Abstract
Aim of the study:
This study examined the response of the free testosterone and cortisol ratio (fTC) to prolonged endurance exercise.
Methods:
A trained sportsman performed treadmill exercise at 70% of maximal oxygen uptake to volitional fatigue. Frequent blood samples were collected over 24 hours and analyzed to determine changes in the fTC ratio.
Results:
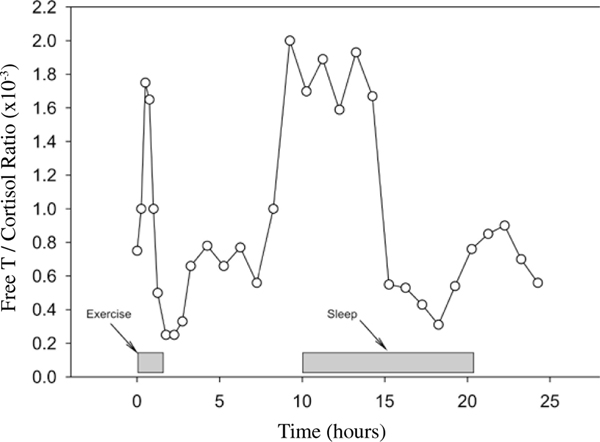
Exercise caused an initial increase in the ratio followed by a rapid decline in the immediate recovery from exercise. Approximately 8 hours into recovery from exercise, there was a pronounced and persistent (~6 hours) secondary increase in the ratio.
Conclusions:
Results suggest that the fTC ratio responds to intensive exercise, but that aspects of the response can be of a delayed nature occurring many hours into recovery. Exercise scientists who wish to monitor this parameter should be aware that the time course by which they choose to conduct their blood sampling could impact upon the interpretation of their data.
Keywords: endocrine, stress, training biomarkers
Introduction
Athletes participating in sporting events subject their bodies to an extreme amount of stress in performing their daily training and sports competitions. This stress is necessary, to a certain degree, in order to facilitate the adaptation process that allows an improvement in human performance (1). When the human body is subjected to stressful events the neuroendocrine system can respond and release a multitude of hormones, which can aid the body in the adaptation process (2).
Many exercise research studies have been conducted to examine the hormonal response to an exercise bout. These studies have provided valuable information on the behavior of the neuroendocrine system. Unfortunately, many of these studies suffer from a major methodological limitation: hormonal assessments based upon an inadequate sampling frequency. This is especially true for studies involving blood sampling. In an attempt to circumvent this problem, less invasive specimens (i.e., urine and saliva) have been obtained. However, these specimens do not allow for as complete a hormonal evaluation as blood specimens (3).
Over 20 years ago, the free testosterone and cortisol (fTC) ratio was proposed as a critical hormonal marker of the anabolic and catabolic status in sportsman (4). Contemporary research suggests the ratio has certain limitations and drawbacks for usage in such a purpose (3,5). Nevertheless, it has remained a useful and popular tool for the monitoring of training stress, especially relative to the development of the Overtraining Syndrome (6,7).
With the above information in mind, our laboratory was presented with what we considered a unique opportunity; i.e., to perform a 24-hour hormonal assessment in an endurance-trained athlete who performed an intensive exercise session that mimicked an intense, prolonged, endurance exercise session. Our purpose in conducting such an evaluation was to provide “reference findings” that might serve as a comparative “normal” fTC ratio hormonal response to such exercise.
Materials and Methods
Hormonal levels were measured in a healthy physically active male (age = 22, height 176 cm, body mass = 72.2kg) who had been involved with exercise training and competitive sport (e.g., running, cycling) for a number of years. His maximal aerobic capacity (VO2max ≈ 60 ml/kg/min) rated him as being highly trained (8). In accord with the Declaration of Helsinki he approved and signed a written informed consent prior to voluntary participation in the study.
Serial blood specimens were collected from the subject for a 24-hour period, following a 12 hr fast. For the day prior to the experiment he performed no physical activity, and for the 3 days before the experiment, he followed a controlled diet to ensure adequate carbohydrate and total caloric intakes were maintained.
On the day of the experiment he reported to our laboratory in the morning and an antecubial venous catheter was inserted into his right arm. The catheter was kept patent with a saline infusion solution. The subject next rested quietly for 30 minutes supine, and then an initial baseline blood specimen was taken, followed by a 5 minute active warm-up period. The subject then began running on a motorized treadmill at an intensity corresponding to ~70% of his VO2max until he reached the point of volitional fatigue. During exercise, blood specimens were taken at 15-minute intervals and additional specimens were obtained at 0.5, 1, 1.5, 2 hours into recovery. Subsequent to this sampling, blood specimens were collected on an hourly basis until a 24-hour period was reached. During recovery the subject remained in the laboratory and rested quietly (supine - reading, television watching) or slept, and consumed water freely as well as evening (see Figure 1, 0800 hours) and breakfast meals (2100 hours; 2500 kcal total intake).
Fig 1.
Free testosterone and cortisol ratio (fTC) versus time during the 24 hour period following the prolonged endurance event. The “0” time point refers to the sample taken immediately pre-exercise.
