Abstract
Background
During the coronavirus disease 2019 (COVID-19) pandemic, concerns have been arisen on the use of renin-angiotensin system inhibitors (RASI) due to the potentially increased expression of Angiotensin-converting-enzyme (ACE)2 and patient's susceptibility to SARS-CoV2 infection. Diabetes mellitus have been recognized favoring the coronavirus infection with consequent increase mortality in COVID-19. No data have been so far reported in diabetic patients suffering from ST-elevation myocardial infarction (STEMI), a very high-risk population deserving of RASI treatment.
Methods
The ISACS-STEMI COVID-19 registry retrospectively assessed STEMI patients treated with primary percutaneous coronary intervention (PPCI) in March/June 2019 and 2020 in 109 European high-volume primary PCI centers. This subanalysis assessed the prognostic impact of chronic RASI therapy at admission on mortality and SARS-CoV2 infection among diabetic patients.
Results
Our population is represented by 3812 diabetic STEMI patients undergoing mechanical reperfusion, 2038 in 2019 and 1774 in 2020. Among 3761 patients with available data on chronic RASI therapy, between those ones with and without treatment there were several differences in baseline characteristics, (similar in both periods) but no difference in the prevalence of SARS-CoV2 infection (1.6% vs 1.3%, respectively, p = 0.786). Considering in-hospital medication, RASI therapy was overall associated with a significantly lower in-hospital mortality (3.3% vs 15.8%, p < 0.0001), consistently both in 2019 and in 2010.
Conclusions
This is first study to investigate the impact of RASI therapy on prognosis and SARS-CoV2 infection of diabetic patients experiencing STEMI and undergoing PPCI during the COVID-19 pandemic. Both pre-admission chronic RASI therapy and in-hospital RASI did not negatively affected patients’ survival during the hospitalization, neither increased the risk of SARS-CoV2 infection.
Trial registration number
Background
The global pandemic of coronavirus disease 2019 (COVID-19) has dramatically impacted on healthcare system worldwide, and its effects are still ongoing. As of 30th June 2021, more than 185 million cases of COVID-19 have been reported in more than 185 countries and territories, resulting in near 4 million deaths, especially in Europe, the United States, India and Latin America.
Increasing interests have been focused on a potential harmful role of angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), jointly named renin-angiotensin system inhibitors (RASI). These drugs were assumed playing a role in the SARS-CoV2 infect action, favoring the virus cell entry through upregulation of angiotensin-converting enzyme 2 (ACE2) [1], [2], [3]. The enhanced expression of ACE2 during RASI treatment, which was suspected to increase the patient's susceptibility to COVID-19, have pulled up several concerns, especially in high risk population as diabetics, that consistently benefit from RASI treatment, especially in light of their high cardiovascular risk profile that significantly impact on long-term survival [4,5]. As stated by international societies guidelines [6], RASI are strongly suggested as first line treatment for hypertension in patients with diabetes mellitus particularly in those with evidence of end-organ damage, like albuminuria and left ventricular hypertrophy [6], [7], [8]. Considering the high prevalence of hypertension among diabetics, > 60% [9], a specific attention has been reserved to these patients, already showing independently enhanced risk of COVID-19 adverse consequences, that could get even worse [10].
Not univocal messages were provided by international professional societies, especially in the first months of the pandemic, regarding recommendation for patients under treatment with a RASI, even if the general agreement was to continue the ongoing therapy until final evidences from sufficiently powered studies [11], [12], [13].
No specific increased risks of COVID-19 or related adverse events for RASI were detected in general population [14], [15], [16], [17]. However, no definite data have been so far reported in diabetics patients suffering from ST-elevation myocardial infarction (STEMI), a very high-risk population with extensive use of RASI.
The International Study on Acute Coronary Syndromes – ST Elevation Myocardial Infarction (ISACS-STEMI) COVID-19 was established in response to the emerging outbreak of COVID-19 to provide a worldwide snapshot and estimates of the true impact of the COVID-19 pandemic on the treatment and outcome of STEMI patients treated by PPCI [18,19]. The aim of the present investigation was to inquire whether chronic use of RASI at the time of admission and their use during the hospitalization impacted on susceptibility to COVID-19 and on mortality risk among diabetic patients with STEMI undergoing primary angioplasty.
Methods
Study design and population
Our study population is represented by patients enrolled in the ISACS-STEMI COVID-19 (NCT 04412655), a retrospective multicenter registry including STEMI patients enrolled by 109 high-volume primary percutaneous coronary intervention (PCI) centers from Europe, Latin America, South-East Asia and North-Africa. This study was conducted to compare STEMI patients treated from March 1st until June 30th of 2019 with those admitted within the same period of 2020.
We collected demographic, clinical, procedural data, data on total ischemia time, door-to-balloon time, referral to primary PCI facility, PCI procedural data, in-hospital mortality. The study was approved by the Ethical Committee of AOU Maggiore della Carità. Novara. Detailed data have previously been provided [18,19].
Statistics
Data analysis was performed by using SPSS Statistics Software 23.0 (IBM SPSS Inc., Chicago, Illinois). Quantitative variables were described using median and interquartile range. Absolute frequencies and percentages were used for qualitative variables. ANOVA or Mann-Whitney and chi-square test were used for continuous and categorical variables, respectively. Normal distribution of continuous variables was tested by the Kolmogorov-Smirnov test.
