Abstract
Introduction:
Existing salvage protocols for infected breast prostheses using negative pressure wound therapy with instillation and dwell (NPWTi-d) require multiple returns to the operating room and prolonged length of stay. We present our expedited salvage protocol and discuss outcomes and associated costs savings.
Methods:
Using a retrospective review, we identified 25 consecutive patients (27 breasts) with peri-prosthetic breast infection. Nine patients (10 breasts) underwent removal of infected breast prostheses followed by autologous or staged implant-based reconstruction. Sixteen patients (17 breasts) underwent our single application salvage protocol. A cost analysis was performed comparing the two groups, and an economic model was used to project the cost savings associated with using single application NPWTi-d protocol.
Results:
Fifteen of the 16 patients (94%) who underwent single application NPWTi-d had successful implant salvage. Average duration of NPWTi-d was 2 days, 7 hours, and average length of stay was 4.43 days. Compared to control, patients who received the single application protocol required significantly fewer hospitalizations and office visits. A total savings of $58,275 could have been achieved by using the single application NPWTi-d protocol in the patients who did not undergo NPWTi-d.
Conclusions:
Single application of NPWTi-d is a simple, safe, and cost-effective technique for salvage of breast prostheses, with 94% success rate, even in immunocompromised patients and severe infection. Compared to previous protocols, ours requires fewer trips to the operating room, shorter length of stay, and more permanent implants placed during salvage. Our protocol is also associated with fewer office visits and fewer returns to the operating room.
Takeaways
Question: Acute infection of breast prostheses is problematic and costly for patients and hospitals, so implant salvage techniques must be improved.
Findings: Fifteen of 16 patients (94%) who underwent this protocol had successful implant salvage. Our patients had a shorter length of stay, fewer operations, fewer office visits, and lower costs of office visits.
Meaning: Single application of NPWTi-d is a simple, safe, and cost-effective technique for breast implant salvage with 94% success rate.
INTRODUCTION
Between 2009 and 2014, the United States had a 62% increase in rate of breast reconstruction for mastectomy.1 This upsurge occurred in the setting of a relatively stable rate of mastectomies performed during this 5-year period, suggesting that more women are choosing to undergo breast reconstruction after mastectomy. This is understandable, as the Breast Cancer Patient Education Act implemented in October 2016 has drastically improved patient education about the availability and insurance coverage of breast reconstruction.2 Prosthesis-based breast reconstruction is still the most common form of breast reconstruction in the United States, but rates of peri-prosthetic breast infection remain unchanged, ranging from 1% to as high as 35%.3–7 Thus, as more women seek breast reconstruction after mastectomy, we must continue to improve reconstructive outcomes and increase success of these seemingly inevitable complications, which can be devastating for patients and costly for hospital systems.
Successful breast implant salvage was first reported in 1965 after Perras et al used antibiotic lavage in a breast pocket to salvage an infected implant after augmentation.8 Recently, innovative techniques using negative pressure wound therapy (NPWT) for implant salvage have been described, which may help improve salvage rates in implant-based breast reconstruction.9,10 Use of negative pressure wound therapy with instillation and dwell (NPWTi-d) in breast implant salvage has been described in the literature, with success. NPWTi-d is an advanced form of NPWT that includes automated volumetric control of instilled topical wound solutions in conjunction with traditional negative pressure therapy.11 V.A.C. VERAFLO (3M/KCI Medical) is a wound management system that allows for cleansing of a wound in a consistent, controlled manner through a programmable cycle of alternating negative pressure and instillation with dwell of any solution of choice. After thorough index operation, each instillation and dwell provides a washout of the breast pocket without the need for repeat operative intervention.12 The V.A.C. VERAFLO system consists of foam that is placed into the wound, sealed, and connected via tubing to a vacuum device that alternates instillation and dwell of choice irrigation with suction. Solutions such as saline, hypochlorite-based solutions, sulfonamides, polyhexanide, and detergents with surfactant are introduced into the wound where they dwell for a preset amount of time, then are removed along with any remaining infectious materials and wound debris, thus promoting clean wound beds. In comparison with NPWT alone, NPWTi-d is associated with improved outcomes in rates of wound healing and decreased length of stay.13 V.A.C. VERAFLO system allows customized control of the solution, the amount, frequency, and duration of suction, dwell time, and volume of fluid, which allows the provider to customize therapy for various types and sizes of wounds.14
In 2017, Meybodi et al published a study of a small series of patients who underwent management for peri-prosthetic infection using NPWTi-d and were able to successfully replace subpectoral tissue expanders in five out of six patients after salvage of the infected breast pocket.15 Similarly, Cheong et al used NPWTi-d with normal saline and frequent dressing changes to successfully salvage implants in five patients.16 More recently, successful use of NPWTi-d in severe infection has been described. Constantine described successful breast prosthesis salvage with use of NPWTi-d in a late mycobacterial infection after mastectomy,17 and Meybodi et al published a protocol with an 83% salvage rate of severe peri-prosthetic infections.18 These authors showed successful salvage in a majority of cases of infected breast reconstructions, thus avoiding total derailment of reconstruction or the need for autologous reconstruction. However, the protocols described involve multiple returns to the operating room, which translate to longer lengths of stay and increased costs to the hospital and the patient.
We present our simple yet extremely effective method of implant salvage that combines the benefits of previously described protocols with a more efficient approach to NPWTi-d. Our protocol allows for a shorter length of stay, fewer trips to the operating room, shorter antibiotic regimens, and immediate placement of implants after salvage (one-stage implant salvage), all while achieving success in clearing peri-prosthetic infection in patients undergoing breast reconstruction or augmentation. When compared with explantation of infected breast prostheses and delayed autologous and/or staged implant-based reconstruction after tissue expansion, single application NPWTi-d salvage has significant monetary and intangible savings for hospitals and patients.
