Abstract
Background
Migrants are not routinely screened for hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) in the Netherlands. We estimated the prevalence and determined factors associated with HBV, HCV and/or HIV infections among undocumented migrants and uninsured legal residents.
Methods
In this cross-sectional study, undocumented migrants and uninsured legal residents were recruited at a non governmental organization (NGO), healthcare facility in the Netherlands and were invited to be tested for hepatitis B surface antigen (HBsAg), anti-hepatitis B core antibodies (anti-HBcAb), HCV-RNA, and anti-HIV antibodies or HIV antigen at a local laboratory.
Results
Of the 1376 patients invited, 784 (57%) participated. Participants originated from Africa (35%), Asia (30%) and North/South America (30%). 451/784 (58%) participants went to the laboratory for testing. Of participants 30% were HBV exposed (anti-HBcAb-positive), with 27% (n = 119/438, 95% CI 23.1% to 31.6%) having resolved HBV infection (HBsAg-negative) and 2.5% (n = 11/438, 95%CI 1.3% to 4.5%, 64% new infection) having chronic HBV infection (HBsAg-positive). Compared to HBV non-exposed, HBV exposed individuals were older (p = 0.034) and more often originated from Africa (p<0.001). Prevalence of chronic HCV infection (HCV-RNA-positive) was 0.7% (n = 3/435, 95%CI 0.1% to 2.0%, all new infections) and HIV infection 1.1% (n = 5/439, 95%CI 0.04% to 2.6%, 40% new infection).
Conclusion
Prevalence of chronic HBV, chronic HCV and HIV infections in our study population is higher compared to the Dutch population, thus emphasizing the importance of case finding for these infections through primary care and public health in this specific group of migrants. Screening uptake could be improved by on-site testing.
Introduction
In the Netherlands, the estimated prevalence of chronic hepatitis B virus (HBV) infection is 0.34% and of chronic hepatitis C virus (HCV) infection 0.16% in 2016. Prevalence of human immunodeficiency virus (HIV) infection was estimated at 0.14% in 2017. Migrants account for 81% of prevalent chronic HBV infections, 60% of prevalent chronic HCV infection [1–3] and 50% of prevalent HIV infections [mostly among men who have sex with men (MSM)] [2,4]. Nevertheless, the prevalence of these infections in migrants depend on a variety of factors, such as the endemicity in their country of origin and susceptibility of acquiring infection while travelling to and staying in Europe [5–7].
Asylum seekers and migrants are not routinely screened for HBV, HCV and HIV in the Netherlands [8]. Guidelines for general practitioners (GP) recommend case finding for infectious diseases during GP consultations, depending on the endemicity of the migrant’s country of origin [9]. However, provider- and patient-related barriers, such as competing priorities and fear of requesting testing, make these recommendations difficult to routinely implement [10]. Furthermore, HBV, HCV and HIV follow a prolonged, asymptomatic phase during the natural course of their infections, which might not lead to suspected infection during routine consultations and thereby could delay diagnosis [11]. Earlier diagnosis could help migrants obtain treatment to control HIV or HBV replication [12] or cure HCV with highly effective, direct-acting antivirals [13]. In recent years, several screening programs have targeted migrant and other key groups to find undiagnosed cases of HBV, HCV and HIV infections [14]. Both integrated (i.e. case finding in the context of other access points to healthcare) and non-integrated screening programs have been shown to be successful, depending on their intended goal [14]. For instance, a study from Bil et al. examined whether integrated screening for HBV, HCV and HIV infections at the tuberculosis (TB) clinics of the Public Health Service of Amsterdam and Gelderland was feasible and effective and although its implementation was successful, no newly diagnosed HIV or chronic HCV infections were found in Amsterdam, whereas all infections were newly diagnosed in Gelderland [15].
Undocumented migrants (including rejected asylum seekers, migrants with expired visas and ‘directly undocumented migrants’, i.e. those who bypassed asylum procedures [16,17]) and uninsured legal residents are thought to represent a considerable fraction of migrants residing in the Netherlands, yet the exact proportion is unknown. In addition, little is known about the infectious disease status of undocumented migrants and uninsured legal residents in the Netherlands A study conducted in Italy found a higher prevalence of HBV, HCV and HIV infections among undocumented migrants compared to the local population [18]. Another study in Denmark observed a higher prevalence of these infections in undocumented compared to documented migrants [19]. These differences in prevalence underline the importance of studying undocumented migrant populations.
This study aimed to assess the prevalence of HBV (chronic or resolved), HCV (chronic or resolved) and HIV infections among undocumented migrants and uninsured legal residents in Amsterdam, the Netherlands. We also intended to study the determinants of infection in this population. Additionally, we determined screening uptake, outcome of referral to specialized care (for those with infection) and HBV vaccination uptake (for non-exposed individuals).
Methods
Study population, design and eligibility criteria
This cross-sectional study was performed at Kruispost, a low-threshold NGO primary care facility for undocumented migrants, homeless and/or uninsured individuals. Data were used from a convenience sample of 1000 patients visiting Kruispost for a general practitioner (GP) consultation between October 2018 and October 2019. Visitors aged 18 years or older who were able to understand study information (provided in Dutch, English, French, Spanish, Arabic and Portuguese) were eligible for participation. Prior to June 20, 2019, we included patients who were born in a country outside of the European Union (EU) or European Economic Area (EEA), or those who were in possession of a citizen service number (CSN) when born in a country within the EU/EEA. After June 20, 2019, patients from a country within the EU/EEA without a CSN were also asked to participate due to a policy change made by the Dutch government that allowed care and treatment reimbursement for this group.
Ethical statement and screening procedure
This study was carried out according to the ethical guidelines of the 1975 Declaration of Helsinki. The medical ethics committee of the Amsterdam University Medical Center (location AMC) decided that the study did not pertain to the Medical Research Involving Human Subjects Act (WMO) and therefore did not require institutional review board approval (W18_164#18.202). After general practitioner (GP) consultation, eligible patients were asked to participate in the study and were provided with study information. Many undocumented migrants are uncomfortable with providing their written name or signature on paper and therefore we requested that all participants provide verbal informed consent to participate. When verbal informed consent was given, it was recorded in our electronic data capture system. All participants were able to withdraw consent at any time during the study without clarification. For those who did not participate, we asked them to fill out a short questionnaire on basic characteristics and reason for non-participation. Data were pseudonymized for further analysis. Participants verbally completed questionnaire 1 (S1 File) together with assistance of the researcher. In questionnaire 1, lifetime risk factors for HBV or HCV infection (admission or treatment in a foreign hospital, surgery abroad, blood transfusion, paying or being paid for sex, sexual contact with men and/or women and injecting drug use) were asked. Afterwards, participants independently filled out questionnaire 2 (S2 File) in which questions were asked about sociodemographic variables (age, sex, country of birth, educational level) and migration history (year of leaving country of origin, year of arrival in the Netherlands, way of entering the Netherlands, housing situation, the number of housemates currently living with).
We conducted the study in accordance with the current screening recommendations from the Dutch government. HIV screening is universal; however, screening HBV and HCV is conducted only if individuals present with risk-factors for viral hepatitis infection (i.e. case finding) [9]. Accordingly, all participants were offered testing for HIV. The researcher determined whether risk factors for HBV and HCV infection were present based on the respondent’s answers to questionnaire 1. If so, the participant was offered testing for both HBV and HCV infections. Participants received one (for HIV testing) or two laboratory forms (for HIV and HBV/HCV testing). With these forms, participants could get tested free of charge at one of 39 blood sampling locations in Amsterdam (Atalmedial). Participants could refuse testing for any of the three infections. All participants were offered an incentive for their participation (a ticket for public transport, socks, disinfectant hand soap and shampoo).
Laboratory analysis
Blood serum samples were first tested for hepatitis B surface antigen (HBsAg), anti-Hepatitis B core (Anti-HBc) antibodies, anti-HCV antibodies, and HIV antigen or antibodies (LIAISON XL MUREX, DiaSorin, Italy) at the Public Health Laboratory of GGD Amsterdam. Samples testing positive for HBsAg or anti-HBc antibodies were further tested for hepatitis B ‘e’ antigen (HBeAg), anti-Hepatitis B ‘e’ antibodies (anti-HBeAb) and anti-HBs antibodies (LIAISON XL, DiaSorin, Italy). Participants with samples testing positive for anti-HCV antibodies were asked for a second blood draw, from which anti-HCV antibody positive status was confirmed and HCV RNA was tested at the clinical virological laboratory of the Amsterdam UMC (HCV Quantitative test, version 2.0, detection limit 10–50 copies/ml, Cobas AmpliPrep/Cobas TaqMan, Roche, Switzerland). Samples testing positive for HIV antigen or anti-HIV antibodies were confirmed by Western blot (INNO-LIA HIV I/II Score, Innogenetics, Belgium) and HIV-1 p24 antigen test (Vidas HIV P24, bioMérieux).
Participants with anti-HBc antibody-positive serology were defined as having been exposed to HBV (i.e. HBV exposed). Based on HBsAg results, these participants were further classified as having resolved infection (HBsAg-negative) or chronic infection (HBsAg-positive). Participants with anti-HCV antibody positive serology were defined as having been exposed to HCV (i.e. HCV exposed). Based on HCV RNA results, these participants were further classified as having resolved infection (undetectable HCV RNA) or chronic infection (detectable HCV RNA). Participants with confirmed HIV antigen- or antibody-positive tests were considered HIV-positive.
