Abstract
Background:
The present study aimed to systematically evaluate the diagnostic value of an isoform of alpha-fetoprotein (AFP), AFP-L3, for early hepatocellular carcinoma (HCC) by a meta-analysis.
Methods:
Diagnostic reports of AFP-L3% in early HCC were searched in the PubMed, Web of Science, Cochrane Library, and Embase databases up to December 2019. The retrieved literature was reviewed, and eligible articles were selected. Data were extracted from the eligible articles, and the risk of bias was evaluated according to the Quality Assessment of Diagnostic Accuracy Studies scale. Statistical analyses were conducted by MetaDiSc1.4 and RevMan5.3 software. The sensitivities, specificities, and diagnostic odds ratios were pooled. The summary receiver operating characteristic curve was drawn, and the area under the curve was calculated.
Results:
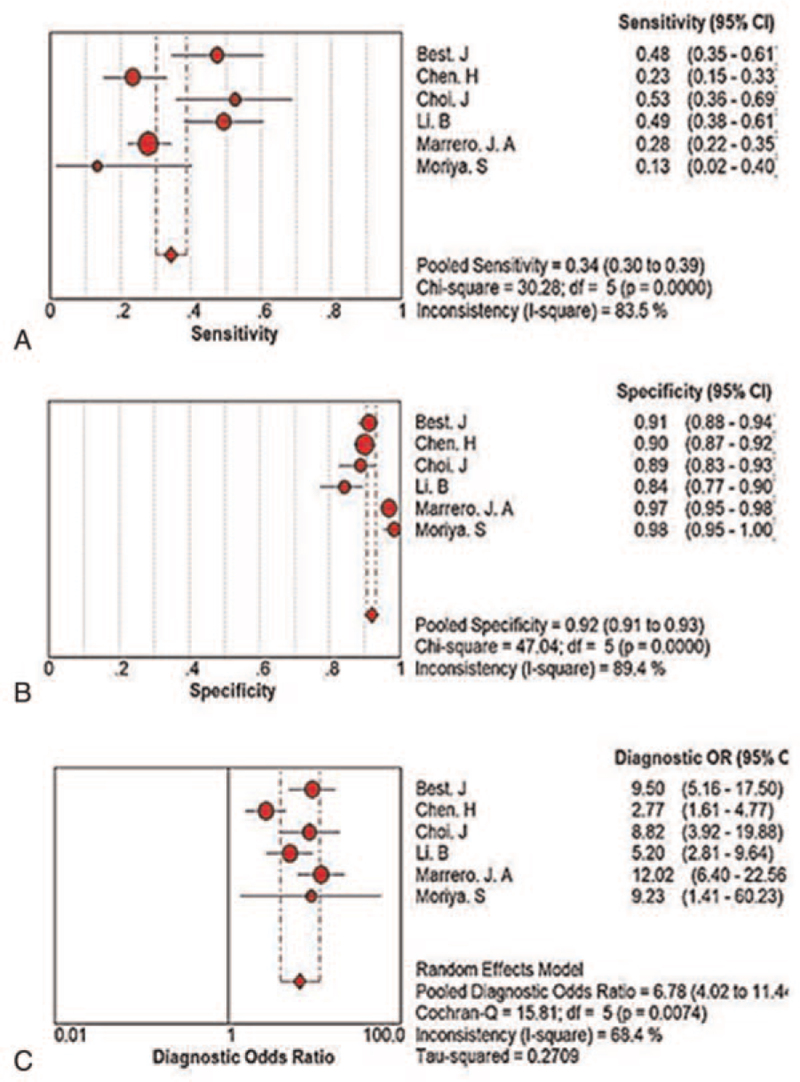
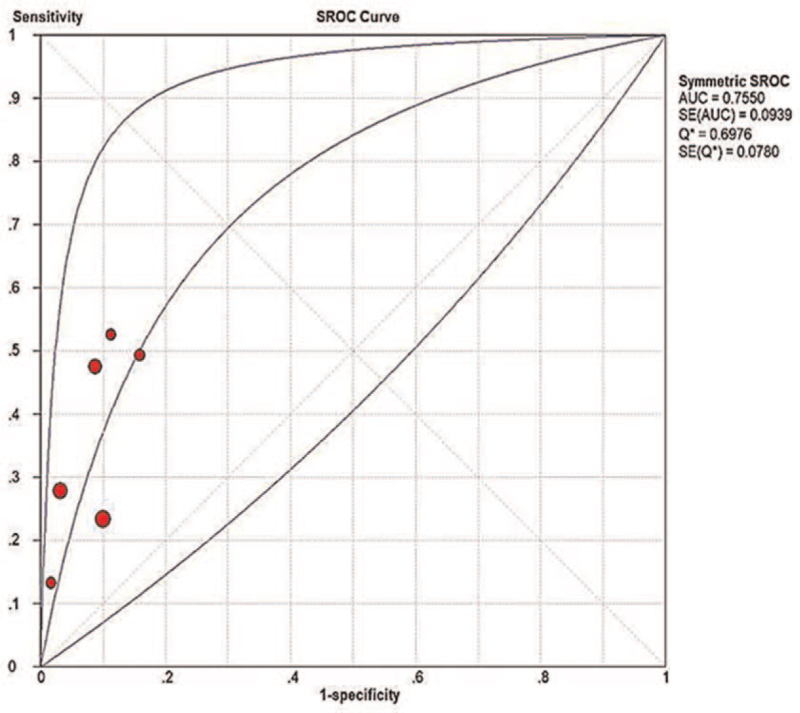
Six studies with acceptable quality were included in the meta-analysis involving 2447 patients. No threshold effect was observed among the 6 studies, but there was obvious heterogeneity. The pooled sensitivity, specificity, and positive and negative likelihood ratios of AFP-L3% for the diagnosis of early HCC were 0.34 (95% CI 0.30–0.39, P < .0001), 0.92 (95% CI 0.91–0.93, P < .0001), 4.46 (95% CI 2.94–6.77, P = .0033), and 0.71 (95% CI 0.61–0.82, P = .0004), respectively. The diagnostic odds ratio was 6.78 (95% CI 4.02–11.44, P = .0074). The the area under the curve of the summary receiver operating characteristic was 0.755 (95% CI 0.57–0.94).
Conclusion:
AFP-L3% has high specificity but low sensitivity for diagnose early HCC, suggesting that AFP-L3% is more valuable for excluding HCC in conditions with elevated AFP than for diagnosing early HCC. In addition, a hypersensitive detection method can improve the diagnostic accuracy of AFP-L3% for early HCC.
Keywords: alpha-fetoprotein isoform L3, biomarker, diagnostic performance, early diagnosis, hepatocellular carcinoma, meta-analysis
1. Introduction