PATIENTS AND METHODS
An IRB-approved retrospective review was performed, which captured 25 consecutive patients (27 breasts) with peri-prosthetic breast infection between October 2015 and November 2019. Nine of these patients (10 breasts) developed such infections between October 2015 and March 2016; their infections were managed by removing the infected prostheses (“going flat”) followed by staged reconstruction with autologous and/or permanent breast implants following interval expansion. Starting in April 2016, the authors adopted a new salvage technique utilizing NPWTi-d. Between April 2016 and November 2019, 16 consecutive patients (17 breasts) underwent a salvage protocol, which entailed immediate operative management [explantation of the prosthesis, debridement and washout of the breast pocket, removal of any non-integrated biologic mesh and/or necrotic tissue, and immediate placement of NPWTi-d (V.A.C. VERAFLO with Prontosan)], followed by definitive removal of NPWTi-d without replacement or VAC changes, and reimplantation of a breast prosthesis. Inclusion criteria for this review included patients who underwent cosmetic breast augmentation or mastectomy followed by immediate or delayed one- and two-stage breast reconstruction, who developed a peri-prosthetic infection requiring removal of the prosthesis and initiation of either staged reconstruction after going flat, or salvage of breast prostheses utilizing NPWTi-d.
This review focuses on the patients who underwent NPWTi-d salvage. These 16 patients were an incredibly heterogenous group, which included one- and two-stage reconstructions, one cosmetic patient, and prostheses placed in sub- and prepectoral planes. All mastectomies were performed by breast surgeons from The Rebecca Fortney Breast Center at Anne Arundel Medical Center in Annapolis, Maryland. Reconstruction and/or augmentation were performed by one of the two plastic surgeons at Anne Arundel Medical Center. When patients presented to either the emergency department or our clinic, with evidence of worrisome peri-prosthetic infection, they were admitted immediately to the hospital for initiation of our protocol, which includes initiation of empiric broad-spectrum IV antibiotics and prompt surgical intervention to remove the prosthesis and any unincorporated mesh and/or necrotic tissue.
Implant Salvage Protocol
We utilized V.A.C. VERAFLO in conjunction with Prontosan Wound Irrigation Solution (B. Braun Medical Inc.), a dual-action aqueous wound cleanser that contains 0.1% polyhexamethylene biguanide (PHMB) and 0.1% Betaine (Surfactant), which together allow for physical removal of wound coatings and biofilms while providing topical antimicrobial therapy against multiple Gram-negative and Gram-positive organisms, which are common culprits of wound infection [eg, Staphylococcus aureus (including MRSA), Staphylococcus epidermidis, Proteus mirabilis, Pseudomonas aeruginosa, Serratia marcesens, VRE].19–21
Initial operative therapy included explantation of the prosthesis and debridement and washout of the breast pocket under general anesthesia. This involves abrading the capsule with a Bovie scratch pad to remove biofilm and irrigating the breast pocket with multiple solutions, including hydrogen peroxide, Betadine, Clorpactin, and/or bacitracin. We also removed any nonintegrated biologic mesh and/or necrotic tissue, then immediately placed the NPWTi-d (V.A.C. VERAFLO) with Prontosan as instillation solution (see Fig. 1). Intraoperative cultures are taken from the wound pocket to narrow antibiotic sensitivities; this is the only time we cultured the wounds in each patient.
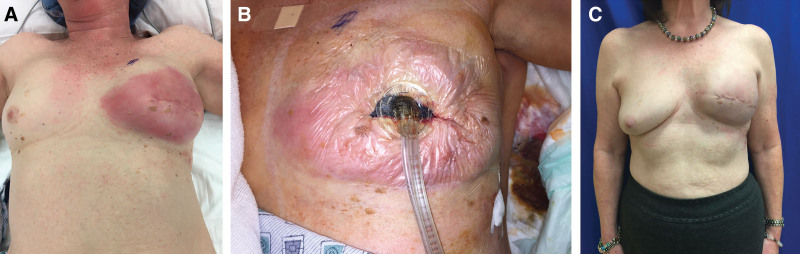
Fig. 1.
Example of surgical intervention in single-application NPWTi-d protocol (case 4 from Tables 1 and 2). Patient presented with left breast cellulitis (A) and underwent immediate washout with removal of prepectoral tissue expander and placement of V.A.C. VERAFLO (B). After 26.5 hours of NPWTi-d therapy, the device was removed and a permanent prepectoral implant was placed. Patient 14 days after NPWTi-d (C).
NPWTi-d settings were customized for each patient based on the size of breast pocket. In this series, we instilled between 80 and 400 mL of Prontosan per instillation into the breast pocket every 1–4 hours, allowing the solution to dwell between 10 and 20 minutes, then resuming suction at −125 mm Hg. Although we were comfortable setting instillation volumes, a surgeon new to this technique can use the fill-assist feature of the V.A.C. ULTA machine (3M/KCI Medical), which will typically choose an appropriate volume for that pocket. All of our patients remained in the inpatient setting and received IV antibiotics. A broad-spectrum antibiotic therapy regimen was initiated, and then antibiotics were narrowed based on results from wound cultures.
Patients underwent NPWTi-d for 1 to 4 days, depending mostly on surgeon preference and operating room schedule. We have found that as long as a patient shows clinical improvement and completes multiple cycles of the NPWTi-d, it is safe to place a new prosthesis in the pocket. The authors have had neither the opportunity nor the need to have rigid criteria for takeback to the operating room, as the duration of our single application protocol is more of a reflection of operating room availability. However, criteria for length of time with NPWTi-d could be developed. It is important to note that time with NPWTi-d can be tailored to span a weekend, for example, or to adjust to surgeon or operating room schedule. In this single application protocol, our patients do not undergo any dressing changes at bedside or in the operating room; initial NPWTi-d application was followed by definitive removal of V.A.C. VERAFLO and placement of new prosthesis. At the time of NPWTi-d removal, we inserted new breast prosthesis (tissue expander or implant) and placed one or two drains (15-French or 19-French Blake). Drains were removed in clinic as outpatients, with timing of removal based upon drain output.
All patients were discharged home on antibiotics. Type of antibiotic, dosage, and duration were decided by medical and infectious disease teams based upon culture growth, sensitivities, and patient tolerance/allergies. Oral antibiotics are preferred, but home infusion of IV antibiotics may be indicated depending on microbiology and recommendations from infectious disease service. Implant salvage was defined as retention of a tissue expander or silicone implant without need for explantation in the following 90 days.
