Abstract
Background:
Group B Streptococcus (GBS) is a common vaginal bacterium and leading cause of invasive fetoplacental infections. GBS in the vagina can invade through the cervix to cause ascending uteroplacental infections or can be transmitted to the neonate during vaginal delivery. Some studies have found that women with a “dysbiotic” polymicrobial and/or Lactobacillus-depleted vaginal microbiota are more likely to harbor GBS. Several studies have shown that Black women are more likely than white women to have polymicrobial vaginal microbiotas and to harbor GBS. Further, life-threatening GBS infections occur at nearly 3-fold higher rates in Black compared to white infants. Irrespective of race, Gardnerella vaginalis is often the most abundant bacteria in the vaginas of women with dysbiosis while being detected at lower levels in most other women. Mouse models of GBS and G. vaginalis colonization have been reported but the two have not, to our knowledge, been studied together.
Objective:
The overarching hypothesis driving this study is that known differences in vaginal microbiota composition between Black and white women may contribute to racial disparities in GBS disease. This hypothesis was tested by examining whether vaginal exposure to G. vaginalis may facilitate colonization and/or invasive infection of the upper reproductive tract by GBS during pregnancy in mice.
Study Design:
Timed-pregnant mice were generated using an allogeneic mating strategy with BALBc males and C57Bl/6 females. Dams were vaginally inoculated at gestational day (E)14 with GBS-alone (using a 10-fold lower dose than previously reported models) or co-inoculated with GBS and G. vaginalis. Bacterial titers were enumerated in vaginal, uterine horn and placental tissues at E17. The presence (Fisher’s exact tests) and levels (Mann-Whitney tests) of bacterial titers were compared between mono- and co-inoculated dams in each compartment. Relative risks were calculated for outcomes that occurred in both groups. Tissue samples were also examined for evidence of pathophysiology.
Results:
Inoculation of pregnant mice with 107 GBS alone did not result in vaginal colonization or ascending infection. In contrast, co-inoculation of GBS with G. vaginalis in pregnant mice resulted in a 10-fold higher risk of GBS vaginal colonization (RR:10.31, 95%CI: 2.710-59.04; P=0.0006, Fishers Exact). Ascending GBS infection of the uterus and placenta occurred in approximately 40% of co-inoculated animals, whereas none of those receiving GBS alone developed uterine or placental infections. Immunofluorescence microscopy revealed GBS in both the maternal and fetal side of the placenta. Histological inflammation and increased pro-inflammatory cytokines were evident in the setting of GBS placental infection. Interestingly, placentas from dams exposed to GBS and G. vaginalis, but without recoverable vaginal or placental bacteria, displayed distinct histopathologic features and cytokine signatures.
Conclusion:
These data suggest that G. vaginalis vaginal exposure can promote GBS vaginal colonization, resulting in greater likelihood of invasive perinatal GBS infections. These findings may be broadly relevant to women colonized by G. vaginalis. Since Black women are more likely to have BV (thus high levels of G. vaginalis), these findings could help explain why Black women and their babies have higher rates of in GBS colonization and invasive disease.
Keywords: ascending infection, bacterial vaginosis, Gardnerella vaginalis, Group B Streptococcus, microbiota, placenta, health disparities, uterus, vagina, vaginal microbiome
Condensation:
Co-inoculation with Gardnerella vaginalis increases the likelihood of sustained vaginal colonization and ascending utero-placental infection by group B Streptococcus in pregnant mice.
Introduction
Group B Streptococcus (GBS) is associated with multiple adverse pregnancy outcomes and life-threatening neonatal infections.1,2 Vaginal colonization with GBS occurs in ~18% of pregnant women worldwide3 and has been linked with preterm birth (PTB),2,4–6 preterm premature rupture of membranes (PPROM)7 and neonatal intensive care unit admission.8 GBS vaginal colonization is a risk factor for neonatal transmission during delivery9,10 and to infants during the postnatal period.11 GBS in the vagina can also cause invasive utero-placental and fetal infections.9,12,13 While results vary, some clinical findings have suggested that GBS colonization is more likely in women with a polymicrobial, “dysbiotic” vaginal microbiota or those with decreased Lactobacillus (Table S1).14–18 Black19–21 and African American22 women were more likely, compared to white women, to have a polymicrobial vaginal microbiota, either defined as bacterial vaginosis (BV) by the Gram-stain based Nugent system, or as community state type (CST)-IV by 16S sequencing. BV affects approximately one-third of U.S. women overall, but rates reach above 50% in black women 23–26. Further, higher rates of GBS vaginal colonization and invasive infections have been reported in Black14,27–29 and African American15,30, compared to white women and their infants. In one study, black women were nearly twice as likely as white women to harbor GBS vaginally 14. Black race was a significant risk factor for early onset GBS disease (OR 1.81) 28.
The factor(s) driving racial disparities in GBS colonization and infection are not clear. Since racial differences in vaginal microbiota composition exist, we postulated that certain vaginal bacteria may facilitate vaginal colonization or ascending uterine and placental infection by GBS. G. vaginalis is an ideal candidate for modeling the effect of the vaginal microbiota on GBS because it is frequently the most abundant member of the polymicrobial vaginal microbiota present in BV31 or CST-IV,19,32 has been found at higher levels during pregnancy in African American compared to white women,33 and has itself been associated with adverse pregnancy outcomes.34 Importantly, we previously demonstrated in non-pregnant mice that G. vaginalis can trigger features of BV on its own (e.g. clue-like cells, sialidase activity, mucus degradation, epithelial exfoliation), and also encourages pathogenesis by other urogenital bacteria.35–37 Here we developed a mouse model of vaginal co-inoculation during pregnancy to test the hypothesis that G. vaginalis may enhance GBS colonization and/or invasive infection during pregnancy.
Methods
Bacterial strains and growth conditions
All experiments utilized a spontaneous streptomycin resistant strain of G. vaginalis JCP8151B-SmR derived from a strain isolated from a woman with BV,38 and a serotype III Group B Streptococcus (GBS) strain COH1 isolated from a case of systemic neonatal infection,39 expressing a plasmid containing an erythromycin resistance cassette (pDC-Erm). Antibiotic-resistant bacteria were used because bacteria endogenous to the mice may be able to grow on non-selective plates with no antibiotics. Use of antibiotic-resistant strains of GBS and G. vaginalis in conjunction with selective plates containing those antibiotics provided confidence that the colonies observed and counted to monitor infection were indeed the inoculated strains. G. vaginalis was grown in NYCIII media at 37 °C in a Coy anaerobic chamber and GBS was grown in TH media supplemented with 100 μg/mL erythromycin at 37 °C aerobically overnight. GBS was grown statically in Todd Hewitt (TH) media supplemented with 10 μg/mL erythromycin at 37 °C aerobically overnight. Following growth, the bacterial cultures were centrifuged, and each pellet was resuspended in PBS. The optical density (OD600) of the bacterial suspensions were measured. The bacterial suspensions were centrifuged and resuspended in the appropriate volume of PBS according to the equation [(OD600 of PBS suspension)*(volume of PBS suspension centrifuged)] / (inoculum target OD600). The target ODs were determined empirically to be: GBS to either OD = 8 (~107 colony forming units (cfu) per 10 uL) or OD = 40 (~108 cfu per 10 uL) and G. vaginalis to OD = 10 (~107 cfu per 10 uL). The actual doses of bacterial inocula were confirmed by serial dilution and plating immediately after performing mouse inoculations.
