Graphical abstract
Abstract
Virus-like particles (VLPs) can play important roles in prevention and therapy for infectious diseases and cancer. Here we describe recent advances in rational construction of VLP assemblies, as well as new approaches to enhance long-lasting antibody and CD8+ T cell responses. DNA origami and computational protein design identified optimal spacing of antigens. Chemical biology advances enabled simple and irreversible VLP decoration with protein or polysaccharide antigens. Mosaic VLPs co-displayed antigens to generate cross-reactive antibodies against different influenza strains and coronaviruses. The mode of action of adjuvants inside VLPs was established through knock-outs and repackaging of innate immune stimuli. VLPs themselves showed their power as adjuvants in cancer models. Finally, landmark clinical results were obtained against malaria and the SARS-CoV-2 pandemic.
Current Opinion in Biotechnology 2022, 73:346–354
This review comes from a themed issue on Nanobiotechnology
Edited by Peter M Tessier and Ravi S Kane
For complete overview of the section, please refer to the article collection, “Nanobiotechnology”
Available online 29th October 2021
https://doi.org/10.1016/j.copbio.2021.09.012
0958-1669/© 2021 Elsevier Ltd. All rights reserved.
Introduction
The study of virus-like particles (VLPs) represents an exciting nexus of nanotechnology, immunology and synthetic biology. VLPs have potential to protect against some of the most important medical challenges, but are also powerful tools for basic understanding of the immune system. Initial work on VLPs hijacked proteins from viral capsids, but it was soon found that similar behavior was achieved with non-viral self-assembling nanocages [1]. Here we focus principally on non-enveloped VLPs and their recent application against infectious disease and cancer. We highlight insights on building different VLP architectures through directed evolution and computational design. The effect of different architectures for induction of the immune response is considered, including the impact of surface glycosylation and interior cargo. There have been striking advances in the use of VLPs to protect against highly variable pathogens, through the use of mosaic VLPs. We also consider how VLPs have been employed against SARS-CoV-2 and momentous advances against malaria.
How different protein architectures affect the immune response
It has long been known that clustering an antigen greatly enhances induction of an immune response [1]. For deeper understanding of the effect of antigen multivalency, an HIV immunogen was injected as a monomer, 4-mer, 8-mer or 60-mer [2]. High multimerization facilitated strong induction of transcription factors interferon regulatory factor 4 and Bcl6 in B cells. High multimerization also led to activation and division of B cells with a broader range of antigen binding affinities. Activating a diverse B cell pool may induce broadly protective immune responses to combat the viral diversity in HIV [2].
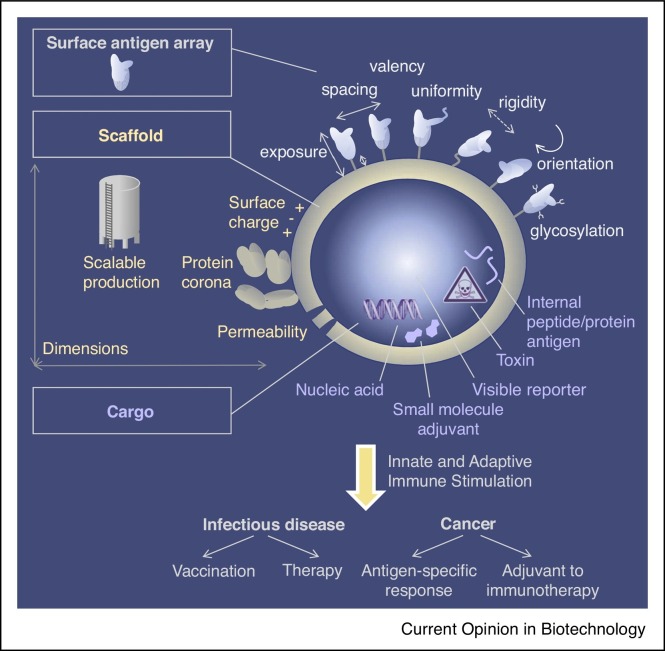
An important question for VLP architecture is what is the optimal spacing between antigens (Figure 1 ) [1]. DNA origami allows excellent spatial control: icosahedral or 6 helical bundle DNA nanoparticles were used for precision display of an HIV antigen. Antigen pairs held rigidly on DNA bundles with 28 nm spacing gave the strongest activation of calcium flux in a B cell line (Figure 2 a). Antigen pairs on flexible single-stranded DNA or polyethylene glycol (PEG) chains were less stimulatory [3••]. The caveat at this stage is that these studies used a B-cell receptor with subnanomolar antigen affinity, not typical during immune priming. Also, the study did not look at immune induction in an animal, where DNases and innate immune activation by DNA may cause further complexity.
Figure 1.
Architectural elements in VLP design. Tuning the surface, underlying scaffold and cargo can all have major effects on VLP biological efficacy.
Figure 2.
Advances in antigen display technologies. (a) DNA origami testing of antigen spacing. 6-helix DNA bundles were prepared with two copies of antigen anchored at different spacing, before testing of B cell activation. Also, DNA icosahedra were constructed with 5 copies of antigen at different spacing. Based on Ref. [3••]. (b) Challenges in matching of symmetry between the nanoparticle and antigen. For modular coupling, display of multimeric antigen (in orange) on the VLP (purple) may lead to different densities of display. There is potential for coupling to destabilize the antigen (misfolding shown in magenta) and for bridging of different VLPs by the antigen. Taken from Ref. [5]. (c) Cobalt porphyrin phospholipid (CoPoP) for anchoring His-tagged antigen to the bilayer of liposomes, taken from Ref. [6]. (d) SnoopLigase (dark gray) directs formation of an isopeptide bond (red) from a lysine of SnoopTagJr peptide (blue) to an asparagine of DogTag peptide (yellow), for linking antigen to nanoparticles. Adapted from Ref. [8].