Liver cancer is a common malignant tumor of the digestive system. According to the Global Cancer Report 2018 released by the International Agency for Research on Cancer (IARC), it is estimated that there were approximately 841,000 new liver cancer cases and 782,000 deaths in 2018, ranking liver cancer as the fourth leading cause of cancer death worldwide.[1] Most of the new cases occurred in underdeveloped countries, especially in China. Hepatocellular carcinoma (HCC) is a major type of primary liver cancer. The prognosis of patients with HCC is related to the tumor stage at the time of diagnosis.[2] Because of the lack of obvious clinical symptoms and signs in the early stages, most HCC is already in advanced stages when diagnosed, and thus, the prognosis is poor. Therefore, one of the key strategies to improving the prognosis of HCC is to diagnose the tumor in the early stages when curative treatment is still possible.
Currently, the most frequently used diagnostic method for HCC is imaging combined with tumor marker evaluations.[3–5] Tumor markers, as simple and inexpensive indicators, have been widely used in clinical practice. Alpha fetoprotein (AFP), which was discovered in 1963, has become the most important serum marker for diagnose HCC and is also widely used in postoperative recurrence monitoring.[6,7] Unfortunately, the positive rate of AFP expression in HCC is only approximately 70%,[8] and nearly 40% of HCC is AFP-negative.[9–11] Due to the elevation of AFP in some active liver diseases, reproductive system tumors and pregnancy, the diagnostic specificity of AFP for HCC is impacted.
In addition to AFP, several new serum biomarkers such as des-carboxy prothrombin (DCP), glypican-3, osteopontin have been proposed for early HCC detection. Serum DCP exhibited sensitivities of 48–62%, specificities of 81–98%, and accuracies of 59–84% for diagnosing HCC.[12,13] Other, more recent studies show glypican-3 levels play a potential role for early HCC detection that can be used for HCC screening. Liu et al.[14] found that the proportion of GPC3-positive was significantly higher in early HCC than AFP (76.4% vs. 64.3%). The similar result also found at Shang et al.[15] study, for early stage HCC, osteopontin had a better sensitivity than AFP (75% vs 46%), but the specificity is lower than AFP (62% vs 93%). Among these new biomarkers, none of them has both high sensitivity and high specificity for early diagnosis of liver cancer. We therefore need to continue efforts to find new serum markers complementary to AFP.
