Abstract
Introduction
High flow nasal cannula (HFNC) is a noninvasive ventilation (NIV) system that has demonstrated promise in the emergency department (ED) setting.
Objective
This narrative review evaluates the utility of HFNC in adult patients with acute hypoxemic respiratory failure in the ED setting.
Discussion
HFNC provides warm (37 °C), humidified (100% relative humidity) oxygen at high flows with a reliable fraction of inspired oxygen (FiO2). HFNC can improve oxygenation, reduce airway resistance, provide humidified flow that can flush anatomical dead space, and provide a low amount of positive end expiratory pressure. Recent literature has demonstrated efficacy in acute hypoxemic respiratory failure, including pneumonia, acute respiratory distress syndrome (ARDS), coronavirus disease 2019 (COVID-19), interstitial lung disease, immunocompromised states, the peri-intubation state, and palliative care, with reduced need for intubation, length of stay, and mortality in some of these conditions. Individual patient factors play an important role in infection control risks with respect to the use of HFNC in patients with COVID-19. Appropriate personal protective equipment, adherence to hand hygiene, surgical mask placement over the HFNC device, and environmental controls promoting adequate room ventilation are the foundation for protecting healthcare personnel. Frequent reassessment of the patient placed on HFNC is necessary; those with severe end organ dysfunction, thoracoabdominal asynchrony, significantly increased respiratory rate, poor oxygenation despite HFNC, and tachycardia are at increased risk of HFNC failure and need for further intervention.
Conclusions
HFNC demonstrates promise in several conditions requiring respiratory support. Further randomized trials are needed in the ED setting.
Keywords: High-flow nasal cannula, Noninvasive ventilation, COVID-19, Acute hypoxemic respiratory failure
1. Introduction
Oxygen supplementation is a key component of resuscitation in critically ill patients with respiratory disease. Conventional delivery systems include low flow such as nasal cannula or high flow systems such as non-rebreather or Venturi mask [[1], [2], [3]]. Unfortunately, these systems are often not well-tolerated due to inadequate warming and humidification of the inspired gas, and they do not provide a reliable fraction of inspired oxygen [[3], [4], [5], [6]]. High-flow nasal cannula (HFNC) is a relatively newer noninvasive ventilation system (NIV) that provides warm (37 °C), humidified (100% relative humidity) oxygen at high flows with reliable fraction of inspired oxygen (FiO2) of 21–100% [1,4,[6], [7], [8], [9]]. This improves oxygenation, reduces airway resistance, provides humidified flow that can flush anatomical dead space, and delivers a low amount of positive end expiratory pressure (PEEP) [[7], [8], [9], [10], [11], [12], [13], [14]]. HFNC has several advantages compared with other airway interventions in that patients can still eat, drink, and speak [7,8]. It is also better tolerated and more comfortable than continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BPAP) devices [9,10].
Several nasal devices are currently available, including Vapotherm® (Exeter, NH) and the Optiflow™ and AIRVO™ 2 devices from Fisher and Paykel Healthcare, Inc. (Auckland, New Zealand). Vapotherm® flow rates can reach 50 L/min, and the Optiflow™ and AIRVO™ 2 devices can provide flow rates of 60 L/min. Various nasal prong sizes are available. These devices all include oxygen with an air source, air‑oxygen blender, active heated humidifier, single limb heated inspiratory circuit, delivery tubing, head strap, and soft and flexible nasal prongs (Fig. 1 ) [[9], [10], [11], [12], [13], [14]].
Fig. 1.
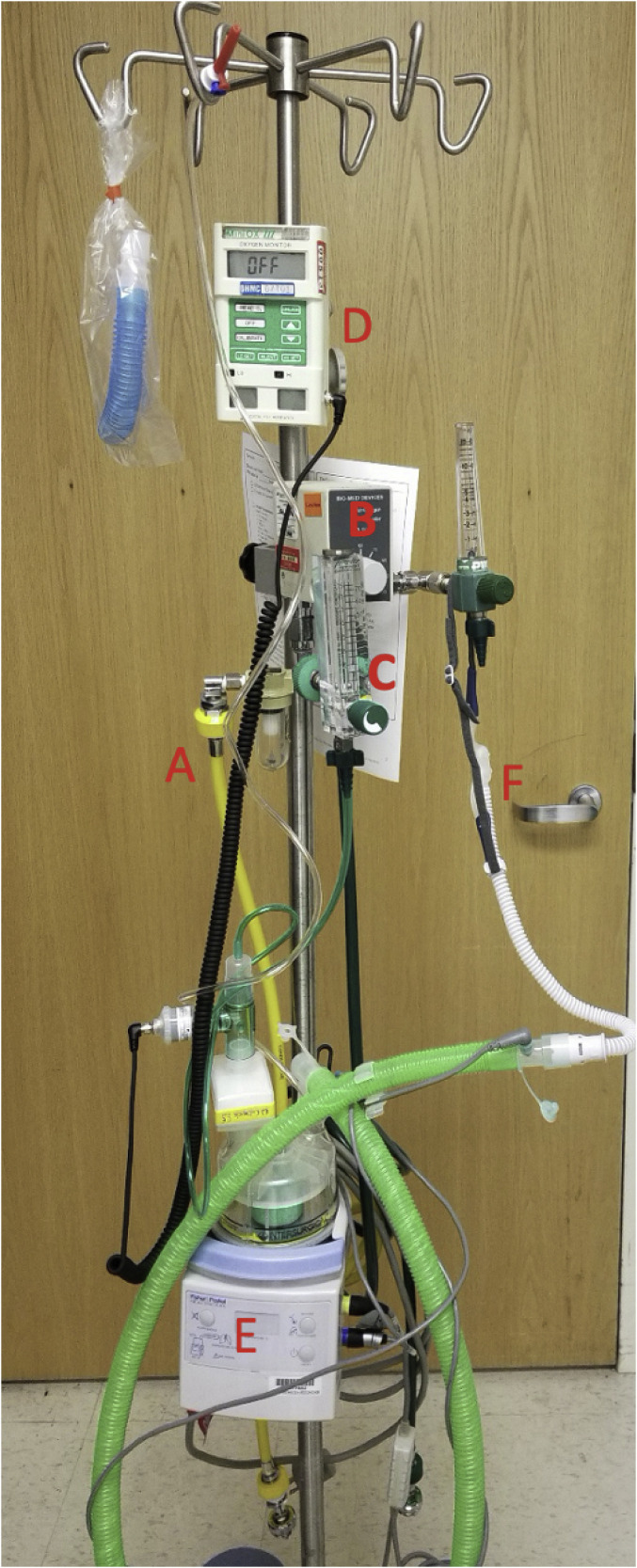
An example of a HFNC delivery setup: (A) Pressurized medical air and oxygen is delivered to an air and oxygen blender where (B) the fraction of oxygen is titrated; a flow meter (C) adjusts the flow of the air and oxygen mixture that is delivered to a heater and humidifier (E). An oxygen monitor (D) can be placed in line for a precise measurement of the fraction of “downstream” oxygen; the humidified air and oxygen mixture is delivered to the patient by a special nasal cannula (F).
HFNC has been evaluated in many different populations and conditions. It was initially developed for neonatal patients and has demonstrated utility in pediatric bronchiolitis. In adults, it has shown promise in acute hypoxemic respiratory failure, preoxygenation and apneic oxygenation for intubation, preventing reintubation, and postoperative recovery [1,9,[11], [12], [13], [14], [15], [16]]. It is feasible to use in the emergency department (ED) and improves respiratory parameters in this setting [[14], [15], [16]]. This narrative review evaluates the utility of HFNC in the ED setting in adults with acute hypoxemic respiratory failure including pneumonia, acute respiratory distress syndrome (ARDS), coronavirus disease 2019 (COVID-19), interstitial lung disease, immunocompromised states, and the peri-intubation state. The review will also provide an overview of infection control considerations surrounding HFNC and predictors of HFNC failure.
