Abstract
Background:
We introduce an innovative and novel technology that achieves scarless skin removal without the use of thermal energy. Microcoring technology (MCT) uses a modified, hollow hypodermic needle to remove skin safely and without a scar. This method is advantageous compared to other fractional devices, given that it has the same benefits as energy-based devices (removal of skin cores without a scar), with the added value of immediate closure along the relaxed skin tension lines, with significantly less thermal energy.
Methods:
Three prospective clinical safety trials analyzing MCT treatment on abdominal and facial skin (short- and long-term) are described.
Results:
MCT treatment of human skin resulted in scarless skin removal that was well tolerated by patients. Healing occurred rapidly, with limited side effects. Skin area reduction (skin tightening) and increase in skin thickness were observed long term.
Conclusions:
MCT treatment of human skin is safe and well tolerated. Although further studies on efficacy are required to evaluate the full potential of MCT in skin rejuvenation, early findings such as skin tightening and increase in skin thickness are encouraging.
Takeaways
Question: Is microcoring technology for scarless skin removal safe?
Findings: MCT treatment of human skin results in scarless skin removal that is well tolerated by patients. Healing occurs rapidly with limited side effects. Although further studies on efficacy are required to evaluate the full potential of MCT in skin rejuvenation, early findings such as skin tightening and increase in skin thickness are encouraging.
Meaning: MCT for scarless skin removal is safe and early data suggests skin tightening, as well as increase in skin thickness.
INTRODUCTION
Scarless skin removal has been a major focus of research, with broad clinical application in aesthetic (wrinkle removal, skin tightening, skin rejuvenation, acne) and reconstructive (scar prevention, scar removal, biopsies) surgery, as well as dermatology.1 Several widely used technologies such as fractional laser, radiofrequency ablation, and microneedling have taken advantage of the ability of the skin to heal and rejuvenate after minor trauma with reasonable safety and efficacy profile.2–4
Despite many advantages, energy-based devices such as fractional laser and radiofrequency ablation lead to epidermal and dermal cell necrosis from thermal injury that inhibits immediate wound closure. Histologic analyses have shown that after treatment with fractional laser, the resultant defect fills with microepidermal necrotic debris (MEND) that is remodeled over time.2 Although fractional lasers and radiofrequency devices demonstrate excellent results in rejuvenation of skin, data on skin tightening is inconclusive.5,6 We suspect that MENDs prevent early closure of microcores and therefore limit reduction of skin surface area and skin tightening. Further, nonenergy-based devices using microneedles have many benefits, including limited side effects and fast patient recovery.4 However, without removal of tissue, significant skin tightening is challenging to achieve and has not been proven in the current literature.7
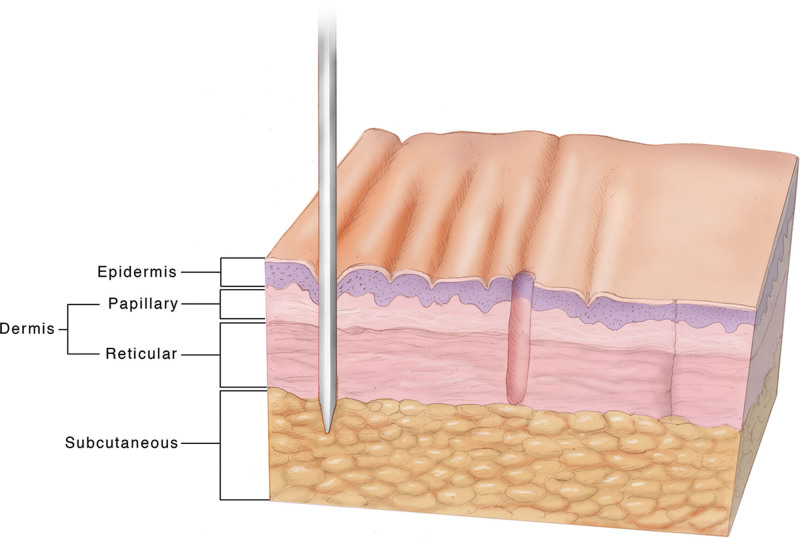
Here, we introduce an innovative and novel technology that achieves scarless skin removal without the use of thermal energy. Microcoring technology (MCT) uses a modified, hollow hypodermic needle to remove skin safely and without scar (see Fig. 1). This method is advantageous when compared with other fractional devices, given that it has the same benefits as laser-based devices (removal of skin cores without scar), with the added value of immediate closure along the relaxed skin tension lines (RSTLs), with significantly less thermal energy.
