Abstract
Studies have shown that low serum albumin (Salb) levels are associated with a high risk of mortality among patients on maintenance hemodialysis (MHD); however, the impact of Salb variability on short-term cardiovascular mortality remains unclear. Herein, we investigated the association between Salb levels and Salb variability on short-term all-cause and cardiovascular-related mortality in patients on MHD.
Eligible patients on MHD at Chongqing General Hospital between June 2017 and June 2020 were recruited in this study. Patients were grouped by Salb levels (normal Salb, ≥3.8 g/dL; low Salb, 3.4–3.8 g/dL; and lower Salb, 2–3.4 g/dL) and Salb variability (decreased, >5% loss; increased, >5% gain; and steady, 5% loss to 5% gain). Associations between Salb levels, Salb variability, and all-cause and cardiovascular-related mortality were analyzed using Cox regression models. A survival analysis was performed using the Kaplan–Meier analysis.
We enrolled a total of 181 patients on MHD with an average age of 65 years (interquartile range [IQR], 53–75 years). The mean Salb level was 3.8 ± 0.6 g/dL (IQR 2.9–4.4 g/dL), and the median Salb variability was 2.6% per year (IQR, −4.1 to 6.5). Fifty-two (29%) patients died, including 31 (17%) patients who died due to cardiovascular-related causes. Compared with the other groups, the lower Salb group had higher all-cause mortality (P < .01). Cox regression analyses revealed that lower Salb levels and decreased Salb variability were independently associated with all-cause mortality (hazard ratio [HR] = 1.95, 95% confidence interval [CI] 1.103–3.452; HR = 2.245, 95% CI 1.084–4.650), whereas increased Salb variability was independently associated with cardiovascular-related mortality (HR = 2.919, 95% CI 1.178–7.234; P < .05).
Lower Salb levels were an independent predictor of all-cause mortality in patients on MHD. Increased Salb variability was strongly associated with cardiovascular-related mortality in the same population, especially in the short-term and in patients with normal Salb levels. Significantly elevated Salb variability should be evaluated to reduce cardiovascular-related mortality.
Keywords: maintenance hemodialysis, mortality, serum albumin, variability
1. Introduction
Vascular calcification (VC) is a common phenomenon among patients on maintenance hemodialysis (MHD)[1,2] and is associated with an increased risk of cardiovascular disease (CVD). VC is strongly related to all-cause mortality and increased cardiovascular-related mortality in patients on MHD.[3] Compared with traditional risk factors, such as age, sex, diabetes mellitus, and dialysis vintage, and nontraditional risk factors, such as abnormal levels of intact parathyroid hormone (i-PTH) in patients with chronic kidney disease (CKD),[4,5] poor nutritional status is strongly associated with the progression of aortic calcification and increased mortality among patients on MHD.[6,7] Malnutrition is prevalent in patients with uremia because of inadequate nutrient intake, poor absorption, and micro-inflammation.[8] There is conflicting evidence on the role of serum albumin (Salb) as an independent predictor of mortality among patients on MHD. Although previous studies have demonstrated that low Salb levels are associated with a high risk of mortality among patients receiving dialysis,[9–11] another study also demonstrated that baseline Salb levels of >3.8 g/dL are associated with a great risk of mortality.[12]
This study examined the effect of Salb levels on all-cause and cardiovascular-related mortality among patients on MHD to identify strategies for the reduction of the risk of mortality.
2. Materials and methods
2.1. Study design and population
This observational cohort study examined 210 patients with CKD who were managed in the HD center of Chongqing General Hospital between June 2017 and June 2020. Patients who were ≥18 years of age, on HD for ≥3 months, and were in stable clinical conditions without acute renal failure, cancers, gastrointestinal hemorrhage, hematological system diseases, and severe infections were included in this study. Patients who were on HD for <3 months, had severe arthralgia, fractures, uncontrolled hypertension (systolic blood pressure and diastolic blood pressure >180 mmHg or 110 mmHg, respectively, for at least 3 measurements taken before HD), hypotension requiring medication, uncontrolled cardiac arrhythmia, hemodynamic instability, surgery, or cardiovascular events or infection within 1 month, primary parathyroid, autoimmune, or liver diseases, malignancy, severe malnutrition, and Salb levels ≤2 g/dL, those receiving peritoneal dialysis, or who underwent renal transplantation were excluded. Patients in whom abdominal aortic calcification (AAC) could not be clearly diagnosed from abdominal radiographs because of constipation and intestinal gas were also excluded.
All patients underwent 4 hours of MHD 3 times weekly. The MHD protocol utilized a dialysate containing the same solute, with dialysate and blood flow rates of 500 mL/min and 200 to 300 mL/min, respectively.
This study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the Chongqing General Hospital of the University of Chinese Academy of Sciences. Informed consent was obtained from all participants.
2.2. Data collection
Demographic and clinical data were retrieved from electronic hospital medical records. Data included age, sex, dialysis vintage, normalized dialysis dose (Kt/V), body mass index (BMI), geriatric nutritional risk index (GNRI) scores, C-reactive protein (CRP), i-PTH, hemoglobin, serum phosphate, serum calcium, alkaline phosphatase (ALP), triglyceride, and total cholesterol levels, AAC scores, CKD etiology, pre-dialysis blood pressure, CVD complications, smoking history, and medications. Laboratory tests were conducted using standardized clinical laboratory methods.
