Abstract
Background
Infections with SARS-CoV-2 continue to cause significant morbidity and mortality. Interleukin (IL)-1 and IL-6 blockade have been proposed as therapeutic strategies in COVID-19, but study outcomes have been conflicting. We sought to study whether blockade of the IL-6 or IL-1 pathway shortened the time to clinical improvement in patients with COVID-19, hypoxic respiratory failure, and signs of systemic cytokine release syndrome.
Methods
We did a prospective, multicentre, open-label, randomised, controlled trial, in hospitalised patients with COVID-19, hypoxia, and signs of a cytokine release syndrome across 16 hospitals in Belgium. Eligible patients had a proven diagnosis of COVID-19 with symptoms between 6 and 16 days, a ratio of the partial pressure of oxygen to the fraction of inspired oxygen (PaO2:FiO2) of less than 350 mm Hg on room air or less than 280 mm Hg on supplemental oxygen, and signs of a cytokine release syndrome in their serum (either a single ferritin measurement of more than 2000 μg/L and immediately requiring high flow oxygen or mechanical ventilation, or a ferritin concentration of more than 1000 μg/L, which had been increasing over the previous 24 h, or lymphopenia below 800/mL with two of the following criteria: an increasing ferritin concentration of more than 700 μg/L, an increasing lactate dehydrogenase concentration of more than 300 international units per L, an increasing C-reactive protein concentration of more than 70 mg/L, or an increasing D-dimers concentration of more than 1000 ng/mL). The COV-AID trial has a 2 × 2 factorial design to evaluate IL-1 blockade versus no IL-1 blockade and IL-6 blockade versus no IL-6 blockade. Patients were randomly assigned by means of permuted block randomisation with varying block size and stratification by centre. In a first randomisation, patients were assigned to receive subcutaneous anakinra once daily (100 mg) for 28 days or until discharge, or to receive no IL-1 blockade (1:2). In a second randomisation step, patients were allocated to receive a single dose of siltuximab (11 mg/kg) intravenously, or a single dose of tocilizumab (8 mg/kg) intravenously, or to receive no IL-6 blockade (1:1:1). The primary outcome was the time to clinical improvement, defined as time from randomisation to an increase of at least two points on a 6-category ordinal scale or to discharge from hospital alive. The primary and supportive efficacy endpoints were assessed in the intention-to-treat population. Safety was assessed in the safety population. This study is registered online with ClinicalTrials.gov (NCT04330638) and EudraCT (2020-001500-41) and is complete.
Findings
Between April 4, and Dec 6, 2020, 342 patients were randomly assigned to IL-1 blockade (n=112) or no IL-1 blockade (n=230) and simultaneously randomly assigned to IL-6 blockade (n=227; 114 for tocilizumab and 113 for siltuximab) or no IL-6 blockade (n=115). Most patients were male (265 [77%] of 342), median age was 65 years (IQR 54–73), and median Systematic Organ Failure Assessment (SOFA) score at randomisation was 3 (2–4). All 342 patients were included in the primary intention-to-treat analysis. The estimated median time to clinical improvement was 12 days (95% CI 10–16) in the IL-1 blockade group versus 12 days (10–15) in the no IL-1 blockade group (hazard ratio [HR] 0·94 [95% CI 0·73–1·21]). For the IL-6 blockade group, the estimated median time to clinical improvement was 11 days (95% CI 10–16) versus 12 days (11–16) in the no IL-6 blockade group (HR 1·00 [0·78–1·29]). 55 patients died during the study, but no evidence for differences in mortality between treatment groups was found. The incidence of serious adverse events and serious infections was similar across study groups.
Interpretation
Drugs targeting IL-1 or IL-6 did not shorten the time to clinical improvement in this sample of patients with COVID-19, hypoxic respiratory failure, low SOFA score, and low baseline mortality risk.
Funding
Belgian Health Care Knowledge Center and VIB Grand Challenges program.
Research in context.
Evidence before this study
We searched PubMed on June 25, 2021, using the following search term (“SARS-CoV-2” OR “COVID-19”) AND (“Siltuximab” OR “Tocilizumab” OR “Anakinra” OR “Interleukin-1” OR “Interleukin-6”) AND (“RCT” OR “Clinical trial” OR “Randomized controlled trial”). We searched for clinical trials published in English assessing the effect of IL-1 blockade or IL-6 blockade in patients with COVID-19 published between database inception and June 25, 2021.
We identified one clinical trial employing the IL-1 receptor antagonist anakinra, which was terminated prematurely for absence of effect following the recruitment of 116 patients. Several randomised controlled trials (RCTs) of IL-6 or IL-6R blockade have been completed, but clinical outcomes of the interventions have been inconsistent. Of these, only three studies showed a decreased risk of mechanical ventilation or an improved survival in severely ill patients treated with an IL-6R antagonist. These discordant outcomes could be due to differences in timing of intervention, patient severity, standard-of-care treatment, which included corticosteroids, measured outcome, or trial design. Few studies prescreened patients for systemic cytokine release syndrome, which could have diluted the efficacy signal.
Added value of this study
Despite a power approaching 90%, we could not detect a benefit for IL-1 or IL-6 pathway blockade on the time to clinical improvement when these drugs were given early in the disease course in a patient population with low to moderate 28-day mortality. We report results of anakinra in a fully powered RCT and also report for the first time on IL-6 blockade using siltuximab in a RCT for COVID-19. As a unique feature, we measured baseline serum IL-1β, IL-1 receptor antagonist (IL-1RA), and IL-6 to identify potential biomarkers. Even in those patients on the high-end of the spectrum of measured cytokines, our multicentre study did not discern an effect of IL-1 or IL-6 blockade.
Our results are clearly at odds with data from the platform trials REMAP-CAP and RECOVERY, despite the fact that patient severity categories and timing of intervention were largely similar. A noticeable difference is that 28-day mortality in the standard-of-care group of the platform trials was more than 30%, whereas in the COV-AID trial it was only around 10%, which is reflective of most acute care settings around the world.
Implications of all the available evidence
The current study does not support blocking IL-1 or IL-6 in patients with COVID-19, but more research is needed to determine whether subgroups of patients might benefit or other biomarkers might identify responders more accurately. Although IL-6 blockade in combination with corticosteroids might be beneficial in the most severely ill patients, it seems less effective when used early in the disease course or in populations with low to moderate mortality.
Introduction
COVID-19 caused by SARS-CoV-2 is a worldwide crisis and despite public health measures and the development of vaccines, re-infections and infections with emerging variants will probably continue to cause significant morbidity and mortality in years to come. Effective therapies are thus needed to improve outcome in the most severe patients.
