Abstract
Introduction
Sex-based clinical outcome differences in sickle cell disease (SCD) remain largely unknown despite evidence that female sex is associated with an increased lifespan. To better characterize sex-based differences in SCD, we assessed pain, treatment characteristics, laboratory measures and complications among males and females currently enrolled in the Sickle Cell Disease Implementation Consortium (SCDIC) registry.
Methods
The SCDIC consists of eight comprehensive SCD centers and one data coordinating center that received funding from the National Heart Lung and Blood Institute to improve outcomes for individuals with SCD. Eligibility criteria included: 15 to 45 years of age and a confirmed diagnosis of SCD. Self-report surveys were completed and data were also abstracted from the participants’ medical records.
Results
A total of 2,124 participants were included (mean age: 27.8 years; 56% female). The majority had hemoglobin SS SCD genotype. Females had worse reports of pain severity (mean (SD) T-score 51.6 (9.6) vs 49.3 (10), p<0.001), more vaso-occlusive episodes (p = 0.01) and a higher occurrence of 3 or more hospital admissions in the past year (30.9% vs. 25.5, p = 0.03). On multivariable analysis, males had higher odds of acute chest syndrome (odds ratio (OR) 1.4, p = 0.002), cardiovascular (OR 1.70, p<0.001) and musculoskeletal (OR 1.33, p = 0.0034) complications and lower odds of depression (OR 0.77, p = 0.0381). Females had higher fetal hemoglobin levels with and without hydroxyurea use (9.6% vs 8.5%, p = 0.03 and 3% vs 2.2%, p = 0.0005, respectively).
Conclusion
Our data suggests that sex differences in clinical outcomes do occur among individuals with SCD. Future research needs to explore the mechanisms underlying these differences.
Introduction
Sickle cell disease (SCD) is a genetically inherited blood disorder that predominantly affects individuals of African descent [1,2]. One in 365 African Americans is born with SCD and 1 in 14 carry the trait [1,2]. The disease is characterized by polymerization of red blood cells into rigid sickle shapes during periods of low oxygen tension [3–6]. The disease is also associated with endothelial damage and chronic inflammation [3–6]. These factors result in vaso-occlusion and multi-organ dysfunction, leading to numerous complications such as vaso-occlusive pain episodes (VOEs), kidney failure and stroke [6].
Despite these complications, people with SCD are living longer [7]. In the United States, the median age of survival has increased from less than 20 years of age in the 1970s to 48 and 54.7 years of age for individuals with HbSS/HbSβ0 and Hb SC/HbSβ+ respectively, in the 2000s [1,8]. The increased median age of survival with SCD can be attributed to interventions to diagnose individuals with the disease much earlier in the life course (e.g., newborn screening) and interventions to prevent common disease-related complications (e.g., pneumococcal vaccination, use of penicillin prophylaxis, increased availability of hydroxyurea, and use of frequent blood transfusions for patients at increased risk of stroke) [9–13]. Of keen interest is that women live longer than their male counterparts [14].
Few studies have investigated sex-based SCD comparisons such as the frequency of VOEs, occurrence of end-organ dysfunction or complications, quality of life and sociodemographic characteristics [15–19]. The majority of those studies have been limited to urologic and obstetric disciplines [16–19]. A case series by Ballas et al [14] reviewed four women with SCD who lived beyond 80 years of age and linked the participants’ increased lifespan and quality of life to milder SCD, long-term family support, a healthy lifestyle and good adherence to medication and clinic appointments [14]. A prospective study by McClish et al [20] on gender differences in pain and healthcare utilization in SCD found an increase in VOEs and healthcare utilization (hospitalizations, doctor visits and ED visits) by men and no gender differences in opioid use and the frequency and intensity of pain [21]. Findings from this study, however, were limited by the small sample size and study scope. These underexplored differences may account for the sex difference in mortality. Therefore, a more comprehensive understanding of the sex differences in pain, treatment characteristics, laboratory measures and complications that exists amongst individuals with SCD is warranted.
To address this gap, we compared differences in sociodemographic and SCD disease characteristics and complications between men and women living with SCD currently enrolled in the Sickle Cell Disease Implementation Consortium (SCDIC) registry. Studying these differences may provide insight into the sex difference in mortality.
Methods
Study population
The study population included participants enrolled in the SCDIC patient registry. The SCDIC is a consortium, funded by the National Heart Lung and Blood Institute (NHLBI), including eight comprehensive sickle cell disease centers across the United States and one data coordinating center [22]. The overall goal of the SCDIC is to translate evidence-based SCD treatments to care [22]. One major priority of the consortium was the establishment of a comprehensive research registry that includes patient-reported and clinical data that can serve as a resource for conducting data queries and identifying gaps in research that inform implementation studies [22]. Participants were eligible for recruitment in the registry based on the following inclusion criteria: 15 to 45 years of age, confirmed diagnosis of SCD (subtypes Hb SS, SC, Sβ-thalassemia, SO, SD, SG, SE or SF), literacy in English and willingness to provide informed consent or assent. Confirmation of SCD diagnosis required laboratory confirmation (such as hemoglobin electrophoresis or newborn screening) of SCD from the participants’ medical records. Participants were excluded if they were unwilling or unable to provide informed consent or assent, had sickle cell trait (Hb AS), or had a successful bone marrow transplant. Recruitment occurred in outpatient clinics (e.g., sickle cell and primary care clinics), hospital inpatient settings, SCD support group meetings and conferences [23].
Ethical approval
Ethical approval was received by the institutional review boards at each of the eight SCDIC study sites prior to any data collection efforts. Written informed consent was obtained before participant recruitment and enrollment in the study. For eligible participants younger than 18 years of age, informed consent was obtained from the parent or legal guardian in conjunction with informed assent from the participant. IRB approval was obtained for this analysis of the existing registry data from the Duke University Health System IRB (Protocol ID: Pro00103703). We analyzed partially anonymized data that was collected in the registry from July 2017 to February 2019. We accessed registry data in October 2019.
Data collection
Data were collected using participant self-report surveys, medical records and laboratory abstraction forms (S1 Table) [24]. The data collection instruments were developed by the SCDIC steering committee, which consisted of at least one SCD expert from each of the eight sites [22]. The survey consisted of validated instruments that assessed socio-demographic information hydroxyurea use, opioid use, as well as pain frequency and severity [24]. Pain frequency and severity were assessed using five items from the pain episode frequency and severity domain of the Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) [24,25]. ASCQ-Me is a validated measure for assessing the health-related quality of life among people with SCD [24].