Fig. 1.
Illustration of microcoring technology. From left to right, the needle is inserted into the skin, removing a microcore of tissue that heals without scarring.
MCT has been evaluated in preclinical animal studies that revealed an excellent safety and efficacy profile.8,9 Studies performed in porcine skin demonstrated that wound healing in the treatment areas was achieved after 1 week and erythema was completely resolved at 2 weeks with no evidence of infection or scarring over a 3-month period.8 At 1 month, a significant increase in epidermal and papillary dermal thickness was seen. Further, collagen content increased by 89% at 3 months.8 Both skin thickening and increased collagen content indicate histologic endpoints of successful skin rejuvenation. In addition, porcine skin treated at 10% density with a 19 Gauge (G) coring needle exhibited a reduction in skin surface area by 9% when compared with 3% in control areas treated with standard hypodermic needles with no tissue removal (P < 0.01). This finding confirms skin tightening after MCT.9
Based on these encouraging findings, we conducted three clinical trials to evaluate the safety of MCT treatment in human skin. The primary goal of all studies was to determine safe MCT treatment parameters, evaluate the healing profile of human skin after MCT, and analyze technical device safety. In addition, limited efficacy variables were assessed. The findings of all three trials are summarized in this study.
METHODS
All three clinical safety trials were approved by the New England Institutional Review Board as nonsignificant risk medical device studies. All subjects signed informed consent adhering to the guidelines outlined by the International Conference on Harmonization Good Clinical Practice. No subjects were lost to follow-up.
Inclusion criteria for all studies included age over 18, Fitzpatrick skin type I-VI, and ability to provide informed consent. The initial trials were completed in White patients given that the Fitzpatrick skin type is amenable to this new technology. Specific inclusion criteria pertinent to each study are outlined below. Exclusion criteria for all studies included silicone, fat, collagen or synthetic material in the treatment area, skin rash in the treatment area, history of keloid formation, history of hypertrophic scarring, history of bleeding disorder or active use of anticoagulation, history of trauma or surgery to the treatment area, scarring in the treatment area, active/chronic or recurrent infection, active smoking status or history of smoking in the 3 months before treatment, compromised immune system, hypersensitivity to analgesic agents, pregnancy or breast feeding, untreated drug and alcohol abuse, any comorbid condition that could limit ability to participate in the study or to comply with follow-up requirements, and treatment with an investigational device or agent within 30 days of treatment. Additional exclusion criteria for the facial skin trials included evidence of malignancy/actinic keratosis/melasma in the treatment area, history of treatment with dermabrasion in the area in the past 12 months, history of injection with Botulinum Toxin in the past 6 months, excessive sun exposure within 30 days before treatment, and treatment with fish oil in the 14 days before treatment.
Subjects received a one-time dose of cefalexin 500 mg before treatment. No antiviral prophylaxis was prescribed, and subjects were not given prophylactic antibiotic or antiviral therapy after treatment. Topical anesthesia with lidocaine/prilocaine cream was provided 30 minutes before the procedures. No dressing was applied to the treated areas. The procedure was performed using a hand-held single needle with one punch. The depth of the needle was manually controllable.
Safety parameters were evaluated for all three clinical trials at all timepoints and consisted of subject reported pain (on a scale of 0–10), bleeding (none, trace, mild, moderate, severe), healing profile (presence of ecchymosis, purpura, fluid accumulation, hyperpigmentation, hypopigmentation, roughness, dryness, inflammation, erythema, crusting on a scale of 0—absent, 1—trace, 2—mild, 3—moderate to 4—severe), scarring (yes/no), and adverse events.
Abdominal Skin Trial
To evaluate MCT for scarless skin removal in human skin, we designed a prospective, randomized controlled in-human feasibility trial that was conducted between October 2013 and October 2014. Five subjects scheduled to undergo abdominoplasty surgery 90 days after enrollment in the study were treated with MCT in the area to be removed during the abdominoplasty operation. The 1 × 1 cm MCT treatment areas and untreated control areas were marked by permanent tattoo before the MCT intervention. Subjects were randomized to MCT needle gauge (G) with diameters ranging from 19 to 24G and treatment density between 10 and 20% of the marked skin surface area.
As an additional safety variable, two subjects underwent tissue biopsy on day 90 to confirm absence of scarring on histology. All safety endpoints were evaluated per treatment area on day 0 and days 1, 7, 30, 60, and 90 post treatment and compared with the untreated control areas.
