Abstract
A critical feature of episodic memory is the ability to remember the order of events as they occurred in time, a capacity shared across species including humans, nonhuman primates, and rodents. Accumulating evidence suggests that this capacity depends on a network of structures including the hippocampus and the prefrontal cortex, but their respective contributions remain poorly understood. As addressing this important issue will require converging evidence from complementary investigative techniques, we developed a cross-species, nonspatial sequence memory task suitable for behavioral and neurophysiological studies in rodents and in humans. The task involves the repeated presentation of sequences of items (odors in rats and images in humans) and requires subjects to make a judgment as to whether each item is presented “in sequence” or “out of sequence.” To shed light on the cognitive processes and sequence representations supporting performance, different types of “out of sequence” probe trials were used including: (i) repeating an item from earlier in the sequence (Repeats; e.g., ABAD), (ii) skipping ahead in the sequence (Skips; e.g., ABD), and (iii) inserting an item from a different sequence into the same ordinal position (Ordinal Transfers; e.g., A2CD). We found a remarkable similarity in the performance of rats and humans, particularly in the pattern of results across probe trial types. Thus, the results suggest that rats and humans not only remember the sequences of events, but also use similar underlying cognitive processes and mnemonic representations. This strong cross-species correspondence validates this task for use in future basic and clinical interdisciplinary studies aimed at examining the neural mechanisms underlying episodic memory.
Keywords: sequence memory, temporal context, order, hippocampus, prefrontal cortex
INTRODUCTION
It is well established that the ability to temporally organize information is fundamental to many perceptual, cognitive, and motor processes (Mauk and Buonomano, 2004; Buhusi and Meck, 2005; Merchant et al., 2013). Temporal organization is also a defining feature of episodic memory as the memory for individual events includes information about when they occurred (Tulving, 1972; Clayton and Dickinson, 1998; Eichenbaum and Fortin, 2005; Kesner and Hunsaker, 2010; Jacobs et al., 2013). The ability to remember sequential relationships among events or stimuli is shared by a variety of species including humans (Cabeza et al., 1997; Ekstrom and Bookheimer, 2007; Jenkins and Ranganath, 2010), nonhuman primates (Swartz et al., 1991; Orlov et al., 2000; Templer and Hampton, 2012), and rodents (Hulse and Dorsky, 1979; Chiba et al., 1994; Fortin et al., 2002; Kesner et al., 2002; DeVito and Eichenbaum, 2011). Accumulating evidence suggests that this capacity depends on a network of structures including the hippocampus and the prefrontal cortex (Agster et al., 2002; Fortin et al., 2002; Kesner et al., 2002; Hannesson et al., 2004; Ekstrom and Bookheimer, 2007; DeVito and Eichenbaum, 2011), and that these fundamental circuits are shared across mammals (Allen and Fortin, 2013). However, despite a wealth of data on the involvement of these structures, the specific nature of their respective contributions, functional interactions, and neuronal mechanisms remains poorly understood.
Solving this problem will require the development of paradigms that can help distinguish potential strategies or processes at the behavioral level, and have a suitable design for cross-species approaches to take advantage of complementary investigative techniques. Although a number of approaches examining the memory for sequences of events have been developed in humans and rodents, none fully satisfy these requirements. Paradigms involving memory for sequences of spatial locations have made important contributions (Chiba et al., 1994; Hopkins et al., 2004; Fouquet et al., 2010). However, these paradigms have the disadvantage of combining spatial and temporal demands, and thus making it impractical to investigate the neural basis of the sequence memory component in isolation. This is particularly problematic for examining the contribution of the hippocampus, a structure known to play a crucial role in spatial processing. In addition, it is important to note that different types of spatial information tend to be used in rodent and human studies (i.e., visited locations in a three-dimensional environment vs. locations on a computer screen, respectively; but see Ekstrom and Bookheimer, 2007), which raises concerns about limitations in cross-species correspondence. Although the use of nonspatial sequence memory tasks circumvents these problems, an integrated approach across species is not yet available. In fact, existing paradigms in rodents (Fortin et al., 2002; Kesner et al., 2002; Hannesson et al., 2004) and humans (Kumaran and Maguire, 2006; Ross et al., 2009) have significant differences in task demands, which preclude a thorough comparative and interdisciplinary examination.
To address this issue, we developed a new cross-species, nonspatial sequence memory task. Critically, our new experimental design features sufficient sampling of each item and sequence, and hence the same paradigm can be used not only in behavioral studies (e.g., lesion studies in rodents, comparing clinical populations in humans), but also in neurophysiological studies (e.g., single-cell recording in rodents, fMRI in humans). The task involves the presentation of sequences of items (pure chemical odorants in rats and fractal images in humans) and requires subjects to make a judgment as to whether each item is presented “in sequence” or “out of sequence.” The task also includes three types of probe trials, which tax different cognitive processes and mnemonic representations. We report that performance is very similar in rats and humans in terms of accuracy, response time (RT), and the response pattern across the different probe trials. These results suggest this form of memory for sequences of events involves comparable cognitive processes and representations in rats and humans, and strongly validates the cross-species sequence task for future interdisciplinary studies.
MATERIALS AND METHODS
This task was developed to test the ability of rats and humans to learn and remember arbitrary sequences of items. This cross-species approach was designed to model the memory for the temporal relationships among events that compose single episodes (Tulving, 1984, 2002; Eichenbaum, 2007; Fig. 1A) and to satisfy the “what–when” aspect of episodic memory (Allen and Fortin, 2013). Briefly, rats and humans were presented with sequences of items (e.g., odors ABCD in rats and images ABCDEF in humans) and had to indicate whether each item was “in sequence” (InSeq; e.g., B correctly presented after A) or “out of sequence” (OutSeq; e.g., C incorrectly presented after A) by holding a response for >1s or <1s, respectively. The fundamental task demands were matched in rats and humans, and, importantly, species-appropriate stimuli (odors in rats and images in humans) and responses (a nose poke in rats and a key press in humans) were used (Fig. 1A). The sequence length and the number of sequences was larger in humans to match overall performance levels with rats (determined by pilot studies), allowing fair comparisons on different OutSeq probe trials.
FIGURE 1.