Enhanced coupling to VLPs
Modular VLP assembly describes when the antigen and scaffold are expressed separately and coupled post-purification. This approach avoids many of the challenges posed by genetic fusion (e.g. misfolding of complex antigens, Figure 1) [4]. Modular coupling can also facilitate coupling of personalized neoantigens for cancer therapy. We previously developed SpyTag/SpyCatcher for plug-and-display VLP coupling. More recently we generated the SpyTag003/SpyCatcher003 system, giving 400-fold faster reaction than the original pair [5]. It was unclear whether antigens of different symmetries could couple efficiently to the VLPs or would provoke catastrophic aggregation (Figure 2b). We found that a panel of antigens of different sizes and symmetries could effectively couple to the SpyCatcher003-mi3 platform, including trimeric influenza hemagglutinin or tetrameric neuraminidase (Figure 2b) [5]. Another emerging conjugation approach is the incorporation of cobalt porphyrin–phospholipid (CoPoP) into a liposome, which facilitates stable interaction with His-tagged proteins (Figure 2c) [6]. The malaria antigen Pfs230 has been coupled to liposomes in this manner and elicited antibodies that inhibit parasite transmission [7].
SnoopLigase allows specific irreversible coupling between the peptides DogTag and SnoopTagJr (Figure 2d). The malarial antigens CyRPA and PfEMP1 or the cancer-associated Telomerase peptide were generated as fusions to SnoopTagJr. SnoopLigase catalyzed the formation of an isopeptide bond to couple the antigen to a DogTag-linked scaffold [8]. The related DogTag/DogCatcher system can proceed to 98% coupling with faster reaction than SnoopLigase. The β-hairpin formed by DogTag is conducive to loop insertions [9]. Modular VLP display from loops rather than termini may help to focus antibody responses to desired regions of the antigen (Figure 1).
Polysaccharides are important antibody targets for protection against a range of bacterial diseases. Polysaccharides on their own do not efficiently induce helper T cells and so are often chemically conjugated to protein antigens, to generate conjugate vaccines. Efficient display of polysaccharides on VLPs could further enhance vaccine potency. The ‘Glycoli platform’ expresses glycosyltransferases in the Escherichia coli cytoplasm, for generation of protein-linked oligosaccharide chains. Glycans could be enzymatically coupled to protein cages that were fused with an acceptor sequence and co-expressed in the cytoplasm [10•]. Synthesis of some bacterial oligosaccharides may be more tractable in the periplasm. Li Zhu’s group expressed PglL in the inner membrane of Shigella flexneri. PglL allowed Shigella oligosaccharides to be coupled onto transfected SpyCatcher bearing a glycosylation sequence in the periplasm. The purified SpyCatcher-oligosaccharide could then react in vitro with SpyTag-fused AP205 or Qβ VLPs. Multimerization of the protein-oligosaccharide conjugate greatly increased the antibody response and protection to Shigella infection [11••].
Building new architectures
In the era of synthetic biology, we should not just rely on nature to furnish us with VLP architectures. The Hilvert group used directed evolution, starting from the lumazine synthase 60-mer that does not bind nucleic acids. Rounds of selection for RNA encapsulation led to an icosahedral 240-mer packaging its encoding mRNA, with spontaneous appearance of stem-loop packaging motifs in the mRNA [12•].
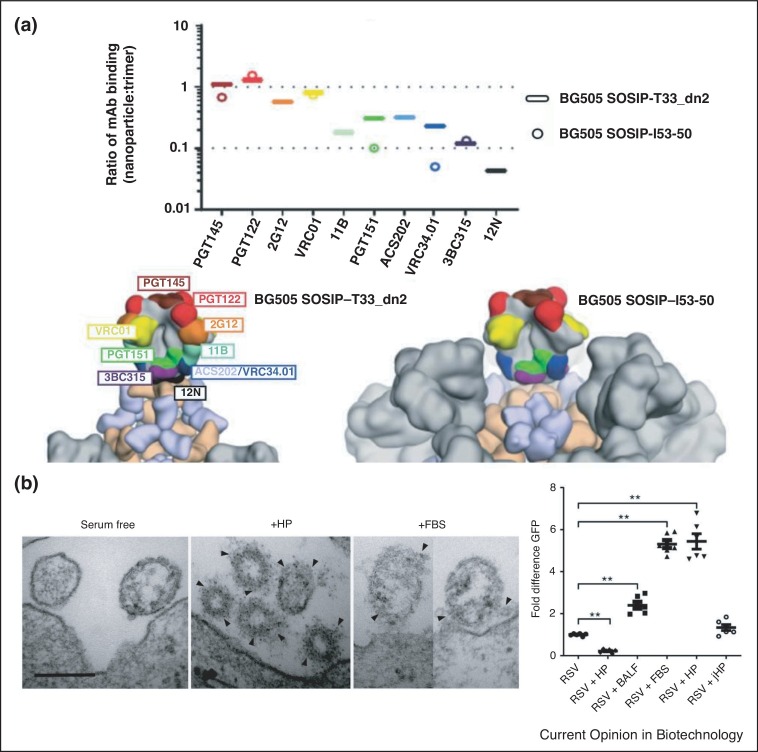
Computational design optimized the assembly of tetrahedral, octahedral, and icosahedral nanoparticles for efficient display of trimeric antigens from HIV, influenza and Respiratory Syncitial Virus (RSV). These scaffolds matched the natural spacing of the antigen termini to maximize antigen stability. In addition, designed scaffolds concealed regions of the antigen, to favor induction of antibodies against the tips of the antigen (Figure 3 a) [13•].
