Abstract
Nearly a decade after becoming formally available in the U.S., HIV pre-exposure prophylaxis (PrEP) remains underutilized by populations at risk for HIV acquisition. The next generation of PrEP research is pivoting toward implementation research in order to identify the most impactful avenues for scaling up PrEP uptake. Rapid identification of patients who may be at risk for HIV in primary care settings and the ability to provide brief consultation and prescription or referral for PrEP could help to increase PrEP uptake. The current study aimed to develop and pilot-test a PrEP screening instrument that could be integrated into the workflow of busy primary care clinics to help facilitate PrEP uptake among at-risk men. During the study, PrEP screening occurred for 12 months in two primary care clinics nested within a large integrated healthcare delivery system in Southern California. An interrupted time series analysis found a significant increase in PrEP referrals overall during the screening intervention period as compared to the preceding 12 months. Findings suggest that brief HIV risk screening in primary care is acceptable, feasible, and shows preliminary effects in increasing PrEP referral rates for Black and Hispanic/Latino men.
Keywords: HIV, Pre-exposure Prophylaxis, PrEP, Primary Care, Implementation, Screening
INTRODUCTION
PrEP is effective for preventing HIV infection among populations at risk for acquiring HIV infection.1–5 However, nearly a decade after FDA approval of the first PrEP drug in 2012, PrEP remains underutilized among populations at risk for HIV.6–8 For example, among those gay, bisexual, and other men who have sex with men (MSM) for whom PrEP is likely to be indicated (i.e., report engaging in condomless anal sex outside of monogamous relationship), only 42% of white MSM, 30% of Hispanic MSM, and 26% of Black MSM reported past-year PrEP use.9 Findings from another recent study suggest that only about 5% of the population at risk for HIV who could benefit from PrEP in 2015 had received a prescription for it.10
With PrEP’s efficacy well-established, the next generation of PrEP research is pivoting toward implementation research in order to identify the most impactful avenues for scaling up PrEP uptake.11 Because PrEP requires a prescription, health care providers are key “gatekeepers”12 for PrEP access and uptake. Most PrEP implementation research thus far has been cross-sectional and focused on barriers and facilitators that are overwhelmingly cognitive in nature (e.g., provider knowledge, attitudes) and there is less research about PrEP implementation especially from a longitudinal perspective,13 and PrEP implementation in primary care settings specifically.14
Primary care providers are an ideal point of entry for HIV-negative persons who are at risk for HIV and could benefit from PrEP,15 and primary care is a feasible setting for PrEP outreach.16 However, general practitioners lag behind specialists, such as HIV and infectious disease specialists, with regard to PrEP knowledge, willingness, and prescriptions written.6,7,17,18 To increase PrEP availability and uptake, there have been calls for greater provider education.15,19,20 However, education is likely necessary but insufficient because it does not address the need to integrate screening into the clinical workflow.15 Without efforts to make PrEP screening and access universal in primary care settings, PrEP prescribing may continue to be isolated to providers who champion it, limiting its reach. For example, over a five-year period in a metropolitan health system, Bien et al.16 found that the majority (55%) of the health system’s PrEP prescriptions originated in primary care, yet a third of primary care sites had never issued PrEP prescriptions.16 This suggests that without a universal and structured approach to implementation in primary care, PrEP remains subject to the myriad factors ranging from individual-level provider characteristics (e.g., knowledge, attitudes) to practice- and system-level characteristics (reimbursement, patient mix), potentially exacerbating primary care inequities in PrEP access.
Embedding PrEP into routine health care, including primary care, may better promote universal access and uptake. As Calabrese et al.21 argue, the routinization of PrEP screening may 1) facilitate access for at-risk persons who would otherwise be “missed,” 2) destigmatize PrEP, 3) promote greater patient-centered care and decision making, and 4) increase PrEP awareness among the broader population of Americans, as individual patients are nodes within their larger social networks.21 Together, these benefits may reduce disparities in PrEP access and uptake, for example those seen among black and Hispanic populations and women, or in the U.S. South.8,10,21,22
Despite established clinical indications (e.g., recent sexual risk behavior), the current clinical paradigm leaves PrEP prescribing up to providers’ knowledge, discretion, and potential biases therein, or the subset of patients who actively request PrEP from their providers.21 Providers’ discomfort with discussing sexual health can be a barrier to PrEP assessment and referral,11,15,23 and it is plausible that standard screening questions and a clear referral process could mitigate this barrier. Embedding PrEP into primary care may also help overcome the oft-cited “purview paradox,” where HIV specialists typically are not providing medical care for HIV-negative patients, yet primary care providers who do not specialize in HIV medicine or prevention may not include HIV screening as part of their routine practice.13,15,19 Geographic provider shortages (e.g., infectious disease clinicians) may also contribute to inequities in PrEP access,13 and embedding screening in existing primary care could make access more universal in these regions.
