Abstract
To estimate the prevalence of the most frequent infections related to device utilization and their antimicrobial sensitivity panel, and to investigate the overall incidence of device associated infection rates per 1000 device days, at the pediatric intensive care unit of the Jordan University of Science and Technology.
This is a retrospective study from a single pediatric intensive care unit. Data were collected in relation to bloodstream infections associated with central venous catheters, pneumonia associated with ventilator endotracheal tubes, and urinary tract infections associated with Foley catheters, between January 2013 and December 2018, according to the center of disease control and prevention protocols.
During the 5-year study, 3195 patients were admitted to the pediatric intensive care unit for a total of 16,487 days. Forty-six patients (1.4%) developed 55 infections, with a median incidence rate of 7.4, 3.7, and 0.7 per 1000 days for central line associated infections, ventilator associated pneumonia, and catheter associated infections, respectively. The commonest isolated microorganisms were gram-negative bacteria in 89.1% of cases, and fungi in 10.9% of cases. Among the resistant bacterial isolates, 59.2% were multidrug resistant, and 32.6% were extended spectrum beta lactamase producers Klebsiella pneumoniae and Eschericia coli. High infection rates were related to Acinetobacter baumannii and K pneumoniae, associated with high resistance to cephalosporins. Susceptibility was highest to tigecycline and imipenem at 42.9% and 32.7% respectively.
Microbial isolates are commonly associated with healthcare device insertions in pediatric intensive care unit, invasive bacterial infections associated with critical morbidity and mortality. Further studies on device associated infections are recommended for regional profiling purposes.
Keywords: device-associated infections, multidrug resistant organisms, pediatric intensive care unit
1. Introduction
Device-associated healthcare-associated infections (DA-HAI) including ventilator-associated pneumonia (VAP), central-line-associated bloodstream infection (CLABSI), and catheter-related urinary tract infection (CAUTI) are considered principal contributors to healthcare hazard and threat to patient safety. They can cause prolonged hospital stay, sepsis, and mortality in intensive care units (ICU). This study intends to characterize DA-HAI in a tertiary care multidisciplinary ICU of a teaching hospital in the north of Jordan.
Healthcare associated infections (HAIs) strongly correlate with morbidity and mortality rates worldwide.[1] Infection control practices is the key to prevent multidrug resistance organisms infections during pediatric intensive care units (PICU) setting by different, HAIs types and frequency could be vary with hospital departments, staying, and population.[2,3]
Infection prevention strategies for managing device associated infections (DAIs) are applied worldwide.[4] Healthcare associated infections in low and middle income countries are higher than in high income countries.[5] The International Nosocomial Infection Control Consortium reported a significant difference in the rates of DAIs at 7 versus 9 per 1000 central line days, 9 versus 4.5 per 1000 ventilator days, and 5.9 versus 3.7 per 1000 urinary catheter days in low versus middle income countries, respectively.[6] In contrast, developing countries’ incidence rates were 18.1, 7.9, and 5.1 per 1000 days in relation to central lines, mechanical ventilators, and urinary catheters respectively.[7] A Japanese study reported rates of 4.3, 3.5, and 13.6 per 1000 device days in relation to central lines, ventilator use, and urinary catheters respectively.[8]
The objectives of this study were to identify the most common reported infections associated with device use at the PICU of the Jordan University of Science and Technology (JUST), the main referral tertiary hospital in the north of Jordan, and to study the antimicrobial sensitivity panel, appropriateness of empirical antibiotic options, length of hospital stay, and mortality rates.
2. Methods
This is a 5-year retrospective study between January 2013 and December 2018. It was conducted at a single closed PICU in a tertiary hospital which is affiliated with the JUST, with a capacity of 10 beds that admits surgical and medical pediatric patients, and accepts transferred cases from other hospitals being a tertiary referral unit.