Figure 3.
Changing the environment around the antigen at the VLP surface. (a) Masking of antigen by surface VLP architecture. Epitopes at the base of HIV antigen (BG505 SOSIP) are less exposed when displayed on I53-50 nanoparticles compared to T33_dn2 nanoparticles, based on binding by different monoclonal antibodies (target sites on antigen color-coded), taken from Ref. [13•]. (b) Protein corona interaction with the viral surface. Transmission electron microscopy of RSV close to the cell surface, incubated in serum-free medium, human plasma (HP) or fetal bovine serum (FBS). Arrows point to protein corona bound at the virus surface. On the right is quantification of RSV infectivity (leading to GFP expression) in serum-free conditions compared to different media (BALF, bronchoalveolar lavage fluid; jHP, juvenile human plasma). Taken from Ref. [27••].
Assembly of cages within a cage was shown by packing ferritin nanoparticles within the 56 nm diameter bacteriophage P22 capsid [14]. Simultaneous control of the interior and exterior decorations of a VLP was shown by Sebyung Kang’s group. Split intein reaction coupled reporter proteins on the inside of encapsulin and SpyTag/SpyCatcher allowed outside functionalization with a cancer-directed affibody [15].
Metagenomic sequences were mined for new single-stranded RNA bacteriophage coat proteins. From E. coli expression of 110 different sequences, including 11 new classes, 80 led to VLP assembly [16].
How VLPs provide adjuvant effects
Injecting antigen alone does not usually induce a strong immune response — it is important to activate the innate immune system. Vaccines are nearly always given with an adjuvant to increase innate stimulation [17]. Beyond multimeric presentation on VLPs, other internal and external VLP features affect the size and quality of the immune response. VLPs from phage capsids would naturally package nucleic acid from the expressing organism, which stimulates the innate immune system. The coat protein of Qβ phage was expressed in E. coli and disassembled with reducing agent, before different RNAs (from E. coli, HEK 293T, or yeast tRNA) were packaged inside. Packaging of bacterial RNA in VLPs induced the greatest protection in an influenza model, altering antibody isotypes. This difference depended on innate immune activation by Toll-like receptor (TLR) 7 and not mitochondrial antiviral signaling protein (MAVS) or retinoic acid-inducible gene-I-like receptor (RIG-I) [18••].
TLR agonists can also facilitate anti-cancer immune responses. A small molecule TLR7/8a agonist linked to neoantigen peptides enhanced CD8+ T cell-mediated responses in different mouse tumor models [19]. TLR5 and the Naip5 inflammasome can be stimulated by flagellin, the building block of the bacterial flagellum. Flagellin attachment to a ferritin-based nano-cage enhanced Th1 immune responses [20].
VLPs can be a potent adjuvant even without antigen loading. Human Papillomavirus-16 VLPs, conjugated with a photoactivatable drug, were injected intravenously. Then the tumor was illuminated with near infra-red light to induce necrotic cell death and stimulate long-lasting CD8+ T cell-mediated anti-tumor responses [21]. Different VLP types were compared for their adjuvant effects following intratumoral injection. Cowpea mosaic virus nanoparticles outperformed other plant, phage or mammalian-derived nanoparticles [22]. TLR reporter assays and knock-out mice indicated that Cowpea mosaic virus nanoparticles activate TLR2, 4 and 7 and promote secretion of type I interferons [23]. The value of recruiting other arms of innate immunity was shown by packaging 2′,3′-cyclic GMP-AMP (cGAMP) in VLPs, which increased CD4+ and CD8+ T cell responses and enhanced influenza protection [24].
To supercharge anti-tumor efficacy, VLPs have even been equipped with micro-motors. Qβ VLPs were coupled onto 25 μm particles containing magnesium metal, which reacts with water to generate hydrogen bubbles and drive propulsion. These motorized assemblies for treatment of ovarian cancer were injected into the mouse peritoneum, where micro-motors increased the Qβ in the tumor [25•].
VLPs certainly can induce good CD8+ T cell responses, but have rarely reached the potency of viral vectors. Recent priming and boosting with different arenavirus vectors induced anti-tumor responses up to 50% of the circulating CD8+ T cell pool [26].
We note that animal studies often focus on inducing the strongest immune response without worrying much about inflammatory side-effects. Clinical translation, at least for infectious disease vaccines, requires a balance of mild side-effects with strong induction of a specific immune response [17].
Fundamental understanding of the immune system and its interaction with VLPs
There has been extensive work on how the localization and biological activity of non-biological nanoparticles (e.g. liposomes, quantum dots) depends on the protein corona — the set of proteins that spontaneously associate with a nanoparticle surface exposed to biological fluids. A protein corona also forms on viruses and influences viral infectivity (Figure 3b) [27••], so this surface complexity is an important concept for VLP engineers.
VLP glycosylation can also be an important signature for the immune system (Figure 1). Increasing the glycosylation on Hepatitis B surface antigen (HBsAg) enhanced immunogenicity, when particles were expressed in a human cell-line and ∼40% of glycans were oligomannose [28]. Mannose-binding lectin recognition of oligomannose-rich surfaces on VLPs activated deposition of complement C3 and enhanced transport to follicular dendritic cells, promoting a strong immune response [29••]. Fluorescent imaging of ferritin-based nanoparticles indicated the involvement of a dendritic cell population expressing SIGN-R1 (specific intercellular adhesion molecule-3-grabbing nonintegrin-related 1) in VLP delivery to follicular dendritic cells. These particles generated activity in a model for therapy of chronic Hepatitis B Virus [30].
