Abstract
Background
Many countries are experiencing a resurgence of COVID-19, driven predominantly by the delta (B.1.617.2) variant of SARS-CoV-2. In response, these countries are considering the administration of a third dose of mRNA COVID-19 vaccine as a booster dose to address potential waning immunity over time and reduced effectiveness against the delta variant. We aimed to use the data repositories of Israel's largest health-care organisation to evaluate the effectiveness of a third dose of the BNT162b2 mRNA vaccine for preventing severe COVID-19 outcomes.
Methods
Using data from Clalit Health Services, which provides mandatory health-care coverage for over half of the Israeli population, individuals receiving a third vaccine dose between July 30, 2020, and Sept 23, 2021, were matched (1:1) to demographically and clinically similar controls who did not receive a third dose. Eligible participants had received the second vaccine dose at least 5 months before the recruitment date, had no previous documented SARS-CoV-2 infection, and had no contact with the health-care system in the 3 days before recruitment. Individuals who are health-care workers, live in long-term care facilities, or are medically confined to their homes were excluded. Primary outcomes were COVID-19-related admission to hospital, severe disease, and COVID-19-related death. The third dose effectiveness for each outcome was estimated as 1 – risk ratio using the Kaplan-Meier estimator.
Findings
1 158 269 individuals were eligible to be included in the third dose group. Following matching, the third dose and control groups each included 728 321 individuals. Participants had a median age of 52 years (IQR 37–68) and 51% were female. The median follow-up time was 13 days (IQR 6–21) in both groups. Vaccine effectiveness evaluated at least 7 days after receipt of the third dose, compared with receiving only two doses at least 5 months ago, was estimated to be 93% (231 events for two doses vs 29 events for three doses; 95% CI 88–97) for admission to hospital, 92% (157 vs 17 events; 82–97) for severe disease, and 81% (44 vs seven events; 59–97) for COVID-19-related death.
Interpretation
Our findings suggest that a third dose of the BNT162b2 mRNA vaccine is effective in protecting individuals against severe COVID-19-related outcomes, compared with receiving only two doses at least 5 months ago.
Funding
The Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
Introduction
Despite the initially promising results of nationwide vaccination campaigns, many countries are currently experiencing a resurgence of COVID-19, dominated by the delta (B.1.617.2) variant of SARS-CoV-2. After several months of low pandemic activity in early 2021, Israel is experiencing its fourth pandemic wave, despite over 55% of the population having been vaccinated with two doses of the BNT162b2 mRNA COVID-19 vaccine. First and second doses were given 21 days apart, as per the pre-approval randomised trials. The increase in infections and hospitalisations of vaccinated individuals likely stems from a combination of waning vaccine immunity over time,1, 2, 3 given that many people in Israel were vaccinated 5–7 months ago, and from potentially reduced effectiveness of the vaccine against the delta variant.4
A standard approach to overcoming waning immunity, also known as secondary vaccine failure, is the administration of an additional vaccine dose—often referred to as a booster dose. Faced with rising rates of COVID-19-related admissions to hospital, and based on initial evidence suggesting a pronounced humoral response to a third dose of the mRNA vaccines,5, 6, 7 the Israeli Ministry of Health announced a campaign to administer a third dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer–BioNTech). This campaign began with immunocompromised patients on July 13, 2021, and was expanded several times to include people aged over 60 years (on July 30), 50 years (on Aug 12), 40 years (on Aug 19), 30 years (on Aug 24), and eventually the entire population over the age of 12 years on Aug 30. The third dose was only given to people who had received the second dose at least 5 months ago. Administration of the third dose progressed rapidly, reaching over half of the population aged at least 60 years within the first 2 weeks. Other countries such as the USA, the UK, Germany, and France are planning or conducting campaigns to provide an additional dose for elderly and vulnerable populations,8, 9, 10 such as immunocompromised patients, who have been shown to mount a lesser immune response to the vaccine.11 On Aug 12, 2021, the US Food and Drug Administration amended the authorisations for both mRNA COVID-19 vaccines (Pfizer–BioNTech's BNT162b2 and Moderna's mRNA-1273) to allow for the use of an additional dose in immunocompromised patients.12 On Sept 22, 2021, the authorisation was extended to include individuals aged 65 years or older, and younger individuals with increased medical or occupational risk.13
Research in context.
Evidence before this study
No formal literature review was done. Several previous publications regarding the effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine have focused on antibody response, showing a pronounced humoral response after administration of the booster. Two recent studies from Israel have focused on clinical outcomes. The first reported a reduction of 90–96% in the risk for severe disease starting from day 12 after the booster dose, but did not adjust for pre-existing clinical conditions related to the risk of severe disease and did not evaluate the effectiveness within subgroups. The second found a reduction of 70–84% in the probability of testing positive for SARS-CoV-2 among vaccinated individuals, but did not estimate effectiveness for more severe outcomes.
