parent patches and normalized. Rho+ genomic DNA was diluted into HS ORI5-1 genomic DNA in five-fold serial dilutions. PCRs of using primer sets recognizing mitochondrial loci were performed from the serial dilutions and run on agarose gels containing ethidium bromide. The first lane contains the ladder and the last lane is a no DNA control.
(TIF)
A. Inserts of four plasmids obtained from the high-copy suppressor screen were PCRed using Q5 (New England Biolabs) and Sanger sequenced using the Yep13 backbone primer set. The resulting sequences were identified via NCBI blast [63] and the genomic boundaries of each plasmid insert are as indicated. Parentheses indicate annotated genes contained within the genomic region with 3’ or 5’ indicating that the genic region was cut off and only the 3’ or 5’ end of the gene was present. B. The suppressive capability of the high-copy PET127 plasmid was tested on HS ORI5 alleles cytoduced into a common recipient strain so as to confirm that the extent of high-copy PET127 suppression is determined by the HS allele and not by a possible nuclear mutation. C. Typical plates following quantitative inheritance assay. “Total Diploid” images taken 2 days after plating on SD-His-Leu diploid selection medium. “Respiratory” images taken 3 days after replica plating to YEPG. Inset showing colony section growth.
(TIF)
Mitochondrial gene expression analysis of A. Intragenic mtDNA regions with and without COX1 as COX1 has much higher expression relative to the other mitochondrial genes. B. Non-coding RNA regions: tRNA and ORI. C. Intergenic mtDNA regions. * indicates a discovery in both comparisons WT to pet127-ND/pet127Δ and WT to pet127Δ via multiple unpaired T-tests with Two-stage step-up (N = 3). “x” indicates discovery in only WT to pet127Δ. + indicates discovery in only WT to pet127-ND/pet127Δ. No discoveries were identified in WT to WT/pet127-ND. Region delineations can be found in Table 4.
(TIF)
Quantitative mtDNA inheritance assay with high-copy pet127-nd. Significance was determined using one-way ANOVA separately on each HS and rho0 cross with means compared to the vector control using Dunnett’s multiple comparison’s test (N = 3). **** indicates adjusted P-value less than 0.0001. All other comparisons were not significantly different.
(TIF)
Diagram of tested PET127 truncation constructs in the bacterial expression and binding assay. First row is full length PET127 with predicted mitochondrial targeting sequence. Second row is with the mitochondrial targeting sequence replaced with 6xHis. All the following rows are truncation alleles to scale. Columns indicate whether the allele was able to express in bacteria or subsequently bind with Rpo41 as in Fig 4B.
(TIF)
Cycling mutant PET127 and TOM70-GFP carrying cells in YEPD were lysed by zymolyase treatment and Dounce homogenization. Mitochondria was enriched by differential centrifugation and 3μg of mitochondria was treated with 50μg/ml proteinase K for 5mins at 37°C. The reaction was stopped by addition of TCA to 12.5%. Samples were immunoblotted as indicated. A low exposure was used for Pet1271-215-HA because it was more abundant than Pet127-HA and Pet127Δ48-215-HA. Arrows indicate the expected size of Pet127 full length and Pet127Δ48-215-HA respectively.
(TIF)
A. RT-qPCR of either COX3, COX2 or ATP9 divided by ACT1 RNA and normalized by the qPCR of the respective gene divided by ACT1 DNA. RT-qPCRs were performed on the high-copy PET127 high-copy RPO41 combination strains collected at high cell density to increase respiration. The left RT-qPCRs are normalized to the Vectors averages. The right column is the same data visualized as a log2 fold change relative to the Vectors control. Significance was determined using one-way ANOVA comparing all values with each other using Tukey’s multiple comparison’s test (N = 2). **** indicates adjusted P-value less than 0.0001, *** indicates an adjusted P-value less than 0.001, ** indicates an adjusted P-value less than 0.01, * indicates an adjusted P-value less than 0.05. B. qPCR of either COX3, COX2, or ATP9 divided by ACT1 qPCR on DNA isolated from high-copy PET127 high-copy RPO41 combination strains. No comparisons were determined as significantly different. Significance was determined using one-way ANOVA comparing all values with each other using Tukey’s multiple comparison’s test (N = 2).