A subtype of AFP, lens culinary agglutinin-reactive fraction of fetoprotein (AFP-L3), is derived from cancerous hepatocytes and considered to be specific to HCC.[16] The proportion of AFP-L3 to total AFP (AFP-L3%) can be used to diagnose early HCC. Early studies reported that the diagnostic sensitivity of AFP-L3% for HCC ranged from 75.0% to 96.9% with a specificity of 90.0–92.0%.[17,18] A few months or even years before the diagnosis of HCC, serum AFP-L3 levels could increase in the blood; a sensitivity of 39% was observed in small HCC (<2 cm).[19] Recent studies showed that newer and simpler methods could be more sensitive in detecting serum AFP-L3%, thus enhancing the potential value of this marker in the diagnosis of early liver cancer.[20,21]
At present, AFP-L3% has not been widely used as a conventional indicator for the clinical diagnosis of HCC. A previous study of AFP-L3% for diagnosing HCC showed a low pooled sensitivity (0.483), but the diagnostic performance of AFP-L3% for early HCC was not evaluated.[22] Hence, we performed a systematic review and meta-analysis of the diagnostic value of AFP-L3% for early HCC.
2. Materials and methods
2.1. Literature retrieval and screening
There is no need for ethical approval because the data used in this study have been published. Articles on AFP-L3% for the diagnosis of early liver cancer were searched from January 1960 to December 2019 in the PubMed, Embase, Cochrane Library, and Web of Science databases. The search terms were liver cancer subject words and free words and AFP-L3 subject words and free words. Two researchers independently screened the eligible literature according to the following inclusion and exclusion criteria.
2.1.1. Inclusion criteria for articles
Studies that are involved in the evaluation of AFP-L3% for diagnose early HCC (defined as HCC patients at Barcelona clinic liver cancer stage 0/A or TNM stage I); and articles that contain the number of true-positive, false-positive, true-negative, and false-negative diagnoses of early liver cancer.
2.1.2. Exclusion criteria for articles
Publications that are considered case reports, reviews, meta-analyses, abstracts, and editorials, animal studies, or cell experiments; articles that do not apply the definition of early HCC mentioned above; articles without the complete data needed to obtain the sensitivity and specificity; and duplicate articles.
2.2. Data extraction and quality evaluation
Two researchers independently reviewed the eligible articles and extracted the following data: basic information (paper title, publication year, first author, sample size, research type), cutoff value of AFP-L3%, true positives, true negatives, false positives, and false negatives. A third party was available to resolve any discrepancies between the sets of extracted data.
The quality of all eligible studies was evaluated and analyzed by 2 authors according to the Quality Assessment of Diagnostic Accuracy Studies scale,[23] and disagreements were discussed and resolved. A third author assisted in the evaluation if the discussion failed.
2.3. Statistical analyses
Review Manager 5.3 software (The Cochrane Collaboration, Copenhagen, Denmark), MetaDisc1.4 software (XI Cochrane Colloquium, Barcelona, Spain) were used for analysis. Spearman correlation analysis and a distribution map of the receiver operating characteristic (ROC) curve were obtained to determine whether there was a threshold effect in the eligible studies. If the correlation coefficient was insignificant (with P > .05) or the ROC curve did not show a shoulder-arm distribution, then there was no threshold effect, and the sensitivity, specificity, and positive and negative likelihood ratios were pooled by Meta-DiSc1.4 software, and the area under the summary ROC (SROC) curve (AUC) was obtained by SROC fitting analysis; otherwise, only SROC fitting analysis was performed. There was no heterogeneity when P > .10 and I2 ≤ 50%, moderate heterogeneity when P ≤ .10 and 50% ≤ I2 ≤ 70%, and high heterogeneity when P ≤ .10 and I2 > 70%. All statistical tests were 2-sided, and P < .05 was considered statistically significant.