Added value of this study
To our knowledge, the present study is the first to estimate the effectiveness of a third dose of an mRNA COVID-19 vaccine—BNT162b2 specifically—against severe outcomes with adjustment for various possible confounders, including comorbidities and behavioural factors, and within subgroups. Our results suggest that that a third dose of the BNT162b2 vaccine is effective in preventing severe COVID-19-related outcomes. Compared with two doses of the vaccine administered at least 5 months ago, receiving a third dose was estimated to have an effectiveness of 93% in preventing COVID-19-related admission to hospital, 92% in preventing severe disease, and 81% in preventing COVID-19-related death.
Implications of all the available evidence
As of October, 2021, many countries are experiencing a resurgence of SARS-CoV-2 infections despite hitherto successful vaccination campaigns. This situation has been suggested to be caused by the greater infectiousness of the delta (B.1.617.2) variant of SARS-CoV-2, and by waning immunity as time passes from earlier vaccination. In the face of the current resurgence, several countries are planning to administer a third booster dose of mRNA COVID-19 vaccine. Our study suggests that a third vaccine dose is effective in reducing severe COVID-19-related outcomes for patients who have received two vaccine doses at least 5 months ago.
We aimed to use the data repositories of Israel's largest health-care organisation to estimate the effectiveness of a third dose of the BNT162b2 COVID-19 vaccine in preventing severe COVID-19-related outcomes.
Methods
Study design and participants
This study was designed to emulate a target trial14 of the effects of a third dose of the BNT162b2 vaccine in a population of individuals who had already received two doses of the vaccine at least 5 months before recruitment. The study design is similar to our previous vaccine effectiveness studies conducted in the same population and setting, which have been described at length.15
Clalit Health Services is the largest of four integrated payer-provider health-care organisations providing mandatory health-care coverage in Israel, insuring over half of the Israeli population. Clalit Health Services information systems are fully digitised and feed into a central data warehouse, covering all aspects of care, including COVID-19. The study period was July 30, 2020, to Sept 23, 2021.
To be included in the study, an individual had to have received the second vaccine dose at least 5 months before the recruitment date, and have been eligible to receive the third vaccine dose as per the guidelines of the Israeli Ministry of Health on at least one of the days of the study period. For those aged 60 years or above, this meant individuals with recruitment potential on or after July 30, 2021; for ages 50–59 years, recruitment potential from Aug 12, 2021; for ages 40–49 years, recruitment potential from Aug 19, 2021; for ages 30–39 years from Aug 24, 2021; and for those aged at least 12 years, from Aug 30, 2021. Additional inclusion criteria were membership in the health organisation for at least 12 months, no previous documented SARS-CoV-2 infection, and no contact with the health-care system in the 3 days before the recruitment date.
Immunocompromised patients who received the third dose before July 30, 2021, were not included in the study, as the focus was on providing vaccine effectiveness estimates applicable to the general population. Individuals who are health-care workers, live in long-term care facilities, or are medically confined to their homes (irrespective of COVID-19) were excluded due to concerns of residual confounding. Individuals with missing body-mass index or residential area data were also excluded. A complete definition of the study variables is provided in the appendix (pp 12–19).
This study was approved by the Clalit Health Services institutional review board and was exempt from requiring written informed consent. The protocol is included in the appendix (pp 3–11).
Procedures
The target trial for this study would compare two treatment strategies: administration of the third dose at recruitment (third dose group) and no administration of the third dose at any time during follow-up (control group). To emulate this target trial, each day during the study period, eligible individuals who received the third dose on that day were matched to eligible controls who were previously vaccinated with two vaccine doses but had not yet received the third dose. Controls matched on a given day who received the third dose on a future date would become newly eligible to be recruited into the third dose group on that future date.
Individuals in the third dose group and the control group were exactly matched on a set of potential confounders: age (categorised into 2-year bins), sex (male or female), place of residence, number of pre-existing chronic conditions considered to be risk factors for severe COVID-19 by the US Centers for Disease Control as of Dec 20, 2020 (divided into four bins),16 calendar month in which each person received the second vaccine dose, and number of SARS-CoV-2 PCR tests performed in the 9 months before the index date (divided into six bins). The latter two matching variables were included as markers of health-seeking behaviour specifically related to vaccination against COVID-19, given that individuals who are more health conscious or concerned about the pandemic chose to be vaccinated sooner and did more PCR tests.