Generation of timed pregnant mice
Female C57BL/6NCR mice were obtained from the National Cancer Institute (now Charles River, Fredericks facility) and male BALB/c mice were obtained from Jackson Labs between January 2015-April 2018. Four days prior to mating Day 0, some urine-soaked bedding from a BALB/c male’s cage was added to the bedding of the females’ cages. The female mouse’s cycle is 4 to 5 days in length, but group housed females often develop cycles that are more irregular as well as longer. The pheromones in the male urine will cause the majority of the group housed females to begin a new estrus cycle by the third day of exposure. On Day 0, females were weighed, and their stage of the estrus cycle determined visually. Females determined to be in estrus were placed in the cage of a Balb/c male (1:1) late in the afternoon of Day 0. The following morning (Day 0.5/Day 1), females were removed from male cages, checked for the presence of a vaginal plug, and returned to their original cage. Females that were both visibly rounded by gestational day (E)13, and weighed a minimum of 120% of their original weight were classified as pregnant and used for infection experiments.
Mouse vaginal co-inoculation model (Fig 1A)
Figure 1. Co-inoculation with G. vaginalis facilitates GBS vaginal colonization in pregnant mice.
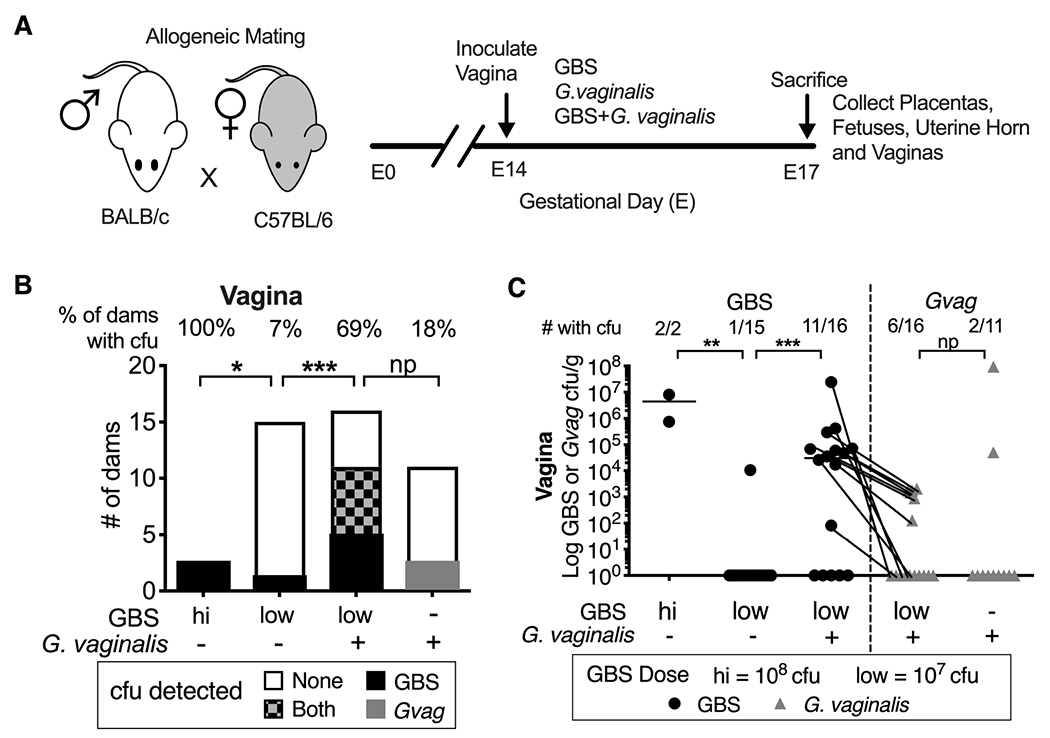
(A) Schematic of allogeneic timed-pregnancy vaginal co-inoculation model. (B) Number of dams without and with bacterial cfu in vaginal homogenates. Percentage of dams with detectable cfu indicated across the top of each bar. Fisher’s exact test *** P < 0.001; *P < 0.05; np = not powered. (C) Bacterial titers in vaginal tissue homogenates. Data points for GBS (circles) and G. vaginalis (triangles) cfu from the same tissues are connected with lines. The fraction of vaginas with detectable cfu is indicated across the top of graph. Bars denote geometric mean. 11 independent experiments. Mann-Whitney ** P < 0.01; *** P < 0.001; ns = not significant.
On E14, dams were restrained and inoculated vaginally with two immediately successive 10 μL inoculations as follows: PBS and PBS (mock vehicle controls); G. vaginalis then PBS; PBS then GBS; G. vaginalis then GBS. This small volume of liquid was entirely taken up into the vagina (it did not pool at the introitus). To further ensure that the inoculum was maintained in the vagina, the mouse was restrained stationary with its tail end raised up for ~30 seconds before being returned to its cage. An initial experiment confirmed that the order of inoculation of the two bacteria did not affect colonization or infection. Previous studies reported that a 108 colony forming units (cfu) inoculum of GBS strain COH1 resulted in vaginal colonization and ascending intrauterine infection in >90% of pregnant mice and intrauterine fetal demise and/or PTB in 16-40% of pups 40–42. As a positive control, we included a small number of mice inoculated with 108 cfu of GBS (GBShigh), but used a ten-fold lower 107 dose of GBS (GBSlow) in our co-inoculation model. The dose of G. vaginalis was ~108 colony forming units (cfu) and the dose of GBS was either 107 or 108 cfu. Dams convalesced undisturbed (so as to not trigger adverse pregnancy outcomes) until they were sacrificed on E17 (see below).
Dams were sacrificed by cervical dislocation under isofluorane anesthesia at E17 to evaluate aseptically dissected tissues from all animals at one time point for bacterial cfu and histological analysis (see supplemental material). In contrast to studies using higher doses or more invasive strains of GBS, we saw no evidence that any dam delivered prior to E17 in 13 independent experiments (although we did not continuously monitor the dams with video surveillance). Further, there was no difference in fetal weight, incidence of intrauterine fetal demise (IUFD) between mono- and co-infected groups (S1 Fig), nor was GBS recovered from amniotic fluid or fetuses.
Tissue collection.