2. Methods
The authors searched PubMed and Google Scholar for articles using a combination of the keywords “high flow nasal cannula” and “hypoxemic respiratory failure” or “pneumonia” or “COVID” or “interstitial disease” or “Acute Respiratory Disease Syndrome”. The search was conducted from database inception to February 20, 2021. PubMed yielded 760 articles. The first 200 articles in Google Scholar were also searched. Authors evaluated case reports and series, retrospective and prospective studies, randomized controlled trials, systematic reviews and meta-analyses, and other narrative reviews. Authors also reviewed guidelines and supporting citations of included articles. The literature search was restricted to studies published in English, with focus on the emergency medicine and critical care literature. Authors decided which studies to include for the review by consensus. When available, systematic reviews and meta-analyses were preferentially selected. These were followed sequentially by randomized controlled trials, prospective studies, retrospective studies, case reports, and other narrative reviews when alternate data were not available. A total of 123 resources were selected for inclusion in this narrative review.
3. Discussion
3.1. Mechanism
HFNC provides respiratory support through a continuous flow of humidified air and a titratable FiO2. Humidification offers comfort, retention, and enhancement of mucociliary function and airway clearance [4,9]. The FiO2 can be adjusted from 21 to 100%. The flow of up to 60 L/min is the source of most of the physiological benefits. The flow is able to approximate a patient's peak inspiratory flow that can range from 30 to >120 L/min when in distress—significantly more than the 15 L/min of conventional oxygen devices [9]. This allows HFNC to deliver a higher and more reliable content of FiO2 with less dilution by room air [9,17,18]. In fact, a non-rebreather mask at 15 L/min delivers 68% FiO2 as compared to >89% on a HFNC device set at 40 L/min [17]. The continuous flow also washes out nasopharyngeal anatomical dead space where exhaled air would otherwise be rebreathed. The effect is a reduction in work of breathing and respiratory rate without causing hypercapnia or reducing alveolar ventilation [4,9,14,19,20].
HFNC delivers a flow of air; in contrast, noninvasive positive pressure ventilation (NIPPV) provides and targets an airway pressure. However, an elevation in mean airway pressure is generated with constant flow and increases with the flow rate [18,19]. The mean airway pressure generated is approximately 1 cm H2O/10 L/min of flow [19]. Effects of the positive airway pressure include an increase in end expiratory lung volume and alveolar recruitment [18,19]. This can reduce atelectasis, increase functional residual capacity, and improve oxygenation.
3.2. Acute hypoxemic respiratory failure
HFNC displays the greatest effectiveness in acute hypoxemic respiratory failure. In this setting it is a first line respiratory support therapy and reduces the rate of intubation [8,11,15,16,21,22]. However, the effect on mortality remains less certain [11,15,16]. International guidelines by the European Society of Intensive Care Medicine strongly recommend HFNC over conventional oxygen therapy in acute hypoxemic respiratory failure [16]. The role of HFNC in patients with acute hypercapnic respiratory failure including chronic obstructive pulmonary disease is less clear, with no improvement in outcomes when compared to NIPPV [[23], [24], [25]]. Currently, NIPPV is recommended as a first-line respiratory support modality in chronic obstructive pulmonary disease exacerbations and cardiogenic pulmonary edema [26,27].
3.2.1. Pneumonia and acute respiratory distress syndrome
HFNC is a first line treatment for those with hypoxemic respiratory failure from pneumonia or early ARDS requiring respiratory support beyond low flow oxygen [8,15,16,28]. The multicenter, randomized FLORALI trial compared treatment with HFNC to conventional oxygen therapy and NIPPV (bilevel pressure settings) in patients with acute hypoxemic respiratory failure and a PaO2:FiO2 ratio of <300 mmHg [8]. This study included 310 patients in the intensive care unit (ICU) setting, the majority of whom had pneumonia. The primary outcome included the proportion of patients intubated at day 28, with a secondary outcome of all-cause mortality. There was no difference in 28 day intubation rates, but there was decreased ICU mortality (HFNC 11% vs. NIPPV 25% vs. conventional oxygen 19%), as well as 90-day mortality (HFNC 12% vs. NIPPV 28% vs. conventional oxygen 23%). The HFNC group on post hoc analysis displayed a reduction in the intubation rate in those with a PaO2:FiO2 ratio of <200 mmHg (HFNC 34.9% vs. NIPPV 58% vs. conventional oxygen 52.7%), as well as reduced ICU mortality and 90-day mortality [8]. Importantly >75% had bilateral infiltrates suggesting that HFNC is effective in those with early ARDS. HFNC has also demonstrated safety and effectiveness in observational trials of respiratory support in ARDS [15,16,28].
Several studies conducted in the ED setting also suggest benefit. A non-blinded randomized controlled trial (RCT) including 100 patients found HFNC compared to standard oxygen therapy reduced respiratory rate at 2 h (66.7% vs. 38.5%) and need for escalation of oxygen therapy (4.2% vs. 19%) [29]. A prospective ED RCT found HFNC improved dyspnea and patient comfort compared with conventional oxygen therapy [30]. Another study found reduced need for escalation of oxygen therapy within the first 24 h [31]. These studies also suggest infrequent, if any, adverse events associated with HFNC. The multiple physiological benefits related to the flow of HFNC (e.g., alveolar recruitment, secretion clearance, reduced work of breathing) explain its superiority to conventional oxygen therapy in pneumonia and ARDS. There are potential risks with NIPPV in early ARDS and pneumonia related to the exacerbation of lung injury by the additional pressure and tidal volume—particularly in those with severe lung injury and a PaO2:FiO2 ratio < 150–200 mmHg [[32], [33], [34], [35], [36]]. The FLORALI trial defines the population that is most likely to benefit from HFNC—those with pure hypoxemic respiratory failure from pneumonia and early ARDS [8].
3.2.2. COVID-19
HFNC is recommended by the National Institutes of Health (NIH) and Surviving Sepsis Campaign guidelines in COVID-19 associated acute hypoxemic respiratory failure requiring support beyond conventional oxygen devices [37,38]. This recommendation is largely based on the FLORALI trial demonstrating mortality benefit with HFNC over NIPPV and conventional oxygen devices in those with acute hypoxemic respiratory failure [8]. The FLORALI trial was published prior to the pandemic; however, severe COVID-19 causes acute hypoxemic respiratory failure similar to the inclusion criteria of the trial [8,37,38]. A randomized trial of 110 patients compared helmet NIPPV (10–12 cm H2O pressure support and 10–12 cm H2O of positive end-expiratory pressure) to HFNC (60 L/min) in patients with COVID-19 associated hypoxemic respiratory failure with a PaO2:FiO2 ≤ 200 mmHg [39]. There was no difference in the primary outcome of days free of respiratory support (HFNC, NIPPV, or invasive mechanical ventilation) or the secondary outcome of mortality. However, the secondary outcome of intubation rate was 30% in the helmet NIPPV group vs. 51% in the HFNC group, and there were more invasive ventilation free days in the helmet NIPPV group (HFNC 25 days vs. helmet NIPPV 28 days) [39]. These data suggest the helmet interface of NIPPV–where available–reduces the rate of intubation without change in the duration or course of the illness. No studies comparing the more common mask interface of NIPPV to HFNC in COVID-19 are currently available at the date of this publication [40].