The route of immunization is also important. To understand the correlates for induction of a strong anti-cancer CD8+ T cell response, intravenous VLP vaccination led to a superior response to subcutaneous delivery. Here neoantigen peptides were induced to self-assemble by conjugation to a hydrophobic polymer linked to a TLR7/8 agonist. T-cell specific transcription factor 1 (TCF1) was identified as an important feature of the arising CD8+ T cells, maintaining a stem cell-like state where cells keep self-renewing [31].
Improved analysis of VLP assembly and composition
In nanotechnology, understanding what has been built is often as much of a challenge as building new structures. Precise antigen conformation is crucial to eliciting protective antibodies. It is now relatively standard to obtain high resolution structures of protein antigens in isolation. However, when antigens are attached to a nanoparticle, it is much harder to use structural analysis. Advanced solid-state nuclear magnetic resonance (NMR) established that the structure of an influenza hemagglutinin variant was retained, following coupling to Qβ particles via thiol-based cross-linking [32]. AFM now enables real-time visualization at the single molecule level of nucleation and reversibility in capsid formation [33]. Many expect perfection in the assembly of a protein nanoparticle. However, cryo-electron microscopy, along with statistical mechanics modeling, showed that mistakes in VLP assembly are normal and hard to avoid, with >15% particles mis-shapen from Hepatitis B Virus capsid [34]. Nonetheless, assembly of computationally designed two component nanoparticles could be uniform and complete, as assessed by dynamic light scattering and native mass spectrometry [35•].
How to improve the antigens displayed on VLPs
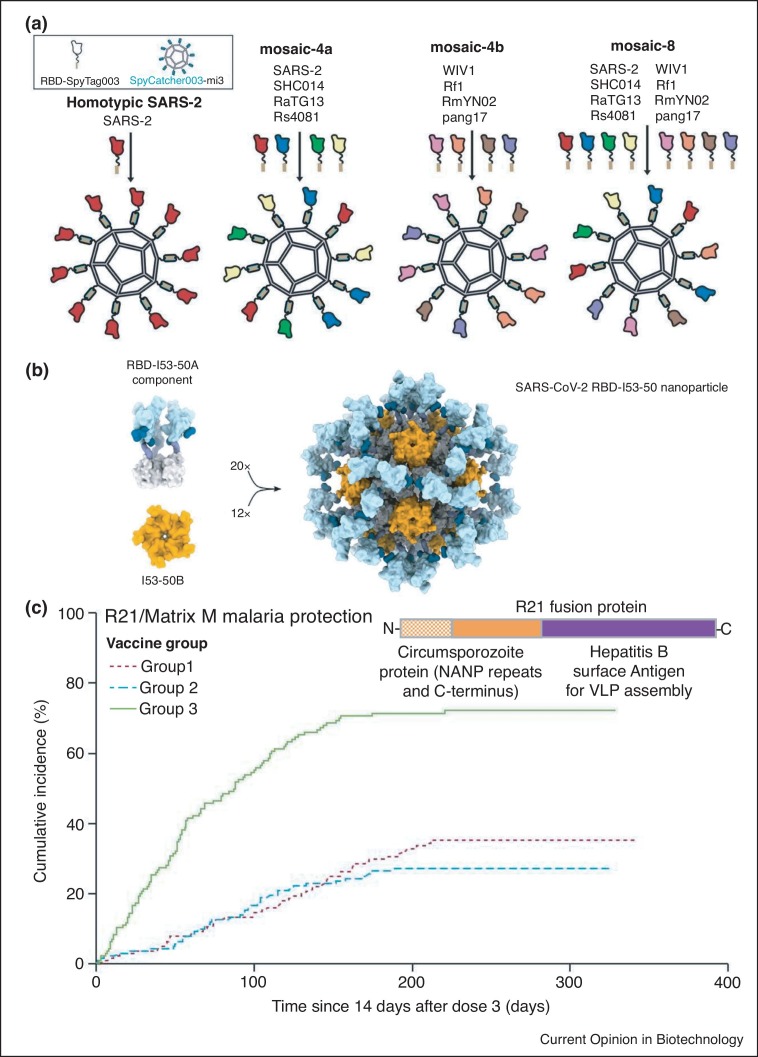
Recent innovations provide strategies to tackle a central challenge — how to generate a vaccine against divergent and evolving pathogens. Mosaic nanoparticles have been produced with several different but related antigens displayed on a single particle. B-cell receptors that bind regions conserved between the different antigens on the VLP should receive the strongest stimulus. Thus mosaic particles encourage the production of antibodies binding a wider variety of pathogen strains [36••]. Mosaic nanoparticles displayed a panel of up to eight different sarbecovirus receptor-binding domains (RBDs) (Figure 4 a). These VLPs elicited a good response against evolutionarily related viruses not represented on the particle, suggesting potential against new SARS-CoV-2 variants and emergent zoonotic coronaviruses [36••]. Mosaic nanoparticles have also been developed for a more broadly neutralizing influenza response, with different hemagglutinin trimers displayed through genetic fusion [37••] or isopeptide coupling [38].
Figure 4.
