Abstract
Recent data suggest that programmed cell death -1 (PD-1) and programmed cell death ligand-1 (PD-L1) are involved in the pathogenesis of Langerhans cell histiocytoma (LCH); however, their contributions are not well established. Also, the involvement of PD-1/PD-L1 molecules in musculoskeletal LCH remains particularly unclear. The current study aims to characterize the involvement of PD-1/PD-L1 immune checkpoint system in the pathogenesis of musculoskeletal LCH. PD-1/PD-L1 expression was evaluated in 6 patients, 3 men and 3 women with a mean age of 13.5 years, with musculoskeletal LCH who were treated at Kindai University Hospital and Osaka Women's and Children's Hospital between November 2005 and December 2020. The median follow-up period for all patients with musculoskeletal LCH was 41 months. We surveyed symptoms, number of lesions, treatment modality, and outcomes. Immunostaining for CD4, CD8, PD-1, and PD-L1 was also performed on pathological specimens obtained by biopsy. Multiple lesions were observed in 5 cases, and a single lesion was observed in 1 case. The chief complaint in 5 cases was pain. Four patients underwent spontaneous regression. The other 2 patients received chemotherapy. The outcomes included continuous disease-free (n = 5) and alive with the disease (n = 1). The CD4-, CD8-, PD-1-, and PD-L1-positive rates among all specimens were 100%, 100%, 16.6%, and 83.3%, respectively. The CD4/PD-L1, CD8/PD-L1, and PD-1/PD-L1 positive rates in all the specimens were 83.3%, 83.3%, and 16.6%, respectively. We believe that the PD-1/PD-L1 immune checkpoint molecules may play some role in the microenvironment of musculoskeletal LCH.
Keywords: immunohistochemistry, Langerhans cell histiocytoma, musculoskeletal system, programmed cell death -1, programmed cell death ligand-1
1. Introduction
Langerhans cell histiocytosis (LCH) is a rare systemic disorder characterized by the accumulation of CD1a+/Langerin+ LCH cells.[1] These LCH cells originate from myeloid dendritic cells rather than skin Langerhans cells.[2] The annual incidence in children is about 5 cases per 1 million population.[3] The male:female ratio is 1.2:1.[3] The annual incidence in adults is 1 to 2 cases per 1 million population.[3] The clinical manifestation of LCH varies from a single organ disease that can spontaneously undergo remission, to a systemic and aggressive disease that can lead to death.[3] Thus, the pathogenesis of LCH is complex and remains unclear. A recent study has shown that immune checkpoint inhibitors are used to alleviate the immunosuppressive state of the tumor microenvironment in malignant neoplasms, such as melanoma, non-small cell lung cancer, and colon cancer by restoring the immune function of T cells and killing tumor cells.[4] There are various genes related to the programmed cell death -1(PD-1)/ programmed cell death ligand-1 (PD-L1) immune checkpoint mechanism, but the main pathway is through T cells.[5] In particular, the CD4/CD8-mediated pathway has been attracting attention in recent years, so we attempted to investigate CD4/CD8 in this study as well.[6] Interestingly, a recent study showed PD-L1 to be expressed in histiocytic and dendritic cell disorders, however, there is insufficient evidence for the expression of PD-1/PD-L1 immune checkpoint molecule in LCH.[7] Moreover, the involvement of PD-1/PD-L1 in the pathogenesis of musculoskeletal LCH remains unclear. Herein, we investigated the expression of PD-1/PD-L1 immune checkpoint molecules including CD4/CD8 molecules in musculoskeletal LCH by using immunohistochemistry and characterized the involvement of the PD-1/PD-L1 immune checkpoint system in the pathogenesis of LCH. Additionally, this is the first report suggesting the involvement of PD-1/PD-L1 immune checkpoint system in the pathogenesis of musculoskeletal LCH.
2. Material and methods
We retrospectively reviewed 6 patients with musculoskeletal LCH treated at Kindai University Hospital and Osaka Women's and Children's Hospital between November 2005 and December 2020. The exclusion criterion was set as patients whose clinical course could not be followed. Informed consent was obtained from the study participants. The study was approved by the Ethics Committee of Kindai University Hospital (Approval number: 31–187; Approved on January 16, 2020).