Follow-up care and treatment
Participants who did not have chronic HBV infection, chronic HCV infection or HIV infection were notified of their results during a telephone call. Participants who did have HBV, HCV and/or HIV infection were verbally informed of their test results by the GP. If necessary, participants were referred to the nearest hospital in Amsterdam for care and treatment, per Dutch GP Guidelines. To determine liver fibrosis levels, transient elastography was measured by a trained technician using FibroScan® and values were converted into METAVIR equivalents. Individuals who were HBsAg-negative and anti-HBc antibody-negative (i.e. non-exposed) were proposed to receive HBV vaccination at the Public Health Service of Amsterdam provided that they were eligible [20] (i.e. MSM and/or participants ever having been paid for sex). These participants received information about free HBV vaccination by telephone from a researcher at the Public Health Service of Amsterdam. In addition, they could discuss HBV vaccination during their consultation with the GP at Kruispost. Information about follow-up and treatment was extracted from the electronic patient files of the GP.
Statistical analyses
Sociodemographic and risk factors for HBV/HCV/HIV were summarized by testing uptake. Comparisons between groups were made using Pearson χ2 or Fisher Exact test for categorical data and by Mann-Whitney U test for continuous data. Prevalence of HBV, HCV and HIV and its corresponding Clopper-Pearson 95% confidence interval (CI) were calculated. We compared characteristics across groups of HBV (no infection, resolved infection, chronic infection), HCV (no infection, resolved infection, chronic infection) and HIV (HIV-negative, HIV-positive) infection. Due to the limited number of infections, we could only perform risk-factor analysis for patients who were HBV exposed versus HBV non-exposed. Odds ratios (OR) comparing odds between HBV exposed and non-exposed across levels of determinants, along with their 95%CI, were calculated using univariable logistic regression. All variables with an associated p-value <0.2 in univariable analyses were included in a full multivariable model. A backwards-stepwise selection procedure was performed, whereby variables with the highest p-value were consecutively removed until only those with a p<0.05 remained. Significance level was set at p<0.05. All analyses were conducted with Stata 15.1 (StataCorp., College Station, Texas, USA).
Results
Characteristics of participants
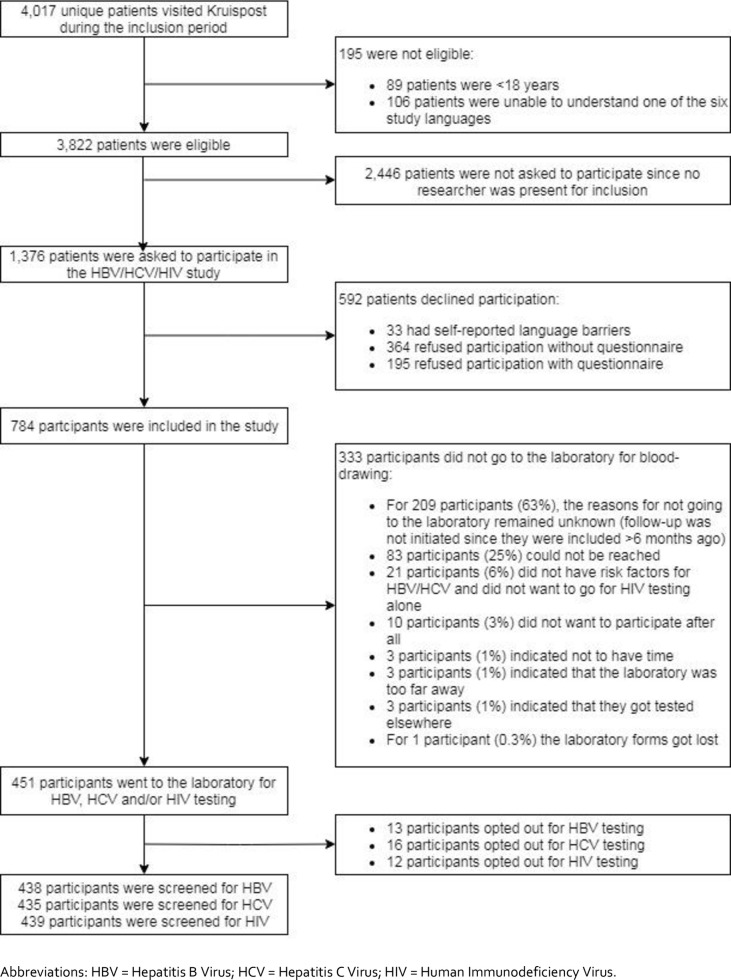
In total, 4,017 patients visited Kruispost during the inclusion period. Of them, 89 (2%) were aged <18 years and 106 (3%) were unable to understand one of the six study languages, and were hence excluded. In total, 3,822 (95%) eligible patients remained. Of them, 1,376 (36%) were invited to participate and 784 (57%; 784/1376) agreed to participate in the study. The reasons for declining participation (n = 592) are described in Fig 1.
Fig 1. Flowchart depicting the recruitment strategy of HBV, HCV and HIV screening offered to undocumented migrants and uninsured legal residents attending Kruispost in Amsterdam, the Netherlands (N = 3,822).
The median age of participants (N = 784) was 40 (interquartile range (IQR) 31–50) and more men than women participated (58% versus 42%, respectively). Sixty participants (8%) were uninsured legal residents and 724 participants (92%) were undocumented migrants. Participants mainly originated from Africa and Asia (35% and 30%, respectively) and the majority of participants completed secondary school (42%) or higher education (36%). Most participants reported to have arrived on a (now expired) tourist visa (45%), or as a rejected asylum seeker (18%). Fourteen percent of male participants reported having sex with men. Moreover, 14% of all participants had ≥3 sexual partners in the last 6 months (n = 104). Half of participants had never been tested for HIV (49%), HBV (55%) or HCV (61%).
Of 784 participants, 451 (58%) went to the laboratory to receive testing for at least one of the three viruses (HBV, HCV and/or HIV) (Fig 1), resulting in an overall percentage participating of 33% (n = 451/1376). Table 1 summarizes the characteristics of participants by testing uptake. Participants who refrained from blood sampling originated significantly more often from North/South America and less often from Africa, were in the Netherlands for a shorter duration, older and had a significantly higher education level than participants who were tested. In addition, participants with at least 3 sexual partners in the last 6 months underwent blood sampling significantly more often. No other variables were significantly associated with testing uptake.
Table 1. Characteristics of undocumented migrants and uninsured legal residents (N = 784) who participated in the Kruispost study to screen for HBV, HCV and HIV, in Amsterdam, the Netherlands, October 2018- October 2019.
| Total (N = 784) | Participants who had blood draw (N = 451) | Participants who refrained from blood draw (N = 333) | P-value* | ||||
|---|---|---|---|---|---|---|---|
| Sociodemographic variables | n | % | n | % | n | % | |
| Sex | .993 | ||||||
| Heterosexual men | 391 | 50 | 224 | 50 | 167 | 50 | |
| Men having sex with men | 65 | 8 | 38 | 8 | 27 | 8 | |
| Female | 326 | 42 | 187 | 42 | 139 | 42 | |
| Other | 1 | 0.1 | 1 | 0.2 | 0 | 0 | |
| Age in years, median (IQR) | 40 | (31–50) | 43 | (33–52) | 38 | (29–47) | < .001 |
| Age, categorized | .001 | ||||||
| <35 years | 262 | 33 | 133 | 30 | 129 | 39 | |
| 35–49 years | 309 | 39 | 173 | 38 | 136 | 41 | |
| 50–64 years | 187 | 24 | 125 | 28 | 62 | 19 | |
| ≥65 years | 26 | 3 | 20 | 4 | 6 | 2 | |
| Kruispost target population | .931 | ||||||
| Undocumented migrants | 724 | 92 | 418 | 93 | 306 | 92 | |
| Uninsured legal residents | 60 | 8 | 33 | 7 | 27 | 8 | |
| Region of birth ** | .001 | ||||||
| Europe | 40 | 5 | 21 | 5 | 19 | 6 | |
| Asia | 234 | 30 | 131 | 29 | 103 | 31 | |
| Africa | 276 | 35 | 184 | 41 | 92 | 28 | |
| North /South America | 233 | 30 | 115 | 26 | 118 | 36 | |
| Educational level | .027 | ||||||
| No school | 36 | 5 | 27 | 6 | 9 | 3 | |
| Primary school | 137 | 18 | 85 | 19 | 52 | 16 | |
| Secondary school | 325 | 42 | 191 | 43 | 134 | 41 | |
| Higher education | 280 | 36 | 146 | 33 | 134 | 41 | |
| Migration history | |||||||
| Year of leaving country of origin, median (IQR) | 2011 | (2003–2016) | 2010 | (2002–2016) | 2012 | (2005–2016) | .025 |
| Year of leaving country of origin | .186 | ||||||
| <2010 | 346 | 45 | 212 | 47 | 134 | 41 | |
| 2010–2017 | 335 | 43 | 182 | 40 | 153 | 47 | |
| ≥2018 | 93 | 12 | 54 | 12 | 39 | 12 | |
| Year of arrival in the Netherlands, median (IQR) | 2013 | (2006–2017) | 2013 | (2004–2017) | 2014 | (2007–2017) | .043 |
| Year of arrival in the Netherlands | .232 | ||||||
| <2010 | 287 | 37 | 177 | 40 | 110 | 34 | |
| 2010–2017 | 316 | 41 | 176 | 39 | 140 | 43 | |
| ≥2018 | 173 | 22 | 95 | 21 | 78 | 24 | |
| Way of entering the Netherlands | .827 | ||||||
| (Expired) tourist/working/student visa | 413 | 53 | 232 | 52 | 181 | 55 | |