A systematic review and meta-analysis demonstrated HFNC—compared to conventional oxygen—may reduce the need for invasive ventilation in COVID-19 (low certainty) (relative risk [RR] 0.85; 95% confidence interval [CI] 0.74–0.99) and need for escalation of oxygen therapy (RR 0.71; 95% CI 0.51–0.98), but no effect on mortality was found [41]. Importantly, this meta-analysis did not include studies on patients with COVID-19. Similarly, a retrospective study showed HFNC reduced intubation rate in COVID-19 and reduced need for mechanical ventilation at day 28 (HFNC 56% vs. early intubation 75%) without a reduction in mortality [42]. A prospective, propensity-matched, observational cohort study of critically ill patients with COVID-19 suggested a HFNC strategy as compared to early intubation increased ventilator free days (mean difference 8 days) and decreased ICU length of stay (mean difference 8.2 days), but had no impact on mortality [43]. The current evidence demonstrates HFNC is safe and reduces the need for intubation in COVID-19 without clear mortality benefit [[37], [38], [39], [40], [41], [42], [43]]. However, high failure rates have been observed with NIPPV and HFNC in COVID-19, with one study finding 61% of patients required endotracheal intubation [44]; close monitoring for signs of failure is warranted.
In practice, HFNC is recommended in those with COVID-19 requiring more support than low flow oxygen but not requiring immediate intubation. Close reassessment is needed for patients with COVID-19 receiving HFNC [[37], [38], [39], [40], [41], [42], [43], [44]].
3.2.3. Interstitial lung disease
Interstitial lung disease (ILD), also known as interstitial pneumonia, is a progressive inflammatory interstitial pulmonary disease associated with poor prognosis. Acute respiratory failure can complicate ILD, with an associated mortality rate of 50% [[45], [46], [47], [48]]. However, positive pressure ventilation, specifically with mechanical ventilation or NIPPV with significant PEEP, may worsen pulmonary function in a restrictive lung disease such as ILD [49,50].
HFNC has demonstrated utility in patients with acute exacerbations in several studies, with the majority demonstrating improved comfort and reduced hospital length of stay and mortality [[51], [52], [53], [54], [55], [56]]. One small, observational, retrospective study suggests reduced mortality with HFNC (23% vs. NIPPV 63%) despite no difference between HFNC and NIPPV in need for intubation [52]. However, patients with a PaO2:FiO2 < 200 mmHg demonstrated reduced intubation rates with HFNC compared to NIPPV. The authors note that the unique attributes of HFNC including low PEEP, humidified flow, and patient comfort are advantageous [52]. Another retrospective analysis of 17 patients admitted to the ICU with ILD found improved survival at 15 days, 30 days, 90 days, and 365 days, with mortality rates falling below 50% with HFNC [56]. Koyauchi et al. evaluated HFNC in 66 patients with acute exacerbations of ILD, finding improved oxygenation, with a pulse oximetry saturation to fraction of inspired oxygen (SpO2/FiO2) ratio > 170.9 at 24 h a significant predictor of successful HFNC therapy [53]. A retrospective study found reduced mortality with HFNC in ILD (27.9% vs. no HFNC 49.1%), reduced need for sedation and analgesia (78.6% vs. no HFNC 31.6%), and improved oral intake (52.8% vs. no HFNC 23.3%) in patients with ILD [54]. A final retrospective study found HFNC improved comfort and was better tolerated by patients with end stage ILD with a Do Not Intubate (DNI) order [57].
Based on the available evidence, HFNC is an optimal respiratory support modality for patients with ILD exacerbation. However, patients with ILD exacerbation receiving HFNC should be monitored closely with evaluation of mental status, hemodynamics, work of breathing, and oxygenation status [56,58,59].
3.2.4. Immunocompromised patients
Immunocompromised patients who require mechanical ventilation for hypoxemic respiratory failure demonstrate high mortality [60,61]. HFNC is a promising respiratory support device in immunocompromised patients. A post hoc analysis of the FLORALI trial–comprised of immunocompromised patients–found the use of HFNC was associated with reduced need for intubation (31% vs. 65%) when compared to NIPPV and conventional oxygen therapy (43%) [62]. Another study including cancer patients found HFNC reduced 28-day mortality (35% vs. 57%) when compared to standard oxygen or NIPPV; a prospective observational study found HFNC reduced mortality (20% vs. 40%) and intubation (35% vs. 55%) when compared to NIPPV [63,64]. In patients with lung transplants, HFNC was associated with reduced mortality (50% vs. 83%) and intubations (59% vs. 89%) compared to conventional oxygen devices [65]. HFNC is also associated with reduced dyspnea in immunocompromised patients [[66], [67], [68], [69]]. A 2017 RCT demonstrated reduced intubations with HFNC but no difference in mortality when compared to NIPPV [70]; a 2018 RCT found no difference in mortality or intubation rate in HFNC vs. NIPPV [71]. However, in this study, delay in identifying acute hypoxemic respiratory failure was associated with high rates of mortality. HFNC may be ineffective if used as rescue therapy after NIPPV. A 2017 post hoc analysis found no difference in intubation rate or 28 day mortality between HFNC and conventional oxygen therapies [72]. Finally, meta-analyses of these studies comparing HFNC and conventional oxygen therapy or NIPPV demonstrate reduced intubation (RR 0.83–0.89), but no difference in mortality or length of stay [[73], [74], [75]]. HFNC is recommended over conventional oxygen therapies in this patient population and can reduce need for intubation. However, current data demonstrate no reduction in mortality when compared to other oxygen therapies. If HFNC is utilized in this population, it should be started early in the patient's course, with close assessment of patient hemodynamic and respiratory status.
3.2.5. Peri-intubation
Preoxygenation is commonly used in ED and critical care settings to prevent desaturation during induction and intubation. However, preoxygenation techniques and strategies vary substantially and are often insufficient. HFNC may be utilized during intubation as an oxygen delivery system [15,16]. Current literature suggests the use of HFNC in the peri-intubation period to reduce risk of hypoxia is controversial with significant heterogeneity [[76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87]]. A meta-analysis of 10 RCTs found HFNC did not affect rates of peri-intubation hypoxemia, apneic times, serious complications, post-intubation oxygenation, or mortality when compared to conventional oxygen therapy [76]. Importantly, many of the current studies included patients in the perioperative period, including emergent surgery, neurosurgery, bariatric surgery, or any surgery [[77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87]]. While there does not appear to be a significant decrease in hypoxemic episodes or mortality with HFNC, undesirable effects of HFNC are trivial at most. In patients on HFNC prior to induction and intubation, HFNC can be continued during the intubation [16]. It can also be kept in place during laryngoscopy, a marked advantage over other types of NIV.
3.2.6. Palliative care
Dyspnea and air hunger are common symptoms in patients at the end of life. NIPPV has been recommended in palliative care, but NIPPV is associated with discomfort, pressure ulcers, and inability to eat or speak [16,36]. Thus, HFNC may be promising in this population. Several observational studies suggest HFNC can improve work of breathing in patients with hypoxemic respiratory failure, advanced disease, and do-not-intubate (DNI) orders [16,57,68,87,88]. While these studies were not exclusively composed of patients receiving palliative care, HFNC is well tolerated in patients with severe end-stage disease and can reduce dyspnea and improve oral intake and communication [16,68]. HFNC should be considered as an adjunct treatment of dyspnea–if within the patient's goals of care–to usual palliative therapies.