To determine a possible skin rejuvenation effect, skin thickness was evaluated with DermaLab Combo on day 90 and compared with the untreated control areas.10 Descriptive statistics and T tests were used for analysis.
Short-term Facial Skin Trial
To determine whether MCT use is safe in the face, a 30 day prospective randomized controlled single-blind clinical trial for use of MCT in the preauricular area was designed and conducted between March 2014 and March 2015. Nine subjects were randomized to MCT treatment in a 2 × 1 cm area of skin in the preauricular area 30 days before excision during facelift surgery. One subject was screened but did not undergo treatment. The MCT treatment area and untreated control areas were marked by permanent tattoo before the intervention. Treatment density was fixed at 10% of the treatment area, whereas needle gauge was randomized to either 22 or 24G.
Based on findings in the abdominal skin trial, erythema and melanin content were evaluated with optical reflectometry as an additional safety variable. All safety endpoints were evaluated per treatment area on day 0 after treatment and days 1, 7, 15, and 30, and compared with the untreated control areas.
Efficacy outcomes included change in skin thickness (DermaLab Combo), and reduction of skin surface area (measured by analysis of the skin surface area between tattooed points via stereo photogrammetry on three-dimensional images obtained with the Canfield Vectra H1 handheld camera). Efficacy endpoints were analyzed at 30 days. Descriptive statistics and paired T tests were performed for analysis.
Long-term Facial Skin Trial
To determine whether MCT treatment is safe long-term, a 90-day prospective single-blind, randomized bilateral paired comparison study in human preauricular facial skin was designed and conducted between October 2015 and October 2016. A total of 15 patients (30 treatment sites) were randomized to MCT treatment in a 2 × 1 cm area of skin in the preauricular area that was not removed surgically. Treatment parameters were randomized to each treatment area, with needle gauge ranging between 22, 24, and 25G and treatment density of either 2.5%, 5%, 7.5%, or 10%.
As an additional safety variable, scarring was evaluated using the Manchester Scar Scale. Safety outcome variables were evaluated at every timepoint. All efficacy endpoints were evaluated at 30 and 90 days.
Efficacy outcome variables were overall aesthetic improvement was measured by subject and investigator reported global aesthetic improvement scale (3 = very much improved; 2 = much improved; 1 = improved; 0 = no change; −1 = worse; −2 = much worse; −3 = very much worse). Study visits were conducted on days 0, 1, 4, 7, 15, 30, 90. Descriptive statistics and paired T tests were performed for analysis.
RESULTS
Demographics
All subjects were White. In the abdominal skin trial, five female subjects with Fitzpatrick skin type 1–3 and a mean age of 46 (±11 years with a minimum age of 34, and maximum age of 58) were included. The short-term facial skin trial included seven female and two male subjects with Fitzpatrick skin type 1–3. Average age was 64.5 (±3.6) with a minimum age of 58 and a maximum age of 71. Eight female and seven male subjects with Fitzpatrick skin type 1–5 were enrolled in the long-term facial skin trial. Mean patient age was 56.2 ± 6 years with a minimum age of 44 and maximum age of 64.
Technical Device Safety
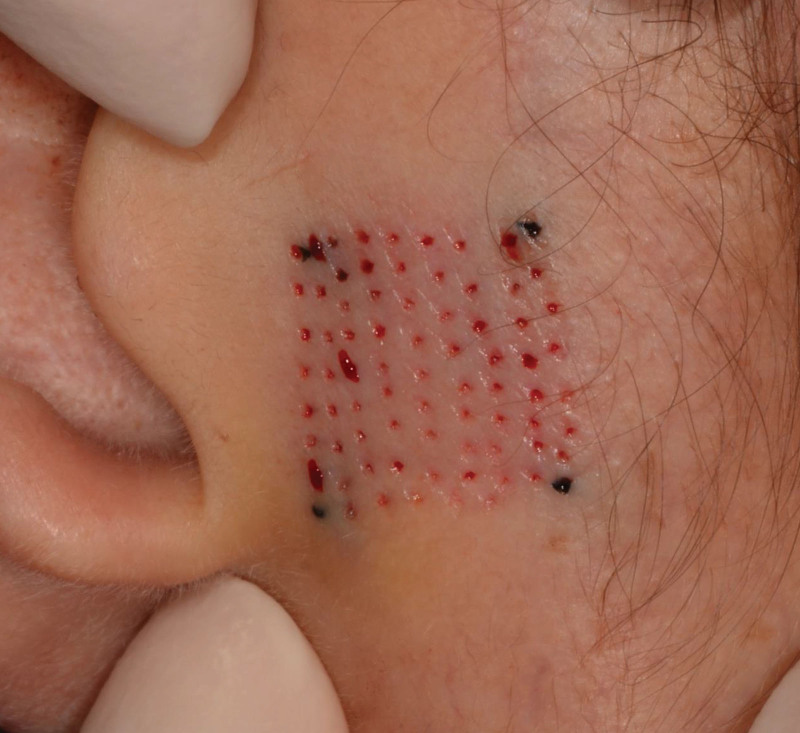
For all studies, the device operated as clinically intended and patterns of microexcisions were generated in abdominal skin, as well as facial skin with needles of 19–24G (abdomen) and 22–25G (face). No device safety events were reported in any of the three clinical trials. See Figure 2 for an example of an MCT treatment area immediately after microcore removal.