3. Results
3.1. Results of literature retrieval and screening and quality of the studies
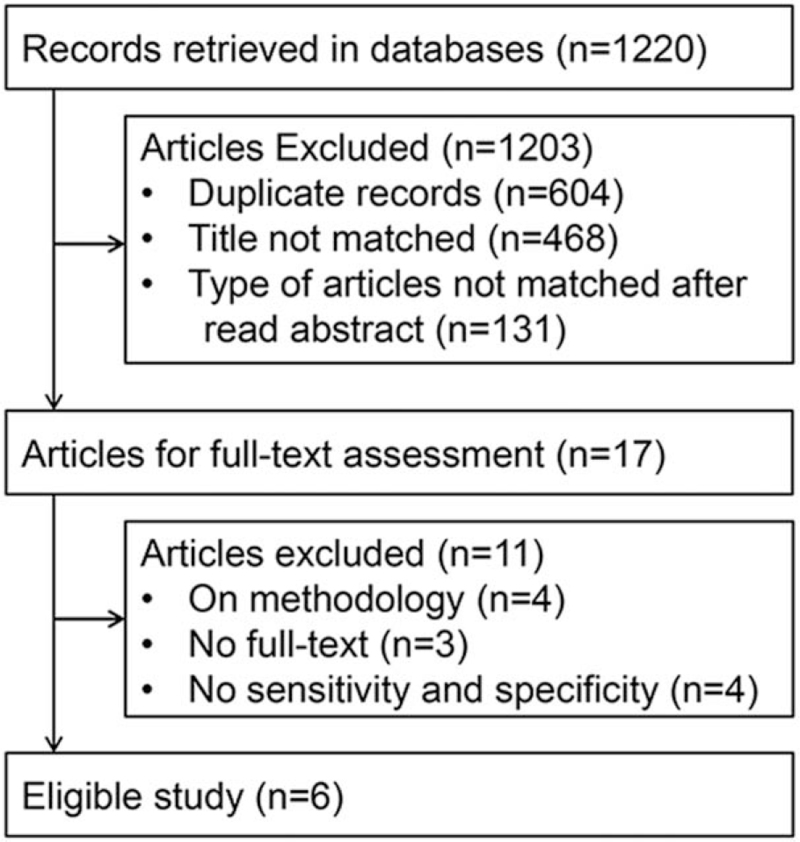
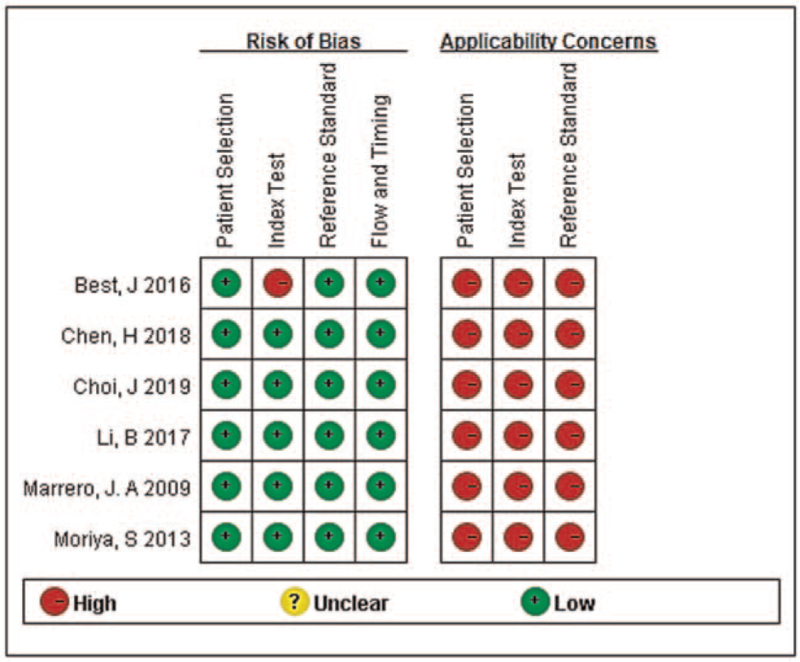
One thousand two hundred and twenty published records were retrieved. After screening according to the inclusion and exclusion criteria, 6 articles were eligible,[24–29] and a total of 2447 patients were included in the analysis. The literature screening process and results are shown in Fig. 1. The basic characteristics of the 6 eligible studies are shown in Table 1, and the quality of the 6 eligible articles evaluated by using the Quality Assessment of Diagnostic Accuracy Studies tool is shown in Fig. 2.
Figure 1.
Flow diagram of the article selection process for meta-analysis.
Table 1.
The basic characteristics of the 6 studies on AFP-L3% for the diagnosis of early hepatocellular carcinoma (HCC).
| Patient number | |||||||||||
| First author | Year | Country | Study design | Method | Cut-off | HCC stage | HCC/Control | TP | FP | FN | TN |
| Best, J | 2016 | Germany | Case control | μTAS | 10% | BCLC 0/A | 61/402 | 29 | 35 | 32 | 367 |
| Chen, H | 2018 | China | Case control | ELISA | NA | TNM I | 94/644 | 22 | 64 | 72 | 580 |
| Choi, J | 2019 | Korea | Case control | uTAS | 4% | BCLC 0/A | 38/152 | 20 | 17 | 18 | 135 |
| Li, B | 2017 | China | Case control | CLIA | 3.84% | BCLC A | 81/152 | 40 | 24 | 41 | 128 |
| Marrero, J. A | 2009 | USA | Case control | NA | 10% | BCLC 0/A | 208/417 | 58 | 13 | 150 | 404 |
| Moriya, S | 2013 | Japan | Case control | ELISA | 10% | pTNM I | 15/183 | 2 | 3 | 13 | 180 |
Figure 2.