Severe disease usually occurs after a few days of symptoms, when viral replication is waning, and excessive inflammation in the lung alveoli causes abnormalities in gas-exchange, ventilation, and blood perfusion of the lung, thus leading to severe hypoxia and acute respiratory distress syndrome (ARDS). Severe disease also has features of the systemic cytokine release syndrome (CRS) presenting with high fever, elevated C-reactive protein (CRP) and ferritin levels, along with peripheral blood cytopenias, although serum cytokine concentrations are much lower than in classical CRS.1 Indeed, the classical pro-inflammatory cytokines interleukin (IL)-1 and IL-6 are increased in the serum of severely ill patients with COVID-19, and elevated concentrations confer increased risk of requiring mechanical ventilation.2, 3, 4, 5, 6, 7, 8, 9 Supporting a role for underlying inflammation, the potent anti-inflammatory drug dexamethasone was shown to improve survival in severely ill patients with COVID-19 and hypoxia.10, 11
Owing to a favourable safety profile in the treatment of other forms of CRS and auto-inflammatory diseases, blockade of the IL-1 and IL-6 pathway have been used as targeted therapies for severely ill patients with COVID-19.12, 13, 14, 15 Others have questioned whether CRS is clinically relevant in COVID-19-associated ARDS, since cytokine concentrations are low and hard to measure clinically.1, 16 A randomised controlled trial (RCT) employing the IL-1 receptor antagonist anakinra has been published, yet this trial was prematurely terminated for absence of effect,17 whereas another RCT (SAVE-MORE) has reported a much more favourable outcome of anakinra treatment compared to standard of care on day 28 mortality in patients selected on the basis of high soluble urokinase plasminogen activator receptor.18 Several RCTs of IL-6 or IL-6R blockade have been completed, but clinical outcomes of the interventions have been inconsistent and have been reviewed in a meta-analysis.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 Only three RCTs showed a decreased risk of mechanical ventilation or an improved survival in severely ill patients treated with an IL-6R antagonist, yet the conclusion of the meta-analysis was that there was a favourable effect of IL6-R blockade in all trials combined.15, 26, 29, 30 Discordant trial outcomes could be due to differences in timing of intervention in relation to clinical deterioration, patient severity and comorbidity, standard-of-care treatment, which included corticosteroids, measured outcome, or methodological differences in trial design.12, 15, 31 Few studies prescreened patients for systemic CRS, which could have diluted the efficacy signal of selected patients, especially those with moderate disease.
We did a randomised, controlled, factorial design trial to study whether blockade of the IL-6 pathway (with tocilizumab antibody directed against IL-6R or siltuximab antibody directed against IL-6) or the IL-1 pathway (with the recombinant IL-1R antagonist anakinra) shortened the time to clinical improvement in patients with COVID-19, hypoxic respiratory failure, and signs of systemic CRS.
Methods
Study design
We did a phase 3, prospective, multicentre, open-label RCT across 16 hospitals in Belgium. The COV-AID trial has a 2 × 2 factorial design, to evaluate IL-1 blockade versus no IL-1 blockade and IL-6 blockade versus no IL-6 blockade. The evaluation of efficacy was done independently for both randomisations. A priori, we assumed no interaction between IL-1 blockade and IL-6 blockade. At the start of the study, there was a shortage of supply of various IL-6 pathway blocking agents and a priori we considered IL-6R blockade with tocilizumab equal to blockade of IL-6 with siltuximab.
The sponsor designed the trial in consultation with the Belgian Health Care Knowledge Center. The trial was approved by the competent authorities and the Ethical Committee of Ghent University Hospital, and the trial was done in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. An independent data safety monitoring board monitored participant safety. Every patient or their legal representative provided written informed consent for participation. All investigators take responsibility for the integrity of the trial and the publication. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Eligible patients were older than 18 years, had a laboratory proven diagnosis of COVID-19 with symptoms between 6 and 16 days, a ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2; P:F ratio) of less than 350 mm Hg on room air or less than 280 mm Hg on supplemental oxygen and bilateral pulmonary infiltrates. There is no widely accepted definition of COVID-19-associated cytokine release, but as surrogate biomarkers, patients needed to have either a single ferritin concentration measurement of more than 2000 μg/L at inclusion when they immediately required high flow oxygen or mechanical ventilation, or a ferritin concentration of more than 1000 μg/L, which had been increasing over the previous 24 h, or lymphopenia below 800/mL with two of the following criteria: an increasing ferritin concentration of more than 700 μg/L; an increasing lactate dehydrogenase concentration of more than 300 international units (IU)/L; an increasing CRP concentration of more than 70 mg/L; or an increasing D-dimers concentration of more than 1000 ng/mL. If the patient had three of the previous criteria at hospital admission with lymphopenia of less than 800/μL, there was no need to document an increase over 24 h.
Exclusion criteria included mechanical ventilation for more than 24 h at randomisation; a clinical frailty score greater than 3 before SARS-CoV-2 infection32; unlikelihood to survive beyond 48 h based on clinical assessment; an active co-infection defined on clinical grounds (positive blood or sputum cultures); thrombocytopenia of less than 50 000/μL or neutropenia of less than 1500/μL; a history of bowel perforation or diverticulitis; or high dose systemic steroid or immunosuppressive drug use for a COVID-19-unrelated disorder. The full list of inclusion and exclusion criteria can be found in the study protocol (appendix) and in the published trial protocol.33
Randomisation and masking
Included patients were randomly assigned by means of permuted block randomisation with varying block size and stratification by centre. Patients were allocated in a 1:2 ratio to anakinra or no IL-1 blockade. Simultaneously, patients were randomly assigned in a 1:1:1 ratio to siltuximab, tocilizumab, or no IL-6 blockade. Randomisation and subsequent data collection were done by means of the webbased system REDCap.34, 35
Procedures
Patients allocated to the IL-1 blockade group received anakinra 100 mg once daily subcutaneously for 28 days or until hospital discharge on top of standard of care. If the glomerular filtration rate fell below 30 mL/min per 1·73 m2, anakinra dosing was lowered to 100 mg once every other day. Patients allocated to the IL-6 blockade group received a single intravenous injection of either siltuximab 11 mg/kg or tocilizumab 8 mg/kg (not exceeding 800 mg) on top of standard of care.
Enrolled patients underwent multiple daily evaluations, which included procalcitonin measurements three times a week. Additional serum and edetic acid samples and arterial blood gas samples were collected on days 1, 6, and 15 since randomisation and on follow-up (10–20 weeks after randomisation).
Most patients (42%) randomly assigned before August, 2020, received hydroxychloroquine as per standard of care and most patients (84%) randomly assigned from August, 2020, onwards received dexamethasone as per standard of care.9
Outcomes
The primary efficacy endpoint was time to clinical improvement, defined as the time in days from randomisation until either an increase of at least two points on a 6-category ordinal scale (compared with the worst status at day of randomisation) or to discharge from the hospital alive, whichever occurred first. The 6-category ordinal scale was defined as 1=death; 2=hospitalised, on invasive mechanical ventilation or extracorporeal membrane oxygenation; 3=hospitalised, on non-invasive ventilation or high-flow oxygen devices; 4=hospitalised, requiring supplemental oxygen; 5=hospitalised, not requiring supplemental oxygen; 6=not hospitalised. Patients who did not have clinical improvement by March 4, 2021, were censored administratively at the date of their last registered day in hospital. Patients who died before clinical improvement were censored at the longest observed follow-up time for this event seen in the study.