To analyze the cost of care, the volume and cost of office visits and hospitalizations were calculated. The volume of office visits to the plastic surgery clinic was manually abstracted, and an average cost of $107 per visit was used for all clinic encounters based on previously published time-driven activity-based unit costs22 and an estimated 25 minutes per encounter. The total variable cost of all hospital encounters was extracted from the institution’s activity-based cost accounting system (CostFlex Systems Inc., Mobile, Ala.). Direct utilization and costs were compared between groups using two-sided independent samples t-tests, and an economic model was developed for projecting the cost savings associated with using V.A.C. VERAFLO in a cohort of patients explanted, taken to flat, and reconstructed without V.A.C. VERAFLO.
RESULTS
Overall, 15 of the 16 patients who underwent single application NPWTi-d had successful implant salvage (94% implant salvage rate). One patient had bilateral infections and successful bilateral salvage; thus, a total 16 of the 17 breasts (94%) were successfully salvaged after acute peri-prosthetic infection. Patient demographics are displayed in Table 1. The average patient age was 47 years (range 35–65) with an average body mass index of 30.4 kg per m2 (range 22.3–39.6). Seven patients had a significant smoking history (7/16, 43.8%): two patients were active smokers at the time of infection (12.5%) and five were former smokers (31.3%). The majority of patients reported at least one medical problem for which they required daily medication (12/16, 75%), including Type 2 diabetes (3/16, 18.8%).
Table 1.
Patient Demographics (in Chronological Order by Case)
| Case | Age (y) | Race/Ethnicity | BMI (kg/m2) | Comorbidities and Notable History | Smoker (Quit Date)? |
|---|---|---|---|---|---|
| 1 | 41 | White | 33 | Hypothyroid | Former (15 mo prior to surgery) |
| 2 | 50 | White | 32.5 | DM2, HLD | No |
| 3 | 40 | White | 34.2 | Celiac disease, history of PE, Factor V Leiden (on Lovenox) | No |
| 4 | 64 | White | 24.8 | HTN | Former (1997) |
| 5 | 42 | White | 23.9 | None | No |
| 6 | 46 | White | 39.6 | HTN | Former (1990) |
| 7 | 46 | White | 23.7 | None | Former (1992) |
| 8 | 64 | African American | 39.5 | DM2, HTN, R breast cancer (2001, s/p adjuvant CT and R SSM with R SP implant reconstruction and matching L reduction mammoplasty) | Former (1997) |
| 9 | 35 | Asian | 22.3 | DM1, PCOS | Yes |
| 10 | 41 | White | 28.3 | Crohn’s disease | Yes |
| 11 | 52 | White | 35.6 | Crohn’s disease, HTN, hypothyroid | No |
| 12 | 36 | White | 32.8 | HTN | No |
| 13 | 51 | White | 31 | Anemia, ESRD s/p kidney transplant (on immunosuppressants), HTN | No |
| 14 | 65 | White | 29.3 | HTN | No |
| 15 | 37 | White | 22.8 | None | No |
| 16 | 51 | White | 33.1 | Hypothyroid | No |
BMI, body mass index; CT, chemotherapy; DM1, type 1 diabetes mellitus; DM2, type 2 diabetes mellitus; ESRD, end-stage renal disease; HLD, hyperlipidemia; HTN, hypertension; L, left; PCOS, polycystic ovarian syndrome; PE, pulmonary embolism; R, right; s/p, status post; SP, subpectoral; SSM, skin-sparing mastectomy.
Summaries of surgical procedures, cancer treatment, infection data, and treatment details for patients who underwent single application NPWTi-d salvage are listed in Table 2. One patient underwent cosmetic breast augmentation, whereas the 15 others underwent breast reconstruction following mastectomy, the majority for breast cancer (14/15, 93%). Types of mastectomy included skin-reducing with Wise pattern (5/15, 33.3%), nipple-sparing (5/15, 33.3%), and skin-sparing mastectomy (5/15, 33.3%). Of the eight patients who underwent bilateral mastectomy for unilateral disease, three developed infection on the prophylactic side (3/8, 37.5%). Fifteen patients experienced unilateral infection (15/16, 93.8%), whereas one patient developed bilateral infections requiring bilateral NPWTi-d (1/16, 6.3%).
Table 2.
Summary of Each Case (in Chronological Order)
| Case | Initial Procedure | Indication | Interval Procedures (Time after Initial Procedure) | RT | CT | Infected Breast | Onset to Infection (Days since Last Surgical Intervention) | Organism | Length of Stay (d) | Length of Time with NPWTi-d (h) | Salvage Successful? | Capsular Contracture (Baker Grade)? | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B/L NSM + PP TE insertion | R IDC | Debridement of B/L mastectomy flaps (6 d) Aspiration of B/L seromas (38 d) | Adjuvant | Neoadjuvant | L | 77 | No growth | 5 | 74 | Yes | Yes (IV) | 59 |
| 2 | B/L SRM + SP TE insertion | R IDC | B/L SP implant exchange (6 mo) | No | Adjuvant | L | 19 | MSSA | 1 | 29.5 | Yes | No | 10 |
| 3 | B/L breast lift + SP implant insertion | Cosmetic | None | No | No | L | 20 | MSSA | 8 | 48 | Yes | No | 4 |
| 4 | L SSM + PP TE insertion | L IDC | L breast margin re-excision and ALND removing 18 nodes (35 d) | No | No | L | 19 | MSSA | 3 | 26.5 | Yes | Yes (III) | 51 |
| 5 | L SRM with Wise pattern + SP TE insertion, matching R reduction mastopexy | L IDC | L implant exchange, R revision mastopexy (7 mo) | No | Neoadjuvant | L | 44 | MSSA | 1 | 24 | Yes | No | 31 |
| 6 | B/L SRM with Wise pattern + PP TE insertion | L IDC | None | No | No | L | 44 | Enterobacter aerogenes | 3 | 74.5 | Yes | No | 11 |