The hospital's microbiology laboratory provides antimicrobial susceptibility testing of clinical isolates using VITEK-2 technique (a fully automated system that performs bacterial identification and antibiotic susceptibility testing, with a unique vacuum filler that provides both safety and the highest level of automation), and manual desk diffusion under Centers for Disease Control and Prevention/National Healthcare Safety Network (CDC/NHSN) protocol.[9]
The targeted population was PICU patients who had at least one invasive device in the form of a central line, endotracheal tube, or Foley catheter. The infection control unit recorded 46 patients with DAIs. Data were collected from the electronic medical records and included age, sex, indication for admission, comorbidities, empirical therapy, identified microorganisms, antibiotics susceptibility tests, admission and discharge dates, and outcome. All data are available upon request from the corresponding author.
Device-associated healthcare-associated infections (DA-HAI), including VAP, CLABSI, and CAUTI, are considered principal contributors to healthcare hazard and threat to patient safety. They can cause prolonged hospital stay, sepsis, and mortality in intensive care units (ICU). The study intended to characterize DA-HAI in a tertiary care multidisciplinary ICU of a teaching hospital in the north of Jordan, and to report the rates of CLABSI, VAP, and CAUTI per 1000 days each, using the standardized surveillance definitions of the CDC/NHSN that are applied by the hospital infection control unit.
The utilized microbiology laboratory provides in-vitro bacteriological diagnoses(gram stain, biochemical tests, and cultures) and susceptibility testing of human body fluids (blood, urine, sputum etc) according to universal clinical and laboratory standards protocols, in which automated (VITEK2 Technique), and manual methods are used (disk diffusion). The laboratory also uses automated Bactec system for detecting microorganisms’ growth in blood culture samples.[10]
For patients to be included in the study, the device related infection had to occur not less than 2 calendar days after insertion, and no >1 calendar day after removal. Cases were confirmed as DA-HAI, based on CDC/NHSN surveillance definition.[11]
Multi drug resistance (MDR) bacteria refer to antimicrobial resistance against one antibiotic in at least 3 different classes. Extended spectrum beta lactamase (ESBL) refers to enzymes produced by bacteria that provide multiresistance to β-lactam antibiotics such as penicillins, cephalosporins, cephamycins, and carbapenems.
Admission and discharge dates or mortality were recorded. Distinction was made between general hospital and PICU length of stay. Mortality rate was calculated as the percentage of death out of the total PICU DA-HAI patients.
The dataset was analyzed using the Statistical Package for Social Sciences (SPSS) version 22. Infection rates were described using summary statistics. The Kaplan–Meier estimator (product limit estimator) was used to estimate the survival function. The number of patients at risk.
The study was approved by the Institutional Review Board of the Faculty of Medicine, JUST.
3. Results
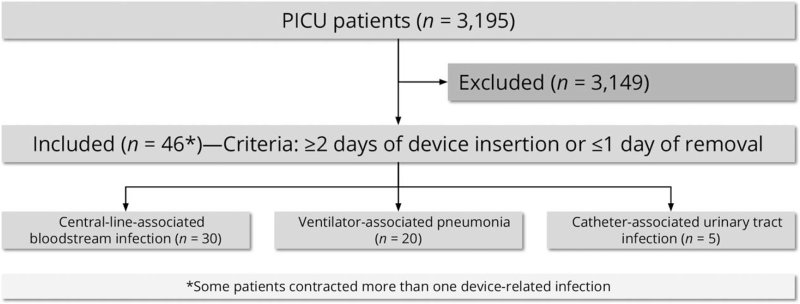
During the study period, 3195 patients were admitted to the PICU for 16,487 patient days, and 12,112 device-days were categorized as 3540 for central venous catheters, 5720 for endotracheal tubes, and 2852 for urinary catheters.
Over the 5 years of study, 46 patients (1.4%) were admitted to the PICU with DA-HAI (Fig. 1). The median age was 1 year (25th percentile 0.25–75th percentile 3.5). Of those, 31 (67.4%) were men, 5 (10.9%) were immunocompromised, 38 (82.6%) had prolonged PICU stay, 4 (8.7%) were infected with >1 device (Table 1). Median PICU length of stay for individual device utilization infection was 30.5, 50, and 42 days for CLABSI, VAP, and CAUTI, respectively.
Figure 1.
Flow diagram of study participants.
Table 1.