Outcomes
Primary outcomes were hospital admission for COVID-19, severe COVID-19 disease (according to US National Institutes of Health criteria17), and COVID-19-related death. Each of these outcomes include the outcomes that precede it. These severe outcomes were chosen because of their greater public health importance, and because they are less likely to be affected by biases stemming from a differential tendency to be tested that is expected to exist between the study groups.
Secondary outcomes were less severe: documented SARS-CoV-2 infection confirmed by positive PCR test and symptomatic infection.
For each outcome, matched pairs of individuals were followed from the start of follow-up until the earliest of: documentation of the outcome, end of the study calendar period (Sept 26, 2021), or death. We also ended the follow-up of a matched pair if the control individual received the third dose. Outcomes were ascertained in the period starting 7 days after receipt of the third dose in the vaccinated, similar to the period used to determine full vaccination after the second dose, and until the end of follow-up.
Statistical analysis
We used the Kaplan-Meier estimator18 to construct cumulative incidence curves and to estimate the risk for each outcome. The risks were compared via ratios and differences. We estimated the risk ratio for each outcome using only matched pairs in which both individuals were still at risk 7 days after receipt of the third vaccine dose in those vaccinated. We analysed outcomes in the full population and in subgroups defined by strata of age, sex, and number of comorbidities. 95% CIs were calculated using the nonparametric percentile bootstrap method with 1000 repetitions. The effectiveness of the third dose was estimated as 1 – risk ratio. As a sensitivity analysis, vaccine effectiveness was also estimated as 1 – incidence rate ratio derived from a Poisson regression using the same dataset, with no further adjustment. Analyses were done using R software (version 4.0.4).
We conducted an ecological analysis in which we plotted daily incidence proportions of SARS-CoV-2 infection (ie, positive PCR test) among the at-risk population by age group around the time the third dose vaccination campaign started.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between July 30, 2020, and Sept 23, 2021, 1 158 269 individuals were eligible to be included in the third dose group (appendix pp 20–21, 23). After matching, the third dose and control groups each included 728 321 individuals, with a median age of 52 years (IQR 37–68) and 51% were female (table 1 ). Baseline demographics were similar between the eligible population (ie, 1 158 269 individuals) and the matched population included in the study. Total follow-up time was 12 632 473 days, with a median follow-up time of 13 days (IQR 6–21) after the first 7 days in both groups, and a maximum follow-up time of 55 days.
Table 1.
Baseline characteristics
| Vaccinated with two doses (n=728 321) | Vaccinated with three doses (n=728 321) | ||
|---|---|---|---|
| Median age (IQR) | 52 (37–68) | 52 (37–68) | |
