Abstract
Background:
Postoperative cognitive dysfunction (POCD) is a very common event in elderly noncardiac surgical patients. The effects of inhalational anaesthetics and propofol on the incidence of POCD and postoperative cognitive status at different time points after surgery are currently unclear.
Methods:
We searched the Embase, Medline, Cochrane Library, and Web of Science databases for randomized controlled trials (RCTs), in which inhalation anaesthesia and propofol anaesthesia were compared. The incidence of POCD or postoperative cognitive status was assessed in elderly patients undergoing noncardiac surgery.
Results:
Fifteen RCTs with 1854 patients were included in this meta-analysis. The incidence of POCD on postoperative Days 2–6 after propofol anaesthesia was markedly lower than that after inhalation anaesthesia (risk ratio (RR): 0.37, 95% confidence interval (CI): 0.15–0.88, P = .025), and Mini-Mental State Examination (MMSE) scores after propofol anaesthesia were substantially higher than those after inhalation anaesthesia (standard mean difference (SMD): 0.59, 95% CI: 0.07–1.11, P = .026). The levels of interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) were much lower after propofol anaesthesia than after inhalation anaesthesia (SMD: -2.027, 95% CI: -3.748– -0.307, P = .021; SMD: -0.68, 95% CI: -0.93– -0.43, P < .001).
Conclusions:
The moderate evidence from this meta-analysis shows that, in elderly noncardiac surgical patients, propofol anaesthesia is superior to inhalation anaesthesia for attenuating of early POCD incidence, and low-level evidence shows that cognitive status is higher and systemic inflammation is less severe after propofol anaesthesia in the early days after surgery.
Limitations:
The sample size was not sufficiently large for systemic inflammation, and the tools to identify POCD were not uniform in the included studies.
Keywords: elderly, inflammation, inhalation anaesthesia, POCD, propofol
Key messages
-
1.
Pooled results showed that the incidence of early POCD after propofol anaesthesia was lower than that after inhalation anaesthesia, and cognitive status (MMSE score) was higher after propofol anaesthesia than after inhalation anaesthesia.
-
2.
Systemic inflammation was less severe after propofol anaesthesia.
-
3.
S-100 β protein, a biomarker of acute cerebral injury, was lower after propofol anaesthesia, but the results were not stable.
-
4.
A well-designed RCT with a large sample size, and uniform diagnostic tool for POCD is needed to demonstrate the superiority of propofol anaesthesia in elderly noncardiac surgical patients.
1. Background
Postoperative cognitive dysfunction (POCD) is a common event in elderly patients undergoing noncardiac surgery,[1] with an incidence of 25–40%, 10% and 1% at 1 week, 3 months, and 1 year after surgery, respectively.[2] POCD prolongs patients’ hospital stays, increases postoperative complications, deteriorates patients’ quality of life, and increases social and economic burdens. Many perioperative risk factors precipitate the development of POCD, including age, surgical stress, and anaesthesia.[3] Animal studies have shown that inhalation anaesthesia with sevoflurane or isoflurane can induce cognitive dysfunction in elderly rats, while propofol cannot[4,5]; however, in a surgical model of elderly rats, cognitive function after inhalation anaesthesia was not different from that after propofol anaesthesia.[6] However, clinical studies have reported conflicting results, with some indicating that inhalation anaesthesia can increase or decrease POCD compared with propofol anaesthesia, or that no difference exists between the two anaesthesia methods.[7–12]
A recent meta-analysis showed that the incidence of POCD was lower in elderly patients undergoing noncardiac surgery after propofol anaesthesia than after inhalation anaesthesia,[13] but the level of evidence was low because only a few articles were included, and the time points of POCD reported in these trials were inconsistent. In clinical practice, POCD mainly consists of early POCD (within 1 week after surgery) and delayed POCD (within 3 months or more after surgery).[2] In addition to assessing the incidence of POCD, the Mini-Mental State Examination (MMSE) score is a very important tool to evaluate the level of perioperative cognition. In recent years, many new studies have been published, and the effect of general anaesthesia on the incidence and level of early and delayed POCD and the level of postoperative cognition requires urgent elucidation.
Surgical procedures cause peripheral inflammation, increase the permeability of the blood–brain barrier, and lead to neuroinflammation, which contributes to the development of POCD.[14] In an animal surgical model, TNF-α in the hippocampus was much higher after isoflurane anaesthesia than after propofol anaesthesia.[6] Few clinical studies have reported the impacts of inhalation and propofol anaesthesia on postoperative systemic inflammation or neuroinflammation in elderly noncardiac surgical patients.[10,12] Whether the effect of general anaesthesia on postoperative inflammation is consistent with the effects on POCD and postoperative cognitive levels needs to be determined.