Findings related to pediatric intensive care unit in relation to device-associated infections.
| Age, yr | Median (25th–75th percentile) | 1 (0.25–3.5) |
| Gender | M/F | 31/15 |
| Medical (%) | 71.7 | |
| Surgical (%) | 28.3 | |
| Comorbidity (%) | ||
| Abdominal disease | 15.2 | |
| Kidney diseases | 8.7 | |
| Brain diseases | 30.4 | |
| Lung diseases | 6.5 | |
| Cancers | 6.5 | |
| ∗Others | 32.6 | |
| Device utilization infections (%) | ||
| ∗CVC | 54.5 | |
| ∗ETT | 36.4 | |
| ∗FC | 9.1 | |
| Stay, days | Median (25th; 75th percentile) | |
| ∗PICU | 21 (14–60.25) | |
| ∗HLOS | 27 (16.75–67) | |
| Crude mortality (%) | ||
| Short | Prolonged | |
| 62.5 | 60.5 |
The mortality rate was 60% with CLABSI, 55% with VAP, and 40% with CAUTI. Kaplan–Meier analysis showed that 50% of mortality was reported by 50 day-stay at PICU (Fig. 2).
Figure 2.
Kaplan–Meier analysis showed that 50% of mortality was reported by 50-day-stay at pediatric intensive care unit.
There were 55 identified DA-HAI isolates in 46 patients, most in children <1 year of age (45.7%). The most common types were CLABSI (30 cases), followed by VAP (20 cases) and CAUTI (5 cases). The median incidence rates, median overall device days, and device utilization ratios are shown in Table 2.
Table 2.
Device-associated infections in pediatric intensive care unit.
| Median (range) | |
| ∗CLABSI | |
| Central line-days | 587.5 (538–625) |
| Central line utilization ratio | 0.21 (0.20–0.27) |
| Catheter associated bloodstream infections/1000 | 7.40 (0.00–13) |
| †% of CLABSI distribution | 54.5 |
| ∗VAP | |
| Ventilator-days | 936.5 (801–1160) |
| Ventilator utilization ratio | 0.37 (0.30–0.43) |
| Ventilator associated Pneumonia/1000 | 3.70 (1.00–7.10) |
| †% of VAP distribution | 63.7 |
| ∗CAUTI | |
| Urinary catheter-days | 498 (210–668) |
| Urinary catheter utilization ratio | 0.16 (0.10–0.25) |
| Catheter associated urinary tract infections/1000 | 0.75 (0.00–3.40) |
| †% of CAUTI distribution | 9.1 |
During the 5-year study period, CLABSI were the most frequent, followed by VAP and CAUTI, alone and in combination with other devices, with 18% of children using >1 invasive device during the same admission (Fig. 3).
Figure 3.
Proportions of common device-associated infections.
Gram-negative bacteria were isolated in 89.1%, and fungal organisms in 10.9% of cases (Fig. 4). There were 30 isolates with primary bloodstream infections. Gram-negative bacteria were the most common pathogens (80%), mainly A baumanii (41.7%). Candida species were found in 20% of cases, of which 33.3% isolates were Candida albicans.
Figure 4.
Distribution of pathogens associated with medical devices utilization.
Concerning healthcare associated pneumonia, found in 20 isolates, only gram-negative bacteria were isolated. Acinetobacter baumanii at 65% was the most common, 20% were Enterobacteriaceae species, 10% were Pseudomonas aeruginosa, and 5% were Stenotrophomonas maltophilia.
Regarding urinary tract infections, found in 5 isolates, 60% were A baumanii; equally distributed between Enterobacteriaceae species and P aeruginosa.
Regarding bacterial resistance profile, 100% of A baumanii isolates were MDR, where 50% resulted from ventilator device usage. Second most prevalent bacteria were Klebsiella pneumoniae, with 100% being ESBL positive.
Central venous catheters contributed to 60% of the mortality rate, with 72.7% isolates, 100% of Eschericia coli species were ESBL positive.
For the isolated P aeruginosa, 100% were resistant to trimethoprim-sulfamethoxazole, while 60% were resistant to meropenem and imipenem, 40% were resistant to minocycline and ticracillin/clavulanic acid, and only 1 out of 5 isolates was MDR. The S maltophilia isolates were only sensitive tolevofloxacin and colistin.