The medical records and laboratory forms collected data on the participants’ SCD genotype, blood transfusion history, number of VOEs in the past year, specialists involved in care, number of hospital admissions in the past year, SCD complications and standard laboratory measures (S1 Table) [24].
Data for the medical and laboratory forms were abstracted from participants’ electronic health records. Laboratory measures were obtained during steady state, which was defined as at least two weeks before or after: 1) hospitalization, 2) a blood transfusion, or 3) a major acute event (e.g., stroke or VOE) [24]. Survey and abstracted health record data were entered into a REDCap database, assessed for completeness and inaccuracies by the data coordinating center, and referred back to each site for correction.
Data analysis
We utilized a cross-sectional study design. Male participants were compared to female participants with regards to sociodemographic characteristics, SCD disease characteristics and complications. Summary statistics were presented as frequencies and percentages for categorical variables, and median and 25th– 75th percentiles (Q1-Q2) or mean and standard deviation for continuous variables. Categorical variables were analyzed using Chi-Square or Fisher exact tests when appropriate. Continuous variables were compared using the Mann-Whitney U test or independent sample t-tests depending on the distribution. For each SCD complication, all variables with p ≤ 0.1 in univariate analysis were included in a multivariable logistic regression with backward elimination. Statistical analysis was performed using SAS version 9.4 (SAS Institute; Cary, NC). A p-value < 0.05 was considered statistically significant. Due to the exploratory and hypothesis generating approach of the study, no adjustment for multiple testing was applied [26].
Results
Demographic characteristics
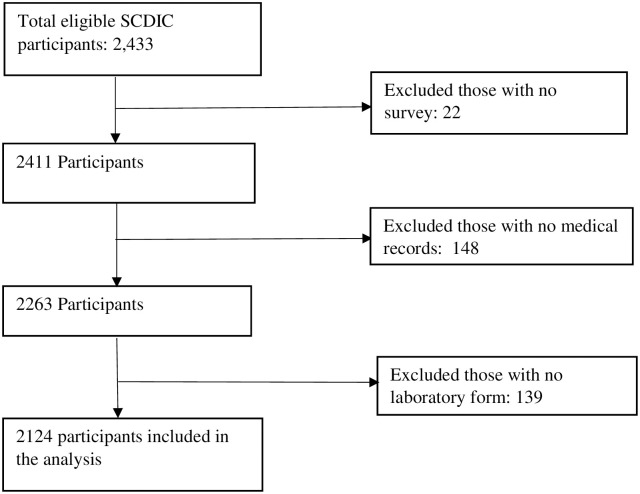
A total of 2124 participants were included in the study; 1190 were female (Fig 1).
Fig 1. Flow diagram of participant inclusion.
Participant demographics are highlighted in Table 1. The mean age of our participants was 27.8 years. The majority were Black or African American (95.6%) and never married (78.4%). There were no statistical differences in the age, ethnicity, race, employment, and marital status between sexes. Females had higher levels of education (25.9% vs 16.8% college graduate and 83.6% vs. 79.7% high school education and higher, p <0.0001), but had a household income less than males (p = 0.04). Although not statistically significant, more women were on government insurance (i.e. Medicare, Medicaid, or other government sponsored programs).
Table 1. Participant socio-demographic characteristics.
| Characteristic | Females (n = 1190) | Males (n = 934) | P-value | Total (n = 2124) |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) years | 27.9 (7.8) | 27.6 (8.0) | 0.3 | 27.8 (7.9) |
| Median (Q1-Q2) | 27 (22–34) | 27 (21–33) | 27 (21–34) | |
| Race, N (%) | ||||
| Black or African American | 1,128 (96.6) | 856 (94.3) | 0.1 | 1,984 (95.6) |
| Multiracial | 28 (2.4) | 41 (4.5) | 69 (3.3) | |
| American Indian/Alaskan | 6 (0.5) | 4 (0.4) | 10 (0.5) | |
| Asian | 3 (0.3) | 3 (0.3) | 6 (0.3) | |
| White | 3 (0.3) | 4 (0.4) | 7 (0.3) | |
| Ethnicity, N (%) | ||||
| Hispanic/ Latino | 44 (3.8) | 45 (4.9) | 0.2 | 89 (4.3) |
| Marital Status, N (%)- adults only | ||||
| Never married | 827 (79.1) | 614 (77.3) | 0.1 | 1,441 (78.4) |
| Married/Living as married | 162 (15.5) | 129 (16.2) | 291 (15.8) | |
| Divorced/separated/widowed | 56 (5.4) | 51 (6.4) | 107 (5.8) | |
| Education, N (%) | ||||
| Less than high school | 31 (2.6) | 33 (3.6) | <0.0001 | 64 (3.1) |
| Some high school | 161 (13.7) | 152 (16.6) | 313 (15.0) | |
| High school graduate or GED equivalent | 285 (24.3) | 305 (33.4) | 590 (28.3) | |
| Some college | 391 (33.4) | 270 (29.5) | 661 (31.7) | |
| College graduate | 303 (25.9) | 154 (16.8) | 457 (21.9) | |
| Employment, N (%) | ||||
| Employed | 413 (35.5) | 339 (37.4) | 0.3 | 752 (36.3) |
| Not employed by choice | 286 (24.6) | 198 (21.9) | 484 (23.4) | |
| Not employed, other | 464 (39.9) | 369 (40.7) | 833 (40.3) | |
| Household income in a year, N (%) | ||||
| $25,000 or less | 610 (56.5) | 412 (50.6) | 0.04 | 1,022 (54.0) |
| $25,001-$50,000 | 234 (21.7) | 194 (23.8) | 428 (22.6) | |
| $50,001-$75,000 | 98 (9.1) | 103 (12.6) | 201 (10.6) | |
| $75,001-$100,000 | 55 (5.1) | 48 (5.9) | 103 (5.4) | |
| >$100,001 | 82 (7.6) | 58 (7.1) | 140 (7.4) | |
| Medical Insurance, N (%) | ||||
| None | 48 (4.0) | 47 (5.0) | 0.2 | 95 (4.5) |
| Medicare, Medicaid or government-sponsored | 826 (69.4) | 618 (66.2) | 1,444 (68.0) | |
| Private | 316 (26.6) | 268 (28.7) | 584 (27.5) |
Missing values were not included in the comparison. The p-values reported represent strength of associations between variables.