Thus, in this meta-analysis, we investigated the effects of inhalation anaesthesia and propofol anaesthesia on the incidence of early and delayed POCD, the level of postoperative cognition at different time points, and postoperative inflammation in elderly patients undergoing noncardiac surgery and tried to determine the optimal anaesthesia method to prevent POCD in elderly patients.
2. Methods
This meta-analysis was conducted in accordance with the recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. The protocol of this systematic review was not registered.
2.1. Literature search and outcome
Several databases (Embase, Medline, Cochrane Library, Web of Science) were searched. The searched items were: inhalation anaesthesia, isoflurane, sevoflurane, desflurane, nitrous oxide, intravenous anaesthesia, total intravenous anaesthesia, propofol, postoperative cognitive dysfunction, POCD, postoperative cognitive impairment, cognition, cognitive, elderly, old and geriatric. The literature search strategy in the Medline database is shown in Supplemental Digital Content (Appendix 1). The references of the retrieved articles were also reviewed to identify any further potential eligible trials. The publication language was English. The end date for the search was June 2020. The primary outcome was postoperative cognitive dysfunction, and the secondary outcomes were MMSE scores, systemic inflammation and neuroinflammation.
2.2. Inclusion and exclusion criteria
The inclusion criteria were defined as follows: elderly patients, RCTs of inhalation versus intravenous anaesthesia, and trials providing data on at least the main outcome. The exclusion criteria were cardiac surgery, the inclusion of patients younger than 60 years, and observational studies.
2.3. Data extraction and quality assessment
Data were extracted according to the predefined inclusion and exclusion criteria by two co-first authors. If necessary, data presented as the medians with quartile were transformed into the mean ± standard deviation (SD) according to the method described by Hozo et al[15] and data presented as graphs were transformed into numbers using Plot-digitizer software. The risk of bias was assessed by the Jadad score; the highest score was 7. A study with a Jadad score greater than or equal to 4 was defined as a high-quality study, and studies were excluded if the Jadad score was less than 4. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology was used to appraise the overall evidence-based quality for each outcome. The risk of bias was evaluated, and any disagreement was resolved by discussion and consensus among all authors.
2.4. Statistical analysis
Meta-analysis was conducted using Stata 12.0 software (STATA, College Station). The effect size for continuous data was expressed as the standard mean difference (SMD) with a 95% confidence interval (CI). The effect size for dichotomous outcomes was expressed as a risk ratio (RR) with a 95% CI. The χ2 test and the I2 value were used to determine the level of heterogeneity; in the case of heterogeneity (P < .1 or I2 ≥ 50%), a random effect model was used, and in the case of homogeneity (P≥ .1 or I2 < 50%), a fixed effect model was used.
2.5. Subgroup analysis and sensitivity analysis
When heterogeneity among studies was statistically significant or I2≥50%, subgroup analysis or sensitivity analysis was performed whenever possible to identify the source of heterogeneity and to test the robustness of uncertainty in the meta-analysis.
2.6. Publication bias
Publication bias was evaluated using Egger's test with Stata 12.0 software, and no significant publication bias was indicated if P > .05.
2.7. Trial sequential analysis
Trial sequential analysis (TSA) was conducted using TSA0.9.5.5 Beta software (www.ctu.dk/tsa), and the required information size (RIS) was estimated using 0.05 for type 1 error, 0.20 for type 2 error, and the relative risk reduction from the control group event rate in low-bias-risk trials included in the meta-analysis. The TSA can be interpreted by viewing the boundaries and assessing whether the cumulative meta-analysis has crossed them.
2.8. Ethical approval and dissemination
Ethics approval and patient consent were not required as this study is a systematic review based on published articles.