| 7 | B/L SRM with Wise pattern + PP TE insertion | R IDC | B/L implant exchange and capsulotomies (6 mo) | No | Adjuvant | R | 22 | MSSA | 7 | 26.5 | No | No | 28 |
| 8 | L SSM + PP TE insertion | L IDC | None | No | No | L | 41 | Proteus mirabilis | 4 | 49 | Yes | No | 31 |
| 9 | B/L NSM + PP TE insertion | R IDC | B/L implant exchange (5 mo) Washout of R breast hematoma and R implant replacement (5.5 mo) | Adjuvant | Adjuvant | R | 21 | Serratia marcesens | 2 | 39 | Yes | No | 22 |
| 10 | B/L NSM + PP TE | R IDC | None | No | Adjuvant | R | 84 | MRSA | 6 | 114 | Yes | No | 33 |
| 11 | B/L SSM + PP TE insertion | R IDC | B/L implant exchange (2 mo) | No | Neoadjuvant | R | 303 | No cultures taken | 8 | 118 | Yes | No | 4 |
| 12 | B/L SSM + PP TE insertion | L IDC | None | Adjuvant | Neoadjuvant | B/L | 38 | Pseudomonas aeruginosa | 2 | 30 | Yes | No | 20 |
| 13 | B/L SRM with Wise pattern + PP TE insertion | L IDC | None | No | Neoadjuvant | L | 84 | Neisseria weaveri | 6 | 99 | Yes | No | 6 |
| 14 | B/L NSM + PP DTI | R IDC | Re-excision of R breast margins, including nipple (3 wk) | No | Adjuvant | R | 14 | Serratia marcesens | 2 | 41 | Yes | No | 18 |
| 15 | R NSM + PP TE insertion | R fibroadenomata, L hypomastia | None | No | No | R | 23 | Pseudomonas aeruginosa | 7 | 27 | Yes | No | 7 |
| 16 | L SSM + PP TE insertion | L IDC | None | No | Neoadjuvant | L | 23 | Pseudomonas aeruginosa | 6 | 67.5 | Yes | No | 16 |
ALND, axillary lymph node dissection; B/L, bilateral; CT, chemotherapy; DTI, direct to implant breast reconstruction; IDC, invasive ductal carcinoma; L, left; MSSA, methicillin sensitive-Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; NPWTi-d, negative pressure wound therapy with instillation and dwell; NSM, nipple-sparing mastectomy; PP, prepectoral; R, right; RT, radiation therapy; s/p, status post; SP, subpectoral; SRM, skin-reducing mastectomy; SSM, skin-sparing mastectomy; TE, tissue expander.
Note: All TE insertions were first stages of planned two-stage breast reconstruction procedures.
Eight patients who developed periprosthetic infection underwent neoadjuvant chemotherapy (8/16, 50%), and two patients were actively on chemotherapy at the time of infection (2/16, 12.55). No patients underwent preoperative radiation therapy.
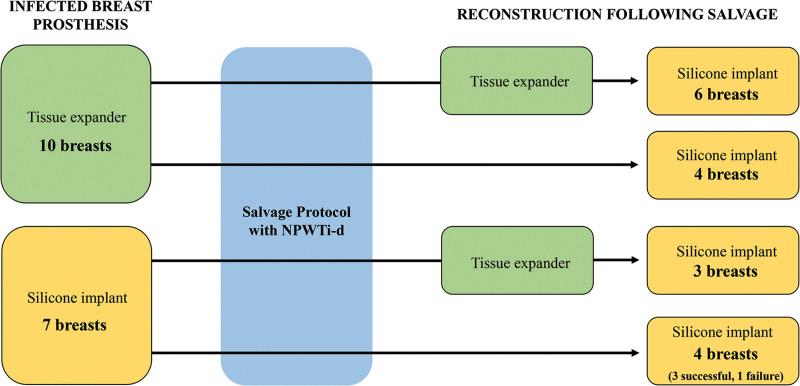
Nine patients (10 breasts) who underwent single application NPWTi-d salvage presented with infected tissue expanders (9/16, 56.4%), whereas seven patients presented with infected breast implants (7/16, 43.8%). For nine patients (9/16, 56.3%), reconstruction after salvage consisted of a two-stage process, with tissue expanders inserted at the time of NPWTi-d removal, then replaced with a silicone implant at a later date after sufficient expansion. Of these nine patients with staged reconstruction, six had originally presented with infected tissue expanders (6/9, 66.7%), whereas three presented with infected implants and required placement of tissue expanders during salvage for subsequent re-expansion (3/9, 33.3%). Seven patients (eight breasts) had permanent implants placed after NPWTi-d removal (7/16, 43.8%). Of these seven patients, three were initially admitted with infected tissue expanders but had silicone implants placed (in a total of four breasts) upon removal of the NPWTi-d with no interval placement of tissue expanders (4/8 breasts, 50%), thus completing their two-stage breast reconstruction before discharge (Fig. 2).
Fig. 2.
Breast implant salvage. Sixteen patients (a total of 17 breasts) presented with peri-prosthetic infection and underwent salvage protocol with NPWTi-d using V.A.C. VERAFLO and Prontosan solution. Seven patients (eight breasts) were discharged home with silicone implants immediately after salvage; three of these patients (four breasts) had tissue expander infections but had successful placement of silicone implants after salvage.
The average duration of NPWTi-d was 55 hours (2 days, 7 hours; range 24–118). Average length of stay was 4.43 days (range 1–8). Methicillin-sensitive Staphylococcus aureus was the most common organism identified in intraoperative cultures (5/16, 31.3%), followed by Pseudomonas aeruginosa (3/16, 18.8%) and Serratia marcesens (2/16, 12.5%). See Table 2 for all isolated organisms. All patients went home on an antibiotic regimen; 14 (14/16, 87.5%) were on oral antibiotics for 5–14 days, whereas two required intravenous antibiotics via peripherally inserted central catheter (2/16, 12.5%).
Implant salvage failed in one patient (case 7 from Tables 1 and 2). This patient was an otherwise healthy 46-year-old who underwent bilateral skin-reducing mastectomies in Wise pattern for IDC of the right breast, with immediate placement of bilateral prepectoral tissue expanders. Six months later, she underwent bilateral implant exchange but developed right breast cellulitis 3 weeks later. Intraoperative cultures grew MSSA. After 25 hours of NPWTi-d, we placed a new silicone implant and she was discharged home on IV antibiotics. At her third postoperative clinic visit (22 days after NPWTi-d removal), our team noted increased right breast erythema and exposed implant with murky peri-prosthetic drainage; so the prosthesis was removed. Five months later, she underwent delayed right breast reconstruction with tissue expansion and subsequent implant exchange with no adverse events or complications, and an excellent final cosmetic result.