A propensity score analysis was performed in order to overcome the potential bias related to differences in baseline characteristics. For each patient, a propensity score indicating the likelihood of having chronic RASI or in-hospital RASI administration was calculated by use of forward logistic regression analysis that identified variables independently associated with RASI prescription.
The discriminatory capacity of the propensity score was assessed by the area under the ROC curve (c-statistic) as an index of model performance. On the basis of the propensity score, the population was divided in four groups (from the lowest to the highest probability to have RASI prescribed) by the use of quartiles. The impact of RASI on mortality and SARS-CoV2 positivity was evaluated for each quartile, separately.
Furthermore, multivariable logistic regression analyses were performed to identify the association of RASI with in-hospital mortality and SARS-CoV2 infection after adjustment for baseline confounding factors between the two groups, including the propensity score. All significant variable (set at a P‐value < 0.1) were entered in block into the model.
A p < 0.05 was considered statistically significant. The data coordinating center was established at the Eastern Piedmont University, Novara, Italy.
Results
Of the 16,077 STEMI patients undergoing mechanical reperfusion included in the study, 3812 (23.7%) were diabetic, in whom complete demographic, clinical, procedural, and outcome data were available as well as information regarding in-hospital RASI therapy. Patients with informative data on RASI treatment at admission were 3761 (98.7%). Higher RASI discontinuation rate after hospital admission was observed was observed in 2019 compared to 2020 (46.2% in 2019 vs 39.0% in 2020, p = 0.001).
Table 1 shows baseline characteristics of patients in overall cohort and with versus without chronic RASI therapy at admission, according to the year of treatment. Almost all differences detected were consistent both in 2019 and 2020 cohort. In particular, patients on RASI were older, more often female, with higher burden of comorbidities including hypertension, hypercholesterolemia, and had already experienced a STEMI, and coronary revascularization, but displayed lower prevalence of active smoking. Patients on RASI had a lower total ischemia time and less often underwent rescue PCI.
Table 1.
Baseline demographic and clinical characteristics according to chronic RASI therapy at admission.
| OVERALL POPULATION (N = 3761) |
YEAR 2019 (N = 2014) |
YEAR 2020 (N = 1747) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RASI (n = 2049) | No RASI (n = 1712) | P value | RASI (n = 1072) | No RASI (n = 942) | P value | RASI (n = 977) | No RASI 2020 (n = 770) | P value | |
| Age (median, IQR) | |||||||||
| Age > 75 year – n. (%) | 525 (25.6) | 344 (20.1) | <0.001 | 289 (27.0) | 196 (20.8) | 0.001 | 236 (24.2) | 148 (19.2) | 0.013 |
| Male gender – n. (%) | 1382 (67.4) | 1269 (74.1) | <0.001 | 734 (68.5) | 709 (75.3) | 0.001 | 648 (66.3) | 560 (72.7) | 0.004 |
| Hypertension- n (%) | 1853 (90.4) | 889 (51.9) | <0.001 | 955 (89.1) | 504 (53.5) | <0.001 | 898 (91.9) | 385 (50.0) | <0.001 |
| Hypercholesterolemia - n (%) | 1174 (57.3) | 774 (45.2) | <0.001 | 601 (56.1) | 422 (44.8) | <0.001 | 573 (58.6) | 352 (45.7) | <0.001 |
| Active Smoker – n (%) | 921 (44.9) | 837 (48.9) | 0.016 | 468 (43.7) | 455 (48.3) | 0.037 | 453 (46.4) | 382 (49.6) | 0.178 |
| Family History of CAD - n (%) | 365 (17.8) | 276 (16.1) | 0.169 | 205 (19.1) | 151 (16.0) | 0.069 | 160 (16.4) | 125 (16.2) | 0.936 |
| Previous STEMI- n (%) | 337 (16.4) | 151 (8.8) | <0.001 | 166 (15.5) | 84 (8.9) | <0.001 | 171 (17.5) | 67 (8.7) | <0.001 |
| Previous PCI – n (%) | 495 (24.2) | 201 (11.7) | <0.001 | 244 (22.8) | 108 (11.5) | <0.001 | 251 (25.7) | 93 (12.1) | <0.001 |
| Previous CABG - n (%) | 78 (3.8) | 38 (2.2) | 0.005 | 38 (3.5) | 24 (2.5) | 0.196 | 40 (4.1) | 14 (1.8) | 0.006 |
| Referral to Primary PCI Hospital | |||||||||
| Ambulance (from community) – n (%) |
880 (42.9) | 753 (44.0) | 0.303 | 462 (43.1) | 408 (43.3) | 0.149 | 418 (42.8) | 345 (44.8) | 0.699 |
| Time delays | |||||||||
| Ischemia time, median [25 - 75th] | |||||||||
| Total Ischemia time > 12 h – n (%) | 240 (11.7) | 266 (15.5) | 0.001 | 105 (9.8) | 137 (14.5) | 0.001 | 135 (13.8) | 129 (16.8) | 0.089 |
| Door-to-balloon time, median [25 - 75th] | |||||||||
| Door-to-balloon time >30 min (%)– n (%) | 1280 (62.5) | 1106 (64.6) | 0.176 | 650 (60.6) | 622 (66.0) | 0.012 | 630 (64.5) | 484 (62.9) | 0.483 |
| Clinical Presentation | |||||||||
| Anterior STEMI – n (%) | 956 (46.7) | 860 (50.2) | 0.029 | 505 (47.1) | 465 (49.4) | 0.312 | 451 (46.2) | 395 (51.3) | 0.033 |
| Out-of-hospital cardiac arrest – n (%) | 89 (4.3) | 80 (4.7) | 0.627 | 49 (4.6) | 41 (4.4) | 0.813 | 40 (4.1) | 39 (5.1) | 0.332 |
| Cardiogenic shock– n (%) | 179 (8.7) | 170 (9.9) | 0.209 | 86 (8.0) | 93 (9.9) | 0.145 | 93 (9.5) | 77 (10.0) | 0.736 |
| Rescue PCI for failed thrombolysis – n (%) | 194 (9.5) | 110 (6.4) | 0.001 | 103 (9.6) | 60 (6.4) | 0.008 | 91 (9.3) | 50 (6.5) | 0.032 |
*Mann-Whitney test.