Risk of bias and applicability concerns of the 6 articles in the meta-analysis.
3.2. Meta-analysis results
3.2.1. Threshold effect
The Spearman correlation coefficient was 0.771, and the P value was .072, indicating that there was no threshold effect. Based on the distribution of each eligible study, there were no obvious “shoulder-arm” distributions, which further indicate that there was no threshold effect.
3.2.2. Heterogeneity test
The sensitivity, specificity, and diagnostic odds ratio of each eligible study were combined with MetaDisc1.4 software and yielded I2 values of 83.5%, 89.4%, and 68.4% (Fig. 3), respectively, indicating the presence of obvious heterogeneity.
Figure 3.
Forest plots of the diagnostic performance of AFP-L3% for early hepatocellular carcinoma. A: Sensitivity and related indicators. B: Specificity and related indicators. C: Diagnostic odds ratio and related indicators. AFP = alpha-fetoprotein.
3.2.3. Quantitative analysis of the merger effect
The random effect model was used to analyze the pooled diagnostic performance because of the high heterogeneity among the studies, and the results are shown in Figures 3 and 4 and summarized in Table 2.
Figure 4.
Summary receiver operating characteristic (SROC) curve of AFP-L3% for the diagnosis of early hepatocellular carcinoma. AFP = alpha-fetoprotein.
Table 2.
The merger effects of AFP-L3 for the diagnosis of early hepatocellular carcinoma.
| Value (95% CI) | P | |
| Pooled sensitivity | 0.34 (0.30–0.39) | <.001 |
| Pooled specificity | 0.92 (0.91–0.93) | <.001 |
| Pooled positive likelihood ratio | 4.46 (2.94–6.77) | .003 |
| Pooled negative likelihood ratio | 0.71 (0.61–0.82) | <.001 |
| Pooled diagnostic odds ratio | 6.78 (4.02–11.44) | .007 |
| Area under summary ROC curve | 0.755 (0.57–0.94) |
4. Discussion
In the present study, 6 articles involving 2447 patients were included. The results showed that AFP-L3% has a high pooled specificity (92%), low pooled sensitivity (34%), and moderate AUC of the summary ROC curve (0.755) for diagnose early HCC, with a pooled diagnostic odds ratio of 6.78.
Most of the articles had a low risk of bias and high applicability of concerns, but obvious heterogeneity was found among the studies, so we chose the random effect model to perform the pooled analysis. To identify the source of heterogeneity, a subgroup analysis was carried out based on the different methods of detecting AFP-L3%. The results showed that the micro total analysis systems (μTAS) method group had no obvious heterogeneity, while the non-μTAS methods still had high heterogeneity, suggesting that the heterogeneity mainly comes from different detection methods. Indeed, compared with other methods, the μTAS method can improve the diagnostic sensitivity and specificity of AFP-L3% for early HCC.[30]
Abdominal ultrasound combined with AFP is widely used in the diagnosis of HCC. However, AFP has not been recommended as an essential indicator for screening early HCC given its suboptimal sensitivity and specificity when used alone. AFP was found to have a sensitivity ranging from 49% to 71% and specificity ranging from 49% to 86% in detecting tumors <5 cm in size when the cutoff value set at 20 ng/mL.[31] Hence, European guidelines recommend ultrasonography alone for HCC screening.[32] Although abdominal ultrasound is recommended for the screening of early HCC, it is not an ideal method in practice and is especially unsatisfactory for the detection of liver lesions in obese patients. A large difference in the sensitivity of abdominal ultrasound for HCC detection (77% vs 21%) was found between BMI groups (<30 vs >30).[33] Therefore, continuous efforts have been made towards the discovery of new serum biomarkers; in the past decades, due to the minimally invasive nature, ease of repetition, and high consistency of these methods, many new serum biomarkers have been reported, although few have been used in clinics.[34,35] Serum chromogranin A and microRNA-107 expression levels could be potential diagnostic markers in early stage HCC.[36,37] Deng et al[38] found that serum pentraxin 3 was an independent risk factor of HCC and could identify hepatitis B virus-associated HCC with an AUC of 0.920 for early-stage HCC. However, further studies are needed to confirm these potential biomarkers.