Antibiotics susceptibility test were analyzed utilizing 3 different medical devices. Ventilator-associated pneumonia was characterized by higher resistance rates against various groups of antibiotics, 70% were multidrug resistant, followed by CAUTI at 60%. Extended spectrum beta lactamases-producers were present in 45.8% of CLABSI, and in 20% of VAP cases (Table 3).
Table 3.
Antibacterial resistance to devices associated infections in pediatric intensive care unit.
| ∗CLABSI | ∗VAP | ∗CAUTI | ||
| Antibiotics names (%) | N = 24 | N = 20 | N = 5 | Total |
| Aminoglycosides | ||||
| Amikacin | 33.3 | 10 | 20 | 22.4 |
| Gentamycin | 25 | 35 | 60 | 32.7 |
| Tobramycin | 12.5 | 15 | 40 | 16.3 |
| Carbapenems | ||||
| Imipenem | 50 | 45 | 60 | 49 |
| Meropenem | 45.8 | 65 | 20 | 51 |
| Cephalosporins | ||||
| Ceftazidime | 41.7 | 35 | 80 | 42.9 |
| Cefepem | 91.7 | 85 | 80 | 87.8 |
| Ceftriaxone | 83.3 | 70 | 60 | 75.5 |
| Cefixime | 70.8 | 60 | 20 | 61.2 |
| Fluoroquinolones | ||||
| Ciprofloxacin | 33.3 | 30 | 60 | 34.7 |
| Levofloxacin | 41.7 | 50 | 20 | 42.9 |
| Moxifloxacin | 33.3 | 20 | – | 24.5 |
| Penicillins | ||||
| Piperacillin | 91.7 | 85 | 40 | 83.7 |
| Penicillins and B inhibitors | ||||
| Ticarcillin/Clavulanic acid | 20.8 | 0 | 0 | 10.2 |
| Piperacillin/Tazobactam | 50 | 40 | 60 | 46.9 |
| Ampicillin/Sulbactam | 66.7 | 60 | 40 | 61.2 |
| Tetracyclines | ||||
| Tetracycline | 29.2 | 45 | – | 32.7 |
| Minocycline | 16.7 | 30 | 20 | 22.4 |
| Tigecycline | 12.5 | 0 | 0 | 6.1 |
| Nitrofurantoin | ||||
| Nitrofurantoin | †– | 5 | 40 | 6.1 |
| Type of resistance (%) | ||||
| MDR | 50 | 70 | 60 | 59.2 |
| ESBL | 45.8 | 20 | 20 | 32.7 |
The gram negative bacterial isolates’ resistance to antibiotics were 87.8% to cefepime, 83.7% to piperacillin, 75.5% to ceftriaxone, 61.2% to ampicillin/sulbactam, 51% to meropenem, 42.9% to levofloxacin, 32.7% to gentamycin, and 32.7% to tetracycline (Table 4).
Table 4.
Antimicrobial susceptibility profiles of gram-negative bacterial isolates.