VLP assemblies against coronavirus and malaria. (a) Mosaic vaccine for broad coronavirus protection. RBD variants from SARS-CoV-2 alone or other sarbecoviruses were expressed bearing SpyTag, allowing covalent coupling onto the SpyCatcher003-mi3 nanoparticle. Display of different RBDs on the same VLP elicited antibodies with broad reactivity to sarbecoviruses. Taken from Ref. [36••]. (b) Two component SARS-CoV-2 VLP vaccine. Model of the SARS-CoV-2 RBD (light blue) fused to the trimeric I53-50A component (gray) and the pentameric I53-50B component (orange). These components are expressed separately and then mixed, to yield a nanoparticle bearing 60 copies of RBD for immunization, taken from Ref. [47]. (c) Malaria VLP vaccine trials results. Incidence of malaria is plotted over time following the third vaccination. Group 1: 5 μg R21/25 μg Matrix M. Group 2: 5 μg R21/50 μg Matrix M. Group 3, rabies control vaccination. The organization of the R21 fusion protein is indicated. Adapted from Ref. [50••].
Antigen has been modified to focus the antibody response, using an innovative strategy called nanopatterning. Zika is an especially difficult vaccine target because non-neutralizing antibodies cause antibody-dependent enhancement (ADE), increasing viral entry into cells. The unnatural amino acid p-azido-L-phenylalanine was introduced at specific sites of Domain III of Zika envelope glycoprotein, allowing click coupling to PEG. When displayed on VLPs, the antibody response was targeted away from the PEGylated region, to favor induction of effective Zika-neutralizing antibodies [39].
Important clinical results from VLPs
The first vaccines approved against SARS-CoV-2 were adenoviral vectors, mRNA vaccines, or inactivated SARS-CoV-2 [40]. A huge range of nanoparticle strategies have been tested towards a COVID-19 vaccine, since VLPs are effective at inducing neutralizing antibodies and may have advantages in stability and scalability [41••,42,43]. NVX-CoV2373 from Novavax is a synthetic lipid nanoparticle displaying SARS-CoV-2 spike, stabilized in pre-fusion conformation, and has successfully completed Phase III trials [41••]. Another lipid nanoparticle candidate employs CoPoP coupling [44•], undergoing Phase I/II [45] (Trial ID: NCT04783311).
Genetic fusions to VLPs have produced promising results against COVID-19. Stabilized spike on ferritin is undergoing Phase I [46] (Trial ID: NCT04784767). RBD was fused to I53-50A and mixed with I53-50B, to assemble into a two-component nanoparticle (Figure 4b) [47] which has entered Phase I/II (Trial ID: NCT04742738). COVID-19 vaccine candidates have been developed using Tag/Catcher ligation, coupling to the protein nanoparticles mi3 [42], AP205 [48] (Trial ID: NCT04839146), or HBsAg [43] (Trial ID: ACTRN12620000817943). Also, VLPs displaying spike trimer, budded from intracellular membranes of Nicotiana benthamiana, entered Phase II/III (Trial ID: NCT04636697) [49].
Against malaria, rational design of VLPs has led to a major advance. R21 is a genetic fusion between a portion of Plasmodium falciparum Circumsporozoite protein and HBsAg, adjuvanted with Matrix-M (Figure 4c). Phase IIb trials demonstrated good safety and a landmark 77% efficacy after 1 year (Figure 4c) [50••]. A Phase III trial is now testing a larger population over a longer time-period (Trial ID: NCT04704830). The antibody response to the NANP repeats correlated to vaccine efficacy [50••]. R21/Matrix-M was previously shown to induce substantial CD4+ T cells but very low CD8+ T cell responses against Circumsporozoite protein [51]. Antibody responses to the HBsAg scaffold (which would provide some protection to Hepatitis B Virus) were very low in response to R21/Matrix-M [51]. Unlike for viral vectors which need to infect cells, the immune response to antigens displayed on VLPs is not blocked by pre-existing antibodies against the VLP scaffold [4,52].
Conclusions
Advances now make it routine to array complex antigens on VLPs, which are scalable for clinical development and induce protective antibody responses for infectious disease vaccination. Data are now accumulating to compare the effect of specific VLP features on the immune response in pre-clinical models. Multiple trials against SARS-CoV-2 will help to uncover how VLP features impact human immunity. Persistent infectious diseases and cancer provide the most challenging targets and it is here that testing of new VLP designs may achieve the greatest benefit.
Conflict of interest statement
M.H. is an author on patents or patent applications for Tag/Catcher/Ligase technologies (UK Intellectual Property Office 1002362.0, 1509782.7, 1705750.6, 1706430.4, 1903479.2, 2104999.4.) and a SpyBiotech co-founder, shareholder and consultant. R.A.H. has no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgement
R.A.H. was funded by the Rhodes Trust.
References
- 1.Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 2.Kato Y., Abbott R.K., Freeman B.L., Haupt S., Groschel B., Silva M., Menis S., Irvine D.J., Schief W.R., Crotty S. Multifaceted effects of antigen valency on B cell response composition and differentiation in vivo. Immunity. 2020;53:548–563.e8. doi: 10.1016/j.immuni.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Veneziano R., Moyer T.J., Stone M.B., Wamhoff E.-C., Read B.J., Mukherjee S., Shepherd T.R., Das J., Schief W.R., Irvine D.J., et al. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat Nanotechnol. 2020;15:716–723. doi: 10.1038/s41565-020-0719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study achieved a new precision in controlling the number and spacing of antigens, presenting the engineered HIV antigen on DNA origami nanoparticles.