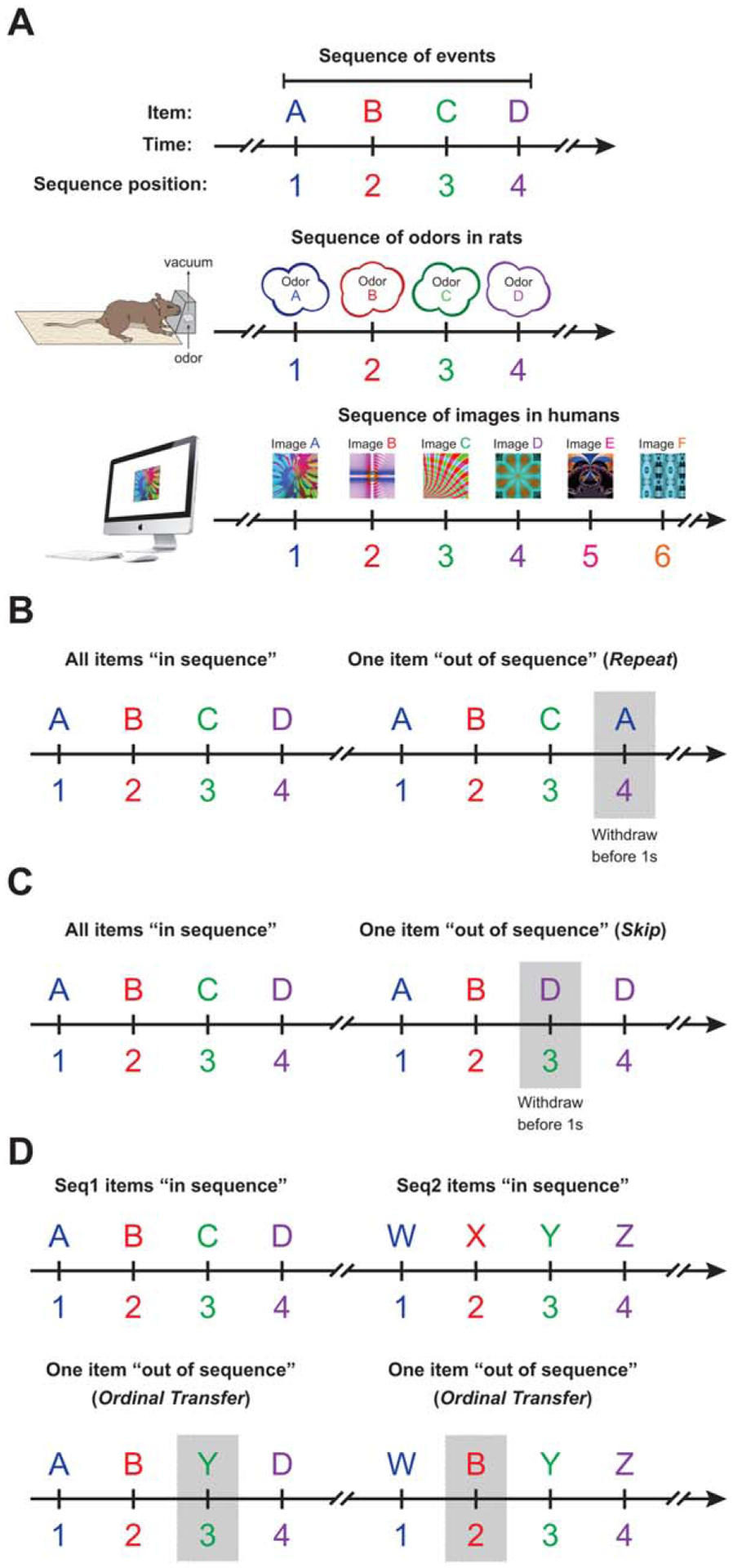
Behavioral design of the cross-species sequence task. A: The task was designed to capture the “flow of events” aspect of episodic memory (Tulving, 1972, 1984, 2002), which conceptualizes an episode as a sequence of events segmented in time. In rats, sequences of four odors were presented through a single-odor port. In humans, sequences of six fractal images were presented in a single location on a computer monitor. Each sequence was presented multiple times within a testing session: 50% of the time the sequence was presented with all items “in sequence” (InSeq) and 50% of the time one item was presented “out of sequence” (OutSeq). Subjects were required to identify each item as InSeq (by holding a response for >1 s) or OutSeq (by holding for <1 s). Three different types of OutSeq probe trials were used during testing (Repeats, Skips, and Ordinal Transfers), which involve different cognitive processes and sequence representations. B: Repeats occurred when an earlier item was presented a second time in the sequence (e.g., ABCA). Repeats can be detected with multiple cognitive strategies and were thus used to define the upper limit of the ability to identify OutSeq items. C: Skips occurred when an item was presented too early in the sequence (e.g., ABD, which skips over item C). Detecting Skips requires accurate predictions of upcoming items and thus performance on these probe trials was used as a sensitive measure of detailed sequence memory. D: Ordinal Transfers occurred when an item from one sequence (e.g., WXYZ) was transferred to another sequence (e.g., ABCD) while retaining the item’s original ordinal position (e.g., ABYD). Ordinal Transfers were used to help identify the type of sequence representations supporting task performance (i.e., sequential item–item associations or item-in-position associations; see Fig. 3C for details).
Subjects
Rats
Twenty male Long–Evans rats, weighing approximately 350–500 g, were used as subjects (Charles River Laboratories, San Diego, CA). Rats were individually housed on an inverse 12-h light/dark cycle, with all training and testing conducted during the dark phase. Rats had free access to food, but access to water was limited to 2–10 min each day after behavioral training. Rats received 3–6 mL of additional water as a reward during daily behavioral sessions (weekdays). On weekends, rats received full access to water for at least 12 h to ensure adequate overall hydration. Hydration levels were monitored daily. All procedures were conducted in accordance with the University of California, Irvine Institutional Animal Care and Use Committee.
Humans
Twenty-four healthy young adults were recruited from the undergraduate population at the University of California, Irvine. Subjects were 18–22 years old (mean = 19.8, SD = 1.2) and included both males and females (M = 7, F = 17). All subjects were compensated for their time. Written consent was obtained in compliance with the University of California, Irvine Institutional Review Board.
Behavioral Equipment and Stimuli
Rats
Subjects were tested in a quiet experimental room with custom-made equipment capable of repeated deliveries of multiple distinct odors. The apparatus consisted of a linear track (length, 150 cm; width, 9 cm), with walls angled outward (30° from vertical; height, 40 cm) and water ports on both ends of the track. A single-odor port was located on one end of the track. The odor port was equipped with photobeam sensors to precisely detect the nose entries and was connected to an odor-delivery system (www.med-associates.com). Timing boards (www.plexon.com) and digital I/O devices (www.ni.com) were used to measure the RTs and to control the hardware. All aspects of the task were automated using custom MATLAB scripts (www.mathworks.com).
Each odor consisted of a pure chemical odorant volatilized with nitrogen and diluted with pure air (flow rate, 2L/min). Eight odors were selected from the Glomerular Activity Response Archive (http://gara.bio.uci.edu/): 1-octanol (Odor A; “sweet”), l-menthone (Odor B; “mint”), acetophenone (Odor C; “rubber”), isobutanol (Odor D; “must”), 5-methyl-2-hexanone (Odor W; “fruit”), beta-pinene (Odor X; “pine”), l-limonene (Odor Y; “lemon”), and l-carvone (Odor Z; “herbaceous”). To prevent crosscontamination, separate Teflon tubing lines were used for each odor, which converged in a single channel at the bottom of the odor port. In addition, an air vacuum located at the top of the odor port provided a constant negative pressure to quickly evacuate odor traces. Readings from a volatile organic compound detector confirmed that odors were cleared from the port 500–750 ms after odor delivery (interodor delay was 1,600 ms on average and limited by software to a minimum of 800 ms).