| ∗S | ∗I | ∗R | ||||
| Antimicrobial agent | Count | % | Count | % | Count | % |
| Amikacin | 7 | 14.3 | 0 | 0 | 11 | 22.4 |
| Gentamycin | 8 | 16.3 | 0 | 0 | 16 | 32.7 |
| Tobramycin | 7 | 14.3 | 3 | 6.1 | 8 | 16.3 |
| Imipenem | 16 | 32.7 | 1 | 2 | 24 | 49 |
| Meropenem | 15 | 30.6 | 0 | 0 | 25 | 51 |
| Ceftazidime | 4 | 8.2 | 0 | 0 | 21 | 42.9 |
| Cefepem | 6 | 12.2 | 0 | 0 | 43 | 87.8 |
| Ceftriaxone | 0 | 0 | 0 | 0 | 37 | 75.5 |
| Cefixime | 0 | 0 | 0 | 0 | 30 | 61.2 |
| Piperacillin | 4 | 8.2 | 1 | 2 | 41 | 83.7 |
| Ciprofloxacin | 8 | 16.3 | 0 | 0 | 17 | 34.7 |
| Levofloxacin | 8 | 16.3 | 1 | 2 | 21 | 42.9 |
| Moxifloxacin | 7 | 14.3 | 1 | 2 | 12 | 24.5 |
| Ticarcillin/Clavulanic Acid | 4 | 8.2 | 1 | 2 | 5 | 10.2 |
| Piperacillin/tazobactam | 6 | 12.2 | 0 | 0 | 23 | 46.9 |
| Ampicillin/Sulbactam | 1 | 2 | 0 | 0 | 30 | 61.2 |
| Tetracycline | 3 | 6.1 | 0 | 0 | 16 | 32.7 |
| Minocycline | 10 | 20.4 | 1 | 2 | 11 | 22.4 |
| Tigecycline | 21 | 42.9 | 1 | 2 | 3 | 6.1 |
| Nitrofurantoin | 0 | 0 | 0 | 0 | 3 | 6.1 |
4. Discussion
To our knowledge there are no reports on DA-HAIs in PICUs in Jordan, in particular, and in the developing world, in general. This study is an attempt at reducing the incidence of HCAIs. It was conducted at a single unit that is the main referral tertiary care center for the north of Jordan. Further national studies could cover the rest of the country. In addition, this study addressed only bacterial and fungal device associated infections, viral respiratory related infections was not included, considering that 14–22% of HAIs are caused by virtual agents.[12]
The median incidence associated with central line invasion and mechanical ventilator were similar to that in developed countries.[8,13,14] In contrast, urinary catheter usage was particularly lower than that reported in other studies.[8,15]
Of interest is the finding that the most common DA-HAIs in this study were CLABSI. This is in contrast to other developing countries where VAP is the most common one. The results of this study matched those of high-income countries.[16]
In this study, the most common causes associated with central line usage were A baumanii. This is in contrast with other studies from Asia and some developed countries in which P aeruginosa and gram positive bacteria were the most frequent.[17,18] In VAP, Acinetobacter baumannii occurred in 65%, followed by K pneumoniae in 15%, P aeruginosa in 10%, S maltophilia in 5%, and E coli in 5%. Similar findings were reported by a study from the north of India.[19] Otherwise, P aeruginosa and Staphylococcus aureus occurred at different rates in different PICUs.[20,21]
The main isolated CAUTI causative agent was also A baumannii. In 80% of PICUs studies, the causative organisms were reported as being gram negative,[22] but an Indian study showed that Candida was the commonest one.[23] The results of this study are compatible with the WHO global priority pathogens catalog of bacteria grouped under priority tiers according to their antibiotic resistance.[24]
In this study, only gram negative bacteria were isolated. This may be related to 10 years of past infection prevention and control practices that focused on gram positive bacteria prevention.[25]
Infections caused by antibiotic-resistant organisms are difficult to treat. In some cases, antibiotic-resistant infections require longer hospital stays, additional follow-up, and extended alternatives. In this study over 80% of the isolates were antibiotic resistant. This high percentage contributed to higher empirical therapy failure, morbidity, and mortality.
Resistant infections cannot be completely avoided, but can be reduced by the application of antibiotic stewardship, providing awareness workshops for healthcare providers, strict rules over antibiotic prescriptions, professional aseptic techniques regarding medical device sterilization and disinfection, reduction in the need for invasive procedures, and applying correct hand hygiene protocols.
This study has some limitations. First, this is a single-center study on a relatively small sample of patients, which limits the generalizability of results. However, there are no reports on DA-HAIs in PICUs in Jordan and the results of this study represent an important first step toward documenting the rates of DA-HAIs in PICUs nationally. Second, although this study reveals the most commonly isolated bacterial species, further studies are needed to document the contribution of viral agents. In spite of these limitations, this study profiles the most common etiological agents and potential risk factors associated with the use of invasive devices.
In conclusion, microbial isolates are commonly associated with healthcare device insertions in pediatric intensive care unit, invasive bacterial infections associated with critical morbidity and mortality. Further studies on device associated infections are recommended for regional profiling purposes.