Disease and treatment characteristics
Most participants (male and female) had the hemoglobin SS genotype and received care from hematologists. The prevalence of hydroxyurea use was higher in males (55.4% vs. 44.6%, p <0.0001) (Table 2). Females had worse ASCQ-Me pain frequency and severity scores (p = 0.0002 and <0.0001, respectively) and had a higher rate of 3 or more admissions in the past year (30.9% vs 25.5, p = 0.03) (Table 2). Females and males had similar rates of taking opioids (80.4% vs. 78.9%). Males had significantly more skin ulcers and respiratory, musculoskeletal, genitourinary and cardiovascular complications. In contrast, females were more likely to have anxiety, depression and autoimmune diseases.
Table 2. Disease characteristics.
| Characteristic | Females (N = 1190) | Males (N = 934) | P-value |
|---|---|---|---|
| Sickle cell genotype, N (%) | |||
| Hb SS | 805 (67.7) | 642 (68.7) | 0.4 |
| Hb SC | 255 (21.4) | 194 (20.8) | |
| Hb S beta+ thalassemia | 75 (6.3) | 45 (4.8) | |
| Hb S beta0 thalassemia | 40 (3.4) | 43 (4.6) | |
| Other variants | 14 (1.2) | 10 (1.1) | |
| Hydroxyurea use, N (%) | |||
| Currently using | 519 (44.6) | 511 (55.4) | <0.0001 |
| Opioid Use, N (%) | |||
| Currently using opioids | 957 (80.4) | 737 (78.9) | 0.4 |
| Number of vaso-occlusive episodes in the past year, N (%) | |||
| 0 | 140 (11.8) | 139 (15.0) | 0.01 |
| 1 | 109 (9.2) | 109 (11.7) | |
| 2 | 151 (12.7) | 134 (14.4) | |
| 3 | 196 (16.5) | 147 (15.8) | |
| 4 or more | 590 (49.7) | 400 (43.1) | |
| Time since most recent vaso-occlusive episode, N (%) | |||
| Currently having one | 150 (12.6) | 108 (11.6) | 0.1 |
| <1 week ago | 204 (17.2) | 127 (13.6) | |
| 1–3 weeks ago | 255 (21.5) | 189 (20.3) | |
| 1–6 months ago | 319 (26.9) | 257 (27.6) | |
| 7–11 months ago | 73 (6.2) | 63 (6.8) | |
| 1–5 years ago | 119 (10.0) | 120 (12.9) | |
| 5+ years ago | 47 (4.0) | 46 (4.9) | |
| Never had a pain attack | 19 (1.6) | 21(2.3) | |
| ASCQ-Me Pain Episode frequency T-score, mean (SD) | 49.2 (11.1) | 47.4 (11.7) | 0.0002 |
| ASCQ-Me Pain Episode severity T-score, mean (SD) | 51.6 (9.6) | 49.3(10) | <0.001 |
| Prior History of Blood Transfusion, N (%) | 1195 (64.0) | 672 (65.4) | 0.2 |
| Number of hospital admissions in the past year, N (%) | |||
| 0 | 344 (35.0) | 318 (41.6) | 0.03 |
| 1 | 215 (21.9) | 172 (22.5) | |
| 2 | 120 (12.2) | 80 (10.5) | |
| 3 | 84 (8.6) | 50 (6.5) | |
| 4+ | 219 (22.3) | 145 (19.0) | |
| Specialists involved in care, N (%) | |||
| PCP only | 26 (2.2) | 8 (0.9) | 0.049 |
| Hematologist only | 700 (59.4) | 579 (62.9) | |
| Co-management (PCP & Hematologist) | 442 (37.5) | 329 (35.7) | |
| Other | 10 (0.8) | 5 (0.5) | |
| Sickle cell disease related complications, N (%) | |||
| Acute chest syndrome | 658 (55.4) | 581 (62.3) | 0.001 |
| Asthma | 319 (26.9) | 253 (27.1) | 0.9 |
| Digestivea | 729 (61.3) | 544 (58.4) | 0.1 |
| Musculoskeletalb | 351 (29.5) | 332 (35.6) | 0.003 |
| Autoimmune/Inflammatoryc | 228 (19.6) | 141 (15.5) | 0.01 |
| Central nervous systemd | 200 (16.8) | 162 (17.3) | 0.7 |
| Genitourinarye | 84 (7.1) | 304 (32.6) | <0.001 |
| Cardiovascularf | 138 (11.6) | 160 (17.1) | 0.0003 |
| Multi-organ failure | 62 (5.2) | 49 (5.2) | 0.9 |
| Pneumococcal sepsis | 40 (3.4) | 47 (5.0) | 0.06 |
| Skin ulcers | 33 (2.8) | 54 (5.8) | 0.0005 |
| Retinopathy | 165 (13.9) | 145 (15.5) | 0.3 |
| Diabetes mellitus | 28 (2.4) | 20 (2.1) | 0.7 |
| Iron overload | 308 (25.9) | 226 (24.2) | 0.4 |
| Chronic refractory pain | 263 (22.1) | 184 (19.7) | 0.2 |
| Anxiety | 174 (14.6) | 104 (11.1) | 0.02 |
| Depression | 259 (21.8) | 154 (16.5) | 0.002 |
| Cancer | 4 (0.3) | 4 (0.4) | 0.7 |
Missing values were not included in the comparison. The p-values reported represent strength of associations between variables.
aDigestive complications: splenomegaly, splenic sequestration, splenic infarcts, hypersplenism, autosplenectomy, gallstones and cholecystitis.
bMusculoskeletal complications: dactylitis, avascular necrosis and osteomyelitis.
cAutoimmune/Inflammatory complications: deep venous thrombosis, lupus, rheumatoid arthritis, gout and sarcoidosis.
dCentral nervous system complications: stroke and intracranial bleeding.
eGenitourinary complications: priapism, chronic kidney disease and end stage renal failure.
fCardiovascular complications: pulmonary arterial hypertension and left ventricular dysfunction.
On multivariable analysis, males had higher odds of acute chest syndrome (odds ratio (OR) 1.4, p = 0.002; Table 3), cardiovascular (OR 1.70, p<0.001) and musculoskeletal (OR 1.33, p = 0.0034) complications and lower odds of depression (OR 0.77, p = 0.0381). Unemployed females had higher odds of anxiety (OR = 1.72, p = 0.0049).