On E17 dams were sacrificed by cervical dislocation under isofluorane anesthesia to evaluate aseptically dissected tissues from all animals at one time point. Vaginas were collected and bisected longitudinally; one half was fixed in methacarn (60% methanol, 30% chloroform, 10% glacial acetic acid) and the other half was homogenized for bacterial cfu determination (see below). A piece of uterine tissue was collected and weighed from each horn immediately adjacent to the cervix and surrounding the most proximal fetus. All placentas and fetuses from both horns were collected and weighed. The uterine tissue and the first two placentas proximal to the cervix from the left horn were homogenized in sterile PBS. Remaining placentas were fixed in methacarn.
Bacterial cfu determination.
To distinguish GBS and G. vaginalis from endogenous mouse vaginal bacteria, cfu were determined by serial dilution in 96-well plates in PBS and plating on selective agar media (supplemented with streptomycin for G. vaginalis and erythromycin for GBS). GBS plates were incubated at 37 °C aerobically overnight. Our prior experiments determined that the plasmid conferring erythromycin resistance in GBS is maintained during in vivo vaginal colonization experiments over extended time periods (data not shown). G. vaginalis plates were grown at 37 °C anaerobically for 48 h. Colonies were counted and reported as cfu per gram of tissue or cfu per placenta.
Histological analysis and placental pathology score.
Histological slide preparation and hematoxylin and eosin (H&E) staining of the fixed vaginal and placental tissue were performed by the Department of Developmental Biology Histology Core at Washington University. Placentas were visualized with a Zeiss Apotome microscope using a 20x objective. A blinded observer scored the pathophysiology (see Fig 5) based on the presence or absence of placental cellular damage and vascular lesions characterized by dark eosin staining and fragmented hematoxylin signals as follows: 0 = absent or barely seen, 1 = occasional, 2 = moderate, 3 = abundant. Vaginas were visualized on an Olympus BX61 microscope using a 10x objective and scored by a blinded observer for epithelial keratinization and exfoliation as follows: 0 = absent, 1 = mild, 2 = severe.
Figure 5. Co-inoculation with GBS and G. vaginalis adversely affects the placenta independent of sustained ascending infection.
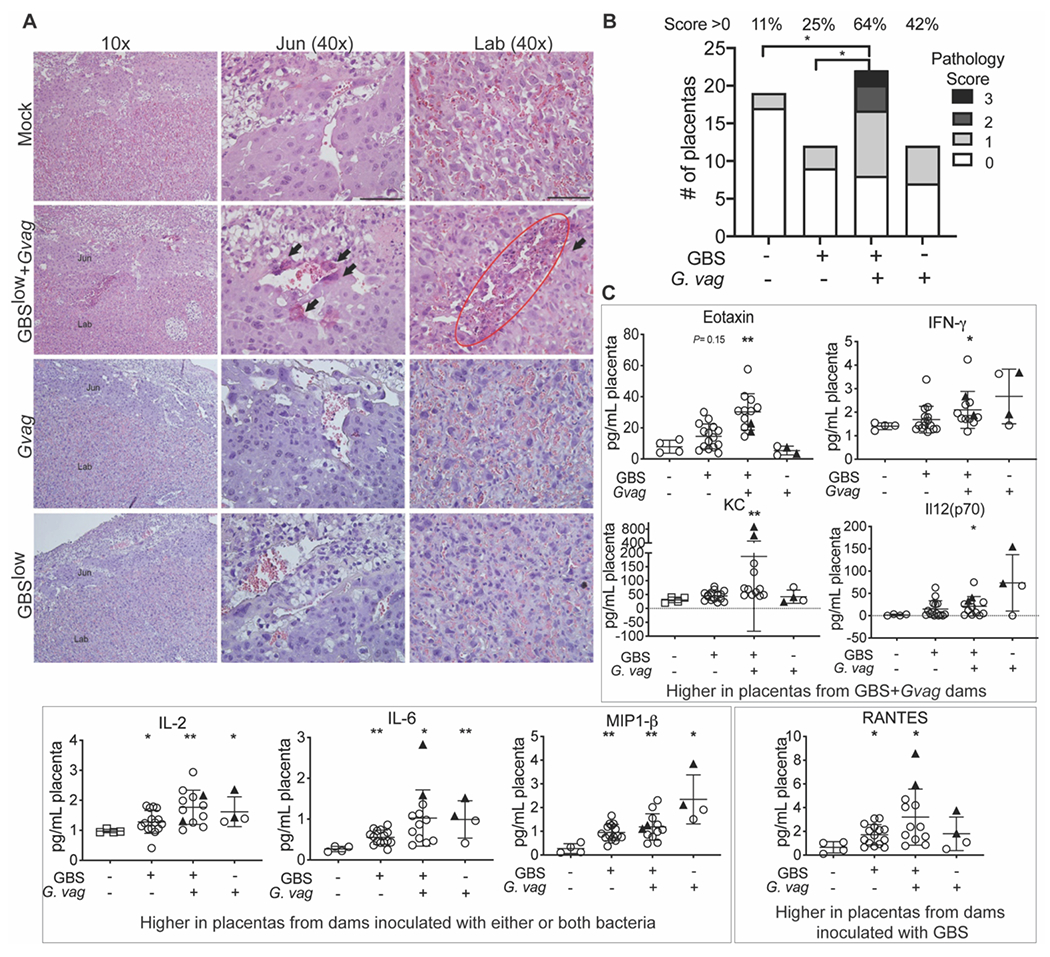
(A) Representative images of H&E stained fixed placental sections from dams inoculated with bacteria or mock controls but without detectable placental infection. Placentas from GBS low+G. vaginalis show cellular damage, characterized by dark eosin staining and fragmented hematoxylin signals (arrows), exclusively within the junctional (Jun) and labyrinthine (Lab) layers. Scale bars 50 μm. (B) Blinded scoring of the placental pathologic features shown in panel A. 2 independent experiments, 3 dams per group. (C) Cytokine/chemokine levels in placenta homogenates. A Kruskal-Wallis test was performed, followed by Dunn’s multiple comparisons test comparing each bacterial group to the PBS control group. * P < 0.05; ** P < 0.01.
Placenta cytokine/chemokine analysis.
Placenta homogenates were centrifuged for 5 min at 12000 g at 4°C and the supernatant was transferred (taking care to avoid the pellet and any fat) to a fresh Eppendorf tube and stored at −20°C until analysis. Supernatants were thawed on ice, centrifuged again at 4°C to remove any remaining particulates and the supernatant collected. Cytokine/chemokine levels were measured using the Bio-Plex-Pro Mouse Cytokine 23-Plex Panel multiplex cytokine bead kit (Bio-Rad), which quantifies 23 different cytokines/chemokines. The assays were performed according to manufacturer instructions, except using 10-fold less standard and half the number of coupled beads and detection antibodies indicated in the protocol.
Immunofluorescence microscopy.