CAD = Coronary Artery Disease; STEMI = ST-segment Elevation Myocardial Infarction; PCI = Percutaneous Coronary Intervention; CABG = Coronary Artery Bypass Graft;.
With regards to treatment during the hospitalization, patients treated with RASI were younger, more often male, suffering of hypercholesterolemia, previous STEMI, coronary revascularization. Patients experienced out-of-hospital cardiac arrest and cardiogenic shock were less frequent under in-hospital RASI therapy (Table 2 ).
Table 2.
Baseline demographic and clinical characteristics according to in-hospital RASI therapy.
| OVERALL POPULATION (N = 3812) |
YEAR 2019 (N = 2038) |
YEAR 2020 (N = 1774) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RASI (n = 2049) | No RASI (n = 1763) | P value | RASI (n = 1034) | No RASI (n = 1004) | P value | RASI (n = 1015) | No RASI (n = 759) | P value | |
| Age (median, IQR) | |||||||||
| Age > 75 year – n. (%) | 434 (21.2) | 446 (25.3) | 0.003 | 230 (22.2) | 260 (25.9) | 0.054 | 204 (20.1) | 186 (24.5) | 0.027 |
| Male gender – n. (%) | 1504 (73.4) | 1182 (67.0) | <0.004 | 773 (74.8) | 685 (68.2) | 0.001 | 731 (72.0) | 497 (65.5) | 0.003 |
| Hypertension- n (%) | 1509 (73.6) | 1268 (71.9) | 0.233 | 742 (71.8) | 733 (73.0) | 0.529 | 767 (75.6) | 535 (70.5) | 0.017 |
| Hypercholesterolemia - n (%) | 1129 (55.1) | 849 (48.2) | <0.001 | 550 (53.2) | 486 (48.4) | 0.031 | 579 (57.0) | 363 (47.8) | <0.001 |
| Active Smoker – n (%) | 1026 (50.1) | 768 (43.6) | <0.001 | 505 (48.8) | 436 (43.4) | 0.014 | 521 (51.3) | 332 (43.7) | 0.002 |
| Family History of CAD - n (%) | 416 (20.3) | 248 (14.1) | <0.001 | 223 (21.6) | 144 (14.3) | <0.001 | 193 (19.0) | 104 (13.7) | 0.003 |
| Previous STEMI- n (%) | 296 (14.4) | 200 (11.3) | 0.005 | 145 (14.0) | 108 (10.8) | 0.025 | 151 (14.9) | 92 (12.1) | 0.095 |
| Previous PCI – n (%) | 408 (19.9) | 297 (16.8) | 0.015 | 189 (18.3) | 166 (16.5) | 0.299 | 219 (21.6) | 131 (17.3) | 0.024 |
| Previous CABG - n (%) | 67 (3.3) | 52 (2.9) | 0.571 | 37 (3.6) | 27 (2.7) | 0.250 | 30 (3.0) | 25 (3.3) | 0.684 |
| Referral to Primary PCI Hospital | |||||||||
| Ambulance (from community) – n (%) |
1011 (49.3) | 659 (37.4) | <0.001 | 508 (49.1) | 379 (37.7) | <0.001 | 503 (49.6) | 280 (36.9) | <0.001 |
| Time delays | |||||||||
| Ischemia time, median [25 - 75th] | |||||||||
| Total Ischemia time > 12 h – n (%) | 267 (13.0) | 248 (14.1) | 0.351 | 124 (12.0) | 122 (12.2) | 0.912 | 143 (14.1) | 126 (16.6) | 0.144 |
| Door-to-balloon time, median [25 - 75th] | |||||||||
| Door-to-balloon time > 30 min - n (%) |
1227 (59.9) | 1167 (66.2) | <0.001 | 611 (59.1) | 664 (66.1) | 0.001 | 616 (60.7) | 503 (66.3) | 0.016 |
| Clinical Presentation | |||||||||
| Anterior STEMI– n (%) | 978 (47.7) | 854 (48.4) | 0.662 | 501 (48.5) | 477 (47.5) | 0.670 | 477 (47.0) | 377 (49.7) | 0.264 |
| Out-of-hospital cardiac arrest – n (%) | 69 (3.4) | 100 (5.7) | 0.001 | 32 (3.1) | 58 (5.8) | 0.003 | 37 (3.6) | 42 (5.5) | 0.056 |
| Cardiogenic shock– n (%) | 123 (6.0) | 226 (12.8) | <0.001 | 53 (5.1) | 126 (12.5) | <0.001 | 70 (6.9) | 100 (13.2) | <0.001 |
| Rescue PCI for failed thrombolysis – n (%) | 143 (7.0) | 161 (9.1) | 0.014 | 79 (7.6) | 84 (8.4) | 0.546 | 64 (6.3) | 77 (10.1) | 0.003 |
*Mann-Whitney test.