Average follow-up time for our 16 patients averaged 22 months (range 4–59 months), from the time of NPWTi-d removal to most recent clinic appointment. Two patients eventually developed capsular contracture following implant salvage (2/16, 12.5%), one of whom underwent postoperative radiation.
Cost Analysis
In comparison with patients whose infected prostheses were removed and underwent delayed autologous or implant-based reconstruction (the No NPWTi-d group), patients who underwent single application NPWTi-d (the NPWTi-d group) required significantly fewer office visits (No NPWTi-d: 24 ± 9 versus NPWTi-d: 11 ± 5, P = 0.002), resulting in lower total office visit costs (No NPWTi-d: $2590 ± 953 versus NPWTi-d: $1164 ± 537). Similarly, patients receiving NPWTi-d underwent fewer hospitalizations (No NPWTi-d: 4 ± 1 versus NPWTi-d: 2 ± 1, P = 0.002). However, no statistically significant differences in cost per hospitalization or total hospitalization cost were observed (Table 3).
Table 3.
Resource Utilization and Cost by Use of Single Application NPWTi-d
| No NPWTi-d (N = 9) | NPWTi-d (N = 16) | P | |
|---|---|---|---|
| No. office visits | 24 ± 9 | 11 ± 5 | 0.002 |
| Total cost office visits | $2590 ± 953 | $1164 ± 537 | 0.002 |
| No. hospitalizations | 4 ± 1 | 2 ± 1 | 0.002 |
| Cost per hospitalization | $7198 ± 2837 | $11,854 ± 8998 | 0.148 |
| Total cost hospitalization | $27,061 ± 14,196 | $21,871 ± 16,422 | 0.435 |
The bolded values are statistically significant.
In the modeled cost analysis, the cost and utilization of the nine patients in the No NPWTi-d group were recalculated using the cost and utilization patterns of the NPWTi-d group. This model resulted in a cost reduction of $6475 per patient, driven by reduced office visits (savings of $1391 per patient) and hospitalizations (savings of $5084 per patient). Based on this model, it is projected that a total cost savings of $58,275 could be achieved by using single application NPWTi-d in this cohort (Table 4).
Table 4.
Modeled Savings if the No NPWTi-d Group Received Single Application NPWTi-d
| Change in Care | Volume Difference per Patient | Total Cost Difference per Patient* | Total Cohort Cost Difference (N = 9) |
|---|---|---|---|
| Reduction in office visits | −13 | ($1391) | ($12,519) |
| Reduction in hospitalizations | −2 | ($5084) | ($45,756) |
| Total | −15 | ($6475) | ($58,275) |
*Calculated as (Avg. cost No NPWTi-d × Avg. Utilization) − (Avg. cost NPWTi-d × Avg. Utilization)
DISCUSSION
Before salvage protocols such as ours, patients with peri-prosthetic breast infections were traditionally managed rather slowly, starting with oral antibiotics, then possibly intravenous antibiotics, followed by ultimate removal of the prosthesis without immediate reconstruction. This delayed approach required starting over with the reconstruction, which was frustrating to the patient and often technically difficult for the surgeon.
Our single application implant salvage protocol is a simple and efficient method to manage peri-prosthetic infection in patients undergoing prepectoral or subpectoral breast reconstruction or augmentation. It allows for a shorter length of stay, fewer trips to the operating room, shorter antibiotic regimens, and, often, immediate placement of implants after salvage. These benefits confer significant cost savings while saving patients the emotional, psychological, and physical stress of undergoing delayed autologous reconstruction.
Materials Used in Salvage of Breast Prostheses
Antibiotic-impregnated materials have been described as successful in implant salvage when placed in the breast pocket along with new prostheses. Lapid et al described a method of implant salvage in which they insert gentamicin-impregnated foams along with a new implant after debridement and pulse lavage of the breast pocket.23 Sherif et al placed calcium sulfate antibiotic beads into the implant pocket along with a new prosthesis at the time of salvage.24 Albright et al utilized polymethylmethacrylate plates and beads impregnated with tobramycin and vancomycin alongside tissue expanders at the time of initial breast pocket washout.25 The polymethylmethacrylate plates and beads eventually required surgical removal after two consecutive negative drain fluid cultures. Xue et al used these studies to suggest an algorithm for implant salvage involving use of antibiotic-impregnated materials.7,8 Although these methods had success (4/4 for Lapid et al and 13/14 success for Albright et al), the addition of these materials—as well as multiple returns to the operating room and repeated cultures—are unnecessary to achieve successful salvage.
Various topical solutions can be used in NPWTi-d therapy in salvage of breast prostheses; the most commonly reported solutions include 1% acetic acid and normal saline.15–17 Kim and Attinger et al found that instillation and dwell using saline in non-implant-based wounds led to rapid promotion of robust granulation tissue.14 Saline is readily available, low-cost, and very safe, making it an attractive solution to use with NPWTi-d. However, granulation tissue formation is not a primary goal in the breast pocket surrounding a prosthesis; thus, solutions and detergents containing surfactant, such as Prontosan, create a milieu with less biofilm while supporting a hospitable environment for an implant and increasing mastectomy flap perfusion.
Timing of Single Application NPWTi-d Therapy
Our protocol with washout and fewer than 5 days of NPWTi-d, in conjunction with inpatient IV antibiotics and outpatient antibiotic regimens not exceeding 14 days, is simple, effective, and is associated with a shorter length of stay and the ability to often directly place a permanent implant following salvage without the need for interim tissue expansion. Our protocol is unique in that we find that it is safe to directly place an implant in the breast pocket after prosthesis salvage. Seven of our patients had implants directly placed after removal of NPWTi-d (six successful). Three of the six patients with successful implant placements were initially admitted with infected tissue expanders; so they essentially had successful implant exchanges during the salvage process. However, this is sometimes not possible due to loss of domain with more extended periods of negative pressure therapy. Thus, over time, we have moved to a much shorter interval with NPWTi-d, with our average length of stay often dictated by operating room availability rather than by time with NPWTi-d. Furthermore, we feel that a shorter interval with NPWTi-d is favorable in that prolonged negative pressure in the breast pocket can result in loss of domain/derecruitment of space. Therefore, the sooner we are able to remove the NPWTi-d, the more likely it is to maintain the volume of the expanded pocket and successfully reconstruct the breast without having to re-expand. We have also tended toward “overfilling” the pocket with foam rather than toward underfilling—or even using a foam-wrapped implant, as in the case example—as this also helps prevent derecruitment of expanded breast skin.