Slides were stained for GBS (rabbit polyclonal antibody 1:200, Abcam: ab 53584) and the cytoskeleton (rabbit monoclonal anti-Vimentin antibody 1:200, Abcam: ab 92547). After three PBS washes at room temperature, antigen-antibody complexes were detected with species-specific Alexa Fluor 488 and 594–conjugated secondary antibodies (1:500, Invitrogen). Slides were counterstained with DAPI (1:1000) for 10 minutes to visualize nuclei and mounted with Prolong Gold (Life Technologies). Images were obtained with a Zeiss Apotome microscope using ×40 or ×60 oil immersion objectives.
Statistics.
Our primary outcome of interest was vaginal colonization by GBS. To determine sample sizes, we anticipated 10% of dams would be colonized vaginally with GBS alone. We required a minimum of 11 mice in each group in order to detect a significant difference (alpha 0.05, beta 0.2, power 80%) if 60% of co-inoculated mice became colonized with GBS (ClinCalc.com). We used 15 and 16 mice in the GBS and GBS+G. vaginalis groups, respectively, which powered us to detect a significant difference if 52% of co-inoculated dams became colonized with GBS. We used a minimum number of animals in the GBS high dose group because we anticipated, based on previous studies, that 100% of dams would become colonized. Thus fewer animals were required to detect a significant difference between GBS high dose and GBS low dose groups. Our study was underpowered (at only 18%) to detect a significant effect of GBS on G. vaginalis since we only had 11 mice in the G. vaginalis alone group. Based on the observed rates of vaginal colonization by G. vaginalis (18% G. vaginalis alone, 38% G. vaginalis+GBS we would require 78 animals per group to detect significance with alpha 0.05, beta 0.2, power 80%, which is well beyond the scope of mouse pregnancy models. GraphPad Prism 8.0 software was used for all statistical analyses; tests used to analyze each dataset are indicated in the figure legends. Relative risk was calculated using the Koopman asymptotic method.
Ethics statement.
Mouse experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals and approved by the Animal Studies Committee of Washington University School of Medicine (Protocol #20140114 and #20170081).
Results
Consistent with previous reports,41,43 a high dose (~108 cfu) of GBS or G. vaginalis alone in our model (Fig 1A) resulted in vaginal colonization (Fig 1B–C). Vaginal colonization was only rarely achieved upon 10-fold reduction of the GBS inoculum (GBS low =107 GBS cfu, hereafter referred to as “GBS-alone”; Fig 1B–C). In contrast, co-inoculation with G. vaginalis resulted in a 10-fold increased risk of dams becoming vaginally colonized by GBS compared to animals inoculated with GBS-alone (69% vs 7%; RR:10.31, 95%CI: 2.710-59.04; P=0.0006, Fishers Exact) (Fig 1).
Infection by GBS in utero is known to be a progressive infection that starts with GBS ascending from the vagina through the cervix, followed by placental invasion, causing chorioamnionitis and bacterial invasion into the amniotic sack. Mice receiving GBS-alone never developed ascending infection of uterine (0/9 dams) or placental tissues (0/23 dams) (Fig 2A–B). Note that relative risk was ∞ because there were zero events of utero-placental infection in the GBS-alone group. Nevertheless, compared to GBS-alone, significantly more of the GBS+G. vaginalis co-inoculated dams had detectable bacterial infections in uterine (42%, P=0.0451, Fishers Exact) and placental tissues (40%, P=0.0178) (Fig 2A–D). Immunofluorescence microscopy of placentas collected in parallel revealed that GBS was present in both maternal and fetal sides of the placenta (Fig 3); however, GBS cfu were not detected in the amniotic fluid or fetus (data not shown). The absence of detectable GBS in amniotic fluid suggests either i) GBS infection had not progressed to the point of invading the amniotic sack, ii) GBS invaded earlier but was cleared from this niche by the time we sacrificed the animals, or iii) GBS was present in amniotic fluid, but at a level lower than our limit of detection. We think the first possibility is the most likely. When GBS cfu were detected in the placenta and/or uterus of co-inoculated dams, GBS cfu were also detected in the vagina (S2 Table). Levels of GBS in the vagina and uterine tissues were significantly correlated (Fig 2E). Some dams from both the GBS-alone and co-inoculated groups showed evidence of keratinization and exfoliation of the vaginal epithelium at E17, but this phenotype did not correlate with vaginal colonization or ascending infection (S3 Fig).
Figure 2. Co-inoculation with G. vaginalis results in increased GBS invasive ascending infection.
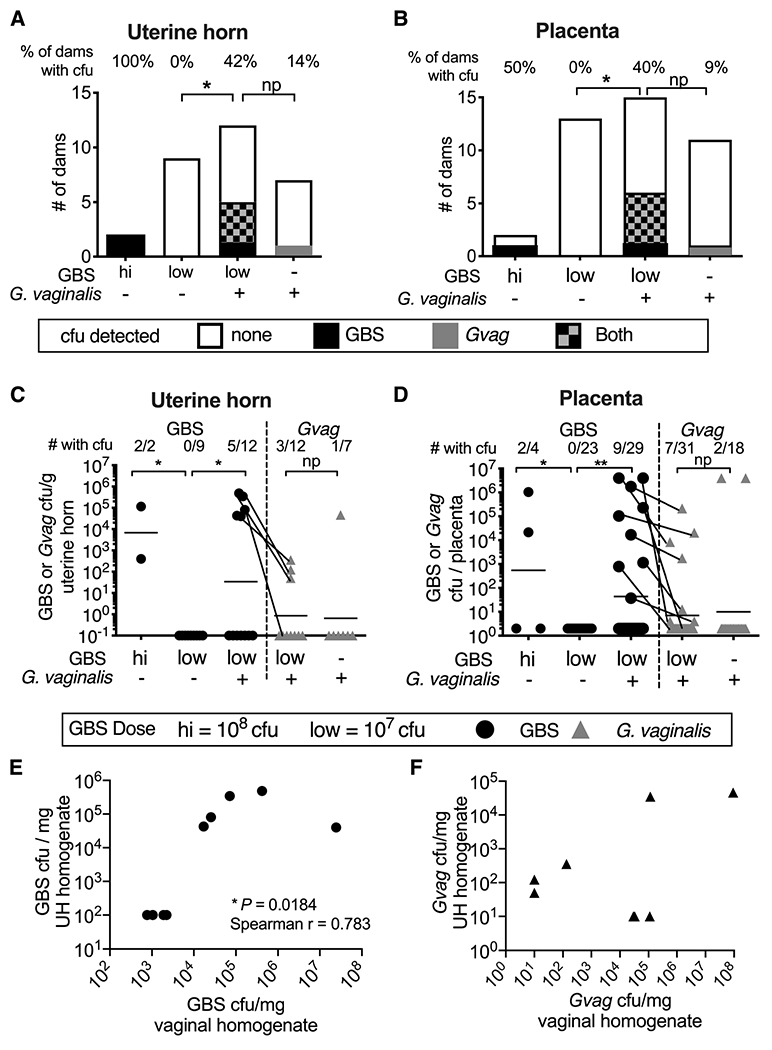
(A-B) Number of dams without or with bacterial cfu in uterine horn (A) or placental (B) tissue homogenates. Percentage of dams with detectable cfu indicated across the top of each bar. Fisher’s exact test *P < 0.05; np = not powered. (C) Bacterial titers in uterine tissue homogenates. The fraction of dams with detectable cfu is indicated across the top of the graph. 11 independent experiments. Uterine tissue was not collected from every dam, therefore, data are from a subset of animals included in Figure 1. (D) Bacterial cfu in placental homogenates. The fraction of placentas with detectable cfu is indicated across the top of the graph. 13 independent experiments. (C-D) Bars denote geometric mean. Data points for GBS (circles) and G. vaginalis (triangles) cfu from the same tissues are connected with lines. Mann-Whitney ** P < 0.01; * P < 0.05; ns= not significant. Mann-Whitney test comparing G. vaginalis titers in placentas from mono vs. co-inoculated dams was 0.07 if the two outliers were excluded from the analysis. (E-F) Correlation of GBS (E) and G. vaginalis (F) cfu in uterine horn (UH) and vaginal homogenates.