Fig. 2.
Immediate result after MCT treatment. Microcores of skin have been removed, resulting in microscopic circular wounds (This picture represents an example of MCT treatment that was performed outside the three clinical trials that are presented in this article. Treatments of facial skin in the short- and long-term facial skin trials were performed in a 2 × 1 cm rectangular treatment area (preauricular), as can be seen in Figure 3).
Clinical Safety Profile
Adverse Events and Serious Adverse Events
In the short-term facial skin trial, one subject developed a superficial wound infection in the preauricular area 21 days after treatment that resolved without intervention. No other adverse events or serious adverse events were reported in any of the three clinical trials.
Pain
Across all treatment areas on abdominal skin, the average pain during treatment was 2.8 ± 1.1 on a scale of 0–10. Pain decreased to 0.4 ± 0.9 on day 1 and 7, and to 0 on all subsequent visits. Average pain in the short-term facial skin trial was 0.4 ± 1 during treatment and 0.4 ± 1.3 on d1, 1 ± 2 on d7 and 0.2 ± 0.7 on day 30. In the long-term facial skin trial, pain during treatment was reduced to 0 ± 0. Mean postprocedure pain was 0.6 ± 0.92 on d1, 0.4 ± 0.8 on d3, 0.07 ± 0.37 on d7, 0.2 ± 0.6 on d15, and 0 ± 0 starting d30 and on all visits thereafter.
Bleeding
Bleeding during treatment of the abdominal skin was trace in two subjects and mild in three subjects. During the short-term facial skin trial, seven subjects experienced mild bleeding during treatment, and two subjects had moderate bleeding. Analysis of the long-term facial skin trial data revealed no bleeding at three (10%), trace at 23 (77%), and moderate at four (13%) treatment sites.
Healing Profile (see Table 1)
Table 1.
Healing Profile
| Abdominal Skin Trial | Ecchymosis | Edema | Crusting | Redness | Roughness | Dryness | Inflammation Hyperpigmentation | Hypopigmentation | |
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | 0.0 | 1.1 | 0.0 | 0.7 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 |
| 1 | 0.1 | 0.4 | 0.0 | 1.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.0 |
| 7 | 0.1 | 0.0 | 0.7 | 0.9 | 1.2 | 0.8 | 0.2 | 0.1 | 0.0 |
| 30 | 0.0 | 0.0 | 0.0 | 2.0 | 0.5 | 0.0 | 0.2 | 0.8 | 0.0 |
| 60 | 0.0 | 0.0 | 0.0 | 1.4 | 0.0 | 0.0 | 0.0 | 0.7 | 0.2 |
| 90 | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 |
| Short-term Facial Skin Trial | |||||||||
| Day 0 | 0.8 | 1.4 | 0.0 | 1.8 | 0.0 | 0.0 | 1.9 | 0.0 | 0.0 |
| 1 | 0.6 | 1.2 | 0.0 | 1.9 | 0.7 | 0.3 | 1.6 | 0.0 | 0.0 |
| 7 | 0.1 | 0.1 | 0.8 | 1.1 | 1.0 | 1.3 | 0.2 | 0.0 | 0.2 |
| 15 | 0.1 | 0.0 | 0.2 | 1.4 | 0.2 | 0.7 | 0.4 | 0.4 | 0.0 |
| 30 | 0.0 | 0.2 | 0.0 | 1.3 | 0.0 | 0.1 | 0.3 | 0.9 | 0.0 |
| Long-term Facial Skin Trial | |||||||||
| Day 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1 | 0.6 | 0.3 | 0.3 | 0.8 | 0.4 | 0.1 | 0.1 | 0.0 | 0.0 |
| 4 | 0.6 | 0.3 | 0.1 | 0.8 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 7 | 0.0 | 0.0 | 0.0 | 0.4 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 |
| 15 | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 30 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 |
| 90 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Average score [on a scale of 0 (absent), 1 (trace), 2 (mild), 3 (moderate) to 4 (severe)] across all subjects per time point.