(TIF)
A. COX3 RNA RT-qPCR, COX3 DNA qPCR, or COX3 RNA RT-qPCR divided by COX3 DNA qPCR on high-copy pGal-Pet127 alleles in rho+ cells normalized to ACT1 RNA and/or DNA 0, 3, and 5 hours after addition of galactose. Significance was determined using one-way ANOVA separately for each time point with means compared to either the vector control or the pGal-mtGFP control using Dunnett’s multiple comparison’s test. *** indicates an adjusted P-value between 0.001 and 0.0001. * indicates an adjusted P-value between 0.05 and 0.01. All other comparisons were not significantly different. B. Gene expression analysis via RNA sequencing of PET127 or mitochondrial genic transcripts in cells carrying high-copy pGal-Pet127 alleles. Values indicate average log2 fold change between 0 hours and 5 hours after addition of galactose for three independent experiments. C. ORI RNA RT-qPCR, ORI DNA qPCR, or ORI RNA RT-qPCR divided by ORI DNA qPCR on high-copy pGal-Pet127 alleles in rho+ cells normalized to ACT1 RNA and/or DNA 0, 3, and 5 hours after addition of galactose. Significance was determined using one-way ANOVA separately for each time point with means compared to either the vector control or the pGal-mtGFP control using Dunnett’s multiple comparison’s test (N = 3). **** indicates an adjusted P-value less than 0.0001. ** indicates an adjusted P-value between 0.01 and 0.001. All other comparisons were not significantly different. D. ORI RNA RT-qPCR on HS ORI5-1 and HS ORI5-2 with pGal-Pet127 RNA normalized to ACT1 0, 3, and 5 hours after addition of galactose. Significance was determined using student’s T-test comparing equivalent time points between strains containing either the same ORI or pGal-PET127 allele (N = 2 or 3). * indicates a P-value between 0.05 and 0.01. All other comparisons were not significant.
(TIF)
A. Left panel: PET127-HA V5-RPO41 cells were grown to high cellular density in YEPD medium and samples were collected and immunoblotted for HA, V5 and VDAC. Right panel: A high density culture of PET127-HA V5-RPO41 cells in YEPD medium was diluted at 0h to 3.3 x 106 cells /ml in YEPD medium and samples were collected over time and immunoblotted for HA, V5 and VDAC. B. PET127-HA V5-RPO41 cells were grown in YEPD medium to mid log, a sample was taken and then the culture was split into YEP medium containing either 2% Glucose, 2% Glycerol, 2% Potassium Acetate, or 2% Ethanol. Samples were taken after 4 hours and samples were stained with Ponceau S and immunoblotted for HA, V5 and VDAC.
(TIF)
Five-fold serial dilutions of strains containing RPO41 alleles beginning at 1.1 x 107 cells/ml plated on SD-His and incubated at either 30°C, 34°C, or 37°C for 2 days.
(TIF)
Acknowledgments
We thank Dmitriy Markov for insight about RPO41 allele design, the MIT Biomicro Center and Stuart Levine for the Illumina sequencing experiments and discussions about experimental design of the SMRT and mitochondrial RNA sequencing, Charlie Whittaker and Duanduan Ma of the Barbara K. Ostrom (1978) Bioinformatics and Computing Facility at the Koch Institute in the Swanson Biotechnology Center for sequencing analysis of the SMRT sequencing and RNA sequencing experiments respectively, Maria Zapp and Ellen Kittler of the UMMS Deep Sequencing and Molecular Biology Core Laboratories and the PacBio Core Enterprise for SMRT sequencing, Xiaoxue Zhou for taking the image of S11 Fig, Stephen P. Bell and Matthew Vander Heiden for mentorship and critical reading of the manuscript, and Hilla Weidberg, Xiaoxue Zhou, and John Replogle for critical reading of the manuscript.
