Abstract
Few pharmaceutical agents for slowing Parkinson's disease (PD) progression existed, especially for perimenopause females. The current general medications are mostly hormone replacement therapy and may have some side effects. Therefore, there is an urgent need for a novel treatment for PD. This study examined the possibility of estradiol plus lithium chloride (LiCl), one of the metal halides used as an alternative to salt. We showed that the combination of LiCl and estradiol could enhance neurogenesis proteins GAP-43 and N-myc in the human neuronal-like cells. We also further confirmed the neurogenesis activity in zebrafish. LiCl and LiCl plus estradiol could enhance 6-OHDA-induced upregulation of TGase-2b and Rho A mRNA expression. Besides, LiCl plus estradiol showed a synergic effect in anti-apoptotic activity. LiCl plus estradiol protected SH-SY5Y cells and zebrafish against 6-OHDA-induced damage on neurons than LiCl or estradiol alone groups via p-P38, p-Akt, Bcl-2, and caspase-3 cascade. The potential for developing this combination as a candidate treatment for PD is discussed.
1. Introduction
Parkinson's disease (PD) is one of the most complex neurodegenerative diseases in the world. By gender, women were observed with a less frequency than in men, about 1 : 1.5. However, Picillo et al. demonstrated that perimenopause might increase the possibility of developing a neurodegenerative disease in female patients [1]. Hence, hormone therapy has become a choice in perimenopause patients. The first study that revealed the neuroprotective role of estrogen came from Hall et al. This study examined the therapeutic efficacy of estrogen in different gender of traumatic brain injury models, and the result showed that female gerbils suffered less than male gerbils [2]. Some further studies used exogenous estrogen to inhibit the volume of thrombosis in the ischemia rat model [3, 4]. Gibson et al. systemically reviewed 161 original articles, including 1304 individuals, and revealed that estrogen had a significant therapeutic effect in neurodegenerative animal models [5]. Then, hormone replacement therapy (HRT) began to be used in patients.
Nowadays, the application of HRT is used chiefly on perimenopause women and combined with progesterone. There were some advantages of HRT. First, it could relieve the symptoms of menopausal syndrome, which helped improve their life quality. In addition, HRT could decrease the incidence of cardiovascular disease in perimenopause women [6]. Some studies also proved that estrogen could improve the symptoms by reducing low-density lipoprotein (LDL) [7, 8]. HRT could also prevent osteoporosis by activating the osteoblast's maturation and inhibiting the osteoclast [9]. However, previous studies revealed that some severe adverse effects showed up after the treatment of HRT. Rossouw et al. demonstrated that HRT could slightly increase the incidence of breast cancer [10]. Besides, Anderson et al. found that HRT could increase the possibility of septic shock in perimenopausal women [11]. Then, Hampton et al. also depicted that HRT would enhance the death rate of lung cancer patients [12]. Due to the adverse effects mentioned above, some studies attempted to combine other agents to reduce the dose of estrogen and further improve menopausal symptoms.
Wimalawansa et al. showed that the combination of estrogen and etidronate could increase patients' bone mineral density (BMD) by about 10.9% in vertebrae and 7.25% in femora in 4 years of treatment compared to the placebo group, respectively. The patients treated with etidronate alone increase by 6.79% and 1.2%, and those treated with estrogen alone increase by 6.78% and 4.01% [13]. Kinn et al. also depicted that the combination of estrogen and phenylpropanolamine could significantly improve symptoms in patients, such as subjective appraisal, micturition schedules, pad-test, and urodynamic methods than either drug alone [14]. Geusens et al. also showed that the osteogenesis effect of combining vitamin D with estrogens is more effective than estrogens alone. They also conclude that the treatment of estrogens could prevent bone loss from ovariectomy in female rats. These studies showed that the feasibility of the combination of estrogen with other agents. They only focused on the osteogenesis effect of estrogen with some compounds. Furthermore, some studies investigated the neuroprotective activity of the combination of estrogen and other agents in experimental models, such as estrogen plus agomelatine, estrogen plus dihydrotestosterone, or estradiol plus testosterone [15–19]. Although these combinations did not apply to clinical PD treatment, none of them were used at the bedside. Thus, our studies intend to find a new combination to improve its neuroprotective effect.
Lithium chloride (LiCl) is one metal halide that was used as an alternative to salt. LiCl was also used as a medication for bipolar disorders [20]. This indication also demonstrated that LiCl might have the ability to cross the blood-brain barrier (BBB). Previous studies also support this hypothesis [21, 22]. Some research then investigated the neuroprotective activity of LiCl. Fan et al. depicted that treatment of LiCl can improve the pathological changes and cognitive outcome via upregulating p-Akt and p-GSK3β expressions in repeated ischemia-reperfusion brain injury [23]. Liu et al. demonstrated that administration of LiCl could attenuate sensorimotor dysfunction and cognitive impairment in intracerebral hemorrhage rats through inhibition of GSK-3β pathway and NF-κB pathway [24]. Boyko et al. also indicated that LiCl could significantly attenuate interleukin-6 and prostaglandin E2 in the rat septic shock model [25]. Doeppner et al. also found that LiCl treatment alleviated the infiltration of white blood cells and activation of microglia cells in the rat septic shock model [26]. The studies mentioned above revealed the possibility of LiCl in the co-treatment of estrogen.
Previous literature demonstrated that some clinical PD drugs, such as rasagiline, amantadine, and talipexole, have been shown to confer neuroprotective activity in the SH-SY5Y cell model with neurotoxins, such as 6-OHDA or 1-methyl-4-phenylpyridinium (MPP+) [27–30]. Even the recent study made by Varier et al. used amantadine as positive control in 6-hydroxydopamine- (6-OHDA-) treated SH-SY5Y model to assess the neuroprotective effect with a tropolone-related compound, Hinokitiol. Hence, we evaluated the neuroprotective activity in 6-OHDA-treated SH-SY5Y cell model. Furthermore, many studies have indicated that the administration of 6-hydroxydopamine to zebrafish specifically destroys the dopamine neurons [31–35]. The study made by Feng et al. used zebrafish as a model to examine some potential compounds or clinical drugs, such as vitamin E, minocycline, and even Sinemet®, to confirm the feasibility of the 6-OHDA-induced PD model [36]. Besides, Cronin et al. showed that zebrafish could reflect preliminary clinical findings for rasagiline and minocycline. Hence, they thought a zebrafish model is suitable for high-throughput screening of putative neuroprotective and neuro-restorative therapies to treat Parkinson's disease [35]. The studies mentioned above revealed that the 6-OHDA-induced zebrafish PD model might provide helpful information on assessing the neuroprotective effect. Thus, our study intended to examine the therapeutic effects of combined estrogen and lithium chloride therapy in these two models. We aimed to confirm the mechanism of action of combined therapy and examine the efficacy of combined therapy with these two agents, respectively. We hope our study could benefit the current treatment situation.
2. Materials and Methods
2.1. Cell Lines
The SH-SY5Y cell line was purchased from American Type Culture Collection (ATCC, No. CRL-2266). The cells were incubated with Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen Corporation, Carlsbad, CA, USA) in a 5% CO2 37°C incubator. Cell numbers for each assay were listed as follows: cell survival assay (trypan blue): 1.8 × 106/well for 6-well microplates (passage 21–24); cell survival assay (AlamarBlue assay): 3 × 104/well for 96-well microplates, 1.8 × 106/well in 6-well culture plate containing coverslips (24 × 24 mm) for TUNEL staining; and 1 × 106/dish in the 10 cm dish for Western blot analysis.