The median follow-up period for all patients with musculoskeletal LCH was 41 (range, 4–73) months. Age, sex, site of origin, symptoms, treatment, and final clinical outcomes were studied. Antibodies against CD1a, S100, and langerin were used for immunohistochemical analyses to determine the immunophenotype of musculoskeletal LCH samples, as previously described.[8]
Immunostaining was performed with specimens obtained using needle biopsies from patients with musculoskeletal LCH treated at Kindai University Hospital and Osaka Women's and Children's Hospital. Tissues were deparaffinized, rehydrated, and subjected to antigen retrieval using 3% hydrogen peroxide. The sections were incubated with the following primary antibodies: CD4 antibody (rabbit monoclonal, ab133616; Abcam) for 32 minutes at 37°C after 60 minutes of high pH (9.0) heat activation; CD8 antibody (rabbit monoclonal, Product No. 05298709001; Roche) for 32 minutes at 37°C after 60 minutes of high pH (9.0) heat activation; PD-1 (mouse monoclonal, ab52587; Abcam, Cambridge, UK) for 30 minutes at 37°C after 30 minutes of low pH (6.0) heat activation; and PD-L1 antibody (rabbit monoclonal, ab205921; Abcam) for 32 minutes at 37°C after 60 minutes of high pH (9.0) heat activation. Sections were incubated with the corresponding secondary antibodies (for CD4, CD8, and PD-L1: Goat anti-rabbit IgG antibody (Cosmo Bio; SA00001–2) and for PD-1: Goat anti-mouse IgG antibody (Cosmo Bio; SA00001–1) for 30 minutes at 37°C. The reaction was visualized using 33′-diaminobenzidine (DAB; DAB Substrate Chromogen System; DAKO, Kyoto, Japan, Code K3467) and counterstained with hematoxylin. Slides were observed under a microscope (BIOREVO BZ-9000; KEYENCE, Osaka, Japan), and brown granules in the cytoplasm or nuclei indicated positive staining. Samples showing presence of CD4, CD8, and PD-1–positive tumor infiltrating lymphocytes were considered positive. We used just secondary antibodies as negative controls. The immune marker staining within the tumor was quantified in 4 representative high-power fields (40x magnification).