The rapid identification of patients for whom PrEP is indicated, and the provision of, or referral for, PrEP treatment by primary care providers remains a challenge.24,25 We developed and pilot-tested a PrEP-screening instrument based on the CDC’s published guidelines for facilitating PrEP uptake among men who are at high-risk for HIV infection. The current study, Project SLIP (Screening and LInkage to PrEP), is the first study to examine the acceptability, feasibility, and test the preliminary effects of a PrEP screening and referral process in two primary care clinics within an integrated health system, Kaiser Permanente Southern California (KPSC).
METHODS
Study Setting and Participant Populations.
KPSC delivers integrated care to about 4.6 million racially and ethnically diverse health plan members that closely mirrors the diverse makeup of Southern California. This pilot study took place within a KPSC medical center and associated medical office buildings. Two primary care clinics associated with this medical center were selected as sites for implementation: one at the Los Angeles medical center with 22 primary care providers, and one at the Pasadena medical office building, with 14 primary care providers. These clinics are highly representative of the racial/ethnic and socioeconomic diversity of the region. The Pasadena clinic was comprised of approximately 32% Hispanic/Latino, 28% White, 20% Asian/Pacific Islander, 7% Black, and 13% mixed race/other race patients. While the Los Angeles clinic served approximately 42% Hispanic/Latino, 24% White, 16% Asian/Pacific Islander, 6% Black, and 12% of mixed race/other race.
Patients who screened eligible for PrEP as part of this pilot were referred to HIV specialty services for PrEP, which was located at the Los Angeles medical center (16 miles from the Pasadena clinic).This process is representative of the system navigation required for the vast majority of Kaiser PrEP patients throughout Southern California.
PrEP Screener Content.
The 7-item PrEP-screener was developed based on the CDC’s 2017 clinical practice guidelines for providing PrEP to at-risk adult males. This pilot study focused on males given that males account for more than 80% of new HIV infections,26 and the anticipated lower rates of eligibility among female primary care patients in this pilot. Accordingly, any adult male who reported 1) having had sex without a condom with another male during the past 6 months, and/or 2) having sex with a known HIV-positive person in the past 6 months, and/or 3) having been diagnosed with a sexually transmitted infection in the past 6 months, and/or 4) injecting drugs in the past 6 months was considered eligible for a brief post-screening consultation and PrEP referral. Two additional items collected demographic (gender identity, sexual orientation) information, and one self-scoring item asked the participant to indicate whether they had indicated “yes” to any of the PrEP indicator items to help staff quickly identify possible PrEP candidates.
Integration of the Screener into Clinic Workflow.
Prior to implementing the screening instrument, the research team held educational training sessions with all participating primary care providers and clinic staff to provide an overview of the PrEP care continuum, including patient indications, best prescribing practices, and how to discuss the screening instrument and PrEP with patients. During the first 6 months of intervention implementation, the brief 7-item self-administered paper screener was handed to patients by nursing staff when the patient was called into the nurses’ station where the patient vitals were collected. During the second 6 months of intervention implementation, the workflow was changed in an attempt to improve screening rates, such that the screener was handed to the incoming patient by front desk staff during the appointment check-in process to be filled out in the waiting room and then given to the nurse when called back for the appointment.
The PrEP screening questionnaire was provided to all male patients between the ages of 18 and 65 years of age who were obtaining healthcare in either of the participating clinics. Patients were instructed to return the completed screener to their nurse when called back. The nurse then ensured that the screeners were brought back to the visit room and left for providers when PrEP was indicated. In turn, primary care providers were instructed to collect the screener from patients during the clinical visit, and to provide brief consultation and referral to HIV Specialty Care for any patient who screened eligible for PrEP based on indication of one or more of the HIV risk criteria. Providers were instructed to simply proceed with the planned medical visit for all ineligible patients or patients who screened eligible but who declined the PrEP referral. Notably, providers reported that many of these visits also included a discussion about the purpose of the screener, PrEP, and a general overview of HIV. All screeners were deposited in a locked box after the patient visit concluded.