Table 3. Association between participant demographics and sickle cell disease complications.
| Variable | P value | OR (95% CI) |
|---|---|---|
| Acute chest syndrome | ||
| Age | < .0001 | 0.96 (0.95–0.97) |
| Sex | 0.002 | |
| Female (ref) | ||
| Male | 1.4 (1.14–1.73) | |
| Hydroxyurea use | 0.05 | |
| No (Ref) | ||
| Yes | 0.8 (0.64–0.99) | |
| Medical insurance | < .0001 | |
| Private (Ref) | ||
| None | 0.92 (0.54–1.55) | |
| Medicare, Medicaid or government-sponsored | 1.8 (1.44–2.27) | |
| SCD genotype | < .001 | |
| Hb SC (Ref) | ||
| Hb SS | 1.73 (1.33–2.26) | |
| Hb S beta+ thalassemia | 0.59 (0.35–0.96) | |
| Hb S beta0 thalassemia | 2.04 (1.14–3.7) | |
| Other variants | 1.04 (0.41–2.6) | |
| Cardiovascular complications | ||
| Age | < .0001 | 1.06 (1.04–1.08) |
| Sex | < .0001 | |
| Female (Ref) | ||
| Male | 1.70 (1.32–2.21) | |
| Medical Insurance | < .0001 | |
| Private (Ref) | ||
| None | 1.17 (0.46–2.56) | |
| Medicare, Medicaid or government-sponsored | 1.93 (1.40–2.72) | |
| SCD genotype | 0.003 | |
| Hb SC (Ref) | ||
| Hb SS | 2.68 (1.82–4.09) | |
| Hb S beta+ thalassemia | 0.95 (0.37–2.14) | |
| Hb S beta0 thalassemia | 1.82 (0.77–3.93) | |
| Other variants | 1.79 (0.40–5.72) | |
| Musculoskeletal complications | ||
| Age | < .0001 | 1.05 (1.03–1.06) |
| Sex | 0.0034 | |
| Female (Ref) | ||
| Male | 1.33 (1.10–1.62) | |
| SCD genotype | 0.006 | |
| Hb SC (Ref) | ||
| Hb SS | 1.57 (1.21–2.05) | |
| Hb S beta+ thalassemia | 1.05 (0.64–1.70) | |
| Hb S beta0 thalassemia | 1.46 (0.85–2.46) | |
| Other variants | 0.81 (0.26–2.12) | |
| Hydroxyurea current use | 0.0083 | |
| No (Ref) | ||
| Yes | 0.76 (0.63–0.93) | |
| Employment | 0.0006 | |
| Employed (Ref) | ||
| Not employed by choice | 1.63 (1.23–2.15) | |
| Not employed, other | 1.41 (1.13–1.76) | |
| Depression | ||
| Age | 0.02 | 1.02 (1.00–1.04) |
| Sex | 0.0381 | |
| Female (Ref) | ||
| Male | 0.77 (0.60–0.99) | |
| Medical Insurance | 0.03 | |
| Private (Ref) | ||
| None | 0.56 (0.23–1.20) | |
| Medicare, Medicaid or government-sponsored | 1.33 (0.98–1.82) | |
| Employment | 0.02 | |
| Not employed, other (Ref) | ||
| Employed | 0.514 | 1.14 (0.77–1.66) |
| Not employed by choice | 0.006 | 1.50 (1.12–2.00) |
| Anxiety | ||
| Employment*sex | 0.02 | |
| Not employed by choice vs Employed gender = Female | 0.8811 | 0.97 (0.60–1.54) |
| Not employed, other vs Employed gender = Female | 0.0049 | 1.72 (1.18–2.51) |
| Not employed by choice vs Employed gender = Male | 0.1717 | 1.46 (0.85–2.51) |
| Not employed, other vs Employed gender = Male | 0.9066 | 1.03 (0.63–1.69) |
Model includes all variables with p<0.1 in univariate analysis.
Interaction between sex and other covariates were not significant and were eliminated from the final model.
Concerning laboratory measures, males had significantly higher blood urea nitrogen (BUN), serum creatinine and liver enzymes (aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP) and albumin (Table 4). Reticulocyte count, white blood cell count and differentials (neutrophils and monocytes) were similar in both sexes. Males had significantly higher mean steady state hemoglobin (10 g/dl vs 9.3 g/dl, p<0.0001). Female participants had significantly higher fetal hemoglobin levels with and without hydroxyurea use (9.6% vs 8.5%, p = 0.03 and 3% vs 2.2%, p = 0.0005, respectively).
Table 4. Laboratory measures.
| Laboratory measure | Females | Males | P-value | ||
|---|---|---|---|---|---|
| n | Median or mean (Q1-Q2 or SD) | n | Median or mean (Q1-Q2 or SD) | ||
| Hemoglobin g/dl, mean (SD) | 1183 | 9.3 (1.70) | 930 | 10.0 (2.10) | <0.0001 |
| Hb A2%, median (Q1-Q2) | 921 | 3.5 (3.0–4.1) | 725 | 3.6 (3.0–4.5) | 0.1 |
| Hb F % with hydroxyurea use, median (Q1-Q2) | 445 | 9.6 (5–16.9) | 451 | 8.5 (3.5–16) | 0.03 |
| Hb F % without hydroxyurea use, median (Q1-Q2) | 521 | 3 (1.3–8) | 306 | 2.2 (1–5.4) | 0.0005 |
| Platelet count 109/l, mean (SD) | 1180 | 345 (141.0) | 926 | 337 (149.0) | 0.2 |
| MCV fl, mean (SD) | 1179 | 88.9 (13.5) | 927 | 90.1 (14.5) | 0.07 |
| MCH pg, mean (SD) | 1182 | 31.0 (5.9) | 928 | 31.5 (5.6) | 0.1 |
| MCHC g/dl, mean (SD) | 1175 | 34.6 (1.5) | 925 | 34.7 (1.6) | 0.1 |
| Reticulocyte Count 103/mm3, median (Q1-Q2) | 588 | 0.5 (0.1–2) | 439 | 0.5 (0.1–2) | 0.15 |
| White Blood Cells 103/mm3, mean (SD) | 1180 | 10.6 (4.5) | 929 | 10.1 (4.1) | 0.01 |
| Neutrophils segmented and band %, mean (SD) | 1116 | 55.9 (12.5) | 889 | 53.4 (12.8) | <0.0001 |
| Lymphocytes %, mean (SD) | 1128 | 31.7 (11.4) | 902 | 31.9 (11.8) | 0.7 |
| Monocytes %, mean (SD) | 1127 | 8.5 (4.5) | 898 | 9.8 (5.1) | <0.0001 |
| Serum BUN mg/dL, median (Q1-Q2) | 1155 | 7.0 (5.0–9.0) | 910 | 9.0 (7–11) | <0.0001 |
| Serum Creatinine mg/dL, median (Q1-Q2) | 1158 | 0.6 (0.5–0.7) | 916 | 0.8 (0.6–0.9) | <0.0001 |
| Bilirubin serum total mg/dL, median (Q1-Q2) | 1153 | 2.0 (1.2–3.2) | 912 | 2.4 (1.5–4.1) | <0.0001 |
| Bilirubin serum direct mg/dL, median (Q1-Q2) | 602 | 0.4 (0.3–0.7) | 460 | 0.4 (0.3–0.7) | 0.8 |
| AST U/L, median (Q1-Q2) | 1150 | 31.0 (22.0–45.0) | 907 | 35.0 (26.0–49.0) | <0.0001 |
| ALT U/L, median (Q1-Q2) | 1150 | 19.0 (13.0–29.0) | 908 | 22.0 (15.0–31.0) | <0.0001 |
| Alkaline Phosphatase U/L, median (Q1-Q2) | 1153 | 75.0 (60.0–100.0) | 907 | 89.0 (70.0–116.0) | <0.0001 |
| Total Protein g/dL, mean (SD) | 1146 | 7.6 (0.70) | 906 | 7.7 (0.7) | 0.07 |
| Albumin g/dL, mean (SD) | 1146 | 4.1 (0.50) | 902 | 4.3 (0.5) | <0.0001 |
Missing values were not included in the comparison. The p-values reported represent strength of associations between variables.