Author contributions
Conceptualization: Ziad Elnasser, Haneen Obeidat.
Data curation: Haneen Obeidat.
Formal analysis: Haneen Obeidat.
Funding acquisition: Ziad Elnasser.
Investigation: Haneen Obeidat.
Methodology: Haneen Obeidat.
Project administration: Ziad Elnasser, Haneen Obeidat.
Resources: Haneen Obeidat.
Software: Haneen Obeidat.
Supervision: Ziad Elnasser.
Validation: Ziad Elnasser, Haneen Obeidat, Zouhair Amarin.
Visualization: Ziad Elnasser, Haneen Obeidat.
Writing – original draft: Haneen Obeidat.
Writing – review & editing: Ziad Elnasser, Haneen Obeidat, Zouhair Amarin.
Footnotes
Abbreviations: CAUTI = catheter-associated urinary tract infection, CDC/NHSN = Centers for Disease Control and Prevention/National Healthcare Safety Network, CLABSI = Central-Line-Associated Bloodstream Infection, DA-HAIs = device-associated healthcare-associated infections, DAIs = device associated infections, ESBL = extended spectrum beta lactamase, HAIs = healthcare associated infections, ICU = intensive care unit, JUST = Jordan University of Science and Technology, MDR = multidrug resistance, PICUs = pediatric intensive care units, VAP = ventilator associated pneumonia.
How to cite this article: Elnasser Z, Obeidat H, Amarin Z. Device-related infections in a pediatric intensive care unit: the Jordan University of Science and Technology experience. Medicine. 2021;100:43(e27651).
This research is fully funded by the Jordan University of Science and Technology Faculty of Research higher council grant number 20190402.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Others include autoimmune hemolytic anemia, lower limb ischemia, hydrops fetalis, hypotonia, familial intrahepatic cholestasis, hydatid cyst, laryngomalacia, down syndrome, cystic fibrosis.
CVC = central venous catheter, ETT = endotracheal tube, FC = Foley catheter, HLOS = hospital length of stay, PICU = pediatric intensive care unit.
Device utilization ratio calculated by dividing device days (Nominator) over patient days (Dominator).
CLABSI: central line associated infection, VAP: ventilator associated pneumonia, CAUTI: catheter associated urinary tract infection.
Rate in percentile.
CLABSI: central line associated infection, VAP: ventilator associated pneumonia, CAUTI: catheter associated urinary tract infection.
Not tested.
Susceptible (S), intermediate (I), resistance (R).
References
- [1].van der Kooi T, Lepape A, Astagneau P, et al. HAI-Net Mortality review study group. Mortality review as a tool to assess the contribution of healthcare-associated infections to death: results of a multicentre validity and reproducibility study, 11 European Union countries, 2017 to 2018. Eurosurveillance 2021;26:2000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wattal C, Oberoi JK. Infections in pediatric intensive care units (PICU). Indian J Pediatr 2012;79:647–9. [DOI] [PubMed] [Google Scholar]
- [3].Atici S, Soysal A, Kadayifci EK, et al. Healthcare-associated infections in a newly opened pediatric intensive care unit in Turkey: results of four-year surveillance. J Infect Dev Ctries 2016;10:254–9. [DOI] [PubMed] [Google Scholar]
- [4].Dudeck MA, Weiner LM, Allen-Bridson K, et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, device-associated module. Am J Infect Control 2013;41:1148–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pettemerides Y, Ghobrial S, Raftopoulos V, Iordanou S. Incidence rate of device-associated, hospital acquired infections in ICUs: a systematic review developed versus developing economies. Int J Caring Sci 2018;11:1931–41. [Google Scholar]
- [6].Rosenthal VD, Jarvis WR, Jamulitrat S, et al. Socioeconomic impact on device-associated infections in pediatric intensive care units of 16 limited-resource countries: International Nosocomial Infection Control Consortium findings. Pediatr Crit Care Med 2012;13:399–406. [DOI] [PubMed] [Google Scholar]