Statistical Analyses.
We collected patient visit data from the two participating primary care clinics between February 1, 2017 and January 31, 2019. The pre-screening intervention period was from February 1, 2017 to January 31, 2018 and the screening intervention period started February 1, 2018 until Jan 31, 2019.
We used interrupted time series (ITS) analysis27,28 with patient visit data to assess changes between the pre-screening intervention period and the screening intervention period. ITS is a quasi-experimental design which allows us to evaluate effects of the pragmatic trial in a real-world setting when a randomized controlled trial is not feasible. We measured rate of referral using an ITS analysis for single outcome series implemented by a SAS macro developed by Caswell. 29
Specifically, the Single-ITS Analysis (SITSA) model is a regression-based method with dummy variables created as predictors to represent the pre- and post-interruption or intervention phases of the series. 28 The analysis is represented by the following equation: , where y represents the outcome variable; α is the intercept; βs are the regression coefficients; T represents time (e.g. 1, 2, 3, …, N); X is study phase (e.g. 0 during pre-interruption and 1 during post-interruption); XT is time after interruption (0 during pre-interruption and 1, 2, 3, …, N during post-interruption); ε is error or residual. The coefficient (β1) for T indicates the pre-interruption slope. The primary coefficients of interest are β2 and β3 (for X and XT) which represent the change in referral rate from pre- to post-interruption and the change in slope of the trend from pre- to post-interruption, respectively. The post-interruption slope can be determined by summing coefficients β1 and β3 with statistical significance obtained using post-estimation procedures.30 The Durbin-Watson test was then utilized for identifying autocorrelation.31,32
RESULTS
From February 2017 to January 2019, we retrieved a total of 29,262 primary care visits to the 36 participating PCPs from members ages 18–65 who were identified as male according to their electronic medical record data from the two sites. Table 1 shows the demographics of our combined sample and by each stage of the intervention. The average age at the primary care visit was 44 (SD=13.26). The majority of participants identified as Hispanic or Latino (39.73%), were currently employed (95.13%), and were covered by commercial health plan insurance (77.59%).
Table 1.
Patient Characteristics Across Subsamples.
| Full sample of the study period* (n = 29,262) |
Patients screened with SLIP screener (n = 1,225) |
Referral made based on SLIP screener (n = 22) |
Prescription filled after being referred (n = 17) |
|
|---|---|---|---|---|
| Age (Mean, SD) | 44 years (13.26) | 42 years (13.20) | 34 years (8.6) | 33 years (8.97) |
| 18–29 | 17.22% (5,039) | 21.55% (264) | 36.36% (8) | 35.29% (6) |
| 30–39 | 22.49% (6,582) | 24.24% (297) | 45.45% (10) | 52.94% (9) |
| 40–49 | 20.84% (6,098) | 21.71% (266) | 9.09% (2) | -- |
| 50–59 | 23.40% (6,846) | 19.43% (238) | 9.09% (2) | 11.76% (2) |
| 60–65 | 16.05% (4,697) | 13.06% (160) | -- | -- |
| Race/ethnicity | ||||
| Hispanic/Latino | 39.73% (11,179) | 41.99% (493) | 45.45% (10) | 58.82% (10) |
| White | 30.85% (8,680) | 30.07% (353) | 27.27% (6) | 23.53% (4) |
| Black/African American | 6.29% (1,771) | 5.62% (66) | 9.09% (2) | 11.76% (2) |
| Asian/Pacific Islander | 18.29% (5,148) | 18.06% (212) | 9.09% (2) | 5.88% (1) |
| Mixed/other | 30.85% (8,680) | 4.26% (50) | 9.09% (2) | -- |
| Employment status | ||||
| Working | 95.13% (22544) | 95.76% (948) | 100% (16) | 100% (14) |
| Retired | 4.87% (1155) | 4.24% (42) | -- | -- |
| Missing (n) | (5563) | (235) | -- | -- |
| Insurance coverage | ||||
| Commercial | 77.59% (22,704) | 76.49% (937) | 72.73% (16) | 82.35% (14) |
| Medicare | 3.35% (980) | 2.37% (29) | -- | -- |
| Medicaid | 4.61% (1,349) | 5.47% (67) | -- | -- |
| Other | 1.51% (442) | 1.31% (16) | -- | -- |
| Out of pocket/no insurance | 12.94% (3,787) | 14.37% (176) | 27.27% (6) | 17.65% (3) |
Total number of primary care visits extracted from 36 PCPs during intervention period
Screening.