[9] The positivity rate was defined as the number of positive cells/total cell number. The positivity rate was quantified using the software provided with BIOREVO-BZ 9000 (Keyence),[9] and staining >5% of total cells was considered positive. Positive staining rates for each immunostaining target (CD4, CD8, PD-1, and PD-L1) were calculated. Adjacent sections stained positive for CD4/PD-L1, CD8/PD-L1, and PD1/PD-L1 were considered co-positive. The co-staining rates for each immune molecule were also examined. The sample was evaluated by 3 observers (K.H., S.N., M.A) to exclude observation bias.
3. Results
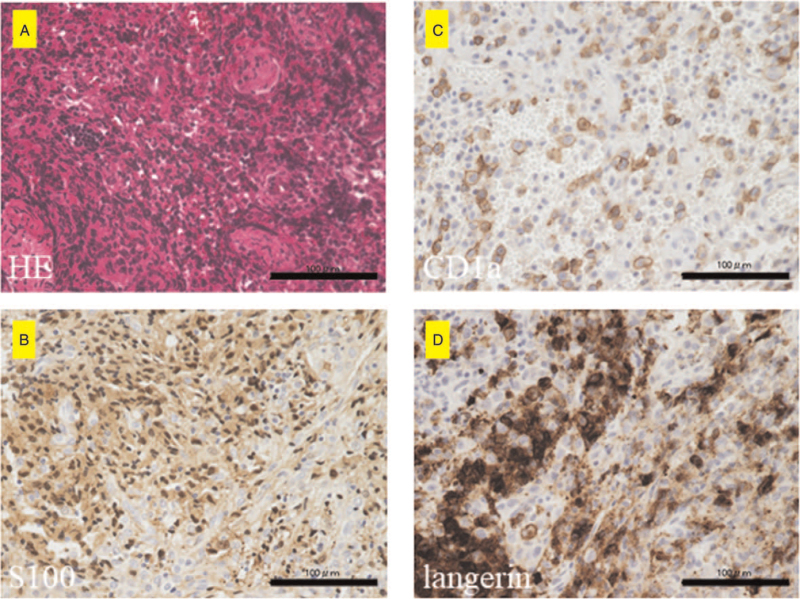
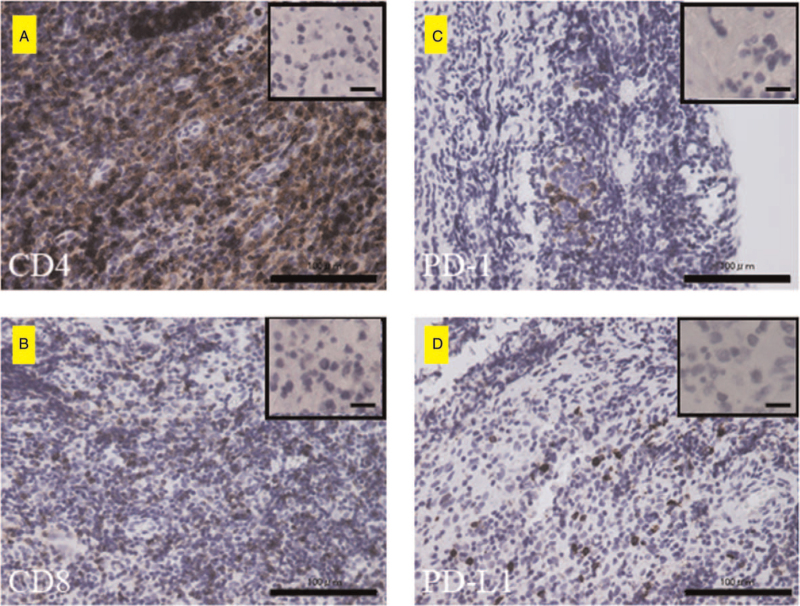
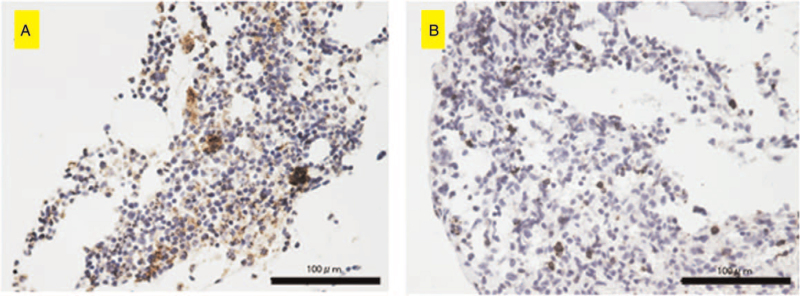
The demographic and clinicopathological characteristics of the 6 patients with musculoskeletal LCH are summarized in Table 1. The cohort consisted of 3 men and 3 women with a mean age of 13.5 (range, 2–60) years. There were 5 cases with multiple lesions and 1 case with a single lesion. Lesions in the trunk such as a rib or lumbar vertebrae were observed in all 3 adults older than 20 years. In contrast, pediatric cases had lesions in the extremities. Five patients were symptomatic at the time of their first visit, while one was asymptomatic. Of the cases with symptoms, pain was the most common complaint. Claudication was a common complaint in patients with limb-onset. Four patients underwent imaging follow-up, and 2 patients were treated with chemotherapy. The final clinical outcome was continuous disease-free in 5 patients and alive with disease in 1 patient. Tissue samples of all 6 cases stained positive for S100, CD1a, and langerin (Fig. 1A–C). Further, the overall immunohistochemistry positive staining rates were 6/6 (100%) for CD4, 6/6 (100%) for CD8, 1/6 (16.6%) for PD-1, and 5/6 (83.3%) for PD-L1. Representative positive histological findings are shown in (Fig. 2A–D). The CD4-positive and PD-L1-positive co-staining rates were 5/6 (83.3%). The CD8-positive and PD-L1-positive co-staining rates were 5/6 (83.3%). The PD-1 and PD-L1 concordance rates were 1/6 (16.6%). Interestingly, CD4 (5/6), CD8 (6/6), PD-1 (0/6), and PD-L1 (2/6) positivity were also observed in lymphocytes (Table 1, Fig. 3A and B).
Table 1.