Figure 3. Co-inoculation with G. vaginalis facilitates GBS maternal-fetal unit invasion.
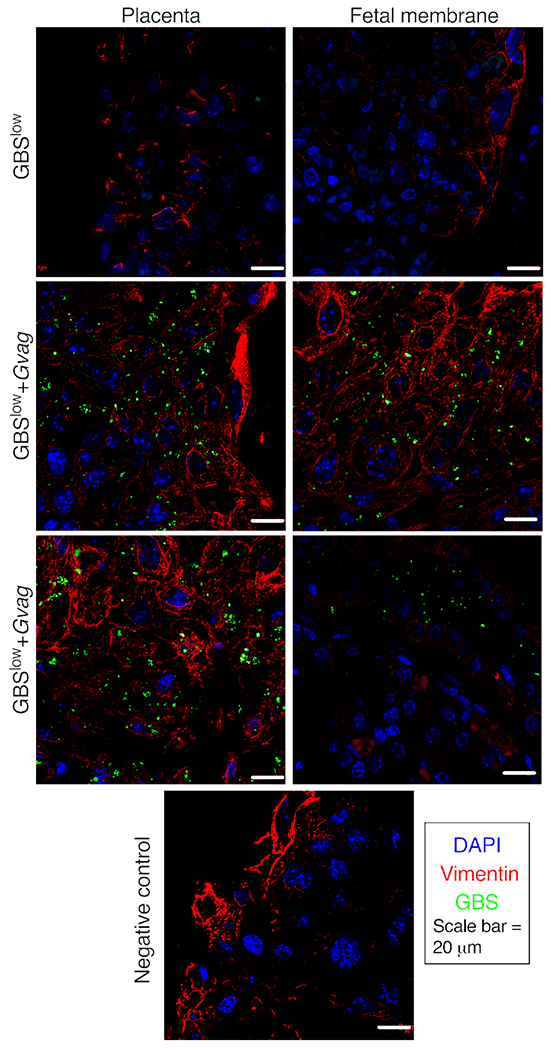
Representative images of immunofluorescence microscopy of the maternal-fetal interface from placentas isolated from dams inoculated with GBSlow or GBSlow+Gvag. GBS bacteria were detected with a monoclonal antibody (green). Sections were counterstained with DAPI (nuclei; blue) and vimentin (vasculature; red). Similar robust GBS staining was observed in placentas that were collected from GBSlow+Gvag dams that had placental infection evident by detectable cfu. The negative control panel (bottom) is a section from a GBSlow+Gvag placenta stained in parallel but omitting the GBS 1° antibody.
Consistent with prior studies using high GBS doses or more invasive GBS strains, 42,44,45 placentas examined for pathology revealed inflammatory infiltrates at the decidual compartment by the junctional zone specifically in co-inoculated dams with detectable placental infections (Fig 4A). Consistent with the presence of inflammatory cells, there were high levels of IL-1β, MIP-1± and G-CSF exclusively in placentas from co-inoculated dams that had detectable titers (triangle symbols), suggesting this response depended on active infection (Fig 4B). Other histopathologic phenotypes were observed within the junctional zone and labyrinth in placentas from co-inoculated mice that did not have signs of histological inflammation and irrespective of whether live placental bacteria were detected (Fig 5A). In a blinded analysis, compared to the mock or GBS-alone groups, placentas from co-inoculated dams were significantly more likely to exhibit histopathology (Fig 5B). Interestingly, certain cytokines (different from those elevated in infected placentas shown in 4B) were elevated in mice vaginally inoculated with bacteria, independent of detectable placental infection (Fig 5C). Placental IL-2, IL-6 and MIP-1β were higher in mice inoculated with bacteria (G. vaginalis, GBS-alone or both) compared to PBS controls. Mice inoculated with GBS-alone, (with or without G. vaginalis), had significantly higher levels of RANTES. Eotaxin, IFN-γ ,IL-12p70 and KC (mouse functional homolog of IL-8) were significantly higher in the GBS+G. vaginalis group. IL-6, RANTES, and KC were highest in placentas with active infection (triangle symbols), but the significant difference between the mock and GBS+G. vaginalis groups remained even if the data from infected placentas were excluded from the analysis. Together, these data suggest that vaginal exposure to bacteria can trigger histopathology and a placental cytokine response that overlaps with, yet is distinct from, the response present during active placental infection.
Figure 4. Placental inflammation is evident in co-inoculated dams with detectable GBS placental infection.
