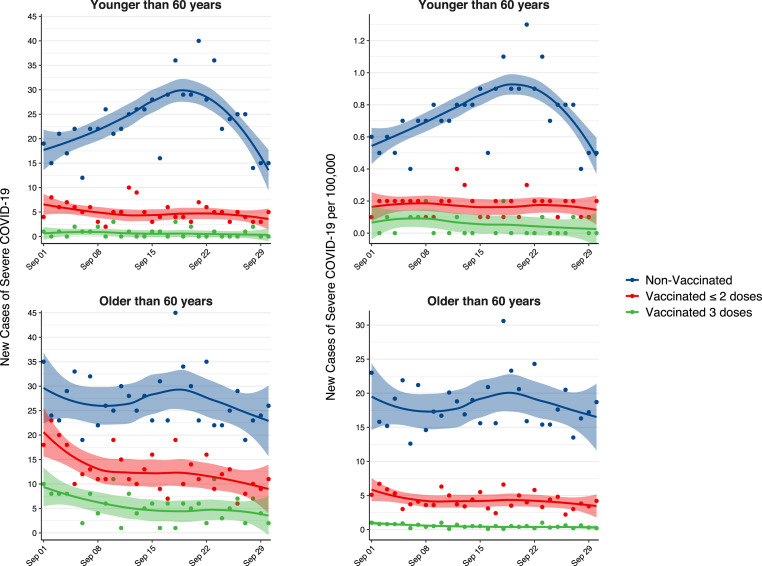
The recent surge in COVID-19 cases led Israeli authorities to recommend a third (booster) anti-SARS-CoV-2 vaccine, initially only in adults aged over 60 years, in late July 2021. The booster program was gradually offered to all individuals 12 years or older, resulting in a nationwide 36% third-dose vaccination rate by the beginning of October 2021. According to a recent Israeli study, the booster reduced the risk of infection by 11-fold and the risk of severe illness by nearly 20-fold in persons 60 years or older [1].
We utilized the Israeli Ministry of Health COVID-19 Dashboard [2], which publishes nationwide daily statistics, to explore the effect of a booster vaccine (BNT162b2) on SARS-CoV-2 infection and severe illness (a proxy for hospitalized patients), during September 2021 (Fig. 1 ). All incidence rates are reported per 100,000. The mean daily incidence of infections in individuals younger than 60 years was 66.9 (95% CI, 57.6–76.2) in the non-booster (1–2 doses) group compared with 14.8 (95% CI, 11.7–17.9) in the booster (3 doses) group, corresponding to an incidence ratio of 0.22 (95% CI, 0.22–0.23). The estimates were generally lower in the elderly (aged over 60 years), with a mean daily incidence of 39.0 (95% CI, 33.9–44.1) and 6.2 (95% CI, 5.2–7.2) in the non-booster and booster groups, respectively (incidence ratio 0.16 [95% CI, 0.15–0.17]). For severe illness, despite a mean daily incidence of 0.18 (95% CI, 0.15–0.21) for individuals under 60 without the booster, there were barely any reports in those vaccinated with the booster (mean 0.06 [95% CI, 0.04–0.09], incidence ratio 0.33 [95% CI, 0.21–0.52]). In fact, there were no new cases of severe illness in the booster group during 50% of the follow-up period. In the elderly, the booster group showed a mean daily incidence of 0.51 (95% CI, 0.41–0.61) severe cases compared to the non-booster group (4.29 [95% CI, 3.86–4.73], incidence ratio 0.12, [95% CI, 0.10–0.14]). Of note, a trend toward less new cases was observed in non-vaccinated young individuals, possibly due to the initial effect of the booster on overall prevalence of infected individuals, or to the containment measures recently implemented in Israel. Unfortunately, these effects cannot be easily disentangled given the dynamic nature of real-world data.
Fig. 1.
Daily incidence of COVID-19 infections or severe illness in the Israeli population following a nationwide booster vaccine program during September 2021. Individuals who received 3 doses of the vaccine at least 7 days earlier, 1–2 doses of the vaccine at least 6 months earlier, or were not vaccinated are represented by green, red, and blue lines, respectively. Upper graphs denote individuals 60 years and younger; lower graphs denote individuals older than 60 years. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The need for a booster vaccine has been heatedly debated in recent months, raising both clinical questions and ethical dilemmas such as equitable vaccine allocation [3]. Recently, national regulators approved a booster dose for Americans aged over 65 or Britons aged over 50, [4] potentially setting the stage for routine booster administration in the case of COVID-19 becoming a seasonal infection [5]. While risk reduction estimates cannot be adjusted for other covariates using aggregated data, our analysis shows an overall remarkable protective effect of a booster vaccine among adults aged under 60 years.
Authors' contributions
Concept and design (ES, RG); Acquisition, analysis, or interpretation of data (DB, ES, RG); Drafting of the manuscript (DB, ES, RG), Critical revision of the manuscript (DB, ES, RG), Statistical analysis (DB), Administrative, technical, or material support (DB, ES, RG).
Funding
This study did not receive funding.
Ethics
This study did not require institutional review board authorization.
Declaration of competing interest
The authors report no conflict of interest.
References
- 1.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N Engl J Med. 2021 doi: 10.1056/NEJMoa2114255. NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Israeli Ministry of Health COVID-19 Data Dashboard https://datadashboard.health.gov.il/COVID-19/general accessed.
- 3.Schaefer G.O., Leland R.J., Emanuel E.J. Making vaccines available to other countries before offering domestic booster vaccinations. J Am Med Assoc. 2021;326:903. doi: 10.1001/jama.2021.13226. [DOI] [PubMed] [Google Scholar]
- 4.Factbox . Reuters; 2021. Countries weigh need for booster COVID-19 shots.https://www.reuters.com/business/healthcare-pharmaceuticals/countries-weigh-need-booster-covid-19-shots-2021-09-24/ published online Sept 24. [Google Scholar]
- 5.Murray C.J.L., Piot P. The potential future of the COVID-19 pandemic: will SARS-CoV-2 become a recurrent seasonal infection? J Am Med Assoc. 2021;325:1249. doi: 10.1001/jama.2021.2828. [DOI] [PubMed] [Google Scholar]