Because of its low pooled sensitivity in the diagnosis of early HCC, AFP-L3% does not seem suitable for first-line screening of early HCC. However, AFP-L3% has been used as a reliable tumor biomarker for HCC and recommended by the Japanese Society of Hepatology.[39] Continuous efforts have been made to improve the performance of AFP-L3% in diagnose HCC, including the development of new methods and combining AFP-L3% with other biomarkers. Some hypersensitive methods have been reported, one of which has a lower detection limit of 12 pg/mL for AFP-L3 detection.[40–42] The GALAD score, based on the combination of sex, age, AFP, AFP-L3, and DCP, had a significantly improved diagnostic performance for early-stage HCC patients that was defined using the Milan criteria, with an AUC of 0.90 (sensitivity 68%; specificity 95.2%).[43,44]
Although AFP-L3% is not highly sensitive for early HCC, it has high specificity that cannot be surpassed by total AFP, with the advantage of differentiating HCC from benign liver diseases in patients with elevated serum AFP. In the differentiation of hepatitis C virus-related HCC and cirrhosis, the high specificity of AFP-L3% persisted in patients with low-level AFP elevation,[45] and a higher incidence of HCC was found in AFP-L3-positive cirrhosis patients than in AFP-positive cirrhosis patients. AFP-L3 was also highly related to biological malignancies of HCC, such as portal vein invasion and intrahepatic metastasis,[46] which may explain the usefulness of AFP-L3 in monitoring treatment response and the recurrence of HCC.[47–49]
There are some limitations in the present study: the data were extracted for subgroups of early HCC in 5 of 6 articles, which may lead to bias in the patient samples. Additionally, all 6 articles were case-control studies, which may present better results than the real world. The cutoff values of AFP-L3% were different among the 6 studies, which may affect the merged diagnostic indicator of AFP-L3% for the diagnosis of early liver cancer. The gold standard diagnostic method used in each study was different, which may produce bias. The analysis of the source of heterogeneity was not sufficient, and only a subgroup analysis of detection methods was performed due to the limited data.
In conclusion, the meta-analysis revealed that AFP-L3% exhibits high specificity and low sensitivity for diagnose early HCC, suggesting that AFP-L3% alone is not ideal for the screening of early HCC but valuable for the discrimination between malignant and benign diseases in patients with elevated total AFP. Among the current methods for AFP-L3 detection, the μTAS method is the best for improving the sensitivity of detection and new highly sensitive methods may provide more favorable results for diagnose HCC. The combination of AFP-L3% with other conventional serum biomarkers could improve its diagnostic performance and enable monitoring applications for AFP-L3% in early HCC. More attention should be paid to the advantage of the high specificity of AFP-L3% for diagnose early HCC, and this is a major direction for the future development of the clinical applications of AFP-L3%.
Author contributions
Conceptualization: Jian-Ming Zhou.
Data curation: Jian-Ming Zhou, Ting Wang, Kun-He Zhang.
Formal analysis: Jian-Ming Zhou.
Methodology: Jian-Ming Zhou, Ting Wang, Kun-He Zhang.
Project administration: Kun-He Zhang.
Software: Jian-Ming Zhou, Ting Wang.
Supervision: Kun-He Zhang.
Validation: Ting Wang.
Writing – original draft: Jian-Ming Zhou.
Writing – review & editing: Ting Wang, Kun-He Zhang.
Footnotes
Abbreviations: μTAS = micro total analysis systems, AFP = alpha-fetoprotein, AUC = the area under the curve, DCP = des-carboxy prothrombin, HCC = hepatocellular carcinoma, ROC = receiver operating characteristic, SROC = summary receiver operating characteristic.
How to cite this article: Zhou JM, Wang T, Zhang KH. AFP-L3 for the diagnosis of early hepatocellular carcinoma: a meta-analysis. Medicine. 2021;100:43(e27673).
Funding Sources: Funding information is not available.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
μTAS = micro total analysis systems, BCLC = Barcelona clinic liver cancer, CLIA = chemiluminescence immunoassay, ELISA = enzyme linked immunosorbent assay, FN = false negative, FP = false positive, NA = not available, TN = true negative, TP = true positive.