When the Salb level was <4 g/dL, it was corrected using the following formula: corrected calcium = total calcium + 0.8 × (4 – Salb). The Kt/V was calculated using the following formula: Kt/V = −ln (Ct/ Co − 0.008 × t) + (4 – 3.5 × Ct/Co) × ΔBW/BW. Ct/Co, t, and BW corresponded to the ratio of post-dialysis to pre-dialysis serum urea nitrogen, dialysis time, and post-dialysis body weight in kilograms, respectively. The GNRI was calculated using the following formula: GNRI = 14.89 × serum albumin (g/dL) + 41.7 × (body weight/ideal body weight), and the ideal weight calculation formula for males and females was height (cm) – 100 - [(height (cm) -150)/4] and height (cm) -100- [(height (cm) -150)/2.5], respectively. The 11th thoracic to 2nd sacral vertebrae were imaged with lateral abdominal radiographs by radiologists in the hospital following the semi-quantitative method reported by Kauppila et al.[15] The average AAC score provided by two radiologists was presented as a categorical score, and the total AAC score ranged from 0 to 24 points.
Twice each quarter, all patients underwent a routine laboratory and physical examination before HD. Salb was measured more than once each month and at least 8 times each year. If <6 Salb measurements were taken within a 1-year period, the year was excluded from the overall analysis of each patient. A 1-year period was defined by either the calendar time or the interval from the date of HD initiation or death. Salb levels were represented by yearly mean values. Variations in Salb levels were analyzed using individual linear regression models through time and were defined by the coefficient of variation (CV). The CV was calculated using the formula: CV = standard deviation/mean.[16] The yearly variability in Salb represented percent changes in Salb per year for the duration of the follow-up period. Salb variability was categorized as positive or negative depending on the slope of the graph.
2.3. Grouping methods
Patients were categorized according to their Salb levels and Salb variability. When Salb levels were considered, patients were categorized into the normal Salb (Salb, ≥3.8 g/dL), low Salb (Salb, 3.4–3.8 g/dL) and lower Salb (Salb, 2–3.4 g/dL) groups. When Salb variability was considered, patients were categorized into the decreased (Salb variability, >5% loss), increased (Salb variability, >5% gain), and steady (Salb variability, 5% loss to 5% gain) Salb variability groups.
2.4. Outcomes
All participants were followed up for 1 year or until the initiation of peritoneal dialysis, renal transplantation, or death due to all-cause mortality and/or cardiovascular-related mortality, or were lost to follow-up. Cardiovascular-related mortality was defined as death due to myocardial infarction, arrhythmia, heart failure, stroke, or ruptured aortic aneurysm.
2.5. Statistical analysis
Statistical analyses were conducted using the R4.0.3 statistical software (The R Project for Statistical Computing, Vienna, Austria). Categorical variables were expressed as frequency and percentage, and were compared using the χ2 test. Normally distributed continuous variables were presented as mean ± standard deviation and were compared using the Student t test or the analysis of variance test. Non-normally distributed variables were expressed as median (interquartile range, IQR) and compared using the Mann–Whitney U test and Kruskal–Wallis H test, as necessary.
A survival analysis was performed using the Kaplan–Meier analysis, and intergroup differences were evaluated using a log-rank test. Several covariates, including baseline Salb levels and Salb variability, were assessed with survival analyses. Factors that were independently associated with cardiovascular-related and all-cause mortality were examined using Cox proportional hazards regression models. Survival analysis data were presented as hazard ratios (HRs) with 95% confidence intervals (CIs). A P value of <.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
A total of 181 patients on MHD were enrolled in this study, and 29 patients were excluded (Fig. 1). The baseline demographic and clinical characteristics of the patients in this study are summarized in Table 1. The median age was 65 years (IQR, 53–75 years), and 65.2% (n = 118) were men. Diabetic nephropathy and primary glomerulonephritis were the primary etiologies of CKD in 63 (34.8%) and 70 (38.7%) patients, respectively. The mean baseline Salb level was 3.8 ± 0.6 g/dL, and the median Salb variability was 2.6% per year (IQR, −4.1 to 6.5). The mean absolute Salb variability value was 4.6% ± 2.1% per year.
Figure 1.
Flow diagram of patient inclusion and exclusion processes.
Table 1.
Baseline characteristics of the patients according to the baseline Salb level.