2.2. Cell Viability
We examined the cell viability with two methods as follows. The cultured cells were incubated with 100 nM β-estradiol (E2) and different concentrations (1 and 5 mM) of lithium chloride (LiCl) for 1 hr before 18-hour incubation of 6-hydroxydopamine (6-OHDA) (Sigma, St. Louis, MO, USA). Then, 10 μl AlamarBlue (Invitrogen, Waltham, MA, USA) in each well was added and measured by ELISA reader (595 nM). Cell viability was calculated as 100 × [(optical density (OD) of 6-OHDA/LiCl plus E2-treated cells − OD of blank-treated cells)/(OD of control cells − OD of blank-treated cells)]. Another method was using trypan blue assay. The cultured cells were treated for 100 nM E2 and 1 mM of LiCl for 1 hr before 18-hour treatment of 20 μM 6-OHDA. The cell numbers were counted by bright field microscopy with 0.4% trypan blue. 10 μl cell suspension was mixed with 10 μl trypan blue and put in a counting chamber. The final concentrations we used in our study are listed below: LiCl (1 or 5 mM), E2 (100 nM), and 6-OHDA (20 μM) in the SH-SY5Y cell model; LiCl (1 mM), E2 (100 nM), and 6-OHDA (250 μM) in the zebrafish model. The 6-OHDA concentrations we used in in vitro and zebrafish model can be referred to in our previous literature [37, 38]. The LiCl concentrations we used in the in vitro model can be referred to in Hou et al. [39], and the E2 concentration we used in the in vitro model can be referred to in Pan et al. [40]. The LiCl and E2 concentrations we used in zebrafish model came from our own test (data not shown).
2.3. TUNEL Stain
The SH-SY5Y cells in 6-well microplates were treated with 100 nM E2 and 1 mM of LiCl for 1 hr before 8-hour treatment of 20 μM 6-OHDA. Then, we used in situ cell death detection kit, POD (Roache Diagnostics, Germany), to assess the cell apoptosis according to the manufacturer. In brief, we washed cells with phosphate-buffered saline (PBS) (NaCl (137 μM), KCl (2.7 mM), Na2HPO4 (10 mM), and KH2PO4 (1.8 mM)) twice and fixed them with 4% paraformaldehyde. Then, the cells were blocked by 3% H2O2 of methanol and stained with a TUNEL reaction mixture (with DAPI) in a 37°C CO2 incubator. We then observed the signal by fluorescence microscopy. Apoptotic cells were validated by fluorescent signal with a microscope (Olympus, Tokyo, Japan, IX-51), and the ratio of apoptotic cells was measured as follows: number of apoptotic cells/total cell number from three random fluorescent images.
2.4. Western Blotting
The cells and zebrafish were treated in 6 cm dish as designed. In cell, the cell pellet was collected and lysed with an equal volume of lysis buffer (Tris-HCl (10 mM, pH = 8.0), EDTA (1 mM), EGTA (0.5 mM), Triton X-100 (1%), sodium deoxycholate (0.1%), SDS (0.1%), and NaCl (140 mM) contained 0.1% protease inhibitor cocktail). The obtained protein was then adjusted to the same concentration with Bradford protein assay and added sample buffer (SDS (2%), glycerol (10%), bromophenol blue (0.1%), 2-mercaptoethanol (2%), and Tris-HCl (50 mM), pH = 7.2). The prepared protein samples (approximately 100 μg for each sample) were processed by the SDS-PAGE electrophoresis separation. The target proteins were then transferred to an activated PVDF membrane. The PVDF membrane was first blocked with 5% non-fat dry milk in Tris-buffered and then incubated with the primary antibody overnight at 4°C. A horseradish peroxidase-conjugated secondary antibody was detected by chemiluminescence (Millipore Corp., Billerica, MA, USA). Images were obtained using the UVP BioChemi Imaging System, and LabWorks 4.0 software (UVP) was used to quantify the relative densitometry. The relative density of protein expressions was normalized by the internal control β-actin.
2.5. Zebrafish Maintenance
We used wild-type zebrafish (AB strain) in this study. The embryos were obtained through natural spawning and screened at the typical stages by standard criteria [41]. The embryos were incubated at 28.5°C in Hank's buffer (NaCl (13.7 mM), KCl (540 μM), Na2HPO4 (25 μM), KH2PO4 (44 μM), CaCl2 (300 μM), MgSO4 (100 μM), and NaHCO3 (420 μM)).
2.6. The 6-OHDA-Induced Zebrafish PD Model
6-OHDA dissolved in 2% freshly prepared L-ascorbic acid (Sigma, St. Louis, MO, USA) [42]. LiCl (1 or 5 mM) and E2 (100 nM) were treated from 9 hours postfertilization (hpf) to 5 days postfertilization (dpf) in 24-well microplates. We then co-treated 6-OHDA (250 μM) from 2 to 5 dpf. The qPCR and protein sample were obtained at 3 dpf and 5 dpf, respectively. The protocol of zebrafish behavior can be referred to in a previous study [36]. In brief, zebrafish larvae were put into the cuvette, and then their behavior was monitored by an automated video tracking system (Singa Technology Co.; catalog no. TM-01). The cuvette size used in this study was 1-1-4.5 cm (L-W-H) and put in front of the camera for 7.5 cm. Every zebrafish in the test was given a 2 min adaptation time; then the swimming pattern and swimming total distance of each fish was recorded for 5 min.
2.7. Zebrafish RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Zebrafish embryos were treated for 87 hr with LiCl (1 or 5 mM) and E2 (100 nM) in the presence or absence of 6-OHDA. Total RNA was extracted from 20 zebrafish larvae of each treatment group using the TRIzol_Reagent (Invitrogen, Waltham, MA, USA). RNA was reverse-transcribed to single-stranded cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA; catalog no. 1708891). The following reverse transcription-polymerase chain reaction (RT-PCR) using the gene expression assay primer for zebrafish was used.
Primers are as follows:
BDNF: forward: 5″- ATAGTAACGAACAGGATGG -3″
reverse: 5″- GCTCAGTCATGGGAGTCC -3″;
Bcl-2: forward: 5″-TCAATAAAGCAGTGGAGGAATC-3″
reverse: 5″- TCAAATGAGGGTCTGAACGAG -3″;
Rho A: forward: 5″-TCCTGAGGTTTACGTTCCCA-3″
reverse: 5″- TGGCCAGCTGTATCCCATAG-3″;
TGase: forward: 5″-CTCGTGGAGCCAGTTATCAACAGCTAC-3″
reverse: 5″- TCTCGAAGTTCACCACCAGCTTGTG-3″;
Caspase-3: forward: 5″-AGGACTCTAGACGGCATCCA-3″
reverse: 5″-CAGTGAGACTTGGTGCAGTG-3″
GAPDH: forward: 5″-GAAGGTGAAGGTCGGAGTC -3″
reverse: 5″- GAAGATGGTGATGGGATTTC -3″.
We then performed real-time PCR using the Fast SYBR™ Green Master Mix (Bio-Rad, Hercules, CA, USA; catalog no. 4385612) for zebrafish in the Bio-Rad real-time PCR system. The expression level of each gene was expressed as a relative fold change (log2 ratio) that was calculated using the comparative Ct method and GAPDH as the internal reference.
2.8. Chemicals and Antibodies
6-Hydroxydopamine (6-OHDA) (Sigma, St. Louis, MO, USA; catalog: H4381)
β-Estradiol (E2) (Sigma, St. Louis, MO, USA; catalog: E8875)
Lithium chloride (LiCl) (Sigma, St. Louis, MO, USA; catalog: L9650)
AlamarBlue (Invitrogen, Waltham, MA, USA; catalog: 786–922)
β-Actin (loading control; Sigma, St. Louis, MO, USA; catalog: A5441; 1 : 3000 dilution)
p-ERK (Cell Signaling Technology, Danvers, MA, USA, Thr202/204; catalog: 9101; 1 : 1000 dilution)
ERK (Cell Signaling Technology, Danvers, MA, USA; catalog: 9102; 1 : 1000 dilution)
p-P38 (Cell Signaling Technology, Danvers, MA, USA; Thr180/Thr182, catalog: 9211; 1 : 1000 dilution)
P38 (Cell Signaling Technology, Danvers, MA, USA; catalog: 9212; 1 : 1000 dilution)
p-Akt (Cell Signaling Technology, Danvers, MA, USA; catalog: 9271; 1 : 1000 dilution)
Akt (Cell Signaling Technology, Danvers, MA, USA; catalog: 9272; 1 : 1000 dilution)
Growth-associated protein 43 (GAP-43) (Sigma-Aldrich, St. Louis, MO, USA; catalog: 951421273; 1 : 1000 dilution)
N-myc (Thermo Fisher Scientific Inc., Waltham, MA, USA; catalog: PIPA5117717; 1 : 1000 dilution)
Tyrosine hydroxylase (TH) (Millipore Corp., Billerica, MA, USA; catalog: Mab318; 1 : 1000 dilution)
2.9. Statistical Analysis
Results were presented as mean ± SEM. For western blotting data, the intensity of each band was expressed as the relative integrated density divided by the average integrated density values from all internal controls. Data were analyzed using one-way analysis of variance followed by Tukey's test. p value less than 0.05 was considered statistically significant.