ROC = receiver operating characteristic.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Ayuso C, Rimola J, Vilana R, et al. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol 2018;101:72–81. [DOI] [PubMed] [Google Scholar]
- [3].Renzulli M, Biselli M, Brocchi S, et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: a new diagnostic algorithm. Gut 2018;67:1674–82. [DOI] [PubMed] [Google Scholar]
- [4].Pinto Marques H, Gomes da Silva S, De Martin E, Agopian VG, Martins PN. Emerging biomarkers in HCC patients: Current status. Int J Surg 2020;82S:70–6. [DOI] [PubMed] [Google Scholar]
- [5].Roberts LR, Sirlin CB, Zaiem F, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology 2018;67:401–21. [DOI] [PubMed] [Google Scholar]
- [6].Nörthen A, Asendorf T, Walson PD, Oellerich M. Diagnostic value of alpha-1-fetoprotein (AFP) as a biomarker for hepatocellular carcinoma recurrence after liver transplantation. Clin Biochem 2018;52:20–5. [DOI] [PubMed] [Google Scholar]
- [7].Nobuoka D, Kato Y, Gotohda N, et al. Postoperative serum alpha-fetoprotein level is a useful predictor of recurrence after hepatectomy for hepatocellular carcinoma. Oncol Rep 2010;24:521–8. [DOI] [PubMed] [Google Scholar]
- [8].Jin J, Zhang X-Y, Shi J-L, et al. Application of AFP whole blood one-step rapid detection kit in screening for HCC in Qidong. Am J Cancer Res 2017;7:1384–8. [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang Z, Zhang Y, Wang Y, Xu L, Xu W. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther 2016;9:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hippo Y, Watanabe K, Watanabe A, et al. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res 2004;64:2418–23. [DOI] [PubMed] [Google Scholar]
- [11].Sun Y, Gao G, Cai J, et al. Annexin A2 is a discriminative serological candidate in early hepatocellular carcinoma. Carcinogenesis 2013;34:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS. Hepatocellualar carcinoma serum markers. Semin Oncol 2012;39:410–33. [DOI] [PubMed] [Google Scholar]
- [13].Debes JD, Romagnoli PA, Prieto J, et al. Serum biomarkers for the prediction of hepatocellular carcinoma. Cancers 2021;13:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu S, Wang M, Zheng C, Zhong Q, Shi Y, Han X. Diagnostic value of serum glypican-3 alone and in combination with AFP as an aid in the diagnosis of liver cancer. Clin Biochem 2020;79:54–60. [DOI] [PubMed] [Google Scholar]
- [15].Shang S, Plymoth A, Ge S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology 2012;55:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lamerz R. AFP isoforms and their clinical significance (overview). Anticancer Res 1997;17:2927–30. [PubMed] [Google Scholar]
- [17].Taketa K, Okada S, Win N, Hlaing NKT, Wind KM. Evaluation of tumor markers for the detection of hepatocellular carcinoma in Yangon General Hospital, Myanmar. Acta Med Okayama 2002;56:317–20. [DOI] [PubMed] [Google Scholar]
- [18].Khien VV, Mao HV, Chinh TT, et al. Clinical evaluation of lentil lectin-reactive alpha-fetoprotein-L3 in histology-proven hepatocellular carcinoma. Int J Biol Markers 2001;16:105–11. [DOI] [PubMed] [Google Scholar]
- [19].Taketa K, Endo Y, Sekiya C, et al. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res 1993;53:5419–23. [PubMed] [Google Scholar]
- [20].Kim H, Sohn A, Yeo I, Yu SJ, Yoon J-H, Kim Y. Clinical assay for AFP-L3 by using multiple reaction monitoring-mass spectrometry for diagnosing hepatocellular carcinoma. Clin Chem 2018;64:1230–8. [DOI] [PubMed] [Google Scholar]
- [21].Ma H, Sun X, Chen L, et al. Multiplex immunochips for high-accuracy detection of AFP-L3% based on surface-enhanced raman scattering: implications for early liver cancer diagnosis. Anal Chem 2017;89:8877–83. [DOI] [PubMed] [Google Scholar]
- [22].Yi X, Yu S, Bao Y. Alpha-fetoprotein-L3 in hepatocellular carcinoma: a meta-analysis. Clin Chim Acta 2013;425:212–20. [DOI] [PubMed] [Google Scholar]
- [23].Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Best J, Bilgi H, Heider D, et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol 2016;54:1296–305. [DOI] [PubMed] [Google Scholar]
- [25].Chen H, Zhang Y, Li S, et al. Direct comparison of five serum biomarkers in early diagnosis of hepatocellular carcinoma. Cancer Manag Res 2018;10:1947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Choi J, Kim G-A, Han S, Lee W, Chun S, Lim Y-S. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology 2019;69:1983–94. [DOI] [PubMed] [Google Scholar]