| Variables | Participants (n = 181) | Lower Salb (n = 38) | Low Salb (n = 45) | Normal Salb (n = 98) | P |
| Age, median (IQR), y | 65.0 (53.0–75.0) | 67.0 (60.0–75.0) | 68.0 (54.0–76.0) | 64.5 (50.3–73.8) | 0.340 |
| Male, n (%) | 118 (65.2) | 23 (60.5) | 34 (75.6) | 61 (62.2) | 0.238 |
| HD. vintage, (y) median (IQR) | 4.40 (3.1–5.9) | 3.35 (2.9–5.6) | 5.40 (4.0–7.2) | 4.10 (2.3–5.6) | 0.002 |
| Deaths, n (%) | 52 (28.7) | 18 (47.4) | 14 (31.1) | 20 (20.4) | 0.007 |
| Cause of CKD, n (%) | |||||
| DMN | 63 (34.8) | 21 (55.2) | 19 (42.2) | 23 (23.5) | 0.001 |
| HTN | 31 (17.1) | 5 (13.1) | 7 (15.6) | 19 (19.4) | 0.653 |
| PGN | 70 (38.7) | 11 (28.9) | 18 (40.0) | 41 (41.8) | 0.375 |
| Others | 7 (3.9) | 2 (5.3) | 2 (4.4) | 3 (3.1) | 0.674 |
| Unknown | 11 (6.1) | 2 (5.3) | 2 (4.4) | 7 (7.1) | 0.916 |
| Medications, n (%) | |||||
| Anti-hypertension drugs | 107 (59.1) | 19 (50.0) | 25 (55.6) | 63 (64.3) | 0.269 |
| Anti-diabetes drugs | 64 (35.4) | 20 (44.4) | 21 (46.6) | 23 (23.5) | 0.001 |
| Vitamin D receptor activation | 92 (50.8) | 18 (47.4) | 23 (51.1) | 51 (52.0) | 0.886 |
| Cinacalcet | 65 (35.9) | 11 (28.9) | 17 (37.8) | 37 (37.8) | 0.602 |
| Non-Ca phosphate binders | 96 (53.0) | 16 (42.1) | 26 (57.8) | 54 (55.1) | 0.302 |
| Statins | 86 (47.5) | 17 (44.7) | 23 (51.1) | 46 (46.9) | 0.834 |
| Hypertension, n (%) | 133 (73.5) | 23 (60.5) | 33 (73.3) | 77 (78.6) | 0.101 |
| Diabetes, n (%) | 65 (36.0) | 21 (55.3) | 21 (46.7) | 23 (23.5) | 0.001 |
| Present smoking Positive, n (%) | 106 (58.6) | 21 (55.2) | 27 (60.0) | 58 (59.1) | 0.202 |
| Hemoglobin, g/L | 113.7 ± 14.5 | 105.8 ± 11.6 | 114.2 ± 11.7 | 116.5 ± 15.6 | <0.001 |
| Total cholesterol, mmol/L | 4.82 ± 0.76 | 4.72 ± 0.74 | 4.84 ± 0.73 | 4.85 ± 0.78 | 0.655 |
| Triglyceride, median (mmol/L) | 1.64 (1.45–1.90) | 1.69 (1.43–1.91) | 1.71 (1.54–1.92) | 1.56 (1.45–1.87) | 0.199 |
| Kt/V | 1.4 ± 0.2 | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.2 | 0.153 |
| GNRI score | 92.3 ± 2.79 | 84.7 ± 3.14 | 89.8 ± 2.87 | 94.6 ± 3.75 | <0.001 |
| BMI, kg/m2 median (IQR) | 20.7 (19.4–21.8) | 19.8 (19.2–20.6) | 20.5 (19.5–21.2) | 21.2 (19.5–23.2) | <0.001 |
| GNRI | 91.7 ± 7.7 | 84.7 ± 3.6 | 87.5 ± 5.9 | 94.6 ± 4.7 | <0.001 |
| CRP, mg/L | 0.79 ± 0.31 | 0.75 ± 0.30 | 0.78 ± 0.31 | 0.82 ± 0.30 | 0.491 |
| ALP, U/L median (IQR) | 110.6 (89.4–138.1) | 105.0 (89.7–126.1) | 137.4 (93.2–168.3) | 108.4 (87.3–132.0) | 0.016 |
| Serum phosphate, mmol/L | 1.67 ± 0.34 | 1.55 ± 0.24 | 1.64 ± 0.26 | 1.73 ± 0.39 | 0.020 |
| Corrected calcium (mmol/L) | 2.21 ± 0.17 | 2.20 ± 0.16 | 2.17 ± 0.21 | 2.24 ± 0.16 | 0.085 |
| i-PTH (pg/mL) median (IQR) | 270.2 (160.4–402.2) | 230.6 (170.5–401.0) | 328.4 (218.6–451.6) | 243.4 (142.07–380.5) | 0.011 |
| AAC score median (IQR) | 9.0 (5.0–12.0) | 8.5 (5.4–11.0) | 11.0 (8.0–15.0) | 7.0 (2.3–11.0) | <0.001 |
3.2. Comparisons of Salb levels and variability
The lower Salb, low Salb, and normal Salb groups had 38 (21%), 45 (24.9%), and 98 (54.1%) patients, respectively (Table 1). Comparison of baseline data demonstrated that patients with lower Salb levels had lower BMI, GNRI scores, hemoglobin levels, and serum phosphate levels (P < .05) but higher ALP levels, i-PTH levels and AAC scores compared to those of patients in the other groups (P < .05). There was no significant difference in the Kt/V, triglyceride levels, total cholesterol levels, corrected calcium levels, and the incidence of hypertension among the 3 groups (P > .05). There was no significant difference in the medications among the three groups (P > .05), except for anti-diabetes medications (P < .05).
The decreased, steady, and increased Salb variability groups had 37 (20.4%), 83 (45.9%), and 61 (33.7%) patients, respectively (Fig. 2). The patients in the decreased and increased Salb variability groups had higher baseline AAC scores compared to those of patients in the steady Salb variability group (P < .05).