3.3. Infection control
A growing body of literature has evaluated the potential for aerosol generation during HFNC therapy. Using a human patient simulator (HPS) to emulate respiration, studies employing laser smoke visualization have shown that HFNC can increase the distance of exhaled air [89,90]. As flow was increased from 10 L/min to 60 L/min in one study, mean exhaled air distance increased from 65 mm to 172 mm with HFNC, although this was less than that encountered with CPAP [90]. In another HPS study using thickened water and yeast to mimic airway secretions in the nasal and oral cavity, dissemination with HFNC at 60 L/min was detected close to the mannequin's face but not in areas ≥60 cm away on water-sensitive paper or microbiological culture [91].
Studies of healthy volunteers measuring aerosol particle concentrations have not found any increase in aerosol production comparing HFNC at various flows to NIPPV, conventional nasal cannula, or breathing room air [92,93]. Photographic particle visualization studies in healthy volunteers using scattering light as well as chemical marker deposition likewise have not demonstrated a relationship between increasing flow and particle formation [94,95]. However, laser aerosol spectrometry in healthy volunteers has demonstrated flow-dependent increases in production of small and large aerosol particles [96]. In a small randomized controlled crossover trial of critically-ill patients with Gram-negative pneumonia, HFNC was not associated with increased contamination by Gram-negative bacteria or total bacteria in air samples collected ≥1 m or environmental surface cultures collected ≥0.4 m from the patient compared to simple oxygen mask [97].
As aerosol generation can vary with individual breathing patterns, HFNC should also be viewed from the perspective of aerosol dispersion [93,98]. Coughing has been associated with significantly increased numbers of aerosol particles emitted from healthy volunteers [93,94,99]. In a simple study using visible food dye, healthy volunteers asked to cough generated droplets that spread a mean distance of 2.5 m at baseline and 2.9 m with HFNC at 60 L/min, with a maximum distance of 4.5 m observed for the latter [100]. Manipulation of loose-fitting nasal prongs and other device connections associated with HFNC can also result in air leakage that could promote aerosol dispersion [90]. The application of a properly fitted surgical mask over the HFNC can reduce the spread of exhaled air and aerosols by more than 80% [94,101,102].
Early in the COVID-19 pandemic, infection control concerns surrounding use of HFNC led many to favor endotracheal intubation in order to limit potential aerosol generation and dispersion. Evaluation of past and emerging data coupled with hard-won clinical experience have dispelled many of these concerns [103,104]. In one healthcare system, implementation of a respiratory protocol increasing the use of HFNC in a dedicated COVID-19 intermediate care unit with non-negative pressure rooms did not result in any significant increase in symptomatic COVID-19 infections among staff compared to those working in COVID-19 units not using HFNC [104]. Healthcare personnel were required to don a N95/K95 respirator during aerosol-generating procedures, including when caring for patients on HFNC; patients were also encouraged to wear a surgical mask. Similarly, no difference in infection rates occurred among Belgian critical care nurses working in a dedicated COVID-19 ICU employing HFNC and NIV compared to those in a non-COVID-19 ICU [105]. In a small observational study of COVID-19 patients, HFNC did not result in greater aerosol particle generation than conventional nasal cannula [103]. Furthermore, SARS-CoV-2 viral RNA was not detected in air samples obtained during HFNC treatment in each patient's negative pressure room [103]. Having the patient wear a surgical mask while receiving HFNC significantly can reduce the concentration of particles ranging from 0.5 to 5 μm in size at 30.5 cm (1 ft) from the patient's face [106]. In a French ICU study, HFNC was not associated with increased risk of environmental contamination with SARS-CoV-2 viral RNA [107].
Individual patient factors play an important role in infection control risks with respect to the use of HFNC in patients with COVID-19. Exertional breathing and coughing are more likely to generate air particles than quiet breathing [93,94,108]. Patients with COVID-19 are more likely to transmit SARS-CoV-2 early in the course of their illness, and many requiring HFNC may exhibit coughing or exertional breathing [108]. Appropriate personal protective equipment, adherence to hand hygiene, and environmental controls promoting adequate room ventilation have been the foundation for protecting healthcare personnel during the COVID-19 pandemic [37,38,40]. Well-fitting nasal prongs and encouraging patients on HFNC to wear a surgical mask over the nasal cannula can further reduce any potential risk for aerosol dispersion [106]. For patients needing aerosolized medications through HFNC, it has been advised to reduce air flow rates during therapy if the patient can tolerate this in addition to application of surgical mask [109]. Finally, vaccination has emerged as a highly effective strategy for protecting healthcare personnel from COVID-19.
3.4. Contraindications and predictors of failure
The majority of patients tolerate HFNC with no adverse outcomes. Contraindications include face, nose, or airway abnormalities that do not allow for appropriate fit of the nasal cannula. These include patients with epistaxis, basilar skull fracture, surgery to the nose or upper aerodigestive tract, nasal obstruction (tumor, nasal fracture, etc.) [1,4,9,16,36,110]. Complications are rare but may include local trauma or discomfort, abdominal distension, aspiration, epistaxis, and barotrauma, though the risks of these complications is lower than other NIV devices [16,36,110]. The most significant complication is that HFNC may lead to a delay in intubation and worse clinical outcomes in patients with acute respiratory failure [111]. Patients on HFNC with acute hypoxemic respiratory failure should be admitted to an intensive care unit setting due to the need for close monitoring.
Several factors are associated with potential failure. As delayed intubation in patients with progressive respiratory failure may result in increased mortality, early identification of patients likely to fail HFNC is paramount [111]. Observational data have demonstrated an association with increased mortality in those with late failure of HFNC–after 48 h [111]. HFNC failure is less likely to increase mortality in the short term (i.e., <24 h) [16,112]. Thus, a trial in the ED under close observation is reasonable. Nevertheless, waiting until the point of failure is inadvisable. The ROX index has been proposed as a tool to predict success and failure of HFNC [58,59]. This index is the ratio of pulse oximetry oxygen saturation over the fraction of inspired oxygen to the respiratory rate [(SpO2/FiO2)/RR]. ROX index values greater than 4.88 at 2, 6, and 12 h are associated with success of HFNC, while values less than 3.85 are associated with HFNC failure and poor outcomes including mortality. If the value is between 3.85 and 4.88, the index should be recalculated in 1–2 h [58,59]. A recent retrospective analysis demonstrated a ROX index of <4.88 at 2 h was predictive of failure in patients with COVID-19 [113]. Beyond the ROX index, several other factors may be associated with HFNC failure. These include nonrespiratory issues such as need for vasopressors, elevated sequential organ failure assessment score, thoracoabdominal asynchrony, significantly increased respiratory rate (≥ 30 breaths per minutes), poor oxygenation, and tachycardia [113,114]. One study suggests cardiovascular failure and no improvement in patient oxygenation at 24 h and 48 h predicted failure of HFNC [114]. Due to these potential risks of deterioration at 24 and 48 h with use of HFNC, patients admitted from the ED should be managed in an ICU or step-down setting with frequent reassessments.
3.5. Future directions and limitations
While the feasibility and efficacy of HFNC use in the ED have been demonstrated in the previously discussed conditions, the benefit of HFNC over other respiratory support devices in the ED setting has not been clearly demonstrated in several diseases [115]. HFNC has been evaluated in patients with obstructive lung disease and hypercapnic respiratory failure, heart failure, and carbon monoxide toxicity [36,[116], [117], [118], [119], [120], [121], [122], [123]]. In patients with hypercapnic respiratory failure associated with obstructive lung disease, studies have noted a decrease in partial pressure of carbon dioxide but no difference in intubation or mortality rates [116,117]. The data are also controversial for acute heart failure, with several studies finding reduction in respiratory rate and degree of dyspnea, but no difference in intubation or mortality when compared with conventional oxygen therapy [36,[118], [119], [120]]. For patients with carbon monoxide toxicity, studies demonstrate greater reduction in carboxyhemoglobin levels with HFNC when compared with conventional oxygen therapy [121,122], with one study finding two thirds of patients had a reduction by half within 40 min of HFNC therapy [123].