3. Results
3.1. Literature search and retrieval
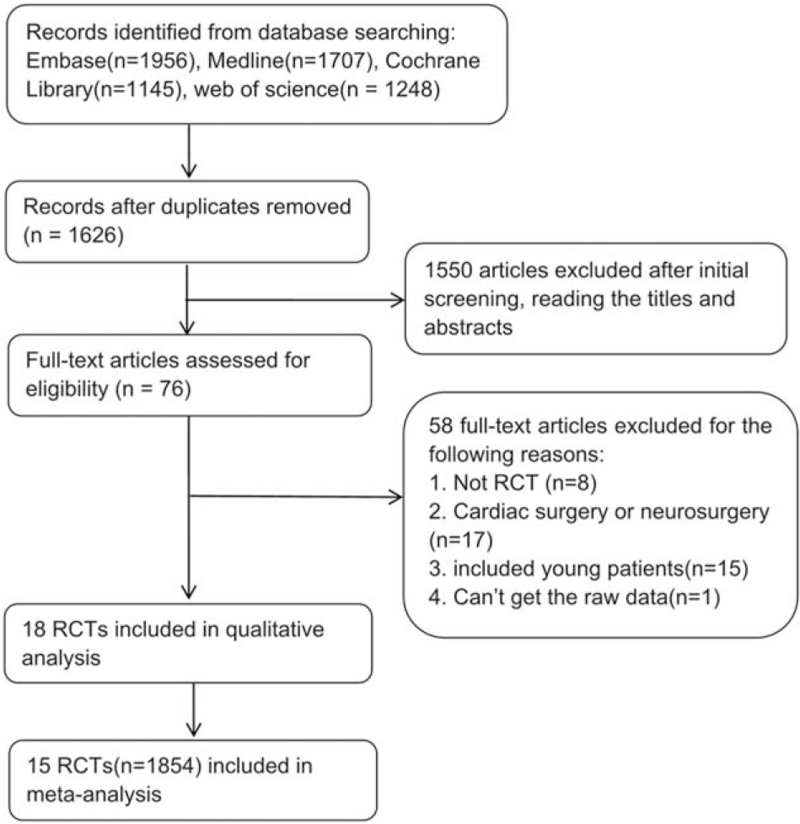
A total of 6056 articles were initially retrieved, and 15 RCTs with 1854 patients were ultimately included in this meta-analysis. Ten studies compared sevoflurane and propofol, 1 study compared isoflurane, sevoflurane and propofol, 1 study compared desflurane, sevoflurane and propofol, 1 study compared desflurane and propofol, 1 study compared isoflurane and propofol, and 1 study compared xenon and propofol. The characteristics and quality evaluation of the included RCTs are presented in Table 1, and the literature screening procedure is shown in Fig. 1.
Table 1.
Characteristics of the trials.
| ID | Country | Surgery | Group (maintenance) | Age (years)∗ | Depth of anesthesia | Outcomes | Jadad |
| Egawa 2016[8] | Japan | Elective lung surgery | Prop (n = 72) | 69 (63∼73) | BIS:40-60 | Incidence of POCD, MMSE on POD 5 and 3 months after surgery | 7 |
| Sev (n = 72) | 72 (63∼72) | ||||||
| Qin 2018[9] | China | Lung cancer surgery | Prop (n = 52) | 60∼77 | BIS:40-60 | MMSE and S-100β on POD 1 and POD 2 | 4 |
| Sev (n = 52) | |||||||
| Micha 2016[10] | Greece | Tumor resection (non-cardiac or neurosurgery) | Prop (n = 36) | 60∼74 | BIS:40-60 | MMSE at 9 months after surgery; incidence of POCD, IL-6 and TNF-α on POD 2 | 7 |
| Sev (n = 37) | |||||||
| Guo 2020[11] | China | Tumor resection | Prop (n = 117) | 69 (66∼72.5) | BIS:40-60 | Incidence of POCD on POD 7 and 3 months after surgery | 7 |
| Sev (n = 117) | 69 (66∼74.0) | ||||||
| Rohan 2005[18] | USA | Minor urological or gynecological surgery | Prop (n = 15) | 72.9 (65∼83) | / | Incidence of POCD, S-100β on POD 1 | 7 |
| Sev (n = 15) | 73.8 (67∼86) | ||||||
| Qiao 2015[12] | China | Resection of esophageal carcinoma | Prop (n = 30) | 65∼75 | BIS:50–60 | MMSE, TNF-α, IL-6 and S-100β on POD 1–7 | 4 |
| Sev (n = 30) | |||||||
| Tang 2014[20] | China | Radical rectal resection | Prop (n = 101) | 69.6 ± 4.8 | BIS:30–60 | Incidence of POCD on POD 7 | 7 |
| Sev (n = 99) | 70.0 ± 4.3 | ||||||
| Zhang 2018[21] | China | Major cancer surgery | Prop (n = 195) | 72.8 ± 5.5 | BIS:40-60 | MMSE, incidence of POCD on POD 7 | 7 |
| Sev (n = 192) | 72.4 ± 5.6 | ||||||
| Geng 2017[16] | China | Laparoscopic cholecystectomy | Prop (n = 50) | ≥ 65 | BIS:40-50 | MMSE, incidence of POCD on POD 1 and POD 3; S-100β, IL-6, TNF-α on POD 1 | 6 |
| Sev (n = 50) | |||||||
| Iso (n = 50) | |||||||
| Rasmussen 2006[19] | Denmark | Knee replacement | Prop (n = 18) | ≥ 60 | / | Incidence of POCD on POD3–5 and 3 months after surgery | 7 |
| Xenon (n = 21) | |||||||
| Tan 2009[17] | China | Upper abdominal surgery | Prop (n = 30) | 61∼80 | / | MMSE on POD 1–2; incidence of POCD on POD1 | 4 |
| Iso (n = 30) | |||||||
| Juvin 1997[22] | France | Major orthopedic surgery | Prop (n = 14) | 75.6 ± 4.2 | / | MMSE on POD1 | 5 |
| Des (n = 14) | 77.3 ± 5 | ||||||
| Iso (n = 15) | 77.4 ± 5.1 | ||||||
| Moffat 1995[23] | USA | Cataract surgery | Prop (n = 20) | 72 (60∼86) | / | MMSE 2 h after surgery | 4 |
| Iso (n = 20) | 77 (64∼88) | ||||||
| Chen 2018[24] | China | Moderate orthopedic surgery | Prop (n = 100) | 66.34 ± 4.5 | / | Recognition test at 30min and 120 min after surgery | 5 |
| Sev (n = 100) | 67.9 ± 7.3 | ||||||
| Tanaka 2017[25] | USA | Total knee arthroplasty | Prop (n = 45) | 70.6 (69.2∼ 72.1) | PSI 30–50 | Recognition test at 6–8h, 48h | 6 |
| Des (n = 45) | 69.8 (68.6∼ 71.1) |
Figure 1.