Characteristics of patients with musculoskeletal Langerhans cell histiocytoma.
| CD4 | CD8 | PD-1 | PD-L1 | ||||||||
| Patient No. | Age | Sex | Site | Symptoms | Treatment | Outcome | Follow-up periods (Months) | +Lymphocytes positivity | |||
| 1 | 24 | F | rt Tibia, left 7th rib | Pain and swelling | Spontaneous regression | CDF | 13 | + | − | + | |
| + | + | − | − | ||||||||
| 2 | 60 | M | 2nd, 3rd, 4th, 5th lumbar, Th10, rt 4th rib, sacrum, pelvic | without symptoms | Chemotherapy (6-mercaptopurine 110 mg, MTX 2.5 mg, PSL 5 mg) | CDF | 63 | + | + | − | + |
| + | + | − | + | ||||||||
| 3 | 30 | F | Rt 4th rib | Pain | Spontaneous regression | AWD | 4 | + | + | − | − |
| + | + | − | − | ||||||||
| 4 | 2 | F | Femur, 8th thoracic vertebra | Pain and claudication | Spontaneous regression | CDF | 27 | + | + | + | + |
| + | + | − | + | ||||||||
| 5 | 2 | M | Femur, Temporal bone, anterior mediastinum | Pain and claudication | Chemotherapy | CDF | 55 | + | + | − | + |
| + | + | − | − | ||||||||
| 6 | 3 | M | Elbow | Pain | Spontaneous regression | CDF | 73 | + | + | − | + |
| − | + | − | − | ||||||||
Figure 1.
Immunohistochemical analyses of S100, CD1a, and langerin in Langerhans cell histiocytosis (LCH) samples. (A) Hematoxylin-Eosin (HE) staining of the representative LCH sample. Proliferation of Langerhans cells is observed. (B–D) Immunohistochemical staining for S100 (B), CD1a (C), and langerin (D). (B–D) indicate S100-, CD1a-, and langerin-positive cells, respectively. Scale bar = 100 μm.
Figure 2.
The histological findings of immunohistochemistry for CD4, CD8, PD-1, and PD-L1. (A–C) indicate CD4-, CD8-, and PD-1-positive lymphocytes, respectively. (D) PD-L1-positive tumor cells. Scale bar = 100 μm. The images in the upper right frame are the negative control in each (Scale bar = 10 μm). The CD4-positive and PD-L1-positive co-staining rates were 5/6 (83.3%). The CD8-positive and PD-L1-positive co-staining rates were 5/6 (83.3%). The PD-1 and PD-L1 concordance rates were 1/6 (16.6%). Interestingly, PD-L1 positivity was observed not only in the tumor cells but also in lymphocytes (Figure 3A and 3B).
Figure 3.
Immunohistochemical staining for PD-L1. The PD-L1–positive tumor cells are observed in (A) and positive lymphocytes are observed in (B). Scale bar = 100 μm.
4. Discussion
The involvement and mechanism of the PD-1/PD-L1 immune checkpoint system in the pathogenesis of LCH remains unclear. Here, we investigated the expression of PD-1/PD-L1 immune checkpoint molecules, including CD4/CD8 components, in musculoskeletal LCH using immunohistochemistry.
A previous study described PD-L1 positive rate of 5% and PD-1 positive rate of 5% to 20% in pulmonary LCH[8]; and, another found a PD-L1 positive rate of 88% in LCH.[10] In the current study, 1/5 (16.6%) positive rate for PD-1 and 5/6 (83.3%) positive rate for PD-L1 were observed. Moreover, the positive rate of CD4/CD8 immune components was high. Therefore, these findings indicate that the PD-1/PD-L1 immune checkpoint system is involved in the pathogenesis of musculoskeletal LCH.
Previous studies have shown a predominance of M2 macrophages, T cells, and regulatory T cells, as well as increased expression of programmed death ligand 1 (PD-L1) in LCH cells.[10–13] It has also been reported that there is a bias towards CD4+ T cells and regulatory T cells compared to CD8+ T cells. Both CD4+ and CD8+ T cells show increased expression of inhibitor receptors such as PD-1, TIM-3, and LAG-3; decreased cytokine production upon stimulation; and reduced effector function It has been reported that T cells showed signs of exhaustion, such as decreased cytokine production upon stimulation and decreased effector function.[10–14]
In the present study, tumor cells and lymphocytes were positive for PD-1/PD-L1 immune checkpoint mechanism molecules; there was no significant difference in the positivity of CD4 and CD8. These findings indicate that the same quantity of CD4 and CD8 are activated in the pathogenesis of musculoskeletal LCH.
Tumor infiltrating CD4+ or CD8+ T cells play an important role in regulating the tumor microenvironment in some cancer types.[15,16] Some studies also showed that the tumor-infiltrating T cells may serve as indicators of prognosis or therapeutic targets.[17–19] In the current study, we observed infiltration of CD4+ or CD8+ T cells in all cases. These findings suggest that infiltrating CD4+ or CD8+ T cells play an important role in the microenvironment of musculoskeletal LCH.