- 4.Brune K.D., Howarth M. New routes and opportunities for modular construction of particulate vaccines: stick, click, and glue. Front Immunol. 2018;9:1432. doi: 10.3389/fimmu.2018.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahikainen R., Rijal P., Tan T.K., Wu H., Andersson A.C., Barrett J.R., Bowden T.A., Draper S.J., Townsend A.R., Howarth M. Overcoming symmetry mismatch in vaccine nanoassembly through spontaneous amidation. Angew Chem Int Ed Engl. 2021;60:321–330. doi: 10.1002/anie.202009663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Federizon J., Feugmo C.G.T., Huang W.-C., He X., Miura K., Razi A., Ortega J., Karttunen M., Lovell J.F. Experimental and computational observations of immunogenic cobalt porphyrin lipid bilayers: nanodomain-enhanced antigen association. Pharmaceutics. 2021;13:98. doi: 10.3390/pharmaceutics13010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang W.-C., Deng B., Seffouh A., Ortega J., Long C.A., Suresh R.V., He X., Miura K., Lee S.-M., Wu Y., et al. Antibody response of a particle-inducing, liposome vaccine adjuvant admixed with a Pfs230 fragment. NPJ Vaccines. 2020;5:23. doi: 10.1038/s41541-020-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson A.-M.C., Buldun C.M., Pattinson D.J., Draper S.J., Howarth M. SnoopLigase peptide-peptide conjugation enables modular vaccine assembly. Sci Rep. 2019;9:4625. doi: 10.1038/s41598-019-40985-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeble A.H., Yadav V.K., Ferla M.P., Bauer C.C., Chuntharpursat-Bon E., Huang J., Bon R.S., Howarth M. DogCatcher allows loop-friendly protein-protein ligation. Cell Chem Biol. 2021 doi: 10.1016/j.chembiol.2021.07.005. S2451-9456(21)00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Tytgat H.L.P., Lin C., Levasseur M.D., Tomek M.B., Rutschmann C., Mock J., Liebscher N., Terasaka N., Azuma Y., Wetter M., et al. Cytoplasmic glycoengineering enables biosynthesis of nanoscale glycoprotein assemblies. Nat Commun. 2019;10 doi: 10.1038/s41467-019-13283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study introduced the ‘Glycoli platform’, which works in the E. coli cytoplasm to synthesize a defined glycan at a specific motif on recombinant proteins.
- 11••.Li X., Pan C., Sun P., Peng Z., Feng E., Wu J., Wang H., Zhu L. Orthogonal modular biosynthesis of nanoscale conjugate vaccines for vaccination against infection. Nano Res. 2021 doi: 10.1007/s12274-021-3713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study established a genetically encodable system for oligosaccharide antigen display on the surface of a VLP, leading to a strong anti-bacterial immune response.
- 12•.Tetter S., Terasaka N., Steinauer A., Bingham R.J., Clark S., Scott A.J.P., Patel N., Leibundgut M., Wroblewski E., Ban N., et al. Evolution of a virus-like architecture and packaging mechanism in a repurposed bacterial protein. Science. 2021;372:1220–1224. doi: 10.1126/science.abg2822. [DOI] [PMC free article] [PubMed] [Google Scholar]; Through directed evolution, a bacterial enzyme incapable of binding nucleic acid became a 240-subunit icosahedral VLP that encapsulated its own encoding mRNA.
- 13•.Ueda G., Antanasijevic A., Fallas J.A., Sheffler W., Copps J., Ellis D., Hutchinson G.B., Moyer A., Yasmeen A., Tsybovsky Y., et al. Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens. eLife. 2020;9 doi: 10.7554/eLife.57659. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper introduces a method for computational design of VLP scaffolds, tuned for the display of specific viral glycoproteins and modulating antigen accessibility.
- 14.Waghwani H.K., Uchida M., Fu C.-Y., LaFrance B., Sharma J., McCoy K., Douglas T. Virus-like particles (VLPs) as a platform for hierarchical compartmentalization. Biomacromolecules. 2020;21:2060–2072. doi: 10.1021/acs.biomac.0c00030. [DOI] [PubMed] [Google Scholar]
- 15.Choi H., Eom S., Kim H., Bae Y., Jung H.S., Kang S. Load and display: engineering encapsulin as a modular nanoplatform for protein-cargo encapsulation and protein-ligand decoration using split intein and SpyTag/SpyCatcher. Biomacromolecules. 2021;22:3028–3039. doi: 10.1021/acs.biomac.1c00481. [DOI] [PubMed] [Google Scholar]
- 16.Liekniņa I., Kalniņš G., Akopjana I., Bogans J., Šišovs M., Jansons J., Rūmnieks J., Tārs K. Production and characterization of novel ssRNA bacteriophage virus-like particles from metagenomic sequencing data. J Nanobiotechnol. 2019;17:61. doi: 10.1186/s12951-019-0497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser B.A., Steinhardt R.C., Escalante-Buendia Y., Boltz D.A., Barker K.M., Cassaidy B.J., Rosenberger M.G., Yoo S., McGonnigal B.G., Esser-Kahn A.P. Increased vaccine tolerability and protection via NF-κB modulation. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Gomes A.C., Roesti E.S., El-Turabi A., Bachmann M.F. Type of RNA packed in VLPs impacts IgG class switching—implications for an influenza vaccine design. Vaccines. 2019;7:47. doi: 10.3390/vaccines7020047. [DOI] [PMC free article] [PubMed] [Google Scholar]; Repackaging of different RNAs in a VLP shows the importance for the production of different IgG subclasses and the overall immune response.