| Sex | |||
| Female | 371 435 (51·0%) | 371 435 (51·0%) | |
| Male | 356 886 (49·0%) | 356 886 (49·0%) | |
| Population sector | |||
| Jewish | 612 006 (84·0%) | 602 402 (82·7%) | |
| Arab | 92 656 (12·7%) | 102 067 (14·0%) | |
| Ultra-Orthodox Jewish | 23 659 (3·2%) | 23 852 (3·3%) | |
| CDC risk factor count | |||
| 0 | 340 607 (46·8%) | 340 607 (46·8%) | |
| 1 | 175 738 (24·1%) | 175 738 (24·1%) | |
| 2 | 90 704 (12·5%) | 90 704 (12·5%) | |
| ≥3 | 121 272 (16·7%) | 121 272 (16·7%) | |
| Number of SARS-CoV-2 PCR tests in the past 9 months | |||
| 0 | 407 815 (56·0%) | 407 815 (56·0%) | |
| 1 | 134 016 (18·4%) | 134 016 (18·4%) | |
| 2 | 84 832 (11·6%) | 84 832 (11·6%) | |
| 3 | 41 962 (5·8%) | 41 962 (5·8%) | |
| 4 | 21 553 (3·0%) | 21 553 (3·0%) | |
| ≥5 | 38 143 (5·2%) | 38 143 (5·2%) | |
| CDC certain risk criteria | |||
| Cancer | 19 773 (2·7%) | 20 621 (2·8%) | |
| Chronic kidney disease | 66 886 (9·2%) | 68 982 (9·5%) | |
| Chronic obstructive pulmonary disease | 20 669 (2·8%) | 22 249 (3·1%) | |
| Heart disease | 71 428 (9·8%) | 71 166 (9·8%) | |
| Solid organ transplant | 431 (<0·1%) | 507 (<0·1%) | |
| Obesity (ie, BMI 30–40) | 147 399 (20·2%) | 145 022 (19·9%) | |
| Severe obesity (ie, BMI ≥40) | 13 438 (1·8%) | 13 405 (1·8%) | |
| Pregnancy | 4 588 (0·6%) | 7 442 (1·0%) | |
| Sickle cell disease | 65 (<0·1%) | 85 (<0·1%) | |
| Smoking | 115 250 (15·8%) | 135 202 (18·6%) | |
| Type 2 diabetes | 115 451 (15·9%) | 115 713 (15·9%) | |
| CDC possible risk criteria | |||
| Asthma | 47 187 (6·5%) | 47 085 (6·5%) | |
| Cerebrovascular disease | 32 822 (4·5%) | 35 262 (4·8%) | |
| Other respiratory disease | 3 771 (0·5%) | 3 768 (0·5%) | |
| Hypertension | 184 317 (25·3%) | 181 157 (24·9%) | |
| Immunosuppression | 26 471 (3·6%) | 26 395 (3·6%) | |
| Neurological disease | 45 483 (6·2%) | 47 904 (6·6%) | |
| Liver disease | 16 074 (2·2%) | 17 283 (2·4%) | |
| Overweight (ie, BMI 25–30) | 260 353 (35·7%) | 255 737 (35·1%) | |
| Thalassaemia | 4085 (0·6%) | 4440 (0·6%) | |
| Type 1 diabetes | 4339 (0·6%) | 4176 (0·6%) | |
CDC=Centers for Disease Control and Prevention. BMI=body-mass index.
198 476 individuals appear in both groups, as they were first recruited as unvaccinated and then, following vaccination, re-recruited as vaccinated.
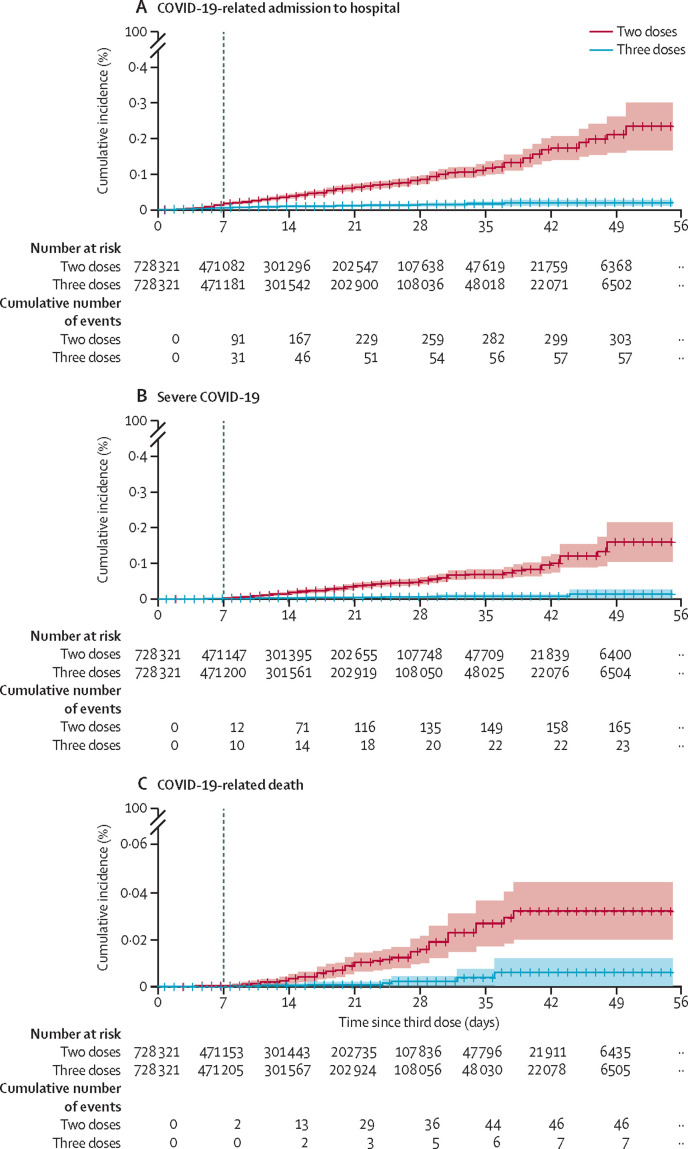
Effectiveness of the third vaccine dose, compared with two doses only, was estimated to be 93% (95% CI 88–97; 231 events for two doses vs 29 events for three doses) against admission to hospital, 92% (82–97; 157 vs 17 events) against severe disease, and 81% (59–97; 44 vs seven events) against COVID-19-related death (table 2 ). Cumulative incidence curves for COVID-19-related admission to hospital began to diverge around 6 days after vaccination; for severe disease and COVID-19-related death, divergence was seen at around 8–9 days after vaccination (figure 1 ). The estimated third-dose vaccine effectiveness against admission to hospital and severe disease was similar between males and females, and between individuals aged 40–69 years and those aged at least 70 years (table 3 ).