Flowchart of study selection.
3.2. Meta-analysis results
3.2.1. Primary outcome: incidence of POCD (Fig. 2)
Figure 2.
Forest plot: comparison in incidence of POCD between intravenous and inhalation anaesthesia. (A): propofol anaesthesia vs sevoflurane anaesthesia; (B): propofol anaesthesia vs isoflurane anaesthesia.[16]
A total of 9 RCTs[8,10,11,16–21] reported the incidence of POCD. Three RCTs[16–18] (n = 284) reported the incidence on postoperative day 1 (POD1), 2 of which studies used the MMSE, while 1 study used the Stroop word test combined with other cognitive tests to identify POCD. The results showed that the incidence of POCD on POD 1 after propofol anaesthesia was not different from that after inhalation anaesthesia (I2 = 64.9%, RR: 0.42, 95% CI: 0.18–1.02, P = .056). No significant publication bias was identified according to Egger's test (P = .286). TSA indicated that the sample size in the meta-analysis was lower than the required sample size (n = 407) (Supplemental Digital Content (Appendix 2)). When the study by Rohan et al[18] was omitted, the data from the other studies using the MMSE were analysed, and the results showed that POCD was significantly decreased after propofol anaesthesia compared with inhalation anaesthesia (I2 = 0%, RR: 0.27, 95% CI: 0.14–0.49, P < .001).
Four RCTs [8,10,16,19] (n = 460) reported the incidence on postoperative day 2–6 (PODs 2–6), 3 of which used the MMSE, while 1 study used the Stroop word test combined with other cognitive tests to identify POCD. The results revealed that the incidence of POCD on PODs 2–6 after propofol anaesthesia was markedly lower than that after inhalation anaesthesia (I2 = 68.2%, RR: 0.37, 95% CI: 0.15–0.88, P = .025). No significant publication bias was identified according to Egger's test (P = .283). TSA indicated that the sample size in the meta-analysis was higher than the required sample size (n = 449) (Supplemental Digital Content (Appendix 3)). When the study by Rasmussen et al[19] was omitted, the data from the other 3 studies using the MMSE were analysed, and the results showed that the incidence of POCD was still lower in the propofol group (I2 = 67.1%, RR: 0.25, 95% CI: 0.08–0.75, P = .013). Sensitivity analysis showed that the results did not change when any study was omitted.
Data on the incidence of POCD on postoperative Day 7 (POD 7) were reported in 3 RCTs[11,20,21] (n = 799). The fixed effect model showed no significant between-group difference in POCD on POD 7 (I2 = 0%, RR: 0.80, 95% CI: 0.63–1.02, P = .077), and no significant publication bias according to Egger's test (P = .543). However, TSA showed that the sample size was lower than the required sample size(n = 1541).
Data on the incidence of POCD at 3 months after surgery were obtained from 3 RCTs[8,11,19] (n = 367). The fixed effect model showed no significant between-group difference (I2 = 0%, RR: 0.77, 95% CI: 0.45–1.30, P = .324), and no significant publication bias according to Egger's test (P = .930). However, TSA showed that the sample size was lower than the required sample size (n = 4534).