Antibiotic Therapy during Single Application NPWTi-d
Many other protocols also involve extended postoperative oral antibiotic regimens between 318 and 6 weeks23 or more following operative salvage. Although all of our patients completed outpatient antibiotic regimens, none extended past 14 days. We cultured the wounds one time—intraoperatively during removal of the infected prosthesis—and did not re-culture any patients. Unless deemed absolutely appropriate by infectious disease consultants, weeks-long courses of antibiotics are likely unnecessary and may have further repercussions such as antibiotic resistance and C. difficile infections in patients who may already be immunocompromised from chemotherapy treatment.
Single Application NPWTd-i in Severe Infection and Complex Patients
Previous studies have suggested that breast implant salvage is contraindicated in patients who present with severe infection. Prince et al did not attempt salvage or replacement of prosthesis if pus was encountered in the pocket,26 and Chun and Schulman reported that prosthesis exposure—or impending exposure—would be the reason to forgo attempted salvage.27 We believe that severity of presentation should not prevent patients from salvage. Our protocol can be used in any patient who presents with any of these severe features of peri-prosthetic breast infection. We successfully salvaged several of these patients, including those with exposed implants, patients with purulent drainage in the breast pocket, and one patient who presented with severe sepsis requiring brief pressor support in the intensive care unit.
Our protocol can also be used in medically complex and immunocompromised patients. Two patients were actively on chemotherapy at the time of infection; neither patient experienced a delay or interruption in therapy, and both healed well without dehiscence or chronic wounds. One patient (Case 13, Fig. 3) was on maintenance immunosuppressant therapy for a kidney transplant at the time of her peri-prosthetic breast infection. She remained on her regimen of prednisone and tacrolimus throughout her NPWTi-d treatment and did not suffer any graft dysfunction.
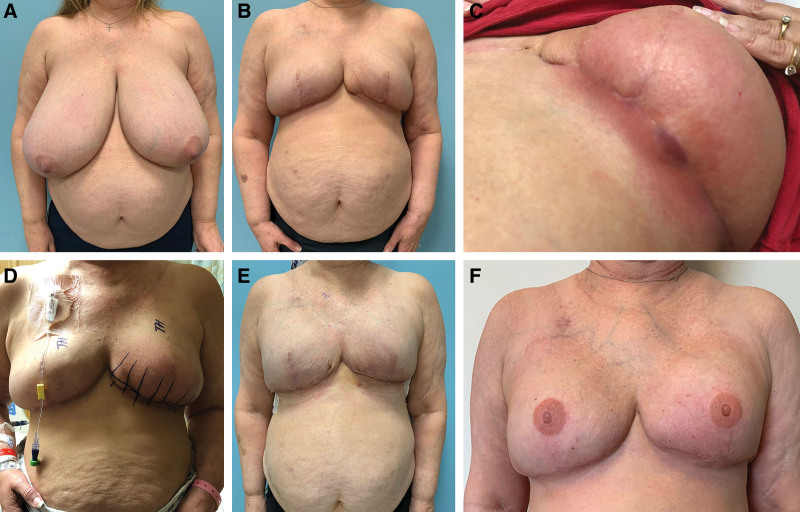
Fig. 3.
Case 13. Preoperative photograph of a 51-year-old woman with left breast IDC (A). The patient underwent a unilateral kidney transplant over 20 years prior, and was on chronic immunosuppressants at the time of infection. She underwent a left therapeutic skin-reducing mastectomy and prophylactic right skin-reducing mastectomy with Ryan flaps and immediate placement of bilateral 800 cm3 prepectoral tissue expanders in March 2019 (B). In June 2019 during adjuvant chemotherapy, she developed moderate to severe cellulitis of the left breast (C) and underwent immediate washout, removal of tissue expander, and placement of V.A.C. VERAFLO (D). After 4 days of IV antibiotic therapy and NPWTi-d with Prontosan, she underwent simultaneous bilateral breast reconstruction with permanent silicone gel implants (E, 11 days postoperative). She recovered well with no further issues and had nipple tattoos in January 2020 (F).
No V.A.C. Changes in Single Application NPWTi-d
Other studies describe successful salvage with week-long NPWTi-d therapy15 and/or multiple V.A.C. changes.9,15,16,18 Cheong et al reported a protocol in which V.A.C. VERAFLO was inserted for a total of 7 days before removal and placement of permanent implants; this week-long therapy involved foam changes in the operating room every 2 days.16 Constantine performed NPWTi-d changes every 48–72 hours.17 Yii et al utilized a modified wound irrigation system using an inflow and outflow drainage system to instill an antibiotic solution for up to 5 days postoperatively.28 Meybodi et al changed the NPWTi-d dressings up to seven times in each patient.15,18 As mentioned, our patients underwent an average of 2 days, 7 hours of NPWTi-d therapy, with no interval washouts or V.A.C. changes. Thus, our average length of stay (4.43 days) is notably shorter when compared with other similar studies, which report an average length of stay from 7 to 12 days (see Table 5).15,16,18
Table 5.
Literature Summary Comparing Periprosthetic Breast Infection Salvage with NPWTi-d
| Study | N (breasts) | Length of Stay (d) | Placement of Permanent Implants Immediately after NPWTi-d Removal | No. Returns to the OR (including Removal of NPWTi-d) | Average Follow-up (mo) | Salvage Rate (Breasts) |
|---|---|---|---|---|---|---|
| Current study | 17 | 4.43 (1–8) | 8/17 (47%)* | 1 | 22 | 94% (16/17) |
| Meybodi et al 201715 | 6 | 12 (7–16) | 0 | 2.3 (1–4) | 9 | 83% (5/6) |
| Meybodi et al 202118 | 30 | 11.5 (6–22) | 5/30 (16.7%) | 3.7 (2–7) | 39.4 | 83% (25/30) |
| Cheong et al 201616 | 5 | ≥7 † | † | ≥2† | † | 100% (5/5) |
*Three patients (four breasts) who presented with infected tissue expanders had permanent breast implants placed immediately following NPWTi-d removal.