There is a significant debate for defining LCH either as immune disorder or neoplasm.[20] A previous study showed that only one case of pulmonary LCH and 2 cases of extrapulmonary LCH expressed PD-1, suggesting PD-1 ligand-mediated immune es-cape to be an unlikely mechanism of disease progression.[7] Here, 83.3% of the LCH specimens expressed PD-L1. Moreover, PD-L1 is typically expressed in tumor cells.[21] Therefore, these findings support the classification of LCH as a neoplasm.
Major advances have been made in the characterization of the tumor microenvironment of soft tissue sarcoma, as “hot tumors” massively infiltrated by immune cells and “cold tumors” with no significant immune infiltration.[22] Moreover, Petitprez et al established an immune-based classification system using the composition of the tumor microenvironment and identified 5 distinct phenotypes: immune-low (A and B), immune-high (D and E), and highly vascularized (C).[22] The report also indicated that the class-E group demonstrated improved survival and high response rate to PD-1 blockade with pembrolizumab in a phase 2 clinical trial.[22] In the present study, the LCH specimens expressed PD-1(1/6: 16.6%)/PD-L1 (5/6: 83.3%) immune checkpoint molecule. Therefore, musculoskeletal LCH can be classified as an immune-high (D or E) tumor.
In general, LCH is a benign tumor and shows favorable prognosis. [2,3]However, some LCH cases have aggressive clinical features, as previously described.[23] Here, 5/6 cases were continuous disease-free and 1/6 cases were alive with disease at the final follow-up. Although no case with an aggressive course was observed in this study, careful follow-up is necessary.
This study has some limitations. First, only 6 patients were enrolled in this study, which may have introduced bias into the analysis of the relationship between PD-L1 and musculoskeletal LCH. Second, this study is not a validation cohort. Therefore, we think it would be better to consider enrolling more patients into the study to reduce bias, or isolating cells from patients’ tissues to confirm that blocking PD1/PD-L1 signaling is beneficial in the treatment of musculoskeletal LCH. Alternatively, a cohort of similar studies could be sought and analyzed to confirm the results. These will be the subjects of future research.
Third, BRAF mutations specific to LCH[8,9] were not investigated. Fourth, we could not investigate the use of anti-PD-1 or an-ti-PD-L1 drugs in these patients because it was a retrospective study. However, despite these limitations, the study findings clearly indicate the involvement of PD-1/PD-L1 immune checkpoint molecules in the pathogenesis of musculoskeletal LCH. Nevertheless, we will attempt to overcome these limitations in future studies.
5. Conclusion
We suggest in this study that musculoskeletal LCH may be a highly immunocompetent neoplasm involving the immune checkpoint molecule, PD-1/PD-L1.
Acknowledgments
We thank Chikoto Tanaka to provide technical assistance.
Author contributions
Conceptualization: Hashimoto Kazuhiko, Shunji Nishimura, Akihisa Sawada, Masao Akagi.
Data curation: Hashimoto Kazuhiko, Shunji Nishimura, Naoki Sakata, Masami Inoue, Akihisa Sawada.
Formal analysis: Hashimoto Kazuhiko, Naoki Sakata, Masami Inoue, Akihisa Sawada.
Investigation: Naoki Sakata, Masami Inoue, Akihisa Sawada.
Methodology: Hashimoto Kazuhiko, Naoki Sakata, Masami Inoue, Akihisa Sawada.
Project administration: Hashimoto Kazuhiko, Masao Akagi.
Resources: Hashimoto Kazuhiko, Masami Inoue.
Software: Hashimoto Kazuhiko.
Supervision: Hashimoto Kazuhiko, Akihisa Sawada.
Validation: Hashimoto Kazuhiko, Akihisa Sawada, Masao Akagi.
Visualization: Hashimoto Kazuhiko, Masao Akagi.
Writing – original draft: Hashimoto Kazuhiko, Shunji Nishimura, Naoki Sakata, Masami Inoue, Akihisa Sawada, Masao Akagi.
Writing – review & editing: Hashimoto Kazuhiko, Shunji Nishimura, Naoki Sakata, Masami Inoue, Akihisa Sawada, Masao Akagi.
Footnotes
Abbreviations: LCH = Langerhans cell histiocytoma, PD-1 = programmed cell death -1, PD-L1 = programmed cell death ligand-1.