| Rejected asylum seeker | 139 | 18 | 81 | 18 | 58 | 18 | |
| EU citizen | 46 | 6 | 27 | 6 | 19 | 6 | |
| Illegally crossing borders | 121 | 16 | 76 | 17 | 45 | 14 | |
| Legally/work/other visa$ | 37 | 5 | 20 | 5 | 17 | 5 | |
| Other/unknown | 19 | 3 | 12 | 3 | 7 | 2 | |
| Housing situation (multiple answers possible) | |||||||
| BBB (Bed Bath Bread) facility# | 58 | 7 | 32 | 7 | 26 | 8 | .706 |
| Friends/family | 411 | 52 | 238 | 53 | 173 | 52 | .820 |
| Illegal rent | 173 | 22 | 98 | 22 | 75 | 23 | .791 |
| Housing provided by charity | 67 | 9 | 45 | 10 | 22 | 7 | .095 |
| Lives on the streets | 63 | 8 | 31 | 7 | 32 | 10 | .164 |
| Lives in other housing## | 53 | 7 | 31 | 7 | 22 | 7 | .883 |
| Number of housemates | .588 | ||||||
| No housemates | 110 | 14 | 66 | 15 | 44 | 14 | |
| <3 | 345 | 45 | 190 | 43 | 78 | 48 | |
| 3–6 | 232 | 30 | 137 | 31 | 153 | 29 | |
| ≥6 | 81 | 11 | 50 | 11 | 13 | 10 | |
| HBV/HCV Risk factors | |||||||
| Ever admitted/treated in a foreign hospital | 276 | 36 | 157 | 35 | 119 | 36 | .781 |
| Ever had surgery abroad | 261 | 33 | 151 | 34 | 110 | 33 | .901 |
| Ever received a blood transfusion | .193 | ||||||
| No | 725 | 93 | 415 | 92 | 310 | 93 | |
| Yes | 45 | 6 | 25 | 6 | 20 | 6 | |
| Unknown | 12 | 2 | 10 | 2 | 2 | 0.6 | |
| More than 3 sexual partners in the previous 6 months | 104 | 14 | 69 | 16 | 35 | 11 | .048 |
| Ever (been) paid for sex | .072 | ||||||
| No, never | 631 | 81 | 351 | 78 | 280 | 84 | |
| Yes, ever paid | 127 | 16 | 82 | 18 | 45 | 14 | |
| Yes, ever been paid | 24 | 3 | 17 | 4 | 7 | 2 | |
| Ever injected drugs | 18 | 2 | 5 | 1 | 13 | 4 | .010 |
| Infectious disease (testing) history | |||||||
| Ever tested for HBV | .023 | ||||||
| No | 434 | 56 | 254 | 56 | 180 | 54 | |
| Unknown | 120 | 15 | 78 | 17 | 42 | 13 | |
| Yes, test was negative | 201 | 26 | 101 | 22 | 100 | 30 | |
| Yes, test was positive | 12 | 2 | 10 | 2 | 2 | 0.6 | |
| Yes, but results of tests are unknown | 15 | 2 | 7 | 2 | 8 | 2 | |
| Ever tested for HCV | .008 | ||||||
| No | 478 | 61 | 287 | 64 | 191 | 58 | |
| Unknown | 173 | 22 | 102 | 23 | 71 | 21 | |
| Yes, test was negative | 126 | 16 | 60 | 13 | 66 | 20 | |
| Yes, test was positive | 4 | 0.5 | 0 | 0 | 4 | 1 | |
| Yes, but results of tests are unknown | 1 | 0.1 | 1 | 0.2 | 0 | 0 | |
| Ever tested for HIV | .683 | ||||||
| No | 387 | 50 | 219 | 49 | 168 | 51 | |
| Unknown | 56 | 7 | 37 | 8 | 19 | 6 | |
| Yes, test was negative | 323 | 41 | 186 | 41 | 137 | 41 | |
| Yes, test was positive | 8 | 1 | 4 | 0.9 | 4 | 1 | |
| Yes, but results of tests are unknown | 8 | 1 | 4 | 0.9 | 4 | 1 | |
| Mother with liver disease | .120 | ||||||
| No | 631 | 81 | 351 | 78 | 280 | 84 | |
| Yes, HBV or HCV | 6 | 0.8 | 4 | 0.9 | 2 | 0.6 | |
| Yes, liver cancer | 6 | 0.8 | 3 | 0.7 | 3 | 0.9 | |
| Unknown | 139 | 18 | 92 | 20 | 47 | 14 | |
| Other family member with liver disease | .149 | ||||||
| No | 593 | 76 | 332 | 74 | 261 | 79 | |
| Yes, HBV or HCV | 36 | 5 | 27 | 6 | 9 | 3 | |
| Yes, liver cancer | 13 | 2 | 8 | 2 | 5 | 2 | |
| Unknown | 140 | 18 | 83 | 18 | 57 | 17 | |
* Differences in variables according to whether or not patients received blood draw were assessed using Fisher’s Exact test for categorical data. Mann-Whitney U test was used for continuous data.
** The most common countries of birth were Brazil (n = 142), Philippines (n = 90), Nigeria (n = 80), Morocco (n = 46), Surinam (n = 43), Egypt (n = 33), Ghana (n = 33), Indonesia (n = 26), Eritrea (n = 23), India (n = 17), Colombia (n = 15), Iran (n = 14), and Somalia (n = 11).
$ Include family visa and Schengen visa.
# The bed, bath, bread regulation arranges basic emergency shelter for rejected asylum seekers provided by the Dutch government.
## Includes legal rent, housing for asylum seekers, boats, employers, hotels, crisis care, winter care and outdoors.
Missing values: Region of birth n = 1; education n = 3; year of leaving country of origin n = 5; year of arrival in the Netherlands 4; method of entering the Netherlands n = 4; number of housemates 8; admitted to foreign hospital 6; surgery abroad 2; blood transfusion 2; biological male having sex with men 1; more than 3 sexual partners in previous 6 months 21; ever (been) paid for sex 2; injected drugs 2; ever tested HBV/HCV/HIV 2; liver disease mother/family 2.
Abbreviations: IQR–inter quartile range; EU–European Union; BBB–bed bath bread; HBV–Hepatitis B Virus; HCV–Hepatitis C Virus; HIV–Human Immunodeficiency Virus.
Prevalence of HBV, HCV and HIV infection and its determinants
A total of 130 of 438 (29,7%) participants were HBV exposed (anti-HBcAb-positive), of whom 11 participants had chronic HBV infection (HBsAg-positive), resulting in a prevalence of 2.5% (n = 11/438). Four (36.4%, n = 4/11) already knew of their chronic HBV infection. Resolved HBV infection (HBsAg-negative) was found in 27% (n = 119/438) of participants. A total of 10 participants were HCV exposed of whom 3 had chronic HCV infection (0.7%, n = 3/435) and 7 resolved HCV infection (1.6%, n = 7/435). No one knew of their HCV infection prior to participation. Five participants tested HIV positive, resulting in a prevalence of 1.1% (n = 5/439). Three participants already knew of their HIV-positive status (Table 2). No co-infections were found.
Table 2. Infectious disease status of participants who had blood draw (N = 435–439*).
| n | % | 95%CI | |
|---|---|---|---|
| Tested for HBV (N = 438) | |||
| HBV exposed (anti-HBc positive) | 130 | 29.7 | 25.4–34.2 |
| Chronic HBV infection (HBsAg positive & anti-HBc positive) | 11 | 2.5 | 1.3–4.5 |
| Resolved HBV infection (HBsAg negative & anti-HBc positive) | 119 | 27.2 | 23.1–31.6 |
| Tested for HCV (N = 435) | |||
| HCV exposed (anti-HCV positive) | 10 | 2.3 | 1.1–4.2 |
| Chronic HCV infection (anti-HCV positive & HCV RNA positive) | 3 | 0.7 | 0.1–2.0 |
| Resolved HCV infection (anti-HCV positive & HCV RNA negative) | 7 | 1.6 | 0.7–3.3 |
| Tested for HIV (N = 439) | |||
| HIV infection | 5 | 1.1 | 0.0–2.6 |
| Known HIV infection | 3 | 0.7 | 0.1–2.0 |
| Newly diagnosed HIV infection | 2 | 0.5 | 0.1–1.6 |
Abbreviations: CI–Confidence Interval; HBV–Hepatitis B Virus; HCV–Hepatitis C Virus; HIV–Human Immunodeficiency Virus.
* Participants could refuse testing for any of the three infections. Participants was only offered testing for both HBV and HCV when risk factors were present.
The prevalence of those HBV/HCV exposed or HIV infected did not differ between undocumented migrants and legal residents.(Tables 3–5) Sex, age, region of birth, educational level, year of leaving country of origin, way of entering the Netherlands, living in housing provided by charity, living on the streets and ever having had surgery abroad significantly differed according to HBV status (Table 3). In multivariable analysis, individuals originating from Africa and older individuals were more likely to be HBV exposed (Table 4). Variables did not differ according to HCV status (Table 5). HIV infection was more frequently observed in individuals who more recently left their country, were MSM, and had more than 3 sexual partners in the past 6 months (Table 6).