Supportive endpoints were median time until discharge, median time until independence from supplemental oxygen or discharge, median time until independence from invasive ventilation, median time until first use of high-flow oxygen device, ventilation, or death, number of days in hospital, number of days in ICU, number of days in ICU in patients ventilated at day of randomisation, number of days in ICU relative to the number of days alive the first 28 days after randomisation, number of days without supplemental oxygen use up to 28 days after randomisation, number of invasive ventilator days, number of invasive ventilator days in patients ventilated at day of randomisation, number of invasive ventilator days relative to number of days alive the first 28 days after randomisation, number of invasive ventilator-free days up to 28 days after randomisation, number of invasive ventilator-free days up to 28 days after randomisation in patients ventilated at day of randomisation. Several subgroups were prespecified on the basis of allocated treatment for the other randomisation, invasive ventilation and serum concentrations of CRP (<130 or ≥130 mg/L), IL-1β (55 or ≥55 fg/mL), and IL-6 (<10 or ≥10 pg/mL) on the day of randomisation. Post-hoc subgroup analyses included serum concentrations of IL-1RA (<915 or ≥915 ng/mL), admission status to ICU or concomitant glucocorticoid use at randomisation. Key safety endpoints were death, serious adverse events, sepsis, and septic shock during hospital stay.
Statistical analysis
To achieve at least 80% power to detect an improvement in median time to clinical improvement from 12 days to 8 days (corresponding to a hazard ratio (HR) of 1·5) at a two-sided significance level of 5%, assuming an allocation ratio of 1:2, 215 clinical improvements are required.
Efficacy analyses were done on the intention-to-treat population. Time to event endpoints were analysed by means of a Cox proportional hazards models stratified for the other randomisation (either IL-1 blockade [yes or no] or IL-6 blockade ([yes or no]). The estimated HR and 95% Wald CI were computed. The exact method was used for construction of the likelihood for tied event times. For time to positive events, patients who died were censored at the longest observed hospital follow-up time seen in the study. The Kaplan-Meier estimated cumulative incidence functions for clinical improvement with pointwise 95% CI were plotted. The two-sided p value from the score test was reported. The reported median time until the event was the earliest time at which at least 50% of patients had reached the respective event. The 95% CI was calculated by means of the log–log approach. A predefined sensitivity analysis tested for interaction between the two randomisations. Negative binomial regression models with a log link function adjusted for the other randomisation were fitted for supportive count outcomes, such as number of days in hospital. The estimated ratio of expected counts and 95% CI was computed. Safety data were analysed descriptively in all randomly assigned patients according to actual treatment received.
A full statistical analysis plan is available in the appendix, p 64). We predefined in the statistical analysis plan that we would first focus and report on the primary endpoint outcomes of the trial. A subsequent publication will focus on all endpoints, including 12–20 week follow-up data. Data monitoring was done by the Health Innovation and Research Institute of UZ Ghent. This study is registered online with ClinicalTrials.gov (NCT04330638) and EudraCT (2020-001500-41).
Role of the funding source
The funder of the study (Belgian Health Care Knowledge Center) was involved in purchasing study medication and study design, but was not involved in data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit. The funder (VIB Grand Challenges) was involved in purchasing reagents for measuring biomarkers.
Results
Between April 4 and Dec 6, 2020, 342 patients were randomly assigned. In the first randomisation, 230 patients were assigned to the no IL-1 blockade group and 112 to the IL-1 blockade group, of whom all received at least one dose of anakinra. In the second randomisation, 115 patients were assigned to the no IL-6 blockade group and 227 to the IL-6 blockade group, of whom 114 were allocated to receive tocilizumab and 113 siltuximab. Tocilizumab was not administered to one patient for unknown reasons and two patients did not receive siltuximab owing to patient withdrawal (figure 1 ). All patients were followed until clinical improvement or death, except for four patients with withdrawal of consent, two patients lost to follow-up owing to transfer to another hospital, and one patient still hospitalised at time of data lock. All patients were included in the intention-to-treat analyses.
Figure 1.
Trial profile
First randomisation: IL-1 blockade (A). Second randomisation: IL-6 blockade. IL=interleukin (B).
Demographic and baseline clinical and biological characteristics of patients are described in table 1 . The median age was 65 years (IQR 54–73) and most patients were men (265 [77%] of 342).
Table 1.
Baseline characteristics
|
IL-1 blockade group |
No IL-1 blockade group |
IL-6 blockade group |
No IL-6 blockade group |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | n (%) or median (IQR) | Number of patients | n (%) or median (IQR) | Number of patients | n (%) or median (IQR) | Number of patients | n (%) or median (IQR) | ||
| Sex | 112 | .. | 230 | .. | 227 | .. | 115 | .. | |
| Female | .. | 25 (22%) | .. | 52 (23%) | .. | 52 (23%) | .. | 25 (22%) | |
| Male | .. | 87 (78%) | .. | 178 (77%) | .. | 175 (77%) | .. | 90 (78%) | |