CAD = Coronary Artery Disease; STEMI = ST-segment Elevation Myocardial Infarction; PCI = Percutaneous Coronary Intervention; CABG = Coronary Artery Bypass Graft;.
Angiographic and procedural characteristics are shown in Table 1S for patients under RASI at admission, while in Table 2S are reported data for patients treated with RASI in hospital. Radial access was performed less frequently in patients with RASI treatment ad admission in the overall cohort and in 2019, while no differences were observed for the same period of 2020. Higher prevalence of in-stent thrombosis was found in patients under RASI, consistently in both 2019 and 2020. Conversely, patients undergoing thrombectomy and DES implantation were more likely treated with RASI during the hospitalization.
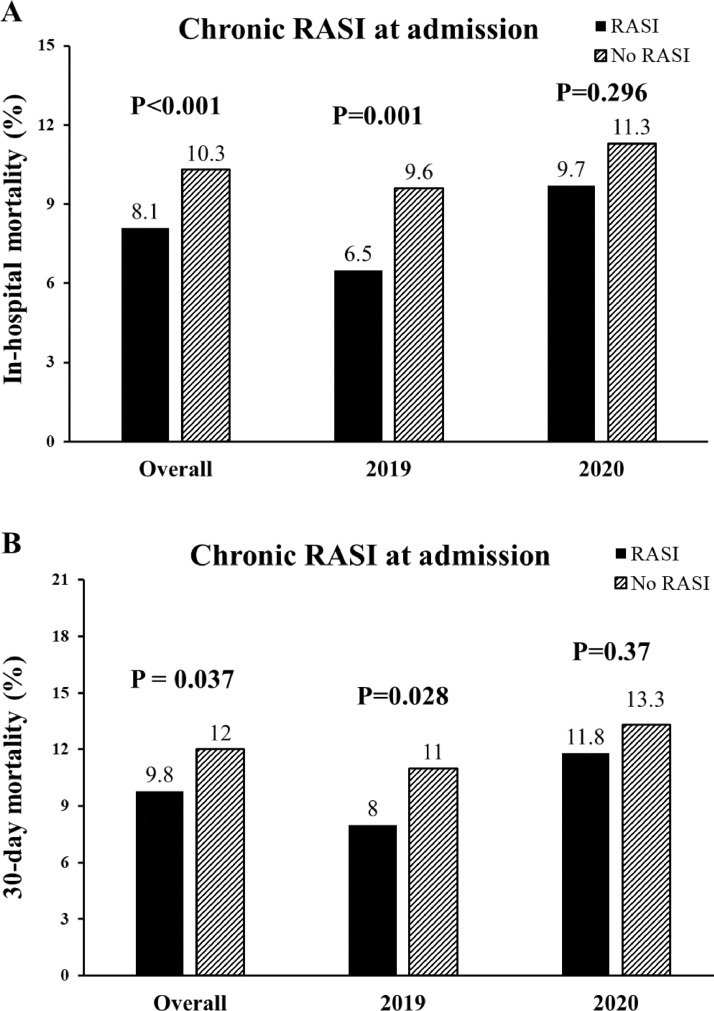
Chronic RASI treatment at hospital admission was overall related to lower in-hospital mortality and 30-day mortality (Fig. 1 ). Results were confirmed in 2019 but in 2020 did not reach the statistical significance (p = 0.296). The benefits were confirmed in almost all the quartiles of the propensity score (Fig. 1S) and confirmed after the adjustment for all the confounding baseline characteristics (gender, hypertension, previous PCI, ischemia time, rescue PCI, radial access, in-hospital RASI,), including the propensity score, for in-hospital (adjusted OR [95% CI] = 0.61[0.47–0.79], p < 0.001) and 30-day mortality (adjusted HR [95% CI] = 0.78[0.62–0.97], p = 0.028).
Fig. 1.
Bar graphs show in-hospital (panel A) and 30-day (panel B) mortality in overall population, in 2019 and 2020 patients according to chronic RASI therapy at admission.