Each odor presentation was initiated by a nose poke (provided 800 ms had elapsed since the last odor) and was terminated after the rat either held for 1 s (signaled by a beep) or pulled its nose out. Water rewards were delivered below the odor port after each correct response (10 μL) and at the opposite end of the track after correct completion of a full sequence (20 μL). After an incorrect response, a buzzing sound was emitted and the sequence was terminated. To enhance the segmentation between each odor sequence (completed correctly or not), rats were required to run to the end of the track opposite the odor port before the next sequence could be presented.
Humans
Subjects were tested in a quiet experimental room on a computer using custom-written MATLAB scripts. Image presentations and response measurements were performed using the Psychophysics Toolbox extensions (http://psychtoolbox.org). Each image presentation (15 cm × 15 cm color fractals on a white background; Fig. 1A) was initiated by pressing and holding down the space bar key. Images disappeared when the spacebar was released, or after 1 s of holding, with no performance feedback. The presentation of the next image in the sequence was self-paced, initiated by again pressing and holding down the space bar key. The statement “press the spacebar to begin the next sequence” was displayed on screen after each sequence to facilitate sequence segmentation.
Initial Training
Rats
Naïve rats were trained on the task in a series of incremental stages over approximately 6 weeks. First, rats were trained to poke and hold their nose in the odor port for a water reward. The required poke duration started at 50 ms and was incrementally increased until rats held reliably for 1.2 s (160–256 pokes per session). Animals reached a criterion of 80% correct over three sessions in 16 ± 3 sessions (mean ± standard deviation). Rats were then habituated to odor presentations in the port (odor A, then odors AB) and required to maintain their poke response for 1 s to receive a reward (3 ± 2 sessions). Second, rats were trained to identify InSeq and OutSeq items (40–64 sequences per session). Rats were initially trained on a two-item sequence in which they were presented with “AB” and “AA” sequences in equal proportions. Although the correct response on the first odor was to hold for >1 s (Odor A was always the first item), the second response required animals to determine whether the second item was InSeq (AB; hold for >1 s to receive reward) or OutSeq (AA; withdraw before 1 s to receive reward). After reaching criterion on two-item sequences (10 ± 3 sessions), rats were trained on three-item sequences (8 ± 5 sessions) and four-item sequences (4 ± 3 sessions). Rats tested on Ordinal Transfer probe trials (n = 7; see below) received the same training procedures, with the exception that they were trained on two sequences (ABCD and WXYZ) simultaneously in alternating sessions.
Humans
Subjects were given verbal instructions to hold the space bar for >1 s for items presented InSeq but to release the space bar before 1 s for items presented OutSeq. Subjects were initially trained to perform a control “no-memory” version of the sequence task to become familiar with the basic parameters. The no-memory version used images of easily predictable sequences of items (e.g., the actual letters “ABCDEF”). Subjects passively viewed four sequences of six images and were then given 80 test sequences (sequences randomly presented).
“Out of Sequence” Probe Trials
To help identify the type of sequence representation used to support task performance, we included three types of OutSeq probe trials: (i) Repeats, (ii) Skips, and (iii) Ordinal Transfers (Figs. 1B–D). It should be noted that probe trials could be presented in any sequence position except the first (i.e., sequences always began with an InSeq item).
Repeats
OutSeq items included “Repeat” presentations of an item that occurred earlier in the sequence (e.g., “ABAD”; Fig. 1B). Repeats are the easiest type of probe trials as they can be detected with multiple cognitive strategies. For example, subjects could use different sequential representations to identify Repeats as OutSeq (e.g., “A should not come after B” or “A should not be 3rd in the sequence”), as well as the ability to hold in mind recently presented items (e.g., “A was already presented in this sequence”). Thus, Repeats are used to define the upper limit of the ability to detect OutSeq items. Although we use the n-back terminology to indicate when the item was previously presented in the sequence, it should be noted that the task is not a traditional working memory task because of the familiarity of the items and predictability of the sequences.
Skips
OutSeq items included items that “skip ahead” in the sequence (e.g., “ABD”; Fig. 1C). Detecting Skips requires a precise knowledge of the sequence because identifying an item that appears too early requires one to accurately predict subsequent items. Therefore, Skips were used as a more sensitive measure of detailed sequence memory.
Ordinal Transfers
OutSeq items also included “Ordinal Transfers,” which were used to help identify the type of mnemonic representations of sequences supporting performance on the task. There are at least two types of sequence representations relevant to our paradigm: (i) a directional associative link with the last item (e.g., B leads to C; a sequential item–item association) or (ii) an association between an item and an ordinal position (e.g., B occurs in the second position; an item-in-position association). The degree to which each representation is used can be evaluated by transferring an item from one sequence set (e.g., WXYZ) into a second sequence set (e.g., ABCD), while retaining the ordinal position (e.g., AXCD; Fig. 1D). If sequential item–item associations alone are representing sequential information, then “X” would be judged as OutSeq as “X” never follows “A.” If item-in-position associations alone are representing sequential information, then “X” would be judged as InSeq.
Sequence Testing Stages
The first stage included Repeats and Skips, but no Ordinal Transfers. Data from 400 item presentations were included in both species, which were collected over four sessions in rats but within a single session in humans. Approximately half of the sequences had one OutSeq item, which could either be a Repeat or a Skip (~20 of each type, randomly selected).
All humans and seven rats were tested on a second stage which included all three probe trial types. In this stage, two-thirds of the sequences had one OutSeq item, and an approximate total of 20 Repeats, 20 Skips, and 40 Ordinal Transfers were used.
Statistics
The basic requirement of the cross-species sequence memory task is to hold for ≥1 s for InSeq items and hold for <1 s for OutSeq items. Thus, performance could be measured in three ways: (i) by RT, (ii) by converting RTs to accuracy, expressed in percent of trials correct, and (iii) by comparing expected and observed response frequencies using G-tests. Importantly, the G-test provides a measure of performance that controls for response bias by taking into account that there are fewer OutSeq than InSeq trials. The G-test is a more robust alternative to the Chi-squared test, especially for data sets including cells with smaller frequencies (Sokal and Rohlf, 1995). To calculate G-tests, we used two-by-two tables to compare the frequencies of accurate responses sorted by trial type (InSeq and OutSeq; Table 1). To be included in the analyses, each subject had to demonstrate evidence of sequence memory as determined by a significant overall G-test. Follow-up G-tests were performed to compare the performance levels by position (e.g., Position 3) and by items (e.g., Item C). This allowed us to test memory across the entire sequence (all positions) and for all items in the sequence (Figs. 2A,B, subplots).
TABLE 1.
Data Table Compiling the Frequencies of Each Response Type (in Columns) for Each Trial Type (in Rows) Used in the Calculation of the G-test and SMI
| RT ≥ 1 s | RT < 1 s | |
|---|---|---|
| InSeq | INcorrect | INincorrect |
| OutSeq | OUTincorrect | OUTcorrect |
FIGURE 2.