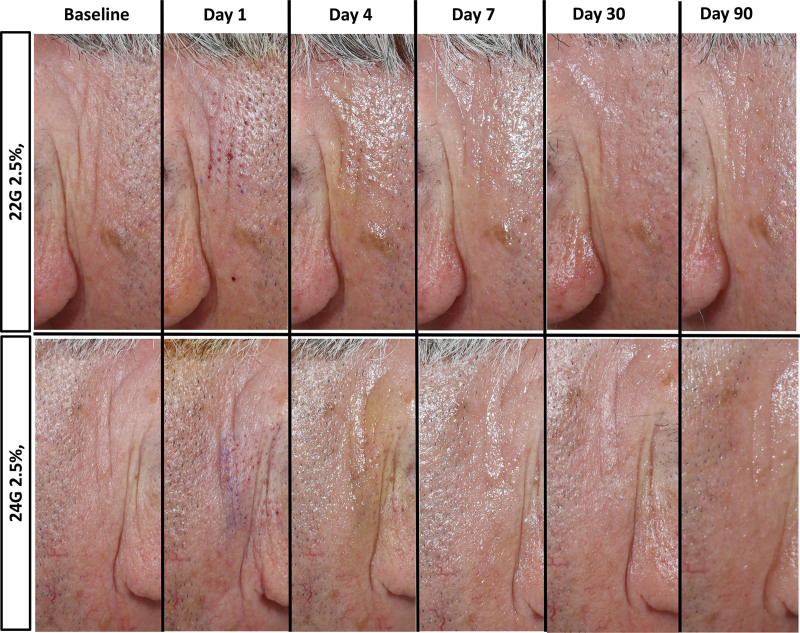
MCT-treated skin healed with no scarring (see Fig. 3). During the abdominal skin trial, trace to mild treatment side effects such as ecchymosis, edema, crusting, roughness, dryness, and inflammation were seen up to day 30. Trace to mild redness was present from day 1 to day 90. Trace hyperpigmentation was seen on days 7–90.
Fig. 3.
Wound healing profile after MCT treatment. Most treatment side effects resolved by day 7. Also note clinical improvement in rhytides on day 90.
The short-term facial skin clinical trial showed trace ecchymosis, crusting, and roughness up to day 15. Trace edema, redness, dryness, inflammation, and hyperpigmentation were present up to day 30.
During the long-term facial skin trial, trace side effects (roughness, dryness, inflammation) were noted up to day 7. Trace redness was seen on days 1–15 and was absent on day 30 and subsequent visits. Trace hyperpigmentation was seen on day 30 that resolved by day 90.
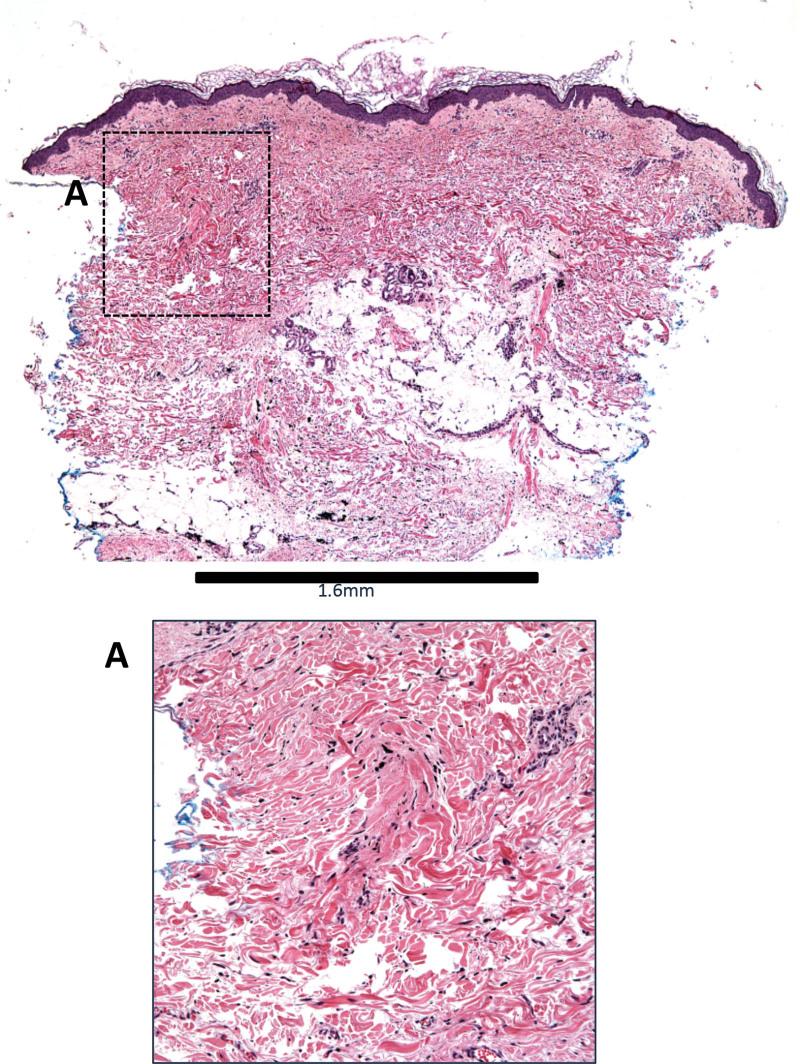
Additional healing parameters included histologic evaluation of biopsies obtained in two patients during the abdominal skin trial at the day 90 visit that confirmed absence of scarring at 10% treatment density (see Fig. 4). Further, the erythema index that calculated as part of the short-term facial skin trial showed no significant difference between treatment (22.7 ± 7.1) and control (25 ± 6.2) groups on day 30 (P = 0.16). In addition, there was no significant difference in melanin index between groups (treatment 40.3 ± 5.7 versus control 43.6 ± 5.3; P = 0.12). In the long-term facial skin trial, Manchester Scar Scale evaluation revealed no scar in any treatment area (n = 30) at needle gauge 22–25 and 2.5%–10% treatment density in the preauricular area.
Fig. 4.
Histologic analysis. At 90 days after treatment, biopsies show no evidence of scarring in treated areas.
Clinical Efficacy Profile
Skin Thickness