3. Results
3.1. Effect of Lithium Chloride Plus Estradiol on 6-OHDA-Induced Apoptosis in SH-SY5Y Neuroblastoma Cell Line
We firstly examined the neuroprotective effect of the combination of lithium chloride and estradiol (E2) on the SH-SY5Y neuroblastoma cell line. The following tests evaluated the anti-apoptotic activity, including cell viability, TUNEL staining, cleavage caspase-3 mRNA, and protein expression. AlamarBlue was used to assess cell viability at 16 hr. The data showed that treatment of 20 μΜ 6-OHDA significantly inhibited cell viability (100.0 ± 4.5% to 32.0 ± 5.3%). Moreover, 100 nM E2, 1 mM, or 5 mM LiCl treatment alone did not affect cell viability. The results demonstrated that 1 mΜ LiCl in the absence or presence of 100 nM E2 could significantly reverse 6-OHDA-induced neuronal toxicity (32.0 ± 5.3% to 60.7 ± 2.4%) (Figure 1(a)). Besides, the co-treatment of 1 mΜ LiCl plus 100 nM E2 showed better therapeutic efficacy than LiCl or E2 alone (32.0 ± 5.3% to 78.6 ± 3.3%). However, the higher dose of LiCl (5 mΜ) showed no neuroprotective activity either in the presence or absence of E2 (Figure 1(a)). In trypan blue assay, 20 μΜ 6-OHDA treatment significantly inhibited cell viability (100.0 ± 9.4% to 25.5 ± 7.5%). The results demonstrated that 1 mΜ LiCl in the absence or presence of 100 nM E2 could significantly reverse 6-OHDA-induced neuronal toxicity (25.5 ± 7.5% to 63.5 ± 8.5%). The therapeutic effect of combination treatment was better than E2 or LiCl alone (Figure 1(b)). TUNEL was further used to detect DNA fragmentation during apoptosis. The data showed that treatment of 20 μM 6-OHDA for 8 hr significantly increased TUNEL staining compared with the control group (3.5 ± 2.9% to 58.5 ± 6.4%), and the co-treatment of 1 mΜ LiCl plus E2 significantly reduced the number of TUNEL-positive cells (58.5 ± 6.4% to 10.5 ± 2.8%). Besides, the treatment of LiCl or E2 in the 6-OHDA-challenge group was 32.4% ± 6.5% and 22.9% ± 3.7%, respectively (Figure 2(a)). Then, we further checked the activated caspase-3 mRNA and protein expression in 6-OHDA-induced SH-SY5Y cells after the treatment of 6-OHDA for 8 hr. The data depicted that treatment of 1 mΜ LiCl or 100 nM E2 significantly attenuated 6-OHDA-induced upregulation of active caspase-3 expression (566.2 ± 33.5% to 390.4 ± 40.3%; 566.2 ± 33.5% to 453.4 ± 32.4%), respectively. Our result demonstrated that 1 mΜ LiCl plus 100 nM E2 significantly attenuated 6-OHDA-induced upregulation of cleaved caspase-3 mRNA expression (566.2 ± 33.5% to 222.5 ± 10.2%) (Figure 2(b)). The protein expression was also consistent with mRNA expression. The co-treatment of 1 mΜ LiCl plus 100 nM E2 significantly attenuated 6-OHDA-induced upregulation of cleaved caspase-3 protein expression (133.4 ± 5.4% to 70.3 ± 10.3%) (Figure 2(c)). In addition, we examined the upstream cascade included p-Akt, p-P38, and p-ERK expression.
Figure 1.

Effect of estradiol (E2) plus lithium chloride (LiCl) on 6-OHDA-induced SH-SY5Y cell death. (a) The SH-SY5Y cells were pretreated with 1 or 5 mM LiCl for 1 hr in the absence or presence of 100 nM E2 and then challenged with 20 μM 6-OHDA for 16 hr. Cell viability of control, 6-OHDA, 6-OHDA plus 1 mM LiCl, 6-OHDA plus 1 mM LiCl and 100 nM E2, 6-OHDA plus 5 mM LiCl, 6-OHDA plus 5 mM LiCl and 100 nM E2, 6-OHDA plus 100 nM E2, 100 nM E2 alone, 1 mM LiCl alone and 5 mM LiCl alone. Cell viability was assessed by AlamarBlue. (b) The SH-SY5Y cells were pretreated with 1 mM LiCl for 1 hr in the absence or presence of 100 nM of E2 and then challenged with 20 μM 6-OHDA for 16 hr. Cell viability of control, 6-OHDA, 6-OHDA plus 1 mM LiCl, 6-OHDA plus 1 mM LiCl and 100 nM E2, and 6-OHDA plus 100 nM E2 by trypan blue. The value corresponds to the mean ± SEM of five independent experiments that have been done in triplicate (n = 15). ∗p < 0.05 compared with the control group; #p < 0.05 compared with the 6-OHDA group.
Figure 2.

The anti-apoptosis effect of E2 plus lithium chloride on 6-OHDA-induced damage in SH-SY5Y cells. (a) The SH-SY5Y cells were pretreated with 1 mM LiCl for 1 hr in the absence or presence of 100 nM E2 and then challenged with 20 μM 6-OHDA for 8 hr. Apoptotic effect of control, 6-OHDA, 6-OHDA plus 1 mM LiCl, 6-OHDA plus 1 mM LiCl, and 100 nM E2 and 6-OHDA plus 100 nM E2 groups. TUNEL staining was performed, and white arrows represent apoptotic cells (scale bar, 100 μm). The quantification result of apoptotic cells is shown. (b) The SH-SY5Y cells were pretreated with 1 mM LiCl for 1 hr in the absence or presence of 100 nM E2 and then challenged with 20 μM 6-OHDA for 8 hr in control, 6-OHDA, 6-OHDA plus 1 mM LiCl, 6-OHDA plus 1 mM LiCl, and 100 nM E2 and 6-OHDA plus 100 nM E2 groups. (c) The SH-SY5Y cells were pretreated with 1 mM LiCl for 1 hr in the absence or presence of 100 nM E2 and then challenged with 20 μM 6-OHDA for 8 hr in control, 6-OHDA, 6-OHDA plus 1 mM LiCl, 6-OHDA plus 1 mM LiCl, and 100 nM E2 and 6-OHDA plus 100 nM E2 groups. Western blotting of cleaved caspase-3 protein and quantification of each group are shown. β-Actin was used as an internal control for normalization. The value corresponds to the mean ± SEM of three independent experiments that have been done in triplicate (n = 9). ∗p < 0.05 compared with the control group; #p < 0.05 compared with the 6-OHDA group.
3.2. Effect of LiCl plus Estradiol on 6-OHDA-Induced Downregulation of p-ERK and p-Akt And Upregulation of p-P38 Expression
The neuroprotective activity by co-treatment of LiCl plus E2 was proven to be mediated via the transient activation of the MAPK pathway. We confirmed the effects of LiCl plus E2 on p-Akt, p-ERK, and p-P38 expressions. The result depicted that 100 nM E2 or 1 mM LiCl treatment alone did not affect p-P38 and p-ERK expressions but increase in p-Akt expression (100.0 ± 4.6% to 123.4 ± 5.6%; 100.0 ± 4.6% to 135.7 ± 4.6%) respectively. Besides, the pretreatment 100 nM E2 or 1 mM LiCl in the 6-OHDA-challenge group could significantly reversed the 6-OHDA-induced down-regulation of p-Akt and p-ERK and enhanced 6-OHDA-induced upregulation of p-P38 expression. Further, the pretreatment of 100 nM E2 plus 1 mΜ LiCl significantly reversed 6-OHDA-induced downregulation p-ERK expressions and enhanced 6-OHDA-induced upregulation of p-P38 than each group (Figure 3(a)). The quantitative results also showed the same trend (Figure 3(b)). Except for apoptosis cascade, we also investigated about the neurogenesis effect of LiCl and E2 on neuronal cell.