- [27].Li B, Li B, Guo T, et al. Artificial neural network models for early diagnosis of hepatocellular carcinoma using serum levels of α-fetoprotein, α-fetoprotein-L3, des-γ-carboxy prothrombin, and Golgi protein 73. Oncotarget 2017;8:80521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009;137:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moriya S, Morimoto M, Numata K, et al. Fucosylated fraction of alpha-fetoprotein as a serological marker of early hepatocellular carcinoma. Anticancer Res 2013;33:997–1001. [PubMed] [Google Scholar]
- [30].Tamura Y, Igarashi M, Kawai H, Suda T, Satomura S, Aoyagi Y. Clinical advantage of highly sensitive on-chip immunoassay for fucosylated fraction of alpha-fetoprotein in patients with hepatocellular carcinoma. Dig Dis Sci 2010;55:3576–83. [DOI] [PubMed] [Google Scholar]
- [31].Song P-P, Xia J-F, Inagaki Y, et al. Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol 2016;22:262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liver EAftSot. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- [33].Esfeh JM, Hajifathalian K, Ansari-Gilani K. Sensitivity of ultrasound in detecting hepatocellular carcinoma in obese patients compared to explant pathology as the gold standard. Clin Mol Hepatol 2020;26:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wen Y, Han J, Chen J, et al. Plasma miRNAs as early biomarkers for detecting hepatocellular carcinoma. Int J Cancer 2015;137:1679–90. [DOI] [PubMed] [Google Scholar]
- [35].Wu J, Xiang Z, Bai L, et al. Diagnostic value of serum PIVKA-II levels for BCLC early hepatocellular carcinoma and correlation with HBV DNA. Cancer Biomark 2018;23:235–42. [DOI] [PubMed] [Google Scholar]
- [36].Biondi A, Malaguarnera G, Vacante M, et al. Elevated serum levels of Chromogranin A in hepatocellular carcinoma. BMC Surg 2012;12: (suppl): S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Loosen SH, Castoldi M, Jördens MS, et al. Serum levels of circulating microRNA-107 are elevated in patients with early-stage HCC. PLoS One 2021;16:e0247917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Deng H, Fan X, Wang X, et al. Serum pentraxin 3 as a biomarker of hepatocellular carcinoma in chronic hepatitis B virus infection. Sci Rep 2020;10:20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011;29:339–64. [DOI] [PubMed] [Google Scholar]
- [40].Wei T, Zhang W, Tan Q, Cui X, Dai Z. Electrochemical assay of the alpha Fetoprotein-L3 isoform ratio to improve the diagnostic accuracy of hepatocellular carcinoma. Anal Chem 2018;90:13051–8. [DOI] [PubMed] [Google Scholar]
- [41].Zhang A, Skog S, Wang S, et al. A chemiluminescent protein microarray method for determining the seroglycoid fucosylation index. Sci Rep 2016;6:31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li J, Gao T, Gu S, Zhi J, Yang J, Li G. An electrochemical biosensor for the assay of alpha-fetoprotein-L3 with practical applications. Biosens Bioelectron 2017;87:352–7. [DOI] [PubMed] [Google Scholar]
- [43].Lim TS, Kim DY, Han K-H, et al. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol 2016;51:344–53. [DOI] [PubMed] [Google Scholar]
- [44].Best J, Bechmann LP, Sowa J-P, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2020;18:728.e4–35.e4. [DOI] [PubMed] [Google Scholar]
- [45].Sterling RK, Jeffers L, Gordon F, et al. Clinical utility of AFP-L3% measurement in North American patients with HCV-related cirrhosis. Am J Gastroenterol 2007;102:2196–205. [DOI] [PubMed] [Google Scholar]
- [46].Tada T, Kumada T, Toyoda H, et al. Relationship between Lens culinaris agglutinin-reactive alpha-fetoprotein and pathologic features of hepatocellular carcinoma. Liver Int 2005;25:848–53. [DOI] [PubMed] [Google Scholar]
- [47].Yamamoto K, Imamura H, Matsuyama Y, et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol 2010;45:1272–82. [DOI] [PubMed] [Google Scholar]
- [48].Kobayashi M, Hosaka T, Ikeda K, et al. Highly sensitive AFP-L3% assay is useful for predicting recurrence of hepatocellular carcinoma after curative treatment pre- and postoperatively. Hepatol Res 2011;41:1036–45. [DOI] [PubMed] [Google Scholar]
- [49].Matsuda M, Asakawa M, Amemiya H, Fujii H. Lens culinaris agglutinin-reactive fraction of AFP is a useful prognostic biomarker for survival after repeat hepatic resection for HCC. J Gastroenterol Hepatol 2011;26:731–8. [DOI] [PubMed] [Google Scholar]