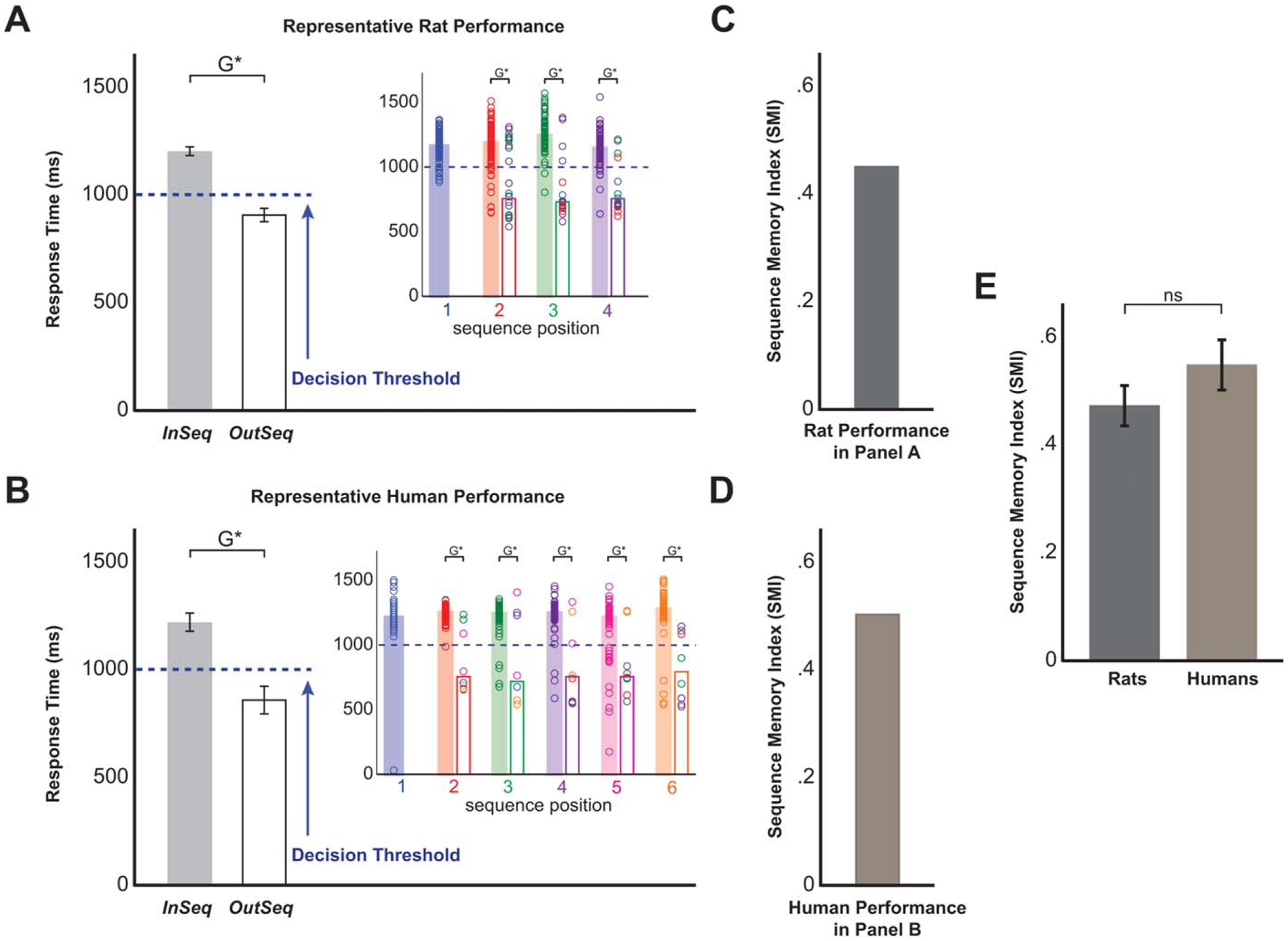
Sequence memory performance was remarkably similar in rats and humans. A: Performance from a representative rat. The main bar graph shows the mean RT (time the rat held in the odor port) on InSeq and OutSeq items, which indicates that rats reliably held for >1 s on InSeq items and for <1 s on OutSeq items (significant G-tests). The inset plot shows the same data sorted by ordinal position in the sequence and by item. The color of individual circles represents the correct sequence position for each odor presentation: blue represents the first sequence position (e.g., A or W), red the second position (e.g., B or X), green the third (e.g., C or Y), and purple the fourth (e.g., D or Z). Bars represent the median RT for each position (filled bar, InSeq; open bar, OutSeq). These data indicate that rats performed well at each sequence position and can identify when each odor was presented InSeq or OutSeq (significant G-tests for each sequence position and odor). It should be noted that only InSeq items are presented on the first position. B: Performance from a representative human subject. As shown in (A), the main bar graph shows the mean RT (time the person held the space bar key) on InSeq and OutSeq items, whereas the inset plot shows the same data sorted by ordinal position and by item. These data indicate that human subjects also performed well at each sequence position and could identify when each image was presented InSeq or OutSeq (significant G-tests). As with rats, the first position always featured an InSeq item. C: SMI [Eq. (1)] for the representative rat shown in (A). The SMI was used to collapse the data into a single normalized measure of sequence memory performance to directly compare the performance across species. D: SMI corresponding to the representative human subject shown in (B). E: Direct comparison of the mean SMI from all rats and humans revealed that both species performed at comparable levels on the task (nonsignificant t-test; power: [1 – β]d = 1 = 0.87). G*, P < 0.05 on G-test; ns, P > 0.05 on independent sample t-test.
Sequence Memory Index
To directly compare sequence memory performance between species, we calculated a Sequence Memory Index (SMI; see Eq. (1)) which controls for small differences in the number of OutSeq items. In essence, the SMI normalized the proportion of InSeq and OutSeq items across sessions and reduced sequence memory performance to a single value ranging from −1 to 1. A score of “1” represents perfect sequence memory in which a subject would have always held for ≥1 s for InSeq items and <1 s for OutSeq items. A score of “0” means chance performance, such as when subjects respond to InSeq and OutSeq items with the exact same response pattern (e.g., holding 90% of the time regardless of the trial type). Although mathematically possible, we did not observe negative (worse than chance) SMI values.
| (1) |
Cross-species comparisons
We used t-tests to compare the performance across species (rats and humans), specific trial types (InSeq and OutSeq), and specific probe trials (Repeats, Skips, and Ordinal Transfers). Tests were considered significant at P < 0.05, two tailed. To maximize statistical power, we refrained from using corrections for multiple comparisons. The statistical power was considered sufficient to limit Type II errors (Cohen, 1988) for all cross-species comparisons [(1 – β)d = 1 = 0.87, except in the case of Ordinal Transfers where (1 – β)d = 1 = 0.60]. Unless otherwise noted, descriptive statistics are presented as the mean ± 1 SEM.