Data Availability
Most relevant data is contained within the manuscript; for a few datasets of raw RNA or DNA sequencing please see the following links: SMRT sequencing of Hypersuppressive mtDNAs: https://doi.org/10.5061/dryad.547d7wm8v RNA sequencing characterizing nuclease dead Pet127 mutants: https://doi.org/10.5061/dryad.cfxpnvx5z RNA sequencing of overexpressed pet127 mutants: https://doi.org/10.5061/dryad.mw6m905xg.
Funding Statement
AA received an award from the National Institute of General Medical Sciences (GM118066) which helped fund this project. https://www.nigms.nih.gov/ AA was an investigator of the Howard Hughes Medical Institute which helped fund this project. https://www.hhmi.org/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Veatch JR, McMurray M a, Nelson, Gottschling DE. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell [Internet]. 2009. Jun 26 [cited 2014 Aug 7];137(7):1247–58. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2759275&tool=pmcentrez&rendertype=abstract doi: 10.1016/j.cell.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schatz G. Mitochondria: beyond oxidative phosphorylation. Biochim Biophys Acta. 1995;1271:123–6. doi: 10.1016/0925-4439(95)00018-y [DOI] [PubMed] [Google Scholar]
- 3.Ernster L, Schatz G. Mitochondria: A historical review. J Cell Biol. 1981;91(3 II). doi: 10.1083/jcb.91.3.227s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing Mitochondrial Proteins: Machineries and Mechanisms. Cell. 2009;138(4):628–44. doi: 10.1016/j.cell.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–49. doi: 10.1146/annurev.biochem.76.052705.163409 [DOI] [PubMed] [Google Scholar]
- 6.Foury F, Roganti T, Lecrenier N, Purnelle B. The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae. FEBS Lett. 1998;440(3):325–31. doi: 10.1016/s0014-5793(98)01467-7 [DOI] [PubMed] [Google Scholar]
- 7.Turk EM, Das V, Seibert RD, Andrulis ED. The Mitochondrial RNA Landscape of Saccharomyces cerevisiae. PLoS One. 2013;8(10):1–21. doi: 10.1371/journal.pone.0078105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad MN, Newlon CS. The regulation of mitochondrial DNA levels in Saccharomyces cerevisiae. Curr Genet. 1982;6(2):147–52. doi: 10.1007/BF00435214 [DOI] [PubMed] [Google Scholar]
- 9.Maleszka R, Skelly PJ, Clark-Walker GD. Rolling circle replication of DNA in yeast mitochondria. EMBO J [Internet]. 1991;10(12):3923–9. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=453131&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dujon B. Mitochondrial genetics and function. In: Strathern JN, Jones EW, Broach JR, editors. The molecular biology of the yeast Saccharomyces: life cycle and inheritance. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 1981. p. 505–635. [Google Scholar]
- 11.Slonimski PP, Perrodin G, Croft JH. Ethidium bromide induced mutation of yeast mitochondria: Complete transformation of cells into respiratory deficient non-chromosomal “petites.” Biochem Biophys Res Commun. 1968;30(3):232–9. doi: 10.1016/0006-291x(68)90440-3 [DOI] [PubMed] [Google Scholar]
- 12.Slonimski P, Ephrussi B. Action de l’acriflavin sur les levures. V. Le système des cytochromes des mutants ‘petite colonie’ de la levure. Ann Inst Pasteur (Paris). 1949;77:47–64. [Google Scholar]