Table 3. Characteristics of undocumented migrants and uninsured legal residents tested for HBV (N = 438) by HBV status, in Amsterdam, the Netherlands, October 2018- October 2019.
| No HBV infection (N = 308) | Resolved HBV infection (N = 119) | Chronic HBV infection (N = 11) | P-value* | ||||
|---|---|---|---|---|---|---|---|
| Sociodemographic variables | n | % | n | % | n | % | |
| Sex | .001 | ||||||
| Heterosexual men | 132 | 43 | 76 | 64 | 9 | 82 | |
| Men having sex with men | 29 | 10 | 9 | 8 | 0 | 0 | |
| Female | 146 | 48 | 34 | 29 | 2 | 18 | |
| Other | 1 | 0.3 | 0 | 0 | 0 | 0 | |
| Age in years, median (IQR) | 39.5 | (32–51) | 46 | (36–54) | 42 | (29–50) | .034 |
| Age, categorized | .176 | ||||||
| <35 years | 102 | 33 | 24 | 20 | 4 | 36 | |
| 35–49 years | 114 | 37 | 48 | 40 | 4 | 36 | |
| 50–64 years | 81 | 26 | 40 | 34 | 3 | 27 | |
| ≥65 years | 11 | 4 | 7 | 6 | 0 | 0 | |
| Kruispost target population | .802 | ||||||
| Undocumented migrants | 286 | 93 | 110 | 92 | 10 | 91 | |
| Uninsured legal residents | 22 | 7 | 9 | 8 | 1 | 9 | |
| Region of birth ** | < .001 | ||||||
| Europe | 16 | 5 | 2 | 2 | 2 | 18 | |
| Asia | 106 | 34 | 20 | 17 | 2 | 18 | |
| Africa | 90 | 29 | 78 | 66 | 7 | 64 | |
| North /South America | 96 | 31 | 19 | 16 | 0 | 0 | |
| Educational level | .008 | ||||||
| No school | 14 | 5 | 9 | 8 | 0 | 0 | |
| Primary school | 48 | 17 | 35 | 29 | 1 | 9 | |
| Secondary school | 141 | 46 | 43 | 36 | 3 | 27 | |
| Higher education | 103 | 34 | 32 | 27 | 7 | 64 | |
| Migration history | |||||||
| Year of leaving country of origin, median (IQR) | 2011 | (2003–2016) | 2007 | (2001–2014) | 2012 | (2000–2016) | .007 |
| Year of leaving country of origin | .019 | ||||||
| <2010 | 131 | 43 | 69 | 58 | 5 | 46 | |
| 2010–2017 | 129 | 42 | 43 | 36 | 5 | 46 | |
| ≥2018 | 46 | 15 | 7 | 6 | 1 | 9 | |
| Year of arrival in the Netherlands, median (IQR) | 2014 | (2006–2017) | 2010 | (2003–2016) | 2012 | (2006–2018) | .101 |
| Year of arrival in the Netherlands | .295 | ||||||
| <2010 | 110 | 36 | 56 | 47 | 4 | 36 | |
| 2010–2017 | 125 | 41 | 42 | 35 | 4 | 36 | |
| ≥2018 | 71 | 23 | 21 | 18 | 3 | 27 | |
| Way of entering the Netherlands | .046 | ||||||
| (Expired) tourist/working/student visa | 176 | 58 | 47 | 39 | 5 | 45 | |
| Rejected asylum seeker | 48 | 16 | 27 | 23 | 3 | 27 | |
| EU citizen | 18 | 6 | 7 | 6 | 1 | 9 | |
| Illegally crossing borders | 40 | 13 | 30 | 25 | 2 | 18 | |
| Legally/work/other visa$ | 14 | 5 | 5 | 4 | 0 | 0 | |
| Other/unknown | 9 | 3 | 3 | 3 | 0 | 0 | |
| Housing situation (multiple answers possible) | |||||||
| BBB (Bed Bath Bread) facility# | 17 | 6 | 11 | 9 | 2 | 18 | .093 |
| friends/family | 165 | 54 | 63 | 53 | 3 | 27 | .242 |
| illegal rent | 70 | 23 | 22 | 18 | 3 | 27 | .526 |
| housing provided by charity | 26 | 8 | 16 | 13 | 3 | 27 | .048 |
| Lives on the streets | 15 | 5 | 15 | 13 | 1 | 9 | .017 |
| Lives in other housing## | 24 | 8 | 6 | 5 | 0 | 0 | .547 |
| Number of housemates | .900 | ||||||
| No housemates | 45 | 15 | 18 | 16 | 2 | 18 | |
| <3 | 129 | 43 | 46 | 40 | 6 | 55 | |
| 3–5 | 97 | 32 | 36 | 31 | 2 | 18 | |
| ≥6 | 32 | 11 | 16 | 14 | 1 | 9 | |
| Risk factors | |||||||
| Ever admitted/treated in a foreign hospital | 115 | 38 | 36 | 31 | 4 | 36 | .353 |
| Ever had surgery abroad | 117 | 38 | 33 | 28 | 1 | 9 | .025 |
| Ever received a blood transfusion | .160 | ||||||
| No/unknown | 285 | 93 | 116 | 97 | 11 | 100 | |
| Yes | 22 | 7 | 3 | 3 | 0 | 0 | |
| More than 3 sexual partners in the previous 6 months | 46 | 16 | 21 | 18 | 2 | 18 | .703 |
| Ever (been) paid for sex | .150 | ||||||
| No, never | 248 | 81 | 85 | 71 | 8 | 73 | |
| Yes, ever paid | 48 | 16 | 29 | 24 | 2 | 18 | |
| Yes, ever been paid | 11 | 4 | 5 | 4 | 1 | 9 | |
| Ever injected drugs | 3 | 1 | 1 | 0.8 | 1 | 9 | .140 |
* Differences in variables according to HBV status were assessed using Fisher exact test for categorical data. Kruskal Wallis test was used for continuous data.
** The most common countries of birth were Brazil (n = 67), Nigeria (n = 59), Philippines (n = 45), Surinam (n = 28), Ghana (n = 26), Morocco (n = 24), Egypt (n = 19), Eritrea (n = 14), and Indonesia (n = 14).
$ Include family visa and Schengen visa.
# The bed, bath, bread regulation arranges basic emergency shelter for rejected asylum seekers provided by the Dutch government.
## Includes legal rent, housing for asylum seekers, boats, employers, hotels, crisis care, winter care and outdoors.
Abbreviations: HBV–hepatitis B virus; IQR–interquartile range; EU–European Union; BBB–bed bath bread.
Table 5. Characteristics of undocumented migrants and uninsured legal residents tested for HCV (N = 435) by HCV status, in Amsterdam, the Netherlands, October 2018- October 2019.
| No HCV infection (N = 425) | Resolved HCV infection (N = 7) | Chronic HCV infection (N = 3) | P-value* | ||||
|---|---|---|---|---|---|---|---|
| Sociodemographic variables | n | % | n | % | n | % | |
| Sex | .273 | ||||||
| Heterosexual men | 207 | 49 | 3 | 43 | 2 | 67 | |
| Men having sex with men | 36 | 8 | 1 | 14 | 1 | 33 | |
| Female | 179 | 42 | 3 | 43 | 0 | 100 | |
| Other | 1 | 0.2 | 0 | 0 | 0 | 0 | |
| Age in years, median (IQR) | 42 | (32–52) | 51 | (34–59) | 52 | (31–61) | .294 |
| Age, categorized | .053 | ||||||
| <35 years | 126 | 30 | 2 | 29 | 1 | 33 | |
| 35–49 years | 166 | 39 | 0 | 0 | 0 | 0 | |
| 50–64 years | 115 | 27 | 5 | 71 | 2 | 67 | |
| ≥65 years | 18 | 4 | 0 | 0 | 0 | 0 | |
| Kruispost target population | .538 | ||||||
| Undocumented migrants | 394 | 93 | 6 | 86 | 3 | 100 | |
| Uninsured legal residents | 31 | 7 | 1 | 14 | 0 | 0 | |
| Region of birth ** | .726 | ||||||
| Europe | 20 | 5 | 0 | 0 | 0 | 0 | |
| Asia | 125 | 29 | 3 | 43 | 1 | 33 | |
| Africa | 169 | 40 | 1 | 14 | 1 | 33 | |
| North /South America | 111 | 26 | 3 | 43 | 1 | 33 | |
| Educational level | .581 | ||||||
| No school | 23 | 5 | 0 | 0 | 0 | 0 | |
| Primary school | 78 | 18 | 1 | 14 | 2 | 67 | |
| Secondary school | 183 | 43 | 3 | 43 | 1 | 33 | |
| Higher education | 139 | 33 | 3 | 43 | 0 | 0 | |
| Migration history | |||||||
| Year of leaving country of origin, median (IQR) | 2010 | (2002–2016) | 1995 | (1988–2015) | 2008 | (2003–2015) | .369 |
| Year of leaving country of origin | .967 | ||||||
| <2010 | 197 | 47 | 4 | 57 | 2 | 67 | |
| 2010–2017 | 172 | 41 | 3 | 43 | 1 | 33 | |
| ≥2018 | 54 | 13 | 0 | 0 | 0 | 0 | |
| Year of arrival in the Netherlands, median (IQR) | 2013 | (2005–2017) | 2015 | (1988–2016) | 2008 | (2003–2015) | .617 |
| Year of arrival in the Netherlands | .958 | ||||||
| <2010 | 163 | 39 | 3 | 43 | 2 | 67 | |
| 2010–2017 | 166 | 39 | 3 | 43 | 1 | 33 | |
| ≥2018 | 94 | 22 | 1 | 14 | 0 | 0 | |
| Way of entering the Netherlands | .398 | ||||||
| (Expired) tourist/working/student visa | 221 | 52 | 5 | 71 | 1 | 33 | |
| Rejected asylum seeker | 76 | 18 | 1 | 14 | 0 | 0 | |
| EU citizen | 25 | 6 | 1 | 14 | 0 | 0 | |
| Illegally crossing borders | 70 | 17 | 0 | 0 | 1 | 33 | |
| Legally/work/other visa$ | 18 | 4 | 0 | 0 | 1 | 33 | |
| Other/unknown | 12 | 3 | 0 | 0 | 0 | 0 | |
| Housing situation (multiple answers possible) | |||||||
| BBB (Bed Bath Bread) facility# | 29 | 7 | 0 | 0 | 0 | 0 | .999 |
| friends/family | 223 | 52 | 4 | 57 | 3 | 100 | .345 |
| illegal rent | 93 | 22 | 1 | 14 | 0 | 0 | .999 |
| housing provided by charity | 45 | 11 | 0 | 0 | 0 | 0 | .999 |
| Lives on the streets | 30 | 7 | 1 | 14 | 0 | 0 | .526 |
| Lives in other housing## | 29 | 7 | 1 | 14 | 0 | 0 | .514 |
| Number of housemates | .528 | ||||||
| No housemates | 63 | 15 | 1 | 14 | 0 | 0 | |
| <3 | 173 | 41 | 5 | 71 | 3 | 100 | |
| 3–5 | 132 | 32 | 1 | 14 | 0 | 0 | |
| ≥6 | 49 | 12 | 0 | 0 | 0 | 0 | |
| Risk factors | |||||||
| Ever admitted/treated in a foreign hospital | 152 | 36 | 2 | 29 | 1 | 33 | .999 |
| Ever had surgery abroad | 149 | 35 | 2 | 29 | 0 | 0 | .746 |
| Ever received a blood transfusion | .999 | ||||||
| No/unknown | 399 | 94 | 7 | 100 | 3 | 100 | |
| Yes | 25 | 6 | 0 | 0 | 0 | 0 | |
| More than 3 sexual partners in the previous 6 months | 67 | 16 | 1 | 17 | 0 | 0 | .999 |
| Ever (been) paid for sex | .527 | ||||||
| No, never | 330 | 78 | 7 | 100 | 2 | 67 | |
| Yes, ever paid | 77 | 18 | 0 | 0 | 1 | 33 | |
| Yes, ever been paid | 17 | 4 | 0 | 0 | 0 | 0 | |
| Ever injected drugs | 5 | 1 | 0 | 0 | 0 | 0 | .999 |
* Differences in variables according to HCV status were assessed using Fisher’s Exact test for categorical data. Kruskal Wallis test was used for continuous data.