Figure 3.

Effect of lithium chloride and E2 on 6-OHDA-induced downregulation of phospho-extracellular signal-regulated kinases (p-ERK), phospho-Akt (p-Akt), and p-P38 in SH-SY5Y cells. (a) SH-SY5Y cells were pretreated with 1 mM LiCl for 1 hr in the absence or presence of 100 nM E2 and then challenged with 20 μM 6-OHDA for 1 hr. Western blotting for p-ERK, p-Akt, and p-P38 of the control, 6-OHDA, 6-OHDA plus 1 mM LiCl, 6-OHDA plus 100 nM E2, 6-OHDA plus 1 mM LiCl and 100 nM E2, 100 nM E2 alone and 1 mM LiCl alone groups. (b) The quantification results of p-ERK, p-Akt, and p-P38 relative density are shown. The value corresponds to the mean ± SEM of three independent experiments that have been done in triplicate (n = 9). ∗p < 0.05 compared with the control group; #p < 0.05 compared with the 6-OHDA group.
3.3. Effect of LiCl plus Estradiol on Neurogenesis Factor Growth Associated Protein 43 (GAP43), N-myc, and TGase Protein Expression
We would like to examine further the neurogenesis-related marker that included growth-associated protein 43 (GAP43), N-myc, and TGase protein expression. Our data showed that the administration of 1 mM LiCl or 100 nM E2 could significantly increase the GAP-43, N-myc, and TGase expression (Figure 4(a)). Besides, the co-administration of LiCl and E2 significantly increase GAP-43 and N-myc protein expression than each group alone. The quantitative result also showed the same trend (Figures 4(b) and 4(c)). Thus, the result depicted that the co-treatment of LiCl and E2 may have a synergic effect on neurogenesis activity. Then, we would like to investigate further the neuroprotective activity in vivo zebrafish PD model.
Figure 4.

Effect of lithium chloride and estradiol on growth associated protein 43 (GAP-43), N-myc, and TGase in SH-SY5Y cells. (a) SH-SY5Y cells were co-treated with 1 mM LiCl in the absence or presence of 100 nM E2 for 24 hr. Western blotting for GAP-43 and N-myc of the control, 1 mM LiCl, 100 nM E2 and 1 mM LiCl plus 100 nM E2 groups. (b) The quantification result of GAP-43 relative density is shown. (c) The quantification result of TGase relative density is shown. (d) The quantification results of N-myc relative density are shown. β-Actin was used as an internal control for normalization. The value corresponds to the mean ± SEM of three independent experiments that have been done in triplicate (n = 9). ∗p < 0.05 compared with the control group; #p < 0.05 compared with the 6-OHDA group.
3.4. Neuroprotective Effect of LiCl plus Estradiol on Zebrafish Locomotor Deficit
We then confirmed the locomotor activity and behavior of zebrafish in the zebrafish PD model. We firstly investigated the neuroprotective effect and dosage of E2 in zebrafish PD model. Zebrafish were pretreated with 10, 100, or 1000 nM E2 (9 hpf to 5 dpf) and in the absence or presence of 250 μM 6-OHDA (2 to 5 dpf). We then analyzed zebrafish locomotor activity at 5, 6, and 7 dpf. The results showed that the treatment of 250 μM 6-OHDA significantly decreased the total swimming distance of zebrafish in 5, 6, and 7 dpf (Figure 5). However, the pretreatment of 100 nM E2 significantly reversed 6-OHDA-induced down-regulation of total swimming distance in 6 and 7 dpf. The pretreatment of 1000 nM E2 only relived 6-OHDA-induced downregulation of total swimming distance at 5 dpf. Hence, we use 100 nM E2 in the follow-up experiment. We then examined the neuroprotective activity of the co-treatment of LiCl plus E2 in the same model. Zebrafish were pretreated with 1 or 5 mM LiCl plus 100 nM E2 (9 hpf to 5 dpf) and in the absence or presence of 250 μM 6-OHDA (2 to 5 dpf). We also analyzed zebrafish locomotor activity at 5, 6, and 7 dpf. Our data demonstrated that administration of 1 mM LiCl with or without 100 nM E2 could significantly reverse 6-OHDA-induced downregulation of locomotor activity at 5, 6, and 7 dpf (Figure 6). Moreover, the combination treatment showed a synergic effect than alone groups only in 6 dpf but not in 5 and 7 dpf. However, the higher dosage of 5 mM LiCl with or without 100 nM E2 showed no neuroprotective effect in 5, 6, and 7dpf (Figure 6). Hence, we would like to use 1 mM LiCl for the further molecular marker examination.
Figure 5.

Effect of estradiol on 6-OHDA-induced locomotor deficit in the zebrafish PD model. Zebrafish were pretreated with 10, 100, and 1000 nM E2 from 9 hpf to 5 dpf and then challenged with 250 μM 6-OHDA from 2 to 5 dpf. The locomotor behavior was measured at 5, 6, and 7 dpf of the control, 6-OHDA, 6-OHDA plus 10 nM E2, 6-OHDA plus 100 nM E2, 6-OHDA plus 1000 nM E2, 10 nM E2, 100 nM E2, and 1000 nM E2 groups. The upper panel demonstrates a representative swimming pattern, and the lower panel shows the average total swimming distance. The value corresponds to the mean ± SEM of two independent experiments that have been done in octuplicate (n = 16). ∗p < 0.05 compared with the control group; #p < 0.05 compared with the 6-OHDA group.
Figure 6.
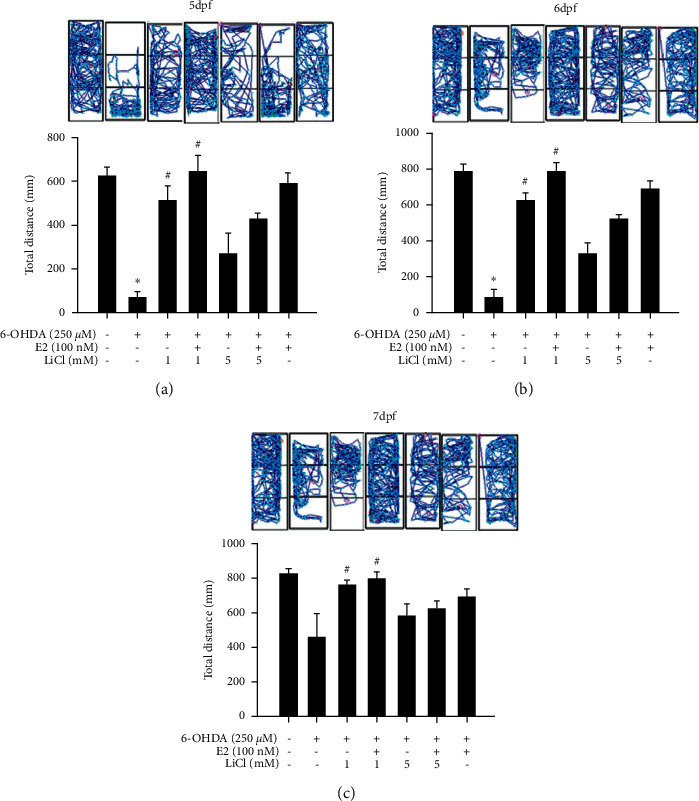
Effect of estradiol plus lithium chloride on 6-OHDA-induced locomotor deficit in the zebrafish PD model. Zebrafish were pretreated 1 or 5 mM LiCl in the absence or presence of 100 nM E2 from 9 hpf to 5 dpf and then challenged with 250 μM 6-OHDA from 2 to 5 dpf of the the control, 6-OHDA, 6-OHDA plus 1 mM LiCl, 6-OHDA plus 100 nM E2, and 6-OHDA plus 1 mM LiCl and 100 nM E2 groups. The upper panel demonstrates a representative swimming pattern, and the lower panel shows the average total swimming distance. The value corresponds to the mean ± SEM of two independent experiments that have been done in octuplicate (n = 16). ∗p < 0.05 compared with the control group; #p < 0.05 compared with the 6-OHDA group.