3.2.2. Secondary outcomes:
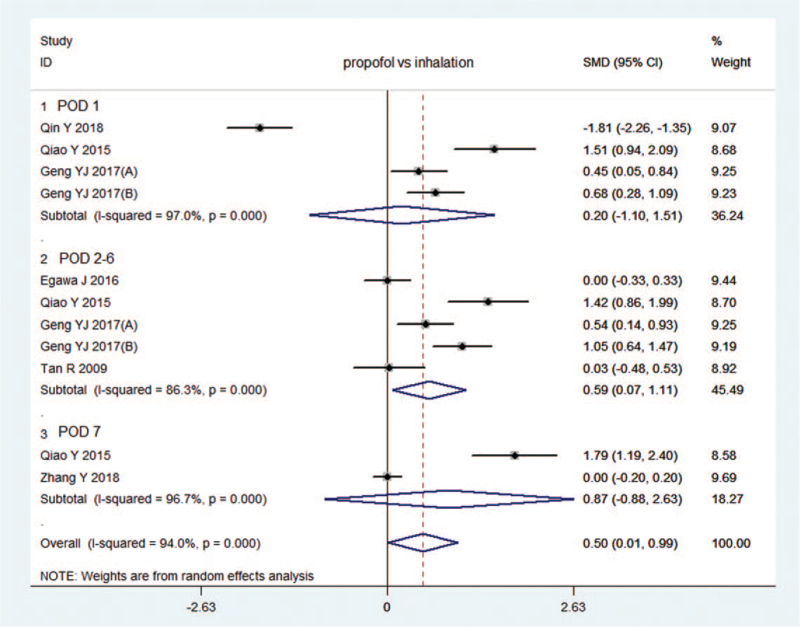
3.2.2.1. MMSE scores (Fig. 3)
Figure 3.
Forest plot: comparison in MMSE score between intravenous and inhalation anaesthesia. (A): propofol anaesthesia vs sevoflurane anaesthesia; (B): propofol anaesthesia vs isoflurane anaesthesia.[16] (A): propofol anaesthesia vs desflurane anaesthesia; (B): propofol anaesthesia vs isoflurane anaesthesia.[22]
A total of 7 RCTs[8,9,12,16,17,21,22] compared MMSE scores between propofol anaesthesia and inhalation anaesthesia. Five studies[9,12,16,17,22] (n = 417) reported MMSE scores on POD 1. The random effect model showed no significant between-group difference (I2 = 94.1%, SMD: 0.13, 95% CI: -0.66–0.93, P = .745), and no significant publication bias according to Egger's test (P = .972). Sensitivity analysis showed that the MMSE score after propofol anaesthesia was substantially higher than that after inhalation anaesthesia when the study by Qin et al[9] was omitted (I2 = 73.2%, SMD: 0.483, 95% CI: 0.064–0.902, P = .024).
Four studies[8,12,16,17] (n = 414) reported MMSE scores on PODs2–6, and the random effect model showed that MMSE score after propofol anaesthesia was much higher than that after inhalation anaesthesia (I2 = 86.3%,SMD: 0.59, 95% CI: 0.07–1.11, P = .026). No significant publication bias was noted according to Egger's test (P = .294). Sensitivity analysis showed that the results did not change when any study was omitted.
Two studies [12,21](n = 491) reported the MMSE score on POD 7, and the random effect model showed no significant between-group difference in MMSE scores on POD 1 (I2 = 96.7%, SMD: 0.87, 95% CI: -0.88–2.63, P = .330).
3.2.2.2. IL-6 and TNF-α levels
Only 2 RCTs[12,16] (n = 210) reported systemic inflammation (IL-6 and TNF-α) on POD 1, and none reported neuroinflammation. The random effect model showed that the IL-6 level in the propofol group was lower than that in the inhalation group (I2 = 96.8%, SMD: -2.027, 95% CI: -3.748– -0.307, P = .021) (Fig. 4). The fixed effect model showed that the TNF-α level was also lower in propofol group (I2 = 0%, SMD: -0.68, 95% CI: -0.93– -0.43, P < .001) (Fig. 5).
Figure 4.
Forest plot: comparison in IL-6 levels between intravenous and inhalation anaesthesia. (A): propofol anaesthesia vs sevoflurane anaesthesia; (B): propofol anaesthesia vs isoflurane anaesthesia.[16]
Figure 5.
Forest plot:comparison in TNF-α levels between intravenous and inhalation anaesthesia. (A): propofol anaesthesia vs sevoflurane anaesthesia; (B): propofol anaesthesia vs isoflurane anaesthesia.[16]
3.2.2.3. S-100β level (Fig. 6)
Figure 6.
Forest plot: comparison in S100β levels between intravenous and inhalation anaesthesia. (A): propofol anaesthesia vs sevoflurane anaesthesia; (B): propofol anaesthesia vs isoflurane anaesthesia.[16]
Four RCTs[9,12,16,18] (n = 344) reported the S-100β level on POD 1, and a meta-analysis showed that it was markedly lower after propofol anaesthesia than after inhalation anaesthesia (I2 = 96.3%, SMD: -1.26, 95% CI: -2.45– -0.07, P = .038) (Fig. 5). No significant publication bias was identified according to Egger's test (P = .116). However, sensitivity analysis showed that when the study by Qiao et al[12] was omitted, the results were changed.