Figure 2.
Comparison of the abdominal aortic calcification (AAC) scores among the serum albumin (Salb) variability groups. Median AAC scores among the Salb variability groups were compared using the Mann–Whitney U test. There are significant associations between the decreased Salb (>5% loss) and steady Salb (5% loss to 5% gain) variability groups (P < .001); between the increased Salb (5% gain group) and steady Salb (5% loss to 5% gain) variability groups (P = .016), and between the increased Salb (>5% gain) and decreased Salb (>5% loss) variability groups (P = .059).
3.3. Outcomes of patients
During a 1-year follow-up period, 52 (28.7%) patients died; 31 (17.1%) patients died from cardiovascular-related causes, including 2 (1.1%), 12 (6.6%), and 17 (9.4%) patients who died of malignant arrhythmia, coronary artery disease (CAD), and heart failure (HF). The median follow-up duration was 12 months (IQR, 8.3–12).
3.4. Survival probability in MHD patients
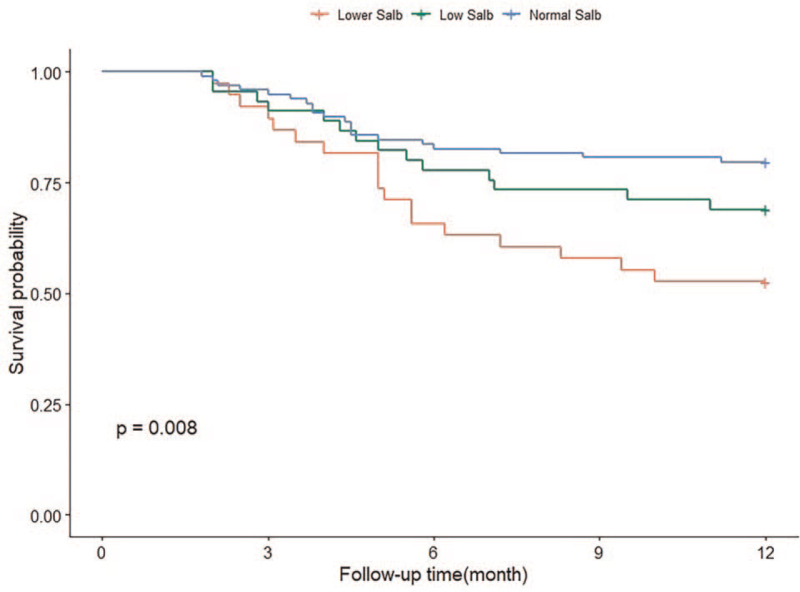
Kaplan–Meier curves revealed a significant tendency for lower survival among patients on MHD with lower Salb levels compared to those in the other groups (P < .01) (Fig. 3), and there was slightly high cardiovascular-related mortality in the lower Salb group, but the difference was not statistically significant (P > .05) (Fig. 4). Our data also demonstrated that decreased Salb variability (loss >5%) was significantly associated with a higher risk of all-cause mortality (P < .01) (Fig. 5). There was a tendency toward higher CVD-related survival among the 3 Salb variability groups, but the tendency was not statistically significant (P > .05) (Fig. 6).
Figure 3.
Kaplan-Meier analyses of the baseline Salb level and its variability on cardiovascular-related and all-cause mortality in maintenance hemodialysis patients. (Figure 3. The Kaplan-Meier analysis of baseline Salb levels and all-cause mortality. Log rank P < .01).
Figure 4.
The Kaplan–Meier analysis of baseline Salb levels and cardiovascular-related mortality. Log lank P > .01.
Figure 5.
The Kaplan-Meier analysis of Salb variability and all-cause mortality. Log lank P < .01.
Figure 6.
The Kaplan–Meier analysis of Salb variability and cardiovascular-related mortality. Log lank P > .01.
3.5. Association of Salb level and variability with mortality
The risk factors associated with all-cause and cardiovascular-related mortality in the study population are summarized in Table 2. After adjustment for various confounders, Cox regression analysis demonstrated that lower Salb levels (HR = 1.952, 95% CI 1.103–3.452) and increased Salb variability (loss >5%) (HR = 2.245, 95% CI 1.084–4.650) were independently associated with all-cause mortality (P < .05). Lower Salb levels were not associated with cardiovascular-related mortality (P > .05), but decreased Salb variability (gain > 5%) was independently associated with an increased risk of cardiovascular-related mortality among patients on MHD, especially in the short-term (HR = 2.919, 95% CI 1.178–7.234; P < .05).
Table 2.
Cox proportional hazards regression analysis for risk factors of all-cause mortality and CVD mortality in MHD patients.