Table 2.
Effectiveness of the third vaccine dose versus two vaccine doses of the BNT162b2 mRNA COVID-19 vaccine
|
Vaccinated with two doses |
Vaccinated with three doses |
1 – risk ratio (95% CI) | Risk difference per 100 000 individuals (95% CI) | |||
|---|---|---|---|---|---|---|
| Events | Risk per 100 000 individuals | Events | Risk per 100 000 individuals | |||
| Admission to hospital | 231 | 220·8 | 29 | 14·4 | 93% (88–97) | 206·4 (146·1–275·1) |
| Severe disease | 157 | 158·9 | 17 | 12·9 | 92% (82–97) | 145·9 (93·1–207·7) |
| Death | 44 | 31·9 | 7 | 6·1 | 81% (59–97) | 25·8 (13·0–38·5) |
Estimates were obtained using the Kaplan-Meier estimator starting from day 7 after receipt of the third dose, in those who received it.
Figure 1.
Cumulative incidence curves comparing COVID-19-related admission to hospital (A), severe disease (B), and death (C) in individuals who received two versus three doses of the BNT162b2 mRNA COVID-19 vaccine
The dashed vertical line indicates day 7, on which the main analysis period begins.
Table 3.
Subgroup analysis of the effectiveness of the third vaccine dose versus two vaccine doses of the BNT162b2 mRNA COVID-19 vaccine
| Total number in analysis (both study groups combined) |
Vaccinated with two doses |
Vaccinated with three doses |
1 – risk ratio (95% CI) | Risk difference per 100 000 individuals (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Events | Risk per 100 000 individuals | Events | Risk per 100 000 individuals | |||||
| Admissions to hospital | ||||||||
| Sex | ||||||||
| Male | 458 552 | 140 | 321·6 | 21 | 25·2 | 92% (85 to 97) | 296·4 (177·2 to 443·2) | |
| Female | 483 548 | 91 | 132·1 | 8 | 5·0 | 96% (93 to 99) | 127·1 (87·2 to 175·9) | |
| Age group, years | ||||||||
| 16–39 | 288 072 | 6 | 7·0 | 1 | 2·1 | 70% (−70 to 100) | 4·9 (−2·1 to 12·3) | |
| 40–69 | 448 366 | 73 | 104·9 | 10 | 8·1 | 92% (83 to 97) | 96·7 (60·1 to 148·7) | |
| ≥70 | 162 958 | 140 | 574·3 | 16 | 41·3 | 93% (87 to 97) | 533·0 (390·1 to 675·3) | |
| Number of coexisting conditions | ||||||||
| 0 | 462 690 | 14 | 13·4 | 2 | 1·5 | 89% (60 to 100) | 11·9 (4·3 to 22·3) | |
| 1–2 | 336 850 | 61 | 111·5 | 7 | 9·7 | 91% (80 to 98) | 101·9 (61·9 to 145·9) | |
| ≥3 | 142 560 | 156 | 689·7 | 20 | 56·3 | 92% (87 to 96) | 633·4 (456·4 to 847·7) | |
| Severe disease | ||||||||
| Sex | ||||||||
| Male | 458 652 | 103 | 233·0 | 13 | 24·8 | 89% (73 to 98) | 208·2 (109·7 to 343·9) | |
| Female | 483 614 | 54 | 93·2 | 4 | 2·8 | 97% (93 to 99) | 90·4 (57·4 to 137·8) | |
| Age group, years | ||||||||
| 16–39 | 288 086 | 2 | 2·5 | 0 | 0·0 | NA | 2·5 (0·7 to 7·5) | |
| 40–69 | 448 410 | 38 | 57·9 | 5 | 3·5 | 94% (85 to 99) | 54·4 (28·0 to 87·6) | |
| ≥70 | 163 054 | 108 | 447·5 | 10 | 35·8 | 92% (83 to 98) | 411·7 (285·9 to 548·7) | |
| Number of coexisting conditions | ||||||||
| 0 | 462 706 | 5 | 3·1 | 0 | 0·0 | NA | 3·1 (0·7 to 6·0) | |
| 1–2 | 336 902 | 39 | 82·0 | 2 | 3·2 | 96% (85 to 100) | 78·8 (39·3 to 126·8) | |
| ≥3 | 142 658 | 113 | 503·5 | 15 | 51·6 | 90% (80 to 96) | 451·9 (322·3 to 605·2) | |
Estimates were obtained using the Kaplan-Meier estimator starting from day 7 after receipt of the third dose in those who received it. Data are listed as NA when one or both of the study groups do not have any events. NA=not available.