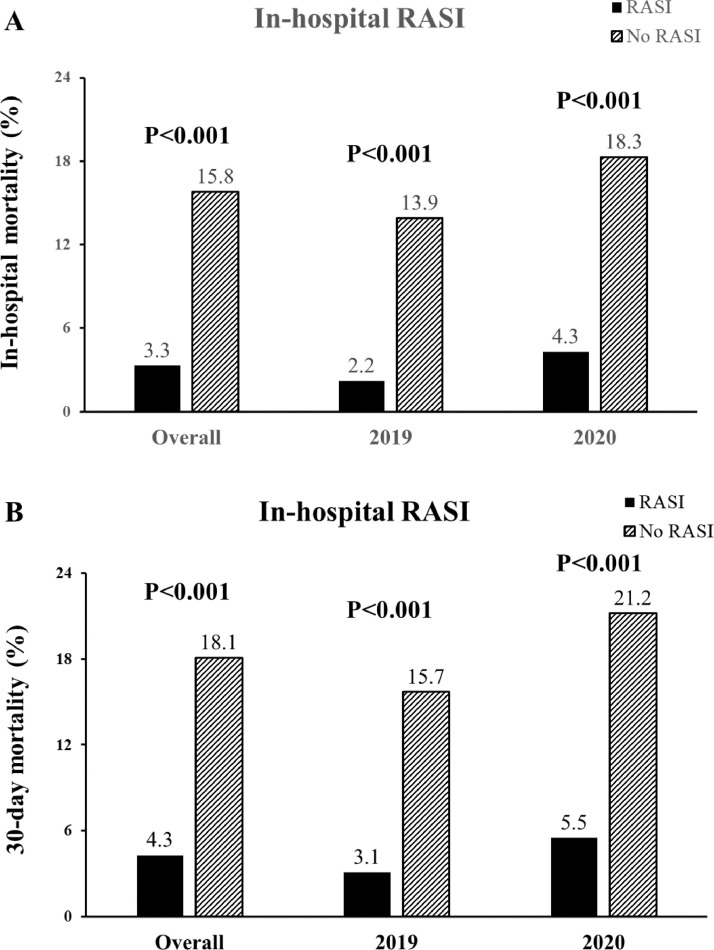
In-hospital treatment with RASI showed significant benefits in-hospital and 30-day mortality that was consistent in both 2019 and 2020 (Fig. 2 ). The benefits were confirmed in almost all the quartiles of the propensity score (Fig. 2S) and confirmed after the adjustment for all the confounding baseline and procedural characteristics (Age > 75 years, Gender, Hypercholesterolemia, Previous PCI, Rescue PCI, instent thrombosis, Radial access, target vessel, multivessel disease, thrombectomy, DES, use of mechanical support devices, bivalirudin, postprocedural TIMI flow, DAPT), including the propensity score, for in-hospital (adjusted OR [95% CI] = 0.21[0.22–0.30], p < 0. 001) and 30-day mortality (adjusted HR [95% CI] = 0.3 [0.23–0.38], p < 0.001).
Fig. 2.
Bar graphs show in-hospital (panel A) and 30-day (panel B) mortality in overall population, in 2019 and 2020 patients according to in-hospital RASI therapy.
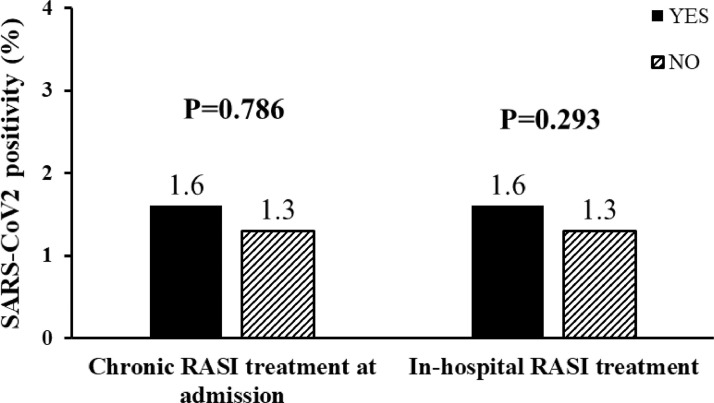
Both chronic treatment and in hospital therapy with RASI did not affect the SARS-CoV2 positivity (n = 26) (Fig. 3 ).
Fig. 3.
Bar graphs show the prevalence of SARS-CoV2 infection in patients treated in 2020 according to chronic RASI therapy at admission (left side) and in-hospital RASI (right side).
Discussion
The main finding of the present study is that among diabetic patients experiencing STEMI and treated with mechanical reperfusion during the COVID-19 pandemic, chronic RASI therapy at admission showed a lower rate of death not raising the statistical significance in 2020, whereas in-hospital treatment with RASI resulted in a significantly lower mortality in both pandemic and prepandemic period. Both chronic treatment and in-hospital therapy with RASI did not affect the SARS-CoV2 positivity.
Diabetes mellitus is a condition enhancing patients’ cardiovascular risk profile due to damages on both microvascular and macrovascular sides [20], [21], [22]. Prolonged glucose homeostasis impairment leads to endothelial dysfunction, atherosclerosis progression and pro-thrombotic environmental, recognizing diabetics as patients at higher risk for cardiovascular events. RASI constitute a cornerstone of treatment in various cardiovascular diseases providing robust evidences in reducing death and morbidity [23], [24], [25], [26]. Their benefits were confirmed also among diabetics, indeed RASI are considered first treatment option in several condition, including hypertension [27].
However, previous animal studies have reported worries on RAS modulation, suggesting that ACE2 expression might play a negative prognostic role in COVID-19 patients: the ACE2 enzyme is a cell membrane protein, that is used by the SARS-CoV2 virus to enter cells [9,28]. Given that experimental studies showed that angiotensin receptor blockers (ARBs) and an ACE inhibitors (ACEIs) could increase ACE2 expression in cardiovascular and renal systems [29,30], favouring the conversion of angiotensin II to angiotensin (1–7) [31], concerns have been raised on potential detrimental consequences driven by ARBs and ACEIs treatment on SARS-CoV2 infection and the severity of the COVID-19 [32].