RESULTS
Overall Sequence Memory Performance
A large majority of rats (19/20) and humans (21/24) performed well in the task, correctly discriminating between InSeq and OutSeq items (representative performance shown in Figs. 2A,B; all G-tests P < 0.05). Importantly, this ability was consistent across items and ordinal positions (subplots in Figs.2A,B; all G-tests P < 0.05), indicating that subjects remembered the entire sequence. Analyses of RTs similarly show that both species held longer on InSeq items than OutSeq items (Rats: RTInSeq = 1.113 ± 0.023 s, RTOutSeq = 0.894 ± 0.030 s, t(18) = 8.450, P < 0.001; Humans: RTInSeq = 1.264 ± 0.024 s, RTOutSeq = 0.891 ± 0.031 s, t(20) = 12.082, P < 0.001). To perform group comparisons on overall performance, we calculated the SMI (Figs. 2C,D). SMI analyses show that rats and humans performed at comparable levels on the sequence memory task (SMIRats = 0.471 ± 0.038; SMIHumans = 0.553 ± 0.045; t(38) = 1.376, P = 0.177; Fig. 2E).
Performance on “Out of Sequence” Probe Trials
Although analyses of SMI and RTs demonstrate strong sequence memory in rats and humans, these measures provide limited insight into the cognitive processing or mnemonic representations used by each species. To investigate this issue, we examined the performance of rats and humans across the three types of probe trials: Repeats, Skips, and Ordinal Transfers. Similar performance patterns across probe trial types would suggest rats and humans solve the sequence memory task using similar cognitive processes and mnemonic representations. It should be noted that performance on probe trials focuses exclusively on OutSeq items and thus G-test and SMI values could not be calculated. Consequently, we used accuracy (percent correct) to quantify the performance on probe trials.
Repeats
Rats and humans identified Repeats with the highest level of accuracy (Fig. 3A). Performance levels were not significantly different between rats and humans (RepeatsRats = 76.4 ± 3.9%, RepeatsHumans = 79.3 ± 3.5%, t(38) = 0.546, P = 0.588). Performance on Repeats was further compared on the specific n-back distances tested in both rats and humans (2-back, 3-back; Fig. 3A, subplot). No statistically significant differences between species were observed on 2-back tests (e.g., ABCB; 2-backRats = 78.3 ± 4.3%, 2-backHumans = 83.9 ± 3.1%, t(38) = 1.080, P = 0.287), nor on 3-back tests (e.g., ABCA; 3-backRats = 73.3 ± 5.7%, 3-backHumans = 81.0 ± 4.9%, t(38) = 1.022, P = 0.313).
FIGURE 3.
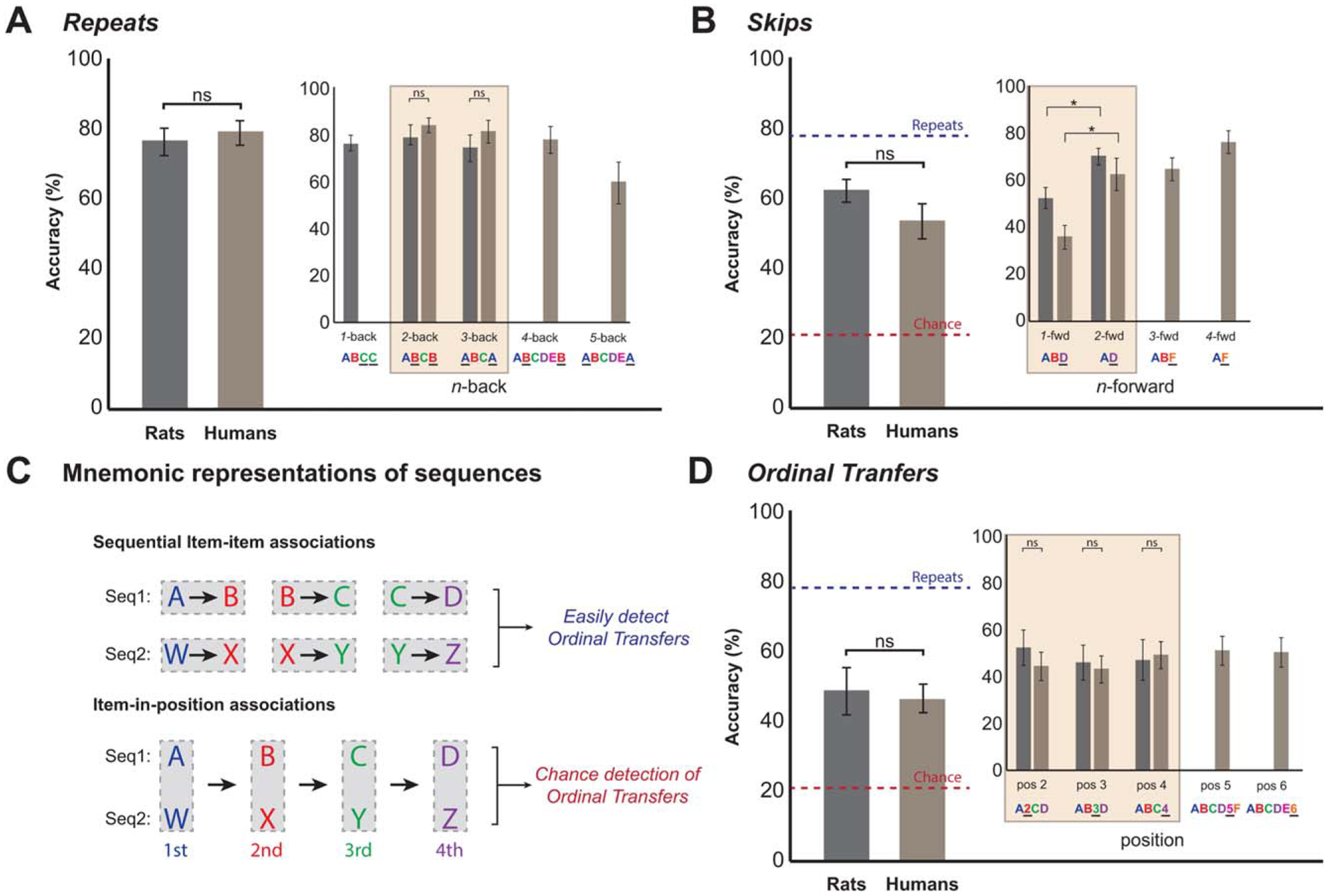
Performance on “out of sequence” probe trials is nearly identical between rats and humans, suggesting the use of similar cognitive processes and sequence representations across species. A: Performance on probe trials in which the OutSeq item was a repeat of an earlier item in the sequence (Repeats). The main bar graph shows accuracy for rats and humans, and the inset graph shows the same data sorted by n-back distance (how many items back did the item first occur; e.g., ABCB is a 2-back Repeat). Subjects performed at a high level on Repeats, with no significant differences observed between the groups (nonsignificant independent sample t-tests). B: Performance on probe trials in which the OutSeq item was presented earlier than its correct position (Skips). The main bar graph shows accuracy for rats and humans, and the inset graph shows the same data sorted by n-forward distance (the number of items skipped; e.g., ABD is a 1-forward Skip). Rats and humans performed similarly on Skips and both species performed significantly better on 2-forward than 1-forward Skips (significant dependent sample t-tests). C: Performance on the task can be supported by two primary representations of sequences in memory: sequential item–item associations and item-in-position associations (Kahana et al., 2010). Importantly, the two representations can be used to identify Repeats and Skips, but predict different outcomes on Ordinal Transfer trials. D: Performance on Ordinal Transfers, probe trials in which an item is presented in the correct ordinal position but in the wrong sequence (e.g., ABYD). If subjects exclusively relied on sequential item–item associations to solve the task, then Ordinal Transfer probes would be easily identified as OutSeq items (as Y should not follow B). Conversely, the same probes would be identified as InSeq items if subjects exclusively relied on item-in-position associations (as Y is in the same ordinal position as C). The main bar graph shows the rate at which subjects identified Ordinal Transfers as OutSeq, and the inset graph shows the data sorted by ordinal position of the transferred item. Rats and humans identified Ordinal Transfers as OutSeq at the same rate across positions, at levels between asymptotic performance (defined by performance on Repeats) and chance. OutSeq chance was defined as the complement to the response bias in each species for statistical tests, (Results), and plotted here as the average of the two species for simplicity. This suggests that rats and humans represent sequences using both item–item and item-in-position associations. Shaded areas in inset graphs of (A, B, and D) highlight the conditions in which data from both species were available. ns, nonsignificant on independent sample t-test; power: (1 – β)d = 1 = 0.87, except in the case of Ordinal Transfers where (1 – β)d = 1 = 0.60; *P < 0.05 on dependent sample t-test.