Discussion
This study identified important sex differences in disease characteristics and complications. In this cohort of 2124 people, females had higher fetal hemoglobin levels despite reporting less hydroxyurea use than males. Females also had more opioid use, significantly worse ASCQ-Me pain frequency and severity scores, and more VOEs and hospitalizations. Despite more hydroxyurea use and less hospitalizations, males had more life-threatening complications; while females had higher rates of anxiety, depression and autoimmune diseases.
Recurrent occurrences of VOEs are the hallmark presentation of SCD and are the leading cause of hospitalization [27]. Almost half of our participants reported having four or more VOEs in the past year, majority of whom were female. A prospective cohort study on life expectancy and risk factors for early death in SCD by Platt et al [1] revealed that patients with SCD have an average of two or more VOEs per year in the absence of treatment [1]. In our study, females reported worse ASCQ-Me pain frequency and severity scores and were taking more opioids. The higher pain and opioid usage in females may be explained by previous literature which identified an increase in VOEs at different stages of the menstrual cycle [28–30]. In addition to the increase of VOEs with menstruation, a study by Brandow et al [31] revealed that females with SCD expressed more neuropathic pain than males [31]. Further investigations on the assessment of pain and the influence of hormonal and physiological changes related to menstruation and the impact they may have on the frequency of VOEs in females with SCD are warranted.
In our registry, more males self-reported taking hydroxyurea than females; however, females had higher levels of fetal hemoglobin. Hydroxyurea use increases levels of fetal hemoglobin and has been associated with a reduction in blood transfusion dependency and mortality in SCD [32]. In addition, higher fetal hemoglobin levels has been associated with increased survival in SCD [1]. Our finding of elevated fetal hemoglobin in females, with and without hydroxyurea use, may contribute to the increased survival in women with SCD. Further studies are needed to determine other causes of elevated fetal hemoglobin in females. Despite the elevated fetal hemoglobin, females reported lower hydroxyurea use. Reasons for lower hydroxyurea use among females identified in the literature include the proven teratogenic effects of the drug in animals; which deters use during pregnancy [33] and has led to recommendations to discontinue hydroxyurea when attempting to become pregnant, during pregnancy or while breast feeding [13]. A key consideration that needs to be taken into account in the interpretation of our findings is that our data on hydroxyurea was self-reported and lacks indicators of adherence. The possibility that males self-reported hydroxyurea use, but may not be adherent cannot be ruled out. Additional investigations into reasons for less hydroxyurea use in females and the association between self-reported hydroxyurea use and adherence in SCD populations are warranted.
Except for autoimmune diseases, females had significantly less prevalence of chronic end-organ complications than their male counterparts [34]. A study by Ngo et al [34] revealed a higher prevalence of autoimmune diseases in females compared to males [34]. The higher prevalence may be attributed to gender specific hormones and the role of those hormones during the various reproductive stages of a woman [34]. Flare up of autoimmune diseases such as systemic lupus erythematosus are influenced by the age of menarche, menopause or the use of oral contraceptives [34]. Gender-specific hormones may account for the higher occurrence of autoimmune complications among females in our registry.
Males had significantly more complications associated with increased mortality, including skin ulcers, acute chest syndrome, musculoskeletal, genitourinary and cardiovascular complications. Our findings are also consistent with Platt et al [1] findings that males have a lower median age at death than females and individuals with symptomatic SCD have higher mortality [1]. For instance, respiratory complications, such as acute chest syndrome, are the leading cause of morbidity and mortality amongst individuals with SCD [35], and more males in our sample had a diagnosis of acute chest syndrome. Males in our sample also had significantly more skin ulcers than females, which has also been previously reported [36]. The higher odds of SCD complications in males could be associated with the higher lab values in males, such as liver enzymes. Although lab values were within normal ranges, this may suggest a higher pro-inflammatory state among men, which has been well reported in SCD [37,38]. Close monitoring of lab values, particularly among males, is warranted to screen for and prevent the occurrence of disease complications that lead to early mortality. Our results support the need for further research to uncover the reasons for a higher incidence of chronic end-organ damage amongst males with SCD and determine if specific social and medical interventions may improve the lifespan of males living with SCD.
Importantly, females had more frequent diagnoses of anxiety and depression than males, consistent with the general population [39,40]. In 2008, using data from the Pain in Sickle Cell Epidemiology Study (PiSCES) study, Levenson et al [41] found that individuals with SCD reporting depressive symptoms and high anxiety had significantly more incidence of pain and also experienced more distress and interference from pain in their daily lives [41]. This is consistent with our findings that females with SCD had significantly more frequent and severe pain in addition to depression and anxiety diagnoses compared to males with SCD. Depression and anxiety is known to exacerbate pain and other physical complications of SCD [41]. In addition to impacts on physical and mental quality of life, depression has also been associated with a significant increase in total health care costs for individuals with SCD ($13,016 for no depression diagnosis vs $30,665 for depression diagnosis p = 0.01) [42]. Despite higher rates of depression and anxiety, we found females were more likely to have higher education. Interestingly, we also found females reported a lower household income than male participants with SCD. Income inequality has been associated with an increased risk of depression [43].