Clinical evidences on the of ACEIs/ARBs in patients with COVID-19 has been controversial in part due to early reports from China, showing higher mortality in hypertensive patients admitted for COVID-19 [33], [34], [35], [36], [37]. Those analysis were hampered by small cohort and main confounding factors such as older age and cardiovascular disease, were collinear with hypertension. Further and larger studies have found no harmful effects or even beneficial effects from RASI therapy[14], [15], [16], [17]. In a large population-based case–control study in the Lombardy region of Italy performed by Mancia et al. [13], a total of 6272 case patients with confirmed SARS-CoV2 infection were matched with more than 30,000 patients according to age, sex and municipality of residence. RASI were detected more frequently used in COVID-19 patients compared to controls, but no significant increased risk of SARS-CoV2 infection was found for chronic RASI treatment.
Diabetes mellitus confers per se a higher risk of mortality and adverse events in patients with cardiovascular disease [27]. Also in COVID-19 an increased death rate was independently associated with diabetes mellitus and metabolic diseases [38], [39], [40]: a retrospective analysis from Bode et al. including 1122 patients showed a 4-fold increased mortality among diabetics compared to non-diabetics, with a further enhanced risk of death in case of uncontrolled hyperglycemia [41]. Main mechanisms, which diabetes mellitus and high glucose promote SARS-CoV2 replication through, are strictly related to enhanced production of reactive oxygen species and pro-inflammatory cytokines [42]. Similarly, are the findings from Zhu et al. that showed significant clinical benefits correlated with improved glycemic control during COVID-19 hospitalization.
Therefore, inquiring the interplay between renin-angiotensin system and diabetes mellitus during COVID-19 results essential to evaluate patients’ prognosis. In fact, the diabetic status favors SARS-CoV2 infection and could be influenced by RASI therapy [43]. The relationship between increased ACE2 expression during RASI treatment and pro-inflammatory environment in diabetics is not completely established, with few studies that addressed their resulting balance. Aghaaliakbari et al. retrospectively investigated the effect of ACEIs and diabetes in COVID-19 patients: among 153 diabetics, ACEIs treatment was found significantly increasing the risk of mortality [44]. Conversely, Ramos-Rincón et al. reported in diabetics with more than 80 years old a potential protective role of chronic treatment with ARB [45]. In a randomized trial testing the continuation versus discontinuation of RASI, authors showed that they can be safely continued in patients admitted for COVID-19, consistently in diabetic and non-diabetics [46].
However, so far no data have been reported in diabetic patients with STEMI, representing a population with a very high adverse event risk and (guideline-recommended) RASI use in the majority of patients [47]. Our report is the first study focused on STEMI patients with diabetes undergoing mechanical reperfusion, including more than 3800 diabetics undergoing mechanical reperfusion. We found that chronic RASI at admission did not affect the prevalence of SARS-CoV2 infection and showed a lower rate of death not raising the statistical significance in 2020. Furthermore, in-hospital RASI treatment was associated with a significantly lower mortality, consistently in 2019 and 2020, without affecting the prevalence of SARS-CoV2 infection. Therefore, confirming our previous report in a general population [48], the present study confirms the overall mortality benefits of RASI among diabetic patients with STEMI. Moreover, it does not suggest a link between RASI therapy and COVID-19 susceptibility or subsequent worse outcomes among these patients.
Professional societies have issued position statements that ACEIs/ARBs should not be discontinued that this study supports [49]. Support of continuing RASI therapy was yielded by the recent randomized BRACE-CORONA Trial, 659 patients with chronic RASI therapy at admission and confirmed diagnosis of COVID-19 were randomly assigned to a temporary 30-day suspension or continuation of RASI therapy. No differences were observed for 30-day mortality (2.8% vs 2.7%) between the two groups [50].
Further randomized trials focused on diabetic patients are needed to clarify the protective or harmful effects of RASI treatment during COVID-19. Benefits provided by RASI seems confirmed in diabetics experiencing STEMI treated with mechanical reperfusion, without increasing the risk for COVID-19 and in-hospital mortality.
Limitations
This study is limited by its retrospective, non-randomized design. As may be expected, diabetics on RASI therapy differed from the other patients in comorbidities, firstly hypertension prevalence, however the results on mortality were not affected by baseline differences.
Moreover, our research was conducted during a pandemic emergency, which was a challenge and expected to encounter some missing data. Nevertheless, considering all adversities, the proportion of patients with missing data regarding in-hospital RASI therapy was tolerable.
The choice of interrupt RASI treatment ad admission as well as its introduction during the hospitalization was made by physicians and at their own discretion: that, together with the uncertainty of RASI harm on SARS-CoV2 infection risk, could have impacted patients’ outcome, even if no increased mortality was found in analyses of both chronic and in-hospital treatments.
As we did not collect information on the type and dosage of RASI, we cannot perform a further in-depth assessment of prognostic implications. Finally, although our database comprised almost 3800 diabetic STEMI patients with known in-hospital pharmacological therapy, procedural features and clinical outcome following mechanical reperfusion procedure, the evaluation of the prognostic impact of RASI treatment among SARS-CoV2-positive patients was limited due to the low prevalence of the infection.
Conclusions
This is the first study investigating the prognostic impact of RASI therapy in patients with diabetes mellitus experiencing STEMI treated with mechanical reperfusion during COVID-19 pandemic. Our main finding is that RASI therapy was associated with an overall significant reduction in mortality, without any negative effect on SARS-CoV2 infection. Therefore, waiting for future dedicated and randomized trials, during the pandemic RASI should not be suspended or omitted among these high-risk patients suffering from STEMI and undergoing mechanical reperfusion.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.deman.2021.100022.