- [7].Becerra MR, Tantaleán JA, Suárez VJ, Alvarado MC, Candela JL, Urcia FC. Epidemiologic surveillance of nosocomial infections in a Pediatric Intensive Care Unit of a developing country. BMC Pediatr 2010;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hatachi T, Tachibana K, Takeuchi M. Incidences and influences of device-associated healthcare-associated infections in a pediatric intensive care unit in Japan: a retrospective surveillance study. J Intensive Care 2015;3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. [DOI] [PubMed] [Google Scholar]
- [10].Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. An Informational Supplement for Global Application Developed through the Clinical and Laboratory Standards Institute, Vol. 33. 2011. [Google Scholar]
- [11].Caston-Gaa A, Curless MS, Gardner J, Gavin MA, Lawson P. Reference Manual for Health Care Facilities with Limited Resources Infection Prevention and Control; 2018. Available at: www.jhpiego.org. [Google Scholar]
- [12].Harris J-AS. Pediatric nosocomial infections: children are not little adults. Infect Control Hosp Epidemiol 1997;18:739–42. [DOI] [PubMed] [Google Scholar]
- [13].Kendirli T, Yaman A, Ödek Ç, et al. Central line-associated bloodstream infections in pediatric intensive care unit. Turkish J Pediatr Emerg Intensive Care Med 2017;4:42–6. [Google Scholar]
- [14].Chomton M, Brossier D, Sauthier M, et al. Ventilator-associated pneumonia and events in pediatric intensive care: a single center study. Pediatr Crit Care Med 2018;19:1106–13. [DOI] [PubMed] [Google Scholar]
- [15].Samraj R, Stalets E, Butcher J, et al. The Impact of catheter-associated Urinary Tract Infection (CA-UTI) in critically ill children in the pediatric intensive care unit. J Pediatr Intensive Care 2015;05:007–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].World Health Organisation. Report on the burden of endemic health care-associated infection worldwide: clean care is safer care. World Heal Organ 2011;01–40. https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf?sequence=1 [Google Scholar]
- [17].Tseng SH, Lee CM, Lin TY, et al. Combating antimicrobial resistance: antimicrobial stewardship program in Taiwan. J Microbiol Immunol Infect 2012;45:79–89. [DOI] [PubMed] [Google Scholar]
- [18].Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2009–2010. Infect Control Hosp Epidemiol 2013;34:01–14. [DOI] [PubMed] [Google Scholar]
- [19].Vijay G, Mandal A, Sankar J, Kapil A, Lodha R, Kabra SK. Ventilator associated pneumonia in pediatric intensive care unit: incidence, risk factors and etiological agents. Indian J Pediatr 2018;85:861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Manjhi M, Das S, Pal M, Saha I, Srinath AR. Incidence, risk factors, clinico-microbiological profile, change in ventilator settings needed and outcome of 135 ventilator associated pneumonia cases in pediatric intensive care unit (PICU) of a tertiary care centre in Eastern India. J Pediatr Neonatal Individ Med 2018;7:e070122. [Google Scholar]
- [21].Porto JP, Mantese OC, Arantes A, Freitas C, Filho PPG, Ribas RM. Nosocomial infections in a pediatric intensive care unit of a developing country: NHSN surveillance. Rev Soc Bras Med Trop 2012;45:475–9. [DOI] [PubMed] [Google Scholar]
- [22].Stockwell JA. Nosocomial infections in the pediatric intensive care unit: affecting the impact on safety and outcome. Pediatr Crit Care Med 2007;8: (2 suppl): S21–37. [DOI] [PubMed] [Google Scholar]
- [23].Brindha SM, Jayashree M, Singhi S, Taneja N. Study of nosocomial urinary tract infections in a pediatric intensive care unit. J Trop Pediatr 2011;57:357–62. [DOI] [PubMed] [Google Scholar]
- [24].World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug Resistant Bacterial Infections, Including Tuberculosis; 2017. doi:WHO reference number: WHO/EMP/IAU/2017.12. [Google Scholar]
- [25].Macvane SH. Antimicrobial resistance in the intensive care unit. J Intensive Care Med 2017;32:25–37. [DOI] [PubMed] [Google Scholar]