Both abdominal and facial skin thickness increased after MCT treatment (see Table 2). Analysis of the abdominal skin treatment sites revealed a significant increase in skin thickness in treated areas of the abdomen when compared with control areas from baseline to 90 days post treatment (P < 0.01). An increase in skin thickness could also be seen for treated facial skin when compared with control (P < 0.05).
Table 2.
Skin Thickness
| Pretreatment Skin Thickness | SD | Posttreatment Skin Thickness | SD | P | |
|---|---|---|---|---|---|
| Abdominal skin trial | |||||
| Treatment areas | 1497 | 268 | 1642 | 234 | <0.01 |
| Control areas | 1562 | 231 | 1577 | 178 | >0.05 |
| Short-term facial skin trial | |||||
| Treatment areas | 1856 | 193 | 2097 | 170 | <0.05 |
| Control areas | 1795 | 160 | 1914 | 186 | >0.05 |
Mean skin thickness (in μm) at 10% density measured with DermaLab Combo pre MCT treatment in abdominal skin on posttreatment day 90 and in facial skin on posttreatment day 30.
Bolded values are significant P values.
Skin Surface Area Reduction
In the short-term facial skin trial, skin surface area reduction at 10% treatment density with needle gauge 22 and 24 was on average −9.4% ± 4.3% (−13.9 ± 7 mm2), which was significant when compared with baseline and control (P < 0.01).
Aesthetic Improvement
Analysis of the long-term facial skin trial data revealed that the mean subject global aesthetic improvement scale score was 2.9 ± 0.6 and mean investigator global aesthetic improvement scale was 2.8 ± 0.5 at 90 days (very much improved). There was a visible reduction of rhytids on clinical examination (see Fig. 3).
DISCUSSION
MCT uses hollow needles to remove skin without formation of scar tissue. This article summarizes the first experience with MCT treatment in human skin by analyzing the results of three prospective clinical safety trials. We demonstrate that MCT treatment of abdominal and facial skin is well tolerated with minimal pain and bleeding during treatment. It is safe with excellent healing profile and shows signs of skin rejuvenation such as increase in abdominal and facial skin thickness, skin surface area reduction (skin tightening), and global aesthetic improvement.