| Ethnicity | 112 | .. | 230 | .. | 227 | .. | 115 | .. | |
| White | .. | 98 (88%) | .. | 180 (78%) | .. | 184 (81%) | .. | 94 (82%) | |
| Middle Eastern–Arabian | .. | 11 (10%) | .. | 29 (13%) | .. | 27 (12%) | .. | 13 (11%) | |
| Black | .. | 1 (1%) | .. | 8 (3%) | .. | 8 (4%) | .. | 1 (1%) | |
| Asian | .. | 1 (1%) | .. | 6 (3%) | .. | 4 (2%) | .. | 3 (3%) | |
| Other | .. | 1 (1%) | .. | 7 (3%) | .. | 4 (2%) | .. | 4 (3%) | |
| Age at randomisation, years | 112 | 67 (56–74) | 230 | 64 (54–72) | 227 | 65 (54–73) | 115 | 64 (55–72) | |
| Body-mass index, kg/m2 | 108 | 28 (26–32) | 222 | 28 (26–32) | 222 | 28 (26–33) | 108 | 28 (26–31) | |
| Smoking | 95 | .. | 186 | .. | 181 | .. | 100 | .. | |
| No | .. | 54 (57%) | .. | 108 (58%) | .. | 101 (56%) | .. | 61 (61%) | |
| Current | .. | 7 (7%) | .. | 11 (6%) | .. | 15 (8%) | .. | 3 (3%) | |
| Former | .. | 34 (36%) | .. | 67 (36%) | .. | 65 (36%) | .. | 36 (36%) | |
| Co-existing conditions | 112 | .. | 230 | .. | 227 | .. | 115 | .. | |
| Arterial hypertension | .. | 57 (51%) | .. | 104 (45%) | .. | 115 (51%) | .. | 46 (40%) | |
| Diabetes | .. | 37 (33%) | .. | 58 (25%) | .. | 59 (26%) | .. | 36 (31%) | |
| Cardiovascular disease | .. | 29 (26%) | .. | 41 (18%) | .. | 46 (20%) | .. | 24 (21%) | |
| Chronic kidney disease | .. | 14 (13%) | .. | 23 (10%) | .. | 25 (11%) | .. | 12 (10%) | |
| 6-category ordinal scale at day of randomisation | 112 | .. | 230 | .. | 227 | .. | 115 | .. | |
| 2 hospitalised, on invasive mechanical ventilation | .. | 17 (15%) | .. | 22 (10%) | .. | 22 (10%) | .. | 17 (15%) | |
| 3 hospitalised, on non-invasive ventilation or high flow oxygen devices | .. | 44 (39%) | .. | 84 (37%) | .. | 89 (39%) | .. | 39 (34%) | |
| 4 hospitalised, requiring supplemental oxygen | .. | 50 (45%) | .. | 119 (52%) | .. | 111 (49%) | .. | 58 (50%) | |
| 5 hospitalised, not requiring supplemental oxygen | .. | 1 (1%) | .. | 5 (2%) | .. | 5 (2%) | .. | 1 (1%) | |
| Mechanical ventilation at day of randomisation | 112 | .. | 230 | .. | 227 | .. | 115 | .. | |
| Invasive | .. | 17 (15%) | .. | 22 (10%) | .. | 22 (10%) | .. | 17 (15%) | |
| Non-invasive or high flow oxygen device | .. | 44 (39%) | .. | 84 (37%) | .. | 89 (39%) | .. | 39 (34%) | |
| ICU at day of randomisation | 112 | .. | 230 | .. | 227 | .. | 115 | .. | |
| Yes | .. | 60 (54%) | .. | 112 (49%) | .. | 113 (50%) | .. | 59 (51%) | |
| SOFA score at day of randomisation | 109 | 3 (2–4) | 207 | 3 (2–4) | 208 | 3 (2–4) | 108 | 3 (2–4) | |
| PaO2/FiO2 ratio at day of randomisation | 110 | 135 (82–233) | 217 | 154 (94–251) | 216 | 155 (92–247) | 111 | 144 (88–250) | |
| PaO2 mm Hg | 110 | 63 (53–75) | 217 | 65 (56–75) | 216 | 64 (56–76) | 111 | 65 (56–74) | |
| PaCO2, mm Hg | 110 | 35 (30–38) | 216 | 35 (31–38) | 215 | 35 (31–38) | 111 | 35 (32–39) | |
| Vasopressor use at day of randomisation | 112 | 10 (9%) | 230 | 11 (5%) | 227 | 12 (5%) | 115 | 9 (8%) | |
| Days of symptoms at randomisation | 100 | 10 (8–11·5) | 214 | 10 (8–12) | 207 | 10 (8–12) | 107 | 10 (9–12) | |
| Days of hospitalisation at randomisation | 112 | 3 (2–4) | 230 | 2 (2–4) | 227 | 3 (2–4) | 115 | 2 (2–4) | |
| Concomitant medication at day of randomisation | 112 | .. | 230 | .. | 227 | .. | 115 | .. | |
| Antibiotics | .. | 58 (52%) | .. | 100 (44%) | .. | 103 (45%) | .. | 55 (48%) | |
| Remdesivir | .. | 6 (5%) | .. | 11 (5%) | .. | 11 (5%) | .. | 6 (5%) | |
| Hydroxychloroquine | .. | 18 (16%) | .. | 22 (10%) | .. | 25 (11%) | .. | 15 (13%) | |
| Glucocorticoids | .. | 72 (64%) | .. | 141 (61%) | .. | 141 (62%) | .. | 72 (63%) | |
| Methylprednisolone equivalents per day, mg | 72 | 32 (32–32) | 141 | 32 (32–32) | 141 | 32 (32–32) | 72 | 32 (32–32) | |
| Duration since randomisation, days | 66 | 8 (5–10) | 133 | 7 (5–9) | 133 | 7 (5–9) | 66 | 8 (5–10) | |
| Laboratory values at day of randomisation | |||||||||
| C-reactive protein, mg/mL | 112 | 148 (86–211) | 226 | 123 (81–183) | 224 | 129 (83–193) | 114 | 129 (81–199) | |
| Lymphocyte count, 103/μL | 108 | 0·7 (0·5–0·9) | 224 | 0·7 (0·5–0·9) | 220 | 0·6 (0·5–0·9) | 112 | 0·7 (0·5–0·9) | |
| Ferritin, μg/L | 109 | 1672 (1187–2509) | 223 | 1688 (1166–2811) | 222 | 1680 (1149–2759) | 110 | 1749 (1205–2619) | |
| D-dimers, ng/mL | 90 | 1091 (510–1847) | 193 | 1000 (560–1490) | 192 | 1000 (550–1600) | 91 | 1099 (567–1780) | |
| Lactate dehydrogenase, IU/L | 111 | 445 (356–537) | 224 | 430 (356–559) | 224 | 435 (356–511) | 111 | 435 (356–589) | |
| IL-1-RA, ng/mL | 108 | 940 (527–1631) | 216 | 903 (525–2150) | 216 | 931 (513–1976) | 108 | 885 (531–1978) | |
| IL-1β, fg/mL | 104 | 44 (11–99) | 213 | 60 (22–127) | 210 | 52 (18–99) | 107 | 74 (20–131) | |
| IL-6, pg/mL | 108 | 8 (3–20) | 216 | 10 (3–23) | 216 | 9 (3–24) | 108 | 8 (3–20) | |
Data are number of patients, n (%), or median (IQR). Sex and ethnicity as reported by study participant. IL=interleukin. ICU=intensive care unit. SOFA=Systematic Organ Failure Assessment. PaO2=partial pressure of arterial oxygen. FiO2=fractional concentration of oxygen in inspired air. PaCO2=partial pressure of arterial carbon dioxide. No patients had an ordinal scale 6 (not hospitalised) or an ordinal scale 1 (death) at day of randomisation.
Overall, 282 (83%) patients experienced the primary endpoint of clinical improvement, more than the anticipated 215 events, leading to a better than anticipated power of more than 89% to detect the HR of clinical interest. Patient enrolment continued beyond reaching the prespecified 215 improvements mark because some time elapsed between event and electronic case report form (eCRF) entry, and some patients continued to improve after enrolment closure.