Skips
These probe trials were more difficult for both rats and humans (Fig. 3B); however, there was no significant difference between species (SkipsRats = 62.1 ± 3.2%, SkipsHumans = 53.3 ± 4.7%, t(38) = 1.512, P = 0.139). Both species performed better on Repeats compared to Skips (Repeats–SkipsRats = 14.3 ± 3.0%, tRepeats vs. Skips(18) = 5.370, P < 0.001; Repeats–SkipsHumans = 26.0 ± 3.0%, tRepeats vs. Skips(20) = 8.766, P < 0.001). Next, the pattern of accuracy on Skips was compared on the specific n-forward distances tested in both species (1-forward, 2-forward; Fig. 3B, subplot). Both rats and humans had the most difficulty skipping ahead one item (e.g., ABD), with rats performing better than humans (1-forwardRats = 52.5 ± 4.4%, 1-forwardHumans = 35.7 ± 5.1%, t(38) = −2.475, P < 0.05, d = 0.78, medium effect size). On 2-forward tests (e.g., AD), rats and humans performed similarly (2-forwardRats = 70.6 ± 3.5%, 2-forwardHumans = 61.9 ± 6.8%, t(38) = −1.096, P = 0.280). Importantly, both rats and humans performed better on 2-forward than 1-forward tests (Rats: difference = 18.1 ± 3.6%, t(18) = 5.078, P < 0.001; Humans: difference = 26.2 ± 6.5%, t(20) = 4.037, P < 0.01). Thus, the overall pattern of performance on Skips is the same in rats and humans: Skips are more difficult for both species and skipping ahead one item in the sequence is the most difficult to detect.
Ordinal transfers
This type of probe trial, in which an item is presented in the correct ordinal position but in the wrong sequence (e.g., ABYD), was used to shed light on the nature of the sequence representations or strategies supporting task performance (Fig. 3C). If subjects exclusively relied on sequential item–item associations to solve the task, then ordinal transfer probes would be identified as OutSeq items (as Y should not follow B). Conversely, the same probes would be identified as InSeq items if subjects exclusively relied on item-in-position associations (as Y is in the same ordinal position as C). Importantly, there were no significant differences between rats and humans overall (OrdinalTransfersRats = 48.6 ± 6.7%, OrdinalTransfersHumans = 47.4 ± 4.8%, t(26) = −0.129, P = 0.899; Fig. 3D) nor across ordinal positions (Position2: t(26) = −0.689, P = 0.497; Position3: t(26) = −0.249, P = 0.805; Position4: t(26) = 0.199, P = 0.843; Fig. 3D, subplot). Both rats and humans performed Ordinal Transfers at greater than chance levels, determined by the complement to the average response bias in each species (%chance = 100 − %trials>1 s; Rats: tOrdinalTranfers vs. Chance(6) = 3.222, P < 0.05, %chance = 26%; Humans: tOrdinalTranfers vs. Chance(20) = 6.293, P < 0.001, %chance = 17%), but performed at lower levels than Repeats (Rats: tOrdinalTranfers vs. Repeats(6) = −4.126, P < 0.01; Humans: tOrdinalTranfers vs. Repeats(20) = −6.619, P < 0.001). These results suggest that rats and humans represent sequences with both item–item and item-in-position associations.
DISCUSSION
We developed a cross-species sequence task to study the memory for sequences of events, a key feature of episodic memory. This approach was carefully designed to match the basic behavioral requirements in rats and humans while using species-appropriate stimuli and responses. Subjects were required to make a decision as to whether each individual item was presented “in sequence” (InSeq) or “out of sequence” (OutSeq). This design allowed the inclusion of different types of OutSeq probe trials to examine underlying cognitive processes and mnemonic representations. We report that rats and humans performed at comparable levels and solved the task in similar ways, suggesting the use of similar neural representations or processes.
Rats and Humans Remember Sequences of Events in the Same Way
The pattern of results suggests that memory of the sequences was fundamentally the same across species. Overall measures of sequence memory indicated that rats and humans performed at comparable levels in the task (Fig. 2). G-tests analyses of single sessions showed that individual rats and humans correctly discriminated between InSeq and OutSeq items (for all items and ordinal positions). SMI analyses demonstrated that performance was not statistically different between rats and humans. RT analyses revealed that rats and humans made InSeq/OutSeq decisions at equivalent latencies.
In addition, the use of different types of OutSeq probe trials allowed us to characterize the performance in further detail and shed light on potential cognitive processes and mnemonic representations. First, we looked at “Repeat” probe trials in which an item that occurred earlier in the sequence is repeated (e.g., ABAD; Fig. 1B). Rats and humans easily identified Repeats as OutSeq (Fig. 3A), and performance did not differ whether the n-back distance involved two or three items (Fig. 3A, inset). Second, we looked at “Skip” probe trials, where an item occurred too early in the sequence (e.g., ABD; Fig. 1C). Skips required subjects to have a detailed memory of the sequence to predict the upcoming item in a sequence and precluded the use of memory strength as a potential strategy. Both rats and humans were able to identify Skips as OutSeq (Fig. 3B) and showed a similar pattern of accuracy that was dependent on the number of items skipped (n-forward distance; Fig. 3B, inset). Specifically, rats and humans had significantly more difficulty with 1-forward (e.g., ABD) than 2-forward (e.g., AD) Skips. Finally, “Ordinal Transfer” probe trials were used to test the mnemonic representation of sequences by transferring an item from one sequence (e.g., WXYZ) into another sequence (e.g., ABCD), while retaining the ordinal position of the transferred item (e.g., ABYD; Fig. 1D). Rats and humans identified Ordinal Transfers as OutSeq at comparable levels (Fig. 3D), and this ability did not depend on the ordinal position of the transferred item (Fig. 3D, inset). Ordinal Transfers were identified as OutSeq at a lower rate than Repeats (asymptotic performance), but at a significantly higher rate than chance. This suggests that both rats and humans represent sequences with sequential item–item associations and item-in-position associations (Fig. 3C). In summary, all three different probe trials resulted in the same response patterns between rats and humans. Thus, rats and humans remember sequences of events with the same cognitive processes and mnemonic representations.