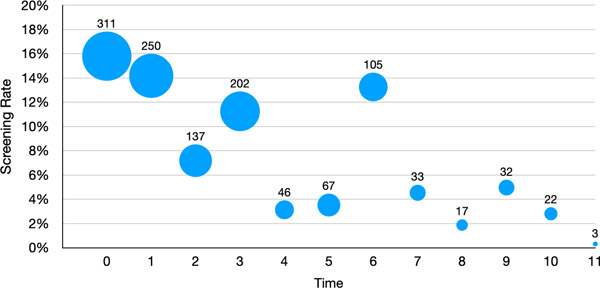
During the intervention period, a total of 1,225 patients were screened for PrEP in primary care with an evaluation in the infectious disease department, where PrEP is provided within the healthcare system. Figure 1 shows the screening rate since the intervention started where time = 0. The screening rate is defined by those visits where the PrEP screener was administered during a linked primary care visit (n=1,225) out of the total number of visits in each month since the intervention began. The bubbles represent the number of patients screened at each month. As shown, screening decreased over time after the launch of the intervention, with an increase again at the 6-month time point, when the procedure was changed from a nurse-administered screener to a self-administered screener provided to patients at check-in by the front desk staff (as described in Methods).
Figure 1:
SLIP Screening Rate and Number of Completed Screenings During the Intervention Period.
Note. Numbers above bubbles indicate the number of men screened at given timepoint. Bubbles are scaled to represent the number of men screened across time points.
Referral.
Overall, 132 patients received referrals for PrEP during the 24 month period (33 before the intervention and 99 during the intervention). Among the 99 patients who were referred after the intervention started, 22 were referred because they were identified as eligible based on their answers on the SLIP screener (22% of those referred). The other 77 were referred at the same period without being screened with the SLIP screener (78% of those referred). Among the 22 men who were referred based on the screening, 17 completed the referral visit and filled a PrEP prescription within 5 days (77% of those referred via screening). Most of these patients filled the PrEP prescription on the same day of the referral visits (n = 11; 65% of those who completed the referral visit), 4 filled the following day, 1 filled on the fourth day and 1 filled on the fifth day. The remaining 5 people who were eligible and received a referral for PrEP either did not make an appointment for the PrEP visit or attended the PrEP visit but did not fill the prescription (23% of those referred via SLIP screening).
Table 2 describes the referral rate across time points, before and during the intervention. During the first three months of the 12 month pre-implementation period, there were no referrals made for PrEP. There was a slight increase in the monthly number of patients being referred over the subsequent 9 months pre-intervention. One month after the intervention launch (time=1), we observed a rapid increase in the referral rate from about 0.51% to about 0.80%. After that time point, we observed a steady but gradual decrease in the referral rate until there was a second spike or rapid increase at the 6th month of the intervention (i.e. time = 5). It was at this month that we switched from having the nursing staff distribute the screener to having the front desk staff distribute the screener. We then again observed a gradual decrease in the referral rate in the following months from the highest point at 6th months (0.89%) to 0.50% in the following month.
Table 2:
Referral Rates by Month
| Phase | Time | Month | Patients referred (n) | Referral rate (%) |
|---|---|---|---|---|
| Pre-Intervention | −12 | 1 | 0 | 0.0% |
| −11 | 2 | 0 | 0.0% | |
| −10 | 3 | 0 | 0.0% | |
| −9 | 4 | 1 | 12.0% | |
| −8 | 5 | 0 | 0.0% | |
| −7 | 6 | 1 | 15.0% | |
| −6 | 7 | 6 | 47.0% | |
| −5 | 8 | 6 | 43.0% | |
| −4 | 9 | 9 | 61.0% | |
| −3 | 10 | 1 | 7.0% | |
| −2 | 11 | 5 | 35.0% | |
| −1 | 12 | 4 | 22.0% | |
| Intervention | 0 | 13 | 10 | 51.0% |
| 1 | 14 | 14 | 79.0% | |
| 2 | 15 | 15 | 78.0% | |
| 3 | 16 | 12 | 66.0% | |
| 4 | 17 | 8 | 54.0% | |
| 5 | 18 | 17 | 89.0% | |
| 6 | 19 | 4 | 50.0% | |
| 7 | 20 | 4 | 54.0% | |
| 8 | 21 | 5 | 54.0% | |
| 9 | 22 | 2 | 31.0% | |
| 10 | 23 | 4 | 50.0% | |
| 11 | 24 | 4 | 41.0% |
We first tested for autocorrelation among the data using the Durbin-Watson (DW) test. Test statistics indicated no statistical significance (p-values) <0.05 for tests of positive or negative autocorrelations. Therefore, we proceeded with ordinary least squares (OLS) regression using the ITSA SAS macro to estimate the SITSA regression coefficients (shown in Equation 1, Methods).