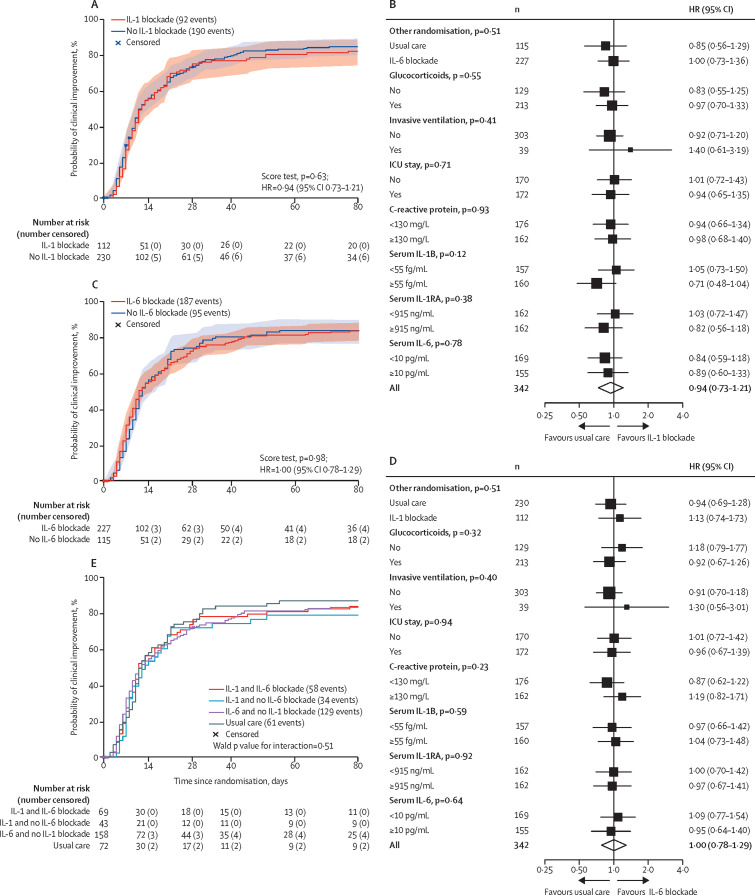
The estimated median time to clinical improvement was 12 days (95% CI 10–16) in the IL-1 blockade (anakinra) group and 12 days (10–15) in the no IL-1 blockade group, corresponding with an estimated HR of 0·94 (95% CI 0·73–1·21; figure 2A ; table 2 ). The estimated probability of having experienced clinical improvement at day 28 was 75% (95% CI 67–83) for the IL-1 blockade and 73% (67–79) for the no IL-1 blockade group. No evidence for a treatment effect of IL-1 blockade could be found in prespecified or post-hoc subgroups, on the basis of allocated treatment for the other randomisation, disease severity (ventilation–ICU stay), concomitant steroid use, or cytokine biomarkers at randomisation (figure 2B).
Figure 2.
Primary endpoint
Kaplan-Meier estimates of the cumulative incidence for clinical improvement according to the allocated treatment for the first randomisation (A) and for the second randomisation (C). Effect of allocation to IL-1 blockade compared with no IL-1 blockade (B) and to IL-6 blockade compared with no IL-6 blockade (D) on time to clinical improvement by baseline characteristics. For defining patients with high or low cytokine biomarker concentrations, the rounded median was used as cutoff. Kaplan-Meier estimates of the cumulative incidence for clinical improvement according to allocated treatment for both randomisations (E). ICU=intensive care unit. IL=interleukin. HR=hazard ratio.
Table 2.
Primary and supportive endpoints in the intention to treat population
| Number included | IL-1 blockade group (95% CI) | No IL-1 blockade group (95% CI) | HR or expected count ratio (95% CI) | IL-6 blockade group (95% CI) | No IL-6 blockade group (95% CI) | HR or expected count ratio (95% CI) | |
|---|---|---|---|---|---|---|---|
| Primary endpoint | |||||||
| Median time until clinical improvement | 342 | 12 days (10–16) | 12 days (10–15) | 0·94 (0·73–1·21) | 11 days (10–16) | 12 days (11–16) | 1·00 (0·78–1·29) |
| Estimated probability of having experienced clinical improvement at day 28 | 342 | 75% (67–83) | 73% (67–79) | .. | 74% (68–79) | 74% (66–82) | .. |
| Supportive endpoints | |||||||
| Median time until discharge | 342 | 14 days (11–19) | 12 days (11–18) | 0·90 (0·70–1·16) | 12 days (11–18) | 13 days (11–19) | 1·02 (0·80–1·31) |
| Median time until independence from supplemental oxygen or discharge | 336 | 12 days (10–20) | 12 days (10–15) | 0·91 (0·71–1·17) | 11 days (10–15) | 12 days (10–15) | 1·00 (0·78–1·28) |
| Median time until independence from invasive ventilation | 39 | 21 days (8–NE) | 27 days (9–NE) | 1·21 (0·54–2·71) | 23 days (8–NE) | 54 days (9–NE) | 1·45 (0·63–3·33) |
| Median time until first use of high-flow oxygen device, ventilation, or death | 175 | <50% reached event | <50% reached event | 0.97 (0·52–1·82) | <50% reached event | <50% reached event | 0·85 (0·47–1·55) |
| Number of days in hospital | 336 | 19 days (17–22) | 19 days (17–21) | 1·01 (0·85–1·21) | 20 days (18–22) | 19 days (16–22) | 1·03 (0·86–1·22) |
| Number of days in ICU | 336 | 11 days (8–15) | 10 days (8–13) | 1·05 (0·69–1·59) | 11 days (8–14) | 10 days (7–15) | 1·03 (0·68–1·56) |
| Number of days in ICU in patients ventilated at day of randomisation | 39 | 20 days (15–27) | 22 days (17–29) | 0·89 (0·60–1·32) | 20 days (16–27) | 22 days (16–29) | 0·94 (0·64–1·40) |
| Number of days in ICU, relative to number of days alive the first 28 days after randomisation | 336 | 42% (31–56) | 36% (29–46) | 1·14 (0·79–1·66) | 38% (30–48) | 40% (29–54) | 0·96 (0·66–1·39) |
| Number of days without supplemental oxygen use up to 28 days after randomisation | 337 | 9 days (7–12) | 9 days (7–11) | 0·97 (0·68–1·38) | 10 days (8–12) | 8 days (6–11) | 1·17 (0·82–1·68) |
| Number of invasive ventilator days | 337 | 5 days (3–9) | 5 days (3–7) | 1·05 (0·54–2·03) | 5 days (3–7) | 5 days (3–9) | 0·89 (0·46–1·72) |
| Number of invasive ventilator days in patients ventilated at day of randomisation | 39 | 15 days (11–20) | 16 days (13–21) | 0·93 (0·63–1·37) | 15 days (12–20) | 16 days (12–22) | 0·96 (0·65–1·42) |
| Number of invasive ventilator days, relative to number of days alive the first 28 days after randomisation | 337 | 23% (14–38) | 21% (14–31) | 1·08 (0·57–2·05) | 21% (14–30) | 23% (14–38) | 0·89 (0·47–1·70) |
| Number of invasive ventilator-free days up to 28 days after randomisation | 337 | 18 days (15–21) | 18 days (16–20) | 1·00 (0·84–1·19) | 18 days (17–20) | 17 days (15–20) | 1·07 (0·90–1·27) |
| Number of invasive ventilator-free days up to 28 days after randomisation in patients ventilated at day of randomisation | 39 | 6 days (3–14) | 6 days (3–13) | 1·01 (0·33–3·07) | 7 days (4–15) | 5 days (2–12) | 1·39 (0·46–4·20) |
Data are n, median time until events (95% CI) with HR (95% CI) or mean number of days (95% CI) with expected count ratio or mean ratio (95% CI). IL=interleukin. HR=hazard ratio. NE=not estimable. ICU=intensive care unit.