Appendix. Supplementary materials
References
- 1.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn J.H., Li W., Choe H., Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci CMLS. 2004;61(21):2738–2743. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Luca G., Verdoia M., Savonitto S., Piatti L., Grosseto D., Morici N., Bossi I., Sganzerla P., Tortorella G., Cacucci M., Murena E., Toso A., Bongioanni S., Ravera A., Corrada E., et al. Impact of diabetes on clinical outcome among elderly patients with acute coronary syndrome treated with percutaneous coronary intervention: insights from the ELDERLY ACS 2 trial. J Cardiovasc Med. 2020;21(6):453–459. doi: 10.2459/JCM.0000000000000978. (Hagerstown) [DOI] [PubMed] [Google Scholar]
- 5.De Luca G., Dirksen M.T., Spaulding C., Kelbæk H., Schalij M., Thuesen L., van der Hoeven B., Vink M.A., Kaiser C., Musto C., Chechi T., Spaziani G., Diaz de la Llera L.S., Pasceri V., Di Lorenzo E., et al. Impact of diabetes on long-term outcome after primary angioplasty: insights from the DESERT cooperation. Diabetes Care. 2013;36(4):1020–1025. doi: 10.2337/dc12-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D.L., Coca A., de Simone G., Dominiczak A., Kahan T., Mahfoud F., Redon J., Ruilope L., Zanchetti A., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. J Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940. the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: the task force for the management of arterial h. [DOI] [PubMed] [Google Scholar]
- 7.Niskanen L., Hedner T., Hansson L., Lanke J., Niklason A., C.A.P.P.P. Study Group Reduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first-line therapy with an ACE inhibitor compared with a diuretic/beta-blocker-based treatment regimen: a subanalysis of the Captopril prevention project. Diabetes Care. 2001;24(12):2091–2096. doi: 10.2337/diacare.24.12.2091. [DOI] [PubMed] [Google Scholar]
- 8.Ostergren J., Poulter N.R., Sever P.S., Dahlöf B., Wedel H., Beevers G., Caulfield M., Collins R., Kjeldsen S.E., Kristinsson A., McInnes G.T., Mehlsen J., Nieminen M., O'Brien E., investigators A.S.C.O.T. The Anglo-Scandinavian cardiac outcomes trial: blood pressure-lowering limb: effects in patients with type II diabetes. J Hypertens. 2008;26(11):2103–2111. doi: 10.1097/HJH.0b013e328310e0d9. [DOI] [PubMed] [Google Scholar]
- 9.Nathan D.M., Bayless M., Cleary P., Genuth S., Gubitosi-Klug R., Lachin J.M., Lorenzi G., Zinman B., DCCT/EDIC Research Group Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62(12):3976–3986. doi: 10.2337/db13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverio A., Di Maio M., Citro R., Esposito L., Iuliano G., Bellino M., Baldi C., De Luca G., Ciccarelli M., Vecchione C., Galasso G. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc Disord. 2021;21(1):23. doi: 10.1186/s12872-020-01816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaduganathan M., Vardeny O., Michel T., McMurray J.J .V, Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P., Carmona-Rubio A.E., Jacob M., Procop G.W., Harrington S., Milinovich A., Svensson L.G., Jehi L., Young J.B., Chung M.K. Association of use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1020. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I., Kunichoff D., Chen Y., Ogedegbe G., et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Abajo F.J., Rodríguez-Martín S., Lerma V., Mejía-Abril G., Aguilar M., García-Luque A., Laredo L., Laosa O., Centeno-Soto G.A., Ángeles Gálvez M., Puerro M., González-Rojano E., Pedraza L., de Pablo I., Abad-Santos F., et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395(10238):1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fosbøl E.L., Butt J.H., Østergaard L., Andersson C., Selmer C., Kragholm K., Schou M., Phelps M., Gislason G.H., Gerds T.A., Torp-Pedersen C., Køber L. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use With COVID-19 diagnosis and mortality. JAMA. 2020;324(2):168–177. doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Luca G., Cercek M., Jensen L.O., Vavlukis M., Calmac L., Johnson T., Roura I Ferrer G., Ganyukov V., Wojakowski W., von Birgelen C., Versaci F., Ten Berg J., Laine M., Dirksen M., Casella G., et al. Impact of COVID-19 pandemic and diabetes on mechanical reperfusion in patients with STEMI: insights from the ISACS STEMI COVID 19 registry. Cardiovasc Diabetol. 2020;19(1):215. doi: 10.1186/s12933-020-01196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Luca G., Verdoia M., Cercek M., Jensen L.O., Vavlukis M., Calmac L., Johnson T., Ferrer G.R., Ganyukov V., Wojakowski W., Kinnaird T., van Birgelen C., Cottin Y., IJsselmuiden A., Tuccillo B., et al. Impact of COVID-19 pandemic on mechanical reperfusion for patients with STEMI. J Am Coll Cardiol. 2020;76(20):2321–2330. doi: 10.1016/j.jacc.2020.09.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tancredi M., Rosengren A., Svensson A.M., Kosiborod M., Pivodic A., Gudbjörnsdottir S., Wedel H., Clements M., Dahlqvist S., Lind M. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 21.Sattar N., Rawshani A., Franzén S., Rawshani A., Svensson A.M., Rosengren A., McGuire D.K., Eliasson B., Gudbjörnsdottir S. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139(19):2228–2237. doi: 10.1161/CIRCULATIONAHA.118.037885. [DOI] [PubMed] [Google Scholar]
- 22.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corrà U., Cosyns B., Deaton C., Graham I., Hall M.S., Hobbs F.D.R., Løchen M.L., Löllgen H., et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. (constituted by representati. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagenais G.R., Pogue J., Fox K., Simoons M.L., Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368(9535):581–588. doi: 10.1016/S0140-6736(06)69201-5. [DOI] [PubMed] [Google Scholar]