Three RCTs [9,12,16] (n = 314) compared the S-100β level on PODs 2–6. Meta-analysis showed no significant between-group difference at PODs 2–6 (I2 = 95.8%, SMD: -1.36, 95% CI: -2.88–0.16, P = .08). No significant publication bias was noted according to Egger's test (P = .166). However, sensitivity analysis showed that when the study by Qin et al[9] was omitted, the results were changed.
3.2.3. Results of studies not eligible for meta-analysis
Three RCTs[23–25] could not be pooled due to a lack of extractable outcomes. No significant between-group differences in cognitive tests were observed 2–8 h after surgery in the studies by Moffat et al[23] and Tanaka et al.[25] Another study reported that a greater number of patients in the propofol group than in the sevoflurane group provided correct answers to the recognition test 30 and 120 min after anaesthesia.[24]
4. Discussion
Our results showed that on postoperative days 2–6 in elderly noncardiac surgical patients, the incidence of POCD was lower after propofol anaesthesia. The sample size was sufficiently large, and the level of evidence was moderate (Table 2). The results indicated that propofol anaesthesia was better than inhalation anaesthesia for the prevention of early POCD in the early days after surgery.
Table 2.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) summary of findings.
Many tools are available to diagnose POCD in clinical practice, including the MMSE, Montreal Cognitive Assessment, Stroop colour word test and other methods, and the tools in different studies are not uniform, but the MMSE is the most commonly used tool. In this meta-analysis, the pooled effects of propofol and inhalation anaesthesia on the incidence of POCD on POD 1 from all the included studies conflicted with the results from the studies using the MMSE. Sensitivity analysis showed that the effects of different anaesthetics on the incidence of POCD and MMSE scores on POD 1 were changed when studies with different cognitive tests were omitted, but the results on PODs 2–6 were stable, indicating that the different tools in these studies might result in different outcomes. However, some bias remained among studies since the heterogeneity was very high.
POCD may be related to many risk factors, such as an imbalance in calcium homeostasis, systemic and neural inflammation from surgical trauma, and deterioration of endogenous neurodegeneration, where role of inflammation is crucial.[26] Surgery causes peripheral tissue damage, and then damage-associated molecular patterns (DAMPs) are released and bind with pattern recognition receptors (PRRs), which can activate NF-κB, promote the synthesis and release of TNF-α and IL-6 from macrophages, and increase the permeability of the blood–brain barrier, eventually leading to neuroinflammation.[27] The impacts of general anaesthetic agents on systemic inflammation and neuroinflammation are very complicated. In animal experiments, propofol did not induce neuroinflammation or cognitive dysfunction, while sevoflurane or isoflurane could directly cause neuroinflammation and cognitive impairment.[6,28,29] None of the clinical trials reported the effects of general anaesthesia on neuroinflammation in elderly patients, and the results regarding the effect on systemic inflammation were conflicting, but only the few studies included in this meta-analysis observed both cognitive function and systemic inflammation.[12,16]
In this meta-analysis, the plasma TNF-α and IL-6 levels were lower on postoperative day 1 after propofol anaesthesia than after inhalation anaesthesia, and neuroinflammation after propofol anaesthesia can be postulated to also be lower. Isoflurane was reported to increase the incidence of POCD in rats through a TNF-α-dependent mechanism.[30] IL-6 can attenuate long-term potentiation (LTP) and inhibit learning and memory,[31] and sevoflurane could increase the permeability of the BBB in an animal study.[32] Thus, higher levels of TNF-α and IL-6 after inhalation anaesthesia may contribute to the higher incidence of POCD and lower cognitive status after noncardiac surgery in elderly patients.
Two meta-analyses reported that inhalation anaesthesia could reduce proinflammatory factors in the alveoli during pneumonectomy and decrease postoperative pulmonary complications.[33,34] However, in this meta-analysis, propofol anaesthesia was better than inhalation anaesthesia for the attenuation of cognitive impairment and systemic inflammation.
S100β is produced by astrocytes, and its increasing concentration in the plasma is indicative of acute brain injury in aged patients.[35] In this meta-analysis, the plasma level of S100β was higher after inhalation anaesthesia than after propofol anaesthesia, but sensitivity analysis showed that the results were not stable; therefore, whether the plasma S100β protein concentration can be used as a biomarker of POCD is still unclear.
This meta-analysis had some limitations. First, the tools used to identify POCD in these included trials were not uniform, and the results for the incidence of POCD by different tools can differ across studies. Second, only a few trials reported the impact of general anaesthesia on both systemic inflammation and POCD in the same literature, and few reported the impact of general anaesthesia on delayed POCD, resulting in low-level evidence. Third, all the included trials reported POCD in elderly patients, but the criteria for elderly patients were not uniform. Some studies set the age criterion above 65 years old, while others set the criterion above 60 years old. Fourth, selective biases existed in some meta-analysis results. Therefore, a large RCT is needed to verify the effects of propofol and inhalation anaesthesia on POCD using a uniform diagnostic tool and systemic inflammation in the near future.