Third-dose vaccine effectiveness against documented SARS-CoV-2 infection was estimated to be 88% (95% CI 87–90; 6131 events for two doses vs 1135 events for three doses) and against symptomatic infection was 91% (89–92; 3345 vs 514 events; table 4 ). Individuals who received the third dose were tested less frequently for SARS-CoV-2 infection during follow-up than those who did not.
Table 4.
Infection outcomes in those who received a third vaccine dose versus two vaccine doses of the BNT162b2 mRNA COVID-19 vaccine
|
Vaccinated with two doses |
Vaccinated with three doses |
1 – risk ratio (95% CI) | Risk difference per 100 000 people (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Tests | Events | Risk per 100 000 people | Tests | Events | Risk per 100 000 people | |||
| Documented infection | 93 566 | 6131 | 3662·3 | 77 184 | 1135 | 422·9 | 88% (87–90) | 3239·4 (3014·6–3468·6) |
| Symptomatic infection | 95 934 | 3345 | 1909·6 | 78 507 | 514 | 178·9 | 91% (89–92) | 1730·7 (1587·6–1923·7) |
Estimates were obtained starting from day 7 after receipt of the third dose in those who received it. Tests were counted during the study follow-up period for each patient.
A sensitivity analysis defining booster effectiveness as 1 – incidence rate ratio yielded similar results: 87% (95% CI 82–92) against admission to hospital, 89% (83–94) against severe disease, and 84% (67–93) against COVID-19-related death (appendix p 22).
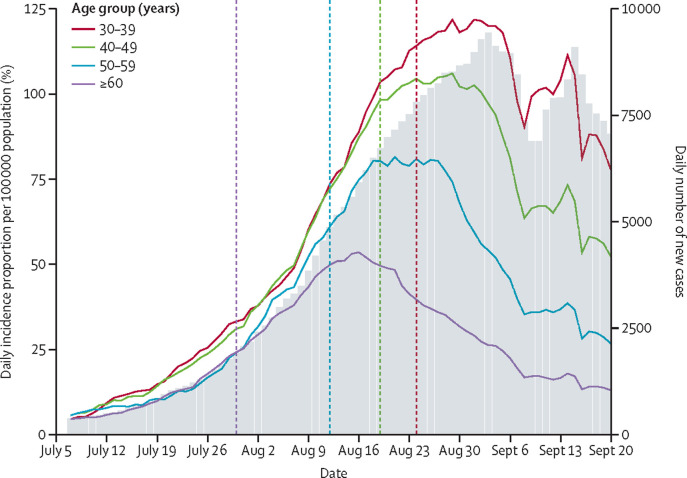
Our ecological analysis showed that, shortly after the third-dose vaccination campaign was initiated in each age group, the incidence trend began to decline in the respective age groups when compared with that of age groups not yet eligible (figure 2 ).
Figure 2.
Daily incidence of SARS-CoV-2 infection for different age groups around initiation of third dose vaccination
Daily incidence proportions of SARS-CoV-2 infection (ie, positive PCR-test) among the at-risk population by age group around the time the third dose vaccination was initiated (left Y axis). For each age group, the vertical dashed line with the same colour is the day that age group became eligible for the third dose. The epidemic curve (daily incidence counts) is shown shaded in the background (right Y axis). All curves were smoothed by using a moving 7-day mean, assigning for each day the value of the mean of the 7 days ending on that day.
Discussion
In this large observational study conducted using nationwide mass vaccination data in Israel, we estimated that a third dose of the BNT162b2 mRNA COVID-19 vaccine is effective in preventing severe COVID-19-related outcomes. Compared with two doses of the vaccine administered at least 5 months before, adding a third dose was estimated to be 93% effective in preventing COVID-19-related admission to hospital, 92% in preventing severe disease, and 81% in preventing COVID-19-related death, as of 7 or more days after the third dose.
Third-dose vaccine effectiveness against admission to hospital and severe disease was estimated to be similar between males and females, and between individuals aged 40–69 years and at least 70 years. In those aged 16–39 years, the rate of these severe outcomes was too small for meaningful estimation of the booster effectiveness. Effectiveness was also similar among groups defined by the number of comorbidities.