While there are studies evaluating use of HFNC in the ED setting, the majority of studies have been conducted in the ICU setting. Further studies in the ED setting are needed evaluating HFNC compared with other respiratory support systems and patient-centered outcomes such as mortality. Further randomized controlled trials are also needed in patients with conditions other than hypoxemic respiratory failure. Table 1 demonstrates pearls and pitfalls in the use of HFNC in the ED setting.
Table 1.
HFNC Problems, Pitfalls, and Pearls.
| Problem | Pitfalls | Pearls |
|---|---|---|
| Starting HFNC therapy | Failure to assess the response and titrate therapy | The support is delivered by the flow and should be increased to the maximum flow if hypoxemia persists or work of breathing remains elevated Reassess frequently for signs of failure (e.g. persistent work of breathing, hypoxemia, a low ROX index) |
| Using HFNC in the wrong patient | Using HFNC instead of bilevel noninvasive ventilation in COPD exacerbation with ventilatory failure | HFNC is a first-line therapy in acute hypoxemic respiratory failure; bilevel noninvasive ventilation is the treatment of choice in COPD exacerbation with acute hypercapnic respiratory failure; HFNC may be beneficial in acute hypercapnic respiratory failure, but the supporting evidence is limited |
| Infection control | Aerosol generation and dispersion during HFNC are possible, particularly with symptomatic patients | Assuring well-fitting nasal prongs, applying a surgical mask over the cannula, and using lower flows when possible are simple measures to minimize risk |
Abbreviations: HFNC - high flow nasal cannula; COPD – chronic obstructive pulmonary disease; ROX index - ratio of pulse oximetry oxygen saturation over the fraction of inspired oxygen to the respiratory rate.
4. Conclusions
HFNC has demonstrated promise in the ED setting. It provides warm, humidified oxygen at high flow rates which may improve outcomes in several deadly conditions including acute hypoxemic respiratory failure, pneumonia, ARDS, COVID-19, interstitial lung disease, immunocompromised states, the peri-intubation state, and palliative care. Individual patient factors play an integral role in infection control risks with respect to the use of HFNC in patients with COVID-19. Patients on HFNC require frequent reassessment as failure can occur, resulting in need for further airway intervention. While promising, further randomized trials evaluating HFNC are needed in emergency care.
Meetings
None.
Grants/Financial support
None.
Declaration of Competing Interest
None.
Acknowledgements
BL, SYL, and SL conceived the idea for this manuscript and contributed substantially to the writing and editing of the review. This manuscript did not utilize any grants, and it has not been presented in abstract form. This clinical review has not been published, it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. This review does not reflect the views or opinions of the U.S. government, Department of Defense, U.S. Army, U.S. Air Force, Brooke Army Medical Center, or SAUSHEC EM Residency Program.
References
- 1.Drake M.G. High-flow nasal cannula oxygen in adults: an evidence-based assessment. Ann Am Thorac Soc. 2018 Feb;15(2):145–155. doi: 10.1513/AnnalsATS.201707-548FR. [DOI] [PubMed] [Google Scholar]
- 2.Waldau T., Larsen V.H., Bonde J. Evaluation of five oxygen delivery devices in spontaneously breathing subjects by oxygraphy. Anaesthesia. 1998;53:256–263. doi: 10.1046/j.1365-2044.1998.00318.x. [DOI] [PubMed] [Google Scholar]
- 3.Puddy A., Younes M. Effect of inspiratory flow rate on respiratory output in normal subjects. Am Rev Respir Dis. 1992;146:787–789. doi: 10.1164/ajrccm/146.3.787. [DOI] [PubMed] [Google Scholar]
- 4.Roca O., Riera J., Torres F., Masclans J.R. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408–413. [PubMed] [Google Scholar]
- 5.Jeffrey A.A., Warren P.M. Should we judge a mask by its cover? Thorax. 1992;47:543–546. doi: 10.1136/thx.47.7.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanques G., Riboulet F., Molinari N., et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol. 2013;79:1344–1355. [PubMed] [Google Scholar]
- 7.Roca O., Hernández G., Díaz-Lobato S., et al. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016;20(1):109. doi: 10.1186/s13054-016-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frat J.P., Thille A.W., Mercat A., FLORALI Study Group, REVA Network, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016 Apr;61(4):529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 10.Spoletini G., Alotaibi M., Blasi F., Hill N.S. Heated humidified high flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148(1):253–261. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 11.Ferreyro B.L., Angriman F., Munshi L., et al. Association of Noninvasive Oxygenation Strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020 Jul 7;324(1):57–67. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X.P., Zhang X.C., Hu S.L., et al. Noninvasive ventilation in acute hypoxemic nonhypercapnic respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017 Jul;45(7):e727–e733. doi: 10.1097/CCM.0000000000002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goligher E.C., Slutsky A.S. Not just oxygen? Mechanisms of benefit from high-flow nasal cannula in hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1128–1131. doi: 10.1164/rccm.201701-0006ED. [DOI] [PubMed] [Google Scholar]
- 14.Lenglet H., Sztrymf B., Leroy C., et al. Humidified high flow nasal oxygen during respiratory failure in the emergency department: feasibility and efficacy. Respir Care. 2012 Nov;57(11):1873–1878. doi: 10.4187/respcare.01575. [DOI] [PubMed] [Google Scholar]
- 15.Rochwerg B., Granton D., Wang D.X., et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019 May;45(5):563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 16.Rochwerg B., Einav S., Chaudhuri D., et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020 Dec;46(12):2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sim M.A., Dean P., Kinsella J., et al. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008 Sep;63(9):938–940. doi: 10.1111/j.1365-2044.2008.05536.x. [DOI] [PubMed] [Google Scholar]
- 18.Ritchie J.E., Williams A.B., Gerard C., Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. 2011 Nov;39(6):1103–1110. doi: 10.1177/0310057X1103900620. [DOI] [PubMed] [Google Scholar]
- 19.Parke R.L., Bloch A., McGuinness S.P. Effect of very-high-flow nasal therapy on airway pressure and end-expiratory lung impedance in healthy volunteers. Respir Care. 2015;60:1397–1403. doi: 10.4187/respcare.04028. [DOI] [PubMed] [Google Scholar]
- 20.Sztrymf B., Messika J., Bertrand F., et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011 Nov;37(11):1780–1786. doi: 10.1007/s00134-011-2354-6. [DOI] [PubMed] [Google Scholar]
- 21.Ricard D., Roca O., Lemiale V., et al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Med. 2020;46:2238–2247. doi: 10.1007/s00134-020-06228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni Y.N., Luo J., Yu H., et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: a systematic review and meta-analysis. Chest. 2017 Apr;151(4):764–775. doi: 10.1016/j.chest.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Lee M.K., Choi J., Park B., et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018 Jun;12(6):2046–2056. doi: 10.1111/crj.12772. [DOI] [PubMed] [Google Scholar]