Our study has several limitations. All the individuals with SCD that participated in the study were recruited through comprehensive SCD care settings where they receive care from sickle cell specialists. Thus, this sample is not representative of individuals who do not have access to SCD experts. Only individuals that had literacy in English were included and our findings are not generalizable to individuals who have low literacy levels or who are non-English speaking. Next, we did not collect information on psychosocial factors (e.g. social support) and lifestyle factors (e.g. smoking, alcohol, diet, exercise) that are linked to quality of life and lifespan [20,44–46]. We were also unable to account for the impact that gender socialization may have on individuals’ behaviors, such as disease self-management. For instance, prior studies have associated femininity with engagement in health promotion [47]. Findings from a recent study by Blake et al [48] suggest that health promotion in SCD is linked to gender socialization; as parents or parental proxies of adolescents with SCD tended to underestimate their male adolescents emotional and social functioning, while overestimating for female adolescents.46 Finally, this study is limited due to the use of a cross-sectional design. The timing of individuals completing the surveys and having their medical records abstracted may not be representative of the sex-based differences in the manifestations and complications of SCD that exists across the lifespan. We abstracted data entered by healthcare providers in the participant’s medical records and could not fully ascertain that there was a gender differential in the measurements and recording of those data in the participants’ medical records.
Despite those limitations, our study has a large sample size and utilizes data from a consortium with eight geographically diverse comprehensive SCD care settings and continues to follow these participants through the prospective registry. Our sample size had sufficient power to detect statistically significant differences between males and females. Additionally, validated instruments were used to collect self-report data.
Conclusion
Our findings suggest key sex differences in the presentation of SCD, with males having more life-threatening chronic end-organ complications and females having higher rates of depression and anxiety. Future research is required to determine how sex influences the mechanisms underpinning clinical outcome differences.
Supporting information
(DOCX)
Acknowledgments
The authors would like to thank all participants who enrolled in this study. We would also like to thank the SCDIC steering committee, the publications committee, the data coordinating center, the study centers and study coordinators.
The SCDIC is governed by the SCDIC steering committee. Members of the steering committee are:
Chair: Alexis Thompson, MD, PhD, Northwestern University Email: a-thompson@northwestern.edu;
Center PI: Robert Gibson, PhD, Augusta University
Center PI: Victor R. Gordeuk, MD, University of Illinois at Chicago
Center PI: Jane Hankins, MD, MS, St. Jude Children’s Research Hospital
Center PI: Allison King, MD, PhD, MPH, Washington University School of Medicine
Center PI: Lynne D. Richardson, MD, FACEP, Icahn School of Medicine at Mount Sinai
Center Co-PI: Paula Tanabe, PhD, MSN, MPH, RN, FAEN, FAAN, Duke University
Center Co-PI: Nirmish Shah, MD, Duke University
Center Co-PI: Marsha Treadwell, PhD, UCSF Benioff Children’s Hospital Oakland
Center Co-PI: Julia Washko (Kanter), MD, Medical University of South Carolina
DCC PI: Barbara L. Kroner, PhD, MPH, RTI International
Program Officer: Phil Tonkins, PhD, National Heart, Lung and Blood Institute
Data Availability
We have ethical restrictions about openly releasing the data set to the public as the nature of the data set would result in loss of participant anonymity. The ethical restrictions were imposed by the Sickle Cell Disease Implementation Consortium (SCDIC). However, data set requests can be made to SCDIC and their data coordinating center at RTI international. Requests will be reviewed by the SCDIC Publications Committee. Data set requests can be sent to SCDIC at scdic-publications-subcommittee@rtiresearch.org or +1 301-230-4674.
Funding Statement
The SCD Implementation Consortium was supported by US Federal Government cooperative agreements 3U01HL133964-04S1, U24HL133948, U01HL133964, U01HL133990, U01HL133996, U01HL133994, U01HL133997, U01HL134004, U01HL134007, and U01HL134042 from the National Heart Lung and Blood Institute and the National Institute on Minority Health and Health Disparities (Bethesda, MD).
References
- 1.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality In Sickle Cell Disease—Life Expectancy and Risk Factors for Early Death. N Engl J Med Boston [Internet]. 1994. Jun 9 [cited 2019 Apr 1];330(23):1639–44. Available from: https://search.proquest.com/docview/1983834366/abstract/E1B961FE1B144C89PQ/1. [DOI] [PubMed] [Google Scholar]
- 2.Hassell KL. Population Estimates of Sickle Cell Disease in the U.S. Am J Prev Med [Internet]. 2010. Apr 1 [cited 2020 Jan 14];38(4, Supplement):S512–21. Available from: http://www.sciencedirect.com/science/article/pii/S074937970900960X. [DOI] [PubMed] [Google Scholar]
- 3.Noguchi CT, Schechter AN, Rodgers GP. 3 Sickle cell disease pathophysiology. Baillières Clin Haematol [Internet]. 1993. Mar 1 [cited 2020 Jan 14];6(1):57–91. Available from: http://www.sciencedirect.com/science/article/pii/S0950353605800666. [DOI] [PubMed] [Google Scholar]