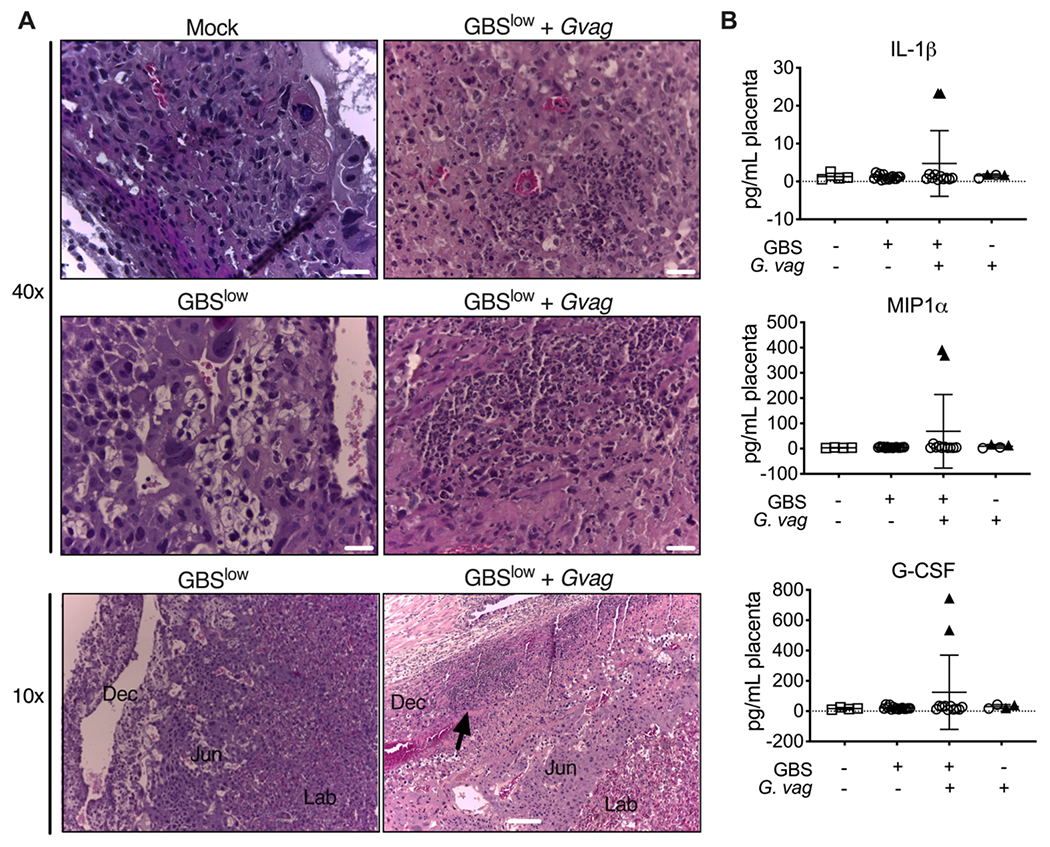
(A) H&E staining reveals prolific PMNs, cell debris and monocytes in the decidual compartment by the junctional zone (black arrow) in placentas from the GBSlow+Gvag dams that had detectable infection. Dec = decidua; Jun = junctional zone; Lab = labryinth. Scale bars 20 μm (top 40x images) and 100 μm (bottom 10x images). (B) Cytokine/chemokine levels in placenta homogenates. Black triangles denote placentas that had detectable bacterial cfu.
To examine if uterine infection was evident at earlier time points, ten dams (n= 5 GBS-alone, n=5 GBS+G. vaginalis) were sacrificed at 24 hours (h) post infection (E15). Dams inoculated with GBS alone cleared the bacteria rather rapidly, with only one of five having detectable GBS cfu in the vagina at 24 h. In contrast, G. vaginalis and GBS were detectable in the vagina of three of five co-inoculated dams. However, none of these dams had detectable ascending infection by either bacterium at 24 h (data not shown), supporting the conclusion that ascending infection by both organisms required longer than 24 h to occur in this model. These data also indicate that the inoculation procedure did not introduce the bacterial inoculum into the upper reproductive tract.
Although the experiments were not originally designed to investigate the effect of GBS on G. vaginalis, we note that 38% of co-inoculated dams had detectable vaginal colonization with G. vaginalis compared to 18% of dams inoculated with G. vaginalis alone (Fig 1). A previous study using a different strain of G. vaginalis inoculated at E12-13 did not detect uterine or placental infection at E15.43 In our model, the rate of G. vaginalis placental infection at E17 was 9% in mice inoculated with G. vaginalis-alone and 30% in mice co-inoculated with GBS (Fig 2B). Although these findings are potentially interesting, we were underpowered to detect significant differences between the proportions for G. vaginalis vaginal colonization and placental infection. Among the co-inoculated dams, two with no detectable vaginal titers of G. vaginalis nonetheless had G. vaginalis cfu in uterine horn tissue, one of which also had placental titers (S2 Table). Similarly, five co-inoculated dams with no detectable G. vaginalis in the vagina nonetheless had evident ascending GBS infection (S2 Table).
Comment
Principal Findings
Co-inoculation of pregnant mice with Gardnerella led to a 10-fold higher relative risk of vaginal colonization with GBS. In this model, invasive infections of uterine and placental tissue by GBS only occurred in co-inoculated animals. Levels of invasive GBS in uterine tissue were proportional to the GBS vaginal burden. Ascending G. vaginalis uterine and placental infection also occurred, including in mice that did not have detectable vaginal titers. This suggests that following ascension of G. vaginalis the mouse was able to clear G. vaginalis from the vagina, but not from the upper reproductive tract. Sustained vaginal colonization by G. vaginalis was also not required to enhance GBS ascending infection. Finally, co-inoculation with GBS and G. vaginalis resulted in placental histopathology even in the absence of placental infection.
Clinical Implications:
Our data highlight G. vaginalis as a potential risk factor that could contribute to GBS neonatal disease by promoting GBS vaginal colonization in women. The results are consistent with some previous data in women showing an association between the vaginal microbiota, particularly BV and Intermediate Nugent score, and GBS colonization.14,15 These findings could help explain why Black and African American women (who are more likely to have a polymicrobial vaginal microbiota and have higher rates of BV)19–22 have higher rates of GBS colonization and neonatal infections.14,15,27–30 As of 2016, the rate of severe GBS infections within the first week of life in the U.S. was 300% higher in Black infants compared to their white counterparts.46 Understanding the factors contributing to racial disparities in GBS colonization is necessary for the development of measures to limit the disproportionate burden of GBS disease. These findings may be even more broadly relevant, since G. vaginalis has been detected, albeit at lower levels than during BV, in the majority of women examined.
Among neonates who develop GBS early onset disease, 40% were born to mothers who tested GBS-negative at antenatal screening, suggesting the women may have become colonized after screening took place.47 A recent study showed that African American women had a higher rate of conversion from GBS-negative to GBS-positive status between the time of normal screening and presentation at the labor and delivery unit (relative risk 2.0; 95%CI 1.02-3.8).48 Future studies are warranted in diverse patient cohorts to determine whether vaginal colonization by G. vaginalis is a risk factor for GBS colonization, becoming GBS-positive following antenatal screening, or for invasive GBS disease. This could translate to improved screening for vaginal colonization and treatment strategies in specific at-risk populations.