- 13.Falkenberg M. Mitochondrial DNA replication in mammalian cells: Overview of the pathway. Essays Biochem. 2018;62(3):287–96. doi: 10.1042/EBC20170100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osman C, Noriega TR, Okreglak V, Fung JC, Walter P. Integrity of the yeast mitochondrial genome, but not its distribution and inheritance, relies on mitochondrial fission and fusion. Proc Natl Acad Sci [Internet]. 2015;201501737. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.1501737112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunnari J, Marshall W, Straight A, Murray A, Sedat J, Walter P. Mitochondrial transmission during mating in S. cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mtDNA. Mol Biol Cell. 1997;8(7):1233–42. doi: 10.1091/mbc.8.7.1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murley A, Lackner LL, Osman C, West M, Voeltz GK, Walter P, et al. ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. Elife [Internet]. 2013. Jan [cited 2014 Sep 15];2:e00422. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3654481&tool=pmcentrez&rendertype=abstract doi: 10.7554/eLife.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labbé K, Murley A, Nunnari J. Determinants and Functions of Mitochondrial Behavior. Annu Rev Cell Dev Biol [Internet]. 2014;30:357–91. Available from: http://www.annualreviews.org/doi/abs/10.1146/annurev-cellbio-101011-155756 [DOI] [PubMed] [Google Scholar]
- 18.Ephrussi B, de Margerie-Hottinguer H, Roman H. Suppressiveness: a New Factor in the Genetic Determinism of the Synthesis of Respiratory Enzymes in Yeast. Proc Natl Acad Sci U S A. 1955;41(12):1065–71. doi: 10.1073/pnas.41.12.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goursot R, de Zamaroczy M, Baldacci G, Bernardi G. Supersuppressive “petite” mutants of yeast. Curr Genet. 1980;1(2):173–6. doi: 10.1007/BF00446963 [DOI] [PubMed] [Google Scholar]
- 20.Ephrussi B, Grandchamp S. ETUDES SUR LA SUPPRESSIVITE DES MUTANTS A DEFICIENCE RESPIRATOIRE DE LA LEVURE. Heredity (Edinb). 1965;20(February):1–7. doi: 10.1038/hdy.1965.1 [DOI] [PubMed] [Google Scholar]
- 21.Mikklos De Zamaroczy Giuseppe Baldacci and GB. Putative Origins of Replication in the Mitochondrial Genome of Yeast. FEBS Lett. 1979;102(2):429–32. [DOI] [PubMed] [Google Scholar]
- 22.Blanc H, Dujon B. Replicator regions of the yeast mitochondrial DNA responsible for suppressiveness. Proc Natl Acad Sci U S A. 1980;77(July 1980):3942–6. doi: 10.1073/pnas.77.7.3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanc H, Dujon B. Replicator regions of the yeast mitochondrial DNA active in vivo and in yeast transformants. In: Slonimski P, Borst P, Attardi G, editors. Mitochondrial genes. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. p. 279–94. [Google Scholar]
- 24.Gingold EB. Genetic analysis of the products of a cross involving a suppressive “petite” mutant of S. cerevisiae. Curr Genet. 1981;3(3):213–20. doi: 10.1007/BF00429823 [DOI] [PubMed] [Google Scholar]
- 25.Schmitt ME, Clayton DA. Conserved features of yeast and mammalian mitochondrial DNA replication. Curr Opin Genet Dev. 1993;3(5):769–74. doi: 10.1016/s0959-437x(05)80097-8 [DOI] [PubMed] [Google Scholar]
- 26.Shadel GS, Clayton DA. Mitochondrial DNA Maintenance in Vertebrates. Annu Rev Biochem. 1997;66:409–35. doi: 10.1146/annurev.biochem.66.1.409 [DOI] [PubMed] [Google Scholar]