- 24.Sun J., Li Y., Ling B., et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study [published correction appears in Int J Chron Obstruct Pulmon Dis. 2019 Jul 15;14:1567] Int J Chron Obstruct Pulmon Dis. 2019;14:1229–1237. doi: 10.2147/COPD.S206567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortegiani A., Longhini F., Madotto F., H. F.-AECOPD study investigators, et al High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. 2020 Dec 14;24(1):692. doi: 10.1186/s13054-020-03409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osadnik C.R., Tee V.S., Carson-Chahhoud K.V., et al. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017 Jul 13;7(7) doi: 10.1002/14651858.CD004104.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berbenetz N., Wang Y., Brown J., et al. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2019 Apr 5;4(4) doi: 10.1002/14651858.CD005351.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messika J., Ben Ahmed K., Gaudry S., et al. Use of high-flow nasal cannula oxygen therapy in subjects with ARDS: a 1-year observational study. Respir Care. 2015 Feb;60(2):162–169. doi: 10.4187/respcare.03423. [DOI] [PubMed] [Google Scholar]
- 29.Bell N., Hutchinson C.L., Green T.C., Rogan E., Bein K.J., Dinh M.M. Randomised control trial of humidified high flow nasal cannulae versusstandard oxygen in the emergency department. Emerg Med Austr EMA. 2015;27(6):537–541. doi: 10.1111/1742-6723.12490. [DOI] [PubMed] [Google Scholar]
- 30.Rittayamai N., Tscheikuna J., Praphruetkit N., Kijpinyochai S. Use of high-flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respir Care. 2015;60(10):1377–1382. doi: 10.4187/respcare.03837. [DOI] [PubMed] [Google Scholar]
- 31.Jones P.G., Kamona S., Doran O., Sawtell F., Wilsher M. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER study. Respir Care. 2016;61(3):291–299. doi: 10.4187/respcare.04252. [DOI] [PubMed] [Google Scholar]
- 32.Grieco D.L., Menga L.S., Eleuteri D., Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019 Sep;85(9):1014–1023. doi: 10.23736/S0375-9393.19.13418-9. [DOI] [PubMed] [Google Scholar]
- 33.Brochard L., Slutsky A., Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017 Feb 15;195(4):438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 34.Bellani G., Laffey J.G., Pham T., et al; LUNG SAFE Investigators; ESICM Trials Group Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017 Jan 1;195(1):67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 35.Thille A.W., Contou D., Fragnoli C., et al. Non-invasive ventilation for acute hypoxemic respiratory failure: intubation rate and risk factors. Crit Care. 2013 Nov 11;17(6):R269. doi: 10.1186/cc13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rochwerg B., Brochard L., Elliott M.W., et al. Members of the task force. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017 Aug 31;50(2):1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 37.COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2021. Coronavirus disease 2019 (COVID-19) treatment guidelines.https://www.covid19treatmentguidelines.nih.gov/ Available at. Accessed [March 23, 2021] [PubMed] [Google Scholar]
- 38.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grieco D.L., Menga L.S., Cesarano M., et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021 doi: 10.1001/jama.2021.4682. Published online March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasa P., Azoulay E., Khanna A.K., et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit Care. 2021 Mar 16;25(1):106. doi: 10.1186/s13054-021-03491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal A., Basmaji J., Muttalib F., et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020 Sep;67(9):1217–1248. doi: 10.1007/s12630-020-01740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demoule A., Vieillard Baron A., Darmon M., et al. High flow nasal cannula in critically ill severe COVID-19 patients. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202005-2007le. Published online August 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellado-Artigas R., Ferreyro B.L., Angriman F., et al; COVID-19 Spanish ICU Network High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit Care. 2021 Feb 11;25(1):58. doi: 10.1186/s13054-021-03469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menga L.S., Cese L.D., Bongiovanni F., et al. High failure rate of noninvasive oxygenation strategies in critically ill subjects with acute hypoxemic respiratory failure due to COVID-19. Respir Care. 2021 Mar 2;66(5):705–714. doi: 10.4187/respcare.08622. Epub 2021 Mar 2. PMID: 33653913. [DOI] [PubMed] [Google Scholar]
- 45.Raghu G., Collard H.R., Egan J.J., et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collard H.R., Ryerson C.J., Corte T.J., et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med. 2016;194:265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 47.Song J.W., Hong S.B., Lim C.M., et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 48.Kishaba T., Tamaki H., Shimaoka Y., et al. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung. 2014;192:141–149. doi: 10.1007/s00408-013-9530-0. [DOI] [PubMed] [Google Scholar]
- 49.Stern J.B., Mal H., Groussard O., et al. Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest. 2001;120:213–219. doi: 10.1378/chest.120.1.213. [DOI] [PubMed] [Google Scholar]
- 50.Anzueto A., Frutos-Vivar F., Esteban A., et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004;30:612–619. doi: 10.1007/s00134-004-2187-7. [DOI] [PubMed] [Google Scholar]
- 51.Horio Y., Takihara T., Niimi K., et al. High-flow nasal cannula oxygen therapy for acute exacerbation of interstitial pneumonia: a case series. Respir Investig. 2016 Mar;54(2):125–129. doi: 10.1016/j.resinv.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Omote N., Matsuda N., Hashimoto N., et al. High-flow nasal cannula therapy for acute respiratory failure in patients with interstitial pneumonia: a retrospective observational study. Nagoya J Med Sci. 2020 May;82(2):301–313. doi: 10.18999/nagjms.82.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koyauchi T., Yasui H., Enomoto N., et al. Pulse oximetric saturation to fraction of inspired oxygen (SpO2/FIO2) ratio 24 hours after high-flow nasal cannula (HFNC) initiation is a good predictor of HFNC therapy in patients with acute exacerbation of interstitial lung disease. Ther Adv Respir Dis. 2020 Jan-Dec;14 doi: 10.1177/1753466620906327. 1753466620906327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito J., Nagata K., Morimoto T., et al. Respiratory management of acute exacerbation of interstitial pneumonia using high-flow nasal cannula oxygen therapy: a single center cohort study. J Thorac Dis. 2019;11:103–112. doi: 10.21037/jtd.2018.12.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J.H., Lim C.M., Koh Y., et al. High-flow nasal cannula oxygen therapy in idiopathic pulmonary fibrosis patients with respiratory failure. J Thorac Dis. 2020 Mar;12(3):966–972. doi: 10.21037/jtd.2019.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vianello A., Arcaro G., Molena B., et al. High-flow nasal cannula oxygen therapy to treat acute respiratory failure in patients with acute exacerbation of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2019;13 doi: 10.1177/1753466619847130. 1753466619847130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koyauchi T., Hasegawa H., Kanata K., et al. Efficacy and tolerability of high-flow nasal cannula oxygen therapy for hypoxemic respiratory failure in patients with interstitial lung disease with do-not-intubate orders: a retrospective single-center study. Respiration. 2018;96(4):323–329. doi: 10.1159/000489890. [DOI] [PubMed] [Google Scholar]
- 58.Roca O., Messika J., Caralt B., et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care. 2016 Oct;35:200–205. doi: 10.1016/j.jcrc.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 59.Roca O., Caralt B., Messika J., et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019 Jun 1;199(11):1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 60.Mokart D., Darmon M., Resche-Rigon M., et al. Prognosis of neutropenic patients admitted to the intensive care unit. Intensive Care Med. 2015;41:296–303. doi: 10.1007/s00134-014-3615-y. [DOI] [PubMed] [Google Scholar]
- 61.Azoulay E., Lemiale V., Mokart D., et al. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med. 2014;40:1106–1114. doi: 10.1007/s00134-014-3354-0. [DOI] [PubMed] [Google Scholar]