The SITSA model results, shown in Table 3, indicate a significant increasing pre-intervention trend (β1 = 0.04, p = 0.02) before the screener was implemented at both sites. That is, the rate was increasing by about 0.04% per month, ranging from 0% to 0.61% with an average of 0.2% before the screening was implemented. After controlling for this pre-intervention trend, there was a significant overall level increase in rate of about 0.37% (β2 = 0.37, p = 0.01) that we observed in the post-intervention period. The referral rate went up to an average of 0.57%, ranging from 0.31% to 0.89%.
Table 3:
Estimated regression coefficients for Single Interrupted Time Series Analysis.
| Indicator | Equation Parameter | β Estimate | Standard error | t | p |
|---|---|---|---|---|---|
| Intercept | β 0 | −0.03 | 0.10 | −0.28 | 0.78 |
| Pre-interruption slope | β 1 | 0.04 | 0.01 | 2.62 | 0.02 |
| Change in referral rate from pre- to post-interruption | β 2 | 0.37 | 0.13 | 2.76 | 0.01 |
| Change in slope from pre- to post-interruption | β 3 | −0.06 | 0.02 | −3.31 | 0.00 |
| Post interruption slope | β1+ β3 | −0.03 | 0.01 | -- | 0.04 |
In addition to the overall increase in rate change, we also observed the change in slope (β3) from pre-intervention (β1 = 0.04) to post-intervention (β1+ β3 = −0.03; p =0.04). This indicated that the post-intervention trend is significantly lower by 0.06% than the pre-intervention period (β3= −0.06, p < 0.01). See Figure 2 for the superimposed trend lines on the observed referral rates.
Figure 2:
Referral Rates Across Pre-Intervention and Intervention Phases, Plotted with Linear Trend Lines.
DISCUSSION
Our analysis sought to understand whether the implementation of a PrEP screening tool in the primary care setting was acceptable to members and could increase screening and referral rates. This study fills an important gap in the HIV prevention landscape, providing evidence for the capacity of health care systems to integrate routine sexual risk assessments into the existing workflows which has large implications for promoting adoption across similar health systems.
Our findings suggest that a standardized HIV risk screening administered to men in a busy primary care clinic can significantly increase the referral rate for a PrEP follow-up visit. Additionally, the majority of men who were referred for a PrEP visit attended the visit and filled their PrEP prescription the same day, or within 5 days of the visit. When the intervention launched, the PrEP referral rate increased dramatically from the prior month (about 29 percentage points). The referral rate remained high for several months, then slowly regressed over time. This may represent the decay of intervention effects over time seen in some primary care screening and referral interventions.33,34 One recent study even found that primary care providers’ orders for breast and colorectal cancer declined over the course of the day, potentially due to decision fatigue as the clinical day wore on.35
When we adjusted the protocol in an attempt to increase the screening rate, such that the front desk distributed the screener to incoming male patients instead of the nursing staff, the referral rate jumped about 35 percentage points. Within about one month, the referral rate returned to a level similar to that immediately before the switch from nurse to receptionist distribution of the screening tool. Regardless of nurse-distribution or receptionist-distribution, we observed regression over time in the screening and referral rates. This suggests a potential need for ongoing “booster” efforts to maintain a newly-implemented screening program, which could consist of additional trainings, internal messaging (e.g., via email or signage), recognition and support from managers and supervisors, staff champions, or patient-facing messaging (e.g., in the waiting room) instructing patients to ask their provider if they have not received a screener.
It important to note that the implementation of the screeners was not uniform across the primary care clinics in these settings. Primary care clinics at KPSC are split into modules which were selected for participation based on having sizable adult male (non-geriatric) populations. As a result, the nurses and staff who rotated between modules were unlikely to have screened at the same rates as nurses and staff who primarily stationed in implementation modules during project training and rollout. There may have been higher screening rates and sustainability of screening if implementation was adopted throughout primary care and if all staff were held accountable for screening.