†Insufficient data to report.
Use of Single Application NPWTi-d in Prepectoral Breast Reconstruction
Of the 16 patients who presented with peri-prosthetic infection and underwent single application NPWTi-d, three presented with subpectoral implants. These subpectoral reconstructions were performed in 2016 and as recently as early 2017, after which both providers transitioned to prepectoral placement of implants. Our practice now exclusively performs prepectoral breast reconstruction. Additionally, the vast majority of our reconstructions are DTI with no need for interval tissue expanders and subsequent implant exchange. As such, our approach is quite different from other studies whose authors only report subpectoral reconstruction.15,16,29,30 Some practices may be tempted to change the plane when responding to infection or capsular contracture. But importantly, we were able to maintain the original plane for all of our patients, which is especially important in prepectoral breast reconstruction.
Capsular Contracture
Two patients developed capsular contracture of the ipsilateral side following implant salvage. The incidence of capsular contracture after breast reconstruction is between 2% and 20% overall,31,32 with rates as high as 33% reported after breast implant infection.33 Although this range is quite broad, it is consistent with the 12.5% incidence in this series of patients with moderate to severe peri-prosthetic infection. Known causes of capsular contracture include acute or subacute peri-prosthetic infection, hematoma, seroma, infection, and radiation.31–33 Three patients in our series underwent adjuvant radiation after resolution of their peri-prosthetic infection, and one of them, to date, has developed capsular contracture (Baker Grade IV). This patient had metastatic disease, which required radiation to her mediastinum and ipsilateral lung. Additionally, in patients who undergo implant salvage, mechanical disturbance of the breast pocket (ie, debridement, cyclic instillation), effects of the detergent, or residual inflammation from the infection itself could potentially lead to histologic conversion to contracted capsule. Patient-specific factors such as smoking and chemotherapy may also contribute to development of capsular contracture; however, no clear link was noted among the three affected patients in our study. Long-term follow-up is especially important in patients who undergo implant salvage; so the surgeon can monitor for this complication.
Cost Analysis of Single Application NPWTi-d
Patients who undergo NPWTi-d salvage have demonstrable cost savings in comparison with patients whose infected prostheses are explanted and then they undergo delayed reconstruction. After infected implants are removed and domain of the breast pocket is lost, patients usually require re-expansion to accommodate an implant or inset of an autologous flap. This derailed path to reconstruction necessitates at least two more returns to the operating room, as well as multiple interval clinic visits for tissue expansion and surgical planning. Thus, it is no surprise that patients whose prostheses were salvaged with NPWTi-d have statistically significantly fewer office visits and hospitalizations. This can result in incalculable savings for the patient when considering other often overlooked costs of frequent clinic appointments and inpatient stays, such as travel, fuel, child care, and time off of work. Although we found no statistically significant differences in cost per hospitalization or total hospitalization cost when comparing our NPWTi-d and delayed reconstruction groups, it is imperative to acknowledge the many intangible advantages of our NPWTi-d protocol, which include a patient’s ability to avoid the emotional stress of delayed reconstruction, the discomfort associated with tissue expansion of a scarred mastectomy flap, and the physical toll of an autologous reconstruction. Furthermore, our protocol allows for quick intervention and salvage, can help keep women on track for starting adjuvant therapy or getting back to “normal” life, and provides tremendous emotional benefits to patients.
When compared with other reports of NPWTi-d salvage for peri-prosthetic breast infection, our average length of stay and number of returns to the OR are notably lower than others (Table 5). Due to cost variation across hospitals, as well as the lack of data to compare costs of care for inpatient stays, medications, materials/supplies, and perioperative costs across medical centers, we were unable to calculate and analyze specific cost savings of our salvage protocol compared with other published NPWTi-d protocols. However, other groups may obtain costs specific to their centers and then utilize the data in Table 5 to extrapolate the potential savings of utilizing our technique. Specifically, we believe that a shorter length of stay and fewer returns to the OR while undergoing the salvage protocol could confer significant cost savings to both patients and hospitals. More research is needed to further explore this notion.
CONCLUSIONS
Our protocol of negative pressure wound therapy with instillation and dwell (NPWTi-d) is a simple, safe, and cost-effective technique for implant salvage with an excellent observed success rate, even in patients with severe infection or exposed prostheses. With this method, we are often able to safely place implants directly in the breast pocket after salvage, even in patients who present with infected tissue expanders. With only one washout and application of NPWTi-d in conjunction with a solution of choice, patients have a shorter length of stay and fewer trips to the OR. Single application of NPWTi-d may also minimize loss of domain and derecruitment of expanded breast tissue, which allows for immediate reconstruction and excellent aesthetic outcomes. Finally, patients who underwent our protocol had significantly fewer office visits and returns to the operating room, which has implications for significant healthcare cost savings for both patients and the hospital.
Footnotes
Published online 29 October 2021.
Disclosure: Dr. Holton is a consultant to 3M/Acelity and Stryker. All the other authors have no financial interests to declare in relation to the content of this article.
References
- 1.Miller AM, Steiner CA, Barrett ML, et al. Breast reconstruction surgery for mastectomy in hospital inpatient and ambulatory settings, 2009–2014. HCUP Statistical Brief #228. October 2017. Rockville, Md.: Agency for Healthcare Research and Quality. Available at www.hcup-us.ahrq.gov/reports/statbriefs/sb228-Breast-Reconstruction-For-Mastectomy.pdf. [PubMed] [Google Scholar]
- 2.Breast Cancer Patient Education Act (BCPEA), Consolidated Appropriations Act, 2016, Public Law No: 114-113. 2016. Available at https://www.congress.gov/bill/114th-congress/house-bill/2029/.