- 62.Frat J.P., Ragot S., Girault C., et al. REVA network. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. Lancet Respir Med. 2016;4:646–652. doi: 10.1016/S2213-2600(16)30093-5. [DOI] [PubMed] [Google Scholar]
- 63.Mokart D., Geay C., Chow-Chine L., et al. High-flow oxygen therapy in cancer patients with acute respiratory failure. Intensive Care Med. 2015;41:2008–2010. doi: 10.1007/s00134-015-3994-8. [DOI] [PubMed] [Google Scholar]
- 64.Coudroy R., Jamet A., Petua P., et al. High-flow nasal cannula oxygen therapy versus noninvasive ventilation in immunocompromised patients with acute respiratory failure: an observational cohort study. Ann Intensive Care. 2016;6:45. doi: 10.1186/s13613-016-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roca O., de Acilu M.G., Caralt B., et al. ICU collaborators Humidified high flow nasal cannula supportive therapy improves outcomes in lung transplant recipients readmitted to the intensive care unit because of acute respiratory failure. Transplantation. 2015;99:1092–1098. doi: 10.1097/TP.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 66.Harada K., Kurosawa S., Hino Y., et al. Clinical utility of high-flow nasal cannula oxygen therapy for acute respiratory failure in patients with hematological disease. Springerplus. 2016;5:512. doi: 10.1186/s40064-016-2161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hui D., Morgado M., Chisholm G., et al. High-flow oxygen and bilevel positive airway pressure for persistent dyspnea in patients with advanced cancer: a phase II randomized trial. J Pain Symptom Manage. 2013;46:463–473. doi: 10.1016/j.jpainsymman.2012.10.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Epstein A.S., Hartridge-Lambert S.K., Ramaker J.S., et al. Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center. J Palliat Med. 2011;14:835–839. doi: 10.1089/jpm.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee H.Y., Rhee C.K., Lee J.W. Feasibility of high-flow nasal cannula oxygen therapy for acute respiratory failure in patients with hematologic malignancies: a retrospective single-center study. J Crit Care. 2015;30:773–777. doi: 10.1016/j.jcrc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Azoulay E., Pickkers P., Soares M., et al; Efraim Investigators and the Nine-I Study Group Acute hypoxemic respiratory failure in immunocompromised patients: the Efraim multinational prospective cohort study. Intensive Care Med. 2017 Dec;43(12):1808–1819. doi: 10.1007/s00134-017-4947-1. [DOI] [PubMed] [Google Scholar]
- 71.Azoulay E., Lemiale V., Mokart D., et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018 Nov 27;320(20):2099–2107. doi: 10.1001/jama.2018.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lemiale V., Resche-Rigon M., Mokart D., et al. High-flow nasal cannula oxygenation in immunocompromised patients with acute hypoxemic respiratory failure: a Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique Study. Crit Care Med. 2017;45:e274–e280. doi: 10.1097/CCM.0000000000002085. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y., Ni Y., Sun J., Liang Z. Use of high-flow nasal cannula for Immunocompromise and acute respiratory failure: a systematic review and meta-analysis. J Emerg Med. 2020 Mar;58(3):413–423. doi: 10.1016/j.jemermed.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 74.Cheng L.C., Chang S.P., Wang J.J., et al. The impact of high-flow nasal cannula on the outcome of immunocompromised patients with acute respiratory failure: a systematic review and meta-analysis. Medicina (Kaunas) 2019;55(10):693. doi: 10.3390/medicina55100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang H., Zhao Z., Tong Z. Effect of high-flow nasal cannula oxygen therapy in immunocompromised subjects with acute respiratory failure. Respir Care. 2020 Mar;65(3):369–376. doi: 10.4187/respcare.07205. [DOI] [PubMed] [Google Scholar]
- 76.Chaudhuri D., Granton D., Wang D.X., et al. Moderate certainty evidence suggests the use of high-flow nasal cannula does not decrease hypoxia when compared with conventional oxygen therapy in the Peri-intubation period: results of a systematic review and meta-analysis. Crit Care Med. 2020 Apr;48(4):571–578. doi: 10.1097/CCM.0000000000004217. [DOI] [PubMed] [Google Scholar]
- 77.Simon M., Wachs C., Braune S., et al. Highflow nasal cannula versus bag-valve-mask for preoxygenation before intubation in subjects with hypoxemic respiratory failure. Respir Care. 2016;61(9):1160–1167. doi: 10.4187/respcare.04413. [DOI] [PubMed] [Google Scholar]
- 78.Frat J.P., Ricard J.D., Quenot J.P., et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. Lancet Respir Med. 2019;7(4):303–312. doi: 10.1016/S2213-2600(19)30048-7. [DOI] [PubMed] [Google Scholar]
- 79.Vourc’h M., Asfar P., Volteau C., et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015;41(9):1538–1548. doi: 10.1007/s00134-015-3796-z. [DOI] [PubMed] [Google Scholar]
- 80.Mir F., Patel A., Iqbal R., et al. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia. 2017;72(4):439–443. doi: 10.1111/anae.13799. [DOI] [PubMed] [Google Scholar]
- 81.Guitton C., Ehrmann S., Volteau C., et al. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient: a randomized clinical trial. Intensive Care Med. 2019;45(4):447–458. doi: 10.1007/s00134-019-05529-w. [DOI] [PubMed] [Google Scholar]
- 82.Sebastian H.T.H., Benedikt S., Stubner B., et al. Benefits of heated and humidified high flow nasal oxygen for preoxygenation in morbidly obese patients undergoing bariatric surgery: a randomized controlled study. J Obes Bariatrics. 2014;1:1–7. [Google Scholar]
- 83.Jaber S., Monnin M., Girard M., et al. Apnoeic oxygenation via highflow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016;42(12):1877–1887. doi: 10.1007/s00134-016-4588-9. [DOI] [PubMed] [Google Scholar]
- 84.Ng I., Krieser R., Mezzavia P., et al. The use of transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) for pre-oxygenation in neurosurgical patients: a randomised controlled trial. Anaesth Intensive Care. 2018;46(4):360–367. doi: 10.1177/0310057X1804600403. [DOI] [PubMed] [Google Scholar]
- 85.Lodenius A., Piehl J., Ostlund A., et al. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) vs facemask breathing pre-oxygenation for rapid sequence induction in adults: a prospective randomised non-blinded clinical trial. Anaesthesia. 2018;73(5):564–571. doi: 10.1111/anae.14215. [DOI] [PubMed] [Google Scholar]
- 86.Vourc’h M., Baud G., Feuillet F., et al. High-flow nasal cannulae versus non-invasive ventilation for preoxygenation of obese patients: the PREOPTIPOP randomized trial. EClinicalMedicine. 2019;13:112–119. doi: 10.1016/j.eclinm.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zemach S., Helviz Y., Shitrit M., Friedman R., Levin P.D. The use of high-flow nasal cannula oxygen outside the ICU. Respir Care. 2019;64(11):1333–1342. doi: 10.4187/respcare.06611. [DOI] [PubMed] [Google Scholar]
- 88.Peters S.G., Holets S.R., Gay P.C. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care. 2013;58(4):597–600. doi: 10.4187/respcare.01887. [DOI] [PubMed] [Google Scholar]