3.5. Neurogenesis and Neuroprotective Effect of LiCl plus Estradiol on Zebrafish PD Model
We confirmed the neurogenesis and neuroprotective effect of LiCl plus E2 in the zebrafish PD model with qPCR and western blotting. Zebrafish were pretreated with 1 mM LiCl plus 100 nM E2 (9 hpf to 5 dpf) and in the absence or presence of 250 μM 6-OHDA (2 to 5 dpf). The qPCR and western blotting sample were collected at 5 dpf. We selected brain-derived neurotrophic factor (BDNF), Bcl-2 as apoptosis biomarker and transglutaminase 2b (TGase-2b), and Ras homolog family member A (Rho A) as neurogenesis biomarker. Our results demonstrated that treatment of 6-OHDA could significantly decrease BDNF and Bcl-2 mRNA expression (Figures 7(a) and 7(b)). At the same time, 6-OHDA could significantly increase TGase-2b and Rho A mRNA expression (Figures 7(c) and 7(d)). However, the treatment of E2 and LiCl plus E2 groups could significantly reverse 6-OHDA-induced downregulation of BDNF and Bcl-2. The combined group did not show a noticeable effect than alone groups because the effect of one compound is maximal. In addition, the LiCl plus E2 group enhanced 6-OHDA-induced upregulation of TGase-2b and Rho A mRNA expression and the other two groups (LiCl or E2 alone group) showed no significant effect in these two markers. Finally, we also use tyrosine hydroxylase (TH) as dopamine neuron biomarker. The data showed that 6-OHDA treatment could significantly decrease expression of TH from 100.0 ± 4.4% to 54.6 ± 8.4%. The pretreatment of LiCl, E2, and LiCl plus E2 could reverse 6-OHDA-induced downregulation of TH expression to 85.5 ± 4.6%, 130.7 ± 4.1%, and 127.9 ± 6.6%, respectively (Figure 7(e)).
Figure 7.

Neuroprotective effect of lithium chloride plus estradiol on the 6-OHDA-induced zebrafish PD model. (a) Zebrafish were pretreated with 1 mM LiCl (9 hpf to 5 dpf) in the absence or presence of 100 nM E2 and then challenged with 250 μM 6-OHDA (2 to 5 dpf). Quantitative PCR of BDNF was performed. (b) Zebrafish were pretreated with 1 mM LiCl (9 hpf to 5 dpf) in the absence or presence of 100 nM E2 and then challenged with 250 μM 6-OHDA (2 to 5 dpf). Quantitative PCR of Bcl-2 was performed. (c) Zebrafish were pretreated with 1 mM LiCl (9 hpf to 5 dpf) in the absence or presence of 100 nM E2 and then challenged with 250 μM 6-OHDA (2 to 5 dpf). Quantitative PCR of Rho A was performed. (d) Zebrafish were pretreated with 1 mM LiCl (9 hpf to 5 dpf) in the absence or presence of 100 nM E2 and then challenged with 250 μM 6-OHDA (2 to 5 dpf). Quantitative PCR of TGase 2b was performed. (e) Zebrafish were pretreated with 1 mM LiCl (9 hpf to 5 dpf) in the absence or presence of 100 nM E2 and then challenged with 250 μM 6-OHDA (2 to 5 dpf). Western blotting of TH was performed. β-Actin was used as an internal control for normalization. The value corresponds to the mean ± SEM of three independent experiments that have been done in triplicate (n = 9). ∗p < 0.05 compared with the control group; #p < 0.05 compared with the 6-OHDA group.
We summarize a putative diagram to describe the effect of LiCl plus E2 in menopausal PD (Figure 8). The treatment of 6-OHDA in neuron cell could increase p-P38, cleaved caspase-3. In zebrafish, treatment of 6-OHDA also decreases apoptotic related marker BDNF, Bcl-2, and increase neurogenesis related markers TGase-2b and Rho A mRNA expression. The treatment of 6-OHDA also decreases TH protein expression. However, the combination of LiCl and E2 alone could enhance neurogenesis proteins GAP-43 and N-myc in the neuronal cell. We also further confirmed the neurogenesis activity in zebrafish. LiCl and LiCl plus E2 could enhance 6-OHDA-induced upregulation of TGase-2b and Rho A mRNA expression. Besides, LiCl plus E2 also showed a synergic effect in anti-apoptotic activity. LiCl plus E2 protected SH-SY5Y cells and zebrafish against 6-OHDA-induced damage on neuron than LiCl or E2 alone groups via p-P38, p-Akt, Bcl-2, and caspase-3 cascade.
Figure 8.

Schematic diagram of lithium chloride (LiCl) plus estradiol (E2) in 6-OHDA-induced neuronal death. 6-OHDA could induce cell apoptosis through increasing of p-Akt and p-ERK expression, decreasing p-P38, and further modulating the downstream apoptotic proteins, such as Bcl-2 and caspase-3. Our studies demonstrated that the co-treatment of LiCl and E2 could enhance neurogenesis via increasing Rho A, GAP-43, and TGase-2b protein expression. Besides, co-treatment of LiCl and E2 attenuated cell apoptosis through reversing 6-OHDA-induced downregulation of p-Akt, p-ERK and attenuating 6-OHDA-induced upregulation of p-P38 protein expression. The downstream cascade was also modulated, such as Bcl-2 and caspase-3. The 6-OHDA-induced upregulating Bcl-2 expression was reversed by the co-treatment of LiCl plus E2 and 6-OHDA-induced upregulating of caspase-3 expression was also attenuated. The neuroprotective effect of LiCl plus E2 significantly protected dopamine neurons against damage.
4. Discussion
Increasing reports depicted that estrogens may protect the nigrostriatal dopaminergic pathway affected in PD [43–45]. Animal studies and human clinical trials revealed that estrogen could increase dopamine synthesis and accelerate the metabolism of dopamine. Besides, the expression of dopamine receptors could also be affected by estrogen [46]. However, some clinical studies showed that the administration of HRT may exacerbate the PD symptoms and the side effects remain ambiguous [47]. Our studies intend to combine LiCl and E2 in the treatment of PD to enhance the neuroprotective activity of estrogen to improve the HRT. Except for our results, previous studies also combine lithium chloride with other reagents to enhance or increase its therapeutic efficacy. Some studies depicted that the combination of lithium chloride and agents could provide a synergic effect [48, 49]. Bai et al. also showed that LiCl and LY294002 (Akt inhibitor) co-treatment could increase osteogenesis activity and decrease osteoclast differentiation and bone absorption capacity compared with each group alone simultaneously. The co-treatment of LiCl and LY294002 significantly enhanced bone alkaline phosphatase levels and significantly decreased PINP, TRACP-5b, and CTX levels. The co-treatment also had the synergic effect [49]. Not only in animal science but also in plant science, Ali et al. demonstrated the synergic effect of LiCl and alkaline hydrogen peroxide in improving biomethanation at corn stalk [50]. The studies mentioned above all revealed that the feasibility of combination treatment of LiCl with other agents. Our study combined LiCl with estrogen and intends to enhance neuroprotective activity. LiCl plus E2 showed a synergic effect in neuroprotection than LiCl or E2 alone, respectively. The locomotor activity of zebrafish also revealed the same trend. The data also proved that the neuroprotective activity might come from neurogenesis and some reports also demonstrated the function of neurogenesis in PD.