How to cite this article: Hashimoto K, Nishimura S, Sakata N, Inoue M, Sawada A, Akagi M. Characterization of PD-1/PD-L1 immune checkpoint expression in the pathogenesis of musculoskeletal Langerhans cell histiocytosis: a retrospective study. Medicine. 2021;100:43(e27650).
This report complies with the Declaration of Helsinki. This study was also approved by the Ethics Committee of Kindai University Hospital (Approval number: 31–187, approved on January 16, 2020, Osaka, Japan).
Written informed consent has been obtained from the patient to publish this paper.
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
CDF = continuous disease-free, AWD = alive with disease, rt = right, MTX = methotrexate, PSL = prednisolone.
References
- [1].Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med 2018;379:856–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol 2018;78:1035–44. [DOI] [PubMed] [Google Scholar]
- [3]. WHO classification of tumors of soft tissue and bone. In Soft Tissue and Bone Tumours, 5th ed. Fletcher, C.D.; Lazar, A.J. eds. Langerhans cell histiocytosis; Pileri SA, Cheuk W, and Picarsic J. IARC Publications, Lyon, France 2020; 492–94. [Google Scholar]
- [4].Dermani FK, Samadi P, Rahmani G, et al. PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J Cell Physiol 2019;234:1313–25. [DOI] [PubMed] [Google Scholar]
- [5].Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 2020;10:727–42. [PMC free article] [PubMed] [Google Scholar]
- [6].Marchevsky AM, Walts AE. PD-L1, PD-1, CD4, and CD8 expression in neoplastic and nonneoplastic thymus. Hum Pathol 2017;60:16–23. [DOI] [PubMed] [Google Scholar]
- [7].Xu J, Sun HH, Fletcher CD, et al. Expression of programmed cell death 1 ligands (PD-L1 and PD-L2) in histiocytic and dendritic cell disorders. Am J Surg Pathol 2016;40:443–53. [DOI] [PubMed] [Google Scholar]
- [8].Wang J, Xie L, Miao Y, et al. Adult pulmonary Langerhans cell histiocytosis might consist of two distinct groups: isolated form and extrapulmonary recidivism type. Ann Transl Med 2021;9:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hashimoto K, Nishimura S, Ito T, Akagi M. Characterization of PD-1/PD-L1 immune checkpoint expression in soft tissue sarcomas. Eur J Histochem 2021;65:3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gatalica Z, Bilalovic N, Palazzo JP, et al. Disseminated histiocytoses biomarkers beyond BRAFV600E: frequent expression of PD-L1. Oncotarget 2015;6:19819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eckstein OS, Visser J, Rodriguez-Galindo C. Allen CE; NACHO-LIBRE study group. Clinical responses and persistent BRAFV600E+ blood cells in children with LCH treated with MAPK pathway inhibition. Blood 2019;133:1691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Paredes SEY, Almeida LY, Trevisan GL, et al. Immunohistochemical characterization of immune cell infiltration in paediatric and adult Langerhans cell histiocytosis. Scand J Immunol 2020;00:e12950. [DOI] [PubMed] [Google Scholar]
- [13].Mitchell JM, Berzins SP, Kannourakis G. A potentially important role for T cells and regulatory T cells in Langerhans cell histiocytosis. Clin Immunol 2018;194:19–25. [DOI] [PubMed] [Google Scholar]
- [14].Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016;44:989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol 2019;234:8509–21. [DOI] [PubMed] [Google Scholar]
- [16].Zander R, Schauder D, Xin G, et al. CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer. Immunity 2019;51:1028–42. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li F, Sun Y, Huang J, et al. CD4/CD8 + T cells, DC subsets, Foxp3, and IDO expression are predictive indictors of gastric cancer prognosis. Cancer Med 2019;8:7330–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li F, Zhao Y, Wei L, et al. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol Ther 2018;19:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Saleh R, Elkord E. FoxP3+ T regulatory cells in cancer: prognostic biomarkers and therapeutic targets. Cancer Lett 2020;490:174–85. [DOI] [PubMed] [Google Scholar]
- [20].Kobayashi M, Tojo A. Langerhans cell histiocytosis in adults: advances in pathophysiology and treatment. Cancer Sci 2018;109:3707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Petitprez F, de Reyniès A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020;577:556–60. [DOI] [PubMed] [Google Scholar]
- [23].Grana N. Langerhans cell histiocytosis. Cancer Control 2014;21:328–34. [DOI] [PubMed] [Google Scholar]