Comparisons to Other Sequence Memory Tasks
It is important to distinguish the cross-species sequence task from other sequence memory paradigms in the literature. First, although our approach can appear similar to serial reaction time tasks (SRT; Nissen and Bullemer, 1987; Schendan et al., 2003; Doyon and Benali, 2005), the behavioral requirements are fundamentally different. Performance on the present task requires memory for sequences of events or stimuli, not memory for motor sequences. The correct series of responses cannot be planned ahead of time and instead reflects a real-time decision for each item presentation that depends on the subject’s memory of the sequential relationships among items.
Second, unlike most sequence memory paradigms in the literature, our task is nonspatial. Many studies have shown that subjects can remember sequences of spatial locations, a capacity that depends on the hippocampus (Chiba et al., 1994, 1997; Hopkins et al., 2004; Howland et al., 2008; Fouquet et al., 2010). However, as the hippocampus is known to play a key role in spatial processing, it is difficult to determine its specific contribution to the temporal components of these tasks. A similar problem applies to single-cell recording studies, which have shown that information about visited sequences of locations is encoded and subsequently replayed by hippocampal neurons (Skaggs and McNaughton, 1996; Lee and Wilson, 2002; Karlsson and Frank, 2009; Remondes and Wilson, 2013). As these locations are adjacent in space and in time, it is difficult to determine whether this form of sequence coding extends beyond the memory for spatial trajectories. Importantly, the nonspatial nature of the cross-species sequence task allows for the use of stimuli not critically processed by the hippocampus (Ranganath, 2010; Feinberg et al., 2012), the dissociation of temporal from spatial demands, and the ability to arbitrarily reorder stimuli to examine underlying mechanisms.
Third, the present task differs from other nonspatial sequence memory tasks, which involve order judgments between simultaneously presented items (Orlov et al., 2000; Fortin et al., 2002; Kesner et al., 2002, DeVito and Eichenbaum, 2011). In these paradigms, subjects are presented with a series of items (e.g., ABCDE) and subsequently tested with two (or more) items from the sequence simultaneously (e.g., B and D). Subjects are typically required to choose the item that occurred earlier in the sequence. This approach has been successful in revealing the critical role of the hippocampus (Fortin et al., 2002; Kesner et al., 2002, DeVito and Eichenbaum, 2011) and prefrontal cortex (Hannesson et al., 2004; Farovik et al., 2010; DeVito and Eichenbaum, 2011), but is not well suited to identify the subjects’ underlying cognitive processes or mnemonic representations. For instance, the extent to which subjects perform direct comparisons between the two items (“B occurred before D”) or rely on temporal information about a single item (“D occurred late in the sequence and is thus likely to be incorrect”) is unclear. Similarly, it is difficult to measure the contribution of different types of mnemonic representations (i.e., sequential item–item, or item-in-position associations) to performance. In contrast, the cross-species sequence task, which requires order judgments about single items and includes different types of probe trials, is better suited to address these issues.
Broader Implications for Episodic Memory Research
Elucidating the neurobiological mechanisms underlying episodic memory will require converging evidence from complementary investigative techniques across species. Accordingly, we designed the cross-species sequence task to be able to investigate the neural basis of the memory for sequences of events using both behavioral and neurophysiological approaches. The task captures the “flow of events” aspect of episodic memory (Tulving, 1972, 1984, 2002), which conceptualizes an episode as a series of events that occur over time (Fig. 1A). This nonspatial design enhances the parallel between the behavioral requirements of paradigms used in rodents and humans and provides an opportunity to specifically investigate memory for the temporal context of events. Furthering our understanding of this capacity is important because it is critical to our ability to distinguish individual episodic memories, many of which have overlapping elements including specific items and locations (Agster et al., 2002; Jacobs et al., 2013).
Temporal pattern separation
Our findings are also pertinent to the emerging literature on pattern separation. It has been proposed that temporal pattern separation is important for the segmentation of episodes and thus may be a critical computation supporting episodic processing (Kesner and Hunsaker, 2010; Ezzyat and Davachi, 2011; Yassa and Stark, 2011; Roberts et al., in press). Our pattern of results is consistent with an underlying process of sequential pattern separation. In both species, 1-forward Skips (e.g., ABD) were more difficult to detect that 2-forward Skips (e.g., AD). This is further supported in the human data in which we have a larger range of n-forward distances, showing that the 3-forward is similar to 2-forward, yet the 4-forward distance is only slightly easier than both (Fig. 3B, inset). That is, when the change in input is small, pattern completion (identifying Skips as InSeq) is more likely to be observed. As the change in input increases, there is a nonlinear change toward pattern separation (detection of Skips as OutSeq). Similarly, the ability to detect Ordinal Transfers as OutSeq may also depend on a pattern separation process. In the present experiment, Ordinal Transfers were presented in the correct ordinal position but in a different sequence. In future studies, we will examine whether changing the ordinal position of the probe leads to a nonlinear change in performance.
Ordinal schema in episodic memories
Tulving (1972, 1984, 2002) proposed that (semantic) schemas, while not critical to episodic memory, normally contribute to the formation of episodic memories. This notion is supported by evidence that spatial schemas can facilitate encoding of single experiences (food–location associations; Tse et al., 2007). We provided evidence that rats and humans use item-in-position representations (in addition to sequential item–item association) suggesting that, as an episode unfolds, individual events become associated with their ordinal position in the sequence (e.g., A is first, B is second, etc.). Therefore, ordinal schemas may be used to remember the order in which events occurred, a hypothesis supported by preliminary evidence of ordinal coding in hippocampal and prefrontal neurons (Allen et al., 2011, 2013).
CONCLUSIONS
A defining feature of episodic memory is the ability to remember the flow of events as they occurred during an experience. Here, we developed a cross-species sequence task that can be used in rats and humans, and possibly in other species, to be able to examine the neural basis of this capacity using complementary investigative techniques. The task has sufficient statistical power to assess sequence memory in individual subjects and includes different types of probe trials which can be employed to shed light on underlying computations. Thus, the cross-species sequence task should be useful for basic, preclinical, and clinical studies (such as in age-related dementia and Alzheimer’s disease). In evaluating the cross-species sequence task, we found a remarkable overall similarity in the performance of humans and rats, across several measures and probe trials, suggesting that memory for sequences of events reflects the same underlying cognitive processes and mnemonic representations in both species. Thus, the task strongly supports the premise that rats and humans not only remember sequences of events, but also use similar underlying strategies in support of such memories.
ACKNOWLEDGMENTS
The authors thank Nathan Lo, Owen Cruz, and Phillip Yu for help testing rats. The authors thank Samantha Rutledge and Kristina Le for their assistance in testing humans. The authors also thank Shauna Stark and Clare Quirk for help with several aspects of the project.