MCT treatment was well tolerated with only mild pain during and after the procedure. Minimal pain was reported during treatment of the abdomen (2.8 ± 1.1) and facial skin (0 ± 0 to 0.4 ± 1.3). The pain profile improved as coring techniques were refined from the abdominal skin trial to the facial skin trials. A recent review demonstrated that patients undergoing microneedling procedures experienced on average 0.2–3.8 out of 10 pain, which is slightly higher than in our MCT cohort.11 Therefore, pain levels during MCT treatment are comparable or lower than pain reported with microneedling. In general, microneedling interventions are very well tolerated by patients, and therefore the MCT pain profile appears to be similarly favorable.12
Further, transient and self-limited bleeding was observed during treatment that was well tolerated by patients and did not require additional hemostasis. Similar to microneedling, pinpoint bleeding is the endpoint of MCT treatment, and the amount of bleeding that was seen in all three clinical trials (trace to moderate) was within the expected range.
MCT-treated skin demonstrated a favorable healing profile with no signs of clinical or histologic scarring. This finding confirms that scarless skin removal with MCT is possible not only in porcine, but also in human skin. Expected treatment side effects were observed with trace to mild severity across all clinical trials. The side effect profile improved with refined treatment technique. During the final long-term facial skin trial with perfected coring technique, trace side effects such as ecchymosis and edema were present up to day seven with only trace redness persistent until day fifteen and one instance of trace hyperpigmentation on day 30 that resolved by day 90. With fractional CO2 laser resurfacing, a similar short-term healing profile can be observed with most patients experiencing side effects for around 14 days.13,14 However, long-term side effects such as hyper- and hypopigmentation seem less common with MCT in this small cohort of patients.15 Further, the most common severe complications seen with fractional laser treatment such as scarring, herpes simplex virus outbreaks, and acneiform eruptions were not seen in MCT-treated areas although no herpes or antibiotic prophylaxis was subscribed.16 Future studies in a larger patient cohort are required to further validate these findings.
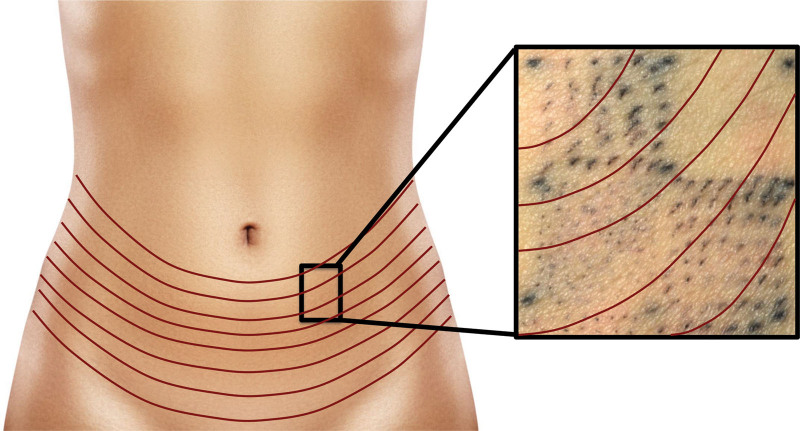
Interestingly, we noted that healing after MCT treatment occurred predominantly along the prevailing RSTLs. After MCT treatment of abdominal skin, the tattooed round cores appeared elliptical along the RTLs at 90 days post treatment (Fig. 5). RSTLs are furrows that are created when the skin is relaxed in the absence of tension. Therefore, surgical incisions are ideally oriented along the RSTLs, as it is well known that wounds heal most inconspicuously under no tension.17 The observation that MCT cores heal along the RTLSs is very encouraging, as this means ideal and aesthetic wound healing occurs.
Fig. 5.
Closure along the RSTLs. Black pigment was used to mark circular cores that were removed at treatment. After 90 days, the tattoo marks appeared as slits along the RSTL, indicating natural closure along the relaxed tension lines with no visible scarring.
Although the primary focus of all three clinical trials was evaluation of safety, notable signs of skin rejuvenation were observed that will be the subject of further studies. Preliminary findings include a significant increase in skin thickness in MCT-treated areas when compared with control in both abdominal and facial skin. One of the main characteristics of aging skin is decreased collagen production, which leads to thinning of the epidermis and dermis.18 Increase in skin thickness suggests an increase in collagen production and reversal of aging effects.19
Further, the average reduction of the facial skin surface area was −9.4% ± 4.3 at 10% treatment density on post procedure day 30, which was significant when compared with baseline and control (P < 0.01). During treatment with fractional devices that use thermal energy, MEND fills the treatment area immediately and is remodeled over time.2 MEND seems to inhibit closure of microwounds. With MCT, closure along the RSTLs occurred within 24 hours without visible interposition of debris. Although these are early results, we hypothesize that absence of MENDS allows for effective reduction in skin surface area and skin tightening.