** The most common countries of birth were Brazil (n = 67), Nigeria (n = 58), Philippines (n = 46), Surinam (n = 28), Ghana (n = 25), Morocco (n = 24), Egypt (n = 19), Eritrea (n = 14), and Indonesia (n = 14).
$ Include family visa and Schengen visa.
# The bed, bath, bread regulation arranges basic emergency shelter for rejected asylum seekers provided by the Dutch government.
## Includes legal rent, housing for asylum seekers, boats, employers, hotels, crisis care, winter care and outdoors.
Abbreviations: HCV–hepatitis C virus; IQR–interquartile range; EU–European Union; BBB–bed bath bread.
Table 4. Determinants of HBV exposure among undocumented migrants and uninsured legal residents that were tested for HBV (n = 130/438) in Amsterdam, the Netherlands, October 2018—October 2019 (univariable and multivariable logistic regression analyses).
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Sociodemographics | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Sex | < .001 | |||||
| Heterosexual men | Ref | |||||
| Men having sex with men | 0.48 | 0.22–1.06 | ||||
| Female | 0.38 | 0.24–0.60 | ||||
| Age in years | .081 | .034 | ||||
| <35 years | Ref | Ref | ||||
| 35–49 years | 1.66 | 0.98–2.83 | 1.68 | 0.96–2.96 | ||
| 50–64 years | 1.93 | 1.11–3.38 | 2.46 | 1.34–4.50 | ||
| ≥65 years | 2.32 | 0.82–6.53 | 2.03 | 0.67–6.17 | ||
| Kruispost target population | .841 | |||||
| Undocumented migrant | Ref | |||||
| Uninsured legal resident | 1.08 | 0.50–2.36 | ||||
| Region of birth * | < .001 | < .001 | ||||
| Europe | Ref | Ref | ||||
| Asia | 0.83 | 0.25–2.72 | 0.67 | 0.20–2.25 | ||
| Africa | 3.78 | 1.21–11.75 | 3.29 | 1.04–10.36 | ||
| North/South America | 0.79 | 0.24–2.63 | 0.63 | 0.19–2.14 | ||
| Educational level | .017 | |||||
| No school | Ref | |||||
| Primary school | 1.17 | 0.45–2.99 | ||||
| Secondary school | 0.51 | 0.21–1.25 | ||||
| Higher education | 0.59 | 0.24–1.47 | ||||
| Migration history | ||||||
| Year of leaving country of origin | .004 | |||||
| <2010 | Ref | |||||
| 2010–2017 | 0.66 | 0.43–1.02 | ||||
| ≥2018 | 0.31 | 0.14–0.69 | ||||
| Year of arrival in the Netherlands | .133 | |||||
| <2010 | Ref | |||||
| 2010–2017 | 0.67 | 0.43–1.07 | ||||
| ≥2018 | 0.62 | 0.35–1.08 | ||||
| Way of entering the Netherlands | .009 | |||||
| Expired tourist/working/student visa | Ref | |||||
| Rejected asylum seeker | 2.12 | 1.22–3.677 | ||||
| EU citizen | 1.50 | 0.62–3.66 | ||||
| Illegally crossing borders | 2.71 | 1.55–4.73 | ||||
| Legally/work/other visa$ | 1.21 | 0.42–3.51 | ||||
| Other/unknown | 1.13 | 0.29–4.32 | ||||
| Housing situation (multiple answers possible) | ||||||
| Lives in BBB (Bed Bath Bread) facility# | 1.90 | 0.90–4.04 | .101 | |||
| Lives with friends/family | 0.89 | 0.59–1.35 | .592 | |||
| Lives in illegal rent | 0.81 | 0.49–1.35 | .413 | |||
| Lives in housing provided by charity | 1.86 | 0.99–3.49 | .059 | |||
| Lives on the streets | 2.74 | 1.31–5.73 | .008 | |||
| Lives in other housing## | 0.57 | 0.23–1.44 | .213 | |||
| Number of housemates | .841 | |||||
| No housemates | Ref | |||||
| <3 | 0.91 | 0.49–1.68 | ||||
| 3–5 | 0.88 | 0.46–1.68 | ||||
| ≥6 | 1.20 | 0.54–2.63 | ||||
| Risk factors | ||||||
| Ever admitted/treated in a foreign hospital | 0.74 | 0.48–1.15 | .173 | |||
| Ever had surgery abroad | 0.58 | 0.37–0.91 | .015 | |||
| Ever received a blood transfusion | .030 | |||||
| No/unknown | Ref | |||||
| Yes | 0.31 | 0.09–1.04 | ||||
| More than 3 sexual partners in the previous 6 months | 1.20 | 0.69–2.08 | .515 | |||
| Ever (been) paid for sex | .106 | |||||
| No, never | Ref | |||||
| Yes, ever paid | 1.72 | 1.03–2.87 | ||||
| Yes, ever been paid | 1.45 | 0.52–4.05 | ||||
| Ever injected drugs | 1.58 | 0.26–9.59 | .624 | |||
* The most common countries of birth were Brazil (n = 67), Nigeria (n = 59), Philippines (n = 45), Surinam (n = 28), Ghana (n = 26), Morocco (n = 24), Egypt (n = 19), Eritrea (n = 14), and Indonesia (n = 14).
$ Includes family visa and Schengen visa.
# The bed, bath, bread regulation arranges basic emergency shelter for rejected asylum seekers provided by the Dutch government.
## Includes legal rent, housing for asylum seekers, boats, employers, hotels, crisis care, winter care, campers and forest huts.
Abbreviations: Ref–reference category; HBV–hepatitis B virus; OR–odds ratio; CI–confidence interval; IQR–inter quartile range; EU–European Union; BBB–bed bath bread.
Table 6. Characteristics of undocumented migrants and uninsured legal residents tested for HIV (N = 439) by HIV status, in Amsterdam, the Netherlands, October 2018- October 2019.