- 24.Fox K.M. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362(9386):782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 25.Braunwald E., Domanski M.J., Fowler S.E., Geller N.L., Gersh B.J., Hsia J., Pfeffer M.A., Rice M.M., Rosenberg Y.D., Rouleau J.L., PEACE Trial Investigators Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351(20):2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeffer M.A., Braunwald E., Moyé L.A., Basta L., Brown E.J., Cuddy T.E., Davis B.R., Geltman E.M., Goldman S., Flaker G.C., Klein M., Lamas G.A., Packer M., Rouleau J., Rouleau J.L., et al. Effect of Captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1992;327(10):669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 27.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., Huikuri H.V., Johansson I., Jüni P., Lettino M., Marx N., et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 28.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Ye Y., Gong H., Wu J., Yuan J., Wang S., Yin P., Ding Z., Kang L., Jiang Q., Zhang W., Li Y., Ge J., Zou Y. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1-7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J Mol Cell Cardiol. 2016;97:180–190. doi: 10.1016/j.yjmcc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Agata J., Ura N., Yoshida H., Shinshi Y., Sasaki H., Hyakkoku M., Taniguchi S., Shimamoto K. Olmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzyme. Hypertens Res. 2006;29(11):865–874. doi: 10.1291/hypres.29.865. official journal of the Japanese Society of Hypertension. [DOI] [PubMed] [Google Scholar]
- 31.Dworakowska D., Grossman A.B. Renin-angiotensin system inhibitors in management of hypertension during the COVID-19 pandemic. J Physiol Pharmacol. 2020;71(2) doi: 10.26402/jpp.2020.2.01. an official journal of the Polish Physiological Society. [DOI] [PubMed] [Google Scholar]
- 32.Watkins J. Preventing a covid-19 pandemic. BMJ. 2020:m810. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 33.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J.N.Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020:m1295. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis IJID. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. official publication of the International Society for Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium. Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 40.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., Ou C.Q., Li L., Chen P.Y., Sang L., Wang W., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., Klonoff D.C. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Codo A.C., Davanzo G.G., Monteiro L de B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C.A.O., Crunfli F., Jimenez Restrepo JL, Vendramini P.H., Reis-de-Oliveira G., Bispo Dos S.K, Toledo-Teixeira D.A., et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32(3):437–446. doi: 10.1016/j.cmet.2020.07.007. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman-Edelstein M., Guetta T., Barnea A., Waldman M., Ben-Dor N., Barak Y., Kornowski R., Arad M., Hochhauser E., Aravot D. Expression of the SARS-CoV-2 receptorACE2 in human heart is associated with uncontrolled diabetes, obesity, and activation of the renin angiotensin system. Cardiovasc Diabetol. 2021;20(1):90. doi: 10.1186/s12933-021-01275-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Rincón J.M., Pérez-Belmonte L.M., Carrasco-Sánchez F.J., Jansen-Chaparro S., De-Sousa-Baena M., Bueno-Fonseca J., Pérez-Aguilar M., Arévalo-Cañas C., Bacete Cebrian M., Méndez-Bailón M., Fiteni M.I, González G.A, Navarro R.F, Tuñón de A.C, Muñiz N.G, et al. Cardiometabolic therapy and mortality in very old patients with diabetes hospitalized due to COVID-19. J Gerontol A Biol Sci Med Sci. 2021 doi: 10.1093/gerona/glab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen J.B., Hanff T.C., William P., Sweitzer N., Rosado-Santander N.R., Medina C., Rodriguez-Mori J.E., Renna N., Chang T.I., Corrales-Medina V., Andrade-Villanueva J.F., Barbagelata A., Cristodulo-Cortez R., Díaz-Cucho O.A., Spaak J., et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9(3):275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., Hindricks G., Kastrati A., Lenzen M.J., Prescott E., Roffi M., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 48.De Luca G., Cercek M., Okkels Jensen L., Bushljetikj O., Calmac L., Johnson T., Gracida Blancas M., Ganyukov V., Wojakowski W., von Birgelen C., IJsselmuiden A., Tuccillo B., Versaci F., Ten Berg J., Laine M., et al. Impact of renin-angiotensin system inhibitors on mortality during the COVID pandemic among STEMI patients undergoing mechanical reperfusion: insight from an international STEMI registry. Biomed Pharmacother. 2021;138 doi: 10.1016/j.biopha.2021.111469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bozkurt B., Kovacs R., Harrington B. Joint HFSA/ACC/AHA statement addresses concerns Re: using RAAS antagonists in COVID-19. J Card Fail. 2020;26(5):370. doi: 10.1016/j.cardfail.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopes R.D., Macedo A.V.S., de Barros E Silva P.G.M., Moll-Bernardes R.J., dos Santos T.M., Mazza L., Feldman A., D'Andréa Saba Arruda G., de Albuquerque D.C., Camiletti A.S., de Sousa A.S., de Paula T.C., Giusti K.G.D., Domiciano R.A.M., Noya-Rabelo M.M., et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and Angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19. JAMA. 2021;325(3):254. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.