5. Conclusions
In conclusion, the moderate evidence from this meta-analysis shows that in elderly noncardiac surgical patients, propofol anaesthesia is superior to inhalation anaesthesia for attenuation of early POCD, and low-level evidence shows that cognitive status (MMSE score) is higher and systemic inflammation is less severe after propofol anaesthesia in the early days after surgery.
Acknowledgments
The authors declare that no acknowledgements have to be made.
Author contributions
Conceptualization: Hongliang Liu.
Data curation: Qian-Yun Pang, Li-Ping Duan.
Formal analysis: Qian-Yun Pang, Li-Ping Duan.
Investigation: Yan Jiang.
Methodology: Qian-Yun Pang, Yan Jiang.
Software: Qian-Yun Pang, Hongliang Liu.
Supervision: Hongliang Liu.
Validation: Qian-Yun Pang.
Visualization: Qian-Yun Pang.
Writing – original draft: Hongliang Liu.
Writing – review & editing: Hongliang Liu.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, IL-6 = interleukin-6, MMSE = Mini-Mental State Examination, POCD = postoperative cognitive dysfunction, POD = postoperative Day, RCTs = randomized controlled trials, RIS = required information size, RR = risk ratio, SMD = standard mean difference, TNF-α = tumor necrosis factor-α, TSA = trial sequential analysis.
How to cite this article: Pang QY, Duan LP, Jiang Y, Liu HL. Effects of inhalation and propofol anaesthesia on postoperative cognitive dysfunction in elderly noncardiac surgical patients: A systematic review and meta-analysis. Medicine. 2021;100:43(e27668).
This research was supported by the Decision Making Consultation and Management Innovation Project in Shapingba District, Chongqing (No:Jcd202036) and the Nature Science Foundation Project of Chongqing (No: cstc2017jcyjBX0043).
Register number: The protocol of this systematic review was not registered.
Ethics approval and patient consent were not required, as this study is a systematic review based on published articles.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
Age (year)∗ = expressed as mean ± standard deviation or median with quartile or the range of age, BIS = bispectral index, Des = desflurane, Iso = isoflurane, MMSE = Mini-Mental State Examination, POCD = postoperative cognitive dysfunction, POD = postoperative Day, Prop = propofol, PSI = patient state index, Sev = sevoflurane.
References
- [1].Berger M, Nadler JW, Browndyke J, et al. Postoperative cognitive dysfunction: minding the gaps in our knowledge of a common postoperative complication in the elderly. Anesthesiol Clin 2015;33:517–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998;351:857–61. [DOI] [PubMed] [Google Scholar]
- [3].Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand 2010;54:951–6. [DOI] [PubMed] [Google Scholar]
- [4].Hu N, Guo D, Wang H, et al. Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res 2014;1551:13–24. [DOI] [PubMed] [Google Scholar]
- [5].Dong Y, Zhang G, Zhang B, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases (-amyloid protein levels. Archives of neurology 2009;66:620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang J, Tan H, Jiang W, Zuo Z. The choice of general anaesthetics may not affect neuroinflammation and impairment of learning and memory after surgery in rats. J Neuroimmune Pharmacol 2015;10:179–89. [DOI] [PubMed] [Google Scholar]
- [7].Konishi Y, Evered LA, Scott DA, Silbert BS. Postoperative cogntive dysfunction after sevoflurane or propofol general anaesthesia in combination with spinal anaesthesia for hip arthroplasty. Anaesth Intensive Care 2018;46:596–600. [DOI] [PubMed] [Google Scholar]
- [8].Egawa J, Inoue S, Nishiwada T, et al. Effects of anaesthetics on early postoperative cognitive outcome and intraoperative cerebral oxygen balance in patients undergoing lung surgery: a randomized clinical trial. Can J Anesth 2016;63:1161–9. [DOI] [PubMed] [Google Scholar]
- [9].Qin Y, Ni J, Kang L, et al. Sevoflurane effect on cognitive function and the expression of oxidative stress response proteins in elderly patients undergoing radical surgery for lung cancer. J Coll Physic Surg Pak 2019;29:12–5. [DOI] [PubMed] [Google Scholar]
- [10].Micha G, Tzimas P, Zalonis I, Kotis K, Papdopoulos G, Arnaoutoglou E. Propofol vs sevoflurane anaesthesia on postoperative cognitive dysfunction in the elderly. A randomized controlled trial. Acta Anaesth Belg 2016;67:129–37. [PubMed] [Google Scholar]