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
| Parameter | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| All-cause mortality | ||||||||
| Salb baseline level (vs normal Salb level) | ||||||||
| Lower Salb | 2.631 (1.391–4.979) | .002 | 2.094 (1.047–4.189) | .036 | 2.079 (1.015–4.252) | .044 | 1.95 (1.103–3.452) | .022 |
| Salb variability per year (vs 5% loss to 5% gain) | ||||||||
| >5% loss | 2.956 (1.474–5.925) | .002 | 2.375 (1.157–4.875) | .018 | 2.313 (1.098–4.870) | .027 | 2.245 (1.084–4.650) | .029 |
| >5% gain | 2.961 (1.427–6.147) | .003 | 2.048 (1.055–3.973) | .034 | 1.982 (0.987–3.983) | .054 | 1.402 (0.689–2.851) | .351 |
| CVD mortality | ||||||||
| Salb baseline level (vs normal Salb level) | ||||||||
| Lower Salb | 3.005 (0.956–9.448) | .059 | 1.468 (0.588–3.672) | .411 | 1.35 (0.513–3.602) | .537 | 1.157 (0.419–3.201) | .778 |
| Salb variability per year (vs 5% loss to 5% gain) | ||||||||
| >5% loss | 1.997 (0.897–4.449) | .090 | 1.683 (0.7236–3.913) | .226 | 1.526 (0.6647–3.505) | .319 | 1.205 (0.465–13.126) | .701 |
| >5% gain | 4.303 (1.571–11.780) | .005 | 4.143 (1.538–11.159) | .005 | 4.112 (1.506–11.229) | .006 | 2.919 (1.178–7.234) | .021 |
Baseline Salb levels and Salb variability were analyzed as categorical variables, and the results are summarized in Table 3. The Cox regression analyses found that lower Salb levels and increased Salb variability (loss > 5%) were significantly associated with an increased risk of all-cause mortality. Their associations were somewhat attenuated after adjustment for relevant clinical factors, such as the etiology of CKD, other comorbidities, and factors associated with malnutrition-inflammation-atherosclerosis syndrome. Among patients who demonstrated decreased Salb variability (>5% loss per year), lower baseline Salb levels were associated higher risks of all-cause death (HR = 5.23, 95% CI, 2.01–12.61; P < .05). In comparison, among patients with increased Salb variability (>5% gain per year), lower Salb levels were associated with higher risks of cardiovascular-related mortality (HR = 1.81, 95% CI 1.17–5.91; P < .05). A similar trend was observed among patients with steady Salb variability (HR = 1.45, 95% CI, 1.08–3.43; P < .05). Our model failed to estimate CVD-free mortality because of the small sample size.
Table 3.
Adjusted HR for all-cause and CVD mortality in MHD patients according to the baseline Salb level and Salb variability by cox regression analysis.
| Baseline Salb level, g/dL | |||
| 2.0–3.4 g/dL | 3.4–3.8 g/dL | ≥3.8 g/dL | |
| Salb variability per year (%) | HR (95% CI) | ||
| (1) All-cause mortality | |||
| >5% loss | 5.23 (2.01–12.61)∗ | 3.56 (1.71–9.54) ∗ | 2.04 (0.78–6.35) |
| 5% loss to 5% gain | 3.17 (1.12–8.47) ∗ | 1.51 (0.95–3.25) | Reference |
| >5% gain | 0.82 (0.54–2.52) | 0.95 (0.51–4.73) | 1.29 (0.74–7.87) |
| (2) CVD mortality | |||
| >5% loss | 2.15 (0.77–5.35) | 1.53 (0.61–7.54) | 1.79 (0.71–8.61) |
| 5% loss to 5% gain | 2.37 (0.82–6.19) | 1.21 (0.51–4.42) | Reference |
| >5% gain | 0.76 (0.63–2.83) | 1.45 (1.08–3.43) ∗ | 1.81 (1.17–5.91) ∗ |
4. Discussion
The study found that lower Salb levels and decreased Salb variability were significantly associated with an increased risk of all-cause death and were independently associated with all-cause mortality among patients on MHD. Salb variability was associated with high AAC scores, and increased Salb variability was independently associated with cardiovascular-related mortality among patients on MHD, especially in the short-term period and in patients with normal Salb levels.
Factors associated with malnutrition, such as low BMI, low GNRI scores, and anemia, showed the prognostic power of lower baseline Salb levels. Our survival analyses data indicated that patients with lower Salb levels had higher all-cause mortality, which corresponded with the results of previous studies.[9,10,14] Several mechanisms may explain the increased risk of mortality among patients with lower Salb levels. Hypoalbuminemia is a marker of malnutrition–inflammation complex syndrome (MICS). Changes in sodium chloride levels induce changes in albumin synthesis and catabolism, which influence the inflammatory and nutritional status of patients on MHD.[12,13] Li et al[17] demonstrated that low Salb was associated with functional falls and independently increased the risk of death among elderly adults on HD. Moreover, studies have shown that changes in nutrition affect albumin synthesis, whereas inflammation and hypoalbuminemia increase the rate of fractional albumin catabolism. Similar to lipoproteins, Salb binds with endotoxins to reduce cytokine cascade activation, which protects patients from CVD. This phenomenon is reduced in patients with low Salb.[18,19] The International Society of Renal Nutrition and Metabolism proposed that protein-energy wasting (PEW) accurately explains the complex mechanism behind malnutrition in patients undergoing HD. Salb is a readily measurable criterion that can be utilized in the clinical diagnosis of protein-energy wasting in patients on MHD, because significantly low Salb levels are strongly associated with worse malnutrition, frailty, and mortality.[20–22] In contrast, Gama-Axelsson et al[23] proposed that Salb may be poorly correlated with nutritional status because chronic inflammation can also reduce Salb. Hence, hypoalbuminemia may be due to poor nutritional intake and the inflammatory process that is common among patients on MHD. Our study demonstrated that patients with lower Salb had lower hemoglobin levels, BMI, and GNRI scores, but had higher CRP levels and all-cause mortality compared to those of other patients; interestingly, malnutrition has the same associations. These results suggested that urgent care is needed for patients with malnutrition on MHD, especially for patients with severe hypoproteinemia.