- 19.Lynn G.M., Sedlik C., Baharom F., Zhu Y., Ramirez-Valdez R.A., Coble V.L., Tobin K., Nichols S.R., Itzkowitz Y., Zaidi N., et al. Peptide–TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat Biotechnol. 2020;38:320–332. doi: 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E.B., Jeon H.-M., Kim C.-U., Park S.M., Cho G., Kim H.-J., Kim Y., Kim D.-J., Kim Y.S., Lee H., et al. Attachment of flagellin enhances the immunostimulatory activity of a hemagglutinin-ferritin nano-cage. Nanomedicine. 2019;17:223–235. doi: 10.1016/j.nano.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Kines R.C., Thompson C.D., Spring S., Li Z., de los Pinos E., Monks S., Schiller J.T. Virus-like particle–drug conjugates induce protective, long-lasting adaptive antitumor immunity in the absence of specifically targeted tumor antigens. Cancer Immunol Res. 2021;9:693–706. doi: 10.1158/2326-6066.CIR-19-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla S., Wang C., Beiss V., Cai H., Washington T., Murray A.A., Gong X., Zhao Z., Masarapu H., Zlotnick A., et al. The unique potency of Cowpea mosaic virus (CPMV) in situ cancer vaccine. Biomater Sci. 2020;8:5489–5503. doi: 10.1039/d0bm01219j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao C., Beiss V., Fields J., Steinmetz N.F., Fiering S. Cowpea mosaic virus stimulates antitumor immunity through recognition by multiple MYD88-dependent toll-like receptors. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauveau L., Bridgeman A., Tan T.K., Beveridge R., Frost J.N., Rijal P., Pedroza-Pacheco I., Partridge T., Gilbert-Jaramillo J., Knight M.L., et al. Inclusion of cGAMP within virus-like particle vaccines enhances their immunogenicity. EMBO Rep. 2021;22 doi: 10.15252/embr.202152447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Wang C., Fernández de Ávila B.E., Mundaca-Uribe R., Lopez-Ramirez M.A., Ramírez-Herrera D.E., Shukla S., Steinmetz N.F., Wang J. Active delivery of VLPs promotes anti-tumor activity in a mouse ovarian tumor model. Small. 2020;16 doi: 10.1002/smll.201907150. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the production of magnesium-based micromotors loaded with Qβ VLPs, where autonomous propulsion enhanced their immunostimulatory effects.
- 26.Bonilla W.V., Kirchhammer N., Marx A.-F., Kallert S.M., Krzyzaniak M.A., Lu M., Darbre S., Schmidt S., Raguz J., Berka U., et al. Heterologous arenavirus vector prime-boost overrules self-tolerance for efficient tumor-specific CD8 T cell attack. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Ezzat K., Pernemalm M., Pålsson S., Roberts T.C., Järver P., Dondalska A., Bestas B., Sobkowiak M.J., Levänen B., Sköld M., et al. The viral protein corona directs viral pathogenesis and amyloid aggregation. Nat Commun. 2019;10 doi: 10.1038/s41467-019-10192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that viruses form a protein corona dependent on the nature of both virus and the biological media, influencing immune cell stimulation and viral infectivity.
- 28.Joe C.C.D., Chatterjee S., Lovrecz G., Adams T.E., Thaysen-Andersen M., Walsh R., Locarnini S.A., Smooker P., Netter H.J. Glycoengineered hepatitis B virus-like particles with enhanced immunogenicity. Vaccine. 2020;38:3892–3901. doi: 10.1016/j.vaccine.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 29••.Tokatlian T., Read B.J., Jones C.A., Kulp D.W., Menis S., Chang J.Y.H., Steichen J.M., Kumari S., Allen J.D., Dane E.L., et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science. 2019;363:649–654. doi: 10.1126/science.aat9120. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study illuminates how immunogen glycosylation engages the innate immune system and affects lymph node responses.
- 30.Wang W., Zhou X., Bian Y., Wang S., Chai Q., Guo Z., Wang Z., Zhu P., Peng H., Yan X., et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat Nanotechnol. 2020;15:406–416. doi: 10.1038/s41565-020-0648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baharom F., Ramirez-Valdez R.A., Tobin K.K.S., Yamane H., Dutertre C.-A., Khalilnezhad A., Reynoso G.V., Coble V.L., Lynn G.M., Mulè M.P., et al. Intravenous nanoparticle vaccination generates stem-like TCF1+ neoantigen-specific CD8+ T cells. Nat Immunol. 2021;22:41–52. doi: 10.1038/s41590-020-00810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaudzems K., Kirsteina A., Schubeis T., Casano G., Ouari O., Bogans J., Kazaks A., Tars K., Lesage A., Pintacuda G. Structural analysis of an antigen chemically coupled on virus-like particles in vaccine formulation. Angew Chem Int Ed. 2021;60:12847–12851. doi: 10.1002/anie.202013189. [DOI] [PubMed] [Google Scholar]
- 33.Valbuena A., Maity S., Mateu M.G., Roos W.H. Visualization of single molecules building a viral capsid protein lattice through stochastic pathways. ACS Nano. 2020;14:8724–8734. doi: 10.1021/acsnano.0c03207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiriti J., Conway J.F., Zuckerman D.M. Should virus capsids assemble perfectly? Theory and observation of defects. Biophys J. 2020;119:1781–1790. doi: 10.1016/j.bpj.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Wargacki A.J., Wörner T.P., van de Waterbeemd M., Ellis D., Heck A.J.R., King N.P. Complete and cooperative in vitro assembly of computationally designed self-assembling protein nanomaterials. Nat Commun. 2021;12 doi: 10.1038/s41467-021-21251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provided deep investigation of the assembly of computationally designed two-component nanoparticles, achieving complete VLP formation in a process that is normally error-prone.