- 27.Baldacci G, Bernardi G. Replication origins are associated with transcription initiation sequences in the mitochondrial genome of yeast. EMBO J. 1982;1(8):987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyck E Van Clayton DA. Transcription-Dependent DNA Transactions in the Mitochondrial Genome of a Yeast Hypersuppressive Petite Mutant. Mol Cell Biol. 1998;18(5):2976–85. doi: 10.1128/MCB.18.5.2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stohl LL, Clayton D a. Saccharomyces cerevisiae contains an RNase MRP that cleaves at a conserved mitochondrial RNA sequence implicated in replication priming. Mol Cell Biol. 1992;12(6):2561–9. doi: 10.1128/mcb.12.6.2561-2569.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Sandoval E, Diaz-Quezada C, Velazquez G, Arroyo-Navarro LF, Almanza-Martinez N, Trasviña-Arenas CH, et al. Yeast mitochondrial RNA polymerase primes mitochondrial DNA polymerase at origins of replication and promoter sequences. Mitochondrion [Internet]. 2015;24:22–31. Available from: http://www.sciencedirect.com/science/article/pii/S1567724915300052 doi: 10.1016/j.mito.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 31.Fangman WL, Henly JW, Churchill G, Brewer BJ. Stable maintenance of a 35-base-pair yeast mitochondrial genome. Mol Cell Biol. 1989;9(5):1917–21. doi: 10.1128/mcb.9.5.1917-1921.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou JY, Leu JY. The Red Queen in mitochondria: Cyto-nuclear co-evolution, hybrid breakdown and human disease. Front Genet. 2015;6(MAY):1–8. doi: 10.3389/fgene.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace DC, Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb Perspect Med. 2013;3(10):1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godeleine Faugeron-Fonty, Caroline Le, Van Kim, et al. A comparative study of the ori sequences from the mitochondrial genomes of twenty wild-type yeast strains. Gene. 1984;32:459–73. doi: 10.1016/0378-1119(84)90020-9 [DOI] [PubMed] [Google Scholar]
- 35.de Zamaroczy M, Faugeron-Fonty G, Baldacci G, Goursot R, Bernardi G. The ori sequences of the mitochondrial genome of a wild-type yeast strain: number, location, orientation and structure. Gene. 1984;32(3):439–57. doi: 10.1016/0378-1119(84)90019-2 [DOI] [PubMed] [Google Scholar]
- 36.Fangman WL, Henly JW, Brewer BJ. RPO41-independent maintenance of [rho-] mitochondrial DNA in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10(1):10–5. doi: 10.1128/mcb.10.1.10-15.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fangman WL, Dujon B. Yeast mitochondrial genomes consisting of only A.T base pairs replicate and exhibit suppressiveness. Proc Natl Acad Sci U S A [Internet]. 1984;81(22):7156–60. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=392096&tool=pmcentrez&rendertype=abstract doi: 10.1073/pnas.81.22.7156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorimer HE, Brewer BJ, Fangman WL. A test of the transcription model for biased inheritance of yeast mitochondrial DNA. Mol Cell Biol. 1995;15(9):4803–9. doi: 10.1128/MCB.15.9.4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling F, Hori A, Shibata T. DNA recombination-initiation plays a role in the extremely biased inheritance of yeast [rho-] mitochondrial DNA that contains the replication origin ori5. Mol Cell Biol. 2007;27(November):1133–45. doi: 10.1128/MCB.00770-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling F, Hori A, Yoshitani A, Niu R, Yoshida M, Shibata T. Din7 and Mhr1 expression levels regulate double-strand-break-induced replication and recombination of mtDNA at ori5 in yeast. Nucleic Acids Res. 2013;41(April):5799–816. doi: 10.1093/nar/gkt273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambers P, Gingold E. A direct study of the relative synthesis of petite and grande petite mutants of Saccharomyces cerevisiae. Curr Genet. 1986;10:565–71. doi: 10.1007/BF00418122 [DOI] [PubMed] [Google Scholar]