There is growing concern regarding the association between malnutrition and CVD. Malnutrition status is considered a predictor of chronic HF.[24] Harada et al[25] showed that GNRI was a significant risk factor for the progression of AAC in nondialyzed patients with CKD and highlighted the utility of GNRI as a screening tool for predicting severe VC.
VC is a common phenomenon among patients with CKD, but the risk of VC increases with HD therapy. VC is an independent predictor of cardiovascular-related mortality among patients on MHD.[26] Previous studies have suggested that poor nutritional status was a critical factor for the progression of aortic calcification.[6,27] Future studies should examine the impact of reducing hypoalbuminemia on the incidence of cardiovascular-related deaths among patients on MHD.
Our data correlated with previous literature that demonstrated that hypoalbuminemia was associated with higher AAC scores and all-cause mortality, particularly in patients on MHD with lower Salb levels. Although our data showed no correlation between lower Salb levels and cardiovascular-related mortality, increased Salb variability was independently associated with higher cardiovascular-related mortality, especially in the short term. We speculated that hypoalbuminemia may increase the risk of arterial calcification among patients on HD, and Salb variability may be a risk factor for VC progression in the short-term. Both hypoalbuminemia and Salb variability increase cardiovascular-related mortality.
Like in atherosclerosis, multiple factors regulate VC, and vascular smooth muscle cells (VSMCs) play a principal role in the pathogenesis of VC in patients with advanced CKD. In patients with end-stage renal disease, VSMCs undergo apoptosis and transform into osteoblast-like cells, which result in bone formation and calcification.[28,29] VC occurs more often in patients on HD because of the combination of traditional factors, such as advanced age, sex, smoking, hypertension, dialysis vintage, calcium phosphate disorders, and diabetes, and nontraditional risk factors, such as excessive calcium intake, mineral metabolism abnormalities, inflammation, malnutrition, oxidative stress, and abnormal levels of i-PTH and bone-related proteins like fetuin-A, bone morphogenetic protein-2, matrix-carboxyglutamic acid protein, and osteoprotegerin.[30,31] At baseline, the vessels involved in HD exhibit increased ALP activity and VSMC loss. Exposure of these vessels to elevated serum calcium and/or phosphate increases the likelihood of injury and calcification.[32]
Our study demonstrated that serum phosphate levels, CRP, and total cholesterol levels were slightly higher in patients with normal Salb levels, whereas serum i-PTH levels were significantly higher in patients with low Salb levels compared to those in the other groups. The increased Salb variability were common in patients with low and normal Salb levels, and we speculated that the rapid increase in Salb disrupted serum calcium phosphate levels and lipid metabolism, which resulted in increased BMI, obesity, poor survival, and cardiovascular-related mortality.[33–35]
Significant increases in Salb variability should be evaluated to improve the cardiovascular prognosis of patients on MHD, especially in the short-term and in patients with normal Salb levels. Other effective interventions and nutritional treatments should be integrated into the management of these patients to reduce all-cause mortality.
This study has several limitations. First, our study was a short-term, observational trial that examined a small sample size, and our conclusions may not be applicable to all patients on MHD. Our follow-up period of 1 year also means that our results cannot be applied to long-term survivors. Second, the diet and protein intake of the patients in this study were not standardized and may have resulted in differences in baseline Salb levels and Salb variability between patients. Goldwasser et al[36] proposed that the increasing Salb levels in patients with worsening renal failure may be due to decreased proteinuria instead of improved nutritional status. We did not examine the etiology of hypoalbuminemia in our patients, particularly in the patients with severe proteinuria, and this lack of this information may have affected the accuracy of our results. Furthermore, this study did not examine the relationship between the degrees of occupational smoke exposure with overall survival or cardiovascular-related mortality. The impact of low or high BMI on mortality was not assessed either. Third, this study did not perform arterial biopsies to definitively demonstrate VC status and did not assess MICS-related laboratory markers, such as the malnutrition inflammation score and serum ferritin, total iron binding capacity, fetuin-A, interleukin-1, and interleukin-6 levels; these markers are particularly important in patients with chronic renal failure.[37,38] Future studies should examine the associations identified in this study in more specific and accurate detail.
5. Conclusions
We demonstrated that lower Salb levels were an independent predictor of all-cause mortality in patients on MHD. Increased Salb variability was strongly associated with cardiovascular-related mortality in patients on MHD, especially in the short-term and in patients with normal Salb levels. Significantly elevated Salb variability should be assessed regularly to reduce cardiovascular-related mortality. Further studies are needed to verify whether Salb supplementation has potential benefits for patients on MHD with normal Salb levels.
Acknowledgments
The authors thank the patients and staff of the Departments of Nephrology and Radiology for contributing their time to this study.
Author contributions
Data curation: Chun Chen, Jing Zhang, Zemei Zhou, Jiguo Liu, Chunyin Li.
Formal analysis: Chun Chen, Zemei Zhou, Jiguo Liu.
Methodology: Chun Chen, Jing Zhang.