| HIV negative (N = 434) | HIV positive (N = 5) | P-value* | |||
|---|---|---|---|---|---|
| Sociodemographic variables | n | % | n | % | |
| Sex | .003 | ||||
| Heterosexual men | 219 | 50 | 0 | 0 | |
| Men having sex with men | 34 | 8 | 3 | 60 | |
| Female | 180 | 42 | 2 | 40 | |
| Other | 1 | 0.2 | 0 | 0 | |
| Age in years, median (IQR) | 43 | (33–52) | 36 | (33–43) | .262 |
| Age, categorized | .523 | ||||
| <35 years | 125 | 29 | 2 | 40 | |
| 35–49 years | 166 | 38 | 3 | 60 | |
| 50–64 years | 123 | 28 | 0 | 0 | |
| ≥65 years | 20 | 5 | 0 | 0 | |
| Kruispost target population | .325 | ||||
| Undocumented migrants | 402 | 93 | 4 | 80 | |
| Uninsured legal residents | 32 | 7 | 1 | 20 | |
| Region of birth ** | .361 | ||||
| Europe | 20 | 5 | 1 | 20 | |
| Asia | 129 | 30 | 1 | 20 | |
| Africa | 173 | 40 | 2 | 40 | |
| North /South America | 112 | 26 | 1 | 20 | |
| Educational level | .237 | ||||
| No school | 27 | 6 | 0 | 0 | |
| Primary school | 82 | 19 | 0 | 0 | |
| Secondary school | 185 | 43 | 1 | 20 | |
| Higher education | 138 | 32 | 4 | 80 | |
| Migration history | |||||
| Year of leaving country of origin, median (IQR) | 2010 | (2002–2016) | 2016 | (2012–2018) | .060 |
| Year of leaving country of origin | .024 | ||||
| <2010 | 207 | 48 | 0 | 0 | |
| 2010–2017 | 173 | 40 | 3 | 60 | |
| ≥2018 | 51 | 12 | 2 | 40 | |
| Year of arrival in the Netherlands, median (IQR) | 2013 | (2004–2017) | 2016 | (2012–2018) | .188 |
| Year of arrival in the Netherlands | .155 | ||||
| <2010 | 173 | 40 | 0 | 0 | |
| 2010–2017 | 171 | 40 | 3 | 60 | |
| ≥2018 | 87 | 20 | 2 | 40 | |
| Way of entering the Netherlands | .523 | ||||
| (Expired) tourist/working/student visa | 225 | 52 | 3 | 60 | |
| Rejected asylum seeker | 76 | 18 | 0 | 0 | |
| EU citizen | 26 | 6 | 1 | 20 | |
| Illegally crossing borders | 72 | 17 | 1 | 20 | |
| Legally/work/other visa$ | 20 | 5 | 0 | 0 | |
| Other/unknown | 12 | 3 | 0 | 0 | |
| Housing situation (multiple answers possible) | |||||
| BBB (Bed Bath Bread) facility# | 31 | 7 | 0 | 0 | .999 |
| friends/family | 231 | 53 | 3 | 60 | .999 |
| illegal rent | 95 | 22 | 1 | 20 | .999 |
| housing provided by charity | 41 | 9 | 1 | 20 | .397 |
| Lives on the streets | 29 | 7 | 0 | 0 | .999 |
| Lives in other housing## | 29 | 7 | 0 | 0 | .999 |
| Number of housemates | .999 | ||||
| No housemates | 62 | 15 | 1 | 20 | |
| <3 | 184 | 43 | 2 | 40 | |
| 3–5 | 133 | 31 | 2 | 40 | |
| ≥6 | 47 | 11 | 0 | 0 | |
| Risk factors | |||||
| Ever admitted/treated in a foreign hospital | 151 | 35 | 0 | 0 | .168 |
| Ever had surgery abroad | 147 | 34 | 1 | 20 | .667 |
| Ever received a blood transfusion | .999 | ||||
| No/unknown | 409 | 94 | 5 | 100 | |
| Yes | 24 | 6 | 0 | 0 | |
| More than 3 sexual partners in the previous 6 months | 65 | 16 | 3 | 60 | .032 |
| Ever (been) paid for sex | .221 | ||||
| No, never | 337 | 78 | 4 | 80 | |
| Yes, ever paid | 80 | 18 | 0 | 0 | |
| Yes, ever been paid | 16 | 4 | 1 | 20 | |
| Ever injected drugs | 4 | 0.9 | 1 | 20 | .056 |
* Differences in variables according to HIV status were assessed using Fisher’s Exact test for categorical data. Mann-Whitney U test was used for continuous data.
** The most common countries of birth were Brazil (n = 67), Nigeria (n = 57), Philippines (n = 46), Morocco (n = 33), Surinam (n = 28), Ghana (n = 24), Egypt (n = 19), Eritrea (n = 13), and Indonesia (n = 13).
$ Include family visa and Schengen visa.
# The bed, bath, bread regulation arranges basic emergency shelter for rejected asylum seekers provided by the Dutch government.
## Includes legal rent, housing for asylum seekers, boats, employers, hotels, crisis care, winter care and outdoors.
Abbreviations: HIV–human immunodeficiency virus; IQR–interquartile range; EU–European Union; BBB–bed bath bread.
Follow-up and treatment of diagnosed patients
Of the 11 participants with chronic HBV infection, nine (36.4%) were diagnosed with HBeAg-negative chronic infection (according to clinical practice guidelines of the European Association for the Study of the Liver [12] and were asked to return for ALT testing every six months and HBsAg testing every three years, according to the Dutch guidelines [9]. One (9.1%) was diagnosed with stage F3-F4 fibrosis and had HBeAg-negative chronic hepatitis. Two (18.2%) did not have liver fibrosis measurements for the following reasons: did not come to their appointment (n = 1) and unable to continue care in the Netherlands because of being expulsed to another country (n = 1).
All three participants newly diagnosed with chronic HCV infection were referred and followed at a hepatology referral center. They had high HCV-RNA viral loads (range 86,846–155,299 IU/mL). Only one had their fibrosis levels measured, which revealed stage F2 fibrosis. Two participants initiated treatment, the third is currently awaiting treatment.
Five persons were HIV-positive. Of the three individuals who were already aware of their HIV-seropositive status, one had never used medication since diagnosis and was referred to an HIV-treatment center. The other two known HIV seropositive persons were already using medication, one of whom was in immediate need for medication refill and was hence referred to an HIV-center for further consultation and antiretroviral prescription. The two individuals with newly diagnosed HIV were both referred to an HIV-treatment center, but only one actually arrived, started treatment, and after treatment, achieved an undetectable HIV viral load. The second person was lost to follow up.
Because of their undocumented status, some participants were initially refused care at the hospital despite efforts from the GPs and research teams and were only able to receive an appointment after extensive mediation from the researchers.
HBV vaccination uptake
A total of 80 participants were MSM (n = 57), had ever been paid for sex (n = 16), or both (n = 7). Of them, 50 (63%) went to the laboratory and were tested for HBV infection. Of these 50 participants, 15 had either resolved or chronic HBV infection and 35 were not HBV exposed. Of the 35 latter participants, 23 were able to be contacted and/or understood the invitation for vaccination. Uptake of ≥1 HBV vaccination dose at the Public Health Service of Amsterdam was 30% (n = 7/23).
Discussion
In this cross-sectional study among undocumented migrants and uninsured legal residents who attended an NGO healthcare facility for GP consultations in Amsterdam, the Netherlands, we found that 29.7% were HBV exposed–with 2.5% having chronic HBV infection, 64% of which were newly diagnosed; 2.3% were HCV exposed–with 0.7% having chronic HCV infection, all of which newly diagnosed; and 1.1% were HIV-positive, 40% of which were newly diagnosed.
The prevalence for chronic HBV and HCV infections found in this study are respectively 7 times and 14 times higher compared to the general Dutch population [15,21], but lower compared to studies in Italy in undocumented migrants where a prevalence of 6–9% for chronic HBV (HBsAg positive) and 3.3% for chronic HCV infection were observed [18,22]. Migrants included in these two studies predominately originated from Eastern-Europe (estimated HBV prevalence ranges from <2%-7.99%) and Sub-Saharan Africa (HBV prevalence >8%), while few individuals came from regions of lower HBV/HCV endemicity, namely North/South America (estimated prevalence <2%) [23]. Moreover, previous studies in the Netherlands that offered testing to documented migrants were targeting specific countries and observed a wide prevalence for chronic HBV and HCV [15,21], which is not comparable with our findings. Most individuals with chronic HBV infection in our study originated from Africa and tend to be older [23]. Older age was also associated with HBV exposure, which likely reflects the rollout of vaccination programs in the country of origin [24]. These findings are in line with a recent study among documented migrants in Amsterdam [3].
We observed an HIV prevalence of 1.1% in our study, which is eight times higher than that of the general Dutch population (0.1%) [4,25] yet comparable to an Italian study among undocumented migrants (1%) [18]. The United Nations goals of 95% of HIV-positive individuals knowing their status, 95% of those who know their status being engaged in care, and 95% of those who are in care having suppressed HIV replication are crucial for reducing onwards transmission [26]. The fact that we observed two newly diagnosed HIV infections and one untreated HIV-positive individual highlights some of the difficulties in achieving these goals for undocumented migrants. However, the limited number of HIV-positive individuals included in this study makes it difficult to draw conclusions on the adequacy of the HIV cascade-of-care for this specific migrant population [26].
Migrants are considered a group at risk for HBV and HCV infection according to the Dutch GP guidelines on viral hepatitis [9]. The relatively high prevalence of HBV, HCV and HIV infections in our study compared to the general Dutch population underscores that undocumented migrants are no exception to these guidelines. Coupled with the fact that screening for chronic hepatitis B and C among migrant populations is cost-effective in low-endemic settings [27], proactive case finding conducted by GPs and other healthcare workers should be considered universally in (undocumented) migrants [28]. MSM are also considered a key population at risk for HBV/HCV/HIV infections [9,29]. Our study included a very small number of migrant MSM and among them, 8% were HIV-positive, 3% had chronic HCV infection and none had chronic HBV infection. The high prevalence for HIV and chronic HCV infections in this group is worrisome, but given the small sample size, it is difficult to generalize to the overall, undocumented migrant population.
From those eligible, 32% participated in our study. Although this percentage was lower than in another integrated screening program of migrants at the tuberculosis clinic in Amsterdam (54%) [15], it was higher compared to previous non-integrated HBV and HCV screening projects targeting migrants in the Netherlands (range: 7–28%) [21,30–32]. The differences in the percentage participating across studies probably highlight the increased effectiveness of proactive HBV, HCV and HIV case finding offered by integrated existing healthcare facilities as opposed to non-integrated programs.