- 42.Bradshaw E, Yoshida M, Ling F. Regulation of Small Mitochondrial DNA Replicative Advantage by Ribonucleotide Reductase in Saccharomyces cerevisiae. Genes|Genomes|Genetics [Internet]. 2017;7(September 2017):3083–90. Available from: http://g3journal.org/lookup/doi/10.1534/g3.117.043851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai-Zhang J, Xiao Y, Mueller DM. Epistatic interactions of deletion mutants in the genes encoding the F1-ATPase in yeast Saccharomyces cerevisiae. EMBO J. 1999;18(1):58–64. doi: 10.1093/emboj/18.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacAlpine DM, Kolesar J, Okamoto K, Butow R a, Perlman PS. Replication and preferential inheritance of hypersuppressive petite mitochondrial DNA. EMBO J [Internet]. 2001. Apr 2;20(7):1807–17. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=145480&tool=pmcentrez&rendertype=abstract doi: 10.1093/emboj/20.7.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeMarini DJ, Adams AEM, Fares H, De Virgilio C, Valle G, Chuang JS, et al. A Septin-based Hierarchy of Proteins Required for Localized Deposition of Chitin in the Saccharomyces cerevisiae Cell Wall. J Cell Biol. 1997;139(1):75–93. doi: 10.1083/jcb.139.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiesenberger G, Fox TD. Pet127p, a membrane-associated protein involved in stability and processing of Saccharomyces cerevisiae mitochondrial RNAs. Mol Cell Biol. 1997;17(5):2816–24. doi: 10.1128/MCB.17.5.2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karavaeva IE, Golyshev SA, Smirnova EA, Sokolov SS, Severin FF, Knorre DA. Mitochondrial depolarization in yeast zygotes inhibits clonal expansion of selfish mtDNA. J Cell Sci. 2017;130(7):1274–84. doi: 10.1242/jcs.197269 [DOI] [PubMed] [Google Scholar]
- 48.Lancashire WE, Mattoon JR. Cytoduction : A Tool for Mitochondrial Genetic Studies in Yeast. 1979;344:333–44. doi: 10.1007/BF00267067 [DOI] [PubMed] [Google Scholar]
- 49.Haffter P, Fox TD. Suppression of carboxy-terminal truncations of the yeast mitochondrial mRNA-specific translational activator PET122 by mutations in two new genes, MRP17 and PET127. MGG Mol Gen Genet. 1992;235(1):64–73. doi: 10.1007/BF00286182 [DOI] [PubMed] [Google Scholar]
- 50.Margelevičius M, Laganeckas M, Venclovas Č. COMA server for protein distant homology search. Bioinformatics. 2010;26(15):1905–6. doi: 10.1093/bioinformatics/btq306 [DOI] [PubMed] [Google Scholar]
- 51.Fekete Z, Ellis TP, Schonauer MS, Dieckmann CL. Pet127 governs a 5’->3’-exonuclease important in maturation of apocytochrome b mRNA in Saccharomyces cerevisiae. J Biol Chem. 2008;283(7):3767–72. doi: 10.1074/jbc.M709617200 [DOI] [PubMed] [Google Scholar]
- 52.Islas-Osuna M a., Ellis TP, Marnell LL, Mittelmeier TM, Dieckmann CL. Cbp1 is required for translation of the mitochondrial cytochrome b mRNA of Saccharomyces cerevisiae. J Biol Chem. 2002;277(41):37987–90. doi: 10.1074/jbc.M206132200 [DOI] [PubMed] [Google Scholar]
- 53.Markov DA, Savkina M, Anikin M, Del Campo M, Ecker K, Lambowitz AM, et al. Identification of proteins associated with the yeast mitochondrial RNA polymerase by tandem affinity purification. Yeast. 2009;26(10):423–40. doi: 10.1002/yea.1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241(3):779–86. doi: 10.1111/j.1432-1033.1996.00779.x [DOI] [PubMed] [Google Scholar]