Supervision: Chun Liu.
Writing – original draft: Chun Chen.
Writing – review & editing: Chun Liu.
Footnotes
Abbreviations: AAC = abdominal aortic calcification, ALP = alkaline phosphatase, BMI = body mass index, CAD = coronary artery disease, CI = confidence interval, CKD = chronic kidney disease, CRP = C-reactive protein, CV = coefficient of variation, CVD = cardiovascular disease, ESRD = end-stage renal disease, GNRI = geriatric nutrition risk index, HD = hemodialysis, HF = heart failure, HR = hazard ratio, i-PTH = intact parathyroid hormone, IQR = interquartile range, ISRNM = International Society for Nutrition and kidney, MHD = maintenance hemodialysis, MICS = malnutrition-inflammation complex syndrome, PEW = protein-energy wasting, Salb = serum albumin, SD = standard deviation, VC = vascular calcification, VSMCs = vascular smooth muscle cells.
How to cite this article: Chen C, Zhang J, Zhou Z, Liu J, Li C, Liu C. Impact of serum albumin level and variability on short-term cardiovascular-related and all-cause mortality in patients on maintenance hemodialysis. Medicine. 2021;100:43(e27666).
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AAC = abdominal aortic calcification, ALP = alkaline phosphatase, BMI = body mass index, CKD = chronic kidney disease, CRP = C-reactive protein, DMN = diabetic nephropathy, GNRI = geriatric nutrition risk Index, HD = hemodialysis, HTN = hypertensive nephropathy, i-PTH = intact parathyroid hormone, IQR = interquartile range, non-Ca phosphate binders = non-calcium containing phosphate binders, PGN = primary glomerulonephritis, Salb = serum albumin.
Variables were assessed using the χ2 test, the ANOVA test (normally distributed data) and the Kruskal–Wallis H test (non-normally distributed data).
AAC = abdominal aortic calcification, BMI = body mass index, CI = confidence interval, CKD = chronic kidney disease, CVD = cardiovascular disease, GNRI = geriatric nutrition risk index, HR = hazard ratio, i-PTH = intact parathyroid hormone, MHD = maintenance hemodialysis, Salb = serum albumin.
Model 1 was adjusted for age, sex, hemodialysis vintage, Kt/V, cause of CKD, hypertension, and type 2 diabetes.
Model 2 was adjusted for Model 1 + levels of total cholesterol, triglyceride, serum corrected calcium, serum phosphate, serum alkaline phosphatase and i-PTH, prescription of non-Ca phosphate binders, cinacalcet hydrochloride, AAC score, history of CVD, medication use, present smoking.
Model 3 was adjusted for Model 2 + levels of C-reactive protein and hemoglobin, BMI, GNRI score, and other comorbidities.
Analyses were performed using a log-rank test.
CI = confidence interval, CVD = cardiovascular disease, HR = Hazard ratio, MHD = maintenance hemodialysis, Salb = serum albumin.
After adjustment for age, sex, hemodialysis vintage, Kt/V, laboratory variables, BMI, GNRI score, AAC score, causes of CKD, present smoking, Medication use, and comorbidities.
Reference baseline Salb level ≥3.8 g/dL and Salb variability 5% loss to 5% gain per year.
Analyses were performed using a log-rank test.
P < .05.
References
- [1].O’Lone E, Viecelli AK, Craig JC, et al. Cardiovascular outcomes reported in hemodialysis trials. J Am Coll Cardiol 2018;71:2802–10. [DOI] [PubMed] [Google Scholar]
- [2].Pencak P, Czerwieńska B, Ficek R, et al. Calcification of coronary arteries and abdominal aorta in relation to traditional and novel risk factors of atherosclerosis in hemodialysis patients. BMC Nephrol 2013;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Komatsu M, Okazaki M, Tsuchiya K, Kawaguchi H, Nitta K. Aortic arch calcification predicts cardiovascular and all-cause mortality in maintenance hemodialysis patients. Kidney Blood Press Res 2014;39:658–67. [DOI] [PubMed] [Google Scholar]
- [4].Vervloet M, Cozzolino M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int 2017;91:808–17. [DOI] [PubMed] [Google Scholar]
- [5].Shanahan CM. Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat Rev Nephrol 2013;9:661–70. [DOI] [PubMed] [Google Scholar]
- [6].Okamoto T, Hatakeyama S, Kodama H, et al. The relationship between poor nutritional status and progression of aortic calcification in patients on maintenance hemodialysis. BMC Nephrol 2018;19:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ikizler TA, Cano NJ, Franch H, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int 2013;84:1096–107. [DOI] [PubMed] [Google Scholar]
- [8].Guarnieri G, Antonione R, Biolo G. Mechanisms of malnutrition in uremia. J Ren Nutr 2003;13:153–7. [DOI] [PubMed] [Google Scholar]
- [9].Zhang K, Cheng G, Cai X, et al. Malnutrition, a new inducer for arterial calcification in hemodialysis patients? J Transl Med 2013;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mehrotra R, Duong U, Jiwakanon S, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am J Kidney Dis 2011;58:418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zager PG, Nikolic J, Brown RH, et al. U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 1998;54:561–9. [DOI] [PubMed] [Google Scholar]