The estimated median time to clinical improvement was 11 days (95% CI 10–16) in the IL-6 blockade group (tocilizumab and siltuximab treated patients combined) and 12 days (11–16) in the no IL-6 blockade group, corresponding with an estimated HR of 1·00 (95% CI 0·78–1·29; figure 2C; table 2). The estimated probability of having experienced clinical improvement at day 28 was 74% (95% CI 68–79) for the IL-6 blockade group and 74% (66–82%) for the no IL-6 blockade group. No evidence for a treatment effect could be found in subgroups based on the basis of allocated treatment for the other randomisation, disease severity (ventilation– ICU stay), concomitant steroid use, or cytokine biomarkers at randomisation (figure 2D).
No evidence for a treatment effect of IL-1 or IL-6 blockade could be found for any of the supportive endpoints listed in table 2. Given the factorial design, we tested for interaction between IL-1 and IL-6 blockade as a sensitivity analysis (figure 2E), but did not find evidence for effect modification (Wald p value for interaction=0·51).
Overall, 55 patients died during the study, of which 43 died during the first 28 days after randomisation. Two patients died after experiencing clinical improvement. We did not find evidence for differences in mortality between study groups (table 3 ; appendix p 2). The probability of death by day 28 in the usual care group was estimated at 10% (Kaplan-Meier; 95% CI 5–20) and by day 90 at 13% (7–23). Compared with usual care, there was no increase in in-hospital sepsis or septic shock frequency in the groups receiving cytokine blockade, nor did we observe excess bacterial or fungal infections in non-lethal serious adverse events of these groups. One anakinra-treated patient developed an anaphylactic reaction requiring epinephrine administration (appendix p 4).
Table 3.
Safety analysis, according to received treatment
| Anakinra (n=44) | Anakinra plus tocilizumab (n=32) | Anakinra plus siltuximab (n=36) | Tocilizumab (n=81) | Siltuximab (n=75) | Usual care (n=74) | |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| Number of deaths | 10 (23%) | 5 (16%) | 6 (17%) | 10 (12%) | 15 (20%) | 9 (12%) |
| Causes of death | ||||||
| COVID-19 | 4 (9%) | 2 (6%) | 4 (11%) | 7 (9%) | 9 (12%) | 5 (7%) |
| Infectious disorder (not COVID-19) | 5 (11%) | 2 (6%) | 2 (6%) | 2 (2%) | 2 (3%) | 3 (4%) |
| Nervous system disorder | 1 (2%) | 1 (3%) | .. | 1 (1%) | .. | 1 (1%) |
| Other | .. | .. | .. | .. | 4 (5) | .. |
| Estimated mortality at day 28 | 16% (8–31) | 13% (5–30) | 17% (8–33) | 11% (6–20) | 13% (7–23) | 10% (5–20) |
| Estimated mortality at day 90 | 23% (13–38) | 16% (7–34) | 17% (8–33) | 12% (7–22) | 19% (12–30) | 13% (7–23) |
| Serious infections* | ||||||
| Sepsis | 5 (11%) | 2 (6%) | 3 (8%) | 4 (5%) | 11 (15%) | 6 (8%) |
| Septic shock | 5 (11%) | 1 (3%) | 3 (8%) | 3 (4%) | 6 (8%) | 3 (4%) |
| Serious adverse events not leading to mortality† | ||||||
| Infectious disorder (not COVID-19) | 1 (2%) | 2 (6%) | 1 (3%) | .. | 4 (5%) | 1 (1%) |
| Bleeding | 2 (5%) | 1 (3%) | .. | 1 (1%) | .. | 1 (1%) |
| Thrombosis | 1 (2%) | .. | 1 (3%) | .. | 1 (1%) | 1 (1%) |
| Acute kidney injury | 1 (2%) | .. | .. | .. | 1 (1%) | 1 (1%) |
| Cardiac disorder | .. | .. | .. | 1 (1%) | 1 (1%) | 1 (1%) |
| Other | .. | 2 (6%) | 2 (6%) | 3 (4%) | 1 (1%) | 1 (1%) |
Data are n (%) or estimated mortality rate (95% CI).
Between randomisation and hospital discharge or death.
Progression and symptoms of COVID-19 were excluded from reporting.
Discussion
In the COV-AID trial, we could not show that IL-1 or IL-6 pathway antagonism shortens the time to clinical improvement or improves supportive endpoints when given early in the disease course of hypoxic patients with COVID-19 with evidence of CRS. There was no increase in infectious adverse events or other safety concerns associated with use of anakinra, siltuximab, or tocilizumab.
Benefit from IL-1 blockade with anakinra was not seen in another RCT in non-ventilated COVID-19 patients with hypoxic respiratory failure and high CRP.17 It is possible that the dose of anakinra chosen was insufficient, given that observational cohort studies with higher doses of this drug reported more favourable effects.36 Interestingly, however, an RCT (SAVE-MORE) published in 2021, which used the same treatment regimen for anakinra as in COV-AID (subcutaneous, 100 mg once daily) reported an impressive 55% improvement in day 28 mortality in patients with moderate and severe COVID-19 preselected for high concentrations of soluble urokinase plasminogen activator receptor.18 Thus patient selection might be crucial to detect those who benefit from anakinra treatment.
Despite high expectations, several trials, which used anti-IL-6 drugs in COVID-19, did not meet their primary endpoint,19, 20, 21, 22, 23, 24 including COV-AID. In the EMPACTA trial, although fewer patients on IL-6 blockade progressed to mechanical ventilation, it did not translate to improved survival.25 Two large platform trials reported improved outcomes with IL-6 blockade. The RECOVERY trial observed an increased survival rate in patients with respiratory failure and a CRP concentration above 75 mg/L treated with tocilizumab.29 The REMAP-CAP trial showed an increased number of organ support-free days at day 21 with tocilizumab or sarilumab in patients who were ventilated or received cardiovascular organ support.26
The conflicting results with anti-IL-6 drugs might be explained by characteristics and clinical severity of enrolled patients, reflected by differences in the outcome and mortality of the standard-of-care group. In the RECOVERY platform trial, 28-day mortality was 35% and in the REMAP-CAP platform trial 28-day mortality was 33% in the standard-of-care groups, whereas the mortality varied between 2% and 20% in the standard-of-care groups of most other clinical trials19, 20, 21, 22, 23 and was 10% in the standard-of-care group of COV-AID. With lower mortality due to deeper understanding of the disease and improvements in standard of care, it becomes increasingly more difficult to show a beneficial effect. In addition to event rate, differences in trial design or type of outcome measure could also affect outcome,12, 31, 37 or IL-6 blockade could be most effective in populations at very high risk of death. However, the baseline characteristics of patients in RECOVERY were similar to COV-AID, including age, duration of symptoms and hospital stay, CRP concentration, and type of respiratory support, whereas those of REMAP-CAP only differed in terms of higher rate of mechanical ventilation.26, 29 There might have been more advanced comorbidity and need for non-ventilatory organ support at randomisation, measurable by the Systemic Organ Failure Assessment (SOFA) score in the platform trials reporting a favourable outcome. The SOFA score of patients in our trial was 2–4 for most patients, yet it was not reported in RECOVERY or REMAP-CAP, and enrolled patients in COV-AID had a low frailty score. In COV-AID, none of the subgroup analyses suggested more benefit in the most severely ill patients (those admitted to the ICU) than in those with mild to moderate disease, although numbers were low. In the RECOVERY trial, anti-IL-6 treatment on top of corticosteroids was more beneficial than anti-IL-6 without corticosteroids.29 Corticosteroids were administered to 82% of patients in the RECOVERY trial and to 93% in the REMAP-CAP trial at baseline, versus 60% in our trial.26, 29 We did a post-hoc subgroup analysis of patients receiving corticosteroids at randomisation, but did not observe a higher efficacy of IL-6 blockade in this subgroup. The emergence of different viral variants is also a potential explanation for conflicting results of different trials that is adding additional complexity to the understanding and evaluation of existing studies and the planning and design of future studies.