Research Implications:
Further studies are needed to delineate the mechanism(s) by which G. vaginalis encourages GBS colonization, define the determinants of ascending infection, examine how exposure to these bacteria adversely affects the placenta independent of active infection, and whether other aspects of pathogenesis are impacted, such as PTB or vertical transfer to the neonate.44,49 Our data suggest that even transient vaginal exposure to G. vaginalis may promote GBS colonization. Further, transient vaginal exposure to GBS or G. vaginalis resulted in distinct placental cytokine signatures and histopathology, even in the absence of detectable placental infection. The findings are consistent with other models in which Gardnerella has been described as a “covert pathogen,” a microbe that can have pathologic effects despite being absent at the time and place disease features manifest.36,37 The presence of G. vaginalis in mouse placental tissue in our model is consistent with studies that have found Gardnerella in human placenta50 and provide a tool for investigating the impact of this bacteria on tissues of the upper reproductive tract during pregnancy. Similar studies in mice could be performed to examine whether additional strains of GBS and G. vaginalis yield similar results. Studies could be expanded to test whether other bacteria associated with BV or CST-IV could have a similar effect as G. vaginalis on GBS colonization and infection. Understanding how different strain backgrounds of GBS or G. vaginalis, or even other members of the vaginal microbiota, contribute to findings in women or in mouse models could also yield insights important for future research efforts aimed at treatment and prevention.
Strengths and Limitations:
Key strengths of our study were the development of a GBS/G. vaginalis co-inoculation model during allogeneic pregnancy.51 Limitations necessitated by the labor and cost-intensive nature of the mouse pregnancy model include that only one strain each of G. vaginalis and GBS were tested, multiple doses or variations in timing of bacterial inoculation were not thoroughly examined, and observations were primarily at a single time point. We used a 10-fold higher dose of G. vaginalis than GBS, therefore co-inoculated dams received a higher total bacteria dose than those inoculated with GBS alone. It has been established that the bacterial load in the vagina is 10 to 100-fold higher in the context of BV than without BV52. Therefore, even if the effect of G. vaginalis on GBS infection in pregnant mice is due to the heightened overall bacterial load of the inoculum used, this is directly relevant to the situation of BV in women. Our primary focus was to examine the effect of G. vaginalis on GBS; thus, our studies included fewer dams inoculated with G. vaginalis alone and were underpowered to detect significant effects of GBS on G. vaginalis. Vaginal inoculation models in pregnant mice have proven valuable,41,43,44,49,53 but they do not recapitulate all features of human pregnancy. Mice have a different endogenous vaginal microbiota than humans and not all studies in women concur with the interpretation that dysbiotic communities are more likely to harbor GBS (Table S1). Interestingly, a recent study showed that the composition of the endogenous vaginal microbiome can impact GBS ascending uterine infection in non-pregnant mice.54 As with previous GBS and G. vaginalis mouse models in pregnancy, it is unknown whether or how the endogenous microbiota in mice may influence the observed phenotypes in our model.
Conclusions:
These data suggest that G. vaginalis plays a causal role in promoting GBS colonization of the vaginal niche during pregnancy.
Supplementary Material
AJOG at a Glance:
The purpose of this study was to develop a murine pregnancy model to test if Gardnerella vaginalis – an abundant vaginal bacterium in certain settings, especially bacterial vaginosis (BV) – facilitates vaginal GBS colonization or invasive intrauterine infection.
Co-inoculation with G. vaginalis promoted sustained GBS vaginal colonization and enhanced the likelihood of uterine and placental GBS infection associated with a strong inflammatory signature in pregnant mice. Sustained vaginal detection of G. vaginalis was dispensable for GBS colonization or invasion. Even in mice without detectable ascending infection or histological inflammation, placentas from those co-exposed to GBS and G. vaginalis displayed other histopathologic features and a distinct cytokine signature.
Gardnerella may be a causal factor that engenders susceptibility to GBS colonization and invasive infections during pregnancy. These findings might help explain persistent disparities in invasive GBS infections as BV is more common among Black women.
Acknowledgements
The authors thank the Department of Developmental Biology at Washington University for histology preparation and Cory Weimer, Bisiayo Fashemi (Mysorekar Lab), and Nadum Member-Meneh for technical assistance.
Sources of funding:
National Institute of Allergy and Infectious Disease grant R01 AI114635 (to ALL), the Burroughs Wellcome fund preterm birth initiative (award 1012547 to ALL), and a March of Dimes, WU Prematurity Research Center Pilot Grant (to ALL), Children’s Discovery Institute fellowship PD-F-2014-395 (to NMG), National Institute of Child Health and Human Development grant R01 HD091218 (to IUM).
Footnotes
Current Address & Affiliation: Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Diego, a1lewis@ucsd.edu
Paper presentation information: This manuscript was presented in part at the 20th Lancefield International Symposium on Streptococci and Streptococcal Diseases and the 66th annual meeting of the Society for Reproductive Investigation.
Conflict of interest statement: The authors declare no conflicts of interest.
References
- 1.Madrid L, Seale AC, Kohli-Lynch M, et al. Infant Group B Streptococcal Disease Incidence and Serotypes Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S160–S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi-Jassir F, Seale AC, Kohli-Lynch M, et al. Preterm Birth Associated With Group B Streptococcus Maternal Colonization Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S133–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell NJ, Seale AC, O’Driscoll M, et al. Maternal Colonization With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S100–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regan JA, Klebanoff MA, Nugent RP, et al. Colonization with group B streptococci in pregnancy and adverse outcome. VIP Study Group. Am J Obstet Gynecol. 1996; 174(4):1354–1360. [DOI] [PubMed] [Google Scholar]
- 5.Allen U, Nimrod C, Macdonald N, Toye B, Stephens D, Marchessault V. Relationship between antenatal group B streptococcal vaginal colonization and premature labour. Paediatr Child Health. 1999;4(7):465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feikin DR, Thorsen P, Zywicki S, Arpi M, Westergaard JG, Schuchat A. Association between colonization with group B streptococci during pregnancy and preterm delivery among Danish women. Am J Obstet Gynecol. 2001;184(3):427–433. [DOI] [PubMed] [Google Scholar]
- 7.Regan JA, Chao S, James LS. Premature rupture of membranes, preterm delivery, and group B streptococcal colonization of mothers. Am J Obstet Gynecol. 1981. ;141(2):184–186. [DOI] [PubMed] [Google Scholar]
- 8.Brigtsen AK, Jacobsen AF, Dedi L, Melby KK, Fugelseth D, Whitelaw A. Maternal Colonization with Group B Streptococcus Is Associated with an Increased Rate of Infants Transferred to the Neonatal Intensive Care Unit. Neonatology. 2015;108(3):157–163. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Wu C, Cao X, et al. Risk factors for neonatal group B streptococcus vertical transmission: a prospective cohort study of 1815 mother-baby pairs. J Perinatol. 2018;38(10):1309–1317. [DOI] [PubMed] [Google Scholar]