This study was also a pilot, using paper screeners which were not entered directly into electronic health records and were thus likely to get overwhelmed by other intake paperwork (e.g., HIPAA forms, consent forms) and also may not have always been collected. Future efforts could examine the integration of an electronic PrEP screening questionnaire. Electronic versions of the screenings instrument could ensure more consistent administration of the screener, and also that a completed screener is linked directed to the patient’s electronic record. These electronic screenings could be administered at the time of check-in (e.g., via check-in tablet), or before arrival at the clinic via patient portals, alongside upcoming appointment reminders. Electronic screenings would also facilitate routine administration, for example, on a yearly basis and could be integrated within a comprehensive sexual health screener for all gender patients. For patients who have not sought office-based care, electronic screenings offered through patient portals could also be used to initiate a clinical visit for persons with potential PrEP indications. These are all areas for future research.
We note that as a whole, the general primary care population is at low risk for HIV, with small subpopulations therein (e.g., MSM) at substantially higher risk for HIV. Therefore, it is not surprising that screening and referral numbers were generally low for this pilot in two urban primary care clinics. Among high risk populations within the primary care setting, a portion of those persons may already be HIV-positive (and thus PrEP would not be applicable) or already taking PrEP. The study was conducted in a large, urban health system where a significant proportion of those at HIV risk were likely already aware of or were taking PrEP. For example, research indicates that 95% of PrEP users are male.8 PrEP uptake rates among California Medi-Cal (Medicaid) beneficiaries have also grown substantially in recent years, and utilization is higher in urban areas, such as Los Angeles.36 This also corroborates our finding that PrEP referral rates were gradually increasing in the pre-implementation phase of the study. This may reflect greater provider awareness of PrEP and willingness to make referrals, as well as patient-initiated requests among men who self-identified themselves as candidates for the PrEP and sought out a prescription.
This study was among the first to introduce a standardized PrEP screening instrument into a busy primary care setting to encourage referrals for PrEP as indicated. We also note that the majority of men at each of the three stages (completed the screener; received a referral; filled a PrEP prescription) were racial and ethnic minority men, particularly Black and Hispanic/Latino men, in line with the general distribution of the overall patient population at the clinics during the study period. Black and Hispanic men, particularly, MSM, experience a disproportionate burden of the HIV epidemic in the U.S.37 Our findings are suggestive of the feasibility and acceptability of primary care PrEP screening and referral for Black and Hispanic/Latino men, who experience lower rates of PrEP conversations with their health care providers and lower rates of actual PrEP use.8,9,21 Conversely, if we had found that a disproportionate proportion of non-Hispanic White men had received screening, referral, and PrEP prescriptions, this would have suggested a need to further research the factors inhibiting the intervention’s reach to Black and Hispanic/Latino men. Future research should assess the feasibility, acceptability and impacts of primary care-based PrEP screening and referral.
Limitations.
Findings from this pilot study should be interpreted with consideration of its limitations. First, this study sought to pilot the acceptability and integration in the workflow of a PrEP screening and referral process among a limited number of modules in two primary care clinics, and therefore is a relatively small population within a large, urban health system. Because this was not a randomized controlled trial, we used ITS analysis to account for natural variation due to the effect of time on PrEP referrals. Further, to reduce the overall burden of screening for busy primary care staff and because we suspected that the overall eligibility level would be lower for women, we piloted the screener with men between the ages of 18–65 only, and our findings should not be generalized to other groups, including adolescent males under the age of 18, males older than 65, or cisgender women. Further work is warranted to assess the overall impact of routine screening for persons of all ages and genders for PrEP in primary care, including nonbinary and transgender populations. Finally, we were unable to run separate analyses for the two primary care sites due to small cell sizes and accompanying large standard error estimates.
Project SLIP was the first step in a body of work that seeks to transform sexual health screening in primary care settings from “rare and uncomfortable” to “routine and replicable.” As access is scaled up, an additional area for future research is the maintenance or persistence of PrEP use among men who have received a prescription.4 Prior research has found varying levels of PrEP retention among PrEP users in the primary care setting, and there is a need for ongoing support and intervention to address these varying patterns of retention and PrEP adherence.38 When long-acting injectable and/or implantable forms of PrEP become available options, this may eliminate barriers to daily pill adherence, but patients will still need to return to the health care setting for additional doses to maintain persistence. The screening and referral protocol piloted here may also have utility for increasing access to, and uptake of, long-acting PrEP.