Biomarkers can identify patients that most benefit from anti-inflammatory drugs in ARDS.38 As a unique feature, we measured baseline serum IL-1β, IL-1RA, and IL-6 concentrations, since these cytokines are associated with poor outcomes in COVID-19.1, 2, 3, 4, 5, 6, 7, 8 Whereas tocilizumab seemed more effective in patients with COVID-19 with the highest IL-6 concentrations in an observational study,14 our prospective data do not support this hypothesis, and further research is required to guide which patients benefit most from anti-inflammatory treatments. Since cytokine concentrations are hard to measure in point-of-care settings, other biomarkers or surrogates of CRS might be more efficient in predicting which patients benefit from a cytokine intervention strategy.18 Another point that should be addressed in future studies is whether the level of cytokine blockade with a particular drug and treatment regimen is adequate or too high, potentially causing excessive toxicity.
This was an academic sponsored trial, and an open-label design was chosen given the logistical and supply challenges at the start of the pandemic, when it was impossible to immediately obtain a placebo format of the prefilled ready-to-use anakinra syringes. Masking for anti-IL-6 is complex owing to the clear anti-pyretic and CRP-lowering effects of tocilizumab and siltuximab. The total number of patients screened for eligibility was not registered in all centres and could not be reported as required by CONSORT guidelines. Therefore, we cannot exclude that selection bias might have influenced the reported results.
Since blocking cytokines can increase risk of bacterial and fungal infection, patients with signs of active co-infection at randomisation were excluded. We did not prespecify how active co-infection should be ruled out, so the interrater reliability might be low. Patients unlikely to survive beyond 48 h were excluded, but since this decision was made by the individual enrolling physician on clinical grounds without objective criteria beyond the use of a frailty scoring index, we cannot rule out selection bias.
The standard of care for patients with COVID-19 changed during the course of this trial. Use of remdesivir and glucocorticoids was balanced in all treatment groups, reducing the risk of bias between groups. A post-hoc analysis (figure 2B and 2D) of patients receiving steroids or not at randomisation did not show differences in outcome of cytokine blockade.
Our trial took place in the Belgian health-care setting. This could limit the extrapolation of our findings to different patient populations. Our patient population was homogeneous and predominantly composed of White men, and further data are needed for generalisability to additional patient populations that differ by gender and ethnic diversity, although data from a meta-analysis of IL-6R blockade showed no effect of ethnicity on effectiveness.30
IL-6 signalling can be modulated in several ways.13 Tocilizumab and sarilumab bind the IL-6 receptor, whereas siltuximab binds the cytokine directly. Since the drugs might have different biological effects, we examined heterogeneity between tocilizumab and siltuximab within the anti-IL-6 group of our trial. Bearing in mind the small size of our study regarding this question, we did not see a major difference between the different anti-IL-6 strategies (appendix p 3).
This was a 2 × 2 factorial design RCT that allowed simultaneous assessment of two cytokine interventions, while minimising the number of patients allocated to the standard-of-care group. Owing to the factorial design, some of the patients received dual cytokine pathway inhibition. Factorial design setup is inherently underpowered to detect interactions between treatment groups. In a predefined sensitivity analysis, however, we did not find evidence for a modification of the anti-IL-1 or anti-IL-6 effect when respectively concomitant tocilizumab–siltuximab or anakinra were given, although much larger numbers of patients would have been required to test for such an interaction.
In our sample of patients with COVID-19 and hypoxic respiratory failure, signs of a cytokine release syndrome, a low 28-day mortality, and low SOFA score, anti-IL-1 or anti-IL-6 drugs given early in the disease course did not shorten the time to clinical improvement. Larger studies or meta-analyses incorporating individual patient characteristics are required to identify which subsets of patients are at risk of increased mortality and could benefit from cytokine blockade treatment.
Data sharing
De-identified individual participant data will be available on approval of a proposal. The shared data can be used for the analyses mentioned in the approved proposal. The study protocol has been published before.33 Proposals should be directed to the corresponding author.