The blood samples were all treated appropriately to insure viable hormonal analysis (specific details are reported elsewhere; 9). Briefly, hormonal concentrations were measured in duplicate and were analyzed with an ultra-sensitive, single-ligand, solid phase methodology radioimmunoassay technique (DPC Inc., Los Angles, CA, USA). Hormones measured were free testosterone as an anabolic index and cortisol as a catabolic index. Additionally, sex hormone binding globulin (SHBG) was measured only in the initial and final resting blood samples. Specific assay sensitivities were; 4.0 pmol·L−1, 5.5 nmol·L−1, and 0.04 nmol·L−1 for free testosterone, cortisol, and SHBG, respectively. The between and within assay coefficients of variation for all assays were ≤8.2% and ≤6.6%, respectively.
Results and Discussion
The subject exercised for a total of 82.2 minutes before he reached volitional fatigue and stopped. The fTC results are depicted in Figure 1. The figure clearly indicates that the exercise caused an initial, substantial rise in the ratio, but was followed by a large decline that continued into early recovery. Most surprisingly was the finding that at approximately 8 hours into recovery there was another substantial and persistent elevation in the ratio (lasting approximately 6 hours). This was followed by another large reduction and a minor elevation during the period of sleep. The hormonal changes far exceeded what would be expected for plasma volume induced changes due to fluids shifts during the exercise (2). The SHBG concentrations in the initial and final blood sample were 36.5 nmol·L−1 and 38.3 nmol·L−1, respectfully. These, as well as, the hormonal values were all within normal clinical reference ranges for physically active males (10).
It is realized that these data are limited due to the case study nature of the study. Nonetheless, the results may prove to be useful to exercise scientists working in the endocrinology area for several reasons. First, the findings suggest that judgments on the effect of exercise to influence the fTC ratio may be dramatically affected by when the timing of the blood sampling occurs following the exercise session. Secondly, relative to the anabolic processes critical to training adaptation (i.e., muscle tissue repair) in the body, the findings suggest it may be essential that the nutritional substrates needed for such hormonal mediated processes be available at the beginning of the delayed elevation in the fTC ratio approximately 8 hours into recovery (2). Finally, from a clinical perspective these results would indicate that certain aspects of the hormonal profiles of an athletic individual, on a day in which they perform intensive exercise, will vary from what is typically considered as a normal hormonal profile. Specifically, at certain times the hormonal levels will be far greater or far lower than the expected range of values as reported in clinical reference textbooks (10).
Another potential limitation to the study involves the methodological aspects of the free testosterone assay which was utilized. Vermeulen et al. (11) has argued that this immunological procedure is not precise enough. This is a legitimate concern, but it is important to point out that the present changes observed in the free testosterone concentrations were substantially large and not of a minute level where precision could have been a critical factor. Furthermore, in the present study select SHBG levels (research funding considerations prevented further analysis) were measured and a free level of testosterone was calculated using the procedures of Nanjee and Wheeler (12). The calculation-derived values were very similar to what had been measured within our assay. Collectively, these points are suggestive that our testosterone data do accurately reflect the physiological status of our subject.
In conclusion, the findings from this case study suggest that the fTC ratio responds to intensive exercise, but that aspects of the response can be of a delayed nature occurring many hours into recovery. Furthermore, scientists monitoring the fTC should be conscious that the time course during which they choose to conduct blood sampling could impact upon the interpretation of their data.
References
- 1.Viru A, Viru M. Cortisol – essential adaptation hormone in exercise. Int J Sports Med 2004; 25: 461–4. [DOI] [PubMed] [Google Scholar]
- 2.McMurray RG, Hackney AC. Endocrine responses to exercise and training. In: Garrett WE, Kirkendall DT., eds. Exercise and Sport Science. Philadelphia, Lippincott Williams & Wilkins; 2000, 135–56. [Google Scholar]
- 3.Viru A, Viru M. Biochemical Monitoring of Sport and Training. Champaign: Human Kinetics, 2001. [Google Scholar]
- 4.Aldercreutz H, Harkonen M, Kuoppasalmi K, et al. Effect of training on plasma anabolic and catabolic steroid hormones and their response during physical exercise. Int J Sports Med 1986; 7(suppl.): 27–8. [DOI] [PubMed] [Google Scholar]
- 5.Fry AC, Steinacker JM, Meeusen R. Endocrinology of overtraining. In: Kraaemer WJ, Rogol AD., eds. The Endocrine System in Sports and Exercise. Oxford, UK: Blackwell Publishing; 2005: 584–93. [Google Scholar]
- 6.Hackney AC. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Reviews in Endocrinology & Metabolism 2006; 1(6): 783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urhausen A, Gabriel H, Kindermann W. Blood hormones as markers for training stress and overtraining. Sports Med 1995; 20(4): 251–76. [DOI] [PubMed] [Google Scholar]
- 8.Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med 1990; 61(1): 3–11. [PubMed] [Google Scholar]
- 9.Daly W, Seegers CA, Rubin DA., et al. Relationship between stress hormones and testosterone with prolonged endurance exercise. Eur J ApplPhysiol 2005; 93: 375–80. [DOI] [PubMed] [Google Scholar]
- 10.Tietz N. Clinical Guide to Laboratory Tests. London: WB Saunders, 1990. [Google Scholar]
- 11.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10): 3666–72. [DOI] [PubMed] [Google Scholar]
- 12.Nanjee MN, Wheeler MJ. Plasma free testosterone - is an index sufficient? Ann Clin Biochem 1985; 22: 387–90. [DOI] [PubMed] [Google Scholar]