Acknowledgments
Funding Sources: This pilot was supported by National Institutes of Health award R03DA043402 (PI: Storholm). Dr. Storholm, Siconolfi, and Huang were also supported by National Institutes of Health award R21DA044073 (PI: Storholm) and Dr. Storholm was also supported by National Institutes of Health award P30MH058107 (PI: Shoptaw).
Footnotes
Conflict of Interest: All authors declare that no conflicts of interest exist.
Compliance with Ethical Standards:
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal-and Community-based sexual health services. JAMA Internal Medicine. 2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack S, Dunn D. Pragmatic open-label randomised trial of preexposure prophylaxis: the PROUD study. Paper presented at: Conference on retroviruses and opportunistic infections (CROI)2015. [Google Scholar]
- 3.Molina JM, Capitant C, Spire B, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. New England Journal of Medicine. 2015;373(23):2237–2246. [DOI] [PubMed] [Google Scholar]
- 4.Mayer KH, Allan-Blitz LT. PrEP 1.0 and Beyond: Optimizing a Biobehavioral Intervention. J Acquir Immune Defic Syndr. 2019;82 Suppl 2:S113–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson PL, Liu A, Buchbinder S, et al. Intracellular tenofovir-diphosphate (TFV-DP) concentrations associated with PrEP efficacy in men who have sex with men (MSM) from iPrEx. 19th Conference on Retroviruses and Opportunistics Infections; 2012. [Google Scholar]
- 6.Zablotska IB, O’Connor CC. Preexposure Prophylaxis of HIV Infection: the Role of Clinical Practices in Ending the HIV Epidemic. Curr HIV/AIDS Rep. 2017;14(6):201–210. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, McMahon J, Fiscella K, et al. HIV Pre-Exposure Prophylaxis Implementation Cascade Among Health Care Professionals in the United States: Implications from a Systematic Review and Meta-Analysis. AIDS Patient Care STDS. 2019;33(12):507–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YA, Zhu W, Smith DK, Harris N, Hoover KW. HIV Preexposure Prophylaxis, by Race and Ethnicity - United States, 2014–2016. MMWR Morb Mortal Wkly Rep. 2018;67(41):1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanny D, Jeffries WLt, Chapin-Bardales J, et al. Racial/Ethnic Disparities in HIV Preexposure Prophylaxis Among Men Who Have Sex with Men - 23 Urban Areas, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(37):801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28(12):850–857 e859. [DOI] [PubMed] [Google Scholar]
- 11.Hillis A, Germain J, Hope V, McVeigh J, Van Hout MC. Pre-exposure Prophylaxis (PrEP) for HIV Prevention Among Men Who Have Sex with Men (MSM): A Scoping Review on PrEP Service Delivery and Programming. AIDS Behav. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krakower DS, Mayer KH. The role of healthcare providers in the roll out of preexposure prophylaxis. Curr Opin HIV AIDS. 2016;11(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto RM, Lacombe-Duncan A, Kay ES, Berringer KR. Expanding Knowledge About Implementation of Pre-exposure Prophylaxis (PrEP): A Methodological Review. AIDS Behav. 2019;23(10):2761–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelman EJ, Moore BA, Calabrese SK, et al. Preferences for implementation of HIV pre-exposure prophylaxis (PrEP): Results from a survey of primary care providers. Prev Med Rep. 2020;17:101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silapaswan A, Krakower D, Mayer KH. Pre-Exposure Prophylaxis: A Narrative Review of Provider Behavior and Interventions to Increase PrEP Implementation in Primary Care. J Gen Intern Med. 2017;32(2):192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bien CH, Patel VV, Blackstock OJ, Felsen UR. Reaching Key Populations: PrEP Uptake in an Urban Health Care System in the Bronx, New York. AIDS Behav. 2017;21(5):1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimiaga MJ, White JM, Krakower DS, Biello KB, Mayer KH. Suboptimal awareness and comprehension of published preexposure prophylaxis efficacy results among physicians in Massachusetts. AIDS Care. 2014;26(6):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumenthal J, Jain S, Krakower D, et al. Knowledge is Power! Increased Provider Knowledge Scores Regarding Pre-exposure Prophylaxis (PrEP) are Associated with Higher Rates of PrEP Prescription and Future Intent to Prescribe PrEP. AIDS Behav. 