Declaration of interests
JD, KFAVD, BM, CB, VB, LH, LN, and EDL have received personal PhD training fellowships from FWO Flanders. SJT and LJMS have received personal postdoctoral fellowships from FWO Flanders and from the Ghent University BOF Fund. HA has received a postdoctoral personal training grant from VLAIO Flanders. US has received a grant from the European Respiratory Society. MH has received a grant from ULB Fonds Erasme on COVID-19 research, and an EU-Horizon DisCoVeRY trial grant and EU-Solid-Act trial grant on COVID-19 therapeutics; she received support for attending meetings from Pfizer and acts as the leader of the Sciensano committee, which produces the national guidelines on COVID-19 therapeutics. ND has received honoraria from Boehringer Ingelheim and support for attending meetings from Pfizer, Johnson & Johnson (J&J), and Merck Sharp & Dohme (MSD) and has served on a data and safety monitoring board (DSMB) for Roche. SA received research grants from Bristol-Myers Squibb (BMS)–Celgene and from European Hematology Association and consultancy fees from BMS–Celgene, J&J, Astellas, and Abbvie and honoraria from Pfizer and Astellas. JvdH received consultancy fess from Novartis and SOBI and honoraria from MSD, Sanofi, and J&J, and support for attending meetings from ViiV; he serves on DSMB boards for Novartis and SOBI. HS has received consultancy fees and support for attending meetings from Roche and Boehringer Ingelheim. CL has received consultancy fees from the sponsor Belgian Health Care Knowledge Center (KCE) to serve on the clinical trial board. MB owns stock of the International Drug Development Institute and Cluepoints. DS has received honoraria and support for attending meetings from AstraZeneca, Chiesi, GSK, Roche, and Merck. FB has received support from the Ghent University Hospital Clinical Research Fund. FHa has received a VIB Grand Challenge research grant, a Ghent University Special Research Fund (BOF) research grant, and a Ghent University Hospital Innovation Research Grant as well as lecture honoraria from CSL Behring. LV has received grants from University Hospital Ghent Innovation Fund, Research Foundation—Flanders–Strategic Basic Research (FWO-SBO) and FWO to do COVID-19 related research; he received consultancy fees and honoraria from Gilead and ViiV Healthcare. EvB received a grant from Ghent University Special Research Fund (BOF) and a research grant from ExeVir to do COVID-19 related research. She received honoraria from Gilead and is a member of the Sciensano committee, which produces the national guidelines on COVID-19 therapeutics. SR has received honoraria and meeting attendance support from BMS, MSD, Pfizer, Bayer, J&J, Astellas, Roche, and Ipsen; she serves on DSMBs organised by Pfizer, J&J, BMS, and MSD. IP has received research grants from FWO and honoraria from UCB Pharma and Galapagos. She serves on advisory boards from Abbvie, Amgen, Argenx, AstraZeneca, BMS, Galapagos, and Novartis. FHu is an employee of KCE The Belgian Healthcare Knowledge center, a public federal agency. BNL received an European Research Council Advanced Grant (ERC-2017-ADG-789384) and several FWO grants, as well as a University of Ghent Methusalem Grant. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The authors acknowledge professional support and committed efforts from various organisations and individuals involved in this trial and thank all trial participants and clinicians involved in patient recruitment at the different participating sites. This study was funded by KCE, and KCE was involved in various aspects of the study design, management, and execution (Nelle Stocquart, Jillian Harrison). The VIB Grand Challenges Program (Sofie Bekaert) funded measurements of cytokines and the Ghent University Special Research Fund (BOF) supported the clinical follow-up of patients at Ghent University Hospital (UZ Ghent). The clinical trial team of the Department of Respiratory Medicine at UZ Ghent (Stefanie Vermeersch, Benedicte Demeyere, Anja Delporte) were involved in protocol development, amendment filing, and eCRF construction. The Health Innovation and Research Institute of UZ Ghent was involved in eCRF design, protocol design, ethical committee reporting, drug dispensing, trial monitoring, data cleaning, and sponsor site management (Charlotte Clauwaert, Dries Loncke, Hanife Kokur, Lieselot Van Landuyt, Joke Tommelein, Hélène De Naeyer). The hospital pharmacy of UZ Ghent dispensed drugs to all study sites (Els Kestens). Team members of the Primary Immune Deficiency laboratory (Karlien Claes, Veronique Debacker, Lisa Roels, Zara Declercq) handled samples from all study sites. The authors acknowledge the insights of the data safety monitoring board (Drs Renaat Peleman, Geert Leroux-Roels, Steven Callens, Frank Vermassen, Piet Hoebeke, Karim Vermaelen, A Dupont, Tomasz Burzykowski, and Marnik Vuylsteke under the chairmanship of SR).
Contributors
BNL, FHu, JD, KFAVD, EDL, BM, CB, IP, CVDS, EUB, and PD conceptualised the study. BNL, IP, JD, KFAVD, EDL, BM, CB, and FHu did the literature research. BNL, FHu, IP, CL, MB, CVDS, SR, and SJT designed the study. BNL, FHu, CL, MB, CVDS, SR, and SJT were responsible for the methodology. JD, KFAVD, EDL, BM, CB, MH, GV, TF, FM, IKD, ND, NDS, EG, SJV, JVL, SA, JvdH, BM, HS, XW, FL, DS, FB, LJMS, HA, US, VB, LH, LN, FHa, LV, EvB, PD, and BNL collected the data. SDB, RC, MB, and CL analysed the data. SDB and RC programmed and extracted the variables derived from the cleaned database, which were then used for data analysis. MH, GV, TF, FM, IKD, ND, NDS, EG, SJV, JVL, SA, JVDH, BM, HS, XW, FL, and BNL verified the data. BNL, SDB, RC, FHu, JD, KFAVD, EDL, BM, and CB interpreted the data. JD, KFAVD, EDL, BM, CB, and BNL wrote the original draft. Data monitoring and cleaning were done by the Health Innovation and Research Institute of UZ Ghent. BNL decided to submit the manuscript.
Supplementary Material
References
- 1.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in covid-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128. doi: 10.1016/j.jaci.2020.05.008. 36.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020;92:2283–2285. doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Pang J, Ji P, et al. Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis. J Med Virol. 2021;93:35–37. doi: 10.1002/jmv.26085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abers MS, Delmonte OM, Ricotta EE, et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021;6 doi: 10.1172/jci.insight.144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagant C, Ponthieux F, Smet J, et al. A score combining early detection of cytokines accurately predicts COVID-19 severity and intensive care unit transfer. Int J Infect Dis. 2020;101:342–345. doi: 10.1016/j.ijid.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am J Respir Crit Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: meta-analysis A. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angriman F, Ferreyro BL, Burry L, et al. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir Med. 2021;9:655–664. doi: 10.1016/S2213-2600(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SA, Hunter CA. Is IL-6 a key cytokine target for therapy in COVID-19? Nat Rev Immunol. 2021;21:337–339. doi: 10.1038/s41577-021-00553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J Allergy Clin Immunol. 2021;147:72–80.e8. doi: 10.1016/j.jaci.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthay MA, Luetkemeyer AF. IL-6 receptor antagonist therapy for patients hospitalized for COVID-19: who, when, and how? JAMA. 2021;326:483–485. doi: 10.1001/jama.2021.11121. [DOI] [PubMed] [Google Scholar]
- 16.Sinha P, Matthay MA, Calfee CS. Is a “Cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 17.Tharaux P-L, Pialoux G, Pavot A, et al. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021 doi: 10.1038/s41591-021-01499-z. published online May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soin AS, Kumar K, Choudhary NS, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021;9:511–521. doi: 10.1016/S2213-2600(21)00081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lescure FX, Honda H, Fowler RA, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:522–532. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Fu B, Peng Z, et al. Tocilizumab in patients with moderate or severe COVID-19: a randomized, controlled, open-label, multicenter trial. Front Med. 2021;15:486–494. doi: 10.1007/s11684-020-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodd LE, Freidlin B, Korn EL. Platform trials—beware the noncomparable control group. N Engl J Med. 2021;384:1572–1573. doi: 10.1056/NEJMc2102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maes B, Bosteels C, De Leeuw E, et al. Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:468. doi: 10.1186/s13063-020-04453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collignon O, Burman CF, Posch M, Schiel A. Collaborative platform trials to fight COVID-19: methodological and regulatory considerations for a better societal outcome. Clin Pharmacol Ther. 2021;110:311–320. doi: 10.1002/cpt.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson JG, Calfee CS. ARDS Subphenotypes: understanding a heterogeneous syndrome. Crit Care. 2020;24:102. doi: 10.1186/s13054-020-2778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data will be available on approval of a proposal. The shared data can be used for the analyses mentioned in the approved proposal. The study protocol has been published before.33 Proposals should be directed to the corresponding author.