- 3.Franchelli S, Pesce M, Baldelli I, et al. Analysis of clinical management of infected breast implants and of factors associated to successful breast pocket salvage in infections occurring after breast reconstruction. Int J Infect Dis. 2018;71:67–72. [DOI] [PubMed] [Google Scholar]
- 4.Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. 2005;5:94–106. [DOI] [PubMed] [Google Scholar]
- 5.Spear SL, Seruya M. Management of the infected or exposed breast prosthesis: a single surgeon’s 15-year experience with 69 patients. Plast Reconstr Surg. 2010;125:1074–1084. [DOI] [PubMed] [Google Scholar]
- 6.Washer LL, Gutowski K. Breast implant infections. Infect Dis Clin North Am. 2012;26:111–125. [DOI] [PubMed] [Google Scholar]
- 7.Xue AS, Volk AS, DeGregorio VL, et al. Follow-up study: one-step salvage of infected prosthetic breast reconstructions using antibiotic-impregnated polymethylmethacrylate plates and concurrent tissue expander exchange. Plast Reconstr Surg. 2020;145:240e–250e. [DOI] [PubMed] [Google Scholar]
- 8.Xue AS, Kania KE, Brown RH, et al. Salvage of infected prosthetic breast reconstructions. Semin Plast Surg. 2016;30:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Accurso A, Rocco N, Accardo G, et al. Innovative management of implant exposure in ADM/implant-based breast reconstruction with negative pressure wound therapy. Aesthetic Plast Surg. 2017;41:36–39. [DOI] [PubMed] [Google Scholar]
- 10.Semsarzadeh NN, Tadisina KK, Maddox J, et al. Closed incision negative-pressure therapy is associated with decreased surgical-site infections: a meta-analysis. Plast Reconstr Surg. 2015;136:592–602. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Gabriel A, Lantis J, et al. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int Wound J. 2016;13:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.3M/KCI. V.A.C. VERAFLO. 2020. Available at https://www.veraflo.com/about-v-a-c-veraflo-therapy/. Accessed August 21, 2020.
- 13.Faust E, Opoku-Agyeman JL, Behnam AB. Use of negative-pressure wound therapy with instillation and dwell time: an overview. Plast Reconstr Surg. 2021;147(1S-1):16S–26S. [DOI] [PubMed] [Google Scholar]
- 14.Kim PJ, Attinger CE, Crist BD, et al. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds. 2015;27:S2–S19. [PubMed] [Google Scholar]
- 15.Meybodi F, Sedaghat N, French J, et al. Implant salvage in breast reconstruction with severe peri-prosthetic infection. ANZ J Surg. 2017;87:E293–E299. [DOI] [PubMed] [Google Scholar]
- 16.Cheong JY, Goltsman D, Warrier S. A new method of salvaging breast reconstruction after breast implant using negative pressure wound therapy and instillation. Aesthetic Plast Surg. 2016;40:745–748. [DOI] [PubMed] [Google Scholar]
- 17.Constantine T. Use of negative-pressure wound therapy with instillation and dwell in breast reconstruction. Plast Reconstr Surg. 2021;147(1S-1):34S–42S. [DOI] [PubMed] [Google Scholar]
- 18.Meybodi F, Sedaghat N, Elder E, et al. Salvaging the unsalvageable: negative pressure wound therapy for severe infection of prosthetic breast reconstruction. Plast Reconstr Surg Glob Open. 2021;9:e3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngaage LM, Elegbede A, Brao K, et al. The efficacy of breast implant irrigant solutions: a comparative analysis using an in vitro model. Plast Reconstr Surg. 2020;146:301–308. [DOI] [PubMed] [Google Scholar]
- 20.Horrocks A. Prontosan wound irrigation and gel: management of chronic wounds. Br J Nurs. 2006;15:1222, 1224–1228. [DOI] [PubMed] [Google Scholar]
- 21.Singh DP, Gowda AU, Chopra K, et al. The effect of negative pressure wound therapy with antiseptic instillation on biofilm formation in a porcine model of infected spinal instrumentation. Wounds. 2017;28:175–180. [PubMed] [Google Scholar]
- 22.Cho J, Sanchez K, Ganor O, et al. Utilizing a physician scribe in a pediatric plastic surgical practice: a time-driven activity-based costing study. Plast Reconstr Surg Glob Open. 2019;7:e2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapid O. Use of gentamicin collagen sponges for the treatment of periprosthetic breast implant infection. J Plast Reconstr Aesthet Surg. 2011;64:e313–e316. [DOI] [PubMed] [Google Scholar]
- 24.Sherif RD, Ingargiola M, Sanati-Mehrizy P, et al. Use of antibiotic beads to salvage infected breast implants. J Plast Reconstr Aesthet Surg. 2017;70:1386–1390. [DOI] [PubMed] [Google Scholar]
- 25.Albright SB, Xue AS, McKnight A, et al. One-step salvage of infected prosthetic breast reconstructions using antibiotic-impregnated polymethylmethacrylate plates and concurrent tissue expander exchange. Ann Plast Surg. 2016;77:280–285. [DOI] [PubMed] [Google Scholar]
- 26.Prince MD, Suber JS, Aya-Ay ML, et al. Prosthesis salvage in breast reconstruction patients with periprosthetic infection and exposure. Plast Reconstr Surg. 2012;129:42–48. [DOI] [PubMed] [Google Scholar]
- 27.Chun JK, Schulman MR. The infected breast prosthesis after mastectomy reconstruction: successful salvage of nine implants in eight consecutive patients. Plast Reconstr Surg. 2007;120:581–589. [DOI] [PubMed] [Google Scholar]
- 28.Yii NW, Khoo CT. Salvage of infected expander prostheses in breast reconstruction. Plast Reconstr Surg. 2003;111:1087–1092. [DOI] [PubMed] [Google Scholar]
- 29.Bennett SP, Fitoussi AD, Berry MG, et al. Management of exposed, infected implant-based breast reconstruction and strategies for salvage. J Plast Reconstr Aesthet Surg. 2011;64:1270–1277. [DOI] [PubMed] [Google Scholar]
- 30.Liao EC, Breuing KH. Breast mound salvage using vacuum-assisted closure device as bridge to reconstruction with inferolateral AlloDerm hammock. Ann Plast Surg. 2007;59:218–224. [DOI] [PubMed] [Google Scholar]
- 31.Headon H, Kasem A, Mokbel K. Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42:532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malahias M, Jordan DJ, Hughes LC, et al. A literature review and summary of capsular contracture: an ongoing challenge to breast surgeons and their patients. Int J Surg Open. 2016;3:1–7. [Google Scholar]
- 33.Reish RG, Damjanovic B, Austen WG, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013;131:1223–1230. [DOI] [PubMed] [Google Scholar]