2015;19(5):802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto RM, Berringer KR, Melendez R, Mmeje O. Improving PrEP Implementation Through Multilevel Interventions: A Synthesis of the Literature. AIDS Behav. 2018;22(11):3681–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henny KD, Duke CC, Geter A, et al. HIV-Related Training and Correlates of Knowledge, HIV Screening and Prescribing of nPEP and PrEP Among Primary Care Providers in Southeast United States, 2017. AIDS Behav. 2019;23(11):2926–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabrese SK, Krakower DS, Mayer KH. Integrating HIV Preexposure Prophylaxis (PrEP) Into Routine Preventive Health Care to Avoid Exacerbating Disparities. Am J Public Health. 2017;107(12):1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamitani E, Johnson WD, Wichser ME, Adegbite AH, Mullins MM, Sipe TA. Growth in Proportion and Disparities of HIV PrEP Use Among Key Populations Identified in the United States National Goals: Systematic Review and Meta-analysis of Published Surveys. J Acquir Immune Defic Syndr. 2020;84(4):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petroll AE, Walsh JL, Owczarzak JL, McAuliffe TL, Bogart LM, Kelly JA. PrEP Awareness, Familiarity, Comfort, and Prescribing Experience among US Primary Care Providers and HIV Specialists. AIDS Behav. 2017;21(5):1256–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karris MY, Beekmann SE, Mehta SR, Anderson CM, Polgreen PM. Are We Prepped for Preexposure Prophylaxis (PrEP)? Provider Opinions on the Real-World Use of PrEP in the United States and Canada. Clinical Infectious Diseases. 2014;58(5):704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mimiaga MJ, White JM, Krakower DS, Biello KB, Mayer KH. Suboptimal awareness and comprehension of published preexposure prophylaxis efficacy results among physicians in Massachusetts. AIDS Care-Psychological and Socio-Medical Aspects of AIDS/HIV. 2014;26(6):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hess KL, Johnson SD, Hu X, et al. Diagnoses of HIV infection in the United States and dependent areas, 2017. 2018.
- 27.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penfold RB, Zhang F. Use of Interrupted Time Series Analysis in Evaluating Health Care Quality Improvements. Academic Pediatrics. 2013;13(6):S38–S44. [DOI] [PubMed] [Google Scholar]
- 29.Caswell JM. Interrupted Time Series Analysis for Single Series and Comparative Designs: A Guide for Beginners with SAS Macro.
- 30.Linden A Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata Journal. 2015;15(2):480–500. [Google Scholar]
- 31.Durbin J, Watson GS. Testing for serial correlation in least squares regression. I. Biometrika. 1950;37(3–4):409–428. [PubMed] [Google Scholar]
- 32.Durbin J, Watson GS. Testing for serial correlation in least squares regression. III. Biometrika. 1971;58(1):1–19. [PubMed] [Google Scholar]
- 33.Carlfjord S, Lindberg M, Andersson A. Sustained use of a tool for lifestyle intervention implemented in primary health care: a 2-year follow-up. J Eval Clin Pract. 2013;19(2):327–334. [DOI] [PubMed] [Google Scholar]
- 34.Wentworth AL, Fox CH, Kahn LS, Glaser K, Cadzow R. Two years after a quality improvement intervention for chronic kidney disease care in a primary care office. Am J Med Qual. 2011;26(3):200–205. [DOI] [PubMed] [Google Scholar]
- 35.Hsiang EY, Mehta SJ, Small DS, et al. Association of Primary Care Clinic Appointment Time With Clinician Ordering and Patient Completion of Breast and Colorectal Cancer Screening. JAMA Netw Open. 2019;2(5):e193403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harawa N, McBride S, Leibowitz A, Pulsipher C, Holloway I. Examining PrEP Uptake among Medi-Cal Beneficiaries in California: Differences by Age, Gender, Race/Ethnicity and Geographic Region. California HIV/AIDS Policy Research Centers;2018. https://www.californiaaidsresearch.org/files/PrEP_Brief_2.8.18.pdf.
- 37.U.S. Centers for Disease Control and Prevention. HIV in the United States and Dependent Areas. 2020; http://www.cdc.gov/hiv/statistics/basics/ataglance.html.
- 38.Spinelli MA, Scott HM, Vittinghoff E, et al. Missed Visits Associated With Future Preexposure Prophylaxis (PrEP) Discontinuation Among PrEP Users in a Municipal Primary Care Health Network. Open Forum Infect Dis. 2019;6(4):ofz101. [DOI] [PMC free article] [PubMed] [Google Scholar]