Most previous studies on this topic have focused on the antibody response elicited by the third dose of mRNA vaccines.5, 6, 7 Two recent studies from Israel reported on the effectiveness of the third dose in preventing clinical outcomes. The first study estimated a reduction of 92–97% in the risk for severe disease starting from day 12 after receipt of the third dose,19 but did not adjust for pre-existing clinical conditions related to the risk of severe disease, and did not evaluate the effectiveness within subgroups. A second study, which adjusted for various confounders and used a test-negative design, estimated a reduction of 70–84% in the probability of testing positive for SARS-CoV-2 among the vaccinated,20 but did not consider more severe outcomes.
The optimal time to achieve maximum protection against SARS-CoV-2-related outcomes after a third vaccine is unknown. In this study, we estimated effectiveness starting from day 7 after the third dose, which is similar to the period used to define full vaccination after the second dose.21 Our choice is supported by high concentrations of antibodies in individuals 7 days after administration of the third dose.6 It is possible, however, that some degree of protection begins earlier. Although an increase in antibody production can be identified on days 3–5 after administration of the second dose of SARS-CoV-2 mRNA vaccines,22 for other vaccines (eg, influenza), antibodies and antibody-secreting cells are detected as early as day 2 after a booster dose.23, 24 Moreover, a rapid response of the immune system can potentially prevent infections in individuals even if they were exposed to the virus shortly before the third dose. Such protection is termed the post-exposure effect25 and is well established in vaccinations for other pathogens, such as varicella,26 measles,27 and hepatitis A.28
Our study has several limitations. First, differing testing frequencies between the groups do not allow unbiased estimates for the less severe secondary outcomes of documented infection and symptomatic infection. Second, as in any observational study, unmeasured confounding might exist. However, this concern is mitigated because our analysis was adjusted for various important possible confounders, including sociodemographic factors, clinical factors, and behavioural factors related to COVID-19. In addition, this study focuses on severe outcomes, which are less likely to be affected by differences in health-seeking behaviours or testing rates between groups. Third, due to the relative scarcity of events in individuals younger than 40 years, we could not evaluate vaccine effectiveness in this age group. Fourth, this vaccine effectiveness study did not explore potential adverse clinical events and excess health-care utilisation associated with the administration of a third dose. Finally, we excluded populations (health-care workers, those living in long-term care facilities, and those medically confined to their homes) that are likely to be targeted early to receive the booster dose.
There is an active debate surrounding the administration of third doses to individuals in some countries while other countries suffer from vaccine shortages.29 It is outside the scope of this epidemiological analysis to address the complex ethical issues involved in this debate, but there is an urgent need for increased vaccine production, distribution, and access worldwide. The present study was designed to use existing observational health data to study vaccine effectiveness in preventing specific COVID-19 outcomes, aiming to expand the scientific evidence base that might be useful in informing this broader discussion.
At the time of writing, many countries are experiencing a resurgence of SARS-CoV-2 infections despite hitherto successful vaccination campaigns, the cause of which is suggested to be the greater infectiousness of the delta variant and waning immunity as time passes from earlier vaccination. Regardless of the cause, these early findings suggest that a third dose of mRNA vaccine is effective in reducing severe COVID-19-related outcomes for patients who have received two doses at least 5 months before.
Data sharing
Due to data privacy regulations, the raw data of this study cannot be shared.
Declaration of interests
NB, ND, and RDB report institutional grants to Clalit Research Institute from Pfizer outside the submitted work and unrelated to COVID-19, with no direct or indirect personal benefits. MAH reports grants from the US National Institutes of Health (NIH) and US Department of Veterans Affairs, and personal fees from Cytel and ProPublica. ML reports grants from Pfizer, NIH, the UK National Institute for Health Research, the US Centers for Disease Control and Prevention, Open Philanthropy Project, the Wellcome Trust, and Pfizer; personal fees from Merck, Bristol Meyers Squibb, Sanofi Pasteur, and Janssen; and unpaid advice given on Covid vaccines or vaccine studies to One Day Sooner, Pfizer, AstraZeneca, Janssen, and COVAXX (United Biosciences), outside the submitted work. BYR reports grants from NIH outside the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute. ML was supported by the Morris-Singer Fund.
Contributors
All authors conceived and designed the study. NB and ND collected and analysed the original data. NB, ND, and RDB had access to and verified the underlying data. All authors wrote the manuscript, critically reviewed the manuscript, and decided to proceed with publication. BYR and RDB supervised the study process. RDB vouches for the data and analysis.