- [12].Villain C, Fouque D, Genet L, et al. Prognostic value of serum albumin changes over time in elderly adults undergoing hemodialysis. J Am Geriatr Soc 2016;64:1353–4. [DOI] [PubMed] [Google Scholar]
- [13].Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, et al. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant 2005;20:1880–8. [DOI] [PubMed] [Google Scholar]
- [14].Chen JB, Cheng BC, Yang CH, Hua MS. An association between time-varying serum albumin level and the mortality rate in maintenance haemodialysis patients: a five-year clinical cohort study. BMC Nephrol 2016;17:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 1997;132:245–50. [DOI] [PubMed] [Google Scholar]
- [16].Nakazato Y, Kurane R, Hirose S, Watanabe A, Shimoyama H. Aging and death-associated changes in serum albumin variability over the course of chronic hemodialysis treatment. PLoS One 2017;12:e0185216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li M, Tomlinson G, Naglie G, Cook WL, Jassal SV. Geriatric comorbidities, such as falls, confer an independent mortality risk to elderly dialysis patients. Nephrol Dial Transplant 2008;23:1396–400. [DOI] [PubMed] [Google Scholar]
- [18].Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet 2000;356:930–3. [DOI] [PubMed] [Google Scholar]
- [19].Jürgens G, Müller M, Garidel P, et al. Investigation into the interaction of recombinant human serum albumin with re-lipopolysaccharide and lipid A. J Endotoxin Res 2002;8:115–26. [DOI] [PubMed] [Google Scholar]
- [20].Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008;73:391–8. [DOI] [PubMed] [Google Scholar]
- [21].Ishii H, Takahashi H, Ito Y, et al. The association of ankle brachial index, protein-energy wasting, and inflammation status with cardiovascular mortality in patients on chronic hemodialysis. Nutrients 2017;9(4.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Takahashi H, Inoue K, Shimizu K, et al. Comparison of nutritional risk scores for predicting mortality in Japanese chronic hemodialysis patients. J Ren Nutr 2017;27:201–6. [DOI] [PubMed] [Google Scholar]
- [23].Gama-Axelsson T, Heimbürger O, Stenvinkel P, Bárány P, Lindholm B, Qureshi AR. Serum albumin as predictor of nutritional status in patients with ESRD. Clin J Am Soc Nephrol 2012;7:1446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Narumi T, Arimoto T, Funayama A, et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol 2013;62:307–13. [DOI] [PubMed] [Google Scholar]
- [25].Harada K, Suzuki S, Ishii H, et al. Nutrition status predicts severity of vascular calcification in non-dialyzed chronic kidney disease. Circ J 2017;81:316–21. [DOI] [PubMed] [Google Scholar]
- [26].Panuccio V, Tripepi R, Tripepi G, et al. Heart valve calcifications, survival, and cardiovascular risk in hemodialysis patients. Am J Kidney Dis 2004;43:479–84. [DOI] [PubMed] [Google Scholar]
- [27].Takahashi H, Ito Y, Ishii H, et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J Cardiol 2014;64:32–6. [DOI] [PubMed] [Google Scholar]
- [28].Chen NX, Duan D, O’Neill KD, et al. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int 2006;70:1046–53. [DOI] [PubMed] [Google Scholar]
- [29].Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol 2009;20:1453–64. [DOI] [PubMed] [Google Scholar]
- [30].Cozzolino M, Mazzaferro S, Pugliese F, Brancaccio D. Vascular calcification and uremia: what do we know? Am J Nephrol 2008;28:339–46. [DOI] [PubMed] [Google Scholar]
- [31].Nitta K, Ogawa T, Hanafusa N, Tsuchiya K. Recent advances in the management of vascular calcification in patients with end-stage renal disease. Contrib Nephrol 2019;198:62–72. [DOI] [PubMed] [Google Scholar]
- [32].Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol 2010;21:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee MJ, Park JT, Park KS, et al. Normal body mass index with central obesity has increased risk of coronary artery calcification in Korean patients with chronic kidney disease. Kidney Int 2016;90:1368–76. [DOI] [PubMed] [Google Scholar]
- [34].Villain C, Ecochard R, Genet L, et al. Impact of BMI variations on survival in elderly hemodialysis patients. J Ren Nutr 2015;25:488–93. [DOI] [PubMed] [Google Scholar]
- [35].Icer MA, Yildiran H. Effects of nutritional status on serum fetuin-A level. Crit Rev Food Sci Nutr 2020;60:1938–46. [DOI] [PubMed] [Google Scholar]
- [36].Goldwasser P, Kaldas AI, Barth RH. Rise in serum albumin and creatinine in the first half year on hemodialysis. Kidney Int 1999;56:2260–8. [DOI] [PubMed] [Google Scholar]
- [37].Jagadeswaran D, Indhumathi E, Hemamalini AJ, Sivakumar V, Soundararajan P, Jayakumar M. Inflammation and nutritional status assessment by malnutrition inflammation score and its outcome in pre-dialysis chronic kidney disease patients. Clin Nutr 2019;38:341–7. [DOI] [PubMed] [Google Scholar]
- [38].Lai J, Akindavyi G, Fu Q, Li Z-L, Wang HM, Wen L-H. Research progress on the relationship between coronary artery calcification and chronic renal failure. Chin Med J 2018;131:608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]