Some research showed that increased neurogenesis could improve PD progress in in vivo or in vitro models [51–54]. Ferrer and Blanco showed that N-myc overexpression in the soma and nuclei of neurons vulnerable PD. The immunoreactivity of N-myc increased in subpopulations of reactive astrocytes in PD. These data suggest that N-myc and c-myc are involved in glial cell differentiation and growth and that this function may also apply to reactive astrocytes in human neurodegenerative disorders [52]. Although some literature reported N-myc as an oncogene in neuroblastoma [55, 56]. The recent study made by Shi in 2021 used N-myc as a biomarker of neurogenesis in SH-SY5Y cell model and other studies considered N-myc as an essential factor in neuronal differentiation. Besides, Hammerling et al. depicted that the mRNA expression of N-myc in SH-SY5Y was found not to change appreciably during the cell cycle and was also unaffected by proliferative inhibition induced by serum starvation or polyamine depletion. Treatment with TPA for 8 days could induce morphological and functional neuronal differentiation [57–59]. Except for N-myc, Park et al. found that the stimulation of IL-1β could lead to the differentiation of neuron cell and could upregulate Wnt5α protein expression via NF-κB. The study also demonstrated that Rho A/ROCK/JNK pathway is involved in neurogenesis and use Rho A as a neurogenesis biomarker [60]. Lopez-Lopez et al. showed that fasudil could attenuate the L-DOPA-induced dyskinesia without decreasing the therapeutic effect of L-DOPA on motor behavior. Besides, the treatment of 6-OHDA significantly increases Rho A protein expression in rat striatum in PD model [61]. Our data showed the same trend that the treatment of 6-OHDA upregulates the expression of Rho A. We further confirmed that the pretreatment of LiCl plus E2 enhanced 6-OHDA-induced upregulation of Rho A expression. Li et al. also showed that the Pilose Antler Extracts (PAEs) reversed 6-OHDA-induced downregulation of the levels of monoamine neurotransmitters and amino acids in the striatum and cerebrospinal fluid in rat PD model. In addition, PAEs also increased the levels of GAP-43 in rat brain tissue, which represents that PAEs may facilitate neuronal growth and plasticity. The result also depicted that PAEs a possible mechanism for the protective effect of PAEs against 6-OHDA-induced neuronal cell death and may tightly correlate with the components in PAEs promoting neurogenesis [51]. Besides, O'Neil et al. also showed an AMPA receptor potentiator can provide neurochemical protection in the rodent PD model. This study also demonstrated that the administration AMPA receptor inhibitor was delayed until after cell death and accompanied by an increase in GAP-43 expression in the striatum provided tantalizing evidence for neurotrophic action [53]. Our data also showed that LiCl plus E2 could significantly increase GAP-43 expression, and the alone group could also do so. Except for the role of neurogenesis in PD progress, some studies also investigated the apoptosis cascade in PD progression.
Many studies demonstrated that the zebrafish animal model is a highly efficient high-throughput screening platform for neuroprotective activity [35, 62–65]. McGrath et al. listed zebrafish are increasingly used for early drug screening studies, especially approaches for assessing drug effects on apoptosis [62]. Cornin et al. showed that zebrafish could be a model suitable for high-throughput drug screening for PD. Their findings for minocycline and rasagiline echoed those studies in rodents and primates, and although, unlike studies in rodents, isradipine was not neuroprotective in zebrafish, which may better reflect the clinical situation. They showed that exposure of zebrafish larvae to 6-OHDA can be used to test for neuroprotection and neuro-restoration in a relatively high throughput manner [35]. Liao et al. also demonstrated that the ShK-like peptide PcShK3 has the ability to confer cardiovascular and neurological protective effects in a zebrafish model of drug screening. The peptide displayed an enjoyable neuroprotective activity that in combination with structurally guided dissection of peptides, PcShK3 suppressed the 6-OHDA-induced neurotoxicity on the locomotive behavior of zebrafish [63]. The same team also used the zebrafish PD model to examine the folded PpVα peptide. They showed the peptide protected PC12 cells against 6-OHDA-induced neurotoxicity via activating heme oxygenase-1 (HO-1) and attenuating inducible nitric oxide synthase (iNOS) protein expression. In vivo, PpVα peptide reversed the 6-OHDA-induced locomotor deficit of zebrafish and prevented the 6-OHDA-induced excessive ROS generation and subsequent dopaminergic neurons loss [64]. Kim et al. also revealed that pretreatment with MgT, and downstream upregulation of glutamate transporter EAAT4, has protective effects on neuronal survival, reduction in cerebral infarction, and preservation of learning and memory in zebrafish following hypoxia. This study also used zebrafish as an in vivo model to examine the neuroprotective activity which MgT could reversed 6-OHDA-induced locomotor deficit in zebrafish PD model [65]. Our study also used zebrafish PD model to examine the neuroprotective and neuro-regenerative activity of LiCl plus E2. The present study depicted that the combination of LiCl and E2 could show synergic protective effect against 6-OHDA damage and neuro-restorative effect than each treatment alone.
To our excitement, our present study shows several advantages of LiCl plus E2. We firstly applied the combination of LiCl plus E2 and intend to use the combination treatment to enhance the neuroprotective effect. The neuroprotective effect is possibly through anti-apoptosis and neurogenesis pathway via the synergic effect of LiCl plus E2. We hope that further experiments and observations could evaluate the specific mechanisms in order to contribute to the clinical application of potential treatments.
5. Conclusions
We firstly examined the neuroprotective activity of LiCl plus E2 in SH-SY5Y in vitro model. The data showed that the combination of LiCl and E2 revealed a better efficacy than LiCl or E2 alone. The results of the TUNEL stain and cleaved caspase-3 protein expression also showed the same trend. The 6-OHDA-induced increase of apoptotic cell number was significantly attenuated by the treatment of LiCl plus E2, and the effect of combination treatment is better than each group alone. LiCl plus E2 protected dopamine neurons against 6-OHDA damage via p-P38, p-Akt, and p-ERK cascade. Besides, LiCl plus E2 also enhanced SH-SY5Y cell neurogenesis related marker included GAP-43, N-myc protein expression. We further used in vivo zebrafish PD model to confirm the neuroprotective activity of LiCl plus E2. The data demonstrated that E2 could protect zebrafish against 6-OHDA damage in locomotor deficit. We further examined LiCl plus E2 in this model. To our excitement, the combination treatment significantly reversed 6-OHDA-induced downregulation of locomotor activity, which is better than each group alone. We also checked some neuroprotection (BDNF, Bcl-2, and TH) and neurogenesis factors (Rho A, TGase-2b) to verify the possible cascade of LiCl plus E2. Except for our results, previous studies also combine lithium chloride with other reagents to enhance or increase its therapeutic efficacy.
Acknowledgments
This research was funded by the Ministry of Science and Technology, Taiwan (109-2314-B-037-101-), the Kaohsiung Medical University Hospital (94-KMUH-022, KMUH109-M910, and KMUH109-9R40) and partially supported by Kaohsiung Medical University Research Center Grant (KMU-TC108A04-0).