- 10.Roca A, Bojang A, Camara B, et al. Maternal colonization with Staphylococcus aureus and Group B streptococcus is associated with colonization in newborns. Clin Microbiol Infect. 2017;23(12):974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tazi A, Plainvert C, Anselem O, et al. Risk Factors for Infant Colonization by Hypervirulent CC17 Group B Streptococcus: Toward the Understanding of Late-onset Disease. Clin Infect Dis. 2019;69(10):1740–1748. [DOI] [PubMed] [Google Scholar]
- 12.Hall J, Adams NH, Bartlett L, et al. Maternal Disease With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S112–S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seale AC, Blencowe H, Bianchi-Jassir F, et al. Stillbirth With Group B Streptococcus Disease Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S125–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyn LA, Krohn MA, Hillier SL. Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am J Obstet Gynecol. 2009;201(1):76 e71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyn LA, Moore DM, Hillier SL, Krohn MA. Association of sexual activity with colonization and vaginal acquisition of group B Streptococcus in nonpregnant women. Am J Epidemiol. 2002;155(10):949–957. [DOI] [PubMed] [Google Scholar]
- 16.Mu X, Zhao C, Yang J, et al. Group B Streptococcus colonization induces Prevotella and Megasphaera abundance-featured vaginal microbiome compositional change in non-pregnant women. PeerJ. 2019;7:e7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen GH, Randis TM, Desai PV, et al. Group B Streptococcus and the Vaginal Microbiota. J Infect Dis. 2017;216(6):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota T, Nojima M, Itoh S. Vaginal bacterial flora of pregnant women colonized with group B Streptococcus. J Infect Chemother. 2002;8(4):326–330. [DOI] [PubMed] [Google Scholar]
- 19.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108Suppl 1:4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex Transm Dis. 2019;46(5):304–311. [DOI] [PubMed] [Google Scholar]
- 21.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34(11):864–869. [DOI] [PubMed] [Google Scholar]
- 22.Fettweis JM, Brooks JP, Serrano MG, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology. 2014;160(Pt 10):2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrazzo JM, Martin DH, Watts DH, et al. Bacterial vaginosis: identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sex Transm Dis. 2010;37(12):732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allsworth jE , Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109(1):114–120. [DOI] [PubMed] [Google Scholar]
- 25.Meis PJ, Goldenberg RL, Mercer B, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1995;173(4):1231–1235. [DOI] [PubMed] [Google Scholar]
- 26.Ness RB, Hillier S, Richter HE, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc. 2003;95(3):201–212. [PMC free article] [PubMed] [Google Scholar]
- 27.Stapleton RD, Kahn JM, Evans LE, Critchlow CW, Gardella CM. Risk factors for group B streptococcal genitourinary tract colonization in pregnant women. Obstet Gynecol. 2005;106(6):1246–1252. [DOI] [PubMed] [Google Scholar]
- 28.Parente V, Clark RH, Ku L, et al. Risk factors for group B streptococcal disease in neonates of mothers with negative antenatal testing. J Perinatol. 2017;37(2):157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatr Infect Dis J. 2011;30(11):937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton ER, Butler MC, Shain RN. Sexual behavior and vaginal colonization by group B Streptococcus among minority women. Obstet Gynecol. 1996;88(4 Pt 1):577–582. [DOI] [PubMed] [Google Scholar]
- 31.Nelson DB, Komaroff E, Nachamkin I, et al. Relationship of selected bacterial vaginosis-associated bacteria to Nugent score bacterial vaginosis among urban women early in pregnancy. Sex Transm Dis. 2013;40(9):721–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014;210(3):338–343. [DOI] [PubMed] [Google Scholar]
- 33.Uscher-Pines L, Hanlon AL, Nelson DB. Racial differences in bacterial vaginosis among pregnant women: the relationship between demographic and behavioral predictors and individual BV-related microorganism levels. Matern Child Health J. 2009;13(4):512–519. [DOI] [PubMed] [Google Scholar]
- 34.Nelson DB, Hanlon A, Hassan S, et al. Preterm labor and bacterial vaginosis-associated bacteria among urban women. J Perinat Med. 2009;37(2):130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert NM, Lewis WG, Lewis AL. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One. 2013;8(3):e59539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert NM, Lewis WG, Li G, Sojka DK, Lubin JB, Lewis AL. Gardnerella vaginalis and Prevotella bivia Trigger Distinct and Overlapping Phenotypes in a Mouse Model of Bacterial Vaginosis. J Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilbert NM, O’Brien VP, Lewis AL. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog. 2017;13(3):e1006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem. 2013;288(17):12067–12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin TR, Rubens CE, Wilson CB. Lung antibacterial defense mechanisms in infant and adult rats: implications for the pathogenesis of group B streptococcal infections in the neonatal lung. J Infect Dis. 1988; 157(1):91–100. [DOI] [PubMed] [Google Scholar]
- 40.Vornhagen J, Armistead B, Santana-Ufret V, et al. Group B Streptococcus exploits vaginal epithelial exfoliation for ascending infection. J Clin Invest. 2018;128(5):1985–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vornhagen J, Quach P, Boldenow E, et al. Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth. MBio. 2016;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiarot E, Spagnuolo A, Maccari S, et al. Protective effect of Group B Streptococcus type-III polysaccharide conjugates against maternal colonization, ascending infection and neonatal transmission in rodent models. Sci Rep. 2018;8(1):2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sierra LJ, Brown AG, Barila GO, et al. Colonization of the cervicovaginal space with Gardnerella vaginalis leads to local inflammation and cervical remodeling in pregnant mice. PLoS One. 2018;13(1):e0191524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randis TM, Gelber SE, Hooven TA, et al. Group B Streptococcus beta-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J Infect Dis. 2014;210(2):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kothary V, Doster RS, Rogers LM, et al. Group B Streptococcus Induces Neutrophil Recruitment to Gestational Tissues and Elaboration of Extracellular Traps and Nutritional Immunity. Front Cell Infect Microbiol. 2017;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Control CfD. Active Bacterial Core Surveillance (ABCs) Report Emerging Infection Program Network group B Streptococcus, 2016. 2018.
- 47.Nanduri SA, Petit S, Smelser C, et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019;173(3):224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiel MH, Hacker MR, Haviland MJ, et al. Racial disparities in intrapartum group B Streptococcus colonization: a higher incidence of conversion in African American women. J Perinatol. 2019;39(3):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrade EB, Magalhaes A, Puga A, et al. A mouse model reproducing the pathophysiology of neonatal group B streptococcal infection. Nat Commun. 2018;9(1):3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cox C, Saxena N, Watt AP, et al. The common vaginal commensal bacterium Ureaplasma parvum is associated with chorioamnionitis in extreme preterm labor. J Matern Fetal Neonatal Med. 2016;29(22):3646–3651. [DOI] [PubMed] [Google Scholar]
- 51.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padubidri V, Daftary Shirish N. Disease of the vagina. In: Shaw’s Textbook fo Gynaecology. 15th ed. New Delhi: Elsevier; 2011. [Google Scholar]
- 53.Pavlidis I, Spiller OB, Sammut Demarco G, et al. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat Commun. 2020;11(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vrbanac A, Riestra AM, Coady A, Knight R, Nizet V, Patras KA. The murine vaginal microbiota and its perturbation by the human pathogen group B Streptococcus. BMC Microbiol. 2018; 18(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