Grant sponsor:
National Science Foundation, National Institute of Health, and the Whitehall Foundation; Grant numbers: IOS-1150292; NIH AG034613.
REFERENCES
- Agster KL, Fortin NJ, Eichenbaum H. 2002. The hippocampus and disambiguation of overlapping sequences. J Neurosci 22:5760–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Fortin NJ. 2013. The evolution of episodic memory. Proc Natl Acad Sci USA 110:10379–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Jacobs N, Feinberg LM, Bharadwaj KR, Wang MX, Fortin NJ. 2011. Prefrontal cortex neurons code for sequences of events. Soc Neurosci Abstr 37:97.19. [Google Scholar]
- Allen TA, Kraus BJ, McKenzie SA, Hasselmo ME, Eichenbaum HB, Fortin NJ. 2013. Neural representations of sequences of events in the hippocampus parallel behavioral performance. Soc Neurosci 39:578.28. [Google Scholar]
- Buhusi CV, Meck WH. 2005. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6:755–765. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh AR, Tulving E. 1997. Brain regions differentially involved in remembering what and when: A PET study. Neuron 19:863–870. [DOI] [PubMed] [Google Scholar]
- Chiba AA, Kesner RP, Reynolds AM. 1994. Memory for spatial location as a function of temporal lag in rats: Role of hippocampus and medial prefrontal cortex. Behav Neural Biol 61:123–131. [DOI] [PubMed] [Google Scholar]
- Chiba AA, Kesner RP, Gibson CJ. 1997. Memory for temporal order of new and familiar spatial location sequences: Role of the medial prefrontal cortex. Learn Mem 4:311–317. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. 1998. Episodic-like memory during cache recovery by scrub jays. Nature 395:272–274. [DOI] [PubMed] [Google Scholar]
- Cohen J. 1988. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, New Jersey: Erlbaum Associates. [Google Scholar]
- DeVito LM, Eichenbaum H. 2011. Memory for the order of events in specific sequences: Contributions of the hippocampus and medial prefrontal cortex. J Neurosci 31:3169–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Benali H. 2005. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15: 161–167. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. 2007. Comparative cognition, hippocampal function, and recollection. Comp Cogn Behav Rev 2:47–66. [Google Scholar]
- Eichenbaum H, Fortin NJ. 2005. Bridging the gap between brain and behavior: Cognitive and neural mechanisms of episodic memory. J Exp Anal Behav 84:619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Bookheimer SY. 2007. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learn Mem 14:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L. 2011. What constitutes an episode in episodic memory? Psychol Sci 22:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Eichenbaum H. 2010. Distinct roles for dorsal CA3 and CA1 in memory for sequences of events. Learn Mem 17:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg LM, Allen TA, Ly D, Fortin NJ. 2012. Recognition memory for social and non-social odors: Differential effects of neurotoxic lesions to the hippocampus and perirhinal cortex. Neurobiol Learn Mem 97:7–16. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. 2002. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci 5: 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet C, Tobin C, Rondi-Reig L. 2010. A new approach for modeling episodic memory from rodents to humans: The temporal order memory. Behav Brain Res 215:172–179. [DOI] [PubMed] [Google Scholar]
- Hannesson DK, Howland JG, Phillips AG. 2004. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci 24:4596–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RO, Waldram K, Kesner RP. 2004. Sequences assessed by declarative and procedural tests of memory in amnesic patients with hippocampal damage. Neuropsychologia 42:1877–1886. [DOI] [PubMed] [Google Scholar]
- Howland JG, Harrison RA, Hannesson DK, Phillips AG. 2008. Ventral hippocampal involvement in temporal order, but not recognition, memory for spatial information. Hippocampus 18:251–257. [DOI] [PubMed] [Google Scholar]
- Hulse SH, Dorsky NP. 1979. Serial pattern learning by rats: Transfer of a formally defined stimulus relationship and the significance of nonreinforcement. Anim Learn Behav 7:211–220. [Google Scholar]
- Jacobs NS, Allen TA, Nguyen N, Fortin NJ. 2013. Critical role of the hippocampus in memory for elapsed time. J Neurosci 33: 13888–13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LJ, Ranganath C. 2010. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci 30:15558–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Mollison MV, Addis KM. 2010. Position cues in serial learning: The spin list technique. Mem Cogn 38:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. 2009. Awake replay of remote experiences in the hippocampus. Nat Neurosci 12:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR. 2010. The temporal attributes of episodic memory. Behav Brain Res 215:299–309. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. 2002. The role of the hippocampus for the temporal order of a sequence of odors. Behav Neurosci 116:286–290. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. 2006. An unexpected sequence of events: Mismatch detection in the human hippocampus. PLoS Biol 4: e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. 2002. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36:1183–1194. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. 2004. The neural basis of temporal processing. Annu Rev Neurosci 27:307–340. [DOI] [PubMed] [Google Scholar]
- Merchant H, Harrington DL, Meck WH. 2013. Neural basis of the perception and estimation of time. Annu Rev Neurosci 36:313–336. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. 1987. Attentional requirements of learning. Evidence from performance measures. Cogn Psychol 19:1–32. [Google Scholar]
- Orlov T, Yakovlev V, Hochstein S, Zohary E. 2000. Macaque monkeys categorize images by their ordinal number. Nature 404:77–80. [DOI] [PubMed] [Google Scholar]
- Ranganath C. 2010. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus 20:1263–1290. [DOI] [PubMed] [Google Scholar]
- Remondes M, Wilson MA. 2013. Cingulate-hippocampus coherence and trajectory coding in a sequential choice task. Neuron 80: 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, Ly M, Muuay E, Yassa MA. 2014. Temporal discrimination deficits as a function of lag interference in older adults. Hippocampus 24:1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Brown TI, Stern CE. 2009. The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus 19:790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. 2003. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron 37:1013–1025. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. 1996. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271:1870–1873. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. 1995. Biometry: The Principles and Practice of Statistics in Biological Research. New York: W. H. Freeman and Company. pp 724–743. [Google Scholar]
- Swartz KB, Chen S, Terrace HS. 1991. Serial learning by rhesus monkeys: I. Acquisition and retention of multiple four-item lists. J Exp Psychol Anim Behav Proces 17:396–410. [DOI] [PubMed] [Google Scholar]
- Templer VL, Hampton RR. 2012. Cognitive mechanisms of memory for order in rhesus monkeys (Macaca mulatta). Hippocampus 23:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. 2007. Schemas and memory consolidation. Science 316:76–82. [DOI] [PubMed] [Google Scholar]
- Tulving E. 1972. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York: Academic Press, Inc. pp 381–402. [Google Scholar]
- Tulving W. 1984. Precis of elements of episodic memory. Behav Brain Sci 7(2) 223–238. [Google Scholar]
- Tulving E. 2002. Episodic memory: From mind to brain. Annu Rev Psychol 53:1–25. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. 2011. Pattern separation in the hippocampus. Trends Neurosci 34:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]