- 55.Mohd Azhar SH, Abdulla R, Jambo SA, Marbawi H, Gansau JA, Mohd Faik AA, et al. Yeasts in sustainable bioethanol production: A review. Biochem Biophys Reports. 2017;10(February):52–61. doi: 10.1016/j.bbrep.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dombek KM, Ingram LO. Ethanol production during batch fermentation with Saccharomyces cerevisiae: Changes in glycolytic enzymes and internal pH. Appl Environ Microbiol. 1987;53(6):1286–91. doi: 10.1128/aem.53.6.1286-1291.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galdieri L, Mehrotra S, Yu S, Vancura A. Transcriptional regulation in yeast during diauxic shift and stationary phase. Omi A J Integr Biol. 2010;14(6):629–38. doi: 10.1089/omi.2010.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenleaf AL, Kelly JL, Lehman IR. Yeast RPO41 gene product is required for transcription and maintenance of the mitochondrial genome. Proc Natl Acad Sci U S A [Internet]. 1986;83(10):3391–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3517858%5Cnhttp://www.ncbi.nlm.nih.gov/pmc/articles/PMC323519/pdf/pnas00314-0350.pdf doi: 10.1073/pnas.83.10.3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dujon B, Slonimski PP, Weill L. Mitochondrial genetics. IX. A model for recombination and segregation of mitochondrial genomes in Saccharomyces cerevisiae. Genetics. 1974;78(1):415–37. doi: 10.1093/genetics/78.1.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blitzblau HG, Chan CS, Hochwagen A, Bell SP. Separation of DNA replication from the assembly of break-competent meiotic chromosomes. PLoS Genet. 2012;8(5). doi: 10.1371/journal.pgen.1002643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamperl S, Bocek MJ, Saldivar JC, Swigut T, Cimprich KA. Transcription-Replication Conflict Orientation Modulates R-Loop Levels and Activates Distinct DNA Damage Responses. Cell [Internet]. 2017;170(4):774–786.e19. Available from: 10.1016/j.cell.2017.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wanrooij PH, Engqvist MKM, Forslund JME, Navarrete C, Nilsson AK, Sedman J, et al. Ribonucleotides incorporated by the yeast mitochondrial DNA polymerase are not repaired. Proc Natl Acad Sci U S A. 2017;114(47):12466–71. doi: 10.1073/pnas.1713085114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000;7(1–2):203–14. doi: 10.1089/10665270050081478 [DOI] [PubMed] [Google Scholar]
- 64.Longtine MS, McKenzie a, Demarini DJ, Shah NG, Wach a, Brachat a, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast [Internet]. 1998. Jul;14(10):953–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9717241 doi: [DOI] [PubMed] [Google Scholar]
- 65.Gibson DG. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 2011;498:349–61. doi: 10.1016/B978-0-12-385120-8.00015-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rose MD, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Schweitzer B, Philippsen P, editors. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 67.Corbi D, Amon A. SMRT sequencing data on four hypersuppressive Saccharomyces cereviciae mitochondrial DNAs. [Internet]. Dryad Digital Repository. Durham (NC): Dryad; 2021. [cited 2021 Oct 11]. Available from: https://datadryad.org/stash/ [Google Scholar]
- 68.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–6. doi: 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corbi D, Amon A. RNA sequencing of nuclease dead PET127 conditions [Internet]. Dryad Digital Repository. Durham (NC): Dryad; 2021. [cited 2021 Oct 11]. Available from: https://datadryad.org/stash/ [Google Scholar]
- 70.Corbi D, Amon A. RNA sequencing of PET127 mutants overexpressed using the galactose inducible promoter [Internet]. Dryad Digital Repository. Durham (NC): Dryad; 2021. [cited 2021 Oct 11]. Available from: https://datadryad.org/stash/ [Google Scholar]
- 71.Pfanner N., Meisinger C., Turcotte B., in Yeast Protocols, Xiao W., Ed. (Springer, 2006), vol. 313 pp. 33–39. Yeast Protocols 2nd Ed. Vol. 1, Methods in molecular Biology. 2006. 408 p. [Google Scholar]