- 4.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol [Internet]. 2009. [cited 2020 Jan 14];84(9):618–25. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conran N, Franco-Penteado CF, Costa FF. Newer Aspects of the Pathophysiology of Sickle Cell Disease Vaso-Occlusion. Hemoglobin [Internet]. 2009. Jan 1 [cited 2020 Jan 14];33(1):1–16. Available from: 10.1080/03630260802625709. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg MH. Management of Sickle Cell Disease. N Engl J Med [Internet]. 1999. Apr 1 [cited 2020 Jan 14];340(13):1021–30. Available from: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 7.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999–2009). Pediatr Blood Cancer [Internet]. 2013. [cited 2020 Jan 14];60(9):1482–6. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- 8.DeBaun MR, Ghafuri DL, Rodeghier M, Maitra P, Chaturvedi S, Kassim A, et al. Decreased median survival of adults with sickle cell disease after adjusting for left truncation bias: a pooled analysis. Blood [Internet]. 2019. Feb 7 [cited 2020 May 4];133(6):615–7. Available from: https://ashpublications.org/blood/article/133/6/615/260539/Decreased-median-survival-of-adults-with-sickle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamkiewicz TV, Silk BJ, Howgate J, Baughman W, Strayhorn G, Sullivan K, et al. Effectiveness of the 7-Valent Pneumococcal Conjugate Vaccine in Children With Sickle Cell Disease in the First Decade of Life. Pediatrics [Internet]. 2008. Mar 1 [cited 2020 Jan 14];121(3):562–9. Available from: https://pediatrics.aappublications.org/content/121/3/562. [DOI] [PubMed] [Google Scholar]
- 10.Halasa NB, Shankar SM, Talbot TR, Arbogast PG, Mitchel EF, Wang WC, et al. Incidence of Invasive Pneumococcal Disease among Individuals with Sickle Cell Disease before and after the Introduction of the Pneumococcal Conjugate Vaccine. Clin Infect Dis [Internet]. 2007. Jun 1 [cited 2020 Jan 14];44(11):1428–33. Available from: https://academic.oup.com/cid/article/44/11/1428/476499. [DOI] [PubMed] [Google Scholar]
- 11.Gaston MH, Verter JI, Woods G, Pegelow C, Kelleher J, Presbury G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986. Jun 19;314(25):1593–9. doi: 10.1056/NEJM198606193142501 [DOI] [PubMed] [Google Scholar]
- 12.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, et al. Effect of Hydroxyurea on Mortality and Morbidity in Adult Sickle Cell Anemia: Risks and Benefits Up to 9 Years of Treatment. JAMA [Internet]. 2003. Apr 2 [cited 2020 Jan 14];289(13):1645–51. Available from: https://jamanetwork.com/journals/jama/fullarticle/196300. [DOI] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute (NHLBI). Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014 | National Heart, Lung, and Blood Institute (NHLBI) [Internet]. 2014 [cited 2020 Jan 20]. https://www.nhlbi.nih.gov/health-topics/all-publications-and-resources/evidence-based-management-sickle-cell-disease-expert-0.
- 14.Ballas SK, Pulte ED, Lobo C, Riddick-Burden G. Case series of octogenarians with sickle cell disease. Blood [Internet]. 2016. Nov 10 [cited 2020 Jan 14];128(19):2367–9. Available from: https://ashpublications.org/blood/article/128/19/2367/35510/Case-series-of-octogenarians-with-sickle-cell. [DOI] [PubMed] [Google Scholar]
- 15.Barton-Gooden A, Grindley M, Knight-Madden J, Asnani M. Gender influences on the health of adolescents with sickle cell disease. Psychol Health Med [Internet]. 2019. Apr 21 [cited 2021 Jul 2];24(4):470–80. Available from: 10.1080/13548506.2018.1533985. [DOI] [PubMed] [Google Scholar]
- 16.Arduini GAO, Trovó de Marqui AB. Prevalence and Characteristics of Priapism in Sickle Cell Disease. Hemoglobin. 2018. Mar;42(2):73–7. doi: 10.1080/03630269.2018.1452760 [DOI] [PubMed] [Google Scholar]
- 17.Boafor TK, Olayemi E, Galadanci N, Hayfron-Benjamin C, Dei-Adomakoh Y, Segbefia C, et al. Pregnancy outcomes in women with sickle-cell disease in low and high income countries: a systematic review and meta-analysis. BJOG Int J Obstet Gynaecol. 2016. Apr;123(5):691–8. doi: 10.1111/1471-0528.13786 [DOI] [PubMed] [Google Scholar]
- 18.Chinegwundoh FI, Smith S, Anie KA. Treatments for priapism in boys and men with sickle cell disease. Cochrane Database Syst Rev [Internet]. 2020. Apr 1 [cited 2021 Jul 2];4:CD004198. Available from: https://europepmc.org/articles/PMC7134865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddad LB, Curtis KM, Legardy-Williams JK, Cwiak C, Jamieson DJ. Contraception for individuals with sickle cell disease: a systematic review of the literature [Internet]. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. Centre for Reviews and Dissemination (UK); 2012. [cited 2021 Jul 2]. http://www.ncbi.nlm.nih.gov/books/NBK98267/. [DOI] [PubMed] [Google Scholar]
- 20.McClish DK, Penberthy LT, Bovbjerg VE, Roberts JD, Aisiku IP, Levenson JL, et al. Health related quality of life in sickle cell patients: the PiSCES project. Health Qual Life Outcomes. 2005. Aug 29;3:50. doi: 10.1186/1477-7525-3-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClish DK, Levenson JL, Penberthy LT, Roseff SD, Bovbjerg VE, Roberts JD, et al. Gender differences in pain and healthcare utilization for adult sickle cell patients: The PiSCES Project. J Womens Health 2002. 2006. Mar;15(2):146–54. [DOI] [PubMed] [Google Scholar]
- 22.DiMartino LD, Baumann AA, Hsu LL, Kanter J, Gordeuk VR, Glassberg J, et al. The Sickle Cell Disease Implementation Consortium: Translating Evidence-Based Guidelines into Practice for Sickle Cell Disease. Am J Hematol [Internet]. 2018. Dec [cited 2020 Jan 14];93(12):E391–5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6503654/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masese RV, DeMartino T, Bonnabeau E, Burns EN, Preiss L, Varughese T, et al. Effective Recruitment Strategies for a Sickle Cell Patient Registry Across Sites from the Sickle Cell Disease Implementation Consortium (SCDIC). J Immigr Minor Health. 2020. Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glassberg JA, Linton EA, Burson K, Hendershot T, Telfair J, Kanter J, et al. Publication of data collection forms from NHLBI funded sickle cell disease implementation consortium (SCDIC) registry. Orphanet J Rare Dis [Internet]. 2020. Jul 7 [cited 2021 Jan 25];15. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7341606/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treadwell MJ, Hassell K, Levine R, Keller S. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): Conceptual Model Based on Review of the Literature and Formative Research. J Pain. 2014. Oct;30(10):902–14. doi: 10.1097/AJP.0000000000000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001. Apr;54(4):343–9. doi: 10.1016/s0895-4356(00)00314-0 [DOI] [PubMed] [Google Scholar]
- 27.Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol [Internet]. 2005. [cited 2020 Jan 28];79(1):17–25. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ajh.20336. [DOI] [PubMed] [Google Scholar]