Participants in our study were required to have their blood drawn at an outside laboratory instead of on-site and 58% of them did make their way to the laboratory to receive testing (n = 451/784). However, on-site testing, particularly rapid testing, at the public NGO healthcare facility has been shown to remove a considerable barrier to testing uptake [33]. Unfortunately, this was not possible at Kruispost due to the absence of trained staff and high workload of nurses/doctors. Opt-out testing has also been shown to be more effective in increasing HIV testing uptake at sexual transmitted infection clinics [34] and HBV and HIV screening of migrants during antenatal care [35]. It should be noted that for many participants, their only source of healthcare was from Kruispost. These participants could view that not participating or not testing could jeopardize receiving healthcare at our center and hence why we chose not to use an opt-out testing strategy for this study. There might be several barriers to testing uptake in our study population. Previous studies among migrants in Western countries have suggested that the decision to test can be influenced by inaccurate knowledge about the disease, or being unaware that treatment options are available. In addition, stigma has also been found to withhold migrants from testing [36].
Notwithstanding the high percentage of patients able to receive appropriate follow-up, there were several noteworthy barriers. As described, some participants were initially refused care due to their undocumented status. A systematic review from 2018 supports our findings that undocumented migrants face particular problems in utilizing healthcare services in Europe [37]. A qualitative study in the Netherlands conducted in undocumented migrant women was able to substantiate personal, language, and institutional barriers to access care from the GP or hospitals for all kind of problems [38]. Failure to engage individuals with infection into care has substantial consequences further down the cascade of care, which involve lack of immediate treatment (for HIV and HCV) or assessment for treatment eligibility (for HBV) and adequate virological and clinical monitoring. Continued replication in these individuals could constitute an important reservoir for onward transmission [39,40]. Taken together, there needs to be more efficient means of ensuring that individuals diagnosed with HBV, HCV or HIV infection can be linked to care. Given the difficulties encountered at participating centers, local protocols specifying how undocumented or uninsured people can access low-threshold services for care and treatment, while including resources to secure healthcare-related funding, are needed for migrants.
HBV vaccination uptake was 30% in this study, which is lower compared to previous studies conducted in the Netherlands among MSM (50–86%) and sex workers (63%), but predominately non-migrants [41,42]. Nevertheless, this study principally aimed at infection screening in migrant populations and not necessarily HBV vaccination, while other studies conducted in the context of HBV screening observed similarly low HBV vaccine uptake [43]. An additional barrier was that participants were required to be vaccinated at the Public Health Service and not at their GP. Two studies among undocumented migrants in Italy found a lower HBV vaccination uptake of 0% to 3.4% [22,44], although they did not focus specifically on MSM or sex workers. The challenge of increasing uptake of vaccination in this migrant population likely stems from language barriers, insecurities and unfamiliarity with the health care system [19,37]. Since there were low numbers of individuals susceptible to HBV infection and the risk for further HBV transmission is unclear in this population, we cannot make any recommendations for targeting these individuals for HBV vaccination.
The main strength of our study is its inclusion of a population that has not yet been examined in previous studies in the Netherlands. We managed to reach a large group of undocumented migrants and uninsured legal residents of Amsterdam with difficult access to routine healthcare. The percentage of participants who had ever been tested for HBV/HCV or HIV support this claim. Study information was available in six different languages, which allowed us to include a very wide group of patients from many different countries. Another noteworthy feature of our study was the particular attention given to ensure linkage-to-care for individuals testing positive, which remains one of the major roadblocks to elimination, particularly for viral hepatitis.
Some limitations need to be addressed. First, we were unable to invite all patients visiting Kruispost during the inclusion period to participate in the study. Since Kruispost is a charity-based organization with limited budget and space, it was not possible to have a research associate present for inclusion during all GP-consultations. Second, only participants who visited the GP were invited, hence the study population could be more frequently in need of care. Some of these individuals might have been seeking care for their HBV, HCV or HIV infection [11,45], which would have biased prevalence estimates to be higher than expected in the target population. Third, screening for HBV and HCV were based on the presence of self-reported risk factors, hence some cases of HBV or HCV could have been missed and prevalence estimates reflect those considered at risk of viral hepatitis. Fourth, 40% of patients declined participation. Especially EU citizens were unable to be included prior to June 20, 2019 because they had to pay for care and treatment. It is therefore unclear whether our results are representative for the larger population of undocumented migrants and uninsured legal residents living in Amsterdam. Fifth, individuals from Africa were more likely to have been tested than those from South-America and given that the prevalence of chronic HBV infection is high in Africa and low to moderate in South-America [23], prevalence of chronic HBV infection could have been overestimated. In addition, MSM were more likely to receive testing than heterosexuals and since MSM were more frequently diagnosed with HIV, the prevalence of HIV-positive individuals could also have been overestimated. Sixth, we only measured anti-HBs antibodies in individuals with HBsAg or anti-HBc antibody positive status and therefore the percentage of (non)-immunized participants is unknown. Furthermore, chronic HBV infection is defined as having two consecutively positive HBsAg tests within at least 6 months. In our study, we only measured HBsAg once and thus we assumed that all those patients with HBsAg-serology had chronic infection. Lastly, absolute numbers of participants infected with HCV or HIV were low, which makes it difficult to examine risk factors. In addition, backwards selection of covariates does present with certain issues [46] and might not be optimal.
Conclusion
To the best of our knowledge, this is the first study to examine the prevalence of chronic or resolved HBV, chronic or resolved HCV and HIV infections among undocumented migrants and uninsured legal residents in the Netherlands. The prevalence of chronic HBV, chronic HCV and HIV infections was substantially higher than in the general Dutch population, and a substantial proportions of infections was newly diagnosed. This study emphasizes the importance of proactive case finding in public health and or primary care settings and that on-site testing could likely increase screening uptake. Health care professionals must also be aware that undocumented migrants or uninsured people face barriers when referred to specialized centers for follow-up care and treatment of HBV/HCV and HIV infections. Quantitative and qualitative research focusing on access to secondary care for undocumented migrants and uninsured patients is needed to map and overcome these barriers.
Supporting information
Questionnaire about lifetime risk factors for HBV or HCV infection. In English.
(PDF)
Questionnaire about lifetime risk factors for HBV or HCV infection. In Dutch.
(PDF)
Questionnaire about sociodemographic variables and migration history. In English.
(PDF)
Questionnaire about sociodemographic variables and migration history. In Dutch.
(PDF)
Acknowledgments
The authors would like to thank Freke Zuure for her role in designing the study. Furthermore, we would like to thank all research assistants for their role in collecting the data.
Abbreviations
- ALT
Alanine aminotransferase
- CI
Confidence Interval
- CSN
Citizen Service Number
- EEA
European Economic Area
- EPD
Electronic Patient Dossier
- EU
European Union
- GP
General practitioner
- HBV
Hepatitis B Virus
- HBsAg
Hepatitis B Surface Antigen
- anti-HBcAb
anti-Hepatitis B Core antibodies
- anti-HBsAb
anti-Hepatitis B Surface antibodies
- anti-HBeAb
anti-Hepatitis B “e” antibodies
- HBeAg
Hepatitis B “e” antigen
- HCV
Hepatitis C Virus
- HIV
Human Immunodeficiency Virus
- IQR
Inter Quartile Range
- MSM
men who have sex with men
- OR
Odds Ratio
- TB
Tuberculosis
- WMO
Medical Research Involving Human Subjects Act
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by regional infectious disease control funds from the Dutch National Institute for Public Health and the Environment (https://www.rivm.nl/regionale-ondersteuning-infectieziektebestrijding). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Koopsen J, van Steenbergen JE, Richardus JH, Prins M, op de Coul ELM, Croes EA, et al. Chronic hepatitis b and c infections in the netherlands: Estimated prevalence in risk groups and the general population. Epidemiology and Infection. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ECDPC/ WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe. End-year report. 2010. [Google Scholar]
- 3.Zuure F, Bil J, Visser M, Snijder M, Boyd A, Blom P, et al. Hepatitis B and C screening needs among different ethnic groups: A population-based study in Amsterdam, the Netherlands. JHEP Reports. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Sighem A.I. BTS, Wit F.W.N.M., Smit C., Matser A. RP. Monitoring Report 2018. Human Immunodeficiency Virus (HIV) Infection in the Netherlands. [Internet]. Amsterdam; 2018. Available from: www.hiv-monitoring.nl. [Google Scholar]