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Picillo M., Nicoletti A., Fetoni V., Garavaglia B., Barone P., Pellecchia M. T. The relevance of gender in Parkinson’s disease: a review. Journal of Neurology . 2017;264(8):1583–1607. doi: 10.1007/s00415-016-8384-9. [DOI] [PubMed] [Google Scholar]
- 2.Hall E. D., Pazara K. E., Linseman K. L. Sex differences in postischemic neuronal necrosis in gerbils. Journal of Cerebral Blood Flow and Metabolism . 1991;11(2):292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 3.Simpkins J. W., Rajakumar G., Zhang Y.-Q., et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. Journal of Neurosurgery . 1997;87(5):724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 4.Dubal D. B., Kashon M. L., Pettigrew L. C., et al. Estradiol protects against ischemic injury. Journal of Cerebral Blood Flow and Metabolism . 1998;18(11):1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Gibson C. L., Gray L. J., Murphy S. P., Bath P. M. Estrogens and experimental ischemic stroke: a systematic review. Journal of Cerebral Blood Flow and Metabolism . 2006;26(9):1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- 6.Mosca L., Collins P., Herrington D. M., et al. Hormone replacement therapy and cardiovascular disease. Circulation . 2001;104(4):499–503. doi: 10.1161/hc2901.092200. [DOI] [PubMed] [Google Scholar]
- 7.Knowlton A. A., Lee A. R. Estrogen and the cardiovascular system. Pharmacology & Therapeutics . 2012;135(1):54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker L., Meldrum K. K., Wang M., et al. The role of estrogen in cardiovascular disease. Journal of Surgical Research . 2003;115(2):325–344. doi: 10.1016/s0022-4804(03)00215-4. [DOI] [PubMed] [Google Scholar]
- 9.Härkönen H., Harkonen P. L. Estrogen and bone metabolism. Maturitas . 1996;23(23):S65–S69. doi: 10.1016/0378-5122(96)01015-8. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw J. E., Anderson G. L., Prentice R. L., et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. Jama . 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Anderson G. L., Limacher M., Assaf A. R., et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Jama . 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 12.Hampton T. Lung cancer mortality higher in women who used combination hormone therapy. Jama . 2009;302(6):615–616. doi: 10.1001/jama.2009.1117. [DOI] [PubMed] [Google Scholar]
- 13.Wimalawansa S. J. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four-year randomized study. The American Journal of Medicine . 1995;99(1):36–42. doi: 10.1016/s0002-9343(99)80102-8. [DOI] [PubMed] [Google Scholar]
- 14.Kinn A.-C., Lindskog M. Estrogens and phenylpropanolamine in combination for stress urinary incontinence in postmenopausal women. Urology . 1988;32(3):273–280. doi: 10.1016/0090-4295(88)90400-1. [DOI] [PubMed] [Google Scholar]
- 15.Peng R., Dai W., Li Y. Neuroprotective effect of a physiological ratio of testosterone and estradiol on corticosterone-induced apoptosis in PC12 cells via Traf6/TAK1 pathway. Toxicology in Vitro . 2018;50:257–263. doi: 10.1016/j.tiv.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim W. W., Safar M. M., Khattab M. M., Agha A. M. 17β-Estradiol augments antidepressant efficacy of escitalopram in ovariectomized rats: neuroprotective and serotonin reuptake transporter modulatory effects. Psychoneuroendocrinology . 2016;74:240–250. doi: 10.1016/j.psyneuen.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Abdelkader N. F., Abd El-Latif A. M., Khattab M. M. Telmisartan/17β-estradiol mitigated cognitive deficit in an ovariectomized rat model of Alzheimer’s disease: modulation of ACE1/ACE2 and AT1/AT2 ratio. Life Sciences . 2020;245 doi: 10.1016/j.lfs.2020.117388.117388 [DOI] [PubMed] [Google Scholar]
- 18.El-Khatib Y. A., Sayed R. H., Sallam N. A., Zaki H. F., Khattab M. M. 17β-Estradiol augments the neuroprotective effect of agomelatine in depressive- and anxiety-like behaviors in ovariectomized rats. Psychopharmacology . 2020;237(9):2873–2886. doi: 10.1007/s00213-020-05580-2. [DOI] [PubMed] [Google Scholar]
- 19.Sengelaub D. R., Han Q., Liu N.-K., et al. Protective effects of estradiol and dihydrotestosterone following spinal cord injury. Journal of Neurotrauma . 2018;35(6):825–841. doi: 10.1089/neu.2017.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curran G., Ravindran A. Lithium for bipolar disorder: a review of the recent literature. Expert Review of Neurotherapeutics . 2014;14(9):1079–1098. doi: 10.1586/14737175.2014.947965. [DOI] [PubMed] [Google Scholar]
- 21.Newman S. A., Pan Y., Short J. L., Nicolazzo J. A. Assessing the impact of lithium chloride on the expression of P-glycoprotein at the blood-brain barrier. Journal of Pharmaceutical Sciences . 2017;106(9):2625–2631. doi: 10.1016/j.xphs.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y., Short J. L., Newman S. A., et al. Cognitive benefits of lithium chloride in APP/PS1 mice are associated with enhanced brain clearance of β-amyloid. Brain, Behavior, and Immunity . 2018;70:36–47. doi: 10.1016/j.bbi.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Fan M., Song C., Wang T., et al. Protective effects of lithium chloride treatment on repeated cerebral ischemia-reperfusion injury in mice. Neurological Sciences . 2015;36(2):315–321. doi: 10.1007/s10072-014-1943-x. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z., Li R., Jiang C., Zhao S., Li W., Tang X. The neuroprotective effect of lithium chloride on cognitive impairment through glycogen synthase kinase-3β inhibition in intracerebral hemorrhage rats. European Journal of Pharmacology . 2018;840:50–59. doi: 10.1016/j.ejphar.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Boyko M., Nassar A., Kaplanski J., Zlotnik A., Sharon-Granit Y., Azab A. N. Effects of acute lithium treatment on brain levels of inflammatory mediators in poststroke rats. BioMed Research International . 2015;2015:8. doi: 10.1155/2015/916234.916234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doeppner T. R., Kaltwasser B., Sanchez-Mendoza E. H., Caglayan A. B., Bähr M., Hermann D. M. Lithium-induced neuroprotection in stroke involves increased miR-124 expression, reduced RE1-silencing transcription factor abundance and decreased protein deubiquitination by GSK3β inhibition-independent pathways. Journal of Cerebral Blood Flow and Metabolism . 2017;37(3):914–926. doi: 10.1177/0271678x16647738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitamura Y., Kosaka T., Kakimura J.-I., et al. Protective effects of the antiparkinsonian drugs talipexole and pramipexole against 1-Methyl-4-phenylpyridinium-Induced apoptotic death in human neuroblastoma SH-SY5Y cells. Molecular Pharmacology . 1998;54(6):1046–1054. doi: 10.1124/mol.54.6.1046. [DOI] [PubMed] [Google Scholar]
- 28.Akao Y., Maruyama W., Yi H., Shamoto-Nagai M., Youdim M. B. H., Naoi M. An anti-Parkinson’s disease drug, N-propargyl-1(R)-aminoindan (rasagiline), enhances expression of anti-apoptotic Bcl-2 in human dopaminergic SH-SY5Y cells. Neuroscience Letters . 2002;326(2):105–108. doi: 10.1016/s0304-3940(02)00332-4. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama W., Takahashi T., Youdim M., Naoi M. The anti-Parkinson drug, rasagiline, prevents apoptotic DNA damage induced by peroxynitrite in human dopaminergic neuroblastoma SH-SY5Y cells. Journal of Neural Transmission . 2002;109(4):467–481. doi: 10.1007/s007020200038. [DOI] [PubMed] [Google Scholar]
- 30.Varier K. M., Sumathi T. Hinokitiol offers neuroprotection against 6-OHDA-induced toxicity in SH-SY5Y neuroblastoma cells by downregulating mRNA expression of MAO/α-Synuclein/LRRK2/PARK7/PINK1/PTEN genes. Neurotoxicity Research . 2019;35(4):945–954. doi: 10.1007/s12640-018-9988-x. [DOI] [PubMed] [Google Scholar]
- 31.Anichtchik O. V., Kaslin J., Peitsaro N., Scheinin M., Panula P. Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Journal of Neurochemistry . 2004;88(2):443–453. doi: 10.1111/j.1471-4159.2004.02190.x. [DOI] [PubMed] [Google Scholar]