- 28.de Abood M, de Castillo Z, Guerrero F, Espino M, Austin KL. Effect of Depo-Provera or Microgynon on the painful crises of sickle cell anemia patients. Contraception. 1997. Nov;56(5):313–6. doi: 10.1016/s0010-7824(97)00156-x [DOI] [PubMed] [Google Scholar]
- 29.Smith-Whitley K. Reproductive issues in sickle cell disease. Blood [Internet]. 2014. Dec 4 [cited 2020 Mar 18];124(24):3538–43. Available from: https://ashpublications.org/blood/article/124/24/3538/33523/Reproductive-issues-in-sickle-cell-disease. [DOI] [PubMed] [Google Scholar]
- 30.Yoong WC, Tuck SM. Menstrual pattern in women with sickle cell anaemia and its association with sickling crises. J Obstet Gynaecol J Inst Obstet Gynaecol. 2002. Jul;22(4):399–401. doi: 10.1080/01443610220141362 [DOI] [PubMed] [Google Scholar]
- 31.Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer. 2014. Mar;61(3):512–7. doi: 10.1002/pbc.24838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010. Jun;3(3):255–60. doi: 10.1586/ehm.10.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballas SK, McCarthy WF, Guo N, DeCastro L, Bellevue R, Barton BA, et al. Exposure to Hydroxyurea and Pregnancy Outcomes in Patients With Sickle Cell Anemia. J Natl Med Assoc [Internet]. 2009. Oct [cited 2020 Mar 18];101(10):1046–51. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0027968415310725. [DOI] [PubMed] [Google Scholar]
- 34.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol [Internet]. 2014. Aug 1 [cited 2021 Jul 2];35(3):347–69. Available from: https://www.sciencedirect.com/science/article/pii/S0091302214000466. [DOI] [PubMed] [Google Scholar]
- 35.Farooq S, Abu Omar M, Salzman GA. Acute chest syndrome in sickle cell disease. Hosp Pract [Internet]. 2018. May 27 [cited 2020 Mar 24];46(3):144–51. Available from: https://www.tandfonline.com/doi/full/10.1080/21548331.2018.1464363. [DOI] [PubMed] [Google Scholar]
- 36.Minniti CP, Eckman J, Sebastiani P, Steinberg MH, Ballas SK. Leg ulcers in sickle cell disease. Am J Hematol [Internet]. 2010. Jul 23 [cited 2020 Mar 24];85(10):831–3. Available from: http://doi.wiley.com/10.1002/ajh.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ataga KI, Moore CG, Hillery CA, Jones S, Whinna HC, Strayhorn D, et al. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica [Internet]. 2008. Jan 1 [cited 2020 Mar 26];93(1):20–6. Available from: http://www.haematologica.org/cgi/doi/10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 38.Torres LS, Okumura JV, Silva DGH, Mimura KKO, Belini-Júnior É, Oliveira RG, et al. Inflammation in Sickle Cell Disease: Differential and Down-Expressed Plasma Levels of Annexin A1 Protein. Connes P, editor. PLOS ONE [Internet]. 2016. Nov 1 [cited 2020 Mar 26];11(11):e0165833. Available from: http://dx.plos.org/10.1371/journal.pone.0165833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender Differences in Anxiety Disorders: Prevalence, Course of Illness, Comorbidity and Burden of Illness. J Psychiatr Res [Internet]. 2011. Aug [cited 2020 Apr 23];45(8):1027–35. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3135672/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci JPN [Internet]. 2015. Jul [cited 2020 Apr 23];40(4):219–21. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4478054/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levenson JL, McClish DK, Dahman BA, Bovbjerg VE, de A. Citero V, Penberthy LT, et al. Depression and Anxiety in Adults With Sickle Cell Disease: The PiSCES Project: Psychosom Med [Internet]. 2008. Feb [cited 2020 Mar 24];70(2):192–6. Available from: http://journals.lww.com/00006842-200802000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Adam SS, Flahiff CM, Kamble S, Telen MJ, Reed SD, De Castro LM. Depression, quality of life, and medical resource utilization in sickle cell disease. Blood Adv [Internet]. 2017. Oct 24 [cited 2020 Mar 24];1(23):1983–92. Available from: https://ashpublications.org/bloodadvances/article/1/23/1983/15709/Depression-quality-of-life-and-medical-resource. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel V, Burns JK, Dhingra M, Tarver L, Kohrt BA, Lund C. Income inequality and depression: a systematic review and meta-analysis of the association and a scoping review of mechanisms. World Psychiatry [Internet]. 2018. Feb 1 [cited 2020 Mar 29];17(1):76–89. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/wps.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palermo TM, Riley CA, Mitchell BA. Daily functioning and quality of life in children with sickle cell disease pain: relationship with family and neighborhood socioeconomic distress. J Pain. 2008. Sep;9(9):833–40. doi: 10.1016/j.jpain.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin C, Pialoux V, Faes C, Charrin E, Skinner S, Connes P. Does physical activity increase or decrease the risk of sickle cell disease complications? Br J Sports Med. 2018. Feb;52(4):214–8. doi: 10.1136/bjsports-2015-095317 [DOI] [PubMed] [Google Scholar]
- 46.Cohen RT, DeBaun MR, Blinder MA, Strunk RC, Field JJ. Smoking is associated with an increased risk of acute chest syndrome and pain among adults with sickle cell disease. Blood. 2010. May 6;115(18):3852–4. doi: 10.1182/blood-2010-01-265819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayor E. Gender roles and traits in stress and health. Front Psychol [Internet]. 2015. Jun 9 [cited 2021 Jul 2];6:779. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4460297/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blake A, Guthrie-Dixon N, Grindley M, Barton-Gooden A, Knight-Madden J, Asnani M. Level of agreement between adolescents’ self-assessment and parent proxy report of health-related quality of life in adolescents with sickle cell disease. Pediatr Blood Cancer. 2020. Apr;67(4):e28198. doi: 10.1002/pbc.28198 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
We have ethical restrictions about openly releasing the data set to the public as the nature of the data set would result in loss of participant anonymity. The ethical restrictions were imposed by the Sickle Cell Disease Implementation Consortium (SCDIC). However, data set requests can be made to SCDIC and their data coordinating center at RTI international. Requests will be reviewed by the SCDIC Publications Committee. Data set requests can be sent to SCDIC at scdic-publications-subcommittee@rtiresearch.org or +1 301-230-4674.