- 5.Coppola N, Alessio L, Onorato L, Sagnelli C, MacEra M, Sagnelli E, et al. Epidemiology and management of hepatitis C virus infections in immigrant populations. Infectious Diseases of Poverty. 2019. doi: 10.1186/s40249-019-0528-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross J, Cunningham CO, Hanna DB. HIV outcomes among migrants from low-income and middle-income countries living in high-income countries: A review of recent evidence. Current Opinion in Infectious Diseases. 2018. doi: 10.1097/QCO.0000000000000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez-Del Arco D, Fakoya I, Thomadakis C, Pantazis N, Touloumi G, Gennotte AF, et al. High levels of postmigration HIV acquisition within nine European countries. AIDS. 2017. doi: 10.1097/QAD.0000000000001571 [DOI] [PubMed] [Google Scholar]
- 8.RIVM. Roadmap for infectious diseases in reception centers for asylum seekers. [In Dutch: Draaiboek infectieziekten in opvangcentra voor asielzoekers] [Internet]. 2016. [cited 2020 Jan 3]. Available from: https://lci.rivm.nl/sites/default/files/2019-03/Draaiboek Infectieziekten in opvangcentra voor asielzoekers.pdf. [Google Scholar]
- 9.Nederlands Huisarts Genootschap. Viral Hepatitis Guideline for General Practitioners. In Dutch [Internet]. 2016. [cited 2019 Oct 15]. Available from: https://www.nhg.org/standaarden/volledig/nhg-standaard-virushepatitis-en-andere-leveraandoeningen. [Google Scholar]
- 10.Mouhebati T, Vleugel van der Y. HIV among migrants. In Dutch. Huisarts en Wetenschap. 2019;september. [Google Scholar]
- 11.Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC, et al. Hepatitis B and C virus infection among 1.2 million persons with access to care: Factors associated with testing and infection prevalence. Clinical Infectious Diseases. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of Hepatology. 2017. doi: 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 13.Pawlotsky JM, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, et al. EASL recommendations on treatment of hepatitis C: Final update of the series☆. Journal of Hepatology. 2020;73(5). doi: 10.1016/j.jhep.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 14.Zuure FR, Urbanus AT, Langendam MW, Helsper CW, van den Berg CHSB, Davidovich U, et al. Outcomes of hepatitis C screening programs targeted at risk groups hidden in the general population: A systematic review. BMC Public Health. 2014. doi: 10.1186/1471-2458-14-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bil JP, Schrooders PA, Prins M, Kouw PM, Klomp JH, Scholing M, et al. Integrating hepatitis B, hepatitis C and HIV screening into tuberculosis entry screening for migrants in the Netherlands, 2013 to 2015. Eurosurveillance. 2018. doi: 10.2807/1560-7917.ES.2018.23.11.17-00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrover M vdLJ, Lucassen L QC. Illegal Migration and Gender in a Global and Historical Perspective. Illegal Migration and Gender in a Global and Historical Perspective. 2008. [Google Scholar]
- 17.Klazinga N.S. BEJJM, Van Buuren J.J.M., Goker E., Kortmann F.A.M., Legemaate J., Pronk C.C., et al. Report from the Medical Care Committee for asylum seekers who are (almost) rejected and illegal migrants. [In Dutch: Arts En Vreemdeling. Rapport van de commissie Medische zorg voor (dreigend) uitgeprocedeerde asielzoekers en illegale vreemdelingen]. Utrecht. [Google Scholar]
- 18.Coppola N, Alessio L, Gualdieri L, Pisaturo M, Sagnelli C, Caprio N, et al. Hepatitis B virus, hepatitis C virus and human immunodeficiency virus infection in undocumented migrants and refugees in southern Italy, january 2012 to june 2013. Eurosurveillance. 2015. doi: 10.2807/1560-7917.ES.2015.20.35.30009 [DOI] [PubMed] [Google Scholar]
- 19.Wendland A, Ehmsen BK, Lenskjold V, Astrup BS, Mohr M, Williams CJ, et al. Undocumented migrant women in Denmark have inadequate access to pregnancy screening and have a higher prevalence Hepatitis B virus infection compared to documented migrants in Denmark: A prevalence study. BMC Public Health. 2016. doi: 10.1186/s12889-016-3096-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RIVM. Hepatitis B vaccination programm risk groups (in Dutch) [Internet]. 2019. [cited 2020 Jul 7]. Available from: https://lci.rivm.nl/draaiboeken/hepatitis-b-vaccinatieprogramma-risicogroepen. [Google Scholar]
- 21.Richter C, Beest G ter, Gisolf EH, van Bentum P, Waegemaekers C, Swanink C, et al. Screening for chronic hepatitis B and C in migrants from Afghanistan, Iran, Iraq, the former Soviet Republics, and Vietnam in the Arnhem region, the Netherlands. Epidemiology and Infection. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Hamad I, Pezzoli MC, Chiari E, Scarcella C, Vassallo F, Puoti M, et al. Point-of-care screening, prevalence, and risk factors for hepatitis B infection among 3,728 mainly undocumented migrants from Non-EU countries in northern Italy. Journal of Travel Medicine. 2015. [DOI] [PubMed] [Google Scholar]
- 23.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. The Lancet. 2015. doi: 10.1016/S0140-6736(15)61412-X [DOI] [PubMed] [Google Scholar]
- 24.Ott JJ, Horn J, Krause G, Mikolajczyk RT. Time trends of chronic HBV infection over prior decades–A global analysis. Journal of Hepatology. 2017;66(1). doi: 10.1016/j.jhep.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 25.Slurink I et al. Sexually transmitted infections in the Netherlands in 2018 [Internet]. 2019. Available from: https://www.rivm.nl/bibliotheek/rapporten/2019-0007.pdf. [Google Scholar]
- 26.United Nations. Political Declaration on HIV and AIDS: On the Fast Track to Accelerating the Fight against HIV and to Ending the AIDS Epidemic by 2030 [Internet]. 2016. Available from: https://www.unaids.org/sites/default/files/media_asset/2016-political-declaration-HIV-AIDS_en.pdf. [Google Scholar]
- 27.Suijkerbuijk AWM, van Hoek AJ, Koopsen J, de Man RA, Mangen MJJ, de Melker HE, et al. Cost-effectiveness of screening for chronic hepatitis B and C among migrant populations in a low endemic country. PLoS ONE. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joore IK. Proactive HIV testing strategies in primary care. 2017. [Google Scholar]
- 29.Nederlands Huisarts Genootschap. STI Guideline for General Practitioners. In Dutch. 2013. [Google Scholar]
- 30.Richter C, Beest G ter, Sancak I, Aydinly R, Bulbul K, Laetemia-Tomata F, et al. Hepatitis B prevalence in the Turkish population of Arnhem: Implications for national screening policy? Epidemiology and Infection. 2012. doi: 10.1017/S0950268811001270 [DOI] [PubMed] [Google Scholar]
- 31.Veldhuijzen IK, Wolter R, Rijckborst V, Mostert M, Voeten HA, Cheung Y, et al. Identification and treatment of chronic hepatitis B in Chinese migrants: Results of a project offering on-site testing in Rotterdam, the Netherlands. Journal of Hepatology. 2012. [DOI] [PubMed] [Google Scholar]
- 32.van der Veen YJJ, van Empelen P, de Zwart O, Visser H, Mackenbach JP, Richardus JH. Cultural tailoring to promote hepatitis B screening in Turkish Dutch: A randomized control study. Health Promotion International. 2014. doi: 10.1093/heapro/dat020 [DOI] [PubMed] [Google Scholar]
- 33.Bottero J, Boyd A, Gozlan J, Carrat F, Nau J, Pauti MD, et al. Simultaneous human immunodeficiency virus-hepatitis B-hepatitis C point-of-care tests improve outcomes in linkage-to-care: Results of a randomized control trial in persons without healthcare coverage. Open Forum Infectious Diseases. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heijman RLJ, Stolte IG, Thiesbrummel HFJ, van Leent E, Coutinho RA, Fennema JSA, et al. Opting out increases HIV testing in a large sexually transmitted infections outpatient clinic. Sexually Transmitted Infections. 2009. [DOI] [PubMed] [Google Scholar]
- 35.op de Coul ELM, Hahné S, van Weert YWM, Oomen P, Smit C, van der Ploeg KPB, et al. Antenatal screening for HIV, hepatitis B and syphilis in the Netherlands is effective. BMC Infectious Diseases. 2011. doi: 10.1186/1471-2334-11-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vedio A, Liu EZH, Lee ACK, Salway S. Improving access to health care for chronic hepatitis B among migrant Chinese populations: A systematic mixed methods review of barriers and enablers. Journal of Viral Hepatitis. 2017;24(7). doi: 10.1111/jvh.12673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winters M, Rechel B, de Jong L, Pavlova M. A systematic review on the use of healthcare services by undocumented migrants in Europe. BMC Health Services Research. 2018. doi: 10.1186/s12913-018-2838-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoevers MA, Loeffen MJ, van den Muijsenbergh ME, Lagro-Janssen ALM. Health care utilisation and problems in accessing health care of female undocumented immigrants in the Netherlands. International Journal of Public Health. 2010. doi: 10.1007/s00038-010-0151-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Global hepatitis report. Geneva; 2017. [Google Scholar]
- 40.(UNAIDS) JUNP on H. The Gap Report. Geneva: UNAIDS. 2014. [Google Scholar]
- 41.Baars JE, Boon BJ, Garretsen HF, van de Mheen D. The reach of a hepatitis B vaccination programme among men who have sex with men. European Journal of Public Health. 2011. doi: 10.1093/eurpub/ckq117 [DOI] [PubMed] [Google Scholar]
- 42.Baars JE, Boon BJF, Garretsen HF, van de Mheen D. Vaccination Uptake and Awareness of a Free Hepatitis B Vaccination Program Among Female Commercial Sex Workers. Women’s Health Issues. 2009. doi: 10.1016/j.whi.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 43.Boyd A, Bottero J, Carrat F, Gozlan J, Rougier H, Girard PM, et al. Testing for hepatitis B virus alone does not increase vaccine coverage in non-immunized persons. World Journal of Gastroenterology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coppola N, Alessio L, Gualdieri L, Pisaturo M, Sagnelli C, Minichini C, et al. Hepatitis B virus infection in undocumented immigrants and refugees in Southern Italy: Demographic, virological, and clinical features. Infectious Diseases of Poverty. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joore IK, Arts DL, Kruijer MJP, van Charante EPM, Geerlings SE, Prins JM, et al. HIV indicator condition-guided testing to reduce the number of undiagnosed patients and prevent late presentation in a high-prevalence area: A case-control study in primary care. Sexually Transmitted Infections. 2015. [DOI] [PubMed] [Google Scholar]
- 46.Smith G. Step away from stepwise. Journal of Big Data. 2018. Dec 15;5(1). doi: 10.1186/s40537-018-0154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Questionnaire about lifetime risk factors for HBV or HCV infection. In English.
(PDF)
Questionnaire about lifetime risk factors for HBV or HCV infection. In Dutch.
(PDF)
Questionnaire about sociodemographic variables and migration history. In English.
(PDF)
Questionnaire about sociodemographic variables and migration history. In Dutch.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.