Supplementary Material
References
- 1.Zipeto D, Carbonare LD, Valenti MT, et al. Antibody response to BTN162b2 mRNA vaccination in naïve versus SARS-CoV-2 infected subjects with and without waning immunity. Res Sq. 2021 doi: 10.21203/rs.3.rs-440410/v1. published online April 30. (preprint). [DOI] [Google Scholar]
- 2.Thompson RN, Hill EM, Gog JR. SARS-CoV-2 incidence and vaccine escape. Lancet Infect Dis. 2021;21:913–914. doi: 10.1016/S1473-3099(21)00202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouwels KB, Pritchard E, Matthews PC, et al. Impact of delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. medRxiv. 2021 doi: 10.1101/2021.08.18.21262237. published online Aug 24. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100:702–704. doi: 10.1016/j.kint.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfizer Second quarter 2021 earnings teleconference. July 28, 2021. https://s21.q4cdn.com/317678438/files/doc_financials/2021/q2/Q2-2021-Earnings-Charts-FINAL.pdf
- 7.Wu K, Choi A, Koch M, et al. Preliminary analysis of safety and immunogenicity of a SARS-CoV-2 variant vaccine booster. medRxiv. 2021 doi: 10.1101/2021.05.05.21256716. published online May 6. (preprint). [DOI] [Google Scholar]
- 8.Fisher L. Vaccine booster shots for 32m to begin next month. Aug 1, 2021. https://www.telegraph.co.uk/politics/2021/08/01/vaccine-booster-shots-32m-begin-next-month/
- 9.Kar-gupta S. Ignoring WHO call, major nations stick to vaccine booster plans. Aug 5, 2021. https://www.reuters.com/world/europe/french-president-macron-third-covid-vaccine-doses-likely-elderly-vulnerable-2021-08-05/
- 10.Restuccia A. Biden administration expected to call for COVID-19 vaccine booster shots. Aug 16, 2021. https://www.wsj.com/articles/biden-administration-to-call-for-COVID-19-vaccine-booster-shots-11629170031
- 11.Kearns P, Siebert S, Willicombe M, et al. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity – the OCTAVE trial. SSRN. 2021 doi: 10.2139/ssrn.3910058. published online Aug 23. (preprint). [DOI] [Google Scholar]
- 12.Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. Aug 12, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised
- 13.Food and Drug Administration FDA authorizes booster dose of Pfizer-BioNTech COVID-19 vaccine for certain populations. Sept 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-COVID-19-vaccine-certain-populations
- 14.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;8:758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Certain medical conditions and risk for severe COVID-19 illness. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [PubMed]
- 17.National Institutes of Health COVID-19 treatment guidelines. Oct 7, 2021. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021 doi: 10.1056/nejmoa2114255. published online Sept 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patalon T, Gazit S, Pitzer VE, Prunas O, Warren JL, Weinberger DM. Short term reduction in the odds of testing positive for SARS-CoV-2; a comparison between two doses and three doses of the BNT162b2 vaccine. medRxiv. 2021 doi: 10.1101/2021.08.29.21262792. published online Aug 31. (preprint). [DOI] [Google Scholar]
- 21.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogata AF, Cheng C-A, Desjardins M, et al. Circulating SARS-CoV-2 vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab465. published online May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171:198–203. doi: 10.1093/infdis/171.1.198. [DOI] [PubMed] [Google Scholar]
- 24.Pyhälä R, Alanko S, Forsten T, et al. Early kinetics of antibody response to inactivated influenza vaccine. Clin Diagn Virol. 1994;1:271–278. doi: 10.1016/0928-0197(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher T, Lipsitch M. Postexposure effects of vaccines on infectious diseases. Epidemiol Rev. 2019;41:13–27. doi: 10.1093/epirev/mxz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson B, Seward J, Yang A, et al. Postexposure effectiveness of varicella vaccine. Pediatrics. 2000;105:84–88. doi: 10.1542/peds.105.1.84. [DOI] [PubMed] [Google Scholar]
- 27.Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting–New York City, 2013. Clin Infect Dis. 2017;65:1843–1847. doi: 10.1093/cid/cix639. [DOI] [PubMed] [Google Scholar]
- 28.Whelan J, Sonder GJ, Bovée L, Speksnijder A, van den Hoek A. Evaluation of hepatitis A vaccine in post-exposure prophylaxis, the Netherlands, 2004–012. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra M. WHO calls for halting COVID-19 vaccine boosters in favor of unvaccinated. Aug 5, 2021. https://www.reuters.com/business/healthcare-pharmaceuticals/who-calls-moratorium-COVID-19-vaccine-booster-doses-until-september-end-2021-08-04/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to data privacy regulations, the raw data of this study cannot be shared.