- 89.Hui D.S., Chow B.K., Chu L., et al. Exhaled air dispersion and removal is influenced by isolation room size and ventilation settings during oxygen delivery via nasal cannula. Respirology. 2011 Aug;16(6):1005–1013. doi: 10.1111/j.1440-1843.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 90.Hui D.S., Chow B.K., Lo T., et al. Exhaled air dispersion during highflow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53(4):1802339. doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 91.Kotoda M., Hishiyama S., Mitsui K., et al. Assessment of the potential for pathogen dispersal during high-flow nasal therapy. J Hosp Infect. 2020 Apr;104(4):534–537. doi: 10.1016/j.jhin.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miller D.C., Beamer P., Billheimer D., et al. Aerosol risk with noninvasive respiratory support in patients with COVID-19. J Am Coll Emerg Physicians Open. 2020 May 21;1(4):521–526. doi: 10.1002/emp2.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaeckle N.T., Lee J., Park Y., et al. Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am J Respir Crit Care Med. 2020 Oct 15;202(8):1115–1124. doi: 10.1164/rccm.202006-2309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takazono T., Yamamoto K., Okamoto R., et al. Effects of surgical masks on droplet dispersion under various oxygen delivery modalities. Crit Care. 2021 Feb 27;25(1):89. doi: 10.1186/s13054-021-03512-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jermy M.C., Spence C.J.T., Kirton R., et al. Assessment of dispersion of airborne particles of oral/nasal fluid by high flow nasal cannula therapy. PLoS One. 2021 Feb 12;16(2) doi: 10.1371/journal.pone.0246123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearce E., Campen M.J., Baca J.T., et al. Aerosol generation with various approaches to oxygenation in healthy volunteers in the emergency department. J Am Coll Emerg Physicians Open. 2021 Mar 2;2(2) doi: 10.1002/emp2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leung C.C.H., Joynt G.M., Gomersall C.D., et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019 Jan;101(1):84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 98.Dhand R., Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS-CoV-2. Am J Respir Crit Care Med. 2020 Sep 1;202(5):651–659. doi: 10.1164/rccm.202004-1263PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson N.M., Marks G.B., Eckhardt A., et al. The effect of respiratory activity, non-invasive respiratory support and facemasks on aerosol generation and its relevance to COVID-19. Anaesthesia. 2021 Mar 30 doi: 10.1111/anae.15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loh N.W., Tan Y., Taculod J., et al. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anaesth. 2020 Jul;67(7):893–894. doi: 10.1007/s12630-020-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leonard S., Strasser W., Whittle J.S., Volakis L.I., et al. Reducing aerosol dispersion by high flow therapy in COVID-19: high resolution computational fluid dynamics simulations of particle behavior during high velocity nasal insufflation with a simple surgical mask. J Am Coll Emerg Physicians Open. 2020 May 29;1(4):578–591. doi: 10.1002/emp2.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leonard S., Atwood C.W., Jr., Walsh B.K., et al. Preliminary findings on control of dispersion of aerosols and droplets during high-velocity nasal insufflation therapy using a simple surgical mask: implications for the high-flow nasal cannula. Chest. 2020 Sep;158(3):1046–1049. doi: 10.1016/j.chest.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J., Fink J.B., Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020 May 14;55(5):2000892. doi: 10.1183/13993003.00892-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Westafer L.M., Soares W.E., 3rd, Salvador D., et al. No evidence of increasing COVID-19 in health care workers after implementation of high flow nasal cannula: a safety evaluation. Am J Emerg Med. 2021 Jan;39:158–161. doi: 10.1016/j.ajem.2020.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lormans P., Blot S., Amerlinck S., et al. COVID-19 acquisition risk among ICU nursing staff with patient-driven use of aerosol-generating respiratory procedures and optimal use of personal protective equipment. Intensive Crit Care Nurs. 2021 Apr;63:102993. doi: 10.1016/j.iccn.2020.102993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li J., Fink J.B., Elshafei A.A., Stewart L.M., et al. Placing a mask on COVID-19 patients during high-flow nasal cannula therapy reduces aerosol particle dispersion. ERJ Open Res. 2021 Jan 25;7(1):00519–02020. doi: 10.1183/23120541.00519-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lomont A., Boubaya M., Khamis W., et al. Environmental contamination related to SARS-CoV-2 in ICU patients. ERJ Open Res. 2020 Nov 10;6(4):00595–02020. doi: 10.1183/23120541.00595-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He X., Lau E.H.Y., Wu P., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020 May;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 109.Ari A., Moody G.B. How to deliver aerosolized medications through high flow nasal cannula safely and effectively in the era of COVID-19 and beyond: a narrative review. Can J Respir Ther. 2021 Mar 1;57:22–25. doi: 10.29390/cjrt-2020-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baudin F., Gagnon S., Crulli B., et al. Modalities and complications associated with the use of high-flow nasal cannula: experience in a pediatric ICU. Respir Care. 2016 Oct;61(10):1305–1310. doi: 10.4187/respcare.04452. [DOI] [PubMed] [Google Scholar]
- 111.Kang B.J., Koh Y., Lim C.M., et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015 Apr;41(4):623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 112.Frat J.P., Ragot S., Coudroy R., et al; REVA Network Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation strategy. Crit Care Med. 2018 Feb;46(2):208–215. doi: 10.1097/CCM.0000000000002818. [DOI] [PubMed] [Google Scholar]
- 113.Fink D.L., Goldman N.R., Cai J., et al. ROX index to guide management of COVID-19 pneumonia. Ann Am Thorac Soc. 2021 Feb 26 doi: 10.1513/AnnalsATS.202008-934RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dantas C.D.F., Cardosa C., Valentina T. HFNC oxygen therapy success and failure predictors: a portuguese ICU experience. Eur Respir J. 2019;54 [Google Scholar]
- 115.Tinelli V., Cabrini L., Fominskiy E., et al. High flow nasal cannula oxygen vs. conventional oxygen therapy and noninvasive ventilation in emergency department patients: a systematic review and meta-analysis. J Emerg Med. 2019 Sep;57(3):322–328. doi: 10.1016/j.jemermed.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 116.Macé J., Marjanovic N., Faranpour F., et al. Early high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure in the ED: a before-after study. Am J Emerg Med. 2019;37(11):2091–2096. doi: 10.1016/j.ajem.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 117.Jeong J.H., Kim D.H., Kim S.C., et al. Changes in arterial blood gases after use of high-flow nasal cannula therapy in the ED. Am J Emerg Med. 2015;33(10):1344–1349. doi: 10.1016/j.ajem.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 118.Makdee O., Monsomboon A., Surabenjawong U., et al. High-flow nasal cannula versus conventional oxygen therapy in emergency department patients with cardiogenic pulmonary edema: a randomized controlled trial. Ann Emerg Med. 2017 Oct;70(4) doi: 10.1016/j.annemergmed.2017.03.028. 465–472.e2. [DOI] [PubMed] [Google Scholar]
- 119.Carratalá Perales J.M., Llorens P., Brouzet B., et al. High-flow therapy via nasal cannula in acute heart failure. Rev Esp Cardiol. 2011;64:723–725. doi: 10.1016/j.recesp.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 120.Roca O., Pérez-Terán P., Masclans J.R., et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care. 2013;28:741–746. doi: 10.1016/j.jcrc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 121.Tomruk O., Karaman K., Erdur B., et al. A new promising treatment strategy for carbon monoxide poisoning: high flow nasal cannula oxygen therapy. Med Sci Monit. 2019;25:605–609. doi: 10.12659/MSM.914800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim Y.M., Shin H.J., Choi D.W., et al. Comparison of high-flow nasal cannula oxygen therapy and conventional reserve-bag oxygen therapy in carbon monoxide intoxication: a pilot study. Am J Emerg Med. 2020;38(8):1621–1626. doi: 10.1016/j.ajem.2019.158451. [published online ahead of print, 2019 Nov 6] Epub 2019 Nov 6. PMID: 31706658. [DOI] [PubMed] [Google Scholar]
- 123.Ozturan I.U., Yaka E., Suner S., et al. Determination of carboxyhemoglobin half-life in patients with carbon monoxide toxicity treated with high flow nasal cannula oxygen therapy. Clin Toxicol (Phila) 2019;57(7):617–623. doi: 10.1080/15563650.2018.1540046. [DOI] [PubMed] [Google Scholar]