- 32.Bretaud S., Lee S., Guo S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicology and Teratology . 2004;26(6):857–864. doi: 10.1016/j.ntt.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Parng C., Roy N. M., Ton C., Lin Y., McGrath P. Neurotoxicity assessment using zebrafish. Journal of Pharmacological and Toxicological Methods . 2007;55(1):103–112. doi: 10.1016/j.vascn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Wang M., Zhang Z., Cheang L. C.-V., Lin Z., Lee S. M.-Y. Eriocaulon buergerianum extract protects PC12 cells and neurons in zebrafish against 6-hydroxydopamine-induced damage. Chinese Medicine . 2011;6(1):p. 16. doi: 10.1186/1749-8546-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cronin A., Grealy M. Neuroprotective and neuro-restorative effects of minocycline and rasagiline in a zebrafish 6-hydroxydopamine model of Parkinson’s disease. Neuroscience . 2017;367:34–46. doi: 10.1016/j.neuroscience.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Feng C.-W., Wen Z.-H., Huang S.-Y., et al. Effects of 6-hydroxydopamine exposure on motor activity and biochemical expression in zebrafish (Danio rerio) larvae. Zebrafish . 2014;11(3):227–239. doi: 10.1089/zeb.2013.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng C. W., Hung H. C., Huang S. Y., et al. Neuroprotective effect of the marine-derived compound 11-dehydrosinulariolide through DJ-1-related pathway in in vitro and in vivo models of Parkinson’s disease. Marine Drugs . 2016;14(10) doi: 10.3390/md14100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng C.-W., Chen N.-F., Chan T.-F., Chen W.-F. Therapeutic role of protein tyrosine phosphatase 1B in Parkinson’s disease via antineuroinflammation and neuroprotection in vitro and in vivo. Parkinson’s Disease . 2020;2020:15. doi: 10.1155/2020/8814236.8814236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou L., Xiong N., Liu L., et al. Lithium protects dopaminergic cells from rotenone toxicity via autophagy enhancement. BMC Neuroscience . 2015;16(1):p. 82. doi: 10.1186/s12868-015-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Q., Guo K., Xue M., Tu Q. Estradiol exerts a neuroprotective effect on SH-SY5Y cells through the miR-106b-5p/TXNIP axis. Journal of Biochemical and Molecular Toxicology . 2015;2015 doi: 10.1002/jbt.22861.e22861 [DOI] [PubMed] [Google Scholar]
- 41.Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. Stages of embryonic development of the zebrafish. Developmental Dynamics . 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Shachar D., Eshel G., Finberg J. P. M., Youdim M. B. H. The iron chelator desferrioxamine (desferal) retards 6-hydroxydopamine-induced degeneration of nigrostriatal dopamine neurons. Journal of Neurochemistry . 1991;56(4):1441–1444. doi: 10.1111/j.1471-4159.1991.tb11444.x. [DOI] [PubMed] [Google Scholar]
- 43.Song Y.-J., Li S.-R., Li X.-W., et al. The effect of estrogen replacement therapy on alzheimer’s disease and Parkinson’s disease in postmenopausal women: a meta-analysis. Frontiers in Neuroscience . 2020;14:p. 157. doi: 10.3389/fnins.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y. H., Cha J., Chung S. J., et al. Beneficial effect of estrogen on nigrostriatal dopaminergic neurons in drug-naïve postmenopausal Parkinson’s disease. Scientific Reports . 2019;9(1) doi: 10.1038/s41598-019-47026-6.10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thadathil N., Xiao J., Hori R., Alway S. E., Khan M. M. Brain selective estrogen treatment protects dopaminergic neurons and preserves behavioral function in MPTP-induced mouse model of Parkinson’s disease. Journal of Neuroimmune Pharmacology . 2020;16 doi: 10.1007/s11481-020-09972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.del Pino J., Moyano P., Ruiz M., et al. Amitraz changes NE, DA and 5-HT biosynthesis and metabolism mediated by alterations in estradiol content in CNS of male rats. Chemosphere . 2017;181:518–529. doi: 10.1016/j.chemosphere.2017.04.113. [DOI] [PubMed] [Google Scholar]
- 47.Cagnacci A., Venier M. The controversial history of hormone replacement therapy. Medicina (Kaunas) . 2019;55(9) doi: 10.3390/medicina55090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schleicher S. B., Zaborski J. J., Riester R., et al. Combined application of arsenic trioxide and lithium chloride augments viability reduction and apoptosis induction in human rhabdomyosarcoma cell lines. PLoS One . 2017;12(6) doi: 10.1371/journal.pone.0178857.e0178857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai J., Xu Y., Dieo Y., Sun G. Combined low-dose LiCl and LY294002 for the treatment of osteoporosis in ovariectomized rats. Journal of Orthopaedic Surgery and Research . 2019;14(1):p. 177. doi: 10.1186/s13018-019-1210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali N., Hamouda H. I., Su H., Li F. L., Lu M. Combinations of alkaline hydrogen peroxide and lithium chloride/N,N-dimethylacetamide pretreatments of corn stalk for improved biomethanation. Environmental Research . 2020;186 doi: 10.1016/j.envres.2020.109563. [DOI] [PubMed] [Google Scholar]
- 51.Li C., Sun Y., Yang W., et al. Pilose antler Extracts (PAEs) protect against neurodegeneration in 6-OHDA-induced Parkinson’s disease rat models. Evidence-Based Complementary and Alternative Medicine . 2019;2019:11. doi: 10.1155/2019/7276407.7276407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrer I., Blanco R. N-myc and c-myc expression in Alzheimer disease, Huntington disease and Parkinson disease. Molecular Brain Research . 2000;77(2):270–276. doi: 10.1016/s0169-328x(00)00062-0. [DOI] [PubMed] [Google Scholar]
- 53.O’Neill M. J., Murray T. K., Whalley K., et al. Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson’s disease. European Journal of Pharmacology . 2004;486(2):163–174. doi: 10.1016/j.ejphar.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 54.Santos N. A., Martins N. M., Sisti F. M., et al. The neuroprotection of cannabidiol against MPP⁺-induced toxicity in PC12 cells involves trkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson’s disease. Toxicology . 2015;30(1):231–240. doi: 10.1016/j.tiv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Breit S., Ashman K., Wilting J., et al. The N-myc oncogene in human neuroblastoma cells: down-regulation of an angiogenesis inhibitor identified as activin A. Cancer Research . 2000;60(16):4596–4601. [PubMed] [Google Scholar]
- 56.Hatzi E., Murphy C., Zoephel A., et al. N-myc oncogene overexpression down-regulates IL-6; evidence that IL-6 inhibits angiogenesis and suppresses neuroblastoma tumor growth. Oncogene . 2002;21(22):3552–3561. doi: 10.1038/sj.onc.1205440. [DOI] [PubMed] [Google Scholar]
- 57.Shi X. L., Yan N., Cui Y. J., Liu Z. P. A unique GSK-3β inhibitor B10 has a direct effect on aβ, targets tau and metal dyshomeostasis, and promotes neuronal neurite outgrowth. Cells . 2020;9(3) doi: 10.3390/cells9030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammerling U., Bjelfman C., Påhlman S. Different regulation of N- and c-myc expression during phorbol ester-induced maturation of human SH-SY5Y neuroblastoma cells. Oncogene . 1987;2:73–77. [PubMed] [Google Scholar]
- 59.Knoepfler P. S., Cheng P. F., Eisenman R. N. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes & Development . 2002;16(20):2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S. Y., Kang M. J., Han J. S. Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells. Molecular Brain . 2018;11 doi: 10.1186/s13041-018-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopez-Lopez A., Labandeira C. M., Labandeira-Garcia J. L., Munoz A. Rho kinase inhibitor fasudil reduces l-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. British Journal of Pharmacology . 2020;177:5622–5641. doi: 10.1111/bph.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGrath P., Seng W. L. Use of zebrafish apoptosis assays for preclinical drug discovery. Expert Opinion on Drug Discovery . 2013;8(10):1191–1202. doi: 10.1517/17460441.2013.825244. [DOI] [PubMed] [Google Scholar]
- 63.Liao Q., Gong G., Siu S. W. I., et al. A novel ShK-like toxic peptide from the transcriptome of the Cnidarian palythoa caribaeorum displays neuroprotection and cardioprotection in zebrafish. Toxins (Basel) . 2018;10(6) doi: 10.3390/toxins10060238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao Q., Li S., Siu S. W. I., et al. Novel neurotoxic peptides from Protopalythoa variabilis virtually interact with voltage-gated sodium channel and display anti-epilepsy and neuroprotective activities in zebrafish. Archives of Toxicology . 2019;93(1):189–206. doi: 10.1007/s00204-018-2334-5. [DOI] [PubMed] [Google Scholar]
- 65.Kim Y.-S., Won Y. J., Lim B. G., Min T. J., Kim Y.-H., Lee I. O. Neuroprotective effects of magnesium l-threonate in a hypoxic zebrafish model. BMC Neuroscience . 2020;21(1):p. 29. doi: 10.1186/s12868-020-00580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.