- [11].Guo L, Lin F, Dai H, et al. Impact of sevoflurane versus propofol anaesthesia on post-operative cognitive dysfunction in elderly cancer patients: a double-blinded randomized controlled trial. Med Sci Monit 2020;26:e919293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Qiao Y, Feng H, Zhao T, Yan H, Zhang H, Zhao X. Postoperative cognitive dysfunction after inhalation anaesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol 2015;15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller D, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non cardiac surgery. Cochrane Database Syst Rev 2018;8:CD012317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Terrando N, Gomez-Galan M, Yang T, et al. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J 2013;27:3564–71. [DOI] [PubMed] [Google Scholar]
- [15].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Geng YJ, Wu QH, Zhang RQ. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: a randomized controlled trial. J Clin Anesth 2017;38:165–71. [DOI] [PubMed] [Google Scholar]
- [17].Tan R. Effect of propofol and isoflurane on surgical stress response and postoperative cognitive function in elderly patients. J South Med Univ 2009;29:1247–8. [PubMed] [Google Scholar]
- [18].Rohan D, Buggy DJ, Crowley S, et al. Increased incidence of postoperative cognitive dysfunction 24 hr after minor surgery in the elderly. Can J Anesth 2005;52:137–42. [DOI] [PubMed] [Google Scholar]
- [19].Rasmussen LS, Schmehl W, Jakobsson J. Comparison of xenon with propofol for supplementary general anaesthesia for knee replacement: a randomized study. Br J Anaesth 2006;97:154–9. [DOI] [PubMed] [Google Scholar]
- [20].Tang N, Ou C, Liu Y, Zuo Y, Bai Y. Effect of inhalational anaesthetic on postoperative cognitive dysfunction following radical rectal resection in elderly patients with mild cognitive impairment. J Int Med Res 2014;42:252–61. [DOI] [PubMed] [Google Scholar]
- [21].Zhang Y, Shan GJ, Zhang YX, et al. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br J Anaesth 2018;121:595–604. [DOI] [PubMed] [Google Scholar]
- [22].Juvin P, Servin F, Giraud O, Desmonts JM. Emergence of elderly patients from prolonged desflurane, isoflurane, or propofol anaesthesia. Anesth Analg 1997;85:647–51. [DOI] [PubMed] [Google Scholar]
- [23].Moffat A, Cullen PM. Comparison of two standard techniques of general anaesthesia for day-case cataract surgery. Br J Anaesth 1995;74:145–8. [DOI] [PubMed] [Google Scholar]
- [24].Chen XD, Xie W, Zhou QH. Effect of propofol and sevoflurane on cognitive function among elderly patients undergoing elective surgery under anaesthesia. Pak J Pharm Sci 2018;31:2909–13. [PubMed] [Google Scholar]
- [25].Tanaka P, Goodman S, Sommer BR, Maloney W, Huddleston J, Lemmens HJ. The effect of desflurane versus propofol anaesthesia on postoperative delirium in elderly obese patients undergoing total knee replacement: a randomized, controlled, double-blinded clinical trial. J Clin Anesth 2017;39:17–22. [DOI] [PubMed] [Google Scholar]
- [26].Hudson AE, Hemmings HC, Jr. Are anaesthetics toxic to the brain? Br J Anaesth 2011;107:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 2011;70:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cui RS, Wang K, Wang ZL. Sevoflurane anaesthesia alters cognitive function by activating inflammation and cell death in rats. Exp Therap Med 2018;15:4127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang ZY, Yuan CX. IL-17A promotes the neuroinflammation and cognitive function in sevoflurane anesthetized aged rats via activation of NF-kB signaling pathway. BMC Anesthesiol 2018;18:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang C, Zhu B, Ding J, Wang ZG. Isoflurane anaesthesia aggravates cognitive impairment in streptozotocin-induced diabetic rats. Int J Clin Exp Med 2014;7:903–10. [PMC free article] [PubMed] [Google Scholar]
- [31].Balschun D, Wetzel W, Del Rey A, Pitossi F, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB J 2004;18:1788–90. [DOI] [PubMed] [Google Scholar]
- [32].Hu N, Guo D, Wang H, et al. Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res 2014;1551:13–24. [DOI] [PubMed] [Google Scholar]
- [33].Sun B, Wang J, Bo L, et al. Effects of volatile vs propofol-based intravenous anaesthetics on the alveolar inflammatory response to one-lung ventilation: a meta-analysis of randomized controlled trilas. J Anesth 2015;29:570–9. [DOI] [PubMed] [Google Scholar]
- [34].Pang QY, An R, Liu HL. Effects of inhalation and intravenous anaesthesia on intraoperative cardiopulmonary function and postoperative complications in patients undergoing thoracic surgery: a systematic review and meta-analysis. Minerva Anesthesiol 2018;84:1287–97. [DOI] [PubMed] [Google Scholar]
- [35].Linstedt U, Meyer O, Kropp P, Berkau A, Tapp E, Zenz M. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anaesthesia in different types of surgery. Acta Anaesthesiol Scand 2002;46:384–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.