- 36••.Cohen A.A., Gnanapragasam P.N.P., Lee Y.E., Hoffman P.R., Ou S., Kakutani L.M., Keeffe J.R., Wu H.-J., Howarth M., West A.P., et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science. 2021;371:735–741. doi: 10.1126/science.abf6840. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mosaic display of multiple coronavirus RBDs on a single nanoparticle induced cross-reactive antibodies, with potential for protective immunization across multiple coronaviruses.
- 37••.Boyoglu-Barnum S., Ellis D., Gillespie R.A., Hutchinson G.B., Park Y.-J., Moin S.M., Acton O.J., Ravichandran R., Murphy M., Pettie D., et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature. 2021;592:623–628. doi: 10.1038/s41586-021-03365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mosaic display of different hemagglutinin trimers on a computationally designed nanoparticle elicited broadly protective antibodies to move beyond seasonal influenza vaccination.
- 38.Cohen A.A., Yang Z., Gnanapragasam P.N.P., Ou S., Dam K.-M.A., Wang H., Bjorkman P.J. Construction, characterization, and immunization of nanoparticles that display a diverse array of influenza HA trimers. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castro A., Carreño J.M., Duehr J., Krammer F., Kane R.S. Refocusing the immune response to selected epitopes on a Zika virus protein antigen by nanopatterning. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21:475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., Chadwick D.R., Clark R., Cosgrove C., Galloway J., et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports the successful phase III clinical trial of the SARS-CoV-2 spike displayed on a lipid nanoparticle.
- 42.Tan T.K., Rijal P., Rahikainen R., Keeble A.H., Schimanski L., Hussain S., Harvey R., Hayes J.W.P., Edwards J.C., McLean R.K., et al. A COVID-19 vaccine candidate using SpyCatcher multimerization of the SARS-CoV-2 spike protein receptor-binding domain induces potent neutralising antibody responses. Nat Commun. 2021;12 doi: 10.1038/s41467-020-20654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalvie N.C., Tostanoski L.H., Rodriguez-Aponte S.A., Kaur K., Bajoria S., Kumru O.S., Martinot A.J., Chandrashekar A., McMahan K., Mercado N.B., et al. A modular protein subunit vaccine candidate produced in yeast confers protection against SARS-CoV-2 in non-human primates. bioRxiv. 2021 doi: 10.1101/2021.07.13.452251. [DOI] [Google Scholar]
- 44•.Huang W.-C., Zhou S., He X., Chiem K., Mabrouk M.T., Nissly R.H., Bird I.M., Strauss M., Sambhara S., Ortega J., et al. SARS-CoV-2 RBD neutralizing antibody induction is enhanced by particulate vaccination. Adv Mater. 2020;32 doi: 10.1002/adma.202005637. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cobalt-porphyrin-phospholipid coupling allowed facile display of the SARS-CoV-2 RBD on a liposome for immunization.
- 45.EuBiologics Co., Ltd . 2021. A Phase 1/2 Dose-exploration, Randomized, Observer-blind, Placebo-controlled Study to Determine Safety, Tolerance and Immunogenicity of EuCorVac-19, a Recombinant Protein Vaccine, for the Prevention of COVID-19 in Healthy Adults.clinicaltrials.gov [Google Scholar]
- 46.Joyce M.G., Chen W.-H., Sankhala R.S., Hajduczki A., Thomas P.V., Choe M., Chang W., Peterson C.E., Martinez E., Morrison E.B., et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. bioRxiv. 2021 doi: 10.1101/2021.05.09.443331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walls A.C., Fiala B., Schäfer A., Wrenn S., Pham M.N., Murphy M., Tse L.V., Shehata L., O’Connor M.A., Chen C., et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell. 2020;183:1367–1382.e17. doi: 10.1016/j.cell.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fougeroux C., Goksøyr L., Idorn M., Soroka V., Myeni S.K., Dagil R., Janitzek C.M., Søgaard M., Aves K.-L., Horsted E.W., et al. Capsid-like particles decorated with the SARS-CoV-2 receptor-binding domain elicit strong virus neutralization activity. Nat Commun. 2021;12 doi: 10.1038/s41467-020-20251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gobeil P., Pillet S., Séguin A., Boulay I., Mahmood A., Vinh D.C., Charland N., Boutet P., Roman F., Most R.V.D., et al. Interim report of a phase 2 randomized trial of a plant-produced virus-like particle vaccine for Covid-19 in healthy adults aged 18-64 and older adults aged 65 and older. medRxiv. 2021 doi: 10.1101/2021.05.14.21257248. [DOI] [Google Scholar]
- 50••.Datoo M.S., Natama M.H., Somé A., Traoré O., Rouamba T., Bellamy D., Yameogo P., Valia D., Tegneri M., Ouedraogo F., et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet. 2021;397:1809–1818. doi: 10.1016/S0140-6736(21)00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; A phase II clinical trial for a VLP vaccine showed strong protection against malaria.
- 51.Collins K.A., Snaith R., Cottingham M.G., Gilbert S.C., Hill A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci Rep. 2017;19:46621. doi: 10.1038/srep46621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marini A., Zhou Y., Li Y., Taylor I.J., Leneghan D.B., Jin J., Zaric M., Mekhaiel D., Long C.A., Miura K., et al. A universal plug-and-display vaccine carrier based on HBsAg VLP to maximize effective antibody response. Front Immunol. 2019;10:2931. doi: 10.3389/fimmu.2019.02931. [DOI] [PMC free article] [PubMed] [Google Scholar]