Finally, both subjects and blinded investigators felt that the overall aesthetic improvement of the MCT treatment areas in the long-term facial skin trial was very much improved, indicating a positive effect overall.
In summary, MCT is an innovative new approach to scarless skin removal that has been shown to be safe for the treatment of abdominal and facial skin. Discomfort during MCT treatment is comparable to microneedling, which is very well tolerated by patients. Further, the healing profile is favorable with only transient trace to mild side effects. Early efficacy results demonstrate promising signs of skin rejuvenation, such as skin tightening and increase in skin thickness after one MCT treatment. We expect increasing efficacy with multiple MCT treatments, which will be the subject of future studies.
CONCLUSIONS
MCT treatment of human skin results in scarless skin removal that is well tolerated by patients. Healing occurs rapidly with limited side effects. Although further studies on MCT efficacy are required to evaluate the full potential of MCT in skin rejuvenation, early findings such as skin tightening and increase in skin thickness are encouraging.
Footnotes
Published online 29 October 2021.
Disclosure: All authors act as consultants for Cytrellis.
References
- 1.Walmsley GG, Maan ZN, Wong VW, et al. Scarless wound healing: chasing the holy grail. Plast Reconstr Surg. 2015;135:907–917. [DOI] [PubMed] [Google Scholar]
- 2.Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426–438. [DOI] [PubMed] [Google Scholar]
- 3.Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32:79–90. [DOI] [PubMed] [Google Scholar]
- 4.Alster TS, Graham PM. Microneedling: a review and practical guide. Dermatol Surg. 2018;44:397–404. [DOI] [PubMed] [Google Scholar]
- 5.Kauvar AN. Fractional nonablative laser resurfacing: is there a skin tightening effect? Dermatol Surg. 2014;40(suppl 12):S157–S163. [DOI] [PubMed] [Google Scholar]
- 6.Nilforoushzadeh MA, Alavi S, Heidari-Kharaji M, et al. Biometric changes of skin parameters in using of microneedling fractional radiofrequency for skin tightening and rejuvenation facial. Skin Res Technol. 2020;26:859–866. [DOI] [PubMed] [Google Scholar]
- 7.Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321–339. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes JR, Samayoa JC, Broelsch GF, et al. Micro-mechanical fractional skin rejuvenation. Plast Reconstr Surg. 2013;131:216–223. [DOI] [PubMed] [Google Scholar]
- 9.Russe E, Purschke M, Farinelli WA, et al. Micro-fractional, directional skin tightening: a porcine model. Lasers Surg Med. 2016;48:264–269. [DOI] [PubMed] [Google Scholar]
- 10.Junker JP, Philip J, Kiwanuka E, et al. Assessing quality of healing in skin: review of available methods and devices. Wound Repair Regen. 2014;22(suppl 1):2–10. [DOI] [PubMed] [Google Scholar]
- 11.Jeong HR, Lee HS, Choi IJ, et al. Considerations in the use of microneedles: pain, convenience, anxiety and safety. J Drug Target. 2017;25:29–40. [DOI] [PubMed] [Google Scholar]
- 12.Iriarte C, Awosika O, Rengifo-Pardo M, et al. Review of applications of microneedling in dermatology. Clin Cosmet Investig Dermatol. 2017;10:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berlin AL, Hussain M, Phelps R, et al. A prospective study of fractional scanned nonsequential carbon dioxide laser resurfacing: a clinical and histopathologic evaluation. Dermatol Surg. 2009;35:222–228. [DOI] [PubMed] [Google Scholar]
- 14.Karsai S, Czarnecka A, Jünger M, et al. Ablative fractional lasers (CO(2) and Er:YAG): a randomized controlled double-blind split-face trial of the treatment of peri-orbital rhytides. Lasers Surg Med. 2010;42:160–167. [DOI] [PubMed] [Google Scholar]
- 15.Ramsdell WM. Fractional CO2 laser resurfacing complications. Semin Plast Surg. 2012;26:137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301–305. [DOI] [PubMed] [Google Scholar]
- 17.Son D, Harijan A. Overview of surgical scar prevention and management. J Korean Med Sci. 2014;29:751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurban RS, Bhawan J. Histologic changes in skin associated with aging. J Dermatol Surg Oncol. 1990;16:908–914. [DOI] [PubMed] [Google Scholar]
- 19.Shin JW, Kwon SH, Choi JY, et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]